Abstract
The National Lung Screening Trial (NLST) demonstrated that screening with low-dose CT versus chest radiography reduced lung cancer mortality by 16% to 20%. More recently, a cost-effectiveness analysis (CEA) of CT screening for lung cancer versus no screening in the NLST was performed. The CEA conformed to the reference-case recommendations of the US Panel on Cost-Effectiveness in Health and Medicine, including the use of the societal perspective and an annual discount rate of 3%. The CEA was based on several important assumptions. In this paper, I review the methods and assumptions used to obtain the base case estimate of $81,000 per quality-adjusted life-year gained. In addition, I show how this estimate varied widely among different subsets and when some of the base case assumptions were changed and speculate on the cost-effectiveness of CT screening for lung cancer outside the NLST.
Keywords: cost-benefit analysis, early diagnosis, lung neoplasms/mortality/radiography, mass screening/economics/methods, quality-adjusted life-years, randomized controlled trials as topic, tomography, spiral computed/methods
Lung cancer is the leading cause of cancer-related deaths in the United States, and about 85% of lung cancers are attributed to cigarette smoking.1 Until recently, no method of screening had been shown to reduce lung cancer mortality. However, the National Lung Screening Trial (NLST) demonstrated that screening with low-dose computed tomography (LDCT) in comparison with screening with chest radiography can statistically significantly reduce mortality from lung cancer by 16% to 20% in those at high risk for the disease.2,3 The NLST also demonstrated a statistically significant reduction in all-cause mortality (6.7%), which had never previously been demonstrated in any other form of screening. Of course these major benefits of CT screening for lung cancer must be weighed against the harms and costs of this intervention. One of the major harms of CT screening for lung cancer are the health effects of false-positive results, which occurred in 37% of NLST participants during 1 or more of the 3 annual screens.2 However, health-related quality of life was not reduced in participants with false-positive results in the NLST,4 and only 3% of false-positive results were followed by an invasive procedure.5 In addition, it appears that CT screening for lung cancer could be cost-effective. It has recently been estimated that, within the NLST, CT screening for lung cancer compared with no screening costs about $81,000 per quality-adjusted life-year (QALY) gained.6 Although no upper limit threshold for cost-effectiveness has been firmly established within the United States, leading health economists have recently recommended a threshold between $100,000 and $150,000 per QALY gained.7
In this article, I will explain the recently published cost-effectiveness analysis (CEA) of the NLST,6 beginning with a brief description of the trial and comparative screening strategies and justification of the societal perspective. Next I will explain how life expectancy (LE), quality-adjusted life expectancy (QALE), and costs were estimated for the different strategies and how the incremental cost-effectiveness ratios (ICERs) were computed. Finally, I will analyze the importance of several assumptions and input variables and speculate on the cost-effectiveness of CT screening outside the NLST.
METHODS
NLST Summary
The NLST was a joint effort of the American College of Radiology Imaging Network (ACRIN) and the Lung Screening Study (LSS), both funded by the National Cancer Institute. From August 2002 through April 2004, 53,452 persons were enrolled and randomly assigned to 3 annual screens with either LDCT or chest radiography.2 Participants were followed up through December 31, 2009. Major eligibility criteria included age between 55 and 74 years and at least 30 pack-years of cigarette smoking. Former smokers had to have quit within the past 15 years. The study protocol was approved by the institutional review board at each of 33 screening centers, and written informed consent was obtained from each participant before randomization.
The vital status was based on questionnaires completed semiannually (ACRIN) or annually (LSS) and, for those lost to follow-up, the National Death Index. Medical records were obtained from participants with positive screening results and/or a diagnosis of lung cancer, and information related to diagnostic procedures and lung cancer staging and treatment was abstracted using forms that were harmonized across ACRIN and LSS. More detailed information related to diagnostic procedures and lung cancer treatment was collected for the ACRIN sites to inform the CEA.6 In addition, health-related quality-of-life data were collected from participants at 16 of the ACRIN screening centers.4
Comparative Screening Strategies
The CEA conformed to the reference-case recommendations of the US Panel on Cost-Effectiveness in Health and Medicine.8 In the NLST CEA, 3 strategies were compared: screening with LDCT, screening with radiography, and no screening. The 2 screening strategies were included because CT screening was the major strategy of interest, and both screening strategies were included in the trial. For these 2 screening strategies, cost and health outcomes were based directly on the trial data. However, previous randomized trials, including the recent Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,9 had failed to show any mortality reduction from screening with chest radiography compared with no screening. Therefore, the most relevant comparison after the completion of NLST was CT screening versus no screening. Because a no-screening arm was not included in the NLST, it had to be simulated for the CEA. For the simulated no-screening strategy, it was assumed that health outcomes would equal those in the radiography group and that costs would equal those for the radiography group minus the costs of the screening examinations and the workup for false-positive results.
Perspective
Economic analyses can be conducted from a variety of perspectives, including those of the payers, providers, or radiology departments. However, the most appropriate perspective for public health decision-making is the societal perspective, for which all costs and all benefits affecting all parties are included.8,10 In the NLST CEA, costs to society for screening and subsequent management and costs to participants and care-givers for time and travel were included. Benefits to participants, in terms of LE and QALE, were also included. The CEA was performed over 2 different time horizons, a within-trial time horizon (effects of screening observed through December 31, 2009) and a lifetime horizon. The advantage of the within-trial horizon is that the costs and health outcomes were actually observed. The lifetime horizon required modeling of costs and health outcomes after the trial but produced more complete estimates of cost and health outcomes relevant for societal decision-making and prioritization. For each strategy, LE, QALE, and expected costs were estimated from the time of randomization (assumed for simplicity to be January 1, 2009 for all participants to account for the most recent Medicare pricing). As is customary, costs and life-years were discounted at an annual rate of 3%.
