Abstract
The hippocampal local field potential (LFP) exhibits three major types of rhythms, theta, sharp wave-ripples and gamma. These rhythms are defined by their frequencies, have behavioral correlates in several species including rats and humans, and have been proposed to perform distinct functions in hippocampal memory processing. However, recent findings have challenged traditional views on these behavioral functions. Here I review our current understanding of the origins and mnemonic functions of hippocampal theta, sharp-wave ripples and gamma rhythms based on findings from rodent studies, and present an updated, synthesized view of their roles and interactions within the hippocampal network.
Introduction
Brain rhythms are periodically fluctuating waves of neuronal activity that are readily observed using local field potential (LFP) recordings. Such rhythms reflect the synchronized activity of large numbers of neurons because synchronous currents sum together to generate large-amplitude fluctuations in LFP, whereas non-synchronized currents do not sum together and thus remain too small to be detected. It is known that individual neurons are not capable of executing complex cognitive operations in isolation, and instead must form functional networks with other neurons1, and so synchronous neuronal activity is thought to be relevant to cognition.
One such cognitive operation, that requires coordination across multiple neurons, is memory. Memories are thought to be represented by distributed ensembles of neurons that are activated concurrently, a concept that can be traced back to Donald Hebb's cell assembly hypothesis2. Brain rhythms are thought to play a key role in memory formation by synchronizing, and thereby coordinating, the activity of distributed neurons during memory operations.
The hippocampus is essential for spatial and episodic memory3 and thus is an ideal region in which to investigate how brain rhythms affect memory operations. In addition, several features of the hippocampus facilitate the study of brain rhythms. The hippocampus contains densely packed neurons that generate large LFPs and, in turn, generate large rhythms. This is especially true for area CA1, because the pyramidal cell dendrites are aligned in parallel. Because of this, synaptic currents flow in the same direction and sum together to produce large amplitude signals that are easily detected in LFP recordings. Accordingly, many studies of the role of brain rhythms in memory have been conducted in the hippocampus.
Another reason why the hippocampus is an excellent model system for studying brain rhythms is because much is known about hippocampal interneurons4. Interneurons are important for rhythm generation because they are able to synchronize activity across large groups of neurons via their highly divergent projections5. Pyramidal cells — the principal excitatory neurons of the hippocampus, many of which exhibit receptive fields for particular locations in space and thus are termed place cells6,7 — are also both modulated by, and participate in the generation of, hippocampal rhythms.
The hippocampus exhibits different types of brain rhythms including theta rhythms8 (~4-12 Hz), sharp wave-ripple complexes9,10 (~110-250 Hz ripples superimposed on ~0.01-3 Hz sharp waves) and gamma rhythms9,11 (~25-100 Hz). Each type of rhythm is observed during particular behaviors, generated by specific mechanisms, and associated with characteristic neuronal firing properties. It is therefore not surprising that these different rhythms are thought to perform distinct functions. Theta rhythms are thought to allow the brain to take in and learn new information12, and sharp wave-ripples are thought to be responsible for stabilizing and consolidating memories13. There is less agreement in the field about the functional significance of gamma rhythms14. Other brain state-dependent rhythms, such as sleep spindles and slow oscillations, also influence hippocampal operations; these rhythms will not be discussed in depth here, but have been reviewed elsewhere15,16.
In this article, I will discuss new findings that have yielded insights and challenged assumptions about the role of hippocampal theta, sharp wave-ripple, and gamma rhythms in memory operations. These rhythms have been observed in humans and non-human primates, as well as lower mammals (BOX 1). However, many of the findings described here have been made in studies carried out in lower mammals, particularly rodents, in part because the measurement of brain rhythms in healthy human subjects within a deep-lying structure such as the hippocampus is difficult. I will discuss the mechanisms underlying hippocampal rhythm generation and provide an overview of what is known about their mnemonic functions.
Theta
Theta rhythms are relatively low frequency, sinusoidal waves that occur in all hippocampal subregions during active exploration and rapid eye movement (REM) sleep8,12. Theta rhythms were first discovered in rabbits17 and have since been recorded in many species including cats, rats, mice, bats, monkeys, and humans8,18-22. Theta rhythms also occur in many cortical and subcortical regions23-27. Theta rhythms have been the most widely studied rhythm in the rodent hippocampus, where high-amplitude theta is readily observed during locomotor behaviors8. The medial septum–diagonal band of Broca has long been thought to act as the pacemaker for theta rhythms since it was first shown that hippocampal theta is abolished by septal lesions18. These results have since been replicated many times, and many further details have been revealed regarding the role of the medial septum–diagonal band of Broca in hippocampal theta generation.
Mechanisms
The GABAergic cells of the medial septum (MS) that act as theta pacemakers target dentate gyrus, CA3, and CA1 interneurons28. These septal interneurons rhythmically disinhibit hippocampal pyramidal cells and thereby promote their theta rhythmic firing. Pacemaking MS interneurons are parvalbumin expressing (PV+), and also express hyperpolarization-activated cyclic nucleotide-gated (HCN) channels29,30 which are likely to promote their pacemaker properties31. However, the mechanisms underlying theta generation are more complicated than this simple summary implies. Septal interneurons terminate on more than one type of hippocampal interneuron28. In addition, distinct classes of hippocampal interneurons are phase-locked in their firing to different phases of the theta cycle32. A recent study of hippocampal interneurons in awake, behaving mice found that PV+ basket cells preferentially fire at an earlier theta phase (when place cells preferentially fire) than do somatostatin-positive (SOM+) oriens lacunosum-moleculare (OLM) interneurons33. These findings suggest that different types of interneurons in the hippocampus serve different functions at distinct phases of the theta cycle.
In support of this view are findings from a study in which different interneuron classes were silenced in head-fixed mice as they ran on a treadmill containing visual and tactile cues34. Silencing of PV+ interneurons, which are comprised largely ofperisomatic-targeting interneurons, increased place cell spiking in the early part of a cell's place field, which corresponds to late phases of theta35,36. By contrast, inhibition of SOM+ interneurons, which largely target dendrites, tended to increase place cell firing in the later part of the place field34, which corresponds to early phases of theta35,36. These findings suggest that PV+ interneurons play a role in selecting which place cells become active during theta. The findings also suggest that SOM+ interneurons could inhibit the activity of place cells as the animal reaches the end of their place fields, thereby allowing the network to update its representation of location according to ongoing behavior and current environmental cues.
Consistent with the findings that different types of interneurons fire at different theta phases, it has been shown that different types of interneuron mechanisms are involved in theta generation. First, theta rhythmic inhibition of interneurons is critical for theta rhythm generation, as described above. In addition, mice lacking functional GABA type A receptors (GABAARs) in PV+ interneurons display attenuated theta rhythms37. Another important interneuron-mediated mechanism of theta generation is the rhythmic suppression of pyramidal cell activity. In support of this mechanism, it was found that specifically silencing theta-modulated PV+ and SOM+ interneurons in behaving mice increased firing rates of place cells within their place fields34. In addition, place cells produced longer bursts of spikes when SOM+ interneurons were silenced34. Moreover, other studies have shown that theta-modulated interneurons not only inhibit spiking but also induce theta synchronized firing in pyramidal cells. In hippocampal slices, theta rhythmic activation of interneurons induced post-inhibitory rebound spiking, producing theta-synchronized firing across multiple pyramidal cells5. Consistent with these findings, it was shown in a recent study of PV+ interneurons in mice that were freely behaving in their home cage that theta rhythmic activation of PV+ interneurons induced rebound spiking and theta rhythmic firing in pyramidal cells38.
