Abstract
Introduction
Cholestasis is a reduction in bile flow that occurs during numerous pathologies. Blockage of the biliary tracts results in hepatic accumulation of bile acids or their conjugate bile salts. The molecular mechanisms behind liver injury associated with cholestasis are extensively studied, but not well understood. Multiple models of obstructive cholestasis result in a significant inflammatory infiltrate at the sites of necrosis that characterize the injury.
Areas Covered
This review will focus on direct bile acid toxicity during cholestasis, bile acid signaling processes and on the development and continuation of inflammation during cholestasis, with a focus on novel proposed molecular mediators of neutrophil recruitment. While significant progress has been made on these molecular mechanisms, a continued focus on how cholestasis and the innate immune system interact is necessary to discover targetable therapeutics that might protect the liver while leaving global immunity intact.
Expert Opinion
While bile acid toxicity likely occurs in humans and other mammals when toxic bile acids accumulate, persistent inflammation is likely responsible for continued liver injury during obstructive cholestasis. Targeting molecular mediators of inflammation may help prevent liver injury during acute cholestasis both in murine models and human patients.
Keywords: Obstructive cholestasis, bile acid toxicity, inflammation, innate immunity, neutrophils
1. Introduction
Cholestasis results from blockage of the biliary tracts at any point or due to inhibition of the bile salt export pump (BSEP) at the canalicular surface of the hepatocyte. This blockade results in accumulation of bile acids (BA) or their conjugate salts in hepatocytes and in serum. While some bile acids or bile salts such as glycochenodeoxycholate (GCDC) are highly toxic [1], many of the bile acids that accumulate in vivo in rodent models during cholestasis are largely non-toxic, even up to biliary (millimolar) concentrations [2]. Exposure of isolated hepatocytes to serum and biliary concentrations of bile acids that are observed during cholestasis in vivo is largely non-toxic, unless the bile acid composition is toxified through the addition of hydrophobic bile acids like GCDC or lithocholic acid (LCA) to the system [3]. As such, new hypotheses have been put forth on the mechanisms behind what causes hepatocyte cytotoxicity during cholestasis [4]. While there are some discrepancies between models dependent on the cause of cholestasis, neutrophils have been implicated as critical to the pathogenesis of multiple models of cholestatic liver injury [4,5,6]. This review will focus largely on obstructive cholestasis and the bile duct ligation (BDL) model of injury and the signaling mechanisms and inflammation associated with this model. While there are other relevant models, a plurality of the papers on cholestatic liver injury take place using the BDL model as it recapitulates many facets of human obstructive cholestasis. It should be noted that while the BDL model is an excellent model for the basic physiology and pathophysiology of the injury, pharmaceutical intervention in the model can be prone to problems, thus, this review will focus mainly on basic science and interventions that use knockout mouse models that do not suffer from this problem [8]. How bile acid accumulation and the innate immune system interact, the makeup of the chemotactic signals that recruit neutrophils to the liver, and the mechanisms behind how neutrophils kill hepatocytes during cholestasis remain unanswered questions. This review will address these questions as well as offer current hypotheses on the role and causes of sterile inflammation during cholestatic liver injury, and review potential therapeutic targets related to bile acid signaling as it is currently understood.
2. Bile Acid Signaling During Cholestasis and its Relation to Innate Immunity
2.1 Bile acid accumulation during cholestasis
A considerable number of papers have focused on bile acid accumulation as the primary mechanism of cellular toxicity during cholestasis in rodents [1]. This hypothesis is based upon the administration of GCDC in rodent hepatocytes [9, 10] or transfected hepatoma lines [11, 12]. Administration of 50–100 µM GCDC to these lines results in well-defined apoptotic cell death that is inhibitable by pan-caspase inhibition [13]. The use of these concentrations of GCDC was originally predicated upon GCDC accumulation during cholestasis in humans, and its recognized toxicity [9,10]. However, more recent reports showed that GCDC accumulation during cholestasis is largely limited to man, and rodents accumulate much higher concentrations of muricholic acid (MCA), a secondary derivative of chenodeoxycholic acid (CDCA) [2, 14]. Other commonly used bile acids to induce toxicity include unconjugated CDCA or DCA; however, 99% or more of the circulating and liver levels of these bile acids found during cholestasis are typically conjugated to either taurine or glycine, thus, the use of micromolar concentrations of unconjugated bile acids may be unwarranted [2]. Also of note, the idea of bile acid-induced apoptosis in rat hepatocytes may be contingent upon unrealistic exposure of hepatocytes to normoxic air, as simulated exposure to physiological hypoxia experienced in the liver resulted in protection against GCDC induced apoptosis [15]. This may be due to the excess oxygen, which is responsible for the creation of reactive oxygen species under normoxic conditions present during in vitro cell culture [16]. Moreover, a majority of bile acids are conjugated to taurine during cholestasis in rodent species, which produces a less toxic bile acid mixture [2]. This is in contrast to man where glycine conjugates, which are far more toxic, occur in approximately equal frequency to taurine conjugates [2, 14, 17]. Taurocholic acid (TCA), MCA and its conjugated derivatives α- and β-tauromuricholic acid compose a majority of the bile acid pool during cholestasis in mice, and display essentially no toxicity in vitro towards hepatocytes, even at millimolar concentrations [2, 18]. In contrast, GCDC levels never accumulate to micromolar levels in serum of rodent models after BDL, nor do they accumulate in the liver to levels on par with toxicity [2]. While rat hepatocytes will undergo apoptosis at concentrations as low as 50µM, human hepatocytes do not undergo apoptosis in response to GCDC, and will only undergo necrosis at concentrations of 500µM and above [19, 20]. These data strongly argue against the idea that exposure of rodent hepatocytes to GCDC mimics the human pathological condition. Instead, in humans biliary concentrations of bile acids associated with hepatic infarction directly induce cell death, which leads to further inflammatory liver injury [20]. Critical to understanding of the injury is how the accumulation of bile acids in hepatocytes leads to a sustained chemotactic signal and predisposes these hepatocytes towards a neutrophilic attack.
2.2 Bile acid signaling in hepatocytes and innate immune cells of the liver
Cholestatic concentrations of bile acids induce a number of genetic changes in hepatocytes [21]. In the BDL model, hepatocytes acutely upregulate export transporters such as the multidrug resistance (Mdr) transporter family and BSEP [22] and downregulate uptake transporters, especially the sodium taurocholate cotransporting polypeptide (Ntcp), in order to reduce hepatic accumulation of bile salts [23]; although, post-transcriptional regulation of Bsep results in a reduction in total activity [24]. This is likely due to an inherent need to reduce bile acid transport into bile because of the lack of bile flow [24]. In Mdr3 (Abcb4)-deficient mice similar results are observed, wherein the mice have increased Bsep mRNA levels after birth; however, total protein expression levels of Bsep are significantly lower [25]. Generally, these compensatory changes are thought to serve as a way to protect hepatocytes from hepatic accumulation of bile acids and shunt excess bile acid levels to the serum where they can potentially be taken up and excreted by the kidneys [24, 26]. A number of these actions take place through autoregulation of bile acid enterohepatic circulation, synthesis, and metabolism via the endogenous bile acid nuclear receptor Farnesoid X Receptor (FXR). Bile acids serve as ligands that activate FXR, which responds by upregulating and downregulating the aforementioned genes, as well as CYP7A1, the rate-limiting enzyme in the classical bile acid synthesis pathway. FXR has been studied extensively and is reviewed in depth elsewhere [27]. FXR is a critical response element to cholestatic concentrations of bile acids, as FXR−/− mice cannot upregulate Bsep to enhance choleresis and undergo increased injury in response to cholestasis [28]. Currently a specific FXR agonist, obeticholic acid, is undergoing clinical trials as a potential therapeutic in the treatment of primary biliary cirrhosis (PBC), a form of autoimmune cholestatic hepatitis [29]. Promisingly, in patients with an inadequate response to UDCA, the current mainline treatment, obeticholic acid treatment resulted in decreases in alkaline phosphatase, γ-glutamyl transpeptidase and alanine aminotransferase, suggesting obeticholic acid can protect hepatocytes, and biliary epithelial cells against PBC-associated liver damage [29]. It should be noted that due to the complete biliary obstruction induced in the BDL model, other choleretic drugs such as ursodeoxycholate (UDCA), the mainline cholestatic therapeutic, can actually worsen injury as they contribute to increased biliary pressure and infarction of the biliary tracts [30, 31]. Doses given in man probably fail to exacerbate the injury due to the smaller quantities, and the protective nature of UDCA towards the mitochondria [19]; thus, these differences should serve as a cautionary note that the BDL model may not always be appropriate to test therapeutics for cholestatic liver injury.