Life Expectancy
In the estimation of LE, it was assumed that screening did not affect LE in those not diagnosed with lung cancer and did not incur costs beyond those for workup of a positive examination (except for potentially significant incidental findings and the very rare radiation-induced lung cancer occurring only in the LDCT group after the trial11). During the trial, there were 116 more lung cancers diagnosed in the CT group than in the radiography group (1109 vs. 993, respectively) (Table 1). For the base case, it was assumed that all the 116 additional cases were due to overdiagnosis rather than some being due to earlier diagnosis of cases that would eventually become clinically evident after the trial. This assumption was justified by the near convergence of lung cancer incidence in the 2 groups in the last 3 years of the trial Fig. S2-1 in Supplementary Appendix of Black et al6) and greatly simplified the analysis. With these assumptions, the LEs of those without a diagnosis of lung cancer in the 2 groups were equal except for the very small deduction for radiation-induced lung cancer in the CT group (below). The LE for each screening strategy was estimated as a weighted average of the LEs of those with and without lung cancer.
TABLE 1.
Lung Cancer Diagnoses and Deaths in NLST
| CT | CXR | Total | |
|---|---|---|---|
| All participants in full NLST | 26,722 | 26,730 | 53,452 |
| With lung cancer diagnosis* | 1109 | 993 | 2102 |
| All exclusions from CEA | 80 | 70 | 150 |
| Lost to FU within 1 d | 46 | 54 | 100 |
| Missing lung cancer information | 33 | 15 | 48 |
| Less than 50 y of age | 1 | 1 | 2 |
| All participants in NLST CEA | 26,642 | 26,660 | 53,302 |
| With lung cancer diagnosis | 1076 | 978 | 2054 |
| All deaths | 1920 | 2044 | 3964 |
| Deaths from lung cancer† | 469 | 552 | 1021 |
| All deaths from other causes | 1451 | 1492 | 2943 |
| Deaths from other causes and lung cancer diagnosis |
49 | 35 | 84 |
| Alive‡ at end of trial | 24,722 | 24,616 | 49,338 |
| Alive‡ at end of trial with lung cancer diagnosis |
558 | 391 | 949 |
| Alive‡ at end of trial without lung cancer diagnosis |
24,164 | 24,225 | 48,389 |
Reprinted with permission from Black et al.6
The numbers are higher than those previously reported,2 because they include lung cancers diagnosed at time of death and a later data cut date (January 31, 2011 vs. September 28, 2010).
The numbers are higher than those previously reported,2 because they result from a later censor date (December 31, 2009 vs. January 15, 2009) and a later data cut date (January 31, 2011 vs. September 28, 2010).
Includes those lost to follow-up before December 31, 2009.
CXR indicates chest radiography; FU, follow-up.
Of the 53,452 randomized in the NLST, 150 were excluded for the following reasons (Table 1): 100 were lost to follow-up within 1 day of randomization or their first screen (46 from the CT group, 54 from the radiography group), 48 were missing lung cancer data to predict survival (33 from the CT group, 15 from the radiography group), and 2 were less than 50 years of age at entry (1 from the CT group, 1 from the radiography group). For each of the remaining 53,302 participants (26,642 in the CT group, 26,660 in the radiography group), life-years were estimated by adding beyond-trial life-years to within-trial life-years. Within-trial life-years were calculated from the date of randomization to the date of death if deceased (3964), to December 31, 2009 if still alive, or to the latest date they were known to be alive if the vital status was missing on December 31, 2009. For the 49,338 participants not known to be deceased on December 31, 2009, beyond-trial life-years were estimated from age on the date they were last known to be alive, sex, smoking status at entry, and lung cancer stage, if any, using 2009 US life tables12 adjusted for smoking status and stage-specific annual probabilities of dying from lung cancer (sections 3 and 4 in the Supplementary Appendix of Black et al6).
For the first 5 years after diagnosis, the observed NLST stage-specific lung cancer mortality rates were used to estimate LE. For subsequent years, when NLST data were sparse, the observed NLST stage-specific lung cancer mortality rates were adjusted to account for the decline in the hazard of lung cancer death from time of diagnosis seen in the Surveillance, Epidemiology and End Results (SEER) Program.13
Quality of Life Adjustment
To adjust LE for quality, utilities were derived from the Short Form Health Survey SF-36,14 which was completed by 11,696 participants from 16 of 23 ACRIN screening sites. By convention, utilities range from 0 (dead) to 1.0 (perfect health). At baseline, the mean utilities were 0.76 and 0.74 for men and women, respectively, and did not differ by age; these utilities were slightly less than those observed in a large US study of health-related quality of life—0.79 and 0.76 for men and women, respectively, ranging in age from 55 to 64 years15—probably because of the greater smoking histories among the NLST population. For those diagnosed with lung cancer during the trial, adjustments were also made for lung cancer stage and time of diagnosis (Table 2). Because there was no difference in utilities between those with false-positive versus true-negative screening results,4 no adjustments for screening results were made in the base case.
TABLE 2.