Non-GABAergic mechanisms are also involved in theta production. First, excitatory currents are essential during theta39,40. A study of head-fixed mice running on a cue-rich treadmill found that silencing interneurons did not affect place cells outside of their place fields34, showing that excitatory inputs are necessary to activate specific place cells within a theta cycle. Second, the intrinsic properties of pyramidal neurons that promote theta resonance may also play a role in theta production. For example, HCN1 is thought to facilitate theta rhythmic firing in neurons41,42, and HCN1 blockade reduced theta entrainment of pyramidal cell spikes induced by rhythmic activation of PV+ interneurons38. However, the precise role of HCN channels in theta production remains unclear because it has also been reported that enhanced theta rhythms occur in HCN1 knockout mice43. Last, cholinergic inputs from the MS also contribute to theta generation. Cholinergic projections from the MS are required for theta rhythms that occur while the animal is not engaged in active behaviors (termed atropine-sensitive theta)44. Cholinergic stimulation may provide excitation45 to the hippocampus during such inactive states, when movement-related inputs that drive atropoine-resistant theta44 are absent. Additionally, a major role for cholinergic inputs in theta generation may be the suppression of other rhythms that function in a manner antagonistic to theta, namely sharp wave-ripples46,47 (BOX 2).
Adding to the range of complex mechanisms known to contribute to theta generation is the discovery that theta rhythms can occur in the absence of MS pacemaker inputs, in a whole hippocampal in vitro preparation48. This finding suggests that theta can also emerge from local circuit interactions in the hippocampus. Nonetheless, it is clear that MS inputs are important for driving theta in behaving animals, as numerous studies have shown that MS lesions or inactivation disrupt theta generation18,49-52.
Functions
In the 1970s, several fundamental studies showed that the extent to which theta was present in an electroencephalogram predicted how quickly animals learned or how well they remembered53-55. These findings led to the theory that theta plays an essential role in learning and memory. Since then, much evidence for this theory has been provided by studies relating theta to mnemonic task performance and synaptic plasticity50,56-67. However, the theory has been called into question by a recent study51 involving recordings from place cells. Place fields emerge as animals gain experience with new environments68-70, and it is often assumed that the development of place cell representations corresponds to spatial learning. It was therefore surprising that this study51 showed that, in rats, place fields were formed when the animal was in novel environments despite theta rhythms and theta entrainment of spikes being blocked by septal inactivation. In addition, flying bats have place cells, even though these place cells exhibit little to no theta rhythmicity in their firing71. These findings demonstrate that theta rhythmicity is not required for the formation of place fields, and thus imply that theta is not required for the formation of spatial memory representations at the single cell level.
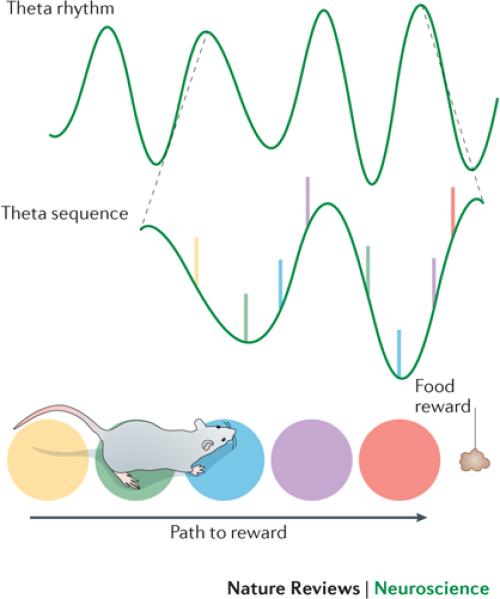
However, it is important to note that memory is a distributed process involving coordinated groups of neurons, not isolated neurons, and, accordingly, a number of studies suggest that theta is required for the formation of memories represented by neuronal ensembles. A recent study in rats showed that theta rhythm blockade by inactivation of the MS impaired performance on a delayed spatial alternation mnemonic task and disrupted organized ensembles of place cells that are activated in a specific order52. Such organized neuronal ensembles are termed “theta sequences”36,72,73 (FIG. 1), and this work suggested that theta sequences, rather than isolated place cells, are important for memory operations. In support of this view, theta sequences have been shown to be experience-dependent74 and to represent meaningful concepts that involve sequences of locations75,76 (such as the path to a reward). In addition, theta sequences do not simply reflect hard-wired connections in the hippocampal network, but rather they change dynamically according to the behavioral intentions of the animal76. These results suggest that theta rhythms link different cells together in functional ensembles that support memory operations by providing integrated representations of complex concepts and experiences.
Figure 1. Theta sequences in the hippocampus during spatial memory operations.
Successive locations in an animal's trajectory are represented by an ordered series of CA3 or CA1 place cell spikes within individual theta cycles, termed a theta sequence. Shown here is a schematic representation of a path to be taken by a rodent in order to reach a reward (bottom), an ongoing theta rhythm (top), and the theta sequence of place cells for two magnified theta cycles (middle), whereby the place cells are colour coded according to the particular point in the path at which their place field is located. For individual place cells within a theta sequence, spikes occur at progressively earlier theta phases across successive theta cycles, a phenomenon termed theta phase precession35. Because of this, spikes at early and late theta phases represent earlier and later locations, respectively, in the trajectory.
Theta may also integrate various types of sensory information received by the hippocampus — such as olfactory and visual input — by linking neurons that code different aspects of the same experience. Theta rhythms are well suited to coordinating multimodal sensory information because they correlate with movements used to acquire sensory stimuli, including sniffing and whisking56,77. These correlations may be important for the optimal intake of sensory information. For example, during an olfactory discrimination task, performance was poor when rats sniffed more slowly than theta frequency78. Moreover, theta rhythm in the hippocampus was not coupled to whisking when rats were merely whisking in air rather than actively sampling olfactory stimuli79. In addition, a recent study reported that saccade generation reset the phase of hippocampal theta in monkeys performing a visual memory task22, suggesting that saccades prime the hippocampus to take in visual information. Together, these results suggest that theta coordination of neuronal activity and active sampling of sensory stimuli produces an integrated representation of the current environment.
Furthermore, each theta cycle may represent a fundamental unit of information within an episodic memory. Support for this idea was provided by studies in rats showing that distinct representations of different environments80, or of the same environment but with the location of rewards changed81, were largely segregated across separate theta cycles. Similar theta segregation occurred across head direction-coding neuronal ensembles in the medial entorhinal cortex (MEC) and parasubiculum of rats82. Head direction cells that fired on the same theta cycles were tuned to similar head directions, whereas cells that fired on separate theta cycles preferred different head directions. Together, these studies suggest that spikes carrying related information are linked within a theta cycle, whereas dissimilar (and thus potentially conflicting) signals are segregated on different theta cycles.
The functions described above are associated with theta that occurs during active states. However, theta is also prominent in the hippocampus during REM sleep, albeit with a lower frequency and a different regional profile than theta that occurs during waking83. Interestingly, place cell spiking patterns that occur during active states have been shown to reoccur during theta during subsequent REM sleep, raising the possibility that REM sleep-associated theta may play a role in memory consolidation84.
Sharp wave-ripples
Sharp wave-ripples are large amplitude, irregularly occurring LFP patterns that are observed in animals during waking immobility and during slow wave sleep, as well as during consummatory behaviors10. These rhythms are largely restricted to the hippocampal network, although they are also observed in the entorhinal cortex85. Sharp wave-ripples are widely presumed to originate in the hippocampus, as they occur persistently in hippocampal slices46,86-88 and transplanted hippocampal grafts89 in which afferents to the hippocampus are missing. Here, I will focus on how mechanisms of generation of sharp wave-ripples relate to their purported mnemonic functions. For additional details on mechanisms of sharp wave-ripple generation, I refer readers to a recent comprehensive review90.
