Abstract
Integrins are activatable adhesion and signaling molecules. Of the 24 known human integrins, three are currently targeted therapeutically by monoclonal antibodies, peptides or small molecules. The platelet αIIbβ3 integrin is targeted by Abciximab, Eptifibatide and Tirofiban, all with indications for preventing thrombotic complications after percutaneous coronary interventions. The lymphocyte α4β1 and α4β7 integrins are targeted by Natalizumab with indications in multiple sclerosis and Crohn’s disease. Although efficacious, use of this antibody is limited by a rare but serious complication, progressive multifocal leukoencephalopathy. Vedolizumab is an antibody to a combinatorial epitope in α4β7 that is approved for use in patients with Crohn’s disease or ulcerative colitis in the United States, Canada and Europe. Progressive multifocal leukoencephalopathy has not been observed in the clinical trials or clinical use of vedolizumab. New antibodies and small molecules targeting β7 integrins (α4β7 and αEβ7) and MAdCAM-1 are in clinical development for treatment of these inflammatory bowel diseases. Overall, integrin-based therapeutics have shown clinically significant benefits in many patients, leading to continued medical interest in the further development of novel integrin inhibitors. Of note, almost all integrin antagonists in use or in late-stage clinical trials target the ligand binding site, or the ligand itself.
Introduction
Integrins are adhesion receptors connecting cells to extracellular matrix ligands and to counter-receptors on other cells. Integrins are obligatory type I αβ heterodimers and molecular machines that undergo large conformational changes in their extracellular domains triggered by signaling molecules inside cells. This process, often referred to as inside-out signaling, is initiated by adaptor molecules that affect the position of the integrin α and β cytoplasmic tails relative to each other and to the plasma membrane. For many, if not all integrins, such conformational changes (“activation”) are required to actuate their adhesive function. Current dogma holds that the ligand binding domain in resting integrins is not readily accessible to adhesive ligands.
The best-known positive regulators of integrin activation are the adaptor molecules, talin-1 1 and the kindlins (kindlin-1, kindlin-2 and kindlin-3) 2. Beyond adhesion, integrins are also signal transduction machines. Once activated, integrins support ligand-dependent cellular signaling, a process called outside-in signaling because it is initiated by the binding of extracellular ligands to the integrins. Outside-in signaling involves, in part, ligand-dependent clustering of integrins that brings signaling domains of integrin-proximal proteins close enough together to initiate intracellular signals. Well-known intracellular events that are dependent on integrin outside-in signaling include activation of the spleen tyrosine kinase Syk 3, 4 and Src family protein tyrosine kinases in platelets 5 and leukocytes 3, and activation of NADPH oxidase in leukocytes 6.
Given their central roles in almost all phases of human biology as well as in the pathobiology of many diseases, integrins have long been the focus of the biotechnology and pharmaceutical industries as potential therapeutic targets. The first integrin-targeted drug, Abciximab, was introduced in 1994. Currently, ClinicalTrials.gov lists 80 clinical trials regarding integrin-based therapeutic drugs, imaging agents or biomarkers.
The purpose of this Opinion piece is to provide a biological context for integrins as drug targets, to highlight integrin antagonists that have shown benefit in patients or promise in late-stage clinical trials, and to review ongoing efforts to develop new integrin-targeted drugs. We focus on mechanisms of action, on what we have learned from successes and failures, and on side effects, both expected and unexpected. Previous reviews on the subject have focused on other aspects including details of integrin structure and allosteric inhibitors 7, leukocyte integrins 8, possible targets in airway hyper-responsiveness 9 and candidate molecules in early-stage trials 10. Not all efforts in this space have proven successful. Ten years ago, high hopes were placed in allosteric inhibitors 7, and large programs to develop such drugs were undertaken by many major pharmaceutical companies.
Integrin biology and drug development
An important lesson from past integrin drug development efforts is that successes are dependent on a combination of deep understanding of basic mechanisms of cell adhesion and unmet clinical need. All integrin antagonists on the market or in late-stage clinical trials target the ligand binding sites of integrins expressed in blood cells: leukocytes or platelets. Leukocyte and platelet integrins undergo conformational changes and “activation”. Both leukocyte and platelet integrins are masters at integrin affinity regulation by inside-out signaling. For example, in leukocyte integrins, the affinity change is thought to be about 10,000-fold 11.
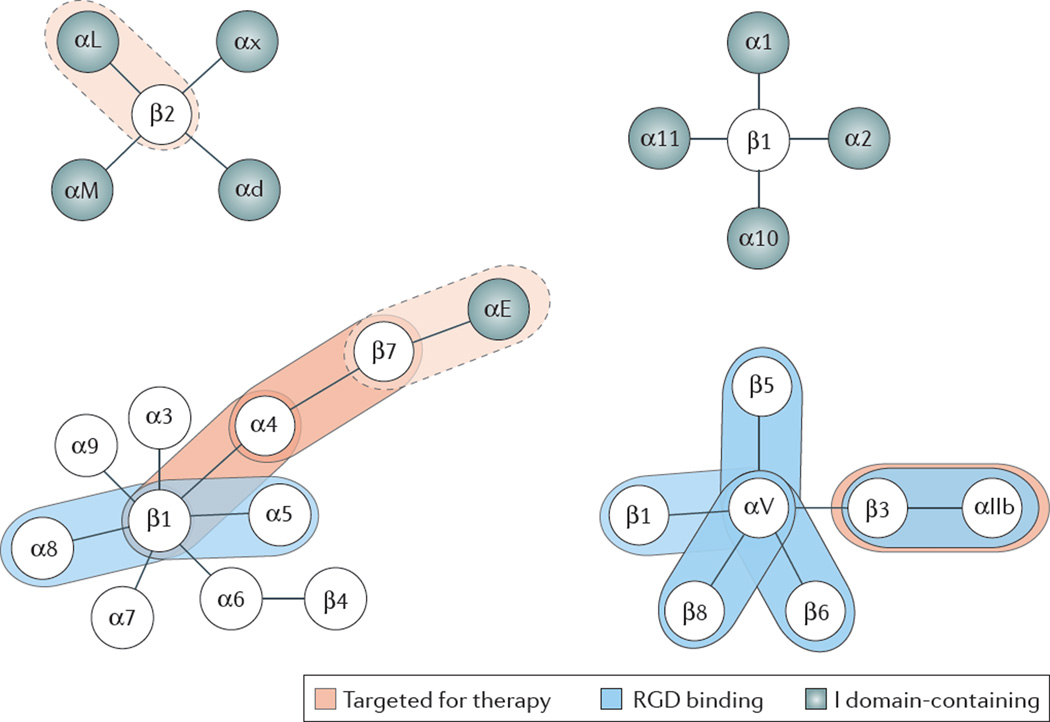
Nine of the 24 human integrins contain an “inserted” or I-domain that has homology to the von Willebrand factor A domain and is found in the extracellular portion of the α subunit 12 (Figure 1). All integrins with an I-domain bind extracellular matrix ligands or counter-receptors on other cells through this domain. These integrins then undergo a conformational change providing an “internal ligand” to the β subunit I-like domain. In contrast, all integrins without an I-domain bind ligand directly in a binding pocket formed by the most N-terminal subunits of both the α and the β polypeptide chains.
Figure 1.
Integrin families. Integrins targeted for therapy circled in red; dotted red circle indicates past therapeutic use (for αLβ2) or unknown effects (antibodies to β7 integrins also target αEβ7, but α4β7 is believed to be the effective target). RGD-binding integrins circled in blue, I-domain containing integrin α subunits circled in green.
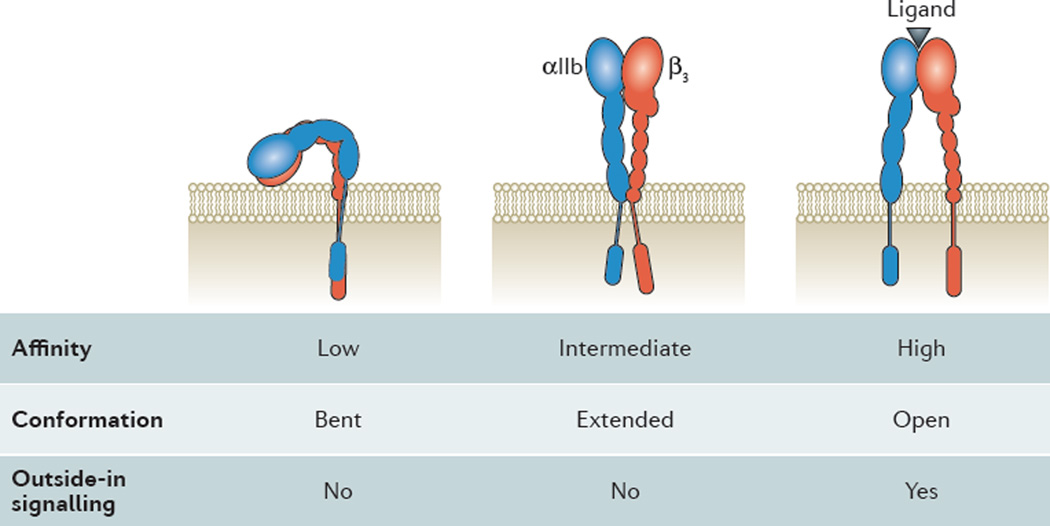
The conformational change during integrin activation (Figure 2) involves extension of the α and β “legs”, rearrangement of the αβ interface in the ligand binding domain, and separation of the α and β “feet” (transmembrane domains). The αL and β2 cytoplasmic tails of LFA-1 have been shown to move apart when LFA-1 is activated 13. This is thought to be a general process associated with integrin activation 14. Several detailed models of integrin activation have been proposed 15, 16.