Base Case Utilities for Lung Cancer by Stage Over Time
| Years Since Diagnosis | ||
|---|---|---|
| Stage | < 12 mo | 12 mo + |
| IA | 0.700 | 0.720 |
| IB | 0.675 | 0.695 |
| II | 0.650 | 0.670 |
| III | 0.625 | 0.645 |
| IV | 0.600 | 0.620 |
Reprinted with permission from Black et al.6
Cost
The expected per person costs were estimated for the same cohort of 53,302 participants used in the estimation of LE. Total per person costs were calculated as the sum of direct medical costs and indirect costs. Direct medical costs were based on utilization related to the screening examination, diagnostic workup for positive screening results and signs or symptoms of lung cancer, and lung cancer treatment and were calculated for each subject each year after randomization. The frequency of screening was based on screening CT and radiography compliance records. In the ACRIN subset, medical utilization related to diagnostic workup and treatment of lung cancer was directly obtained by medical record abstraction (section 6 in the Supplementary Appendix of Black et al6). Cost data were obtained from 5133 of the 18,840 participants in the ACRIN subset, including almost all who had a positive screen and/or diagnosis of lung cancer. Costs for LSS participants and ACRIN participants without cost data were imputed using variables collected on all participants. Medicare prices for procedures were based on the 2009 Current Procedural Terminology and Physician Fee Schedule16 for both professional and technical components (Table 3). The prices for screening CT and chest radiography were based on Current Procedural Terminologies 71250 and 71010, respectively. Medicare prices for hospitalizations were based on Medical Severity Diagnosis-related Groups17 (Table 4).
TABLE 3.
Most Common Current Procedural Terminology (CPT) Procedures
| CPT Description | CPT Code | Price* |
|---|---|---|
| MRI brain w/o and w/dye | 70553 | 852.61 |
| Chest x-ray (single view, frontal) | 71010 | 23.80 |
| Chest x-ray (2 views, frontal and lateral) | 71020 | 31.74 |
| CT thorax w/o dye | 71250 | 284.93 |
| CT thorax w/dye | 71260 | 341.91 |
| CT pelvis w/dye | 72193 | 324.60 |
| CT abdomen w/dye | 74160 | 363.55 |
| Tumor image PET/CT skull-thigh | 78815 | 1284.00 |
All prices based on 2009 national pricing for Medicare16 except for PET imaging (CPTs 78811–78816), which were based on Medicare payment at Dartmouth-Hitchcock Medical Center, because no national prices existed.
MRI indicates magnetic resonance imaging; PET, positron emission tomography.
TABLE 4.
Most Common Medical Severity Diagnosis-related Groups (MS-DRGs)
| MS-DRG Description | Code | Weight17 | Price* |
|---|---|---|---|
| Major chest procedures w MCC | 163 | 4.9978 | $36,632 |
| Major chest procedures w CC | 164 | 2.5953 | $19,022 |
| Major chest procedures w/o CC/MCC | 165 | 1.8036 | $13,220 |
| Other resp system OR procedures w MCC |
166 | 3.6912 | $27,055 |
| Other resp system OR procedures w CC | 167 | 2.0264 | $14,853 |
| Respiratory neoplasms w MCC | 180 | 1.6950 | $12,424 |
| Respiratory neoplasms w CC | 181 | 1.2316 | $9027 |
| Respiratory neoplasms w/o CC/MCC | 182 | 0.8736 | $6403 |
Price = Weight × $7329.64 (weights have been rounded to 4 decimal places).
CC indicates complications or comorbidities; MCC, major complications or comorbidities.
Indirect medical costs were based on time and travel for the subject and caregiver using a previously reported methodology for colon cancer screening.18 Time costs were based on total subject and caregiver time expended for workup and treatment of lung cancer and the mean hourly total compensation costs for civilian workers in 2009 ($29.37).19 The cost of travel included estimated time and round trip mileage at the US government automobile reimbursement rate per mile ($0.55).20 A 50-mile round trip for each medical encounter was assumed. Using this approach, the nondiscounted costs of time and travel for each screening, workup, and surgical visit was $101, for each radiation therapy visit was $175, and for each chemotherapy visit was $381.
There is no reliable information on the costs of managing potentially significant incidental findings; however, 2 recent studies of lung cancer screening with CT in Italy and Canada reported radiologic costs of $12921 and $9522 per finding. In a study of incidental findings in CT colonography in the United States, total medical costs were about $1600 per finding, only about one third of which was due to radiologic imaging. For the base case, it was assumed that 15% of participants in the LDCT group had at least 1 potentially significant incidental finding2 and that the total (direct and indirect) cost of managing the findings was $500 per participant. This cost was incorporated into the cost of the LDCT screening examination (but not in the radiography or no-screening groups). Given that participants in the LDCT group had on average about 2.8 screening examinations, $26.68 ($500 × 0.15/2.8) was added to the cost of each LDCT screening examination. The cost of managing potentially significant findings was varied from zero to $2500 in the sensitivity analysis.
In the base case, medical care (during and after the trial) for lung cancers diagnosed during the trial and for future hypothetical lung cancers induced by radiation from CT screening was included.
Adjustments for Radiation-induced Lung Cancer
Because of the uncertainty and complexity of modeling radiation-induced lung cancer from CT screening at the individual level, adjustments to the LE, QALE, and expected cost were made at the cohort level. On the basis of previous modeling,11,23 it was assumed that CT screening would cause 24 lung cancer deaths for every 521 prevented. It was also assumed that the mean years of lost life for a radiation-induced lung cancer was about 9 years on the basis of a lag time of 10 years and life table methods,6,24 and the mean cost for treatment of lung cancer was about $32,000 (section 7 in the Supplementary Appendix of Black et al6). After discounting, radiation-induced lung cancer reduced the LE and QALE in the CT screening arm by 0.00060 years and 0.00045 QALYs, respectively, and added $3.49 in per person cost. These adjustments were made for the base case.
