Abstract
Posttraumatic stress disorder (PTSD) – a debilitating disorder characterized by severe deficits in emotion regulation – is prevalent among U.S. military veterans. Research into the pathophysiology of PTSD has focused primarily on emotional reactivity, showing evidence of heightened neural response during negative affect provocation. By comparison, studies of brain functioning during the voluntary regulation of negative affect are limited. In the current study, combat-exposed U.S. military veterans with (n = 25) and without (n = 25) PTSD performed an emotion regulation task during electroencephalographic (EEG) recording. The late positive potential (LPP) was used as a measure of sustained attention toward, and processing of, negative and neutral pictures, and was scored prior to and after instructions to either maintain or down-regulate emotional response using the strategy of cognitive reappraisal. Results showed that groups did not differ in picture-elicited LPP amplitude either prior to or during cognitive reappraisal; reappraisal reduced the LPP in both groups over time. Time-dependent increases in LPP amplitude as a function of emotional reactivity maintenance were evident in the non-PTSD group only. This latter finding may signal PTSD-related deficits in sustained engagement with emotion-processing over the course of several seconds.
Keywords: ERP, late positive potential, PTSD, IAPS
1. Introduction
Lifetime prevalence of posttraumatic stress disorder (PTSD) is approximately 7% among U.S. adults, but the disorder is far more common among U.S. military veterans. Some estimates indicate that up to 13% of veterans meet PTSD diagnostic criteria in the months that follow combat deployment (Castro, 2014), making the disorder one of the most common injuries suffered by U.S. veterans returning from overseas (Thomas et al., 2010). PTSD is characterized by symptom heterogeneity, including reoccurrence of trauma memories, emotional withdrawal, alterations in arousal and emotional reactivity, and persistent negative changes in cognition and mood (American Psychiatric Association, 2013). In spite of PTSD’s varied presentation, recent meta-analyses and review papers endorse emotion dysregulation as a core feature of the disorder (Etkin and Wager, 2007; Frewen and Lanius, 2006). In addition, clinical studies have found that self-reported deficiency in emotion regulation in PTSD is predictive of increased symptom severity (Badour and Feldner, 2013; Boden et al., 2012) and inferior social functioning (Klemanski et al., 2012). Together, this research suggests that the study of emotion dysregulation may be central in understanding PTSD; however more information is needed on the biological mechanisms that support this aspect of the disorder.
Much of the neuroimaging work in PTSD to-date has sought to determine whether individuals with PTSD exhibit heightened neural activation in response to aversive stimuli (Bryant et al., 2008; El Khoury-Malhame et al., 2011; Felmingham et al., 2010; Hayes et al., 2012; Hendler et al., 2003; Morey et al., 2009; Protopopescu et al., 2005; Rauch et al., 2006; Shin and Liberzon, 2010; Simmons and Matthews, 2012; White et al., 2014). For instance, compared to non-traumatized (Bryant et al., 2009; El Khoury-Malhame et al., 2011; Felmingham et al., 2010; Protopopescu et al., 2005) and traumatized peers without PTSD (Hendler et al., 2003; Morey et al., 2009), those with PTSD have been found to exhibit heightened amygdala activity when exposed to both generic and trauma-related aversive stimuli. Greater engagement of the amygdala has also been found to correlate with severity of PTSD symptoms (White et al., 2014). Based on these findings, one possibility is that symptoms of emotion dysregulation in PTSD stem from heightened reactivity to aversive information. Yet another possibility is that symptoms of emotion dysregulation result from deficient regulatory control over emotional stimulus processing. Therefore, to fully understand the mechanisms of emotion dysregulation in PTSD, additional work on neural functioning during the voluntary regulation of emotional reactivity is needed.
Voluntary emotion regulation refers to a conscious attempt to change the intensity and/or duration of an emotional reaction (Gross and Thompson, 2007). Cognitive reappraisal is a highly effective emotion regulation strategy in which individuals modulate the emotional salience of a stimulus by changing its meaning (Ochsner et al., 2002). In uncovering the neural correlates of the strategy, studies in healthy individuals have found that cognitive reappraisal is associated with increased engagement of multiple regions in the prefrontal cortex (PFC), including the dorsolateral PFC (dlPFC) (Goldin et al., 2008; Phan et al., 2005), ventrolateral PFC (vlPFC) (Ochsner et al., 2002; Wager et al., 2008), dorsomedial PFC (dmPFC) (Banks et al., 2007; Phan et al., 2005), and ventromedial PFC (vmPFC) (Urry et al., 2006) in addition to the anterior cingulate cortex (ACC) (Eippert et al., 2007; Hermann et al., 2014) (see Buhle et al., 2014; Ochsner et al., 2012 for reviews). During reappraisal, amygdala activation is also reduced (Buhle et al., 2014) and reductions in amygdala responding have been found to correlate with reappraisal-related increases in prefrontal cortical activity (Banks et al., 2007). These data suggest that cognitive reappraisal may exert its effects on emotional reactivity by way of PFC engagement.
Neuroimaging studies of cognitive reappraisal in PTSD have found that PTSD may be characterized by deficits in prefrontal regions implicated in the successful down-regulation of negative affect. For instance, Rabinak and colleagues (2014) found that, compared to combat-exposed U.S. military veterans without PTSD, those with PTSD showed a focal deficit in dlPFC engagement when asked to reduce their response to negative pictures using cognitive reappraisal (Rabinak et al., 2014). In an earlier study, New and colleagues (2009) found that, compared to their non-traumatized counterparts, traumatized females showed reduced recruitment of prefrontal brain regions during cognitive reappraisal; however, recruitment of these regions did not differ for traumatized individuals with versus without PTSD (New et al., 2009). In this study, individuals with PTSD also showed less activation of the PFC when asked to increase negative affect, compared to traumatized individuals without PTSD and healthy controls. This prior work then suggests that PTSD and/or trauma-exposure may be characterized by reduced recruitment of prefrontal regions during the cognitive reappraisal of negative stimuli. Additionally, PTSD may be uniquely characterized by aberrant neural activation during the up-regulation of emotional response.