Bile acids can also activate extracellular receptors. Bile acids have been shown to activate both receptor tyrosine kinases and G-protein coupled receptors (GPCRs) on hepatocytes [32, 33] and also activate downstream MAPK proteins via activation of the epidermal growth factor receptor [34]. While many of these signaling cascades seem to be dependent on ROS generation and injury in rat hepatocytes [33], it is not well known if these mechanisms are recapitulated in other mammals. Bile acids also act as ligands for sphingosine 1 phosphate receptor 2 (S1PR2) in isolated rat hepatocytes, which activates MAPK signaling in hepatocytes [35]. Although S1PR2 inhibition was not protective against bile acid-induced cytotoxicity, inhibition of sphingosine kinase or administration of exogenous sphingosine 1 phosphate (S1P), were both protective against cell death in vitro [36]. Interestingly, while S1PR3 antagonism has not been examined in vitro, inhibition of S1PR3 has been shown to inhibit S1P mediated bone marrow derived cell homing and reduce fibrosis and injury during cholestasis in vivo in the BDL model [37]. Although the detailed mechanisms of how this occurs have yet to be determined, S1PR activity may be a potential therapeutic target to reduce neutrophil accumulation during cholestasis without compromising global immunity.
The recently discovered G-protein coupled bile acid receptor (GPBAR or hereafter referred to as TGR5) is a novel receptor for bile acids expressed on multiple different cell types [38]. TGR5 is expressed on monocytes, sinusoidal endothelial cells, Kupffer cells, cholangiocytes, and to a lesser degree, hepatocytes in the liver [38–40]. Due to the diverse expression pattern, TGR5 activation by bile acids has pleiotropic effects, including varied effects in multiple models of liver injury such as cholestasis [41], LPS-induced inflammation [42], and partial hepatectomy [43], in addition to native physiological functions such as gallbladder filling [44]. TGR5 activation is thought to be generally protective against liver injury in multiple ways. TGR5 activation on sinusoidal endothelial cells induces endothelial nitric oxide synthase and nitric oxide (NO) production via cyclic adenosine monophosphate (cAMP) [45]. NO is a critical mediator of vasodilation which may serve to prevent portal hypertension, one of the most serious complications of cirrhosis caused by long term cholestasis. TGR5 activation in macrophages and Kupffer cells antagonizes NF-kB mediated increases in pathogenic cytokines during cholestasis [42]. This leads to protection against injury, as TGR5 KO mice have exacerbated inflammation after BDL or partial hepatectomy, particularly, an increase in recruited neutrophils [43]. Kupffer cell depletion through clodronated liposomes attenuated the TGR5 KO phenotype, suggesting a portion of this phenotype is due to TGR5 mediated ablation of pro-inflammatory Kupffer cell activity [43]. TGR5 is also known to enhance biliary excretion and bile flow by enhancing bicarbonate output, and thus helps control bile acid overload in models of cholestasis and liver regeneration [41, 43]. In addition to obeticholic acid, a TGR5/FXR dual agonist (INT-767) is in development that might not only enhance choleresis, but also limit inflammation that occurs concurrently during liver disease. As macrophages are constantly exposed to serum bile acids levels, TGR5 activation by excess serum bile acids may be beneficial in the greater context of injury. Treatments that push bile acids outside of the enterohepatic circulation and into systemic circulation not only bypass cholestatic, damaged areas, but may also serve to enhance systemic bile acid elimination via the kidneys and activate protective measures through TGR5 on macrophages. It should be noted though, that chronic cholestasis and enhanced renal bile acid excretion has been associated with cholemic nephropathy [26]. Thus, more studies would be required to determine if renal excretion is a viable route to enhance elimination of excess bile acids levels without renal toxicity.
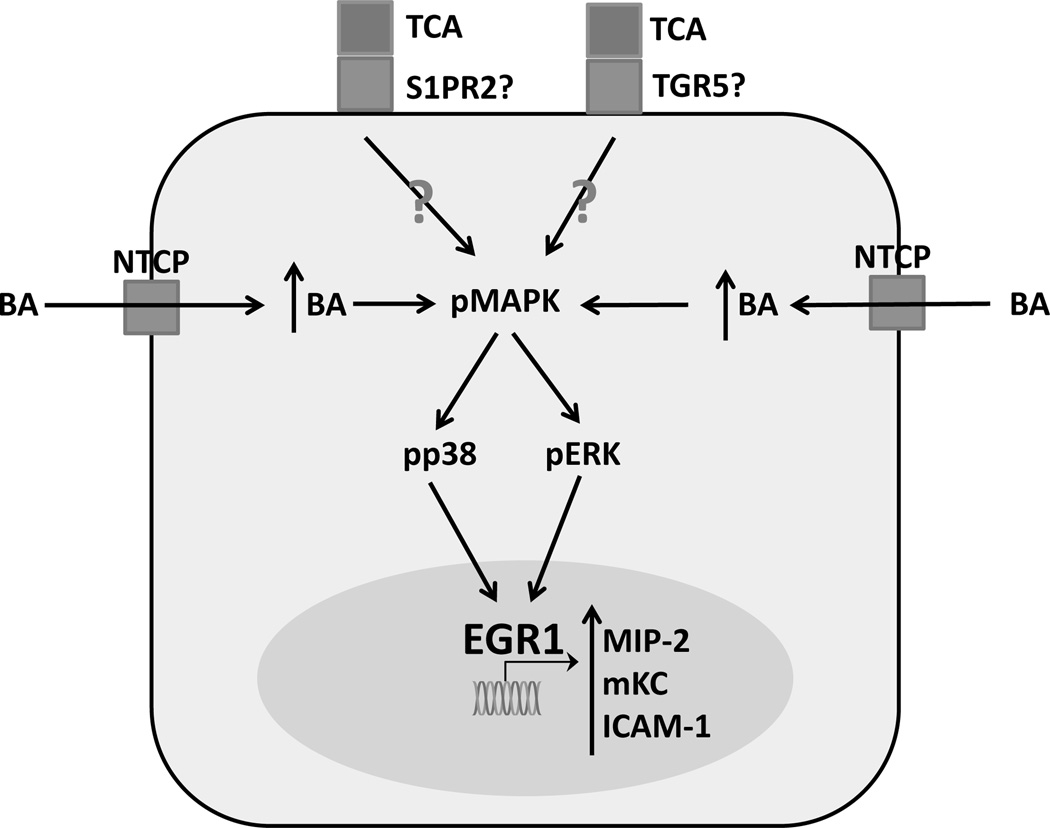
Recent reports suggest that bile acids themselves directly cause innate immune activation and recruitment in addition to their other processes [18, 46]. Mouse hepatocytes exposed to pathophysiological levels of multiple conjugated bile acids respond by upregulating C-X-C motif chemokines such as CXCL-1 (referred to as mKC) and CXCL-2 (referred to as MIP-2) in an FXR-independent manner [18]. This response is potentially dependent on bile acid uptake as this response is lost if hepatocytes are allowed to adhere for 24 hours before the initial exposure, (Woolbright and Jaeschke, unpublished observation) a period during which cultured primary rodent hepatocytes naturally dedifferentiate, and downregulate Ntcp and other uptake transporters [47, 48]; although, dedifferentiation of hepatocytes could have other additional unknown effects independent of the decrease in Ntcp that might account for this loss of response. In addition to cytokines, bile acids can directly upregulate the transcription factor early growth response protein 1 (Egr-1) [49]. This action is mediated by MAP kinases, and thus may be linked to the aforementioned activation of cell surface receptors [49]. Egr-1 is also upregulated by BDL, and is essential for inflammation after BDL, as Egr-1−/− mice are largely protected from BDL-induced liver injury [50]. Egr-1−/− mice fail to upregulate cytokines in response to bile acid exposure, linking the in vitro upregulation of cytokines and pro-inflammatory proteins like intercellular adhesion molecule-1 (ICAM-1) to obstructive cholestasis and inflammation [18]. IL-17A may act on the same axis as it synergistically enhances Egr-1 signaling, increasing cytokine production [46]. IL-17 has been shown to enhance both immune activity and progression of fibrosis during obstructive cholestasis [51], potentially linking bile acid-induced inflammation directly to downstream fibrosis. Thus, Egr-1 may play a central role in the inflammatory process during cholestasis and is a potential therapeutic target for inflammation during cholestatic liver injury. However, it should be noted that studies in human hepatocytes have indicated a considerably blunted inflammatory response compared to mouse hepatocytes [20]. These findings are illustrated in Figure 1.