Incremental Cost-effectiveness
The 3 strategies were ordered by their baseline costs, from high to low. The incremental costs, LEs, QALEs, and ICERs were calculated after first excluding any dominated strategy (a strategy is considered dominated if it costs more but provides no health benefit compared with another strategy). The ICER is defined as the ratio of the incremental cost to the incremental health benefit. Statistical uncertainty was estimated with equal-tail 95% bootstrap confidence intervals (CIs).25,26 Random samples of size 26,642 from the CT group and 26,660 from the radiography group were drawn with replacement 10,000 times to generate a scatterplot of incremental QALY and incremental cost and a cost-effectiveness acceptability curve.27
Subset and Sensitivity Analyses
Subset analyses were performed for 5-year age groups, sex, smoking status (current vs. former), and quintiles of lung cancer risk on the basis of a recently validated model.28
To assess the internal validity of the analysis, 3 of the conservative base case assumptions were individually varied. The first assumption was that screening with LDCT did not affect other-cause mortality. To account for the possibility that the observed reduction in non–lung cancer mortality in the CT group was due to screening, through incidental detection of other abnormalities or something else related to the CT screening process, the incremental gain in QALE was multiplied by the ratio of number of fewer total deaths to number of fewer lung cancer deaths in the CT group, that is, 124/83 (Table 1).
The second assumption was that all 116 excess cases of lung cancer in the LDCT group were due to over-diagnosis.29,30 This assumption was varied to allow for the possibility that some of the excess lung cancers diagnosis in the CT group would have eventually become clinically evident after the trial. There was an excess lung cancer incidence in the radiography group of about 1.0/1000 person-years during the last 1.5 years of the NLST, and modeling has shown that about one half of the cumulative excess in non–small cell lung cancer (NSCLC) in the LDCT group would disappear with lifetime follow-up.31
The third assumption was that screening with chest radiography was ineffective in reducing lung cancer mortality in comparison with no screening (section 7 in the Supplementary Appendix of Black et al6). This assumption was varied to allow for the possibility that screening with chest radiography might reduce lung cancer mortality to some degree. In PLCO, the 95% CIs around the relative risk included 1.0, but the point estimate was 0.94.
In addition, because of the large disparity across groups in the numbers of participants with stage IA NSCLC alive at the end of the trial—324 in CT versus 140 in radiography—and the uncertainty in their survival beyond 5 years from diagnosis, the analysis was repeated for optimistic and pessimistic assumptions about their long-term survival (section 4 in the Supplementary Appendix of Black et al6).
Sensitivity analyses related to the internal validity of the analysis were also performed on the following variables: the quality of life after a positive screening result and lung cancer diagnosis, overdiagnosis in the radiography group (compared with no screening), which would have violated the base case assumptions about the no-screening group, and radiation-induced lung cancer deaths. For the latter variable, the number of deaths was varied from 0 to twice the base case estimate.
To assess the generalizability of the results, a sensitivity analysis was performed on the risk of developing lung cancer relative to those in the NLST. Additional sensitivity analyses were performed on surgical mortality and on several costs, including the costs of screening with LDCT examination, CT follow-up examinations, surgical resection, chemotherapy, radiation therapy, time and travel, management of incidental findings, and future medical care. To assess the impact of the new American College of Radiology (ACR) reporting system for lung cancer screening, LungRADS v.1.0,32 it was assumed that the reduction in frequency of positive CT screening examinations would decrease the cost of screening by decreasing the number of follow-up chest CTs, which was by far the most common procedure performed to evaluate a positive CT screening examination. It was further assumed that the reduction in the frequency of positive CT screens would have no effect on LE or QALE. That is, it was assumed that LungRADS would not result in missing any lung cancers that would reduce LE or QALE.
RESULTS
Lung Cancer Diagnoses and Deaths
Among the 53,302 participants in the CEA, 1076 participants in the CT group and 978 participants in the radiography group received a lung cancer diagnosis through December 31, 2009 (Table 1).6 Among those with lung cancer in the CT group, 469 died from lung cancer and 49 from other causes. Among those in the radiography group, 552 died from lung cancer and 35 from other causes.
LE and QALE
Discounted LE and QALE were higher in the CT group than in the radiography group. However, the differences were greater over the lifetime horizon (14.7386 vs. 14.7071 y and 10.9692 vs. 10.9491 QALYs) than the within-trial horizon, because the former accounted for life-years saved after the trial, which greatly exceeded the life-years gained during the trial (Table 5). For the approximately 4% of participants with a lung cancer diagnosis, the incremental LE was about 1.6 years.
TABLE 5.
Life-years and QALY Per Person (Discounted at 3%)
| LE | QALE | |||
|---|---|---|---|---|
| CT | Radiography† | CT | Radiography† | |
| Within trial | 5.7846 | 5.7775 | 4.3390 | 4.3351 |
| With lung cancer | 4.9013 | 4.6029 | 3.5603 | 3.3596 |
| Without lung cancer* | 5.8228 | 5.8228 | 4.3728 | 4.3728 |
| Lifetime | 14.7386 | 14.7071 | 10.9692 | 10.9491 |
| With lung cancer | 8.4792 | 6.8479 | 6.0521 | 4.8981 |
| Without lung cancer* | 15.0097 | 15.0103 | 11.1821 | 11.1825 |
Reprinted with permission from Black et al.6
Assumed same for CT and radiography groups except for adjustment for radiation-induced lung cancer occurring after the trial.
Results for no screening were assumed to be the same as for radiography.
Costs
Discounted per person costs were much higher in the CT group than in the radiography group—$3074 versus $1911 (Table 6)6—mainly because of screening and the much higher Medicare price for noncontrast chest CT scan than for chest radiography—$285 versus $24 (Table 3). Per person costs of workup and surgery were also higher in the CT group, but per person costs of chemotherapy and radiation therapy were lower. Future medical costs, used only in the sensitivity analysis, were slightly higher for the CT group than for the radiography group—$171,018 versus $170,248 (not shown)—because a higher proportion of participants were still alive at the end of the trial in the CT group. By assumption, per person costs in the no-screening strategy were the same as those in the radiography group minus the costs for screening and workup of false-positive screens.
TABLE 6.