Figure 2.
Inside-out activation of αIIbβ3 platelet integrin. Drawing of the bent (left), extended (middle) and extended-open (right) conformation of αIIbβ3. αIIb in blue, β3 in red. Ligand binding site indicated by black triangle in extended-open integrin. Note the movement of the transmembrane and cytoplasmic domains with integrin activation. Binding of cytoplasmic adaptor molecules (not shown here) are thought to drive the conformational changes in the ectodomains. Ligand binding affinity, conformation and outside-in signaling noted below each conformation.
Most of the integrins without αI-domains but none of the integrins with αI-domains bind the short peptide sequence arginine-glycine-aspartic acid (RGD), first discovered by Pierschbacher and Ruoslahti 17 (Figure 1). Some of the drugs targeting platelet αIIbβ3 are based on this RGD sequence. Another short amino acid recognition sequence was identified for α4β1 integrin: ILDV in the type III CS-1 segment of fibronectin 18. The other integrins do not bind consensus peptide sequences; the recognition site(s) in their ligands may be non-linear. A few integrins like Mac-1 (αMβ2) have also been reported to bind non-protein ligands (glycans and glycolipids), but this appears to be the exception rather than the rule. All integrins that have been targeted so far for therapeutic purposes normally bind protein ligands, and the antibody, peptide or small molecule antagonists that have made it to market all target the ligand binding site. Since integrins undergo large conformational changes during activation, allosteric inhibitors of the activation process have been proposed as drug targets 7. Small molecules that act as allosteric inhibitors have been developed by pharmaceutical industry 19, but none of them have made it to market. It is likely that allosteric inhibitors would have limited specificity and would have effects on multiple integrins.
Integrins have several divalent cation binding sites in their extracellular domains. Under physiologic conditions, these sites are occupied by Ca2+ and Mg2+. Mg2+ binding promotes the “open” or high-affinity conformation and Ca2+ promotes the “closed” or low-affinity conformation 20. In vitro, absence of Ca2+ and presence of Mg2+ or (even more powerfully but artificially) Mn2+ can induce the high affinity conformation(s), but at physiologic levels of calcium and magnesium, integrins can exist in all three conformations shown in Figure 2. The two activated forms are thought to be transient and can revert back to the low affinity conformation after seconds to minutes.
The canonical model of integrin activation posits that integrin extension is mechanically linked to open headpiece (high affinity binding) 11. As shown in figure 2, this would predict three conformations: bent with low affinity headpiece, extended with low affinity headpiece and extended with high affinity headpiece. Indeed, these conformations have been shown to exist on primary cells and the extended conformation with low affinity can be stabilized by certain allosteric antagonists 21. This conformation appears to support neutrophil rolling, but not firm adhesion 22–24. Although a large number of allosteric antagonists have been made that effectively inhibit either extension or the high affinity conformation 7, 19, 25, these have not been successful as systemic therapeutics. We speculate that either the specificity of these molecules was insufficient, i.e., each blocker would block multiple integrins, or unexpected systemic toxicity may have occurred. Alternatively, or in addition, the proposed conformational changes during activation, which have mainly been determined for αVβ3, αIIbβ3 and the β2 integrins, may not apply directly to α4β1 and α4β7 integrins so that the allosteric inhibitors would not work in some therapeutically relevant integrins. A few allosteric inhibitors for α4β1 have been described in preclinical studies 26, 27, but there is no evidence that any have been developed further or gone into clinical trials.
The clinically successful integrin drugs target αIIbβ3 (table 1), α4integrins (table 2) and α4β7 (table 3). Therefore, the remainder of this opinion piece is organized along these targets with a focus on new drugs.
Table 1.
Clinical trials evaluating drugs targeting αIIbβ3 in cardiovascular disease
| Drug | Indication | Major findings | Major adverse effects |
|---|---|---|---|
| Abciximab (monoclonal antibody to αIIbβ3 near MIDAS site, blocks binding of fibrinogen and other RGD ligands) |
In combination with heparin and aspirin: prevention of cardiac ischemic complications in patients with ACS undergoing PCI |
EPIC (2,099 patients) 35% fewer events |
Major bleeding (2x more common than placebo in CAPTURE, no difference in EPISTENT), thrombocytopenia by patient antibodies against murine sequences in Abciximab |
| EPILOG (2,972 patients): 65% fewer events, sustained benefit at 1 year | |||
| CAPTURE (1,265 patients) 29% fewer events | |||
| EPISTENT (2,399 patients) 51% fewer events | |||
| Eptifibatide (disulfide- linked cyclic heptapeptide, blocks binding of fibrinogen and other RGD ligands) |
NSTEMI patients undergoing elective, urgent or emergency PCI |
IMPACT-II (4,010 patients) 19% fewer events |
Major bleeding unchanged, minor bleeding slightly increased in IMPACT-II. Vascular access site complications reduced by early sheath removal and limiting heparin dose |
| PURSUIT (10,948 patients) 10% fewer events, sustained benefit at 6 months | |||
| ESPRIT (2,064 patients) significantly fewer events, sustained at six months and one year | |||
| Tirofiban (small molecule, blocks binding of fibrinogen and other RGD ligands) |
Unstable angina or NSTEMI patients undergoing PCI |
PRISM (3,232 patients) 32% fewer events |
Major bleeding similar in PRISM, PRISM-PLUS and RESTORE. Reversible thrombocytopenia 3x more common in PRISM, no difference in RESTORE |
| PRISM-PLUS (1,915 patients) 28% fewer events (compared to heparin/aspirin alone) | |||
| RESTORE (2,212 patients) significant reduction of events at 2 and 7, but not 30 or 180 days |
ACS: acute coronary syndrome, includes unstable angina, non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI)
PCI: percutaneous coronary intervention
Events: primary composite cardiovascular endpoint events, including death, myocardial infarction, repeat PCI, stent or bypass at 30 days, reduction compared to placebo
Table 2.
Clinical trials evaluating Natalizumab targeting α4 integrins in MS and CD
| Drug | Indication | Major findings | Major adverse effects |
|---|---|---|---|
| Natalizumab (monoclonal antibody to α4 ligand binding site, blocks VCAM-1 binding) |
MS patients who experienced at least one clinical relapse |
reduced relapses by 68% vs. placebo in 2 trials106 |
PML, a potentially fatal complication, estimated risk 2:1000 for patients treated for more than two years 102 6% of individuals receiving Natalizumab develop efficacy-reducing antibodies 75 |
| MS patients treated with Natalizumab in combination with interferon-β |
Reduced relapse and disability progression more than with interferon-β alone 107 |
||
| Patients with relapsing MS |
reduced visual loss 108 improved assessments of health-related quality of life 109, 110 MRI evidence that the formation of new lesions was prevented 111 |
||
|
Natalizumab (monoclonal antibody to α4 ligand binding site, blocks VCAM-1 and MAdCAM-1 binding) |
Patients with mild to moderate, active CD (151 <CDAI <450), 30 patients |
CDAI at week 2 after infusion significantly reduced112 compared to placebo, induction of clinical remission, as defined by a CDAI <150 |
Significant increase in circulating B and T lymphocytes at 1, 2, and 4 weeks |
| Patients with moderate to severe CD (220 <CDAI <450), 248 patients |
significantly higher chance of being in remission by week 4 to 12 113 |
No difference between treatment and placebo groups |
|
| Patients with moderate-to-severe CD (220 <CDAI <450): ENACT study. Induction and maintenance arms in patients with CD 114 905 patients |
clinical response at week 10, defined as a decrease in CDAI score of at least 70 points. Patients on continuous Natalizumab treatment had sustained clinical response through week 60 |
||
| CD patients with objective evidence of inflammation (elevated CRP): ENCORE trial115, 509 Patients |
clinical response (reduction of CDAI by 70 points from baseline) at week 8 significantly higher than placebo. Effect maintained through week 12 |
||
| FDA-approved as a remission-inductive, maintenance- sustaining and steroid-sparing therapy for CD. Patients must be monitored for PML in the TOUCH program |
Because of the PML risk, Natalizumab has effectively been displaced by drugs targeting β7 integrins |
||
Table 3.