Figure 1.
Bile acid signaling in murine hepatocytes. Cultured hepatocytes exposed to conjugated bile acids such as taurocholic acid active a signaling pathway that results in dramatic increases in expression of pro-inflammatory mediators. TCA – taurocholic acid, S1PR2 – sphingosine 1 phosphate receptor 2, GPBAR-1 – G-protein bile acid coupled receptor-1, BA – bile acid, NTCP – sodium taurocholate co-transporting polypeptide, pMAPK – phosphorylated mitogen activated protein kinase, pp38 – phosphorylated protein-38, pERK – phosphorylated extracellular signal regulated kinase, Egr1 – early growth response factor 1, MIP-2 – macrophage inflammatory protein 2, mKC – mouse keratinocyte factor, ICAM-1 – intercellular adhesion molecule-1, OATP – organic anion transporting polypeptide, TGR5/GPBAR-1 – G-protein coupled bile acid receptor
3. Inflammation as a Mechanism of Injury during Cholestasis
3.1 The innate immune system
The primary role of the immune system is host defense against microbial pathogens. Multiple components of the innate immune system rapidly respond to microbial infection to identify, phagocytose, and kill microbial components. The liver is a critical organ in the innate immune defense response, as Kupffer cells in the liver filter a substantial amount of bacteria, LPS, and viral components from the blood stream. Sterile inflammation parallels host defense, wherein the inflammatory system activates against normal cellular components that are perceived as indicators of cellular damage termed damage associated molecular patterns (DAMPs), instead of activating against microbial pathogens. This duality is critical to understand. Global immunosuppression to prevent injury during liver disease is not a realistic therapeutic option, as the risk of infection can become greater than the therapeutic benefit. Thus, there is an impetus to identify therapeutic targets that prevent inflammation during cholestatic liver injury without compromising the global immune response. This section will focus on new mediators of neutrophil extravasation with the potential to inhibit inflammation after cholestasis while minimizing global impact, and also discuss current mechanisms of innate immune cell recruitment and injury.
3.2 The role of neutrophils during cholestatic liver injury
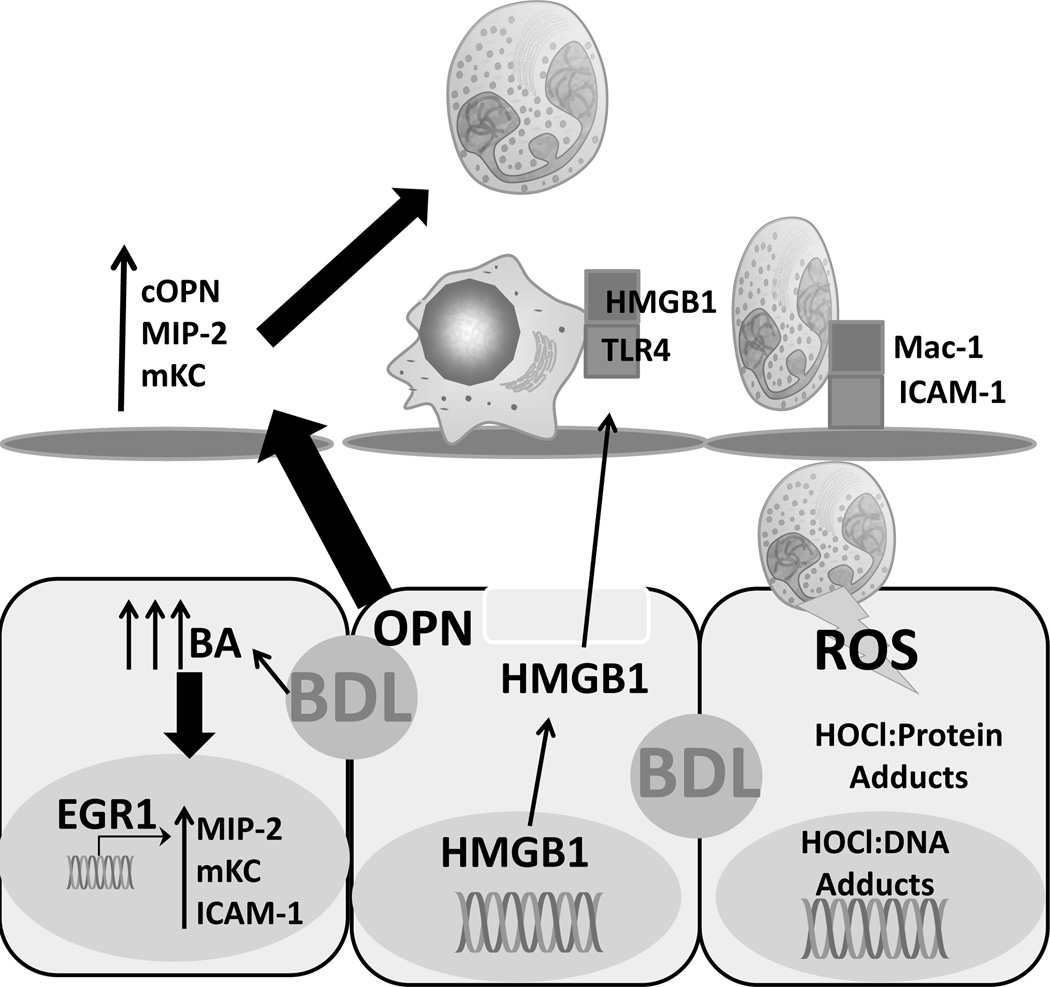
Neutrophils are a critical component of the innate immune system and the most populous granulocytes in mice and man. Initially, neutrophils were shown to correlate with the areas of biliary infarction and focal necrosis that characterize obstructive cholestasis [52]. Subsequently, BDL was shown to cause an increase in chemoattractants in the liver [53]. Mice deficient in ICAM-1 or CD18, which are important for neutrophil extravasation and cytotoxicity [54], are both protected against BDL-induced liver injury [5, 55]. In addition, depletion of P-selectin glycoprotein ligand 1 attenuated neutrophil-mediated injury after BDL [56]. Anti-inflammatory glucocorticoids are protective against BDL in rats [57]. Neutrophils have also been found to mediate injury in some models of intrahepatic cholestasis [6, 58]. In neutrophil-depleted mice fed alpha-naphthylisothiocyanate (ANIT) there was a substantial decrease in injury that could be directly attributed towards protection of hepatocytes, and not protection of cholangiocytes, in line with data in the BDL mouse [6]. Neutrophils express myeloperoxidase, which is responsible for the generation of hypochlorous acid, a potent oxidant. The areas of biliary infarction that characterize the focal necrosis immunostain positive for chlorotyrosine adducts, indicative of release of the highly toxic hypochlorous acid in the region [5, 55, 59]. While these data support a hypothesis where neutrophils are the predominant source of injury, the idea that neutrophils caused cholestatic liver injury was controversial. Previously, the injury was thought to be largely apoptotic through a mitochondrial-lysosomal axis, as both cathepsin B−/− mice and fas receptor-deficient lpr mice were protected against BDL [60, 61]. Of note, a reduction in neutrophil recruitment was observed in the cathepsin B−/− mouse, though the mechanism was never discerned [61]. Furthermore, the lpr mouse was found to have a blunted inflammatory response to BDL, and pan-caspase inhibition was shown to have no effect on the injury [62]. These findings suggested necrosis is the dominant mode of cell death. Subsequent studies investigating the modality of cell death further confirmed focal necrosis, eliminating the idea that apoptosis could be the primary mechanism of injury in vivo [63–65]. Together, these data strongly indicate that neutrophils are the primary source of toxicity after BDL. Given that bile acid concentrations in murine bile are insufficient to result in toxicity [2, 18] the current hypothesis is that neutrophils are recruited to the liver, where they target, and kill, injured hepatocytes. This mechanism involves bile acids as pro-inflammatory but not directly cytotoxic mediators [4]. These findings are illustrated in Figure 2.