Costs Per Person (Discounted at 3%)
| CT | Radiography | No Screening |
|
|---|---|---|---|
| Total | $3074 | $1911 | $1443 |
| Screening | $1130† | $336 | $0 |
| Workup | $835 | $645 | $512 |
| Treatment | $1106 | $931 | $931 |
| Surgery | $736 | $470 | $470 |
| Chemotherapy | $282 | $351 | $351 |
| Radiation therapy | $88 | $110 | $110 |
| Radiation-induced lung cancer |
$3 | $0 | $0 |
Reprinted with permission from Black et al.6
Costs include time and travel, which were $101 for each screening, workup, and surgical visit. The time and travel cost for each radiation therapy visit was $175 and for each chemotherapy visit was $381.
Includes cost of managing potentially significant incidental findings.
Incremental Cost-effectiveness
In the base case, screening with radiography was dominated, because it was more expensive than no screening but provided no health benefit (Table 7). Compared with no screening, screening with LDCT cost an additional $1631 (95% CI, $1557–$1709) per person and provided an additional 0.0316 (95% CI, 0.0154–0.0478) life-years per person and 0.0201 (95% CI, 0.0088–0.0314) QALYs per person.6 The corresponding ICERs were $52,000 (95% CI, $34,000–$106,000) per life-year gained and $81,000 (95% CI: $52,000–$186,000) per QALY gained.
TABLE 7.
Incremental Cost-effectiveness (Base Case)
| Strategy | Cost | LE | QALE | Incremental Costs* | Incremental Lys | Incremental QALYs | $/LY | 95% CI | $/QALY | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| CT | $3074 | 14.7386 | 10.9692 | $1631 | 0.0316 | 0.0201 | 52,000 | 34,000–106,000 | 81,000 | 52,000–186,000 |
| Radiography | $1911 | 14.7071 | 10.9491 | $469 | 0 | 0 | Dom | Dom | ||
| None† | $1443 | 14.7071 | 10.9491 | — | — | — | — | — |
Reprinted with permission from Black et al.6
Incremental costs are in reference to the None strategy because the Radiography strategy is dominated.
Cost in the None strategy includes diagnosis and treatment of lung cancer without LDCT or radiographic screening.
Dom indicates dominated.
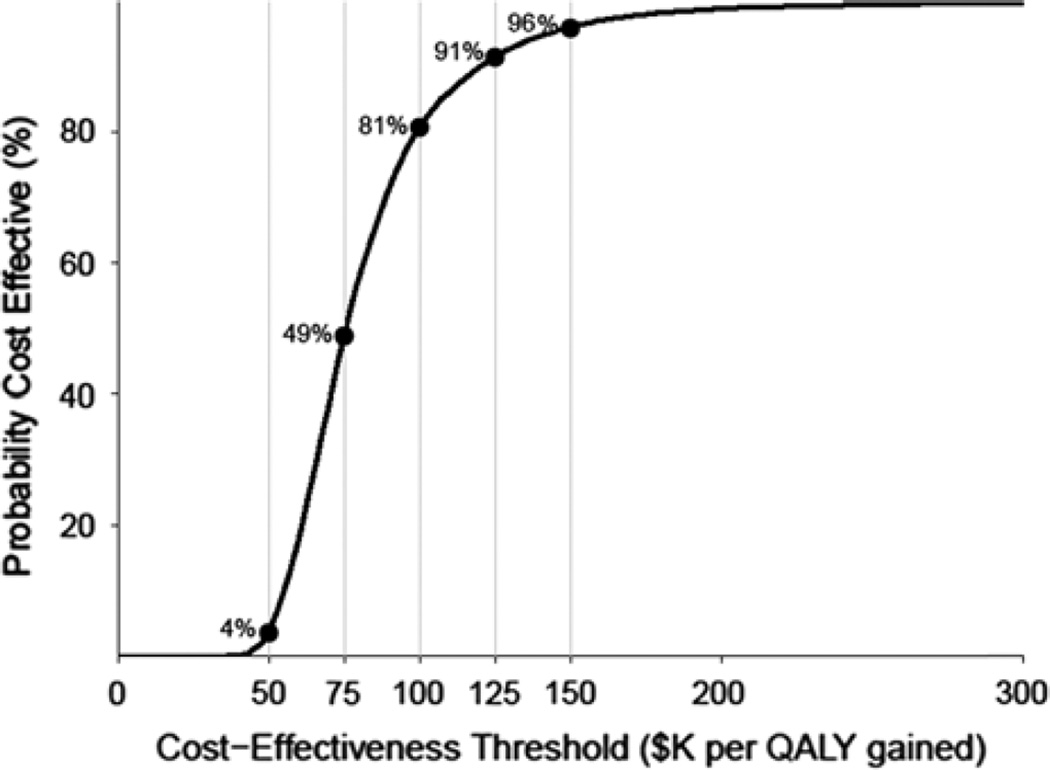
A scatterplot of incremental QALY and a incremental cost (Fig. 1) and cost-effectiveness acceptability curve (Fig. 2) were generated from 10,000 bootstrap samplings.
FIGURE 1.
Scatterplot of incremental QALY and incremental cost obtained by 10,000 bootstrap samplings.
FIGURE 2.
Cost-effectiveness acceptability curve (CEAC). As the cost-effectiveness threshold increases, the probability that CT screening for lung cancer is cost-effective also increases.
Subset Analyses
The incremental costs were relatively stable among the subsets, ranging from $1453 to $1905 (Table 8). However, the incremental QALYs ranged almost 20-fold, from 0.0027 to 0.0515. Consequently, the ICERs also ranged almost 20-fold, from $32,000 to $615,000 per QALY gained. The ICER was much lower for women than for men—$46,000 versus $147,000 per QALY gained—and for current than former smokers—$43,000 versus $615,000 per QALY gained—for the 3 older age groups than the youngest and for the 2 highest-risk quintiles than 3 lowest-risk quintiles. The wide variation among subsets is at least partially explained by the relatively small number of lung cancers in the subgroups compared with the full sample.
