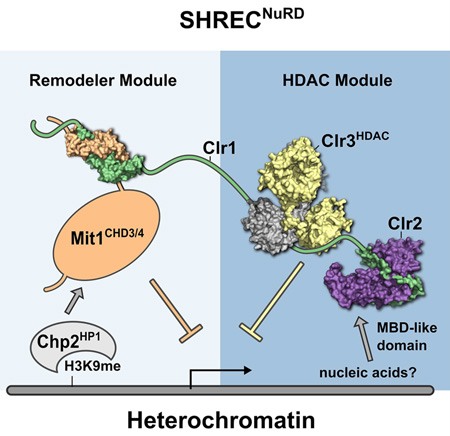
Summary
Nucleosome remodeling and deacetylation (NuRD) complexes are co-transcriptional regulators implicated in differentiation, development and diseases. Methyl-CpG Binding Domain (MBD) proteins play an essential role in recruitment of NuRD complexes to their target sites in chromatin. The related SHREC complex in fission yeast drives transcriptional gene silencing in heterochromatin through cooperation with HP1 proteins. How remodeler and histone deacetylase (HDAC) cooperate within NuRD complexes remains unresolved. We determined that in SHREC the two modules occupy distant sites on the scaffold protein Clr1, and that repressive activity of SHREC can be modulated by the expression level of the HDAC-associated Clr1 domain alone. Moreover, the crystal structure of Clr2 reveals an MBD-like domain mediating recruitment of the HDAC module to heterochromatin. Thus SHREC bi-functionality is organized in two separate modules with separate recruitment mechanisms, which work together to elicit transcriptional silencing at heterochromatic loci.
Keywords: Heterochromatin, gene silencing, centromere, x-ray crystallography, histone deacetylase, Schizosaccharomyces pombe, chromatin remodeling, NuRD complexes, Methyl-CpG-binding proteins
Introduction
Nucleosome remodeling and deacetylase (NuRD) complexes complexes play important roles in development (Hu and Wade, 2012) and aging (Pegoraro et al., 2009), as they sustain specific gene expression programs required for lineage specification. Gaining insight into the regulation and function of NuRD like complexes is important since NuRD components are frequently mutated in cancer (Lai and Wade, 2011), and play key roles in regulation of stem cell function and in the reprogramming of induced pluripotent stem cells (Rais et al., 2013).
The NuRD complexes in mammalian systems form a family of diverse complexes that share two histone deacetylases (HDAC) and two chromatin remodeling subunits as well as several subunits involved in recruitment and structural aspects, amongst them the metastasis associated proteins (MTA) and methyl-CpG-binding domain (MBD) proteins (Allen et al., 2013; Torchy et al., 2015). This structural heterogeneity makes higher eukaryotic NuRD complexes difficult to characterize, and consequently the overall structure of NuRD complexes and their mechanism of action are poorly understood. A fundamental question is why remodeling and HDAC activities are physically linked in NuRD complexes, and how complex formation affects their function.
The Snf2/Hdac Repressive Complex (SHREC) is the fission yeast NuRD equivalent, and plays a major role in transcriptional gene silencing (TGS) within S. pombe heterochromatin. SHREC consists of the chromatin remodeler Mit1, the HDAC Clr3 and the poorly understood Clr1 and Clr2 proteins (Sugiyama et al., 2007). It is recruited to heterochromatin through the HP1 homolog Chp2 (Motamedi et al., 2008) (Fig. 1A). SHREC is a promising model system for NuRD complexes due to its simplicity. However, its mechanism of recruitment through HP1 proteins sets it apart from animal and plant NuRD complexes.
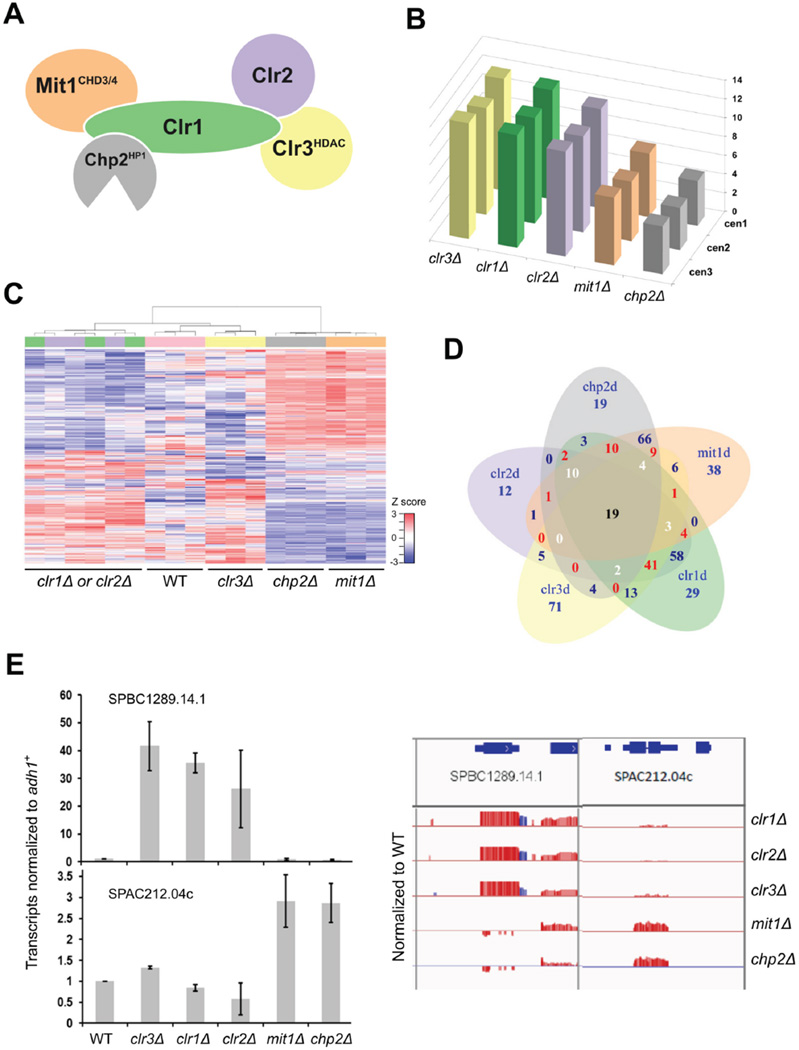
Figure 1. SHREC subunits show separation of function in gene expression.
(A) Scheme of SHREC complex. (B–E) RNA-seq analysis of strains deleted for individual SHREC components. (B) Representation of number of reads from repetitive sequences located at the centromeres in different genetic backgrounds normalized to WT cells. (C) Unsupervised Hierarchical Clustering Analysis of the top 1000 most variable genes based on MAD score (median absolute deviation). Blue lines represent individual transcripts whose expression is low, and red those that are highly expressed. (D) Venn diagram showing overlap in differentially regulated genes (>1.5 fold difference, <0.05 FDR). Blue numbers reflect overlap between 2 genotypes, red between 3 genotypes, white between 4 genotypes and black between 5 genotypes. (E) Representative genes whose transcripts are differentially regulated between clr1Δ, clr2Δ and clr3Δ compared with mit1Δ and chp2Δ (>1.5 fold change and FDR <0.05). Q-RT-PCR (left panel) confirms RNA-seq patterns (right panel). Red are sense and blue are antisense transcript ratios shown as log2 ratio, with a scale of −2 to +2. Signal above the line represents overexpression, and below is repression relative to WT. See also Fig. S1.
Heterochromatin in S. pombe depends on both the chromatin machinery and the RNAi system (Creamer and Partridge, 2011). While the RNAi machinery is mainly involved in degradation of transcripts (Bühler and Moazed, 2007), it is the chromatin machinery including the Clr4 H3K9 methyltransferase, HP1 proteins and SHREC that promote transcriptional gene silencing (Alper et al., 2012; Grewal and Jia, 2007). Clr3 plays a central role in this system by preventing RNA polymerase access to heterochromatin (Alper et al., 2013; Buscaino et al., 2013; Garcia et al., 2010; Yamada et al., 2005). Clr3 is further involved in stabilization of heterochromatin by protecting nucleosomes from the histone turnover machinery (Aygün et al., 2013).
Mutation of SHREC subunits leads to a small to moderate derepression of centromeric and telomeric transcripts, with stronger effects at the silent mating type loci specifically for clr1Δ, clr2Δ and clr3Δ (Bjerling et al., 2004; Ekwall and Ruusala, 1994; Motamedi et al., 2008; Sugiyama et al., 2007; Thon and Klar, 1992; Thon et al., 1994). Nucleosome occupancy in heterochromatin also depends on SHREC subunits, but the effects on individual genomic loci show large variations between different SHREC mutant strains (Garcia et al., 2010). These data raise the possibility that the SHREC complex consists of distinct sub-entities that can function both independently of the whole complex or coordinately as the holo-complex. To test this hypothesis, we performed a detailed functional and structural characterization of the SHREC complex.
Results
SHREC subunits show separation of function in transcriptional regulation
To examine whether SHREC consists of functionally independent modules, we monitored transcriptional effects caused by deletion of individual components of SHREC by global profiling using RNA-seq. Previous studies have shown similarity in gene expression profiles for clr1Δ and clr3Δ (Hansen et al., 2005). All mutants showed upregulation of expression in regions of constitutive heterochromatin compared with wild type cells as previously observed (Fig. 1B), but we also detected altered expression of transcripts from euchromatic regions in some mutant backgrounds compared to wild type. We note that some of the differentially expressed genes are non-coding, and many are expressed at low levels in vegetative cells (Wilhelm et al., 2008).