Figure 2.
Inflammation after surgically induced cholestasis. After bile duct ligation (BDL), numerous cytokines, including osteopontin present in bile duct epithelial cells, are produced that actively recruit cytotoxic neutrophils. These neutrophil extravasate through the sinusoidal endothelial cell layer and release ROS that actively kill damaged, but alive hepatocytes. BA – bile acid, MIP-2 – macrophage inflammatory protein 2, mKC – mouse keratinocyte factor, ICAM-1 – intercellular adhesion molecule-1, Egr1 – early growth response factor 1, BDL – bile duct ligation, OPN – osteopontin, cOPN – cleaved ostepontin, HMGB1 – high mobility group box-1, Mac-1 – macrophage 1 antigen (CD11b/CD18), TLR-4 – toll-like receptor 4, HOCl – hypochlorous acid, ROS – reactive oxygen species
While neutrophils are the predominant innate immune cell during cholestasis, there are significant interactions with other components of the innate immune system. While Kupffer cell inactivation with gadolinium chloride was initially thought to be protective against BDL-induced injury [66], this was later attributed to be an effect of gadolinium chloride itself and likely unrelated to Kupffer cell activation, as depletion of Kupffer cells with the relatively more specific clodronate liposomes resulted in an enhancement of BDL injury [67]. Kupffer cells activate NF-κB which results in IL-6 production during early cholestasis that can protect against injury [67]. Adaptive immune cells contribute to the inflammatory signal as well, as both natural killer cells and invariant natural killer T cells help to suppress cholestatic liver injury through this same function, as they activate protective effects in Kupffer cells [68, 69]. A majority of the effects of these other immune components are likely achieved through modulation of neutrophil recruitment though, as neutrophils carry out the actual cell killing processes.
3.3 Novel Mediators of Neutrophil Recruitment in Cholestasis
As global inflammatory inhibition is not a viable therapeutic avenue, identifying novel pathways of neutrophil recruitment during cholestasis are necessary in order to find specific pharmaceutical targets. Numerous advances have occurred recently in the field of sterile inflammation. Amongst these is the identification of receptors of DAMPs that result in activation of immune cells and stimulation of sterile inflammatory response [70]. Numerous DAMPs have been identified thus far, as well as nine receptors (toll-like receptors, TLRs) for these ligands [70]. Many DAMPs are released passively from necrotic cells, such as mitochondrial DNA, nuclear DNA fragments, and ATP [71–74]. DAMP release has been proposed as both a clinical and a mechanistic biomarker that may give additional insight into clinical injury, without the need for directly sampling the injured tissue [72, 73, 75–76]. Since acute liver injury compromises the coagulation system and poses a serious bleeding risk, the use of serum biomarkers provides an interesting and suitable alternative to tissue studies in man. One such serum biomarker is HMGB1, which binds and activates TLR4 [71]. HMGB1 is a DNA associated histone deacetylase that translocates to the cytosol during injury [77]. Necrosis during cholestasis results in release of HMGB1 into serum as early as 6 hours after injury [65]. In addition to native HMGB1, an acetylated form is actively secreted from macrophages that may serve as a pro-inflammatory signal [78]. The ratio of acetylated to hypoacetylated HMGB1 increases during cholestasis throughout the first 72 hours of injury when inflammation begins to peak [65], and is increased in human patients with cholestatic liver injury [20]. This acetylated form of HMGB1 may serve as a potent chemokine for neutrophil recruitment during cholestasis [79]. TLR expression levels increase in both hepatocytes and cholangiocytes of chronically cholestatic patients [80, 81]. Moreover, activation of TLRs on liver immune cells stimulates local NK cells to attack and kill bile ducts during primary biliary cirrhosis [82]. As blockade of TLR4 signaling via TLR4 depletion in hematopoietic derived cells [83] or the use of neutralizing antibodies to deplete HMGB-1 [84] has previously been shown to be protective against sterile inflammatory injury in the hepatic ischemia reperfusion model, further studies investigating the role of TLR signaling, HMGB1 and its acetylated form, acetyl-HMGB1, are indicated. In the mouse BDL model, endotoxin-resistant animals, which have a defect in TLR4 signaling [85], are not protected suggesting that neither gut-derived endotoxin nor other TLR4 ligands (e.g. HMGB1) are critical for the inflammatory response [18]. However, these studies are complicated by the fact that HMGB1 has additional receptors with additional functions. Amongst these is the receptor for advanced glycation end products (RAGE) [86]. The delineation of RAGE mediated and TLR mediated effects of HMGB1 or acetylated HMGB1 are necessary to understand both in the context of cholestatic liver injury, and during general inflammation, before therapeutic options become a reality in this field, although blockade of these receptors or their ligands may serve as a therapeutic target in the future.