Mechanisms
Although sharp waves and ripples are coupled, they are thought to be separate events with distinct origins. Sharp waves are excitatory events that are transmitted from CA3 to CA110,91. By contrast, ripples are generated locally in CA1 by ripple-frequency spiking of perisomatically targeting basket cell interneurons92-94. It is unlikely that CA1 spiking during ripples is entrained by CA3, as CA1 place cells spikes are phase-locked to CA1 ripples, but CA3 spikes are not91,95. In addition, ripples are not coherent between CA3 and CA1; specifically, CA1 ripples are of higher frequency than CA3 ripples91,95.
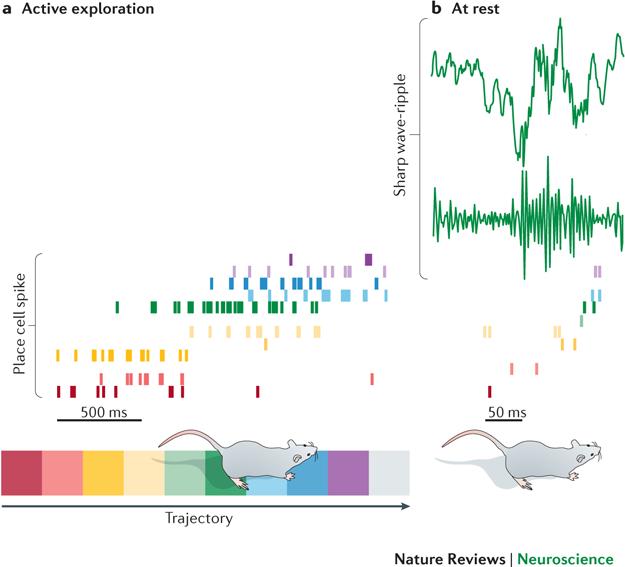
Nonetheless, sharp wave-associated spiking in CA3 is likely to affect CA1 cell firing during ripples, as shown by studies of place cell “replay” or “reactivation”. Replay refers to the phenomenon in which place cell firing patterns that occurred during active exploration are reactivated during subsequent sharp wave-ripples96-99 (FIG. 2). A study has investigated replay in CA3 and CA1 place cells of rats, when ripples were detected in CA1 during wakefulness100. The data from this study implies that CA3 and CA1 place cells concurrently replay the same spatial memories. Moreover, another study has found that suppression of CA3 inputs to CA1 in transgenic mice impaired ripple-associated reactivation of CA1 place cell pairs101. Consistent with the idea that ripples are generated locally, the rate of ripple occurrence in CA1 did not change significantly when CA3 inputs were suppressed, although ripple frequency was lower.
Figure 2. Replay during sharp wave-ripples.
a| Shown here are spikes from successively activated place cells (top) as a rodent passes through the cells’ place fields in a particular trajectory on a linear track (bottom). Each row of colored tick marks represents spikes from a different place cell (calibration: 500 ms). b| Shown here is an example of a sharp wave-ripple (top), recorded during subsequent rest at the end of the linear track; a bandpass filtered (150-300 Hz) version of the sharp wave-ripple is shown immediately below the raw recording. Spikes from the place cell ensemble are shown to reactivate during the sharp wave-ripple in the same order as in exploration but on a faster time scale. Calibration: 50 ms.
In addition, CA1 ripple frequency in sleeping rats has been found to positively correlate with sharp wave magnitude91, suggesting that stronger excitation from CA3 produces faster ripples in CA1. In this study, CA1 cell firing was phase-locked to ripples during sleep91, and thus faster ripples would be expected to promote shorter interspike intervals in CA1. This could allow CA1 to send a more powerful output to its downstream targets. In support of this idea, hippocampal ripple activity has been reported to peak before neocortical spindles peak102,103. Thus, ripples could facilitate transfer of memories to neocortex during memory consolidation, which is a commonly hypothesized function of sharp wave-ripples96-99,101,104-107. However, another study reported that neocortical spindles precede hippocampal ripples, suggesting that neocortical projections also influence the content of hippocampal ripples108.
Mechanisms of ripple formation also support the idea that ripples have a memory consolidation function. During sharp wave-ripples, CA1 pyramidal cells are not only depolarized by sharp waves but also receive strong shunting inhibition from ripples109. This shunting inhibition raises spike thresholds and prevents most cells from firing. This suggests that ripples may serve to select only the most strongly encoded memories for consolidation. That is, only the cells that receive sufficient excitation, which are likely to be those with synapses that were potentiated during earlier learning, would be able to overcome this inhibition and send their memory traces to long-term storage. While memories are being transferred to neocortical storage sites, the neural mechanisms encoding the memories may be depotentiated in synapses between CA3 and CA1. In support of this notion, long-term potentiation at the Schaffer collateral synapse was found to decay over time in hippocampal slices that exhibited sharp wave-ripples87. In addition, reactivation of events in CA1 does not increase, but rather decreases, over the course of slow wave sleep following the events96. Moreover, experience-dependent plasticity in rat place cells has been shown to persist for days in CA3 but disappear overnight in CA1110.
Functions
Any hypothesized functions of sharp wave-ripples must be consistent with the unconscious nature of slow-wave sleep, the state during which sharp wave-ripples most often occur10. Accordingly, sharp wave-ripples have not been thought to be involved in the active sampling of incoming sensory information and the corresponding encoding of memories of these sensory experiences. Instead, sharp wave-ripples have been traditionally hypothesized to perform off-line mnemonic functions, including memory consolidation96-99,101-107,111 and erasure of hippocampal memory traces87,112.
However, recent evidence suggests that sharp wave-ripples play a key role in certain aspects of active spatial navigation111,113,114. At times, place cells must convey representations of locations that are distinct from an animal's current location. During such times, place cells cannot simply respond to inputs driven by stimuli in the immediate environment but instead must be activated by another form of excitatory input. Sharp waves have long been recognized as excitatory events10, and recent intracellular recordings from behaving mice confirmed the presence of depolarizing events in pyramidal cells during sharp waves109. Moreover, in rats performing spatial memory tasks, place cells have been shown to represent locations distinct from the animal's current location, while sharp waves are occurring between bouts of active navigation99,115. Thus, sharp waves are likely to provide the excitation that allows place cells to fire outside of their place fields, during waking behaviors that require representations of distant locations.
Planning of future trajectories requires the internal representation of maps of distant locations, and new evidence indicates that sharp wave-ripples are important for retrieving such maps. One recent study found that blockade of sharp wave-ripples in rats that were learning a spatial memory task impaired task performance, suggesting that sharp wave-ripples in awake animals are involved in memory-guided trajectory planning114. Another study examined sharp wave-ripples during a spatial memory task in which rats foraged for a randomly placed reward and then returned to a predictable goal location116. During rest periods of the task, sequences of place cell spikes during sharp wave-ripples were found to represent paths towards the goals. Importantly, paths represented during sharp wave-ripples matched the paths that were subsequently taken to reach the goals. Another recent study investigated sharp wave-ripples in rats that were learning a spatial alternation maze117. Sharp wave-related spiking was examined during periods when rats were deciding which path to take. As task performance improved, it was found that pairs of place cells representing maze locations were more likely to co-activate during sharp waves that occurred before correct choices were made than during sharp waves that occurred before incorrect choices were made.
It is likely that spatial route planning is not the only waking function in which sharp wave-ripples play a role. It is possible that sharp wave-ripples emerge when the hippocampal network engages in intrinsically driven processes, but not during processes driven by the immediate sensory environment. Such intrinsic operations may include memory retrieval100,113, envisioning the future (such as in trajectory planning, as discussed above), or imagining experiences that have never happened. With respect to the latter example, it has been shown that place cell sequences during sharp wave-ripples can represent paths that were not previously experienced or subsequently traversed115. The temporal compression of spatial memories during sharp wave-ripples97,98,118 is consistent with this set of functions, because memories are retrieved (or imagined) on a faster time scale than the time scale in which they were experienced113.