Event-related potentials (ERPs) have excellent temporal resolution and can be used to assess the neural correlates of affective processing. For instance, the late positive potential (LPP) is an ERP component that begins approximately 300 milliseconds (ms) after stimulus onset and is larger for emotional than neutral stimuli (Codispoti et al., 2006; Cuthbert et al., 2000; Foti et al., 2009; Hajcak et al., 2012; MacNamara et al., 2009; Schupp et al., 2000). The LPP persists throughout stimulus presentation duration, lasting up to several seconds or longer (Cuthbert et al., 2000; Hajcak and Olvet, 2008). The LPP is initially evident at centro-parietal regions but becomes more frontal later on during stimulus presentation (Hajcak et al., 2012; MacNamara et al., 2009). In addition to being sensitive to the overall emotional nature of stimuli, the LPP is also sensitive to individual differences in the perceived salience of stimuli. For example, the LPP is larger for personally-salient stimuli such as pictures of food among food-deprived individuals (Stockburger et al., 2009) and pictures of one’s own face (Tacikowski and Nowicka, 2010) or that of a relative or close friend (Grasso and Simons, 2011). Among individuals with anxiety, the LPP may be larger in response to threat-related (Kujawa et al., 2015; MacNamara and Hajcak, 2010) and disorder-specific stimuli (e.g., images of spiders in spider phobia) (Leutgeb et al., 2009; Michalowski et al., 2009). Further, the LPP is also sensitive to “top-down” manipulations of stimulus meaning. For instance, the LPP is smaller for neutrally- as compared to negatively-described pictures (Foti and Hajcak, 2008; MacNamara et al., 2009) and is smaller when participants are asked to reduce the emotional salience of pictures using the strategy of cognitive reappraisal (Hajcak and Nieuwenhuis, 2006; Moran et al., 2013; Moser et al., 2010, 2009; Parvaz et al., 2012). Some studies also document larger LPPs when participants are asked to increase emotional picture salience (Gardener et al., 2013; Moser et al., 2010, 2009; Sarlo et al., 2013). Given this research, the LPP appears as a valid neural ‘assay’ by which to assess emotion regulation in the context of both healthy and emotion-dysregulated individuals. However, despite its widespread use in healthy samples (Hajcak and Nieuwenhuis, 2006; MacNamara et al., 2009; Moran et al., 2013; Moser et al., 2010, 2009; Parvaz et al., 2012), only one study to date has employed the LPP as a measure of emotion-processing during willful emotion regulation in PTSD.
Recently, Woodward and colleagues (2015) examined group differences in LPP amplitude during voluntary regulation in military veterans with PTSD and non-traumatized controls. In this study, participants viewed negative and neutral pictures; prior to the presentation of each negative picture, participants were shown a color-coded cue that either instructed them to “think of something to tell yourself that helps you feel less negative about the photo” (Woodward et al., 2015; p. 669 ‘rationalize’ condition) or “notice your beating heart and your angry or fearful thoughts, and do not resist these reactions in any way… let the emotion flow over you like a wave” (Woodward et al., 2015; p.669 ‘notice’ condition). In other words, in the rationalize condition, participants were instructed to cognitively down-regulate their emotional response, though the specific strategy (e.g., distraction, reappraisal) was not indicated. By contrast, in the notice condition, participants were instructed to attend to their emotional response – a phenomenon that has been shown to increase the LPP (Hajcak et al., 2009). In a third, control, condition, participants were instructed to “respond freely” to negative pictures (i.e., to refrain from changing their affective response in any way). Results showed that there was no effect of group and no group × condition interaction. Importantly, however, there was also no overall effect of condition on the LPP. That is, the LPP was not modulated by condition in either the PTSD or the non-PTSD group. Without evidence that the regulation strategies used by participants in the Woodward and colleagues study (2015) modulated the LPP, it is difficult to interpret the absence of a group effect. Further, despite a picture presentation duration of 10 seconds, Woodward and colleagues (2015) assessed the LPP using peak amplitudes corresponding to the LPP within a 250 ms – 750 ms window post-picture presentation, thus also limiting understanding of the time-course of sustained emotion regulation.
In order to address these gaps in knowledge and more broadly address the relative paucity of emotion regulation work in PTSD, the current study examined the effects of cognitive reappraisal on the LPP elicited by negative and neutral pictures in a group of combat-exposed U.S. military veterans with and without PTSD (combat-exposed controls; CEC). To assess the effects of emotion generation (i.e., passive picture processing) as distinct from the effects of cognitive reappraisal, we used a dynamic task published in prior work (Parvaz et al., 2012), in which regulation instructions were delivered after initial picture presentation. Specifically, participants viewed negative and neutral pictures for 1 second prior to an auditory instruction that instructed participants to continue viewing the picture without trying to change their emotional reaction (‘maintain’ condition) or to reduce their emotional response to pictures via the strategy of cognitive reappraisal (‘reappraise’ condition). Based on prior work in healthy individuals (Hajcak and Nieuwenhuis, 2006; Moran et al., 2013; Moser et al., 2010, 2009; Parvaz et al., 2012), we believed that cognitive reappraisal would successfully reduce the LPP elicited by negative pictures. Moreover, given some evidence that prefrontal brain regions involved in the down-regulation of negative affect may be insufficiently recruited in PTSD (Rabinak et al., 2014), we hypothesized that the LPP – a measure of the sustained processing of emotional stimuli – would be less modulated by cognitive reappraisal in PTSD.
2. Methods
2.1. Participants
A total of 84 Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and/or Operation New Dawn (OND) male and female combat-exposed veterans with and without PTSD were recruited from the Veterans Affairs (VA) Ann Arbor Healthcare System (Ann Arbor, MI) and the Jesse Brown VA Medical Center (Chicago, IL). Participants with PTSD were required to have a primary diagnosis of PTSD while participants in the CEC group could not meet criteria for PTSD. Inclusion criteria for all participants included discharge from active military service, aged 18–55 years, absence of head trauma that involved a loss of consciousness, and ability to provide written consent. Exclusion criteria were presence of a clinically-significant medical or neurologic illness, or life history of bipolar disorder, schizophrenia, or pervasive developmental disorder. Twelve participants from the initial sample were excluded from analysis (n = 10 PTSD) in order to match groups on incidence of combat exposure, as assessed by the Combat Exposure Scale (CES; Keane et al., 1989).