Clinical trials evaluating Vedolizumab targeting α4β7 in CD and UC
| Drug | Indication | Major findings | Major adverse effects |
|---|---|---|---|
| MLN02 (IgG1 monoclonal antibody to a combinatorial epitope requiring α4 and β7, blocks MAdCAM-p1 binding, manufactured in mouse myeloma cell line). MLN02 was an early version of Vedolizumab. |
Patients with active UC |
Significant improvement by 6 weeks, sustained at 10 and 14 weeks |
No deaths, no evidence of PML (more than 2,700 patients treated: zero PML events, upper level of 95% confidence interval), no increase in opportunistic infections 117, 118 Significant anti-drug antibody formation118 one significant infusion reaction |
| 29 patients with moderately severe UC 119 |
|||
| 181 patients with moderate-to-severe UC (UCCS 5 to 7) phase II study 120 |
Clinical remission (UCCS 0 to 2, absence of rectal bleeding) significantly higher than placebo in the 0.5mg and 2.0 mg groups |
||
| 185 patients with moderate-to-severe CD, double-blind placebo controlled trial 121 |
Significantly higher clinical remission (CDAI score < 150) in the 2.0 mg/kg group than placebo |
||
|
Vedolizumab (humanized IgG1 monoclonal antibody to a combinatorial epitope requiring α4 and β7, blocks MAdCAM-p1 binding, manufactured in Chinese hamster ovary cells) |
Phase III UC trial (GEMINI 1), 374 patients 122 |
Clinical response at week 6 significantly better than placebo, significantly higher remission rates than placebo, higher mucosal healing rates |
no evidence of PML (more than 2,700 patients treated: zero PML events, upper level of 95% confidence interval) low anti-drug antibody formation118 safety data not different between drug and placebo |
| Active CD: phase III CD trial (GEMINI 2), 368 patients 123 |
clinical remission (CDAI score of ≤150 points) and CDAI-100 response (≥100-point decrease in the CDAI score from baseline) at week 6 significantly better than placebo, maintained at week 52 |
||
| 416 patients with CD, most with previously failed anti-TNF therapy. Multicenter double- blind phase 3 study |
Remission rates and rates of CDAI-100 response at week 6 and 10 in anti-TNF non- responders significantly higher than placebo 117, 124 |
UCCS: Ulcerative Colitis Clinical Score
Platelet integrins
αIIbβ3
Inherited deficiency or dysfunction of αIIbβ328 causes a rare but serious bleeding disorder, Glanzmann thrombasthenia, due to the inability of activated platelets to aggregate in a ligand-dependent manner. Platelets also express four other integrins, including α2β1, which binds collagen, but its signaling function in stimulating platelet aggregation and secretion is minor compared to that of the (non-integrin) collagen receptor GPVI 29. αIIbβ3 has some affinity for immobilized fibrinogen even without deliberate platelet activation. When platelets are fully activated, αIIbβ3 can bind soluble fibrinogen, von Willebrand factor, fibronectin and vitronectin 28, 30 in a manner that depends on the presence of one or more of the RGD sequences in the ligands, or in the case of fibrinogen, on the carboxy-terminus of the γ chain 31, 32. The dimeric fibrinogen molecule mediates platelet aggregation by serving as a bridge between, αIIbβ3 receptors on adjacent platelets.
Inside-out activation of αIIbβ3 is very well studied 14. A key element is binding of talin-1 to a membrane-proximal region and an NPXY motif in the β3 cytoplasmic domain 1. Identifying the gene responsible for a rare inherited bleeding disorder in which αIIbβ3 cannot be activated has led to the recognition that kindlin-3 is also required for this process 33,34. The precise mechanism(s) by which kindlin-3 influences integrin activation is incompletely understood but appears to involve, at least in part, kindlin-3 interaction with the C-terminal region of the β3 cytoplasmic domain and clustering of αIIbβ3 heterodimers into oligomers 35. All reported patients with a null mutation in kindlin-3 also exhibit defective activation of their leukocyte integrins 34, resulting in recurrent infections. The syndrome is therefore known as leukocyte adhesion deficiency (LAD) type III (or LAD type I variant).
After blood cell development, αIIbβ3 is expressed exclusively in megakaryocytes and platelets. This restricted expression and the obligatory requirement for ligand binding to αIIbβ3 in platelet aggregation during hemostasis and thrombosis led investigators to consider this integrin as a potential therapeutic target for development of anti-platelet, anti-thrombotic drugs 28, 30. Abciximab, the Fab fragment of a chimeric mouse-human monoclonal antibody to αIIbβ3, was the first integrin antagonist in clinical medicine 36. Two additional parenteral, non-antibody αIIbβ3 antagonists, Eptifibatide 37 and Tirofiban 38, quickly followed for similar indications, and all three drugs work by directly blocking ligand binding to αIIbβ3. As knowledge pertaining to mechanisms of αIIbβ3 signaling has increased in recent years, therapeutic blockade of specific intracellular facets of inside-out and/or outside-in αIIbβ3 signaling remains an appealing, if only theoretical, possibility 39.
αVβ3
In contrast to αIIbβ3, αVβ3 is more widely expressed in tissues, particularly in proliferative endothelial cells, where it has been implicated in aspects of angiogenesis, and in vascular smooth muscle cells, monocyte/macrophages and some tumor cells 40. αVβ3 can interact with many of the same RGD-containing adhesive proteins as αIIbβ3, but with different affinities, and it can interact with a number of non-RGD-containing proteins in the extracellular matrix. Despite substantial efforts at development by the pharmaceutical industry 10, an αV antagonist, Cilengitide, blocks the binding of vitronectin to αVβ3 but has not shown efficacy in clinical trials aimed at limiting tumor angiogenesis and progression in patients with glioblastoma 41. Its failure in this context may be due to complexities in the dose- and timing-dependent mechanism of action of Cilengitide administration as shown in mouse models 42 as well as the inherent difficulties of treating a notoriously resistant neoplasm with a single targeted drug 43.
αIIbβ3 in cardiovascular medicine
Abciximab36, 44 binds to αIIbβ3 with nanomolar affinity and inhibits the binding of fibrinogen, von Willebrand factor and other RGD-containing adhesive ligands to human αIIbβ3. As a result, Abciximab blocks agonist-induced aggregation of human platelets as well as downstream platelet responses dependent on aggregation 28, 44. The epitope for this antibody is in the specificity-determining loop of β3, close to the β3 “MIDAS” metal ion-dependent adhesion site 45. In addition to αIIbβ3, Abciximab has been reported to bind to αVβ3 and, to a lesser extent, αMβ2, and to inhibit αVβ3-dependent endothelial cell spreading in vitro 46, 47. The role, if any, of αVβ3 or αMβ2 binding by Abciximab in its anti-thrombotic efficacy in humans is unclear. Abciximab is indicated for use with heparin and aspirin as an adjunct for the prevention of cardiac ischemic complications, either in patients undergoing PCI or in patients with unstable angina not responding to conventional medical therapy in whom PCI is planned within 24 hours (table 1).
Eptifibatide is a disulfide-linked, cyclic heptapeptide containing a 1-mercaptopropionyl residue and is based on the amino acid sequence Lys-Gly-Asp (KGD) in the snake venom, barbourin.37, 44, 48 Eptifibatide is highly selective for αIIbβ3 and binds to the integrin at the ligand-binding pocket in a divalent cation-sensitive manner to reversibly inhibit adhesive ligand binding and platelet aggregation. Eptifibatide is indicated for patients with non-ST elevation myocardial infarction (NSTEMI), including those who are to be managed medically and those undergoing PCI (table 1).
Tirofiban is a highly selective small molecule (N(butylsulfonyl)-O-(4-(4-piperidinyl)butyl)-L-tyrosine monohydrochloride monohydrate) inhibitor of αIIbβ3 that was approved for human use in 1999 38, 44. Like Eptifibatide, it is highly selective for αIIbβ3 and blocks ADP-induced platelet aggregation. Tirofiban is indicated to reduce the rate of thrombotic cardiovascular events in patients with NSTEMI who are undergoing PCI (table 1).