Unsupervised hierarchical clustering (UHC) and multidimensional scaling analyses of the gene expression profiles revealed two distinct groups, with clr1Δ, clr2Δ and clr3Δ cells clustered with wild type, and chp2Δ clustered with mit1Δ cells (Fig. 1C and Fig. S1A). We found that gene expression patterns were similarly altered in clr1Δ and clr2Δ cells compared with wild type, and that clr3Δ showed a broader range of gene expression differences, but substantial overlap with clr1Δ and clr2Δ. In contrast, a quite distinct gene expression profile was seen for cells lacking mit1+ or chp2+.
Using false discovery rate of 5%, and a cut-off of 1.5 fold change, we found 60–100 upas well as down-regulated genes for each mutant (Fig. S1B and Table S1). As observed in UHC (Fig. 1C), a substantial number of differentially expressed genes overlap between clr1 Δ, clr2Δ and clr3Δ (Fig. 1D, 65 genes affected by all three), and 119 genes shared between mit1 Δ and chp2Δ. Interestingly, only 19 genes showed overlap of differential expression across all 5 genotypes, and the majority of these (17/19) mapped to heterochromatic loci such as subtelomeric regions or non-repetitive sequences within centromeres. This data suggests that although there is partial overlap in the gene targets of individual SHREC components, there is clustering of the genetic targets for Clr1, Clr2 and Clr3 and for Chp2 and Mit1.
We noted that this trend of differential regulation between SHREC components was also seen for centromeric transcripts. As fission yeast has large repetitive centromeric regions, we counted the number of non-unique reads that could be mapped to each one of the three centromeres. Total read counts for the three centromeric regions in clr1 Δ, clr2Δ and clr3Δ were twice the number counted for mit1Δ and chp2Δ (Fig 1B). This indicates that Clr1, Clr2 and Clr3 exert stronger effects on transcriptional silencing at centromeres than Chp2 and Mit1, implying separation of function between SHREC subunits at centromeres as well as non-centromeric loci.
To independently assess the trends seen in the RNA-seq analysis, we performed real time PCR using cDNA prepared from two additional sets of RNA samples. We confirmed the regulation patterns for several genes that were either upregulated in all backgrounds, upregulated in clr1 Δ, clr2Δ and clr3Δ, or upregulated in mit1Δ and chp2Δ (Fig. 1E and Fig. S1C). Our data reveals that SHREC components do not act as a single entity to promote transcriptional regulation, and suggests that there is a functional division of labor between Mit1 and its associated protein Chp2, and the other components of SHREC, Clr1, Clr2 and Clr3.
Overexpression of the Clr1 C-terminus restores silencing of centromeric heterochromatin
Published mass spectrometry results for SHREC suggest Clr1 provides a backbone upon which remodeler and HP1 protein associate N-terminally, while HDAC and Clr2 associate C-terminally (Fig. 1A) (Motamedi et al., 2008). Accordingly, the 1238 amino acid long and mainly unstructured Clr1 (Fig. S2A) is suggested to provide the physical link between remodeling and HDAC activities. We tested this model by mapping the domains of Clr1 required for the silencing function of SHREC using heterochromatin mediated silencing of a cen∷ura4+ marker at the centromere as a functional assay (Allshire et al., 1995). clr1Δ cells lose silencing of cen∷ura4+, and ectopic expression of Clr1 with a C terminal-3xHA tag from a low strength nmt1 (pREP81) promoter restores silencing to wild type levels, allowing growth of cells on FOA-containing media (Fig. 2A). We note that clr1+ is ~ 18 fold overexpressed from this plasmid when transcript levels are compared with endogenous clr1+ transcripts (Fig. S2B). Using a collection of truncation mutants that were stably expressed (Fig. S2C), we found that Clr1 deleted for N terminal or central sequences could rescue function, but that deletion of the C terminus of Clr1 impaired function. Unexpectedly, overexpression of just the C-terminal 268 amino acids of Clr1 (Clr1T) was capable of fully rescuing function (Fig. 2A). Since Clr1T includes the only annotated domain of Clr1, namely three zinc fingers harboring a tandem CWCH2 motif (Hatayama and Aruga, 2010), we tested their role in Clr1T function. Subdivision of Clr1T produced non-functional fragments and indicates that the zinc fingers as well as the very C-terminus of Clr1 are functionally important (Fig. S2D, S2E). These results demonstrate that the C-terminal 268 amino acids of Clr1 are required and have the potential to restore Clr1’s function in TGS.
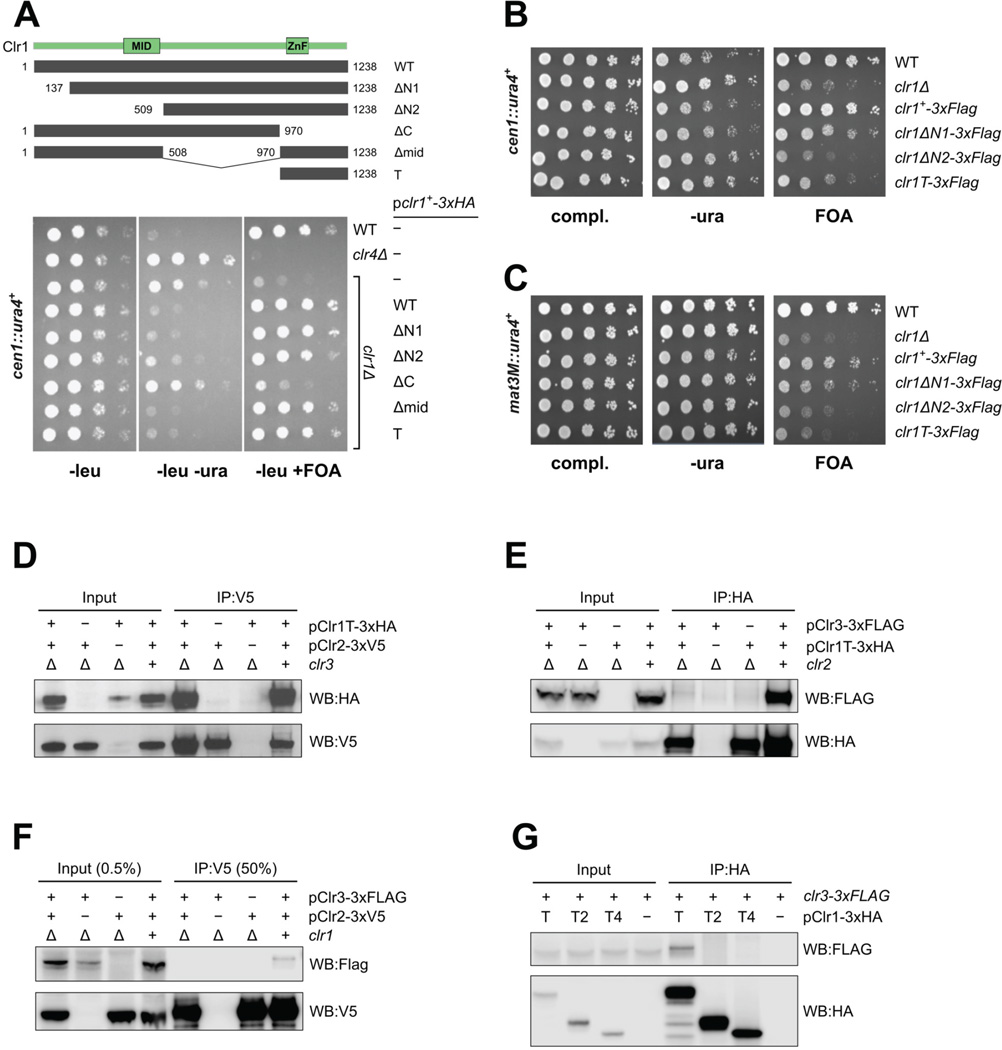
Figure 2. The C-terminal end of Clr1 is the assembly platform for the SHREC HDAC module.
(A) Serial dilution growth assay of clr1Δ cells expressing various domains of Clr1 (grey bars). Strains were assessed for growth on PMG media lacking leucine (PMG-leu) to select for maintenance of the plasmid, PMG-leu-ura to monitor cen∷ura4+ expression and PMG-leu+FOA to monitor silencing of cen1∷ura4+. The overexpressed Clr1T fragment is required and sufficient for silencing at cen1∷ura4+. (B–C) Serial dilution assay of strains bearing genomic clr1 truncation mutants assessed for silencing of (B) cen1∷ura4+ and (C) mat3M∷ura4+.clr1 truncations expressed at endogenous levels do not confer proper silencing at either locus. (D–G) Immunoprecipitation and western blot analyses reveal (D) Clr2 interacts strongly with Clr1T even in the absence of Clr3. (E) Clr1T binding to Clr3 is dependent on the presence of Clr2. (F) Clr3 binding to Clr2 is dependent on the presence of Clr1. (G) Clr3 binding appears to depend on both Zn finger and C terminal domains of Clr1T. See also Fig. S2.
In order to gauge Clr1 function in a more physiological setting, Clr1 truncation mutants were generated at the clr1 locus and silencing of ura4+ reporter genes was tested at centromeric (Fig 2B) and mating type loci (Fig 2C). These experiments show that expression of Clr1T at endogenous levels is not sufficient for silencing at either locus. This suggests that at endogenous levels of SHREC components, there is a dependence on both remodeler and HDAC modules for heterochromatin silencing, but that in principle the two activities are separable and do not strictly depend on a physical link between each other for silencing at the centromere.