Exclusion of participants with unusable EEG data following data collection resulted in a final sample size of N = 50 (n = 25 PTSD; see Table 1 for sample demographics). Across PTSD and CEC groups, comorbidities included major depressive disorder (MDD), panic disorder, generalized anxiety disorder (GAD), obsessive-compulsive disorder (OCD), alcohol dependence, and agoraphobia without panic. Upon enrollment, 41 participants were medication free while all others were taking a stable single or combination regimen consisting of antidepressants (Trazodone, Sertraline, Buproprion, Mirtazepine, Amitriptyline), mood stabilizers (Divalproex, Topiramate), benzodiazepines (Clonazepam), or antipsychotics (Abilify, Risperidone). Group usage did not differ with respect to class of medication (all p’s > 0.14) and ERP effects did not change substantially when this variable was added as a covariate in analyses; therefore, results are reported without controlling for medication status. All participants provided written informed consent for study participation and were monetarily compensated for their time as approved by the Institutional Review Boards of VA Ann Arbor, the University of Michigan Medical School, and the University of Illinois at Chicago.
Table 1.
Sample Demographics and Clinical Characteristics
| CEC (n = 25) M (SD) |
PTSD (n = 25) M (SD) |
test statistic
|
p
|
|
|---|---|---|---|---|
| Age | 32.84 (7.77) | 30.16 (7.80) | 1.22 | 0.23 |
| Years of Education | 15.00 (2.20) | 12.90 (1.21) | 4.19 | < .001 |
| CES | 18.16 (5.02) | 20.44 (5.63) | −1.51 | 0.14 |
| CAPS | 10.88 (16.08) | 68.76 (19.34) | −11.71 | < .001 |
| Intrusive | 2.12 (3.47) | 10.52 (11.25) | −3.57 | < .01 |
| Avoidance | 3.28 (7.09) | 15.40 (14.61) | −3.73 | < .01 |
| Hyperarousal | 3.92 (7.11) | 13.28 (11.26) | −3.51 | < .01 |
| PCL-M | 25.68 (11.01) | 54.20 (12.67) | −8.50 | < .001 |
| Ham-A | 3.80 (5.09) | 13.24 (6.60) | −5.66 | < .001 |
| Ham-D | 4.00 (5.40) | 11.56 (5.18) | −5.05 | < .001 |
| BDI | 6.68 (7.73) | 22.28 (10.09) | −6.13 | < .001 |
|
n (%)
|
n (%)
|
|||
| Gender (male) | 22 (88%) | 24 (96%) | 1.09 | 0.30 |
| Ethnicity (Caucasian) | 13 (52%) | 19 (76%) | 4.88 | 0.14 |
| Comorbidity | ||||
| None | 19 (76%) | 15 (60%) | 1.47 | 0.36 |
| MDD | 0 (0%) | 3 (12%) | 3.19 | 0.24 |
| Alcohol Dependence | 4 (16%) | 1 (4%) | 2.00 | 0.35 |
| Agoraphobia without Panic | 0 (0%) | 2 (8%) | 2.08 | 0.49 |
| Multiplea | 2 (8%) | 4 (16%) | 0.76 | 0.67 |
| Medication Status | ||||
| None | 23 (92%) | 18 (72%) | 3.39 | 0.14 |
| Antidepressant | 1 (4%) | 3 (12%) | 1.09 | 0.61 |
| Mood stabilizer | 0 (0%) | 1 (4%) | 1.02 | 0.99 |
| Combination regimenb | 1 (4%) | 3 (12%) | 1.09 | 0.61 |
Note. CEC = combat exposed controls; PTSD = posttraumatic stress disorder; CES = Combat Exposure Scale; CAPS = Clinician Administered PTSD Scale; PCL-M = PTSD Checklist – Military version; Ham-A = Hamilton Rating Scale for Anxiety; Ham-D = Hamilton Rating Scale for Depression; BDI = Beck Depression Inventory; MDD = Major Depression Disorder. Group comparisons were performed using independent t-tests except gender, ethnicity, comorbidity, and medication status which were calculated using Fisher’s Exact Test due to low cell count.
consists of a combination of MDD, Alcohol Dependence, Agoraphobia without Panic, Panic Disorder, Generalized Anxiety Disorder, or Obsessive Compulsive Disorder;
consists of a combination of antidepressants, mood stabilizers, benzodiazepines or antipsychotics.
2.2. Materials
Diagnostic criteria were assessed according to Diagnostic and Statistical Manual of Mental disorders (DSM-IV) criteria and using either the Structured Clinical Interview for DSM Disorders (SCID; First et al., 2002) or Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Participants were also assessed on standardized clinical rating scales including the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995), the PTSD Checklist: Military (PCL-M; Blanchard et al., 1996), the Hamilton Anxiety Scale (HAM-A; Hamilton, 1959) the Hamilton Depression Inventory (HAM-D; Williams, 1998), and the Beck Depression Inventory (BDI-II; Beck et al., 1996). All diagnostic and clinical interviews were performed by one of two trained clinicians: (1) a board-certified research psychiatrist (KLP) or (2) a licensed social worker (AEK).
2.3. ERT
Participants completed an Emotion Regulation Task (ERT) during continuous EEG recording (Parvaz et al., 2012). Task stimuli consisted of 50 negative (valence: M = 2.51, SD = 0.78; arousal: M = 5.78, SD = 0.68) and 50 neutral (valence: M = 5.02, SD = .44; arousal: M = 3.44, SD = 0.41; higher numbers indicate more pleasant and higher arousal ratings) images taken from the International Affective Picture System (IAPS; Lang et al., 2008). Each image was presented only once during the entirety of the task. Participants were seated approximately 60 cm in front of a computer screen; images occupied 40° of visual angle horizontally and vertically and were shown serially. Images were grouped in blocks of negative and neutral with each block consisting of 25 images. Following picture onset, an auditory instruction was given at 1,000 ms. For negative pictures, participants were instructed to either “maintain” (i.e., continue viewing the picture without trying to change their emotional reaction) or to “reappraise” (i.e., reduce negative affect by making the image appear less emotional). For neutral images, participants received an auditory instruction to “look” (i.e., continue picture viewing as they normally would). In all three conditions, the image remained on-screen for an additional 6,000 ms after the auditory instruction, resulting in a total image duration of 7,000 ms. After picture offset, participants viewed a white fixation cross presented in the center of the screen for 1,000 ms, prior to the beginning of the next trial. In total, there were 25 “maintain” trials, 25 “reappraise” trials, and 50 “look” trials. Block order was pseudo-randomized for each participant. Negative and neutral picture order was also pseudorandomized across each of the 2 blocks of that type, and – for negative blocks – the order of “maintain” and “reappraise” trials was pseudorandomized across both negative blocks. Participants were given a self-timed rest period between blocks.