Adverse events with αIIbβ3 antagonists
Unsurprisingly, the most serious side effects common to all parenteral αIIbβ3 antagonists are bleeding and thrombocytopenia. The main underlying mechanism is immunological but appears to vary in detail depending on the drug 49. In the case of Abciximab, thrombocytopenia appears to be caused in most cases by the development of antibodies to murine sequences in the chimeric Fab fragment. In individuals receiving Eptifibatide or Tirofiban, thrombocytopenia typically occurs during the drug infusion and appears to be caused in most cases by antibodies to extracellular epitopes in αIIbβ3 that are exposed by binding of the drug. These antibodies may be pre-formed and naturally occurring in these individuals 49, 50. When thrombocytopenia occurs with any αIIbβ3 antagonist, the drug must be discontinued and platelet transfusion given if clinically indicated.
Impact of the availability of newer anti-platelet and anti-thrombotic drugs
Currently, no active clinical trials testing new inhibitors of αIIbβ3 are listed in clinicaltrials.gov. This is owing largely to the introduction of P2Y12 and PAR1 thrombin receptor inhibitors, which has led to a reduction in the market for αIIbβ3 integrin antagonists. In ISAR-REACT-2, Abciximab reduced the risk of adverse events in patients with NSTEMI undergoing PCI, even after optimal pre-treatment with 600 mg of clopidogrel 51. However, with the increased availability of efficacious newer parenteral anticoagulants (e.g., bivalirudin) and newer P2Y12 receptor antagonists that are more potent and/or more rapdily acting than clopidogrel (e.g., cangrelor and the orally bioavailable prasugrel and ticagrelor), the use of αIIbβ3 antagonists has decreased 52. Nonetheless, the αIIbβ3 antagonists appropriately remain in the cardiologist’s arsenal, particularly for use in high-risk individuals undergoing PCI, including those in whom the use of P2Y12 antagonists might have been delayed or are likely to be relatively ineffective.
Failure of oral αIIbβ3 antagonists
Given the efficacy of parenteral αIIbβ3 antagonists in the setting of acute coronary syndromes (which include NSTEMI, ST-elevation myocardial infarction and unstable angina) and PCI, several oral agents selective for αIIbβ3 were developed and tested in phase III trials. These studies showed that the use of the oral agents was associated with excess bleeding and mortality, the latter primarily due to cardiovascular events 28, 30, 53. The reasons for this failure are still debated, although three issues seem germane 54. First, the successful studies with parenteral αIIbβ3 antagonists employed drug doses and infusion schemes that resulted in high-grade, continued occupancy of αIIbβ3 (>80%) during the treatment phase. This degree and continuity of receptor inhibition might be very hard to maintain with an oral αIIbβ3 antagonist, such that periods of αIIbβ3 availability to fibrinogen and other adhesive ligands would be expected to occur, enabling aggregation of activated platelets at sites of vascular pathology. Second, most oral αIIbβ3 antagonists are expected to change the conformation of the receptor upon binding, possibly leading to an unintended partial agonist effect on platelets 55. The failure of oral αIIbβ3 inhibitors is a good example of how perfectly sound mechanistic reasoning (these drugs should have worked) paired with unfavorable pharmacokinetics and unexpected allosteric effects (conformation change) can result in adverse outcomes that were completely unpredictable until the clinical trial data became available.
Future prospects for αIIbβ3 antagonists
Although αIIbβ3 is a proven therapeutic target, the initial wave of failures of oral αIIbβ3 antagonists effectively eliminated pharmaceutical companies from this development space. Recently, however, Coller and colleagues identified an αIIbβ3-selective compound from a high-throughput drug screen that does not induce a detectable conformational change in αIIbβ3 when it binds to the RGD binding site in the receptor. Subsequent structure-based drug design and development of a water-soluble congener, RUC-4, demonstrated a sub-micromolar IC50 for ADP-induced platelet aggregation, both in vitro and after intramuscular administration to non-human primates 56. RUC-4 may be further evaluated for potential administration to patients with acute coronary syndromes in the pre-hospital setting, because the drug could be available in a formulation for intramuscular administration, a clear advantage over intravenous αIIbβ3 antagonists in an ambulance setting.
Leukocyte integrins
Six integrins are expressed exclusively in leukocytes: LFA-1 (αLβ2), Mac-1 (αMβ2), p150, 95 (αxβ2), αdβ2, α4β7 and αEβ7. Five of these contain αI–domains (Figure 1). LFA-1 (αLβ2) has been shown to undergo very large conformational changes secondary to inside-out signaling as revealed by cryo-electron microscopy 57 and crystallography58, which is associated with ~10,000 fold increases in ligand binding affinity11. Patients with Leukocyte Adhesion Deficiency Type I (LAD-I) have hypomorphic or null mutations in the β2 chain, also known as CD18, common to αLβ2, αMβ2, αxβ2 and αdβ2 and exhibit mild to severe inflammatory defects 59. There are no known human genetic defects in the four individual α chains or in either of the β7 integrins.
Leukocyte integrins play a prominent role in inflammation and immunity. Specifically, the β2 integrin LFA-1 is required for the formation of the immunological synapse 60. The association with lymphocyte function is actually what gave it its name. αL knockout mice have reduced lymphocyte numbers in their secondary lymphoid organs and a mild defect in inflammation 61. Mac-1 (αMβ2) plays a major role in host defense, especially against bacterial and fungal infections. Mac-1 is also known as complement receptor CR3 and is a major molecule recognizing complement C3bi-opsonized particles. αM knockout mice have a defect in neutrophil apoptosis 62 and reduced proteinuria in a mouse model of immune complex-induced kidney disease 63. A single nucleotide polymorphism in the human ITGAM gene encoding the αM subunit of αMβ2 is highly associated with lupus erythemaosus 64, 65. αxβ2 is also known as CR4. The knockout mouse has no spontaneous phenotype, but was shown to play a role in a model of atherosclerosis 66. Combined knockout of all four β2 integrins by targeting the β2 subunit Itgb2 results in a very severe inflammatory disease with high neutrophil numbers, spontanmeous infections and an inability of neutrophils to assemble the NADPH oxidase 6.
Among the leukocyte-specific integrins, both α4β7 and αEβ7 direct lymphocyte trafficking to the intestinal tissues. α4β7 is the major and defining determinant of gut-homing lymphocytes 67, 68. αEβ7 binds to E-cadherin and places lymphocytes known as intraepithelial lymphocytes (IEL) near or inside the epithelial monolayer lining the intestine. α4β1 was originally identified on lymphocytes activated for extended periods and named very late antigen-4 (VLA-4) 69. It binds VCAM-1 70 and other ligands on endothelial cells and is involved in adhesion of effector, effector-memory and central memory cells to many, if not all, inflamed organs.
The rationale for targeting leukocyte integrins is to modulate inflammation. An early major observation in the field was that antibodies to α4β1 can cure EAE, a mouse model of multiple sclerosis, which spawned the clinical development of α4 antagonists (see below and table 2). Mice lacking all four β2 integrins or individual β2 integrins, or mice in which β2 integrins are blocked by antibodies are protected in many models of ischemia and reperfusion 71, 72.
α4β7 is targeted by the antibody Vedolizumab, which has recently proven useful in the treatment of inflammatory bowel diseases, as well as by another antibody, AMG181. α4β7 does not contain an I-domain and binds predominantly to MAdCAM-1, which is expressed on endothelial cells in tissues of the gastrointestinal tract 73. The α4β7 ligand MAdCAM-1 is the target of a new antibody to treat inflammatory bowel disease (see below).
αEβ7 binds E-cadherin and is thought to be involved in localizing leukocytes to gut epithelial cells. An antibody targeting β7 is in late-stage clinical development for inflammatory bowel diseases (see below). A seventh leukocyte integrin, α4β1, is expressed on monocytes and lymphocytes, but, unlike the other six, is also expressed on many other cells. It binds both a splice variant of fibronectin containing the peptide sequence ILDV as well as vascular endothelial cell adhesion molecule-1, VCAM-1 70, which supports slow rolling, adhesion and transmigration as well as pro-inflammatory signaling into the endothelial cells. α4β1 has no I-domain and is targeted by the antibody Natalizumab, with indications in multiple sclerosis (MS) and Crohn’s disease (CD) (see below).