However, it is important to note that current sensory stimuli can nonetheless influence which place cells fire during sharp wave-ripples. For example, in rats, paths that are represented by place cell sequences in both forward and reverse order during waking sharp waves often begin at or near an animal's current location115,118-120. Moreover, sensory stimuli can bias selection of the place cell ensembles that fire during sharp waves while the animal is sleeping. In a recent study it was found that in rats performing an auditory-spatial association task, auditory cues were associated with particular place cell firing patterns during waking121. The subsequent presentation of these auditory cues during non-REM sleep led to preferential reactivation of the place cell ensembles that were activated by the cues during earlier learning.
Gamma
Gamma rhythms are recorded in the hippocampus during a variety of behaviors but are of a lower amplitude than the theta rhythms and sharp waves with which they occur9,122. Perhaps for this reason, gamma rhythms have received less experimental attention than theta rhythms and sharp wave-ripples, at least in vivo. The high variability of gamma frequency11,123 also complicates its measurement in behaving animals’
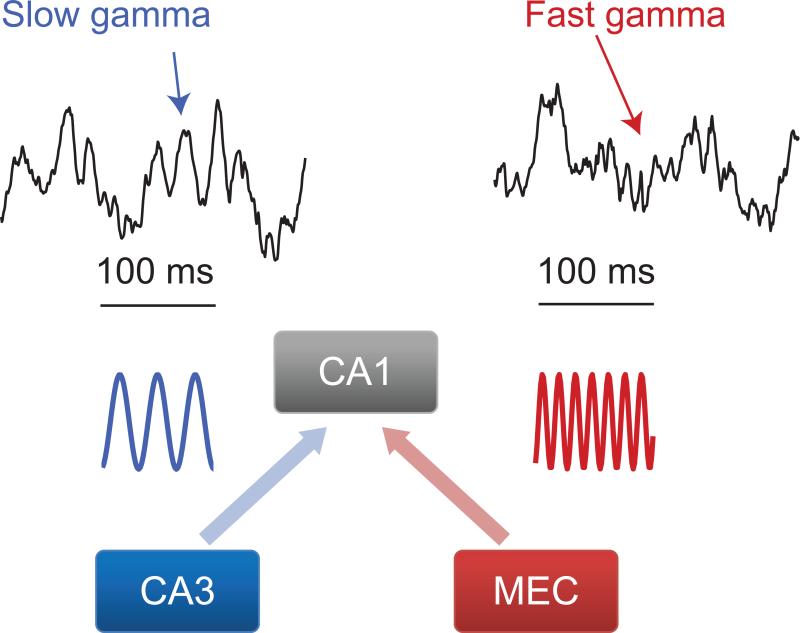
Recently, multiple researchers have begun to agree that rhythmic activity across the broad range of frequencies that are defined as gamma (~25-100 Hz) in CA1 actually encompasses more than one type of brain rhythm (FIG. 3). Activity in the lower end of the frequency range (~25-55 Hz) has been termed “low gamma” or “slow gamma” and is driven by CA3123-126. A second type of gamma rhythm exhibits a higher range of frequencies (~60-100 Hz) and is entrained by inputs from MEC123-126; this type of activity has been termed “fast gamma” in some studies123,125 and “midfrequency gamma” in others124,126 (the former terminology will be used here). Gamma rhythms also occur in many other brain regions besides the hippocampus, with similar gamma subtypes exhibiting low and high frequencies127-131.
Figure 3. Slow and fast gamma rhythms.
Two distinct subtypes of gamma rhythms, termed slow and fast gamma, occur in the hippocampal network. Slow gamma rhythms couple activity in hippocampal subregion CA1 to inputs from CA3. By contrast, fast gamma rhythms in CA1 are entrained by inputs from MEC. Typically, the two subtypes of gamma rhythm do not occur at the same time within CA1123. Slow and fast gamma rhythms are also observed in CA3, where fast gamma rhythms are also entrained by inputs from MEC123. Examples of slow and fast gamma rhythms recorded from CA1 in a freely behaving rat are shown at the top (calibration: 100 ms) in order to demonstrate their somewhat irregular appearance, while stylized versions of the rhythms are shown below.
Mechanisms
There is much evidence to indicate that inhibitory interneurons are critical for gamma generation14,132. Gamma rhythms in the LFP of rats have been shown to reflect inhibitory events recorded intracellularly in CA3 and CA1 pyramidal cells133 and in dentate gyrus granule cells40. In addition, interneuron spikes consistently phase-lock to gamma oscillations, whereas pyramidal cell spikes do not9,122-124,134,135. Moreover, CA1 basket cells recorded intracellularly in anesthetized rats were found to fire bursts of action potentials at gamma frequencies that were locked to gamma rhythms in the LFP136. Gamma phase-locked firing has been observed in several types of hippocampal interneurons including axo-axonic cells, bistratified cells, PV+ basket cells, and cholecystokinin-expressing (CCK+) cells134. Together, these findings suggest that gamma-frequency spiking of fast-spiking interneurons produces the observed gamma rhythms.
Gamma amplitude in freely behaving rats is largest during theta states122, and gamma-generating mechanisms can also be modulated by theta; that is, gamma amplitude11,123,133,136 and gamma phase124,137 are coupled to theta phase. Theta phase modulation of gamma amplitude may involve inhibition of gamma-generating interneurons at a particular theta phase. In support of this idea, theta phase–gamma amplitude coupling was decreased when GABA-A mediated conductances in PV+ interneurons were blocked in freely moving transgenic mice37. Gamma-generating circuits are also likely to be influenced by theta-modulated excitatory inputs. Theta–gamma phase-phase locking, which has been observed in rats during both running- and REM-associated theta124, is consistent with the notion that excitation at a particular theta phase triggers the onset of gamma by inducing gamma-frequency bursting in interneurons.
It should be noted that most of the studies described above did not differentiate between different frequency variants of gamma, and it is possible that slow and fast gamma are generated by different mechanisms. Recordings in freely exploring rats have shown that slow and fast gamma rhythms of CA1 exhibit phase synchrony with CA3 and MEC, respectively123. Accordingly, different gamma frequencies have been shown to be prevalent in different layers of CA1 in rats performing spatial navigation tasks126. Specifically, slow gamma (~30-80 Hz) activity dominated in stratum radiatum, whereas ~60-100 Hz gamma dominated in stratum lacunosum-moleculare. In addition, different CA1 interneurons have been found to be preferentially modulated by different frequencies of gamma in both behaving and anesthetized rats126,138. These findings suggest that CA3-activated interneurons drive slow gamma, and MEC-activated interneurons drive fast gamma. In further support of this, fast gamma rhythms were reduced when the MEC projection to CA1 was blocked in mice performing a spatial memory task139. Moreover, in mouse brain slices, optogenetic stimulation of MEC at theta frequency generated bursts of fast gamma oscillations that corresponded to fast gamma spiking in MEC interneurons140.
Nonetheless, it has been shown that a substantial proportion of CA1 interneurons phase-lock to both slow and fast gamma123,126. Such interneurons may be part of a network that can generate either slow or fast gamma, depending on the state of the network. Theoretical work has shown that the frequency of gamma rhythms generated by networks of hippocampal interneurons can vary depending on the strength of excitatory and inhibitory inputs141. Specifically, in simulations of gamma activity in a model neuron network, gamma frequency was found to increase with increased excitatory drive onto the interneurons, and was found to decrease with increased GABA conductances in the interneurons. The increased excitatory drive onto interneurons needed to produce fast gamma may be provided by increased spiking in MEC projection neurons. In support of this idea, both spiking activity in MEC neurons in rats and mice142-144 and the power of fast gamma in rats125,142,145 have been shown to increase with increased running speed. However, a different mechanism may promote slow gamma firing in gamma-generating interneurons. One possibility is that a certain class of interneurons projects to gamma-generating interneurons, thereby increasing their GABA conductances and reducing their spike rates to slow gamma frequencies. However, these hypotheses are tentative, and much work remains to be done to uncover mechanisms underlying slow and fast gamma rhythms.