The SHREC HDAC module assembles on the C-terminal end of Clr1
In order to characterize the composition of the SHREC subcomplex associated with Clr1T we performed immunoprecipitation (IP) experiments with Clr1T, Clr2 and Clr3 from fission yeast lysates (Fig. 2D, E, F). The IPs show that Clr1T efficiently pulls down Clr2, and this interaction is independent of Clr3 (Fig. 2D). In contrast, Clr3 association with the complex relies on both Clr2 (Fig. 2E) and Clr1 (Fig. 2F). Interaction between Clr3 and Clr2 is likely to be Clr1 mediated, since the interaction is Clr1 dependent and relatively weak, probably due to the low levels of endogenous Clr1 protein that mediates the interaction (Fig. 2F). We attempted to further map sub-domains of Clr1T that bind Clr3 in fission yeast cell extracts. However, neither the Zn finger domain Clr1T2 nor the C terminal domain Clr1T4 (Fig. S2D, S2E) were sufficient to pull down Clr3 (Fig. 2G). In agreement with our RNA-seq data and the published IP-MS data, these experiments reveal that the C-terminal 268 amino acids including the zinc fingers of Clr1 are at the core of the SHREC HDAC module, assembling the HDAC Clr3, and the subunit of unknown function Clr2.
The Mit1 C-terminus connects the remodeler with the Clr1 N-terminal half
We next sought to determine how the Mit1 remodeler gets integrated into SHREC by mapping interacting domains using the baculovirus expression system (Schalch et al., 2011). Screening of interactions between Mit1 and Clr1 fragments revealed that the C-terminal 261 amino acids of Mit1 (1156–1417) bind to a region of Clr1 between residues 357–500 (Fig. 3A, S3A, B).
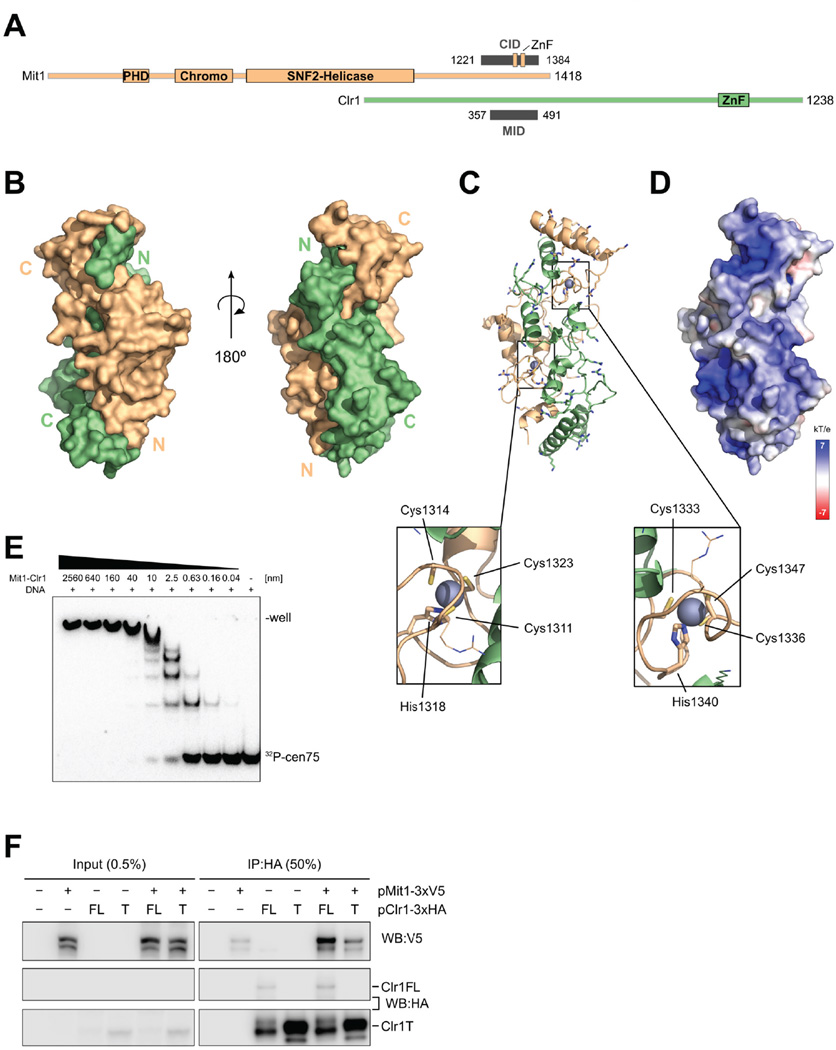
Figure 3. Mit1 interacts with Clr1 350–500 using C-terminal zinc finger motifs.
(A) Schematic view of domains forming the interaction between Mit1 and Clr1. Residues observed in the crystal structure as Mit1 interaction domain (MID) on Clr1 and Clr1 interaction domain (CID) on Mit1 are shown as gray bars. The two zinc fingers (ZnF) identified as part of the Mit1 CID are indicated. (B) Surface representation of the Mit1-Clr1 interface shows the two proteins Mit1 (orange) and Clr1 (green) twisting around each other. (C) Basic amino acids covering the surface of the Clr1-Mit1 complex are shown in stick representation (orientation as in (B), right side). Zoom outs highlight the two zinc fingers of the Mit1 fold. (D) Electrostatic surface representation of Clr1-Mit1 complex in same orientation as in (C). (E) EMSA assay of radiolabelled cen75 DNA analyzed on 5% polyacrylamide gel. (F) Immunoprecipitation and western blot analyses reveal Mit1 binds to full length but not to Clr1T. See also Fig. S3.
We determined the structure of the identified Clr1-Mit1 interaction domains by x-ray crystallography at 2.8 Å using the single anomalous dispersion (SAD) method (Fig. 3A, B, Table 1). This was facilitated by the presence of endogenously bound zinc ions in the crystal structure.
Table 1.
Data Collection and Refinement Statistics
| Clr1-Mit1 | Clr1-Clr2 |
Clr3 | ||||
|---|---|---|---|---|---|---|
| Native | Native | Peak | Inflection | High remote | Native | |
| Data Collection | ||||||
| Space group | C121 | P31 2 1 | P31 2 1 | P31 2 1 | P31 2 1 | C 2 2 21 |
| Cell dimensions | ||||||
| a, b, c (Å, °) | 105.7, 52.9, 82.0 |
103.5, 103.5, 135.1 |
103.7, 103.7,136.0 |
103.5, 103.5, 135.1 |
103.7, 103.7, 136.0 |
60.5, 187.3, 142.6 |
| α, β, γ | 90, 115.6, 90 | 90, 90, 120 | 90, 90, 120 | 90, 90,120 | 90, 90, 120 | 90, 90, 90 |
| Resolution range (Å) | 47.64–2.80 (2.95–2.80)a |
48.33–2.3 (2.38–2.30) |
45.33–2.60 (2.72–2.60) |
48.33–2.3 (2.38–2.30) |
45.33–2.60 (2.72–2.60) |
46.81–2.40 (2.46–2.40) |
| Wavelength (Å) | 1.00004 | 0.9763 | 0.9792 | 0.9763 | 0.9792 | 0.97924 |
| Rmerge | 0.059 (0.377) | 0.042 (0.845) | 0.091 (1.775) | 0.042 (0.845) | 0.091 (1.775) | 0.072 (0.60) |
| Mn(I) half-set correlation CC(1/2) |
0.99 (0.96) | 1.00 (0.77) | 1.00 (0.32) | 1.00 (0.77) | 1.00 (0.32) | 1.00 (0.88) |
| Mean I/σ(I) | 21.0 (4.5) | 24.9 (2.2) | 19.7 (0.9) | 24.9 (2.2) | 19.7 (0.9) | 14.86 (2.50) |
| Completeness (%) | 99.8 (98.8) | 100.0 (100.0) | 99.4 (95.1) | 100.0 (100.0) | 99.4 (95.1) | 98.9 (87.7) |
| Multiplicity | 6.6 (6.5) | 7.6 (7.9) | 9.8 (5.8) | 7.6 (7.9) | 9.8 (5.8) | 6.6 (5.9) |
| Wilson B (Å2) | 55.96 | 54.9 | 63.7 | 54.9 | 63.7 | 52.3 |
| Refinement | ||||||
| Resolution range | 47.64 – 2.80 | 48.3–2.3 | 46.81–2.40 | |||
| Number of reflections | 10217 | 37690 | 31826 | |||
| Number of reflections in test set |
1983 | 3588 | 1535 | |||
| R factor (%) | 20.30 | 21.4 | 0.187 | |||
| Rfree (%) | 24.69 | 24.3 | 0.228 | |||
| No. of atoms - total | 2305 | 4507 | 4952 | |||
| Macromolecule | 2271 | 4403 | 4705 | |||
| Ligands | 4 | 0 | 34 | |||
| Solvent | 30 | 104 | 213 | |||
| No. of protein residues | 285 | 532 | 582 | |||
| R.m.s.d., bonds (Å) | 0.0026 | 0.002 | 0.005 | |||
| R.m.s.d., angles (°) | 0.468 | 0.523 | 0.65 | |||
| Ramachandran favored (%) |
98.2 | 97.54 | 97.8 | |||
| Ramachandran outliers (%) |
0.0 | 2.27 | 0.0 | |||
| Clash score | 1.30 | 0.19 | 0.64 | |||
| Average B factor (Å2) | 67.70 | 1.39 | 38.20 | |||
| Macromolecule | 67.94 | 70.2 | 38.5 | |||
| Ligands | 52.67 | 79.4 | 53.4 | |||
| Solvent | 51.17 | 62.0 | 28.6 | |||
Values in parentheses are for highest-resolution shell.