Targeting leukocyte integrins
Targeting leukocyte integrins has proven applications in diseases such as multiple sclerosis and the inflammatory bowel diseases Crohn’s disease (CD) and ulcerative colitis (UC). Four leukocyte integrins, αLβ2, α4β1, α4β7 and αEβ7 have been targeted by monoclonal antibodies in patients. αL is the target of efalizumab, which was previously on the market for psoriasis but was withdrawn in 2009 because of association with a fatal brain infection, progressive multifocal leukoencephalopathy (PML, see box 1). A topical LFA-1 inhibitor, lifitegrast, recently successfully completed a phase III trial, the SONATA study, examining lifitegrast ophthalmic solution in patients with dry eye (see Further information) Lifitegrast is a small-molecule integrin antagonist designed to reduce inflammation. It binds to αLβ2 integrin (LFA-1) and blocks the interaction of LFA-1 with its cognate ligand ICAM-1. ICAM-1 is over-expressed in corneal and conjunctival tissues in dry eye disease. In the SONATA study, adverse events occurred in 53.6% of patients in the lifitegrast group and 32.4% of patients in the placebo group, but there were no serious ocular adverse events or systemic toxicity and discontinuation due to adverse events was infrequent. The FDA granted lifitegrast priority review status on April 9, 2015.
Box 1: Progressive Multifocal Leukoencephalopathy: A Major Complication.
The widespread use of anti-integrin monoclonal antibodies for the treatment of IBD, multiple sclerosis or psoriasis has been hampered by the occurrence of a rare but potentially fatal complication, PML 102. This condition is the result of reactivation of a polyoma virus, which is designated John Cunningham (JC) virus. The risk for developing PML after treatment with Natalizumab has been estimated to be approximately 2:1000 for patients treated for more than two years. Until 2009, four cases were described within a cohort of 6000 patients that had received Efalizumab for psoriasis. The unexpected development of PML in patients treated with Natalizumab triggered its voluntary withdrawal from the market in February 2005 (it returned in July 2006 under TOUCH monitoring) and of Efalizumab in 2009. PML appears to be a true drug-effect, as neither MS, CD nor psoriasis, per se, have been associated with PML. There is no known treatment, prevention or cure for PML. The infection usually leads to death or severe disability.
The pathogenesis of PML in patients receiving Natalizumab is largely unknown. Nevertheless, it may be primarily associated with the blockade of α4β1/VCAM-1 interactions by Natalizumab. This may result in the blockade of migration of JCV-specific lymphocytes to the central nervous system, including cytolytic T-lymphocytes, which have been associated with increased survival from PML 103. Alternative pathogenic mechanisms may also participate, such as mobilization of JC-infected pre-B-cells from the bone marrow due to α4β1 blockade 104. If blockade of α4β1/VCAM-1 interactions is mainly responsible for PML development in the central nervous system, then it should be expected that selective blockade of α4β7 may not be associated with this complication. Indeed, there have been no cases of PML to date in patients treated with the specific anti- α4β7 antibody vedolizumab.
It is not clear whether PML is a “class” adverse effect. Cases of PML have also been reported in patients receiving rituximab 105, an anti-CD20 monoclonal antibody that primarily targets B-cells. Nevertheless, a causal association between this drug and PML cannot be directly established, since some of the conditions for which rituximab was administered (lymphoproliferative disorders, systemic lupus erythematosus and rheumatoid arthritis) may inherently increase the risk for developing PML. The frequency of PML in patients who are negative for JC virus (around 50% of patients) is near zero, thus it is possible that anti-integrin antibodies might be safely used in subsets of JC-seronegative patients.
The association between anti-integrin monoclonal antibodies and PML has been a significant impediment for their widespread use in IBD clinical practice. Current strategies to overcome this problem have focused on the careful pre-testing for JC virus antibodies in treatment candidates and thorough monitoring of JC serologic conversion in actively treated patients.
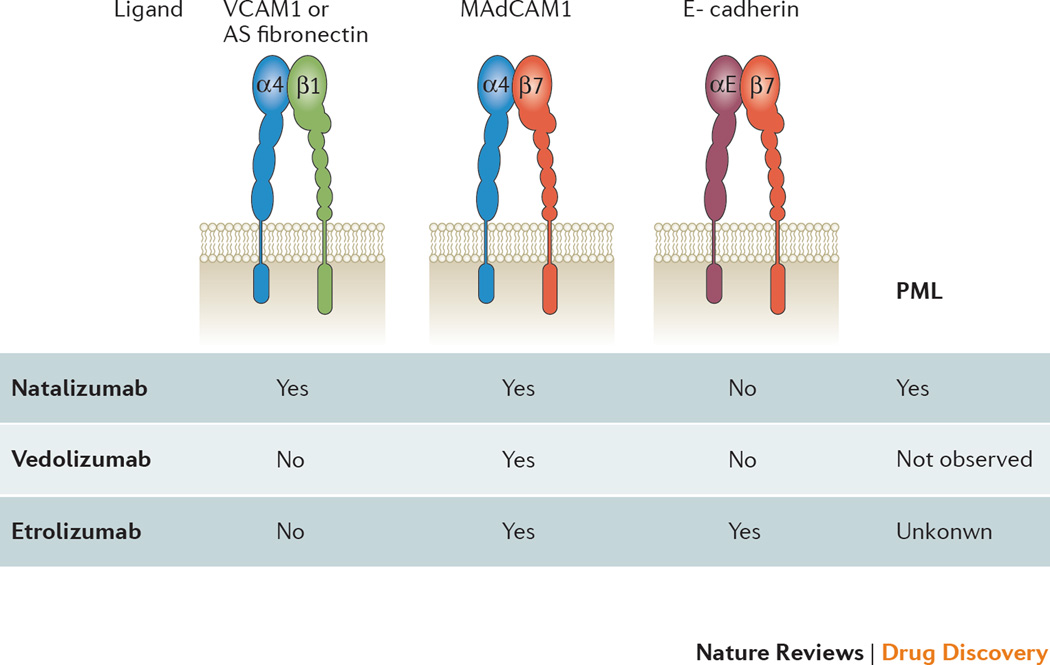
The first widely successful drug targeting leukocyte integrins was natalizumab with indications in multiple sclerosis and Crohn’s disease. All integrin-targeting drugs clinically approved for IBD are humanized monoclonal antibodies that target α4 integrins or the α4β7 heterodimer (Figure 3). Antibodies that target the β7 integrin subunit and the MAdCAM-1 ligand are currently in clinical trials.
Figure 3.
The three integrins α4β1, α4β7 and αEβ7 targeted by therapeutic α4 and β7 antibodies. α4 (blue) and thus α4β1 and α4β7 are targeted by natalizumab. β7 (purple) and thus α4β7 and αEβ7 are targeted by etrolizumab. Vedalizumab and AMG-181 recognize an epitope formed by both α4 and β7 and thus is monospecific. The αE subunit (orange) contains an I domain (I). Main ligands for each integrin noted above. PML: progressive multifocal leukoencephalopathy.
Targeting α4 integrins in MS
α4 can pair with β1 to form VLA-4 or with β7 to form α4β7 (Figures 1, 3). Therefore, drugs targeting α4 effectively inhibit two integrins, α4β1 and α4β7 (Figure 3). Natalizumab is a recombinant humanized IgG4κ monoclonal antibody that binds to α4 integrins and blocks the binding of physiological ligands. α4β1 integrin binds VCAM-1, which is expressed on inflamed endothelial cells, macrophages and other cells, and alternatively spliced fibronectin, an extracellular matrix component. α4β7 binds MAdCAM-1, expressed on intestinal endothelial cells (Figure 3). These properties were demonstrated in vitro, as Natalizumab effectively prevented adhesion of human Jurkat cells that expressed α4β1 to purified recombinant VCAM-1 and of RPMI-8866 cells that expressed α4β7 to recombinant MAdCAM-1. These data were complemented by in vivo studies of experimental allergic encephalomyelitis (EAE) in rodents. This model is mediated by T-lymphocytes that infiltrate regions of the central nervous system via α4β1/VCAM-1-mediated migration. A monoclonal α4 antibody prevented leukocytes from crossing the blood-brain barrier and prevented the development of neurological manifestations of EAE and reversed established disease 74. In all, these results provided a direct proof for the efficacy of natalizumab-like antibodies as an anti-adhesion drug in animal models.
Natalizumab is effective in treating patients with multiple sclerosis (clinical trials summarized in table 2). Approximately 6% of individuals receiving Natalizumab have been found to develop efficacy-reducing antibodies to the drug 75. Natalizumab is approved in the United States and the European Union as monotherapy for the treatment of highly active relapsing and remitting MS in spite of prior treatments. The unexpected development of PML (box 1) in patients treated with natalizumab triggered a voluntary withdrawal of the drug from the market in February 2005. Remarkably, MS patient advocacy groups lobbied the US Food and Drug Administration (FDA) to make Natalizumab available again, because the benefits were so significant. Natalizumab returned in July 2006 under a strict TYSABRI Outreach: Unified Commitment to Health (TOUCH) monitoring program. More than 100,000 MS patients have been treated with Natalizumab.