Osteopontin (OPN) is another recently discovered mediator of chemotaxis during cholestasis [87]. Osteopontin is a pleiotropic molecule with a role in numerous cellular activities. Secreted OPN acts as chemokine that binds integrin receptors and functions as a potent neutrophil chemoattractant [88]. Osteopontin is localized to cholangiocytes in normal liver [89]; however, chronic injury induces expression of OPN in hepatocytes [90]. Release of OPN from bile duct epithelial cells (BDECs), presumably due to the increased biliary pressure during obstructive cholestasis, mediates a substantial portion of the early immune response [87]. OPN−/− mice were protected against BDL-induced injury and hepatic neutrophil recruitment during cholestasis and nearly completely protected against the focal necrosis associated with bile infarcts at very early time points [87]. Activation of OPN is apparently required, as prevention of OPN cleavage by inhibition of matrix metalloproteinases in bile was protective against the early injury [87]. However, this protection was temporary, as injury returned to wild-type levels in mice by 72 hours post BDL [87]. Thus, there are likely redundancies in place to ensure neutrophil chemotaxis during cholestasis. In fact, the second wave of neutrophil chemotaxis is most likely caused by CXC chemokines [18]. These pro-inflammatory mediators are generated by hepatocytes when exposed to biliary levels of bile acids, which come from ruptured bile ducts during obstructive cholestasis [18].
Despite the strong chemotactic gradient present in the liver during BDL, it is unlikely that long term protection could be achieved through inhibition of single components of chemotaxis. Alternative options would be to inhibit upstream signaling components of cholestasis in cholangiocytes or hepatocytes to prevent activation of the inflammatory system, or to prevent the injury that occurs due to neutrophil inflammation by protecting hepatocytes against neutrophil-mediated oxidative stress through enhancement of anti-oxidant capabilities. These avenues are currently being pursued.
3.4 Innate Immune-Mediated Oxidant Stress during Cholestasis
Neutrophils execute their cytotoxicity mainly through generation of reactive oxygen species (ROS) [91]. While ROS are generated intracellularly in all cells during mitochondrial respiration, these species are largely detoxified through intracellular antioxidants. In contrast, neutrophils and other innate immune cells generate significant quantities of highly cytotoxic ROS that they use to kill hepatocytes during sterile inflammation. This requires activation of neutrophils and then extravasation into the hepatic parenchyma where release of these toxic mediators can directly damage hepatocytes [92]. Activation of neutrophils involves increased surface expression of the Mac-1 complex (CD11b/CD18), shedding of L-selectin and priming for ROS formation [93]. Neutrophil activation has been reported in response to cytokines, chemokines and complement factors [93], and also to HMGB1 [94], and bile acids [95], which are elevated in serum during cholestasis [2, 20]. After extravasation, neutrophil adhere to target cells through CD18-ICAM-1 interactions, which triggers a long-lasting adherence-dependent oxidant stress [92]. Neutrophils produce superoxide anion through NADPH oxidase (Nox2), which then dismutates into oxygen and hydrogen peroxide [91]. The neutrophilic enzyme myeloperoxidase converts chloride anion and hydrogen peroxide into the highly toxic hypochlorous acid. While Kupffer cells are capable of producing oxidant stress, and do so in other models of sterile inflammation such as the hepatic ischemia-reperfusion model [96], the predominant oxidative stress during cholestasis likely comes from neutrophils. Kupffer cell and monocyte levels stay fairly consistent during the first two weeks of cholestasis and depletion of Kupffer cells worsens the injury due to a reduction in protective acute phase cytokines and protein production [67]. More importantly, chlorotyrosine-protein adducts are prevalent in the areas of necrosis after acute BDL injury [5, 55, 59]. These chlorotyrosine-protein adducts are indicative of an intracellular oxidant stress in hepatocytes and nearby endothelium, suggesting that neutrophil-derived hypochlorous acid must diffuse into local hepatocytes, where it causes toxicity [91]. As hypochlorous acid is a potent oxidant, it can bind and damage various components of the cell. Hypochlorous acid has been shown to form adducts with DNA and protein, and is particularly predisposed towards binding thiol groups on proteins [97], and towards forming toxic chloramines [98]. It is likely hepatocytes exposed to neutrophilic ROS are already compromised, as even low levels of toxic bile acids impair mitochondrial function without killing the cell [99]. Further exposure to ROS would then result in cell death due to aggravated mitochondrial impairment. Ablation of the mitochondrial permeability transition pore (MPTP) with NIM-811, a cyclosporine analogue, is protective against the early BDL injury, although it does not protect against later fibrosis [100]. Additionally, preservation or enhancement of intracellular antioxidants is protective against BDL. For example, kelch-like-ECH-associated-protein-1 (Keap-1) knockdown mice with a constitutively active nuclear factor E2 related factor-2 (Nrf2) are protected against BDL-induced liver injury, although some portion of this may be through enhanced choleresis [101]. More information is required in this area to assess if targeting the neutrophilic oxidant stress can prevent cholestatic liver injury. However, a general impairment of the neutrophil’s cytotoxicity, which is possible using interventions against NADPH oxidase or β2 integrins (CD18) may be effective but bears the risk of increased susceptibility to infections. Thus, strengthening of hepatocellular antioxidant systems specifically against a neutrophilic oxidant stress may be a more viable option.