The crystal structure reveals that the two domains embrace each other (Fig 3B), thereby burying a total solvent accessible surface of 6250 Å2. The interacting domains are entirely α-helical, burying two zinc-binding motifs found in the C-terminus of Mit1 onto which the α-helices from both Clr1 and Mit1 pack (Fig. 3C). The zinc motifs correspond to CCHC zinc fingers, are very similar to one another and show strong similarity to the DNA binding zinc finger of the HIV-1 nucleocapsid (South and Summers, 1993) (Fig. S3C). The surface that the HIV zinc finger uses to bind to DNA is occluded for both Mit1 zinc fingers by α-helices of Clr1. Nevertheless, the surface of the Clr1-Mit1 complex is covered with basic amino acids, and one side of the complex has a pronounced positively charged surface potential (Fig. 3C, D), suggesting that it may bind to DNA. We have tested this possibility by performing EMSA experiments with the Clr1-Mit1 complex and a probe comprising 75 bp of S. pombe centromere dh repeat DNA (cen75) (Ishida et al., 2012) (Fig. 3E). Albeit non-specifically, the Clr1-Mit1 interface produces well defined shifts with an apparent dissociation constant of low nanomolar concentrations. This experiment shows that the C-terminal domain of Mit1, as well as its chromodomain (Creamer et al., 2014), has a significant potential for binding nucleic acids.
In summary, the Clr1-Mit1 pull-down experiments and crystal structure show that the Mit1 chromatin remodeler uses its C-terminal domain to intimately bind to the N-terminal half of Clr1 around residues 350–500 to integrate into the SHREC complex. Mit1 is thus separated by 470 amino acids from the Clr1 domain (Clr1T) that recruits the HDAC module, suggesting that the remodeler and HDAC modules of SHREC can silence centromeric heterochromatin when Clr1T is overexpressed, without a physical link between them.
In order to test connectivity between the remodeler and the HDAC directly in fission yeast, we evaluated whether Mit1 association with Clr1 was dependent on sequences N-terminal to Clr1T. We co-expressed plasmids encoding fully functional V5-tagged Mit1 (Creamer et al., 2014) and HA-tagged full length Clr1 or Clr1T in mit1Δ clr1Δ cells. IP experiments showed that Mit1 associated with full length Clr1 but binding dropped to background levels with Clr1T (Fig. 3F), confirming the importance of N terminal sequences of Clr1 for Mit1 association. This data shows Clr1 provides the physical link between HDAC and remodeling modules. However, the fact that Clr1T lacks association with Mit1 but can suffice for centromeric silencing when overexpressed, shows that the principal function of Clr1 is not just the linking of remodeling and HDAC activities, but assembly of the functionally important HDAC module on its C terminus.
Clr2 structure reveals methyl-CpG-binding (MBD), chromo barrel and bromo-adjacent homology (BAH) domains
The enigmatic Clr2 subunit of the SHREC module is required for silencing of heterochromatin (Bjerling et al., 2004; Ekwall and Ruusala, 1994; Thon et al., 1994). Based on the common phenotypes observed for Clr1T, Clr2 and Clr3, we hypothesized that chromatin recruitment of the HDAC module might require Clr2. To test this idea we decided to elucidate the Clr2 structure by X-ray crystallography.
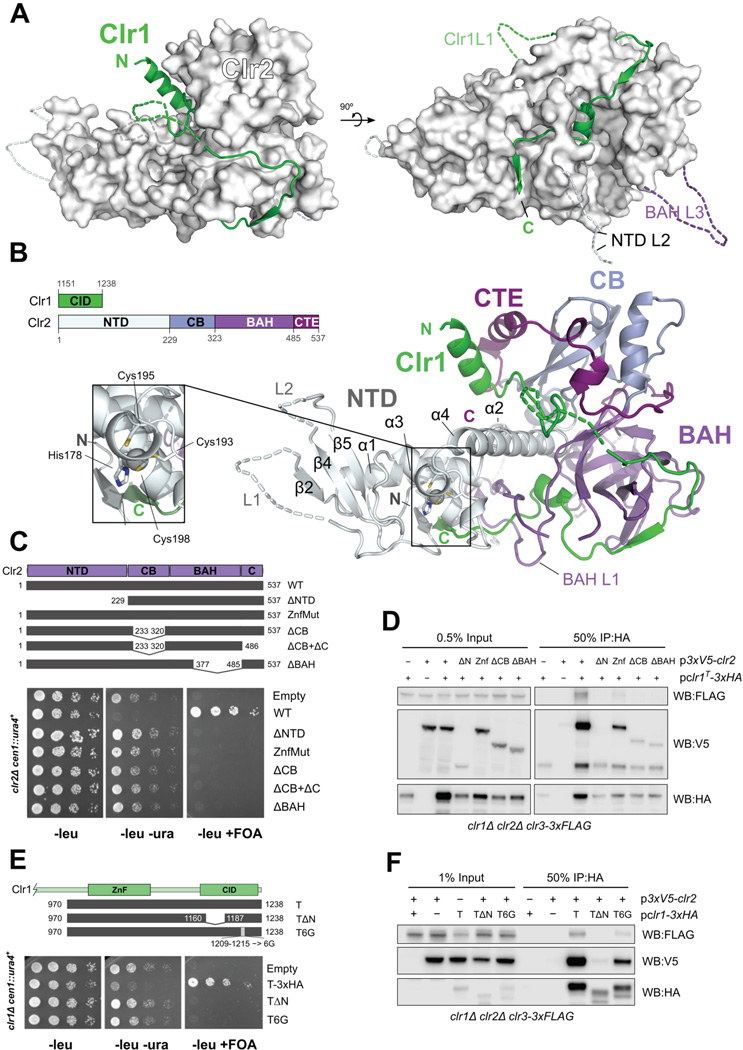
The Clr1 C-terminus (aa 1151–1238) associates strongly with Clr2 (Fig. S4A), and we solved the crystal structure of the corresponding Clr1-Clr2 complex to 2.3 Å (Table 1). The structure of the Clr1-Clr2 complex reveals a unique multi-domain assembly that resembles the shape of a jester's shoe (Fig. 4A, B) with Clr2 providing the structural core of the complex and Clr1 threading along the surface starting like shoelaces from the top part of the shoe down to the heel and along the sole towards the tip of the Clr2 shoe. Remarkably, the very C-terminus of Clr1 threads through a loop of Clr2 (Fig. 4A, B) and ends next to the Clr2 N-terminus. Clr2's structure can be divided into three tightly interdigitated domains (Fig. 4B, S4B): an N-terminal domain (NTD) and two internal domains followed by a C-terminal extension (CTE).
Figure 4. Crystal structure of the Clr1-Clr2 complex shows Clr1 wrapping around the multidomain protein Clr2.
(A) Surface representation of Clr2 in white with Clr1 in cartoon representation shown in green. Dashed lines indicate loops not observed in the electron density. (B) Clr1-Clr2 structure in cartoon representation colored by domain assignment as defined in the linear schematic (left). Zoom out shows structural zinc motif inside the Clr2 NTD.
(C) Serial dilution growth assays of clr2Δ cells expressing Clr2 truncation constructs (indicated schematically by grey bars) show that all Clr2 domains are required for function. (D) IP of Clr2 constructs in (C) by Clr1T and probing for interaction of Clr2 and Clr3 by Western blot. (E) Serial dilution assays of Clr1T mutants (indicated schematically) expressed in clr1Δ cells show that α1 and β1 domains are required for function. (F) IP of Clr1T constructs in (E) and probing for interaction with Clr2 and Clr3 by Western blot. See also Fig. S4.
The Clr2 NTD, which makes up the front of Clr2's shoe shape, contains a structural zinc ion, and has a mixed α/β fold (Fig. 4B). A bundle of three α-helices (NTD α1, α3, α5) with α3 in the center form the base onto which a small three-stranded β-sheet assembles (NTD β2,β4,β5), forming the tip of Clr2 jester's shoe. The zinc ion is coordinated by His178, Cys193, Cys195 and Cys198. His178 is part of the α3 helix while the cysteines are contributed by the loop between α3 and α4 (Fig. 4B, S4B). The long α4 helix anchors the N-terminal domain deep into a cleft between the two internal domains. α4 thereby bridges over the BAH domain's L1 loop that lassos the Clr1 C-terminus.
The first internal domain spanning residues 228–322 strongly resembles a chromo barrel (CB) domain (Nielsen et al., 2005). This Clr2 CB domain is highly similar to the CB domain of MOF and the chromodomain of MRG2 (Fig. S4C, Table S2). Many of these domains bind to methylated histone tail lysine residues, for example MRG2 binds H3K36me3 and H3K4me3 (Liu and Huang). However, Clr2 does not possess the aromatic cage residues required for methyl-lysine recognition (Fig. S4C) and is therefore unlikely to bind methylated histone tails. Encircled by the C-terminal extension (CTE) of Clr2, the CB domain forms the core of the ankle of the Clr2 shoe, sitting on top of the C-terminus of the NTD's α4 helix.
The second internal domain of Clr2 (residues 323–485), forms a warped β-sheet that resembles a BAH domain (Yang and Xu, 2013) (Table S3). The BAH domain of Clr2 extensively interacts with Clr1 and contains a long loop (L1) that encircles the Clr1 C-terminus (Fig. S4B, D), and makes up the heel of the shoe underneath the CB domain. The Clr2 BAH domain closely resembles the BAH domain of mouse Orc1 which binds the H4K20me2 histone tail. When superimposed, it becomes clear that the Clr2 BAH domain uses the same binding mode for binding the extended Clr1 peptide as Orc1 uses for binding the H4 tail (Fig. S4D). However, the methyllysine binding pocket does not exist in Clr2 since the BAH L2 loop between β4 and β5 occupies that space.