Prior to the task, participants were trained in the technique of cognitive reappraisal. For reappraisal, participants were instructed by trained research staff (KLP, AEK, CAR) to either (1) conceptualize the depicted scenario in a less negative way (e.g., women crying outside of a church could be attending a wedding instead of a funeral); or (2) objectify the content of the pictures (e.g., a woman with facial bruises could be an actor in a movie). For training in maintaining emotional reactivity, participants were instructed to passively process the negative images they were seeing (e.g., “view the picture without trying to change their emotional reaction”). Participants then performed eight trials with IAPS images not used in the actual task to practice implementing the “reappraise”, “maintain”, and “look” instructions. While participants viewed each practice image and in particular for negative images, the research staff asked them to verbalize emotion modification strategies and provided feedback to ensure participants understood task instructions and used appropriate strategies to cognitively reappraise emotional content of the images.
2.4. Electroencephalogram recording and initial data reduction
Continuous EEG recording during the task was completed using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, The Netherlands). Thirty-four electrode sites were used, based on the 10/20 system (standard 32 channel montage, as well as FCz and Iz). The voltage from each active electrode was referenced online with respect to a common mode sense (CMS) active electrode, producing a monopolar (non-differential) channel. Two electrodes were placed on the left and right mastoids, while an additional four facial electrodes were used to record the electrooculogram (EOG) generated from eye blinks and eye movements: two of these electrodes were located approximately one cm outside the outer edge of the right and left eyes and two electrodes were placed approximately one cm above and below the left eye. The EEG and EOG were low-pass filtered using a fifth order sinc filter with a half-power cutoff of 204.8 Hz and then digitized at 1024 Hz with 24 bits of resolution.
2.5. Offline data reduction and statistical analyses
Offline data processing was performed using Brain Vision Analyzer 2 software (BVA, Brain Products, Gilching, Germany). Data were re-referenced offline to the average of the two mastoids, and band-pass filtered from 0.01 to 30 Hz. Trials were segmented beginning 200 ms prior to picture onset and ending 7,000 ms after picture onset for a total segment duration of 7,200 ms. Following segmentation of data, eye blink and ocular corrections were made according to the method developed by Miller, Gratton and Yee (1988). Artifact analysis was used to identify a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100 ms intervals. Trials were also inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-by-trial basis. Only individuals retaining at least 80% or more data on each electrode channel were included in group averages. For participants included in the final analysis, we found no difference in data quality between PTSD and CEC groups as assessed by number of artifacts per condition (all p’s > 0.09). Trials were averaged separately for each condition and baseline correction was performed using the 200 ms period prior to picture presentation.
Based on visual inspection and on prior work (Dennis and Hajcak, 2009; Foti and Hajcak, 2008; Hajcak and Olvet, 2008), two electrode poolings were used for extraction: first, at a central-parietal location consisting of Pz, CP1, and CP2 and, second, at a frontal location consisting of Fz, AF3, and AF4. The pre-instruction LPP was calculated using mean amplitudes from 400 – 1,000 ms post-picture onset. The post-instruction LPP was calculated using mean amplitudes in each of three time windows: early (1,500 – 3,000 ms post-picture onset), middle (3,000 – 5,000 ms post-picture onset), and late (5,000 – 7,000 ms post-picture onset), in agreement with prior work (Dunning and Hajcak, 2009; Parvaz et al., 2012). For figures, a digital low-pass filter (12 Hz) was applied offline for plotting grand-averaged waveforms while electrophysiological activity using original filter settings was used for all statistical analyses.
Pre-instruction LPP amplitudes were analyzed separately at the centro-parietal and frontal electrode poolings using a 2 (group: PTSD, CEC) × 3 (condition: look, maintain, reappraise) mixed measures analysis of variance (ANOVA) while post-instruction amplitudes were analyzed using a 2 (group: PTSD, CEC) × 3 (condition: look, maintain, reappraise) × 3 (time: early, middle, late) mixed measures ANOVA. Interactions involving the effect of time were explored using linear contrasts to assess for changes in the LPP throughout picture presentation. Greenhouse-Geisser corrections were used in instances where assumptions of sphericity were violated. All analyses were performed using IBM SPSS Statistics (Version 22.0).
3. Results
Figure 1 presents grand-averaged waveforms, shown separately for each LPP pooling and group. Figure 2 depicts the topographical distribution of the LPP by group at both pre- and post-instruction. Mean LPP amplitudes are presented in Table 2.
Figure 1.
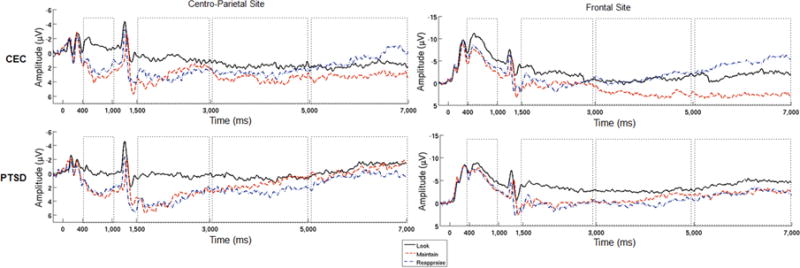
Grand-averaged waveforms at centro-parietal and frontal sites at which the LPP was scored, shown separately for each group and condition. Note. Stimulus onset occurred at 0 ms, task instruction occurred at 1,000 ms. Dashed boxes indicate the time-windows in which the LPP was analyzed. On the y-axis, positive amplitude is plotted down. CEC = combat exposed controls; PTSD = posttraumatic stress disorder.
Figure 2.