Functions
Recently, several studies have begun to address the question of whether slow and fast gamma perform different functions, and a few main hypotheses have emerged from these studies. Evidence for and against each of these hypotheses is discussed below.
The entorhinal cortex processes sensory information and transmits this information to the hippocampus146, and fast gamma is driven by inputs from MEC in rats and mice123-126,139. Therefore one plausible function for fast gamma is the encoding of current sensory information in memory, and some results from recent studies are consistent with such a function. In one study, systemic administration of scopolamine, a muscarinic antagonist that blocks memory encoding, was shown to reduce fast gamma in rats running on a circular track147. It has also been shown that in rats running on a linear track, place cells tended to encode recent locations and ongoing trajectories during fast gamma, rather than predicting future locations137,148. In addition, several studies have linked changes in fast gamma to variations in ongoing behavior. Studies have shown that in rats, fast gamma frequency increases as a function of running speed during random foraging, performance of a spatial alternation task, or running on a linear track142,145. This may allow spatial representations within gamma cycles to transition more quickly across successive locations as running speeds increase. In addition, fast gamma power increased relative to slow gamma power when mice used cues in the external environment to navigate toward the location of a reward149. In another study, fast gamma power was found to be high when rats attended to stimuli that signaled the beginning of a trial in a spatial alternation task150. However, other results do not support the hypothesis that fast gamma has a memory encoding function. For example, a recent study has found that fast gamma is linked to working memory, but not memory encoding, in mice performing a delayed nonmatching-to-place task139.
Another plausible hypothesis is that slow gamma promotes memory retrieval. Memories are thought to be stored in and retrieved from the CA3 network151-154, and it is known that slow gamma rhythms in CA1 of behaving rats are entrained by inputs from CA3123-126. It has been shown in rats performing an associative memory based task that coupling between theta phase and slow gamma amplitude in CA3, at the time when memory retrieval was expected to occur, was correlated with task performance155. A subsequent study also found that coordination between slow gamma and theta was related to successful memory retrieval in rats156. In a recent study in which rats learned an odor-place association, ~20-40 Hz coupling between lateral entorhinal cortex (LEC) and CA1 was found to develop as animals learned the task157. The LEC-CA1 coupling was observed during the odor sampling period, which is likely to correspond to the time when animals recalled the place association, and was not seen during error trials. However, the observed ~20-40 Hz activity overlapped with both beta and slow gamma frequency bands and thus may reflect either beta (which has been reported in the rat hippocampus during odor sampling158), slow gamma, or a combination of both.
Recent findings from studies of place cell ensemble activity suggest that slow gamma facilitates the activation of previously stored representations of spatial sequences, providing further support for the hypothesis that slow gamma is involved in memory retrieval. In rats traversing a familiar linear track, place cell ensembles preferentially represented upcoming locations, rather than recent locations, during periods of slow gamma148. Additionally, sequences of locations were represented in a temporally compressed manner during slow gamma, which may help explain how events are experienced in real time but subsequently retrieved in a time-compressed form137. In another study, slow gamma coupling between CA3 and CA1 was observed during sharp wave-ripples in awake resting rats, and the fidelity of replay increased as this slow gamma coupling increased100. A subsequent study also investigated replay during awake rest in rats and found that representations of sequences of locations jumped from one discrete location to another across slow gamma cycles within sharp waves159. These findings suggest that slow gamma is important in memory retrieval, since replay is thought to reflect reactivation of memories of earlier experiences.
However, not all findings from studies of slow gamma fit with this memory retrieval hypothesis. Specifically, some studies have described novelty-related increases in slow gamma in rats125,160,161. One possible explanation for these observations is that slow gamma promotes interactions between CA3 and CA1 in general, and that these interactions are heightened when the animal is in novel environments. These apparent incongruities across studies highlight the need for further investigation into the functions of slow gamma rhythms.
Conclusions and future directions
Although findings from recent studies have provided many important insights into the functional significance and origins of hippocampal rhythms, some important questions remain. For one, what exactly is the functional significance of variations in theta frequency? Specifically, is there any functional significance to the lower frequency of theta that occurs during novel experiences compared to familiar ones?162 Furthermore, sharp wave-ripple replay often occurs in an order that is reversed relative to the order of cell activation from earlier behavior119,163, particularly for memories of recent experiences164. Why would memories be stored or consolidated in reverse order? Do forward and reverse replay serve different functions? Another critical gap in knowledge exists regarding the memory consolidation hypothesis of replay function. According to this hypothesis, replay serves to transfer memories from the hippocampus to the neocortex. However, it remains unknown whether coordinated sequence replay occurs between the hippocampus and its major downstream cortical target, the deep layers of the entorhinal cortex. Lastly, do different frequencies of gamma represent different functional states of the hippocampal network, as proposed above, and, if so, are different circuits involved in their generation or is the same circuitry simply entrained by different inputs?
Recent innovations in experimental methods provide us with the tools to address these and other important questions. For example, optogenetic activation of septal pacemaker interneurons will allow researchers to drive theta at particular frequencies in order to determine how memories are affected by theta frequency variations. In addition, researchers are now able to examine large numbers (that is, >100) of simultaneously recorded neurons across different hippocampal sites in rats159, which allows for better and more precise decoding of neuronal activation patterns. Experiments employing such high density recordings can determine whether coordinated replay occurs across different regions. Furthemore, optogenetic and pharmacogenetic techniques permit selective silencing of particular interneurons. Such manipulations should reveal whether slow and fast gamma rhythms are mechanistically distinct. If so, these same tools can then be used to selectively silence slow or fast gamma in order to shed light on their functions. Such results may pave the way toward novel treatments for diseases associated with aberrant rhythms165 (BOX 3).
BOX 1| Hippocampal rhythms in humans and non-human primates.
The study of hippocampal rhythms in healthy humans using non-invasive techniques is challenging because of the large distance between hippocampal sources (which are deep in the brain) and sensors (which are on or outside of the head), leading to questions of whether the same hippocampus rhythms occur in humans and rodents. However, findings from studies of patients undergoing neurosurgery that have had electrodes implanted directly in the hippocampus support the conclusion that hippocampal rhythms in humans share many similarities with those in rodents. Moreover, in non-human primates it is possible to implant depth electrodes, and thereby confirm the presence of oscillations in healthy primate hippocampi.
In human epilepsy patients, hippocampal theta occurs as a traveling wave166, as it does in rodents167,168, and is associated with movement. However, human theta recorded during virtual movement is lower in frequency, less sinusoidal, and less continuous20,169,170 than theta in freely moving rats. Short bouts of theta rhythms also occur in the monkey hippocampus and correlate with eye movements22. Studies of hippocampus in individuals with epilepsy have shown that sharp wave-ripples occur in the hippocampal network during rest and slow-wave sleep171, as in rats, and may promote memory consolidation172. Moreover, hippocampal ripples are coupled with neocortical spindles in human epilepsy patients103,173, as they are in rats102,108. In monkey hippocampus, sharp wave-ripples that appear very similar to their counterparts in rodents also occur during inactive and active states174,175. Finally, separate slow and fast gamma rhythms may occur in primates, as in rodents. In human epilepsy patients, lower and higher frequency gamma oscillations have been reported in the hippocampus, with the higher frequency variant associated with memory encoding176. In monkey hippocampus, it has been shown that neuronal spiking was coordinated by separate slow and fast gamma rhythms, and that coordination by fast gamma was associated with successful memory encoding177.
BOX 2| Interactions between different hippocampal rhythms.