The interaction between Clr1 and Clr2 buries a large interface of 7180 Å2. Thus the Clr1 C-terminus and Clr2 form an intimate complex that involves all domains of Clr2, with the BAH domain contributing the largest part (3990 Å2).
Clr2 NTD is critical for chromatin targeting and TGS
We tested the role of the structurally determined Clr2 domains by engineering mutations into plasmid expressed 3xV5-clr2+ and assessing functional complementation in a clr2Δ cen∷ura4+ strain (Fig. 4C). We found that all domains (NTD, CB and BAH) are required for silencing. We next used IP assays to query whether Clr2's domains are required to bind Clr1. These experiments revealed that while most mutant Clr2 proteins were stably expressed (Fig S4E), all mutations weakened binding of Clr2 to Clr1T (Fig. 4D).
In a reciprocal experiment, we investigated the functional role of Clr1's structural motifs on silencing and complex formation. We first tested the impact of removal of the first α-helix (α1) and adjacent residues from Clr1T (Clr1TΔN), which removes the “shoelaces” and possible interactions with the CTE/CB of Clr2. Second, we tested the effect of weakening the interaction of Clr1 with the Clr2 BAH domain by replacement of β1 of Clr1T with glycines (Clr1T6G). Both mutants were stably expressed, albeit at lower levels than Clr1T, but completely disrupted the ability of Clr1T to silence the cen∷ura4+ transgene (Fig. 4E, F). Co-immunoprecipitation experiments revealed that Clr2 and Clr3 could not bind Clr1TΔN, but maintained association with Clr1T when the β-sheet at the back of the heel of the shoe was mutated (Fig. 4F). These results suggest that β1 of Clr1T (or heel of the shoe) may play a role in heterochromatin assembly distinct from maintaining integrity of the Clr1-2–3 complex. This notion is further supported by a mild defect in silencing of the cen∷ura4+ reporter (Fig. S4F, G) of the E376A,R373A mutant which maps close to the β1 interaction.
We also specifically tested the role of the structural zinc motif within the Clr2 NTD. Mutation of this motif (H178A, C193A, C195A, C198A) caused a loss of silencing of cen∷ura4+ (Fig. 4C), but maintained Clr1T and some Clr3 association (Fig. 4D). This data suggests that the zinc-stabilized structure of the NTD of Clr2 plays a role in heterochromatin function other than maintenance of Clr1-2–3 complex formation.
The mutational analysis shows that association of Clr2 with Clr1 is distributed across all domains of Clr2 and that integrity of the SHREC HDAC module is tightly linked to silencing function. Mutants in the zinc binding motif of Clr2’s NTD domain and the β1 motif of Clr1 maintain Clr1T-Clr2-Clr3 interaction but cause loss of function of the HDAC module. These motifs may play an important role in targeting the HDAC complex to chromatin.
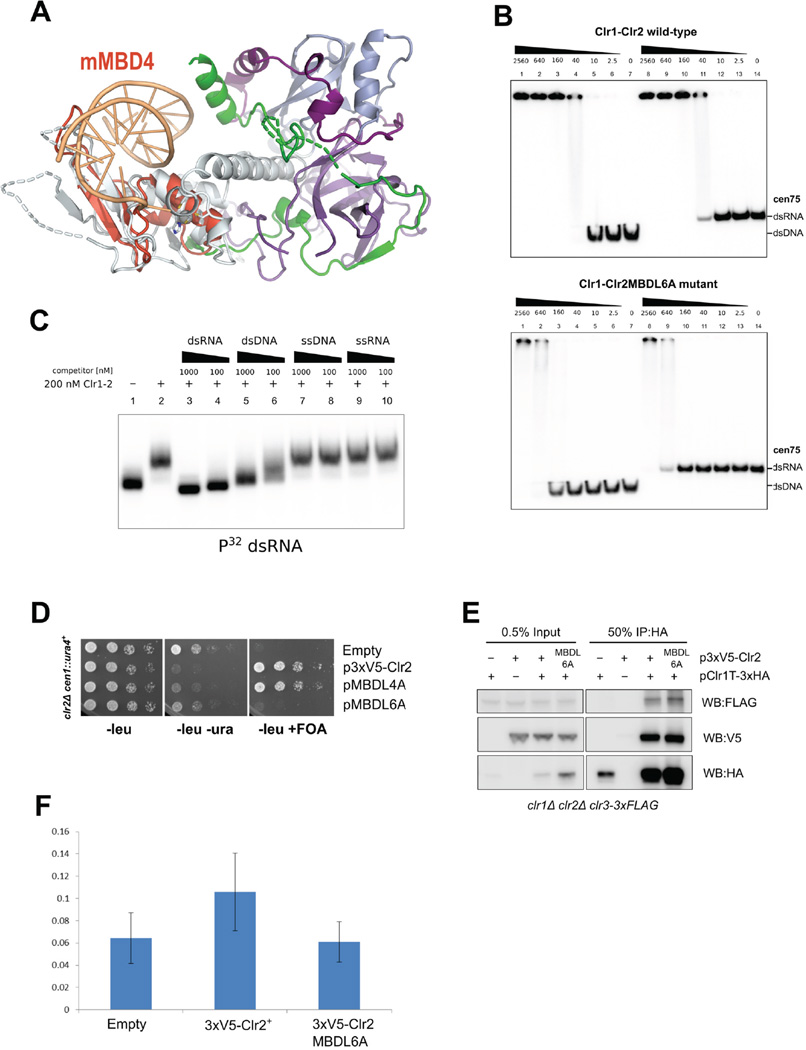
The Clr1–2 complex binds RNA and DNA through an MBD-like domain
Further examination of the Clr2 NTD revealed that the motif composed of β4, β5 and α3 (part of the zinc binding motif) shows strong similarity to the Methyl-CpG Binding Domain 4 (MBD4) protein (Otani et al., 2013) (Fig. 5A, Table S4). MBD proteins are small domains consisting of two β-strands and one α-helix that can specifically recognize methylated-CpG dinucleotides in double stranded DNA (Hendrich and Bird, 1998). MBD2 and MBD3 are mutually exclusive core members of the mammalian NuRD complexes (Le Guezennec et al., 2006) and it is striking to find an MBD-like (MBDL) domain in Clr2, especially since S. pombe has no DNA methylation (Capuano et al., 2014; Wilkinson et al., 1995). Sequence alignment of MBD domains with Clr2 (Fig. S5A) shows that critical residues important for DNA binding are conserved. It is thus likely that the Clr1-Clr2 complex (Clr1–2) has affinity for nucleic acids, and we tested nucleic acid binding of Clr1–2 by EMSA. We found that Clr1–2 bound to a library of random 21-mer DNA sequence embedded in 70-mer DNA oligos or to the cen75 sequence with apparent affinities around 40 nM (Fig. 5B, S5B). However, no defined complex is formed since no well defined shift is discernible and at saturating concentrations of Clr1–2, DNA-bound complexes are retained within the well of the gel.
Figure 5. Clr1-2 contains a MBD-like domain and binds to dsDNA and RNA.
(A) MBD4 structure with DNA (PDB ID: 3VYQ, orange) superimposes onto Clr2 N-terminal domain with RMDS of 2.8 Å. (B) EMS A of the wild type and the MBD6A mutant Clr1-Clr2 complex on 5% acrylamide gel. Nucleic acid substrates are 5'-P32 labeled cen75 dsDNA or dsRNA. (C) Competition of 32P labeled cen75 dsRNA with non-labeled dsRNA and DNA and single stranded RNA and DNA. (D) Serial dilution growth assays of Clr2 MBDL mutants show that the MBDL6A mutant loses silencing function. (E) IP and Western blot analysis shows that the Clr2MBDL6A mutant maintains interaction with Clr1T and Clr3. (F) ChIP for Clr2 at centromeric dh repeat shows that Clr2 is recruited by MBDL domain. See also Fig. S5.
In order to test if the nucleic acid binding activity of Clr1–2 originates from the MBDL domain we made a compound mutant of six basic residues that decorate the MBDL domain and are predicted to be in contact with DNA (Fig. S5A and S5C). This Clr2 MBDL6A mutant was stably expressed and purified from baculovirus, but significantly lost binding to dsDNA and dsRNA (Fig. 5B, S5B) and thus shows that the MBDL domain harbors the nucleic acid binding activity. We compared dsRNA and dsDNA affinity directly by binding the Clr1–2 complex to cen75 sequence that was prepared as dsDNA and dsRNA. Figure 5B shows that both dsDNA and dsRNA are bound by Clr1-Clr2, and quantitation yields estimates of apparent Kd of 20–30 nM (Fig. S5D). However, the Clr1–2 MBDL6A mutant was significantly more defective for RNA binding than DNA binding (32-fold vs. 13-fold loss). This indicates that the MBDL domain might bind to RNA with higher specificity. We further performed a competition assay for RNA binding. The cold dsRNA is a very efficient competitor while dsDNA is weaker, and neither single stranded DNA nor single stranded RNA competes with the binding (Fig. 5C).