4. Conclusions
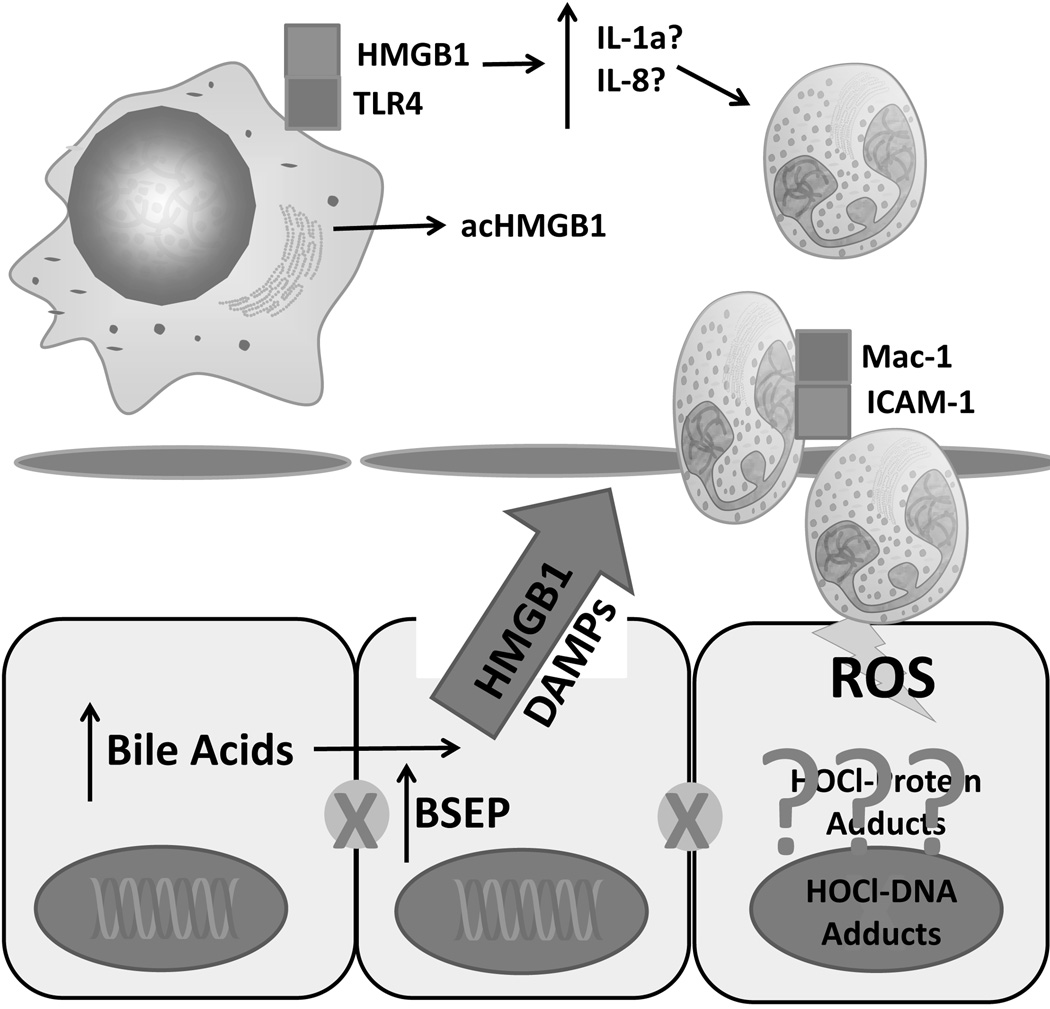
Cholestasis is a complex, multi-cellular pathology involving multiple biological compartments in the liver. Modeling inflammation during cholestasis increases the complexity further as one includes additional cell types, molecular mediators and signals, and cellular stress to the pathophysiology. Studies in the past have focused largely on the role of bile acids in cholestasis; however, new data suggest the injury is far more complex. Novel roles of bile acids have recently been identified and must be explored further in appropriate rodent models of cholestasis. Future studies on the mechanisms and signaling agents involved in cholestatic liver injury should focus not only on the development of the cholestasis itself, but the subsequent inflammation, keeping in mind that blocking inflammation is only productive when it can be done specifically. Most importantly, there is a significant gap in the amount of information available in rodent models versus human patients (Figure 3). More translational work is required in the area to discern which, if any, of these same mechanisms are relevant for the human pathophysiology.
Figure 3.
Model for bile acid-induced necrosis in man. Increases in cellular bile acid levels leads directly to cell death when exposed to biliary bile acid concentrations. This results in release of damage associated molecular patterns such as HMGB1 and potentially other DAMPs. Current research is attempting to determine if the subsequent recruitment of neutrophils is mediated by specific cytokines, and if neutrophils worsen injury after cholestasis. BA – bile acid, HMGB1 – high mobility group box-1, DAMP – damage associated molecular pattern, acHMGB1 – acetylated HMGB1, TLR-4, toll-like receptor 4, IL-1α – interleukin 1α, IL-8 - interleukin-8, ROS – reactive oxygen species, HOCl – hypochlorous acid, Mac-1- macrophage 1 antigen (CD11b/CD18), ICAM-1 – intercellular adhesion molecule 1
5. Expert Opinion
Considerable efforts went into better understanding cell death processes associated with cholestasis. The mainline therapeutic, ursodeoxycholic acid, has been in use clinically for over two decades, and while it reduces transaminase levels in some patients, it lacks efficacy when it comes to long-term improvement [102]. Newer formulations of UDCA, including nor-ursodeoxycholic acid (nor-UDCA), may offer an improvement over classical UDCA, as nor-UDCA has shown increased efficacy in a number of murine models [103]. A majority of the current models, such as BDL, are very good models of obstructive cholestasis; however, they fail to recapitulate aspects of more complex chronic diseases. Moreover, while classical cholestasis, such as obstruction of the common bile duct, can be treated with endoscopy, new therapeutic targets are needed for successful treatment of advanced cholestatic disorders such as primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC), both of which prominently feature inflammation. While the therapeutic targets discussed in this review may serve as viable targets for pure cholestatic disorders, better models of both PSC and PBC are needed where these targets can be further validated. More effort should be placed towards understanding the mechanisms behind these clinically relevant disease states, and currently understood targets should be re-evaluated in the context of more advanced models and when possible, in patients with PSC and PBC.
One concept this review attempts to address is the idea of differential susceptibility to bile acids between species, and how inherent bile acid production and accumulation is likely to be partially responsible for this difference. While we have previously argued for a limited role for bile acid toxicity during cholestatic liver injury, recent data from primary human hepatocytes suggests human hepatocytes are acutely susceptible to bile acid-induced liver injury, but only in the context of biliary concentrations of bile acids [20]. These data are supported by the fact that human hepatocytes do not undergo toxicity until bile acid concentrations of 500µM or more are added for an extended period [19, 20]. Hepatocytes may be exposed to these concentrations when local biliary tracts rupture due to the increased pressure associated with cholestasis [7, 20, 30]. This is consistent with the presence of extra-biliary bile in patients with acute extrahepatic cholestasis [20]; although, patients with chronic intrahepatic cholestasis do not always recapitulate this facet of cholestatic liver injury. This may be due to chronic compensation and subsequent alteration of relevant transporter levels, and thus inflammation may play a greater role in these disease states. One important caveat is the idea of intracellular bile acid accumulation and what role it plays in bile acid-induced toxicity, especially in the context of chronic exposure to elevated bile acid levels in patients. Measurements of bile acids from liver tissue do not reflect measurements in hepatocytes themselves, as any biopsy would also include multiple other cell types including blood vessels, biliary tracts, and other hepatic architecture. Experiments measuring retention of bile acids in isolated hepatocytes after exposure to mixed or single bile acid concentrations may yield novel and important information about the kinetics of acute or chronic bile acid exposure, and how this effects toxicity. Isolated primary human hepatocytes and HepaRG cells will likely make good models for this as they retain transporter activity for longer periods in vitro [20, 104, 105].
Recent data from our group and others suggest a re-evaluation of the levels of bile acids used during in vitro experimentation [2, 3, 14, 20]. For example, studies have repeatedly demonstrated that unconjugated levels of bile acids rarely accumulate to micromolar concentrations in vivo. Chenodeoxycholic acid is a potent activator of FXR; however, levels of unconjugated chenodeoxycholic acid in serum are typically two orders of magnitude below concentrations routinely used in vitro. As hepatocytes are unaccustomed to these high concentrations of bile acids, this may yield artifacts specific to the in vitro system. Potentially the best example of this is the profound susceptibility of rat hepatocytes to bile acid-induced apoptosis as compared to either mouse or human hepatocytes.
It should be noted that while this review focused on bile acid signaling in hepatocytes and the subsequent inflammatory response, bile acid signaling in cholangiocytes may be another potential therapeutic area. Recently, it was established that cholangiocytes protect themselves against bile acid induced injury through a protective glycocalyx featuring an “umbrella” of bicarbonate secretion that lowers local pH values and reduces bile acid uptake through the anion exchange 1 transporter [106]. This new therapeutic area holds a great deal of potential as drugs specifically targeted towards the bile may be especially effective at preventing the adverse reactions associated with bile acid-induced cholangiocyte damage.