Targeting α4 integrins in IBD
Natalizumab is also approved by the FDA as a remission-inductive and maintenance-sustaining therapy for CD. The success of natalizumab in patients with CD (see table 2 for clinical trials) provided a strong incentive to develop more specific drugs targeting α4 integrins in the intestinal tract. This was achieved by targeting α4β7, β7 and MAdCAM-1.
Targeting α4β7 in IBD
Pre-clinical studies in cotton top tamarins with spontaneous colitis provided evidence for an anti-inflammatory effect of α4β7 blockade in experimental intestinal inflammation 76, 77. Based on these results, Vedolizumab (previously known as MLN-02, LDP-02, MLN0002, brand name Entyvio), a humanized IgG-1 monoclonal antibody, was developed. This antibody blocks binding of MAdCAM-1 to α4β7 integrin by binding to the integrin heterodimer The clinical trials of Vedolizumab in CD and UC are summarized in table 3 and the drug is approved by the FDA for the treatment of UC and CD.
As PML risk is the limiting factor for the use of Natalizumab, Vedolizumab has effectively replaced Natalizumab in clinical practice for CD and UC. At the same time, these encouraging results have spawned studies into new indications and new drugs in the β7 integrin space. Potential new indications for Vedolizumab include primary sclerosing cholangitis, based on the expression of MAdCAM-1 in chronically inflamed liver 78. Refractory pouchitis is another potential new indication. No published clinical data are available at this time.
New leukocyte integrin antagonists for IBD
AMG 181
AMG 181 is a human monoclonal antibody (IgG2) against the α4β7 integrin heterodimer. AMG 181 is conceptually similar to vedolizumab, because it also binds a combinatorial epitope, which means it binds neither α4 nor β7 in isolation. The drug was generated at Amgen by expression in CHO cells 79 and is administered via the subcutaneous route. Safety data was published in 2014 80 . No cases of PML have been observed. There is no published data regarding similarities or differences between AMG 181 and Vedolizumab in terms of their binding site. One study, NCT01290042, comparing four escalating doses of AMG 181 administered as multiple doses in healthy subjects and in subjects with active UC has been completed but not yet published. A phase I study (NCT01164904) in healthy volunteers and patients with UC was terminated. The reason for termination is not available. Two phase 2 studies in UC (NCT01694485) and CD (NCT01696396) are listed as active at http://clinicaltrials.gov.
Etrolizumab
Etrolizumab is a humanized IgG1 monoclonal antibody that is directed against the β7 integrin subunit, thus targeting both αEβ7 and the α4β7 (Figure 3) and blocking their interactions with MAdCAM-1 and E-cadherin, respectively. It is not known whether additional aspects of immunology would be targeted by etrolizumab, but theoretically it should target intestinal intraepithelial lymphocytes (IELs), which express αEβ7. A subset of dendritic cells that produce anti-inflammatory retinoic acid and support the development of regulatory T cells also express αEβ7 81 and thus might be targeted by Etrolizumab. Pre-clinical studies showed that Etrolizumab effectively inhibits migration of T-cells to mucosal sites, without affecting their homing to non-mucosal tissue 82. In a randomized, phase I study on the use of Etrolizumab (PRO145223) in moderate to severe UC, the drug was safe and well tolerated 83. Serious adverse effects included exacerbation of UC and impaired wound healing in two patients who underwent colectomy. There was a decrease in “availability” of β7 receptors on target CD4+ lymphocytes, suggesting that Etrolizumab administration might decrease the number of lymphocytes homing to the gut.
The results of a double-blind, placebo-controlled randomized, phase 2 study on the use of etrolizumab in patients with UC were recently reported 84. Etrolizumab was safe and well tolerated, and no serious opportunistic infections were reported. In that study, Etrolizumab was more likely than placebo to lead to clinical remission at week 10, however the high dose did not provide added benefit. Interestingly, anti-TNF non-responders fared worse than anti-TNF-naïve. Although the biologic basis for this observation is unknown at the present time, we may speculate that the anti-TNF non-responder population is enriched for those with the most therapy-resistant diseaseThe mechanisms of non-response are not clearly understood, but have tentatively been attributed to anti-drug antibodies or pharmacokinetics.
The study additionally found increased CD4+β7+ T cells in peripheral blood, which consistent with the hypothesis that Etrolizumab interferes with effector T cell recruitment into the intestine. However, they found no change in β7, β1 or αE mRNA levels in intestinal biopsies, which would have further supported this possibility. Interestingly, when the investigators looked at subsets of patients that were either αE high or αElow they observed that most patients with clinical remission at day one were αEhigh. This is interesting as this αEβ7 heterodimer has been more commonly associated with tolerogenic/immunoregulatory cells in populations of both T cells and dendritic cells. We could speculate that different doses of this drug might result in distinct levels of the drug in tissues that interfere with the α4β7 heterodimer at one level and with the αEβ7 heterodimer at another level. This could have consequences that would not be expected based on the clinical experience with vedolizumab, which does not bind to the αEβ7 heterodimer. αEβ7 has been most recently implicated on tissue resident memory cells (TRM cells), 85 however, both the functional role of αEβ7 in TRM cells and the role of TRM cells in chronic inflammatory processes are poorly understood.
AJM 300
AJM 300 is an oral compound that acts as an antagonist of α4 integrins. All information regarding molecular structure and binding site remains unpublished by Ajinomoto Inc. Kawasaki Japan. Several studies have reported the efficacy of this small molecule in animal models of IBD. A manuscript was submitted and later withdrawn as the authors did not comply with journal requirements for publishing the molecular structure 86. Similarly, the results of a randomized, double-blind, placebo controlled trial in Japanese patients with active CD was presented during the Digestive Diseases Week meeting in 2009 87 AJM 300 was safe and well tolerated and showed a statistically significant improvement in clinical response rate in patients with moderately active UC. However, this drug is likely to cause PML at a rate similar to natalizumab which would be an unacceptable risk in UC patients. Further evaluation of the potential safety of AJM 300 in IBD will be required.
Targeting integrin ligands
Since targeting α4β7 has been so successful, MAdCAM-1 became an obvious target. MAdCAM-1 is normally expressed in the mesentric lymph node and Peyers patches, but becomes more widely expressed in other venules of the intestinal wall during inflammation. Many of the endothelial ligands for integrins share structural and genetic features with immunoglobulin molecules; they contain at least one immunoglobulin domain, comprising two β-pleated sheets held together by a disulfide bond. Among the many members of the immunoglobulin superfamily, several have established pathogenetic roles in IBD. Intercellular adhesion molecule-1 (ICAM-1, CD54), Vascular Cell Adhesion Molecule-1 (VCAM-1, CD106) and MAdCAM-1 are all known to be involved in IBD, but only MAdCAM-1 is gut-specific.
MAdCAM-1: the endothelial α4β7 integrin ligand
MAdCAM-1 levels are increased in the colon of animal models of colitis 88 and in humans with IBD the number of intestinal mucosal vessels that stain positive for MAdCAM-1 is increased89. TNF-α and IL-1 are abundant in areas of active CD or UC and have been shown to upregulate MAdCAM-1 expression in the intestine, colon and MLN 88, 90. MAdCAM-1 is detected at extra-intestinal sites, such as the joints, eyes, skin and liver 91. As these organs are frequently affected in patients with IBD, the aberrant expression of this gut-homing molecule may attract pathogenic cells and induce extra-intestinal inflammation. MAdCAM-1 expression is increased on inflamed venules in other chronic inflammatory conditions such as diabetes, primary sclerosing cholangitis, and cirrhosis 92.
PF-00547659 is a fully human IgG2K monoclonal antibody that binds specifically to human MAdCAM-1. In functional assays the drug blocked the adhesion of cells expressing α4β7 integrin to MAdCAM-1 93. The results of the phase II TURANDOT study were presented at the Digestive Diseases Week Meeting 2015 94 The primary endpoint of clinical remission was significantly greater in the three lowest dose groups compared with placebo. The secondary endpoint of mucosal healing was significantly greater in the 22.5 mg and 75 mg dose groups compared with placebo, while response was greater for the 22.5 mg and 225 mg groups. This study was not powered to compare the different doses of drug, and so it is unclear whether the observed lower remission rates in the 225 mg group was statistical play of chance, or whether there is a biologic basis for this observation. There was no evidence of increased infections in mucosal tissues (gastrointestinal, nasal, spleen, bladder, uterus and lung) and no cases of progressive multifocal leukoencephalopathy (PML) were observed. Of note, a consistent finding for all endpoints was that the second-lowest of the four doses tested was the most effective.