To test if the nucleic acid binding activity is important for function of Clr2, we expressed the 3xV5-Clr2 MBDL6A mutant in S. pombe and assayed for silencing of the cen∷ura4+ reporter in a clr2Δ strain. Despite being stably expressed (Fig. S5E) the MBDL6A was not able to rescue the clr2 deletion. These results indicate that the nucleic acid binding of Clr2 is required for SHREC function. We assessed whether the MBDL6A mutation affected the integrity of the Clr1-2–3 complex by IP, and found no significant difference in Clr1T or Clr3 association of the mutant compared to wild type protein (Fig. 5E). We further tested whether the mutant caused a defect in recruitment of Clr2 to centromeres by chromatin IP (ChIP) (Fig. 5F). Whereas WT 3xV5-Clr2 was recruited to centromeric sequences, the MBDL6A mutant was not. These data highlight the importance of the MBD-like domain in targeting the HDAC submodule of SHREC to centromeric sequences.
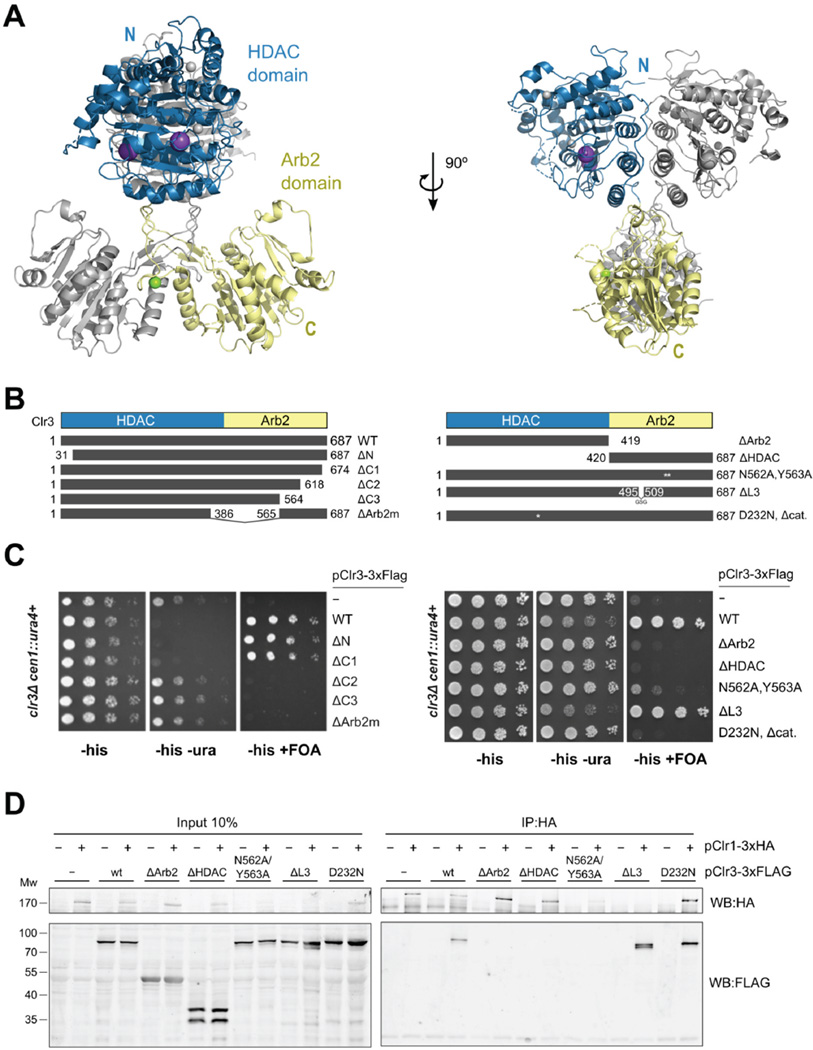
HDAC Clr3 dimer requires its C-terminal α/β-hydrolase fold for incorporation into SHREC
Our functional and biochemical characterization shows that the HDAC Clr3 assembles with Clr1T and Clr2 into a functional unit. The central catalytic activity of this module is Clr3, but structural information on Clr3 or other fungal class II HDACs is lacking. To understand Clr3 and how it integrates into the SHREC complex we have determined its crystal structure. Clr3 has the characteristic domain architecture of fungal class II HDACs and is a close homologue of the founding member of Class II HDACs, S. cerevisiae Hda1 (Fig. S6A) (Grewal et al., 1998; Yamada et al., 2005). Besides the catalytic HDAC domain, the fungal members of this class of HDACs have a C-terminal extension, called the Arb2 domain based on sequence homology to the Argonaute-binding protein-2 (Arb2) protein of the S. pombe ARC complex (Buker et al., 2007).
We solved the structure of fully functional Clr3 (aa31–687) by molecular replacement at 2.4 Å (Table 1). The structure of Clr3 shows a dimeric architecture with separation between HDAC (residues 1–420) and Arb2 domains (residues 421–687) (Fig. 6A). Clr3 forms dimers both in solution and in the crystal structure (Fig. S6B). Both the HDAC domains and Arb2 domains contribute to an extensive dimerization interface, burying 7580 Å2, with the larger part provided by the Arb2 domains (4010 Å2). While the HDAC domains pack against each other using a large, flat interface, the Arb2 domains use a heavily intertwined interface. This interface is made up of a loop protruding from the core of the domain that forms a hollow tubular structure together with the sequence linking the HDAC domain to the core of the Arb2 domain. This hollow tube docks against the HDAC dimer and thus resembles a neck attached to two HDAC ‘heads’ (Fig. 6A).
Figure 6. Clr3 HDAC dimer uses the α/β-hydrolase-based Arb2 domain for silencing through SHREC.
(A) Clr3 structure with HDAC domains on top and Arb2 domains at the bottom. One of the Clr3 monomers (the asymmetric unit) is colored blue (HDAC) and yellow (Arb2 domain). Zinc ions are shown as gray, potassium as purple and magnesium as green spheres. (B) Schematic of Clr3-3xFLAG tagged mutants. (C) Comparative growth assay of serially diluted clr3Δ strains bearing a cen∷ura4+ reporter, which have been transformed with Clr3-3xFLAG tagged mutants. Strains were assessed for growth on PMG media lacking histidine (PMG-HIS) to select for maintenance of the plasmid, PMG-HIS-URA to monitor cen∷ura4+ expression and PMG-HIS+FOA to monitor silencing of cen∷ura4+. Truncation mutants (left panel) and structure-based mutants (right panel) were assayed. Integrity of Arb2 domain is required for silencing. (D) IP of Clr1 and analysis by Western blot identifies Arb2 mutants losing interaction with Clr1. See also Fig. S6.
Our structure reveals that the Arb2 domain has clear homology to α/β-hydrolases, but that it is lacking the catalytic triad of these enzymes (Fig. S6C). Mapping of conservation onto the Clr3 structure shows highly conserved patches around the HDAC active site and on the outside of the Arb2 domain (Fig. S6D). The Arb2 patch does not show features of an active site, and we hypothesise that it is used for protein-protein interactions.
We performed functional analysis of mutants that perturb specific features observed in the crystal structure, using a fully functional 3xFLAG tagged Clr3 expression construct (Fig. 6B, S6E, 6C). We found that the Arb2 domain is necessary for centromeric heterochromatin silencing (Fig. 6B, C) since loss of the Arb2 domain (ΔArb2), the Arb2 homology motif (ΔArb2m) and C-terminal truncations of 70 amino acids and larger (ΔC2, ΔC3) are not tolerated. Interestingly, the conserved patch on the surface of the Arb2 domain (N652, Y563) is also required for silencing. In contrast, the neck structure that could potentially transmit allosteric signals from the Arb2 domain to the HDAC domain is not required for silencing (ΔL3). Based on the silencing results we conclude that the Arb2 domain may act as an anchor for recruitment of Clr3 to the SHREC complex for silencing.
To test this hypothesis we assessed the interaction of Clr3 mutants with Clr1 through co-IP from fission yeast lysates (Fig. 6D). The IP results show that mutants that lack the HDAC domain (ΔHDAC), but not those lacking HDAC enzymatic activity (D232N) lose the interaction with Clr1. That the Arb2 domain alone (ΔHDAC) is not sufficient for interaction with Clr1 may be due to compromised structural integrity of the isolated Arb2 domain dimer, since a prominent breakdown product is seen in lysates of yeast expressing the ΔHDAC mutant (Fig. 6D) and expression of isolated Arb2 domains in insect cells produces heterogeneous protein preparations. The ΔL3 mutant retains association with Clr1, whereas both ΔArb2, and the Arb2 conserved patch mutant (N562A, Y563A) fail to be pulled down by Clr1. This lends support to the model that the Arb2 domain, through residues N562 and Y563, acts as an anchor that connects the HDAC activity of Clr3 to the SHREC complex.