Hipppocampal rhythms interact with each other in various ways. Sharp wave-ripples occur persistently in the absence of extrinsic input to the network46,86-89. As an animal shifts from inactive to active behaviors, extrinsic inputs, such as cholinergic inputs, may serve to suppress sharp wave-ripples10,46,47, allowing theta rhythms to predominate. Theta-rhythmic inhibitory events demarcate theta cycles, which correlate with actions such as sniffing and eye movements that serve to take in sensory information. In this way, each theta cycle may provide the hippocampus with a “snapshot” of the current environment as an animal actively samples external stimuli and the hippocampus encodes information about these stimuli. However, theta rhythms are also present during internal hippocampal operations such as the retrieval of previously stored memories64,66 and envisaging future events76. During such intrinsic hippocampal network operations, each theta cycle may activate a coordinated ensemble of cells that represents a previously stored memory or an anticipated future trajectory. The switch between extrinsically and intrinsically driven network operations may depend on which type of gamma rhythms are nested within the theta cycle, with fast gamma promoting extrinsic inputs from MEC and slow gamma promoting intrinsic signals from CA3. Consistent with the latter, slow gamma rhythms also occur during sharp wave-ripples100,148 suggesting that slow gamma may promote any state in which CA3 transmits information to CA1. For both fast and slow variants of gamma, the inhibitory events that comprise gamma cycles may serve to select those cells receiving the most excitatory input178, and thus carrying the most salient information at that time, while also filtering out noisy irrelevant inputs.
BOX 3| Abnormal brain rhythms in schizophrenia.
Deficits in neuronal synchrony have been reported in several human brain disorders, including schizophrenia and Alzheimer's disease165. Brain rhythm abnormalities have also been reported in rodent models of these diseases, suggesting that their underlying mechanisms can be discovered and treated. Rodent models of schizophrenia are particularly informative in this regard.
In the Df(16)A+/− mouse model of schizophrenia, hippocampal and medial prefrontal cortex (mPFC) theta rhythms were less correlated than in wild-type mice179. These mutant mice also showed working memory impairments that correlated with hippocampal-mPFC theta synchrony impairments. In another study, rats prenatally exposed to maternal immune activation180, a risk factor for schizophrenia, were found to have reduced theta and slow gamma synchrony between hippocampus and mPFC, and these slow gamma deficits correlated with deficits in sensorimotor gating. Another study used a rat model of schizophrenia that induces schizophrenia-like cognitive deficits by lesion of the ventral hippocampus181. The rats were trained on a slowly rotating arena on which they had to learn to avoid a shock zone that was defined according to distal room cues, not local arena cues. Successful performance of this task requires the animals to attend to relevant stimuli while ignoring irrelevant stimuli, and it is known that such attentional functions are impaired in schizophrenia. Performance on this task was impaired in the rat model of schizophrenia, and theta oscillations were less coordinated across the hippocampus in rats that showed impaired task performance. Remarkably, deficits in both task performance and hippocampal synchrony were prevented by pre-training rats on the task during adolescence.
Abnormalities in sharp wave-ripples may also occur in individuals with schizophrenia, based on evidence from animal models. Animal models that exhibit neuronal deficits (for example, PV+ neuron dysfunction) or behavioral abnormalities associated with schizophrenia display augmented ripples and impaired reactivation182,183. Together, these findings suggest that oscillatory coordination in the hippocampal network is disrupted in schizophrenia and that treatments that increase oscillatory coordination may alleviate cognitive deficits.
Online Summary.
The hippocampus exhibits three main classes of rhythms: theta (~4-12 Hz), sharp-wave ripples (~150-200 Hz ripples superimposed on ~0.01-3 Hz sharp waves) and gamma (~25-100 Hz).
Theta rhythm generation involves a variety of mechanisms, including theta rhythmic firing in septal and hippocampal interneurons, excitatory inputs to hippocampus, and intrinsic properties of hippocampal neurons.
Theta rhythms are likely to be important for the formation of memories of sequences of events.
Sharp wave-ripple complexes are composed of two distinct network patterns, sharp waves (excitatory events that propagate from CA3 to CA1) and ripples (which reflect high frequency firing in hippocampal interneurons).
Accumulating evidence suggests that sharp wave-ripples are important for intrinsic hippocampal operations, including off-line memory processing, retrieval of previously stored memories, and planning of future behaviors.
The class of brain rhythms traditionally defined as gamma probably contains at least two different variants of oscillatory activity.
Recent findings suggest that slow (~25-55 Hz) and fast (~60-100 Hz) variants of gamma have different origins and may serve different functions.
Acknowledgements
The author thanks Kevin Bieri, Ernie Hwaun, and Chenguang Zheng for assistance with data collection and/or figure preparation. The work of L.L.C. is supported by the Klingenstein Fund, the Whitehall Foundation, Alzheimer's Association grant NIRP-14-305205, NIMH grant 1R01MH102450-01A1, the Office of Naval Research Young Investigator Program award N00014-14-1-0322, and NSF CAREER award #1453756.
Glossary
- Neuronal ensembles
Co-active neurons that work together to carry out neuronal computations and perform operations such as stimuli coding or memory storage.
- Sleep spindles
7-14 Hz thalamocortical oscillations that occur in bouts lasting for a few seconds and recurring approximately once every ten seconds during slow wave sleep, particularly around the onset of sleep.
- Slow oscillations
~0.5-1 Hz cortically generated oscillations, consisting of alternating depolarizing “up” states and hyperpolarizing “down” states that regulate the occurrence of other oscillations, including sleep spindles, during slow wave sleep.
- Theta sequences
Ordered series of place cell spikes that occur within theta cycles and represent the succession of locations traversed during active behaviors.
- Head direction cells
Neurons that fire when an animal's head is pointing in a particular direction and are found in several brain areas including the postsubiculum, the thalamus, and the medial entorhinal cortex.
- Replay
The phenomenon by which ordered place cell spike trains that occur during exploratory theta-related behaviors later reactivate in a temporally compressed manner during sharp wave-ripples while the animal is at rest and during slow wave-sleep.
- Stratum radiatum
Apical dendritic layer in CA3 and CA1 in which axons from CA3 pyramidal neurons terminate.
- Stratum lacunosum-moleculare
Distal apical dendritic layer in CA3 and CA1 in which perforant pathway fibers from the entorhinal cortex terminate.
- Delayed nonmatching-to-place task
A behavioral task that assesses memory by first allowing animals to visit one of two goal locations and then, after a delay period, requiring animals to navigate to the other goal location in order to receive a reward.
Biography
Laura Lee Colgin received her Ph.D. from the University of California at Irvine, where she worked in Gary Lynch's laboratory. She did her postdoctoral work with Edvard and May-Britt Moser at the Norwegian University of Science and Technology. She is currently an Assistant Professor in the Center for Learning and Memory and the Department of Neuroscience at the University of Texas at Austin. Her research investigates how brain rhythms affect neuronal activity and behavior during learning and memory processing.