Finally, increased effort needs to be placed on understanding the complex role of inflammation during cholestasis. A number of different DAMPS and cytokines likely have integral roles in the pathogenesis and recruitment of inflammatory cells of cholestatic disorders including molecules such as HMGB-1 [20], osteopontin [87], IL-17 [46], complement C5 [107], and IL-33 [108]. Interventions against inflammation are protective in multiple models of cholestatic liver injury [5, 6, 109], and a number of different inflammatory populations have been indicated as potentially pathogenic in multiple models of cholestasis [5, 6, 55, 109, 110]. Even so, limited resources have been placed on understanding the role of specific mediators of inflammation in the context of advanced cholestatic diseases such as PSC and PBC, or in childhood major cholestatic disorders such as biliary atresia. More studies designed at understanding the unique role of individual inflammatory mediators, both cellular macromolecules and inflammatory cell themselves, is needed to progress in the understanding of this field.
Highlights.
Cholestasis results in elevated bile acid levels in multiple tissue compartments.
Hydrophobic bile acids may directly cause injury to hepatocytes and other cells at biliary (millimolar) concentrations; this effect is species-dependent.
Neutrophil recruitment and neutrophil mediated liver injury is common sequelae in cholestatic liver injury.
Bile acids themselves may be pro-inflammatory molecules at high concentrations.
A number of different therapeutic targets including nuclear receptors, G-protein coupled cell surface receptors and more have been identified that may alter bile acid induced signaling.
Acknowledgments
This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # UL1TR000001 (formerly #UL1RR033179). In addition, this work was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 (to H.J.), and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to B.L.W.) from the National Institute of Environmental Health Sciences.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interest.
References
- 1.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Hong JY, Rockwell CE, et al. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolbright BL, Li F, Xie Y, et al. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett. 2014;228:56–66. doi: 10.1016/j.toxlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2013;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gujral JS, Farhood A, Bajt ML, et al. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. ** Neutrophils mediate bile duct ligation induced liver injury in mice.
- 6.Kodali P, Wu P, Lahiji PA, et al. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–G363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- 7.Fickert P, Stöger U, Fuchsbichler A, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fickert P. Time to say goodbye to the drug or the model? – why do drugs fail to live up to their promise in bile duct ligated mice? J Hepatol. 2014;60:12–15. doi: 10.1016/j.jhep.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 9. Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;2:17–24. doi: 10.1172/JCI116546. * First report of apoptosis in rat hepatocytes exposed to GCDCA.
- 10.Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denk GU, Kleiss CP, Wimmer R, et al. Tauro-β-muricholic acid restricts bile acid-induced hepatocellular apoptosis by preserving the mitochondrial membrane potential. Biochem Biophys Res Commun. 2012;424:758–764. doi: 10.1016/j.bbrc.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Denk GU, Maitz S, Wimmer R, et al. Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid-induced cholestasis in rat liver. Hepatology. 2010;52:1758–1768. doi: 10.1002/hep.23911. [DOI] [PubMed] [Google Scholar]
- 13.Jones B, Roberts PJ, Faubion WA, et al. Cystatin A expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am J Physiol. 1998;275:G723–G730. doi: 10.1152/ajpgi.1998.275.4.G723. [DOI] [PubMed] [Google Scholar]
- 14.Trottier J, Białek A, Caron P, et al. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS One. 2011;6:e22094. doi: 10.1371/journal.pone.0022094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohenester S, Vennegeerts T, Wagner M, et al. Physiological hypoxia prevents bile salt-induced apoptosis in human and rat hepatocytes. Liver Int. 2014;34:1224–1231. doi: 10.1111/liv.12368. [DOI] [PubMed] [Google Scholar]
- 16.Yan HM, Ramachandran A, Bajt ML, et al. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Bijsmans IT, van Mil SW, et al. Toxicity and intracellular accumulation of bile acids in sandwich-cultured rat hepatocytes: role of glycine conjugates. Toxicol In Vitro. 2014;28:218–230. doi: 10.1016/j.tiv.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 18. Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. **Bile acids induce expression of inflammatory mediators in murine hepatocytes.
- 19.Galle PR, Theilmann L, Raedsch R, et al. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- 20. Woolbright BL, Dorko K, Antoine DJ, et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168–177. doi: 10.1016/j.taap.2015.01.015. * Indicated human hepatocytes are likely resistant to serum bile acid concentrations and may function very differently than rats.
- 21.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2010;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slitt AL, Allen K, Morrone J, et al. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768:637–647. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Gartung C, Ananthanarayanan M, Rahman MA, et al. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- 24.Donner MG, Schumacher S, Warskulat U, et al. Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2014;293:G1134–G1146. doi: 10.1152/ajpgi.00079.2007. [DOI] [PubMed] [Google Scholar]; Cai SY, Mennone A, Soroka CJ, et al. Altered expression and function of canalicular transporters during early development of cholestatic liver injury in Abcb4-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G670–G676. doi: 10.1152/ajpgi.00334.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fickert P, Krones E, Pollheimer MJ, et al. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056–2069. doi: 10.1002/hep.26599. [DOI] [PubMed] [Google Scholar]
- 26.Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Fickert P, Zollner G, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 28. Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761. doi: 10.1053/j.gastro.2014.12.005. **First report on efficacy of obeticholic acid in PBC.
- 29. Fickert P, Zollner G, Fuchsbichler A, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. *Elevated bile acid levels may exacerbate obstructive cholestasis.
- 30.Weerachayaphorn J, Luo Y, Mennone A, et al. Deleterious effect of oltipraz on extrahepatic cholestasis in bile duct-ligated mice. J Hepatol. 2014;60:160–166. doi: 10.1016/j.jhep.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang Y, Han SI, Mitchell C, et al. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40:961–971. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 32.Dent P, Fang Y, Gupta S, et al. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 2005;42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- 33.Rao YP, Studer EJ, Stravitz RT, et al. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology. 2002;35:307–314. doi: 10.1053/jhep.2002.31104. [DOI] [PubMed] [Google Scholar]
- 34.Studer E, Zhou X, Zhao R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karimian G, Buist-Homan M, Schmidt M, et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochim Biophys Acta. 2013;1832:1922–1929. doi: 10.1016/j.bbadis.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Jiang X, Yang L, et al. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175:1464–1472. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. **TGR5/GPBAR is a receptor for bile acids.
