Abstract
Glutaraldehyde-fixed bioprosthetic heart valves (GBHVs), derived from pigs or cows, undergo structural valve deterioration (SVD) over time, with calcification and eventual failure. It is generally accepted that SVD is due to chemical processes between glutaraldehyde and free calcium ions in the blood. Valve companies have made significant progress in decreasing SVD from calcification through various valve chemical treatments. However, there are still groups of patients (e.g., children and young adults) that have accelerated SVD of GBHV. Unfortunately, these patients are not ideal patients for valve replacement with mechanical heart valve prostheses as they are at high long-term risk from complications of the mandatory anticoagulation that is required. Thus, there is no “ideal” heart valve replacement for children and young adults. GBHVs represent a form of xenotransplantation, and there is increasing evidence that SVD seen in these valves is at least in part associated with xenograft rejection. We review the evidence that suggests that xenograft rejection of GBHVs is occurring, and that calcification of the valve may be related to this rejection. Furthermore, we review recent research into the transplantation of live porcine organs in nonhuman primates that may be applicable to GBHVs, and consider the potential use of genetically-modified pigs as sources of bioprosthetic heart valves.
Keywords: calcification; children/young adults; heart valves; pigs, genetically-modified; xenograft rejection; glutaraldehyde; Gal
Introduction
Approximately 20% of all cardiac surgery is for the treatment of valvular heart disease, and worldwide, more than 250,000 heart valves are replaced each year (1). Options for valvular heart surgery include repairing the valve or replacing the valve. Replacement of diseased heart valves has been taking place clinically for >50 years (2). Replacement of the diseased valve is with either a mechanical valve prosthesis (MHV) or a glutaraldehyde-fixed bioprosthetic heart valve (GBHV), usually of porcine or bovine origin. Worldwide, approximately 55% of replacements are with a MHV and 45% with a GBHV (1).
The decision as to which valve type to implant in a patient depends on a number of variables. Whenever possible, a GBHV is the preferred choice since it does not require long-term anticoagulant therapy, with its potential risks, which is mandatory after insertion of a MHV. However, GBHVs tend to have limited durability, especially in certain patient populations, such as children and young adults (1, 3). Thus, if a GBHV is implanted in these patients, they may require repeat cardiac surgery within months to a few years. Repeat cardiac surgery is associated with a higher rate of complications and a 2-3-fold higher risk of death than the initial operation (4). Hence, many young patients will often receive a MHV if adequate valve repair is not possible.
Assuming there are no complications, e.g., pannus build-up on the valve or infection of the valve, MHVs have demonstrated long-term durability. However, patients with a MHV need life-long, closely-regulated anticoagulation, with its associated risks of spontaneous bleeding, thrombosis, or thromboembolism, any of which can be fatal. The cumulative annual risk of bleeding (associated with therapeutic anticoagulation) is approximately 1% per year (1), with valve thrombosis (from inadequate anticoagulation) being 0.1-5.7% per patient year (1). Thus, if a patient receives a MHV at age 25, and assuming a 1% bleeding risk and 1% thrombotic risk annually (i.e., a total risk of 2% per year), there is a >99% cumulative chance of either complication by the age of 75 years (i.e., within 50 years of the valve surgery). Young patients are also more likely to experience these complications as (i) they are more likely to be active (work/sports) which puts them at greater risk for injury and bleeding; (ii) are less reliable in taking medications (including anticoagulants) regularly; (iii) are less likely to adhere to restrictions to their diet (which is necessary as certain foods can modify the effectiveness of anticoagulants); and (iv) young women who are menstruating or become pregnant may have problems with warfarin (currently used for anticoagulation) due to bleeding and the potential of birth defects in the child. Though newer anticoagulants are now available, which do not have as many restrictions and do not require monitoring (5), their safety profile and efficacy as they relate to a MHV are unknown, and they are currently not approved for routine use with a MHV (6).