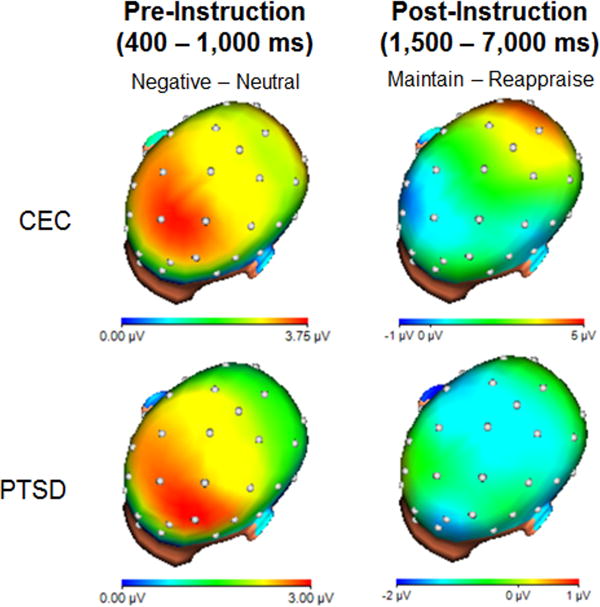
Headmaps depicting the spatial distribution of voltage differences for negative images minus neutral images, from 400 to 1,000 ms after picture onset (LPP during initial reactivity; left), and the maintain condition minus the reappraise condition, from 1,500 to 7,000 ms after picture onset (LPP post-instruction; right) for CEC and PTSD groups.
Table 2.
LPP Amplitudes by Condition, Group and Electrode Pooling Sites
| Centro-Parietal Sites
|
Frontal Sites
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Look
|
Maintain
|
Reappraise
|
Look
|
Maintain
|
Reappraise
|
|||||||
| CEC M μV (SD) |
PTSD M μV (SD) |
CEC M μV (SD) |
PTSD M μV (SD) |
CEC M μV (SD) |
PTSD M μV (SD) |
CEC M μV (SD) |
PTSD M μV (SD) |
CEC M μV (SD) |
PTSD M μV (SD) |
CEC M μV (SD) |
PTSD M μV (SD) |
|
| Time window | ||||||||||||
| Pre-Instruction (400 – 1,000 ms) |
−1.06 (3.72) |
−0.12 (2.82) |
2.98 (3.95) |
2.75 (4.18) |
2.16 (5.24) |
2.35 (4.53) |
−8.33 (5.70) |
−6.83 (4.29) |
−5.11 (4.74) |
−5.56 (5.73) |
−5.83 (6.56) |
−5.49 (5.67) |
| Early Post-Instruction (1,500 – 3,000 ms) |
0.83 (5.22) |
0.30 (4.53) |
2.97 (8.49) |
3.74 (5.99) |
3.05 (8.09) |
3.77 (5.27) |
−2.12 (6.99) |
−3.39 (4.26) |
0.33 (6.88) |
0.01 (6.46) |
−0.03 (6.70) |
0.33 (6.36) |
| Middle Post-Instruction (3,000 – 5,000 ms) |
1.79 (5.76) |
0.61 (5.47) |
3.58 (9.59) |
1.55 (6.66) |
2.77 (10.69) |
2.51 (5.28) |
−0.68 (6.82) |
−2.66 (6.37) |
2.41 (7.53) |
−0.92 (9.27) |
−1.03 (9.16) |
0.01 (5.31) |
| Late Post-Instruction (5,000 – 7,000 ms) |
1.76 (7.03) |
−0.78 (5.15) |
3.20 (7.82) |
−0.34 (7.20) |
0.84 (7.97) |
0.53 (5.41) |
−1.80 (7.21) |
−4.35 (6.63) |
2.76 (7.67) |
−2.27 (8.87) |
−4.40 (9.36) |
−2.21 (5.11) |
Note. LPP = late positive potential; CEC = combat exposed controls; PTSD = posttraumatic stress disorder.
3.1. Pre-instruction LPP
There was a significant main effect of condition at the centro-parietal pooling (F(2, 96) = 20.76, p < 0.001, = 0.30). Follow-up tests revealed no difference in LPP amplitude between maintain and reappraise conditions during this window prior to actual task instruction (F(1, 49) = 0.99, p = 0.33, = 0.02). However, compared to the look condition, a larger LPP was evident during both maintain (F(1, 49) = 39.21, p < 0.001, = 0.44) and reappraise (F(1, 49) = 27.39, p < 0.001, = 0.36) conditions, suggesting that – as expected – the aversive/emotionally-negative images elicited a greater LPP than non-emotional images. There was no effect of group and no group × condition interaction (all p’s > 0.59). To note, early ERPs (e.g., < 300 ms) at this location appeared smaller in the PTSD group compared to the CEC group.
Similar results were found at the frontal pooling site: there was a significant main effect of condition (F(2, 96) = 5.70, p = 0.01, = 0.11). Follow-up tests showed no difference between maintain and reappraise conditions (F(1, 49) = 0.27, p = 0.61, = 0.01). However, larger LPP’s were found during both the maintain and reappraise conditions in comparison to the look condition (F(1, 49) = 8.75, p = 0.01, = 0.15 and F(1, 49) = 6.55, p = 0.01, = 0.12, respectively). Again there was no effect of group and no group × condition interaction (p’s > 0.70).
3.2. Post-instruction LPP
At the centro-parietal pooling, there was a significant main effect of time (F(1.37, 65.83) = 5.52, p = 0.01, = 0.10). Follow-up comparisons determined a linear decrease in LPP amplitude across groups and conditions (early > middle > late; F(1, 48) = 6.19, p = 0.02, = 0.11). No other significant effects were found (all p’s > 0.06).
At the frontal pooling, we observed a significant main effect of time (F(1.27, 60.99) = 5.05, p = 0.02, = 0.10), a condition × time interaction (F(2.80, 134.33) = 5.03, p < 0.01, = 0.10) and a group × condition × time interaction (F(2.80, 134.34) = 3.47, p = 0.02, = 0.07). No other effects reached significance (all p’s > 0.06).