TABLE 8.
Subset Analyses
| Subset | No. Subjects | Incremental Costs | Incremental QALYs | $/QALY |
|---|---|---|---|---|
| Sex | ||||
| Men | 31,446 | $1683 | 0.0115 | 147,000 |
| Women | 21,856 | $1557 | 0.0340 | 46,000 |
| Age (years at entry) | ||||
| 55–59 | 22,773 | $1541 | 0.0101 | 152,000 |
| 60–64 | 16,333 | $1520 | 0.0320 | 48,000 |
| 65–69 | 9504 | $1900 | 0.0351 | 54,000 |
| 70–74 | 4685 | $1905 | 0.0163 | 117,000 |
| Smoking status | ||||
| Former | 27,643 | $1661 | 0.0027 | 615,000 |
| Current | 25,659 | $1601 | 0.0369 | 43,000 |
| Lung cancer risk | ||||
| First quintile | 10,660 | $1453 | 0.0086 | 169,000 |
| Second quintile | 10,661 | $1454 | 0.0118 | 123,000 |
| Third quintile | 10,660 | $1651 | 0.0061 | 269,000 |
| Fourth quintile | 10,661 | $1672 | 0.0515 | 32,000 |
| Fifth quintile | 10,660 | $1851 | 0.0354 | 52,000 |
Reprinted with permission from Black et al.6
Sensitivity Analyses
The results were highly sensitive to several of the base case assumptions (Table 9). When the reduction of non–lung cancer mortality was included as a health benefit, the ICER fell from $81,000 to $54,000 per QALY gained. When only half (58) rather than all (116) of the excess lung cancers in the CT group were attributed to overdiagnosis, the ICER fell to $55,000 per QALY gained. When the risk of lung cancer death with chest radiography screening relative to no screening was reduced to 0.94, the point estimate in the PLCO,9 the ICER fell to $62,000 per QALY gained.
TABLE 9.
Sensitivity Analyses (Base Case Assumptions in Parentheses)
| Scenario | $/QALY |
|---|---|
| Base case | 81,000 |
| Inclusion of non–lung cancer deaths | 54,000 |
| Relative risk of screening with radiography to no screening (1.0) |
|
| 0.8 | 40,000 |
| 0.94* | 62,000 |
| 1.1 | 171,000 |
| No. future excess cases (0) | |
| 29 | 66,000 |
| 58 | 55,000 |
| Survival for stage IA NSCLC (intermediate) | |
| Low | 67,000 |
| High | 108,000 |
| Cost of screening with LDCT examination ($285) | |
| $100 | 56,000 |
| $500 | 110,000 |
| Multiplier for number of follow-up | |
| LDCT examinations (1) | |
| 0.5 | 78,000 |
| 5 | 110,000 |
| Multiplier for cost of surgery ($22,000) | |
| 0.5 | 73,000 |
| 3 | 114,000 |
| Surgical mortality (1.2%) | |
| 0.0% | 79,000 |
| 8.0% | 96,000 |
| Future health care costs (0, 0) $171,018 for CT screening $170,248 for no screening |
120,000 |
| Reduction in quality of life after positive screen (0) 0.05 |
116,000 |
| Reduction in quality of life after diagnosis of stage IA lung cancer (0.03) 0.07 |
101,000 |
| Cost of managing potentially significant incidental finding (500) |
|
| $0 | 78,000 |
| $2500 | 96,000 |
| Radiation-induced lung cancer deaths per lung cancer death prevented (0.046) |
|
| 0 | 79,000 |
| 0.092 | 83,000 |
The ICER fell much more modestly with the reduction in the relative number of follow-up CT screens for positive CT screening examinations (Table 9). In the extreme case that all follow-up CT scans were eliminated, the ICER fell to $74,000 per QALY gained. In the more realistic case that the number of positive screens were reduced by one half, the ICER fell to $78,000 per QALY gained.
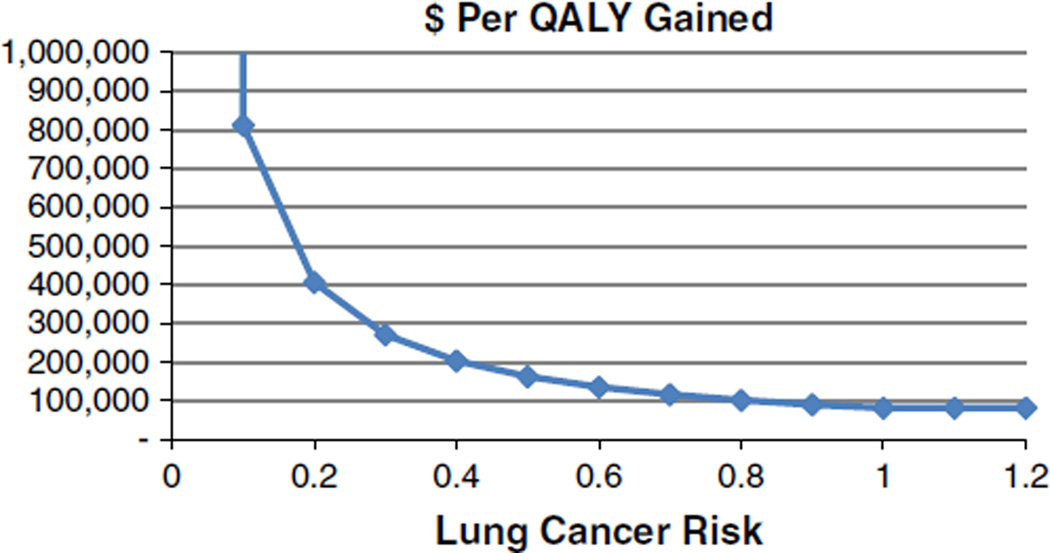
However, the ICER approached or exceeded $100,000 per QALY gained when the risk of developing lung cancer relative to those in NLST fell below 0.8 (Fig. 3), when future health care costs were included, when costs for the screening examination, follow-up, or surgery were increased several fold, when the pessimistic survival with stage IA NSCLC was assumed, and when small reductions in quality of life–related positive screening results and the diagnosis of stage IA lung cancer were included. The ICER varied only slightly over the range of radiation-induced lung cancer deaths, from $79,000 to $83,000 per QALY gained.