References
- 1.Quiroga RQ. Gnostic cells in the 21st century. Acta Neurobiol Exp (Wars) 2013;73:463–471. doi: 10.55782/ane-2013-1952. [DOI] [PubMed] [Google Scholar]
- 2.Hebb DO. The organization of behavior; a neuropsychological theory. Wiley; 1949. [Google Scholar]
- 3.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 4.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 6.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 8.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [This classic study showed that theta rhythms occur in the rat hippocampus during active movements and REM sleep.] [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [This study described behavioral correlates of sharp waves and explained how sharp waves in the hippocampus are generated.] [DOI] [PubMed] [Google Scholar]
- 11.Bragin A, et al. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Luthi A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist. 2014;20:243–256. doi: 10.1177/1073858413500854. [DOI] [PubMed] [Google Scholar]
- 17.Jung R, Kornmüller AE. Eine Methodik der ableitung lokalisierter Potentialschwankungen aus subcorticalen Hirngebieten. Arch Psychiat Nervenkr. 1938;109:1–30. [Google Scholar]
- 18.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 19.Grastyan E, Lissak K, Madarasz I, Donhoffer H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr Clin Neurophysiol. 1959;11:409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrom AD, et al. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- 21.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nature Neuroscience. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 22.Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc Natl Acad Sci U S A. 2013;110:13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell SJ, Ranck JB. Generation of Theta Rhythm in Medial Entorhinal Cortex of Freely Moving Rats. Brain Research. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- 24.Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- 25.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Meer MA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. J Neurosci. 2011;31:2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [This paper showed that GABAergic cells in the medial septum project to interneurons in the hippocampus.] [DOI] [PubMed] [Google Scholar]
- 29.Varga V, et al. The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum. Journal of Physiology-London. 2008;586:3893–3915. doi: 10.1113/jphysiol.2008.155242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V. GABAergic Neurons of the Medial Septum Lead the Hippocampal Network during Theta Activity. Journal of Neuroscience. 2009;29:8094–8102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 32.Somogyi P, Katona L, Klausberger T, Lasztoczi B, Viney TJ. Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120518. doi: 10.1098/rstb.2012.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci U S A. 2012;109:E2726–2734. doi: 10.1073/pnas.1210929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [This study describes the discovery of theta phase precession in hippocampal place cells.] [DOI] [PubMed] [Google Scholar]
- 36.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Wulff P, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark E, et al. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–1276. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Pernia-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron. 2014;81:140–152. doi: 10.1016/j.neuron.2013.09.046. [In this study, whole cell recordings from dentate gyrus granule cells in vivo revealed excitatory inputs from the entorhinal cortex that were coherent with theta rhythms, as well as inhibitory currents that were coherent with gamma rhythms.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickson CT, et al. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nolan MF, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima Y, Nakajima S, Leonard RJ, Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1986;83:3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubota D, Colgin LL, Casale M, Brucher FA, Lynch G. Endogenous waves in hippocampal slices. J Neurophysiol. 2003;89:81–89. doi: 10.1152/jn.00542.2002. [DOI] [PubMed] [Google Scholar]
- 47.Vandecasteele M, et al. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci U S A. 2014;111:13535–13540. doi: 10.1073/pnas.1411233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goutagny R, Jackson J, Williams S. Self-generated theta oscillations in the hippocampus. Nat Neurosci. 2009;12:1491–1493. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- 49.Petsche H, Stumpf C, Gogolak G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- 50.Mizumori SJ, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res. 1990;528:12–20. doi: 10.1016/0006-8993(90)90188-h. [DOI] [PubMed] [Google Scholar]
- 51.Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [This paper showed that stable place fields emerged in a new environment when theta rhythms were suppressed.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci. 2015;18:282–288. doi: 10.1038/nn.3904. [This paper showed that “episode fields”, which normally emerge as animals run on a wheel during delays in a memory task, were abolished when theta rhythms were blocked by inactivation of the medial septum.] [DOI] [PubMed] [Google Scholar]
- 53.Landfield PW, McGaugh JL, Tusa RJ. Theta rhythm: a temporal correlate of memory storage processes in the rat. Science. 1972;175:87–89. doi: 10.1126/science.175.4017.87. [DOI] [PubMed] [Google Scholar]
- 54.Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- 55.Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- 56.Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci. 1982;2:1705–1717. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci. 1982;2:292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 59.M'Harzi M, Jarrard LE. Effects of medial and lateral septal lesions on acquisition of a place and cue radial maze task. Behav Brain Res. 1992;49:159–165. doi: 10.1016/s0166-4328(05)80160-3. [DOI] [PubMed] [Google Scholar]
- 60.Orr G, Rao G, Houston FP, McNaughton BL, Barnes CA. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus. 2001;11:647–654. doi: 10.1002/hipo.1079. [DOI] [PubMed] [Google Scholar]
- 61.Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci. 2004;118:403–411. doi: 10.1037/0735-7044.118.2.403. [DOI] [PubMed] [Google Scholar]
- 63.McNaughton N, Ruan M, Woodnorth MA. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- 64.Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol Learn Mem. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 66.Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. Elife. 2014;3:e03061. doi: 10.7554/eLife.03061. [This study used optogenetic techniques to inhibit CA1 activity at particular phases of theta and showed that memory encoding and memory retrieval were differentially affected, depending on the theta phase at which CA1 was inhibited.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belchior H, Lopes-Dos-Santos V, Tort AB, Ribeiro S. Increase in hippocampal theta oscillations during spatial decision making. Hippocampus. 2014;24:693–702. doi: 10.1002/hipo.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 69.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 70.Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24:7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yartsev MM, Ulanovsky N. Representation of three-dimensional space in the hippocampus of flying bats. Science. 2013;340:367–372. doi: 10.1126/science.1235338. [DOI] [PubMed] [Google Scholar]
- 72.Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 73.Foster DJ, Wilson MA. Hippocampal theta sequences. Hippocampus. 2007;17:1093–1099. doi: 10.1002/hipo.20345. [DOI] [PubMed] [Google Scholar]
- 74.Feng T, Silva D, Foster DJ. Dissociation between the experience-dependent development of hippocampal theta sequences and single-trial phase precession. J Neurosci. 2015;35:4890–4902. doi: 10.1523/JNEUROSCI.2614-14.2015. [This study revealed that theta phase precession was present immediately in a novel environment but that theta sequences developed with experience.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wikenheiser AM, Redish AD. Hippocampal theta sequences reflect current goals. Nat Neurosci. 2015;18:289–294. doi: 10.1038/nn.3909. [This paper showed that theta sequences dynamically represented paths extending ahead of an animal's location toward upcoming goal locations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komisaruk BR. Synchrony between limbic system theta activity and rhythmical behavior in rats. J Comp Physiol Psychol. 1970;70:482–492. doi: 10.1037/h0028709. [DOI] [PubMed] [Google Scholar]
- 78.Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- 79.Berg RW, Whitmer D, Kleinfeld D. Exploratory whisking by rat is not phase locked to the hippocampal theta rhythm. J Neurosci. 2006;26:6518–6522. doi: 10.1523/JNEUROSCI.0190-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478:246–249. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- 81.Dupret D, O'Neill J, Csicsvari J. Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron. 2013;78:166–180. doi: 10.1016/j.neuron.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brandon MP, Bogaard AR, Schultheiss NW, Hasselmo ME. Segregation of cortical head direction cell assemblies on alternating theta cycles. Nat Neurosci. 2013;16:739–748. doi: 10.1038/nn.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 85.Chrobak JJ, Buzsaki G. Selective Activation of Deep Layer (V-Vi) Retrohippocampal Cortical-Neurons during Hippocampal Sharp Waves in the Behaving Rat. Journal of Neuroscience. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol. 2003;550:873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colgin LL, Kubota D, Jia Y, Rex CS, Lynch G. Long-term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. J Physiol. 2004;558:953–961. doi: 10.1113/jphysiol.2004.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papatheodoropoulos C, Koniaris E. alpha5GABAA receptors regulate hippocampal sharp wave-ripple activity in vitro. Neuropharmacology. 2011;60:662–673. doi: 10.1016/j.neuropharm.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 89.Buzsaki G, Gage FH, Kellenyi L, Bjorklund A. Behavioral dependence of the electrical activity of intracerebrally transplanted fetal hippocampus. Brain Res. 1987;400:321–333. doi: 10.1016/0006-8993(87)90631-7. doi:0006-8993(87)90631-7 [pii] [DOI] [PubMed] [Google Scholar]