To explore the significant three-way interaction, we conducted separate group × time mixed-measures ANOVAs within each condition. For the look condition, no significant main or interaction effects were found (all p’s > 0.13). In the reappraise condition, a main effect of time (F(1.41, 67.87) = 13.42, p < 0.001, = 0.22) was driven by a linear decrease in amplitude (early > middle > late, F(1, 48) = 14.49, p < 0.001, = 0.23); no other effects reached significance (all p’s > 0.40). That is, across the groups, the LPP decreased over time during reappraise trials. In the maintain condition, there was no overall effect of time or group (all p’s > 0.16), but a significant group × time interaction (F(1.43, 68.69) = 4.64, p = 0.02, = 0.09). Follow-up tests showed that in the PTSD group there was no significant effect of time (F(1.46, 34.96) = 1.62, p = 0.22, = 0.06). In contrast, in the CEC group, there was a main effect of time (F(1.32, 31.56) = 4.18, p = 0.04, = 0.15) that was explained by a linear increase in the LPP across time (F(1, 24) = 4.17, p = 0.05, = 0.15). Therefore, we found that the LPP increased during maintain trials in the CEC group and that this effect was absent within the PTSD group.1
4. Discussion
The current study examined differences in LPP amplitude during cognitive reappraisal of negative pictures in a group of OEF/OIF/OND combat-exposed veterans with and without PTSD. As expected, negative images elicited an enhanced LPP and cognitive reappraisal reduced the LPP to negative pictures across participants. Moreover, this effect did not differentiate individuals with from those without PTSD. In other words, both groups effectively decreased the LPP as a function of cognitive reappraisal. Group differences in the LPP to negative pictures prior to instruction onset – i.e., during passive picture viewing – were not observed, suggesting that both groups reacted similarly in response to negative emotion evocation. However, veterans without PTSD showed an increase in the LPP over time as a function of sustained maintenance of emotional reactivity during the post-instruction period, whereas those with PTSD did not.
To our knowledge, this is the first study to document that combat-exposed veterans both with and without PTSD are able to down-regulate the LPP via cognitive reappraisal. Prior work (Woodward et al., 2015) examined group differences in LPP amplitude as a function of varied voluntary regulation strategies, however results showed that down-regulation did not modulate the LPP in either controls or veterans with PTSD, making it difficult to draw conclusions about the absence of group differences observed in that study. In the Woodward and colleagues’ study (2015), the “rationalize” condition instructed participants to reduce the emotional salience of pictures using a cognitive strategy (i.e., by thinking about something else), however the nature of this strategy seems not to have been specified (e.g., both distraction and reappraisal involve thinking about something else). The “notice” strategy asked participants to focus on their emotional reactions, a technique that may be more akin to up-regulation than down-regulation (Hajcak et al., 2009). Therefore, the emotion regulation techniques used by Woodward and colleagues (2015) are quite different than those used in the present study, which are consistent with those used in a wide body of prior work (Hajcak and Nieuwenhuis, 2006; New et al., 2009; Parvaz et al., 2012; Rabinak et al., 2014). Moreover, Woodward and colleagues (2015) analyzed the notice and rationalize conditions together, which could have confounded up- and down-regulatory effects. The present study adds to this prior work – first, by showing successful modulation of the LPP via cognitive reappraisal in veterans with and without PTSD and, second, by showing that these effects do not differ between groups.
The absence of a group difference in reappraisal, while contrary to our hypothesis, is broadly in line with prior fMRI work that found no difference in reappraisal-related brain activity for traumatized women with versus without PTSD (New et al., 2009). However, in contrast to New and colleagues’ (2009) results, Rabinak and colleagues (2014) found that military veterans with versus without PTSD showed reduced recruitment localized to the dlPFC during reappraisal to negative imagery. Both studies suggest that PFC alterations in PTSD during emotion regulation are not profuse. Interestingly, neither study found group differences in amygdala activation as a result of down-regulation, suggesting that differential recruitment of prefrontal regions in the PTSD group across these samples may not necessarily translate into discrepant modulation of affective responding. One possibility is that individuals with PTSD compensate for reduced PFC engagement during reappraisal by recruiting different brain regions to moderate emotional processing, though this hypothesis will need to be tested in future work. Another possible explanation for the absence of group differences observed here may be that the PTSD group was differentially impacted by the cognitive reappraisal training, building off the observation that this group exhibited qualitatively smaller early ERPs prior to task instruction (e.g., < 300 ms). To note, the bulk of PTSD studies report heightened early ERPs (50 – 3000 ms) during the viewing of trauma-related imagery (see Javanbakht et al., 2011 for a review).
Although we did not observe a group difference in the LPP elicited by negative stimuli prior to instruction onset, we did observe a post-instruction group difference in the LPP elicited by negative pictures presented in the maintain condition. Specifically, veterans with PTSD showed no change in the LPP during the maintenance of negative reactivity whereas controls showed an increase in the LPP over time in this condition. These results are in line with prior work suggesting that sustaining attention towards negative imagery increases the LPP among psychiatrically-healthy individuals (Dunning and Hajcak, 2009; Hajcak et al., 2006) (though not as much as when up-regulation is used (Gardener et al., 2013; Moser et al., 2010, 2009)). Interestingly, veterans with PTSD appear to have been more successful in maintaining (rather than increasing) attention toward negative pictures in the maintain condition, though the functional significance of this finding is as of yet unclear. For instance, maintaining an emotional response may require top-down direction from the PFC, which – given prior evidence of PFC deficits in PTSD (Rabinak et al., 2014) – might explain why the PTSD group showed smaller amplitudes than the CEC group in this condition. Avoidance is also a cardinal symptom of PTSD and could have played a role in blunting a maintain-related increase in the LPP. On the other hand, increased LPPs in the CEC group may reflect processes related to resiliency in this traumatized group that did not develop PTSD. To better characterize “normative” versus abnormal responding during emotional maintenance, future work may wish to include non-traumatized and traumatized control groups as well as a PTSD group. In addition, future studies directly comparing groups during down-regulation and up-regulation versus sustained affect maintenance and neutral picture viewing are warranted.