FIGURE 3.
ICER of CT screening for lung cancer versus lung cancer risk relative to participants in NLST.
DISCUSSION
The base case estimate of the ICERs for screening with LDCT versus no screening, that is, $81,000 per QALY gained, was below the recently recommended $100,000 per QALY gained threshold.7 However, the 95% CIs around this estimate were wide, that is, $52,000 to $186,000 per QALY gained, and the estimate varied widely in the subset and in the sensitivity analyses pertaining to several of the base case assumptions.6
Much of this variation in the subset analysis can probably be explained by variation in the risk of developing lung cancer and the smaller number of lung cancers in subgroups than in the total study population. Although screening with LDCT appeared much more cost-effective for women than for men, this may be largely due to the chance distribution of the histologic subtypes among men and women.3 Similarly, the much higher ICER among former smokers than current smokers may be partly due to a combination of a chance distribution of “excess” lung cancers in the CT groups and the conservative assumption that all excess cases were due to overdiagnosis. If and how these subset findings should affect CT screening recommendations will require further investigation and analysis.
The ICER fell substantially when each of the 3 conservative base case assumptions was changed. The first conservative assumption was that CT screening did not affect other-cause mortality in the NLST when the observed reduction in non–lung cancer deaths (41) was nearly one half the reduction in lung cancer deaths (83). However, this reduction in non–lung cancer deaths was not statistically significant, and there is no well-defined hypothesis for causation. Although CT screening did detect many potentially significant findings of cardiac nature, there was no reduction in cardiac mortality. The second conservative assumption was that all excess lung cancers in the CT group were due to overdiagnosis, whereas modeling suggests that only about one half of the cumulative excess was due to overdiagnosis.31 A planned posttrial follow-up of NLST participants should provide an update on the magnitude of overdiagnosis and allow a reestimation of the base case ICER. The third conservative assumption was that screening with chest radiography was ineffective when the point estimate of the relative risk in the NLST-eligible subset of the PLCO was 0.94. Given that screening with LDCT decreased lung cancer mortality by about 20% compared with screening with chest radiography, it is plausible that screening with chest radiography might reduce lung cancer mortality to some lesser degree compared with no screening.
Conversely, the ICER rose substantially when each of the 2 base case optimistic assumptions was changed. When future health care costs of survivors were included, the ICER increased to about $130,000 per QALY gained. The rationale for not including future health care costs in the base case was that they had not been included in prior cost-effectiveness analyses of lung cancer screening.33–38 However, whether they should be included remains controversial in cost-effectiveness research.8 The ICER also rose substantially with small decrements in quality of life related to positive screens. Although no decrease was observed in the quality of life after positive screening results using generic quality-of-life instruments,4 NELSON investigators have observed decreases using disease-specific quality-of-life instruments.39
Although the CEA suggests that screening with LDCT in the NLST costs <$100,000 per QALY gained, screening outside the trial may not be, depending on how exactly it is implemented. One of the most important factors is who would receive screening, specifically with regard to their risk of developing lung cancer, which is directly proportional to the health benefit and thus inversely proportional to the ICER. The NLST smoking eligibility criteria were fairly stringent. If they were to be broadened to ever-smokers 50 to 79 years of age, for example, then the population risk would decrease to about one half of that in NLST,40 and the ICER would increase to about $160,000 per QALY gained. Even if the NLST eligibility were rigorously enforced, the ICER could still rise substantially if the proportions of younger or former smokers among those actually screened were to increase in comparison with NLST. Another important factor is counseling regarding the potential benefits and harms of screening and the significance of positive screening results. Counseling or shared decision-making, which has been strongly recommended by the US Preventive Services Task Force (USPSTF)11 and others,41 was a major component of the NLST and may have prevented the expected decrease in the quality of life after a positive screening result. Without counseling, even a small decrease in quality of life could substantially decrease the net health benefit and substantially increase the ICER given the high positivity rate in NLST. Critics of lung cancer screening have argued that the positivity rate outside NLST might be even higher. Although this is a legitimate concern, the ACR has recently developed a reporting system, LungRADS,32 which appears to substantively decrease the positivity rate, by increasing the nodule threshold size from 4 to 6 mm, without increasing the number of missed lung cancers.42 Uniform application of LungRADS could reduce the cost of follow-up CT by about half and modestly reduce the ICER by several thousand dollars per QALY gained. A somewhat related factor is the reporting and management of potentially significant incidental findings, which are known to vary considerably across the United States and even within radiology departments.43 However, efforts by the ACR to standardize reporting and management of incidental findings since the completion of NLST should help mitigate this expense of screening. Finally, the effectiveness and safety of surgical resection are critical to the effectiveness and cost-effectiveness of screening. In the NLST, the surgical mortality rate of 1% was much lower than that reported nationally.2 Ideally, metrics for all of these factors related to cost-effectiveness should be developed and monitored in future screening.