Another anti-MAdCAM-1 antibody, PF-00547659, has been investigated for the treatment of Crohn’s Disease. The results of a randomized, multicenter double-blind, placebo-controlled study were presented at the Digestive Diseases week 2015 95 Although the primary endpoint disease score was not significantly different between any of PF-00547659 doses and placebo, remission at week 12 appeared to be substantially higher in those patients with a median baseline CRP level >18. The primary endpoint was not met due to a high placebo response. However, PF-00547659 was pharmacologically active as shown by a dose-related increase in circulating β7+ T lymphocytes and a sustained dose-related decrease in soluble MAdCAM-1 in the blood MAdCAM-1 levels remained low during the study in patients who received drug. Circulating β7 CD4+ central memory T-lymphocytes increased at weeks 8 and 12 in patients treated with PF-00547659 in a dose-dependent manner. This suggests that MAdCAM-1 is relevant in rolling and adhesion of α4β7+ lymphocytes in these patients and blocking MAdCAM-1 releases these lymphocytes into the circulation. Interestingly, higher rates of remission in patients with high CRP levels were also observed in the Natalizumab trials, completed nearly a decade ago. Objective outcomes other than disease score will be needed for future CD trials. Endoscopic, histologic and magnetic resonance imaging outcomes are being extensively discussed in the field.
Other ligands that could be targeted include ICAM-1, a ligand for LFA-1 and Mac-1, and VCAM-1, a ligand for α4β1. ICAM-1 was targeted by mAb RR6.5 (Enlimomab, Boehringer Ingelheim) early on 96, but this agent was not effective in a clinical trial of 625 patients with ischemic stroke that were treated within 6 hours of stroke onset 97. VCAM-1 is expressed broadly on endothelial cells but also in macrophages. Notably, as VCAM-1 is the main ligand of α4β1, blocking VCAM-1 would be expected to have a PML liability.
The experience with integrin-targeted drugs in cancer is limited to αVβ3, which is associated with angiogenic endothelium in some cancers but not others. Theoretically, targeting β2 integrins could limit the infiltration of myeloid-derived suppressor cells (MDSCs), which are known to enhance tumor growth and metastasis. However, the lessons from β2 integrin null mice and people (LAD-I) suggest that severe host defense and inflammation issues would arise. The fact that only molecules targeting the ligand binding site or the ligand have been successful suggests that we do not know enough about integrin conformation change during activation to successfully construct allosteric inhibitors. Theoretically, it might be possible to target talin-1, an adaptor molecule that regulates the affinity of β2 and β3 integrins. However, knockout of talin-1 in mice is lethal 98, suggesting that talin-1 has other important functions. Another adaptor molecule involved in integrin-mediated leukocyte and platelet adhesion is kindlin-3. Kindlin-3 knockout mice 99 and people (LAD-III) 34 have severe bleeding and an infectious diathesis. Once integrins bind their ligands, outside-in signaling ensues 4, 100, and there are known drug targets in this signaling pathway, for example Syk 101 and Src kinases 100. Targeting these tyrosine kinases cannot be expected to be specific for integrin signaling, because they are involved in Fc receptor and B cell receptor signaling as well.
Conclusions
The development and demonstrated efficacy of integrin antagonists are a prime example of translational medicine whereby a deep fundamental knowledge of integrin biology has informed the design of antibody, peptide and small molecule drugs that were successful in Phase III clinical trials. In turn, the results and adverse events observed in these trials have informed our understanding of pathophysiology. Integrin-targeting drugs have found four main indications: thrombosis prevention after PCI (αIIbβ3 integrin), ulcerative colitis and Crohn’s disease (α4β7 integrin) and multiple sclerosis (α4β7 and α4β1 integrins). All approved drugs prevent the target integrin from binding its ligand(s). With the exception of Natalizumab, which carries a significant risk for PML, the other integrin-targeting drugs have proven remarkably safe and effective. Hundreds of thousands of patients have benefited from these drugs. In the future, one can anticipate expanded indications for existing integrin antagonists, particularly those in the α4 and β7 space. Antibody drugs targeting integrin ligands are emerging, as exemplified by the antibodies to MAdCAM-1.
Acknowledgments
Supported by National Institutes of Health (DK108670) and BLRD VA Merit Review award (1I01BX001051) to J.R.-N., National Institutes of Health (HL 56595 and HL 78784) to S.S., National Institutes of Health (DK091222 and HL078784) to K.L.
Footnotes
Further information
Competing interests
The authors declare competing interests. See website for further details.
Online competing interests statement: W.J.S. participates in clinical trials in this area. The other authors declare no competing interests.
References
- 1.Tadokoro S, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 2.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 3.Mocsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Salgado EG, et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharffetter-Kochanek K, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 8.Mitroulis I, et al. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright DB, Meurs H, Dekkers BG. Integrins: therapeutic targets in airway hyperresponsiveness and remodelling? Trends Pharmacol Sci. 2014;35:567–574. doi: 10.1016/j.tips.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Shimaoka M, et al. Structures of the alphaL I Domain and Its Complex with ICAM-1 Reveal a Shape-Shifting Pathway for Integrin Regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 14.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. 619-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119:26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 18.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J. Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA. Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 2003;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 20.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salas A, et al. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/s1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116:617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin mediated rolling on Intercellular Adhesion Molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefort CT, et al. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 2012;119:4275–4283. doi: 10.1182/blood-2011-08-373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weitz-Schmidt G, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 26.Chigaev A, et al. Real-time analysis of the inside-out regulation of lymphocyte function-associated antigen-1 revealed similarities and differences with very late antigen-4. J. Biol. Chem. 2011;286:20375–20386. doi: 10.1074/jbc.M110.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chigaev A, Wu Y, Williams DB, Smagley Y, Sklar LA. Discovery of very late antigen-4 (VLA-4, alpha4beta1 integrin) allosteric antagonists. J Biol Chem. 2011;286:5455–5463. doi: 10.1074/jbc.M110.162636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coller BS, Shattil SJ. The GP IIb/IIIa (integrin αIIbβ3) odyssey: a technology driven saga of a receptor with twists, turns and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 30.Bledzka K, Smyth SS, Plow EF. Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circ Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawiger J, Timmons S, Kloczewiak M, Strong DD, Doolittle RF. Gamma and alpha chains of human fibrinogen possess sites reactive with human platelet receptors. Proc.Natl.Acad.Sci.U.S.A. 1982;79:2068–2071. doi: 10.1073/pnas.79.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plow EF, Pierschbacher MD, Ruoslahti E, Marguerie GA, Ginsberg MH. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc.Natl.Acad.Sci.U.S.A. 1985;82:8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 34.Kuijpers TW, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009;113:4740–4746. doi: 10.1182/blood-2008-10-182154. [DOI] [PubMed] [Google Scholar]
- 35.Ye F, et al. The Mechanism of Kindlin-Mediated Activation of Integrin alphaIIbbeta3. Curr. Biol. 2013;23:2288–2295. doi: 10.1016/j.cub.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coller BS. Platelet GPIIb/IIIa antagonists: The first anti-integrin receptor therapeutics. J.Clin.Invest. 1997;99:1467–1471. doi: 10.1172/JCI119307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol. 1997;80:11B–20B. doi: 10.1016/s0002-9149(97)00572-9. [DOI] [PubMed] [Google Scholar]
- 38.Cook JJ, et al. Tirofiban (Aggrastat(R)) Cardiovasc.Drug Rev. 1999;17:199–224. [Google Scholar]
- 39.Estevez B, Shen B, Du X. Targeting Integrin and Integrin Signaling in Treating Thrombosis. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 41.Chinot OL. Cilengitide in glioblastoma: when did it fail? Lancet Oncol. 2014;15:1044–1045. doi: 10.1016/S1470-2045(14)70403-6. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 43.Wong PP, et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27:123–137. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Coller BS. Anti-GPIIb/IIIa drugs: current strategies and future directions. Thromb Haemost. 2001;86:427–443. [PubMed] [Google Scholar]
- 45.Artoni A, et al. Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc Natl Acad Sci U S A. 2004;101:13114–13120. doi: 10.1073/pnas.0404201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and avb3 integrins. Circulation. 1998;98:1085–1091. doi: 10.1161/01.cir.98.11.1085. [DOI] [PubMed] [Google Scholar]
- 47.Kintscher U, et al. Effects of abciximab and tirofiban on vitronectin receptors in human endothelial and smooth muscle cells. Eur.J.Pharmacol. 2000;390:75–87. doi: 10.1016/s0014-2999(99)00912-7. [DOI] [PubMed] [Google Scholar]
- 48.Scarborough RM. Structure-activity relationships of b-amino acid-containing integrin antagonists. Curr.Med.Chem. 1999;6:971–981. [PubMed] [Google Scholar]
- 49.Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest. 2005;127:53S–59S. doi: 10.1378/chest.127.2_suppl.53S. [DOI] [PubMed] [Google Scholar]