In the present study, condition effects were specific to the frontal sites while group effects (for the maintain condition) were specific to the late time window at frontal sites. Prior work suggests that the effects of cognitive reappraisal on the LPP are evident primarily during later portions of picture viewing (i.e., several seconds in; Foti et al., 2008; MacNamara et al., 2009; Parvaz et al., 2012), when the LPP is more likely to be maximal at frontal electrode sites (i.e., due to a topographic shift in the component; Foti et al., 2009; Hajcak et al., 2012; MacNamara et al., 2009). The late latency group difference in the maintain condition suggests that individuals with PTSD may show aberrant sustained processing of negative stimuli, though as noted above, more work is needed to differentiate emotional maintenance from up-regulation and to ascertain whether CEC effects observed are comparable to those observed in a non-traumatized control group.
An absence of group differences in the LPP during initial emotion processing (i.e. prior to regulation instruction) does not, at first glance, fit with prior findings of increased amygdala activation to negative or trauma-related stimuli reported in several studies of PTSD (Rauch et al., 2000; Shin et al., 2006, 2005). However, this finding has been inconsistently observed and other studies have either failed to find evidence of amygdala hyper-activation in PTSD (Bremner et al., 2003, 1999a, 1999b, Sakamoto et al., 2005; Shin et al., 1999, Yang et al., 2004) or have found evidence of reduced amygdala activation during fear processing in PTSD (Britton et al., 2005; Phan et al., 2006). Along these lines, a growing body of psychophysiological work has found evidence of emotional blunting in this population (D’Andrea et al., 2013; Felmingham et al., 2014; Kemp et al., 2009; MacNamara et al., 2013; McTeague et al., 2010; Shepherd and Wild, 2014; Tso et al., 2011; Woodward et al., 2015; Zaba et al., 2015). For instance, recent work by our group found that military veterans with PTSD showed smaller LPPs to angry faces, compared to their non-PTSD peers (MacNamara et al., 2013) (see also Tso et al., 2011). In another study, PTSD symptoms in a trauma-exposed sample were associated with increased difficulty enhancing emotional response to negative imagery as measured by skin conductance response, a measure of peripheral arousal (Shepherd and Wild, 2014). Together, this evidence suggests that exaggerated fear reactivity may not fully characterize PTSD (see also Lanius et al., 2012).
Findings from the current study should be considered in light of several limitations. First, the lack of a non-traumatized control group limits interpretation of our results; however both CEC and PTSD groups exhibited increased the LPP to negative pictures and decreased the LPP during reappraisal, similar to prior findings observed in healthy, non-traumatized volunteers. New and colleagues (2009) found evidence of trauma-related but not PTSD-related effects on reappraisal-related brain functioning; therefore future work may wish to determine whether traumatized individuals as a group (i.e., with and/or without PTSD) might show reduced reappraisal-related modulation of the LPP compared to non-traumatized controls. Second, because participants were not asked to make subjective ratings during the reappraisal task, we were unable to investigate group differences in subjective emotional responding initially or after instruction to reappraise/down-regulate the negative emotion. In the New and colleagues’ (2009) study, participants with PTSD showed less effective modulation of emotional arousal, as evidenced by subjective report; therefore, it is possible that we might have observed differences between groups using this measure (but see Rabinak et al., 2014 in which no group differences in subjective response were observed during the fMRI version of this task). Further, the results reflect a null effect perhaps due to insufficient power to detect group differences; therefore, future work may wish to replicate these results in a larger sample. In addition, given previous research suggesting that personalized stimuli impact the LPP, examination of the impact of individualized trauma stimuli on these processes in trauma survivors with and without PTSD is also warranted and may clarify the null effect observed in the current design.
In conclusion, the current study found that reappraisal-related reductions in the LPP did not differ for U.S. military veterans with versus without PTSD, implying broadly that this population does not display measurable deficits in this capacity, at least as far as this specific neural measure of emotional “output” is concerned (see also prior work that failed to find group differences in reappraisal-related modulation of other emotional outputs (i.e., amygdala reactivity in PTSD; New et al., 2009; Rabinak et al., 2014). This study also replicates prior PTSD work that used the LPP (Woodward et al., 2015) but – by showing for the first time that reappraisal effectively modulates the LPP in both veterans with and without PTSD – expands this work and demonstrates that groups do not differ in this regard. Finally, veterans with PTSD did not show increased LPPs during the sustained processing of negative stimuli when asked to maintain their response to these stimuli – a result which may fit with prior evidence of emotional blunting in PTSD (D’Andrea et al., 2013; Felmingham et al., 2014; Kemp et al., 2009; MacNamara et al., 2013; McTeague et al., 2010; Shepherd and Wild, 2014; Tso et al., 2011; Woodward et al., 2015; Zaba et al., 2015) and which opens avenues for future investigation.
Acknowledgments
The authors would like to acknowledge the OEF/OIF/OND veterans for their participation in this research study and, more importantly, for their dedication and service to the United States of America.
Disclosures
This research was supported by Award Number I01BX007080 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (KLP) and by a VA Merit Review Program Award (KLP). JMF is supported by T32 MH067631 and AM is supported by K23 MH105553. JAD, AEK, CR, RP, JG, EP, SAMR, and GH have no disclosures to report.