Discussion
SHREC is composed of remodeling and HDAC modules
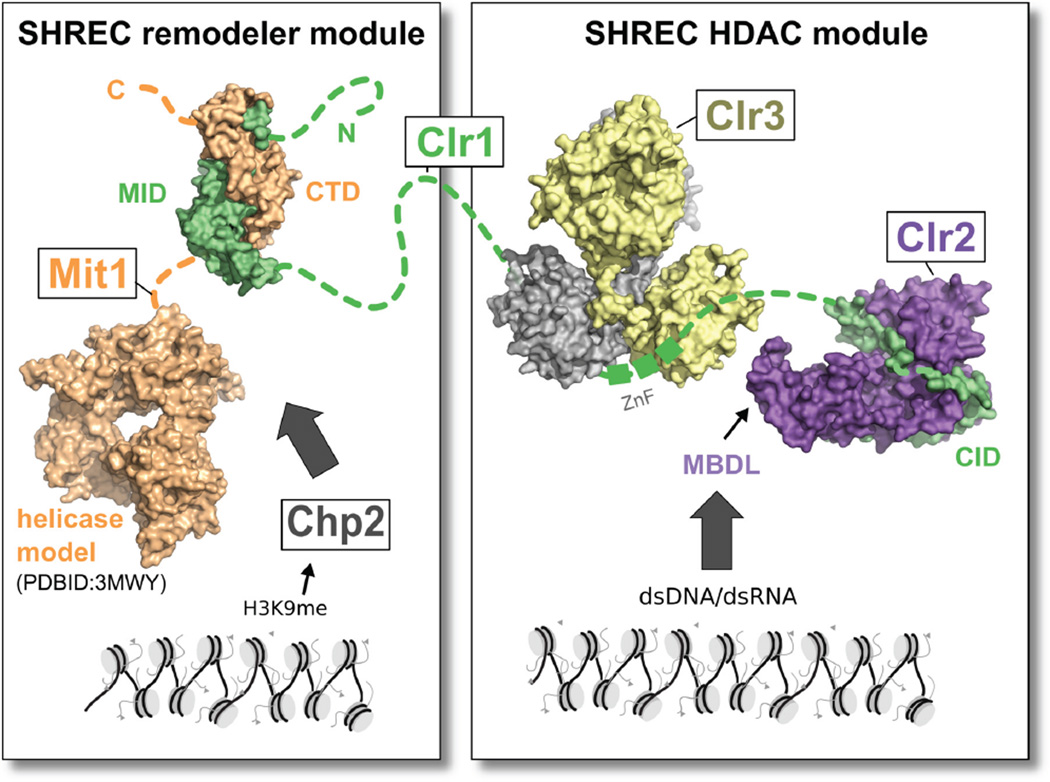
Our analysis reveals that SHREC consists of two sub-complexes: a chromatin remodeler module and an HDAC module. These modules exert different effects on the transcriptional program of fission yeast, but are held together in the SHREC complex by the long flexible Clr1 scaffold protein that binds the remodeler at its N terminus, and the HDAC module at its C terminal end (Fig. 7). The remodeler module is made up of Mit1 and its associated HP1 protein Chp2 (Motamedi et al., 2008), while the HDAC module comprising Clr2 and Clr3 is tied together by the Clr1 C terminus (Clr1T). The portion of Clr1 that links Mit1 and Clr3 is at least 470 amino acids long and predicted to be unstructured, suggesting that the two modules exert a high degree of independence even within the context of SHREC. Remarkably, the physically separated modules are capable of eliciting transcriptional silencing at centromeres when the HDAC-associated Clr1T fragment is overexpressed, suggesting they are recruited to heterochromatin through distinct mechanisms. We postulate that Chp2 binding to H3K9me is primarily responsible for recruitment of Mit1, while the HDAC module can be independently recruited to nucleic acids through Clr2's MBDL domain. These findings raise the possibility that whilst the remodeler is recruited to pre-assembled heterochromatin, recruitment of the HDAC domain to nucleic acid may be targeting a specific sequence or nucleic acid-based structural motif.
Figure 7. SHREC is a flexibly linked assembly of functional modules.
Model of the SHREC complex assembled from the crystal structures presented in this work and the structure of the chromo and helicase domains of Chd1 (Hauk et al. 2010). SHREC structurally and functionally divides into a remodeling module and an HDAC module linked by Clr1. While the remodeling module is recruited through Chp2 the HDAC module is recruited through the nucleic acid binding activity of the MBDL of Clr2.
A long-standing question in NuRD research has been why chromatin remodeler and HDAC activities are combined in evolutionarily well conserved complexes. There is some support for cooperation of the two activities by the observed stimulation of HDAC activity on nucleosome substrates on addition of ATP to purified human NuRD complex (Xue et al., 1998). On the other hand, CLOCK-BMAL and PER have been reported to sequentially recruit NuRD as remodeler and HDAC modules for increasing repression of the circadian clock genes (Kim et al., 2014). Both Mit1 and Clr3 catalytic activities are required for heterochromatin formation (Grewal et al., 1998; Sugiyama et al., 2007). However, our results show that in S. pombe the physical connection between the two activities is not necessary for centromeric heterochromatin silencing when the HDAC bound Clr1 fragment is overexpressed. Under normal levels of expression, Mit1 remodeling activity may regulate Clr3 HDAC activity or vice versa since linkage between the activities is required for SHREC to silence heterochromatic loci. The two modules may act through an additive mechanism by co-ordinately affecting nucleosome substrates in close spatial and temporal proximity. Since both modules have distinct recruitment mechanisms the strength of each module might be different depending on the specific chromatin environment, an idea that is supported by the wide range of responses to SHREC mutants observed in the RNA-seq experiments, and different dependence of different genomic loci to Mit1- mediated chromatin remodeling activity (Creamer et al., 2014; Garcia et al., 2010).
Multivalent recruitment of the Mit1 remodeler
We show that the Mit1 C-terminal domain interacts with Clr1 and thereby forms a potential DNA binding interface. Some CHD-type remodelers harbor a DNA binding domain within their C-terminus, but the Mit1 homologues CHD3 and CHD4 instead use their C-terminal domains for interaction with various recruitment factors such as for example the heterochromatin organizer KAP-1 (Schultz et al., 2001). The Mit1-Clr1 complex seems to incorporate both these mechanisms. Thus, besides HP1-based recruitment to heterochromatin, Mit1 uses Clr1 interaction and potentially DNA binding for targeting of the remodeling module of SHREC.
MBD-like and BAH domains in SHREC
While similarity between SHREC and NuRD complexes has always been evident by their combination of HDAC and remodeling activity, two aspects of our study significantly deepen this similarity. First, we identify an MBD-like domain in the NTD of Clr2 and demonstrate that nucleic acid binding activity of this domain is critical for SHREC recruitment. Second, we identify a BAH domain in Clr2 that is reminiscent of the BAH domain found in the MTA subunits of NuRD, and show that it is crucial for the integrity of the HDAC submodule of SHREC. MBD proteins in NuRD contribute to NuRD localization to regions of heterochromatin through affinity for meCpG. Since S. pombe is devoid of DNA methylation this poses the interesting question of what the role of Clr2 might be. We show that the MBDL domain has relatively high affinity for dsRNA or DNA and is required for silencing and targeting of Clr2 to centromeres. With all likelihood, Clr2 is a DNA or RNA anchor with modest specificity for di- or trinucleotide motifs. The nature of this specificity needs further investigation, and might shed light on the non-methyl-DNA binding MBDs, in particular the NuRD subunit MBD3 (Hendrich and Bird, 1998).
The role of the BAH domain in MTA proteins is also not well understood. Here we show that the BAH domain of Clr2 is an integral part of Clr2's structure and crucial for complex formation with Clr1. The Clr2 BAH domain is surrounded by deep grooves and it is conceivable that other functionality is located there. This notion is supported by the silencing phenotype of the E376G (Steinhauf et al., 2014) and E376A, R373A mutants which target the groove between the BAH and CB domains (Fig. S4F). In addition, the silencing defective Clr1T6G mutant also maps to the same region and maintains Clr1–2–3 association, further arguing for the importance of this surface in a distinct aspect of heterochromatic silencing.
Our structural and functional dissection of SHREC significantly advance our understanding of the molecular mechanisms that are working in this simple chromatin remodeling and deacetylation complex. We find that the structurally distinct remodeler and HDAC modules are both endowed with their own recruitment mechanism. Our data suggest that expression levels of the Clr1 scaffold are finely tuned to ensure concerted action of the HDAC and remodeling activities of SHREC. Future studies will determine whether metazoan NuRD complexes show similar linkage of autonomous modules to drive complex patterns of gene regulation.
Experimental Procedures
Protein expression and purification
All recombinant protein constructs were expressed using baculovirus expression system in insect cells. Expression and purification procedures are outlined in Extended Experimental Procedures
Crystallization
Mit1-Clr1 was solved by single-wavelength anomalous dispersion. The Clr1-Clr2 complex was solved by multi-wavelength anomalous dispersion and the Clr3 structure was solved by molecular replacement with a composite model of known HDAC structures. Details for crystallization, data collection and refinement are given in Extended Experimental Procedures and Table 1.
Generation of S. pombe strains and plasmids
S. pombe strains and expression plasmids were constructed and manipulated as described in the Extended Experimental Procedures. Plasmids used for these studies are listed in Table S5 and strains in Table S6.
Biochemical assays
Details on EMSA analysis, western blotting, IP and ChIP are outlined in Extended Experimental Procedures.
RNA extraction and RNA-seq analysis
RNA extraction and RNA-seq analysis were performed as outlined in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Leemor Joshua-Tor for support and Leemor, Charles Roberts, Thanos Halazonetis, Ueli Schibler and Kevin Creamer for comments on the manuscript. We are grateful to Bo Wang, Wenbin Du and Hannah Henson for preliminary work. We thank Susan Forsburg, Pernilla Bjerling, Genevieve Thon, Karl Ekwall, Rashmi Prasad and Robin Allshire for gifts of plasmids and strains that enabled generation of reagents used in this study. Thanks to St. Jude Hartwell Center staff for DNA sequencing and for library generation for RNA-seq. Thanks to Stéphane Thore for help with X-ray equipment. We acknowledge the European Synchrotron Radiation Facility (ESRF) and the Swiss Light Source at the Paul Scherrer Institut, Villigen (SLS), Switzerland for provision of synchrotron radiation facilities and thank Daniele de Sanctis, Stéphanie Monaco and Vincent Olieric for assistance in using ESRF beam lines ID23-1, ID29 and PXIII respectively.