- 38.Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 39.Keitel V, Häussinger D. TGR5 in cholangiocytes. Curr Opin Gastroenterol. 2013;29:299–304. doi: 10.1097/MOG.0b013e32835f3f14. [DOI] [PubMed] [Google Scholar]
- 40.Baghdasaryan A, Claudel T, Gumhold J, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO−3 output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YD, Chen WD, Yu D, et al. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Péan N, Doignon I, Garcin I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien KM, Allen KM, Rockwell CE, et al. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183:1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang D, Hagenbuch B, Stieger B, et al. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology. 1993;18:1162–1166. [PubMed] [Google Scholar]
- 47.Rippin SJ, Hagenbuch B, Meier PJ, et al. Cholestatic expression pattern of sinusoidal and canalicular organic anion transport systems in primary cultured rat hepatocytes. Hepatology. 2001;33:776–782. doi: 10.1053/jhep.2001.23433. [DOI] [PubMed] [Google Scholar]
- 48.Allen K, Kim ND, Moon JO, et al. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010;243:63–67. doi: 10.1016/j.taap.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim ND, Moon JO, Slitt AL, et al. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010;243:63–67. doi: 10.1016/j.taap.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. doi: 10.1053/j.gastro.2012.05.049. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koeppel TA, Trauner M, Baas JC, et al. Extrahepatic biliary obstruction impairs microvascular perfusion and increases leukocyte adhesion in rat liver. Hepatology. 1997;26:1085–1091. doi: 10.1002/hep.510260501. [DOI] [PubMed] [Google Scholar]
- 52.Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- 53.Jaeschke H. Cellular adhesion molecules: regulation and functional significance in the pathogenesis of liver diseases. Am J Physiol. 1997 Sep;273(3 Pt 1):G602–G611. doi: 10.1152/ajpgi.1997.273.3.G602. [DOI] [PubMed] [Google Scholar]
- 54.Gujral JS, Liu J, Farhood A, et al. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004a;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 55.Dold S, Laschke MW, Zhau Y, et al. P-selectin glycoprotein ligand-1-mediated leukocyte recruitment regulates hepatocellular damage in acute obstructive cholestasis in mice. Inflamm Res. 2010;59:291–298. doi: 10.1007/s00011-009-0099-2. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh CS, Wang PW, Lee SY, et al. Glucocorticoid pretreatment suppresses chemokine expression and inflammatory cell infiltration in cholestatic rats receiving biliary intervention. J Pediatr Surg. 2006;41:1669–1675. doi: 10.1016/j.jpedsurg.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 57.Luyendyk JP, Flanagan KC, Williams CD, et al. Tissue factor contributes to neutrophil CD11b expression in alpha-naphthylisothiocyanate-treated mice. Toxicol Appl Pharmacol. 2011;250:256–262. doi: 10.1016/j.taap.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004;14(3 Suppl 1):S48. doi: 10.1186/1476-5926-2-S1-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyoshi H, Rust C, Roberts PJ, et al. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 60.Canbay A, Guicciardi ME, Higuchi H, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gujral JS, Liu J, Farhood A, et al. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J–lpr mice after bile duct ligation. Hepatology. 2004b;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- 62.Fickert P, Trauner M, Fuchsbichler A, et al. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 63.Nalapareddy P, Schüngel S, Hong JY, et al. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175:1077–1085. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woolbright BL, Antoine DJ, Jenkins RE, et al. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 66.Gehring S, Dickson EM, San Martin ME, van Rooijen N, Papa EF, Harty MW, et al. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Cheng CW, Duwaerts CC, Rooijen Nv, et al. NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J Hepatol. 2011;54:746–752. doi: 10.1016/j.jhep.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duwaerts CC, Sun EP, Cheng CW, et al. Cross-activating invariant NKT cells and Kupffer cells suppress cholestatic liver injury in a mouse model of biliary obstruction. PLoS One. 2013;8:e79702. doi: 10.1371/journal.pone.0079702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 71.McGill MR, Sharpe MR, Williams CD, et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGill MR, Staggs VS, Sharpe MR, et al. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60:1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;(26 Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 74.Antoine DJ, Jenkins RE, Dear JW, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.McGill MR, Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014;10:1005–1017. doi: 10.1517/17425255.2014.920823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 77.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 78.Penzo M, Molteni R, Suda T, et al. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis [corrected] J Immunol. 2010;184:4497–4509. doi: 10.4049/jimmunol.0903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang AP, Migita K, Ito M, et al. Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis. J Autoimmun. 2005;25:85–91. doi: 10.1016/j.jaut.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Benias PC, Gopal K, Bodenheimer H, Jr, et al. Hepatic expression of toll-like receptors 3, 4, and 9 in primary biliary cirrhosis and chronic hepatitis C. Clin Res Hepatol Gastroenterol. 2012;36:448–454. doi: 10.1016/j.clinre.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Shimoda S, Harada K, Niiro H, et al. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 83.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poltorak A, He X, Smirnova I. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 85.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Yang M, Ramachandran A, Yan HM, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- 88.Lorena D, Darby IA, Gadeau AP, et al. Osteopontin expression in normal and fibrotic liver. Altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383–390. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 89.Apte UM, Banerjee A, McRee R, et al. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2005;207:25–38. doi: 10.1016/j.taap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 90.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 91.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 92.Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- 93.Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 94.Dahm LJ, Roth RA. Differential effects of lithocholate on rat neutrophil activation. J Leukoc Biol. 1990;47:551–560. doi: 10.1002/jlb.47.6.551. [DOI] [PubMed] [Google Scholar]
- 95.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 96.Pattison DI, Davies MJ. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry. 2006;45:8152–8162. doi: 10.1021/bi060348s. [DOI] [PubMed] [Google Scholar]
- 97.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 98.Sokol RJ, Dahl R, Devereaux MW, et al. Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr. 2005;41:235–243. doi: 10.1097/01.mpg.0000170600.80640.88. [DOI] [PubMed] [Google Scholar]
- 99.Rehman H, Ramshesh VK, Theruvath TP, et al. NIM811 (N-methyl-4-isoleucine cyclosporine), a mitochondrial permeability transition inhibitor, attenuates cholestatic liver injury but not fibrosis in mice. J Pharmacol Exp Ther. 2008;327:699–706. doi: 10.1124/jpet.108.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okada K, Shoda J, Taguchi K, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- 101.Carey EJ, Lindor KD. Current pharmacotherapy for cholestatic liver disease. Expert Opin Pharmacother. 2012;13:2473–2484. doi: 10.1517/14656566.2012.736491. [DOI] [PubMed] [Google Scholar]
- 102.Trauner M, Halilbasic E, Claudel T, et al. Potential of nor -Ursodeoxycholic Acid in Cholestatic and Metabolic Disorders. Dig Dis. 2015;33:433–439. doi: 10.1159/000371904. [DOI] [PubMed] [Google Scholar]
- 103.Szabo M, Veres Z, Baranyai Z, et al. Comparison of human hepatoma HepaRG cells with human and rat hepatocytes in uptake transport assays in order to predict a risk of drug induced hepatotoxicity. PLoS One. 2013;8:e59432. doi: 10.1371/journal.pone.0059432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woolbright BL, McGill MR, Yan H, et al. Bile acid-induced toxicity in HepaRG cells recapitulates the response in primary human hepatocytes. Basic Clin Pharmacol Toxicol. 2015 Jul 14; doi: 10.1111/bcpt.12449. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hohenester S, Maillette de Buy Wenniger L, Jefferson DM, et al. Biliary bicarbonate secretion constitutes a protective mechanism against bile acid-induced injury in man. Dig Dis. 2011;29:62–65. doi: 10.1159/000324687. [DOI] [PubMed] [Google Scholar]
- 106.Schmitt J, Roderfeld M, Sabrane K, et al. Complement factor C5 deficiency significantly delays the progression of biliary fibrosis in bile duct-ligated mice. Biochem Biophys Res Commun. 2012;418:445–450. doi: 10.1016/j.bbrc.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 107.Li J, Razumilava N, Gores GJ, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Licata LA, Nguyen CT, Burga RA, et al. Biliary obstruction results in PD-1-dependent liver T cell dysfunction and acute inflammation mediated by Th17 cells and neutrophils. J Leukoc Biol. 2013;94:813–823. doi: 10.1189/jlb.0313137. [DOI] [PMC free article] [PubMed] [Google Scholar]