Footnotes
A significant group × condition × time interaction was also evident at the frontal electrode pooling when conditions reflected the use of two difference waves: reappraise – look versus maintain – look (F(1.48, 70.85) = 7.50, p < 0.01, = 0.14), which was later qualified by a significant group × condition interaction at the late time point (F(1, 48) = 4.78, p = 0.03). When groups were directly compared on difference waves at this time point, no significant effects emerged (all p’s > 0.06). However, follow-up tests demonstrated that the LPP was larger during the maintain-look difference wave compared to the reappraise – look difference wave but only for the CEC group (t(24) = −2.87, p = 0.01). To note, it is not possible to attribute this effect in the CEC group to a reduction of the LPP in the regulation condition as this effect is driven by a combination of a maintain-related increase in the LPP and a reappraisal-related decrease. No significant group × condition × time interaction was evident at the centro-parietal electrode pooling (p > 0.12)
Contributions
Data analysis and manuscript preparation was completed by JMF with assistance from AM and KLP. The original study was designed by KLP with consultation from CR, SAMR, and GH. JAD, AEK, RP, JG, and EP assisted with data collection.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Publishing, Inc; Arlington, VA: 2013. [Google Scholar]
- Badour CL, Feldner MT. Trauma-related reactivity and regulation of emotion: associations with posttraumatic symptoms. J Behav Ther Exp Psychiatry. 2013;44:69–76. doi: 10.1016/j.jbtep.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Boden MT, Bonn-Miller MO, Kashdan TB, Alvarez J, Gross JJ. The interactive effects of emotional clarity and cognitive reappraisal in Posttraumatic Stress Disorder. J Anxiety Disord. 2012;26:233–238. doi: 10.1016/j.janxdis.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999a;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999b;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AC. The US framework for understanding, preventing, and caring for the mental health needs of service members who served in combat in Afghanistan and Iraq: a brief review of the issues and the research. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.24713. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Res. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- D’Andrea W, Pole N, DePierro J, Freed S, Wallace DB. Heterogeneity of defensive responses after exposure to trauma: blunted autonomic reactivity in response to startling sounds. Int J Psychophysiol. 2013;90:80–89. doi: 10.1016/j.ijpsycho.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J Child Psychol Psychiatry. 2009;50:1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning JP, Hajcak G. See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46:28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb N, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, Gellato C, Eric F, Lefebvre MN, Rouby F, Samuelian JC, Anton JL, Blin O, Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Falconer EM, Williams L, Kemp AH, Allen A, Peduto A, Bryant RA. Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One. 2014;9:e103653. doi: 10.1371/journal.pone.0103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20:977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation. Ann N Y Acad Sci. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Gardener EK, Carr AR, Macgregor A, Felmingham KL. Sex differences and emotion regulation: an event-related potential study. PLoS One. 2013;8:e73475. doi: 10.1371/journal.pone.0073475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, Simons RF. Perceived parental support predicts enhancedlate positive event-related brain potentials to parent faces. Biol Psychol. 2011;86:26–30. doi: 10.1016/j.biopsycho.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. Guilford Press; New York, NY: 2007. [Google Scholar]
- Hajcak H, Dunning JP, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clin Neurophysiol. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet D. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. Oxford University Press; New York, NY: 2012. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Brit J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Seeing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Hermann A, Keck T, Stark R. Dispositional cognitive reappraisal modulates the neural correlates of fear acquisition and extinction. Neurobiol Learn Mem. 2014;13:115–124. doi: 10.1016/j.nlm.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Javanbakht A, Liberzon I, Amirsadri A, Gjini K, Boutros NN. Event-related potential studies of post-traumatic stress disorder: a critical review and synthesis. Biol Mood Anxiety Disord. 2011;1 doi: 10.1186/2045-5380-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. J Consult Clin Psych. 1989;1:53–55. [Google Scholar]
- Kemp AH, Felmingham KL, Falconer E, Liddell BJ, Bryant RA, Williams LM. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: an fMRI study. Psychiatry Res. 2009;174:158–161. doi: 10.1016/j.pscychresns.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Klemanski DH, Mennin DS, Borelli JL, Morrissey PM, Aikins DE. Emotion-related regulatory difficulties contribute to negative psychological outcomes in active-duty Iraq war soldiers with and without posttraumatic stress disorder. Depress Anxiety. 2012;29:621–628. doi: 10.1002/da.21914. [DOI] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, Phan KL. Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. J Abnorm Child Psychol. 2015;43:1493–1501. doi: 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, Technical Report A-8. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biol Psychol. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depress Anxiety. 2010;27:234–243. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, Phan KL. Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biol Psychol. 2013;94:441–449. doi: 10.1016/j.biopsycho.2013.08.009. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowski JM, Melzig CA, Weike AI, Stockburger J, Schupp HT, Hamm AO. Brain dynamics in spider-phobic individuals exposed to phobia-relevant and other emotional stimuli. Emotion. 2009;9:306–315. doi: 10.1037/a0015550. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM. Generalized implementation of an eye movement correction procedure. Psychophysiology. 1988;25:241–243. [Google Scholar]
- Moran TP, Jendrusina AA, Moser JS. The psychometric properties of the late positive potential during emotion processing and regulation. Brain Res. 2013;1516:66–75. doi: 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, LaBar KS, McCarthy G. The role of trauma–related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, Simons RF. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Most SB, Simons RF. Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioral and electrophysiological study. Cogn Affect Behav Neurosci. 2010;10:195–207. doi: 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, Hajcak G. Event-related induced alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cogn Affect Behav Neurosci. 2012;12:730–740. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 2014;31:851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Ubel S, Leutgeb V, Schienle A. Cognitive reappraisal fails when attempting to reduce the appetitive value of food: an ERP study. Biol Psychol. 2013;94:507–512. doi: 10.1016/j.biopsycho.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shepherd L, Wild J. Emotion regulation, physiological arousal and PTSD symptoms in trauma-exposed individuals. J Behav Ther Exp Psychiatry. 2014;45:360–367. doi: 10.1016/j.jbtep.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC. Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology. 2012;62:598–606. doi: 10.1016/j.neuropharm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Stockburger J, Schmälzle R, Flaisch T, Bublatzky F, Schupp HT. The impact of hunger on food cue processing: an event-related brain potential study. Neuroimage. 2009;47:1819–1829. doi: 10.1016/j.neuroimage.2009.04.071. [DOI] [PubMed] [Google Scholar]
- Tacikowski P, Nowicka A. Allocation of attention to self-name and self-face: an ERP study. Biol Psychol. 2010;84:318–324. doi: 10.1016/j.biopsycho.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67:614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Tso IF, Chiu PH, King-Casas BR, Deldin PJ. Alterations in affective processing of attack images following September 11, 2001. J Trauma Stress. 2011;24:538–545. doi: 10.1002/jts.20678. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Costanzo ME, Blair JR, Roy MJ. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage Clin. 2014;7:19–27. doi: 10.1016/j.nicl.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Shurick AA, Alvarez J, Kuo J, Nonyieva Y, Blechert J, McRae K, Gross JJ. A psychophysiological investigation of emotion regulation in chronic severe posttraumatic stress disorder. Psychophysiology. 2015;52:667–678. doi: 10.1111/psyp.12392. [DOI] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schubert CF, Novak B, Huber C, Köhler K, Holsboer F, Pütz B, Müller-Myhsok B, Höhne N, Uhr M, Ising M, Herrmann L, Schmidt U. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 2015;55:102–115. doi: 10.1016/j.psyneuen.2015.02.005. [DOI] [PubMed] [Google Scholar]