Funding
T.S.: Swiss National Science Foundation SNF Professorship (PP00P3_139137), Cold Spring Harbor Laboratory, Fondation Ernst et Lucie Schmidheiny, Fonds Constantin Topali and Société Académique de Genève.
C.B.: EMBO Short Term Fellowship (ASTF 255–2014)
G.J, T.X, B.R.L, S.S. and J.F.P: NIH R01 grant (GM084045), Cancer Center support grant (CCSG 2 P30 CA21765), and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, J.F.P and T.S; Methodology, G.J.,T.X.,Y.P., J.F.P., T.S.; Formal Analysis, C.Q.; Investigation, G.J.,C.B.,T.X., B.R.L.,Y.P.,S.S.,J.I.B.S., J.F.P., T.S.; Writing – Original Draft, J.F.P and T.S; Writing – Review & Editing, J.F.P and T.S.; Resources, J.F.P and T.S.; Funding Acquisition, J.F.P and T.S.; Supervision, J.F.P. and T.S.
Accession codes
Coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 5IKF, 5IKJ, 5IKK.
RNA-seq data has been deposited at the NCBI Gene Expression Omnibus (GEO) accession number GSE73359.
References
- Allen HF, Wade PA, Kutateladze TG. The NuRD architecture. Cell. Mol. Life Sci. 2013;70:3513–3524. doi: 10.1007/s00018-012-1256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Alper BJ, Lowe BR, Partridge JF. Centromeric heterochromatin assembly in fission yeast--balancing transcription, RNA interference and chromatin modification. Chromosome Res. 2012;20:521–534. doi: 10.1007/s10577-012-9288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper BJ, Job G, Yadav RK, Shanker S, Lowe BR, Partridge JF. Sir2 is required for Clr4 to initiate centromeric heterochromatin assembly in fission yeast. EMBO J. 2013;32:2321–2335. doi: 10.1038/emboj.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aygün O, Mehta S, Grewal SIS. HDAC-mediated suppression of histone turnover promotes epigenetic stability of heterochromatin. Nat. Struct. Mol. Biol. 2013;20:547–554. doi: 10.1038/nsmb.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerling P, Ekwall K, Egel R, Thon G. A novel type of silencing factor, Clr2, is necessary for transcriptional silencing at various chromosomal locations in the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res. 2004;32:4421–4428. doi: 10.1093/nar/gkh780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler M, Moazed D. Transcription and RNAi in heterochromatic gene silencing. Nat. Struct. Mol. Biol. 2007;14:1041–1048. doi: 10.1038/nsmb1315. [DOI] [PubMed] [Google Scholar]
- Buker SM, Iida T, Bühler M, Villén J, Gygi SP, Nakayama J-I, Moazed D. Two different Argonaute complexes are required for siRNA generation and heterochromatin assembly in fission yeast. Nat. Struct. Mol. Biol. 2007;14:200–207. doi: 10.1038/nsmb1211. [DOI] [PubMed] [Google Scholar]
- Buscaino A, Lejeune E, Audergon P, Hamilton G, Pidoux A, Allshire RC. Distinct roles for Sir2 and RNAi in centromeric heterochromatin nucleation, spreading and maintenance. EMBO J. 2013;32:1250–1264. doi: 10.1038/emboj.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano F, Mülleder M, Kok R, Blom HJ, Ralser M. Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. Anal. Chem. 2014;86:3697–3702. doi: 10.1021/ac500447w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer KM, Partridge JF. RITS-connecting transcription, RNA interference, and heterochromatin assembly in fission yeast. Wiley Interdiscip. Rev. RNA. 2011;2:632–646. doi: 10.1002/wrna.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer KM, Job G, Shanker S, Neale GA, Lin Y-C, Bartholomew B, Partridge JF. The Mi-2 homolog Mit1 actively positions nucleosomes within heterochromatin to suppress transcription. Mol. Cell. Biol. 2014;34:2046–2061. doi: 10.1128/MCB.01609-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K, Ruusala T. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics. 1994;136:53–64. doi: 10.1093/genetics/136.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JF, Dumesic PA, Hartley PD, El-Samad H, Madhani HD. Combinatorial, site-specific requirement for heterochromatic silencing factors in the elimination of nucleosome-free regions. Genes Dev. 2010;24:1758–1771. doi: 10.1101/gad.1946410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SIS, Jia S. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Grewal SI, Bonaduce MJ, Klar AJ. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics. 1998;150:563–576. doi: 10.1093/genetics/150.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Burns G, Mata J, Volpe TA, Martienssen RA, Bähler J, Thon G. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol. Cell. Biol. 2005;25:590–601. doi: 10.1128/MCB.25.2.590-601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama M, Aruga J. Characterization of the tandem CWCH2 sequence motif: a hallmark of inter-zinc finger interactions. BMC Evol. Biol. 2010;10:53. doi: 10.1186/1471-2148-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk G, McKnight JN, Nodelman IM, Bowman GD. The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol. Cell. 2010;39:711–723. doi: 10.1016/j.molcel.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wade PA. NuRD and pluripotency: a complex balancing act. Cell Stem Cell. 2012;10:497–503. doi: 10.1016/j.stem.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M, Shimojo H, Hayashi A, Kawaguchi R, Ohtani Y, Uegaki K, Nishimura Y, Nakayama J-I. Intrinsic nucleic acid-binding activity of Chp1 chromodomain is required for heterochromatic gene silencing. Mol. Cell. 2012;47:228–241. doi: 10.1016/j.molcel.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kwak PB, Weitz CJ. Specificity in circadian clock feedback from targeted reconstitution of the NuRD corepressor. Mol. Cell. 2014;56:738–748. doi: 10.1016/j.molcel.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Lai AY, Wade PA. Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat. Rev. Cancer. 2011;11:588–596. doi: 10.1038/nrc3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guezennec X, Vermeulen M, Brinkman AB, Hoeijmakers WAM, Cohen A, Lasonder E, Stunnenberg HG. MBD2/NuRD and MBD3/NuRD, Two Distinct Complexes with Different Biochemical and Functional Properties. Mol. Cell. Biol. 2006;26:843–851. doi: 10.1128/MCB.26.3.843-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Huang Y. Structure of the chromodomain of MRG2 in complex with H3K4me3. PDB [Google Scholar]
- Motamedi MR, Hong E-JE, Li X, Gerber S, Denison C, Gygi S, Moazed D. HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol. Cell. 2008;32:778–790. doi: 10.1016/j.molcel.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Buscaino A, Warner RJ, Akhtar A, Murzin AG, Murzina NV, Laue ED. Structure of the Chromo Barrel Domain from the MOF Acetyltransferase. J. Biol. Chem. 2005;280:32326–32331. doi: 10.1074/jbc.M501347200. [DOI] [PubMed] [Google Scholar]
- Otani J, Arita K, Kato T, Kinoshita M, Kimura H, Suetake I, Tajima S, Ariyoshi M, Shirakawa M. Structural basis of the versatile DNA recognition ability of the methyl-CpG binding domain of methyl-CpG binding domain protein 4. J. Biol. Chem. 2013;288:6351–6362. doi: 10.1074/jbc.M112.431098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat. Cell Biol. 2009;11:1261–1267. doi: 10.1038/ncb1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- Schalch T, Job G, Shanker S, Partridge JF, Joshua-Tor L. The Chp1-Tas3 core is a multifunctional platform critical for gene silencing by RITS. Nat. Struct. Mol. Biol. 2011;18:1351–1357. doi: 10.1038/nsmb.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DC, Friedman JR, Rauscher FJ., 3rd Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 2001;15:428–443. doi: 10.1101/gad.869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South TL, Summers MF. Zinc- and sequence-dependent binding to nucleic acids by the N-terminal zinc finger of the HIV-1 nucleocapsid protein: NMR structure of the complex with the Psi-site analog, dACGCC. Protein Sci. 1993;2:3–19. doi: 10.1002/pro.5560020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauf D, Rodriguez A, Vlachakis D, Virgo G, Maksimov V, Kristell C, Olsson I, Linder T, Kossida S, Bongcam-Rudloff E, et al. Silencing motifs in the Clr2 protein from fission yeast, Schizosaccharomyces pombe. PLoS One. 2014;9:e86948. doi: 10.1371/journal.pone.0086948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Cam HP, Sugiyama R, Noma K-I, Zofall M, Kobayashi R, Grewal SIS. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Thon G, Klar AJ. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics. 1992;131:287–296. doi: 10.1093/genetics/131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Cohen A, Klar AJ. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics. 1994;138:29–38. doi: 10.1093/genetics/138.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchy MP, Hamiche A, Klaholz BP. Structure and function insights into the NuRD chromatin remodeling complex. Cell. Mol. Life Sci. 2015;72:2491–2507. doi: 10.1007/s00018-015-1880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BT, Marguerat S, Watt S, Schubert F, Wood V, Goodhead I, Penkett CJ, Rogers J, Bähler J. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453:1239–1243. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Bartlett R, Nurse P, Bird AP. The fission yeast gene pmt1+ encodes a DNA methyltransferase homologue. Nucleic Acids Res. 1995;23:203–210. doi: 10.1093/nar/23.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Wong J, Moreno GT, Young MK, Côté J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- Yamada T, Fischle W, Sugiyama T, Allis CD, Grewal SIS. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol. Cell. 2005;20:173–185. doi: 10.1016/j.molcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yang N, Xu R-M. Structure and function of the BAH domain in chromatin biology. Crit. Rev. Biochem. Mol. Biol. 2013;48:211–221. doi: 10.3109/10409238.2012.742035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.