- 50.Bougie DW, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–2076. [PubMed] [Google Scholar]
- 51.Kastrati A, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006;295:1531–1538. doi: 10.1001/jama.295.13.joc60034. [DOI] [PubMed] [Google Scholar]
- 52.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 53.Kristensen SD, et al. Contemporary use of glycoprotein IIb/IIIa inhibitors. Thromb Haemost. 2012;107:215–224. doi: 10.1160/TH11-07-0468. [DOI] [PubMed] [Google Scholar]
- 54.Cox D. Oral GPIIb/IIIa antagonists: what went wrong? Curr Pharm Des. 2004;10:1587–1596. doi: 10.2174/1381612043384673. [DOI] [PubMed] [Google Scholar]
- 55.Bassler N, et al. A mechanistic model for paradoxical platelet activation by ligand-mimetic alphaIIb beta3 (GPIIb/IIIa) antagonists. Arterioscler Thromb Vasc Biol. 2007;27:e9–e15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]
- 56.Li J, et al. RUC-4: a novel alphaIIbbeta3 antagonist for prehospital therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34:2321–2329. doi: 10.1161/ATVBAHA.114.303724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takagi J, Petre B, Walz T, Springer T. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 58.Xie C, et al. Structure of an integrin with an alphaI domain, complement receptor type 4. EMBO J. 2010;29:666–679. doi: 10.1038/emboj.2009.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Springer TA, Thompson WS, Miller LJ, Schmalstieg FC, Anderson DC. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J. Exp. Med. 1984;160:1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 61.Henderson RB, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J. Exp. Med. 2001;194:219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coxon A, et al. A novel role for the beta-2 integrin CD11b/CD18 in neutrophil apoptosis - a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 63.Tang T, et al. A role for Mac-1 (CD11b/CD18) in immune complex-stimulated neutrophil function in vivo - Mac-1 deficiency abrogates sustained Fc-gamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J. Exp. Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hom G, et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 65.Nath SK, et al. A nonsynonymous functional variant in integrin-alpha(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat Genet. 2008;40:152–154. doi: 10.1038/ng.71. [DOI] [PubMed] [Google Scholar]
- 66.Wu H, et al. Functional Role of CD11c+ Monocytes in Atherogenesis Associated With Hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berlin C, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 68.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Hemler ME, Huang C, Schwarz L. The VLA protein family: characterization of five distinct cell surface heterodimers each with a common 130,000 Mr subunit. J. Biol. Chem. 1987;262:3300–3309. [PubMed] [Google Scholar]
- 70.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 71.Vedder NB, et al. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J. Clin. Invest. 1988;81:939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J. Leukoc. Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- 73.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 74.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 75.Calabresi PA, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69:1391–1403. doi: 10.1212/01.wnl.0000277457.17420.b5. [DOI] [PubMed] [Google Scholar]
- 76.Hesterberg PE, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin α4β7 . Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 77.Picarella D, et al. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- 78.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 79.Pan WJ, et al. Pharmacology of AMG 181, a human anti-alpha4 beta7 antibody that specifically alters trafficking of gut-homing T cells. Br J Pharmacol. 2013;169:51–68. doi: 10.1111/bph.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan WJ, et al. Clinical pharmacology of AMG 181, a gut-specific human anti-alpha4beta7 monoclonal antibody, for treating inflammatory bowel diseases. Br J Clin Pharmacol. 2014;78:1315–1333. doi: 10.1111/bcp.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 82.Stefanich EG, et al. A humanized monoclonal antibody targeting the beta7 integrin selectively blocks intestinal homing of T lymphocytes. Br J Pharmacol. 2011;162:1855–1870. doi: 10.1111/j.1476-5381.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rutgeerts PJ, et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut. 2012 doi: 10.1136/gutjnl-2011-301769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vermeire S, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–318. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 85.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugiura T, et al. A novel, orally active alpha 4 integrin antagonist, AJM300 prevents the development of experimental colitis induced by adoptive transfer of IL-10 deficient CD4(+) T cells in mice. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.193946. [DOI] [PubMed] [Google Scholar]
- 87.Takazoe M. Oral alpha-4 integrin inhibitor (AJM300) in patients with active Crohn’s disease - a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;136 [Google Scholar]
- 88.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM- 1) in acute and chronic inflammation. J. Leukocyte Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 89.Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 90.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-α and IL- 1. J. Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 91.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 92.Salmi M, Andrew DP, Butcher EC, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J. Exp. Med. 1995;181:137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pullen N, et al. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281–293. doi: 10.1111/j.1476-5381.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reinisch W. A Randomized, Multicenter Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Anti-MAdCAM Antibody PF-00547659 (PF) in Patients With Moderate to Severe Ulcerative Colitis: Results of the TURANDOT Study. Gastroenterology. 2015;148 [Google Scholar]
- 95.Sandborn WJ. Anti-MAdCAM-1 Antibody (PF-00547659) for Active Refractory Crohn’s Disease: Results of the OPERA Study. Gastroenterology. 2015;148 [Google Scholar]
- 96.Lazaar AL, et al. T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med. 1994;180:807–816. doi: 10.1084/jem.180.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enlimomab Acute Stroke Trial, I. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 98.Monkley SJ, et al. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 99.Moser M, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nature Medicine. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 100.Shattil SJ. Integrins and Src: dynamic duo of adhesion signaling. Trends Cell Biol. 2005;15:399–403. doi: 10.1016/j.tcb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berger JR, Houff SA. Neurological infections: the year of PML and influenza. Lancet Neurol. 2010;9:14–17. doi: 10.1016/S1474-4422(09)70337-0. [DOI] [PubMed] [Google Scholar]
- 103.Du Pasquier RA, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 104.Krumbholz M, Meinl I, Kumpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology. 2008;71:1350–1354. doi: 10.1212/01.wnl.0000327671.91357.96. [DOI] [PubMed] [Google Scholar]
- 105.Carson KR, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 106.Polman CH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 107.Natalizumab: new drug. Multiple sclerosis: risky market approval. Prescrire Int. 2008;17:7–10. [PubMed] [Google Scholar]
- 108.Balcer LJ, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68:1299–1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 109.Rudick RA, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- 110.Rudick RA, Miller DM. Health-related quality of life in multiple sclerosis: current evidence, measurement and effects of disease severity and treatment. CNS Drugs. 2008;22:827–839. doi: 10.2165/00023210-200822100-00004. [DOI] [PubMed] [Google Scholar]
- 111.Miller DH, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 112.Gordon FH, et al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther. 2002;16:699–705. doi: 10.1046/j.1365-2036.2002.01205.x. [DOI] [PubMed] [Google Scholar]
- 113.Ghosh S, et al. Natalizumab for active Crohn’s disease. New England Journal of Medicine. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 114.Sandborn WJ, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 115.Targan SR, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 116.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 117.Sands Bruce E, Paul Rutgeerts BGF, Colombel Jean-Frédéric, Sandborn William J., Sy Richmond, D’Haens Geert, Ben-Horin Shomron, Xu Jing, Rosario Maria, Fox Irving, Parikh Asit, Milch Catherine, Hanauer Stephen. Vedolizumab Induction Therapy for Patients With Crohn’s Disease Who Failed Tumor Necrosis Factor Antagonist Treatment. 2013 doi: 10.1053/j.gastro.2014.05.008. (In Press) [DOI] [PubMed] [Google Scholar]
- 118.Parikh A, et al. Vedolizumab for the treatment of active ulcerative colitis: A randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2011;18:1470–1479. doi: 10.1002/ibd.21896. ** An important phase-IIb study that provided significant insight into the pharmacological profile of vedolizumab. [DOI] [PubMed] [Google Scholar]
- 119.Brian G, Feagan JM, Gordon Greenberg, Gary Wild, Pierre Pare, Richard Fedorak. Steven B Landau, Lee R Brettman. Gastroenterology. 2000;118(4 suppl 2):A874. [Google Scholar]
- 120.Feagan BG, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. ** The first study to examine the effects of inhibiting alpha4beta7 as a therapeutic target for inflammatory bowel disease. [DOI] [PubMed] [Google Scholar]
- 121.Feagan BG, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. ** A pivotal phase-II study. [DOI] [PubMed] [Google Scholar]
- 122.Feagan BG, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 123.Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 124.Sands BE, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627. doi: 10.1053/j.gastro.2014.05.008. e3. [DOI] [PubMed] [Google Scholar]