The preceding analysis was based on the societal perspective, because it is the most appropriate perspective for decisions involving public health. However, the successful implementation of lung cancer screening will require that financial incentives be properly aligned. Because CT screening for lung cancer has received a grade B recommendation from the USPSTF, new private health plans will be required to cover the costs of screening examinations for patients who meet USPSTF eligibility criteria without any copays. In addition, the Centers for Medicare and Medicaid (CMS) has recently announced its proposed decision to cover counseling and screening under a certain set of conditions.44 After a public comment period, CMS is expected to issue its final decision for Medicare patients in February 2015. Regardless, reimbursement for the CT screening examination may not be sufficient to cover the full costs of screening in the manner advocated by the USPSTF. Accordingly, a properly designed screening program should ensure eligibility, inform eligible patients of the potential benefits and harms, provide smoking cessation counseling for current smokers, standardize CT performance and interpretation, ensure follow-up of screening results, participate in a registry, and validate overall performance against the NLST.45 How much these other components of screening will cost and who will pay for them have yet to be determined.
In conclusion, screening with LDCT for lung cancer as performed in the NLST appears to have cost about $81,000 per QALY gained. Continued follow-up of NLST participants should allow a reestimation of the base case ICER. Whether screening outside the trial will be cost-effective will depend on exactly how screening is implemented.
Acknowledgments
Funded by the National Cancer Institute; National Lung Screening Trial ClinicalTrials.gov number, NCT00047385.
William C. Black served on various committees of the National Lung Screening Trial, including the Executive Committee and was the site Principal Investigator at Dartmouth-Hitchcock Medical Center.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figure. Atlanta, GA: 2014. [Accessed October 23, 2014]. Available at: http://www.cancer.org/cancer/lungcancer/index. [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119:3976–3983. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gareen IF, Duan F, Greco EM, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer. 2014;120:3401–3409. doi: 10.1002/cncr.28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsky PF, Gierada DS, Hocking W, et al. National lung screening trial findings by age: medicare-eligible versus under-65 population. Ann Intern Med. 2014;161:627–633. doi: 10.7326/M14-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–1802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 8.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- 9.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 10.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 11.Moyer VA. Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 12.Arias E. United States life tables, 2009. Natl Vital Stat Rep. 2014;62:1–63. [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) [Accessed April 2013];SEER*Stat Database: Incidence - SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (1973–2010 varying), April 2013. Available at: http://www.seer.cancer.gov/data/seerstat/nov2012.
- 14.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 15.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Physician Fee Schedule Look-up tool. [Accessed Mar 1, 2011]; Available at: http://www.cms.hhs.gov/pfslookup/02_PFSsearch.asp.
- 17.Centers for Medicare & Medicaid Services. Acute Inpatient PPS. [Accessed August 20, 2013]; Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
- 18.Heitman SJ, Au F, Manns BJ, et al. Nonmedical costs of colorectal cancer screening using CT colonography. J Am Radiol. 2010;7:943–948. doi: 10.1016/j.jacr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Employer costs for employee compensation – December 2009. [Accessed March 1, 2011];2010 Available at: http://www.bls.gov/news.release/archives/ecec_03102010.pdf.
- 20.US General Services Administration. Privately owned vehicle (POV) mileage reimbursement rates. 2012 Available at: http://www.gsa.gov/portal/content/103969.
- 21.Priola AM, Priola SM, Giaj-Levra M, et al. Clinical implications and added costs of incidental findings in an early detection study of lung cancer by using low-dose spiral computed tomography. Clin Lung Cancer. 2013;14:139–148. doi: 10.1016/j.cllc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Kucharczyk MJ, Menezes RJ, McGregor A, et al. Assessing the impact of incidental findings in a lung cancer screening study by using low-dose computed tomography. Can Assoc Radiol J. 2011;62:141–145. doi: 10.1016/j.carj.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrington de Gonzalez A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen. 2008;15:153–158. doi: 10.1258/jms.2008.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Stinnett AA. Adjusting for bias in C/E ratio estimates. Health Econ. 1996;5:470–472. doi: 10.1002/(SICI)1099-1050(199609)5:5<470::AID-HEC224>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Fenwick E, Marshall DA, Levy AR, et al. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6:52. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2012;157:776–784. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 31.Patz EF, Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Int Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Radiology. Lung Imaging Reporting and Data System (Lung-RADS®) [Accessed November 14, 2014];2014 Available at: http://www.acr.org/Quality-Safety/Resources/LungRADS. [Google Scholar]
- 33.Mahadevia PJ, Fleisher LA, Frick KD, et al. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003;289:313–322. doi: 10.1001/jama.289.3.313. [DOI] [PubMed] [Google Scholar]
- 34.Manser R, Dalton A, Carter R, et al. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer. 2005;48:171–185. doi: 10.1016/j.lungcan.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Marshall D, Simpson KN, Earle CC, et al. Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer. 2001;32:227–236. doi: 10.1016/s0169-5002(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 36.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff. 2012;31:770–779. doi: 10.1377/hlthaff.2011.0814. [DOI] [PubMed] [Google Scholar]
- 38.Wisnivesky JP, Mushlin AI, Sicherman N, et al. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest. 2003;124:614–621. doi: 10.1378/chest.124.2.614. [DOI] [PubMed] [Google Scholar]
- 39.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON) Br J Cancer. 2010;102:27–34. doi: 10.1038/sj.bjc.6605459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–156. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 41.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKee BJ, Regis SM, McKee AB, et al. Performance of ACR Lung-RADS in a Clinical CT Lung Screening Program. J Am Coll Radiol. 2014 doi: 10.1016/j.jacr.2014.08.004. DOI: http://dx.doi.org/10.1016/j.jacr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754–773. doi: 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Proposed decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) [Accessed November 10, 2014]; Available at: http://www.cms.gov/medicare-coverage-database/details/nca-details.aspx?NCAId=274&bc=AiAAAAAAAAAAAA%3d%3d&.2014.
- 45.Detterbeck FC, Unger M. Screening for lung cancer: moving into a new era. Ann Intern Med. 2014;160:363–364. doi: 10.7326/M13-2904. [DOI] [PubMed] [Google Scholar]