- 90.Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sullivan D, et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci. 2011;31:8605–8616. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ylinen A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klausberger T, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 94.Schlingloff D, Kali S, Freund TF, Hajos N, Gulyas AI. Mechanisms of sharp wave initiation and ripple generation. J Neurosci. 2014;34:11385–11398. doi: 10.1523/JNEUROSCI.0867-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 99.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 103.Clemens Z, et al. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 104.Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS One. 2009;4:e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 106.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.English DF, et al. Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J Neurosci. 2014;34:16509–16517. doi: 10.1523/JNEUROSCI.2600-14.2014. [In this study, intracellular recordings in behaving mice showed that pyramidal cells are depolarized during sharp wave-ripples but their spiking is suppressed by ripple-associated shunting inhibition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;42:803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 111.Roumis DK, Frank LM. Hippocampal sharp-wave ripples in waking and sleeping states. Curr Opin Neurobiol. 2015;35:6–12. doi: 10.1016/j.conb.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mehta MR. Cortico-hippocampal interaction during up-down states and memory consolidation. Nat Neurosci. 2007;10:13–15. doi: 10.1038/nn0107-13. [DOI] [PubMed] [Google Scholar]
- 113.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [This study disrupted sharp wave-ripples in rats while they performed spatial memory task and showed that task performance was impaired, suggesting that sharp wave-ripples in awake animals play a role in memory-guided trajectory planning.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [In this paper, sequences of place cell spikes observed during sharp wave-ripples while the animal was at rest were shown to represent paths that animals subsequently traversed to reach goal locations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 120.Csicsvari J, O'Neill J, Allen K, Senior T. Place-selective firing contributes to the reverse-order reactivation of CA1 pyramidal cells during sharp waves in open-field exploration. Eur J Neurosci. 2007;26:704–716. doi: 10.1111/j.1460-9568.2007.05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 123.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 124.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kemere C, Carr MF, Karlsson MP, Frank LM. Rapid and continuous modulation of hippocampal network state during exploration of new places. PLoS One. 2013;8:e73114. doi: 10.1371/journal.pone.0073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schomburg EW, et al. Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron. 2014;84:470–485. doi: 10.1016/j.neuron.2014.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- 128.van der Meer MA, Redish AD. Low and High Gamma Oscillations in Rat Ventral Striatum have Distinct Relationships to Behavior, Reward, and Spiking Activity on a Learned Spatial Decision Task. Front Integr Neurosci. 2009;3:9. doi: 10.3389/neuro.07.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manabe H, Mori K. Sniff rhythm-paced fast and slow gamma-oscillations in the olfactory bulb: relation to tufted and mitral cells and behavioral states. Journal of Neurophysiology. 2013;110:1593–1599. doi: 10.1152/jn.00379.2013. [DOI] [PubMed] [Google Scholar]
- 130.Bastos AM, et al. Visual Areas Exert Feedforward and Feedback Influences through Distinct Frequency Channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 131.Zheng CG, Colgin LL. Beta and Gamma Rhythms Go with the Flow. Neuron. 2015;85:236–237. doi: 10.1016/j.neuron.2014.12.067. doi:10.1016/j.neuron.2014.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 133.Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- 134.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Senior TJ, Huxter JR, Allen K, O'Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 137.Zheng C, Bieri KW, Hsiao YT, Colgin LL. Spatial Sequence Coding Differs during Slow and Fast Gamma Rhythms in the Hippocampus. Neuron. 2016;89:398–408. doi: 10.1016/j.neuron.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lasztoczi B, Klausberger T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 2014;81:1126–1139. doi: 10.1016/j.neuron.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 139.Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–857. doi: 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 140.Pastoll H, Solanka L, van Rossum MC, Nolan MF. Feedback inhibition enables theta-nested gamma oscillations and grid firing fields. Neuron. 2013;77:141–154. doi: 10.1016/j.neuron.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 141.Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng C, Bieri KW, Trettel SG, Colgin LL. The relationship between gamma frequency and running speed differs for slow and fast gamma rhythms in freely behaving rats. Hippocampus. 2015;25:924–938. doi: 10.1002/hipo.22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature. 2015;523:419–424. doi: 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- 144.Sun C, et al. Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc Natl Acad Sci U S A. 2015;112:9466–9471. doi: 10.1073/pnas.1511668112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed OJ, Mehta MR. Running speed alters the frequency of hippocampal gamma oscillations. J Neurosci. 2012;32:7373–7383. doi: 10.1523/JNEUROSCI.5110-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J Neurosci. 2013;33:19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bieri KW, Bobbitt KN, Colgin LL. Slow and fast gamma rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron. 2014;82:670–681. doi: 10.1016/j.neuron.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cabral HO, et al. Oscillatory dynamics and place field maps reflect hippocampal ensemble processing of sequence and place memory under NMDA receptor control. Neuron. 2014;81:402–415. doi: 10.1016/j.neuron.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi M, Nishida H, Redish AD, Lauwereyns J. Theta phase shift in spike timing and modulation of gamma oscillation: a dynamic code for spatial alternation during fixation in rat hippocampal area CA1. J Neurophysiol. 2014;111:1601–1614. doi: 10.1152/jn.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 152.Brun VH, et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- 153.Steffenach HA, Sloviter RS, Moser EI, Moser MB. Impaired retention of spatial memory after transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proc Natl Acad Sci U S A. 2002;99:3194–3198. doi: 10.1073/pnas.042700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci U S A. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- 158.Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 159.Pfeiffer BE, Foster DJ. PLACE CELLS. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science. 2015;349:180–183. doi: 10.1126/science.aaa9633. [DOI] [PubMed] [Google Scholar]
- 160.Trimper JB, Stefanescu RA, Manns JR. Recognition memory and theta-gamma interactions in the hippocampus. Hippocampus. 2014;24:341–353. doi: 10.1002/hipo.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kitanishi T, et al. Novelty-Induced Phase-Locked Firing to Slow Gamma Oscillations in the Hippocampus: Requirement of Synaptic Plasticity. Neuron. 2015;86:1265–1276. doi: 10.1016/j.neuron.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 162.Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2008;18:340–348. doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu X, Foster DJ. Hippocampal replay captures the unique topological structure of a novel environment. J Neurosci. 2014;34:6459–6469. doi: 10.1523/JNEUROSCI.3414-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 166.Zhang H, Jacobs J. Traveling Theta Waves in the Human Hippocampus. J Neurosci. 2015;35:12477–12487. doi: 10.1523/JNEUROSCI.5102-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- 168.Patel J, Fujisawa S, Berenyi A, Royer S, Buzsaki G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron. 2012;75:410–417. doi: 10.1016/j.neuron.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Watrous AJ, et al. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23:656–661. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jacobs J. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130304. doi: 10.1098/rstb.2013.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bragin A, Engel J, Jr., Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 172.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 173.Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18:1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Skaggs WE, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 175.Leonard TK, et al. Sharp Wave Ripples during Visual Exploration in the Primate Hippocampus. J Neurosci. 2015;35:14771–14782. doi: 10.1523/JNEUROSCI.0864-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sederberg PB, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- 177.Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. J Neurosci. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.de Almeida L, Idiart M, Lisman JE. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. J Neurosci. 2009;29:7497–7503. doi: 10.1523/JNEUROSCI.6044-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–U139. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dickerson DD, Wolff AR, Bilkey DK. Abnormal Long-Range Neural Synchrony in a Maternal Immune Activation Animal Model of Schizophrenia. Journal of Neuroscience. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee H, et al. Early Cognitive Experience Prevents Adult Deficits in a Neurodevelopmental Schizophrenia Model. Neuron. 2012;75:714–724. doi: 10.1016/j.neuron.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Racz A, Ponomarenko AA, Fuchs EC, Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J Neurosci. 2009;29:2563–2568. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suh J, Foster DJ, Davoudi H, Wilson MA, Tonegawa S. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron. 2013;80:484–493. doi: 10.1016/j.neuron.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]