GBHVs are constructed from porcine heart valves or from bovine pericardium that have been glutaraldehyde-treated to help preserve the tissues and decrease their immunogenicity (1, 2). A GBHV undergoes structural valve deterioration (SVD) whereby the valve thickens and calcifies. This process narrows the valve opening, leading to flow-limiting stenosis; in addition, the valve leaflets can tear, which can result in incompetence (leaking) (Figure 1). The process of calcification is seen in other inflammatory conditions, such as tuberculosis, and atherosclerosis, and in these inflammatory conditions macrophages and giant cells can be seen on microscopic examination (7, 8). Furthermore, it is possible that the chronic inflammation seen in diseases leads to calcification through secretion of cytokines by macrophages, such as osteopontin (9, 10). As GBHVs are glutaraldehyde-fixed, making them difficult to destroy, and are a xenotransplant, there may be chronic inflammation in these valves that leads to calcification.
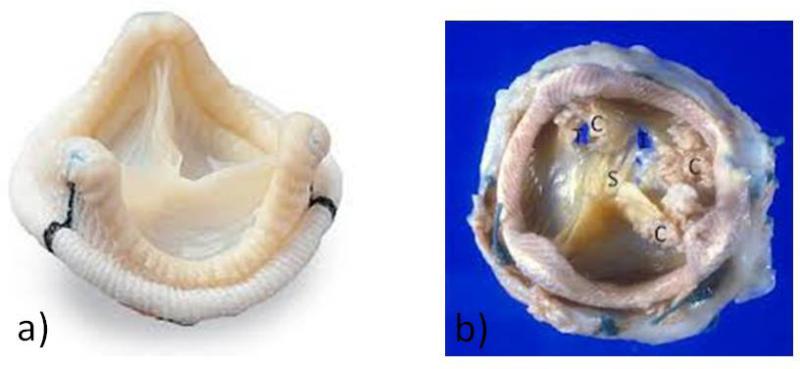
Figure 1. Comparison of an unused glutaraldehyde-fixed bioprosthetic heart valve (GBHV; “off the shelf”) with a GBHV removed from a patient for structural valve deterioration.
a) Porcine bioprosthetic heart valve prior to implantation in patient. There is no calcification and the valve leaflets are thin and clean. b) Porcine bioprosthetic heart valve removed for structural valve deterioration, showing calcification (C), stenosis of the valve orifice (S), and cusp tears (T).
Structural valve deterioration is age-dependent. In patients >65 years of age, <10% of GBHVs fail within 10 years, but there is a significant rate of failure within 5 years in patients <35 years of age (1). It is generally accepted that calcification of GBHVs occurs from chemical interactions between phospholipids, free aldehyde groups, and other components of the valve with calcium ions in the blood (11). The various manufacturers of GBHVs have been able to decrease the rate and extent of calcification through several ‘anti-calcification’ chemical processes (11), and have successfully avoided the development of calcification-related SVD in elderly patients. However, children and young adults continue to develop early calcification and failure of GBHVs despite these anti-calcification treatments. One intuitive difference between non-infant pediatric patients and young adults compared to the elderly (in whom GBHVs functional longevity is three to four times that in young patients) is the immune competence of the groups (12) and the more rapid metabolism, e.g., of calcium, in younger people (1, 11).
We here review studies suggesting that there is an immune response to a GBHV, and we highlight links between the immune response and calcification. We also review research into the transplantation of live pig organs into nonhuman primates as it relates to outcomes after the implantation of a GBHV. Finally, we discuss developments in the production of genetically-engineered pigs that may prove to be future sources of GBHVs that offer some protection from the human immune response and SVD.
Studies Suggesting an Immune Response to a GBHV
Clinical replacement of a diseased heart valve was pioneered in the early 1960s, but was accomplished with increasing frequency in the 1970s. In the 1970s and 1980s, there were reports of inflammatory changes (pseudointimal proliferation, fibrocalcific and thrombotic changes, presence of activated mononuclear cells) on histological examination of GBHVs removed from children and young adults (2-20 years-old) whose GBHVs failed within 4 years of implantation (2, 3, 13-17). As early as 1982, Talbert and Wright suggested that rejection was occurring in a GBHV (a porcine valve xenograft) (13).
Electron microscopy and immunohistochemical studies by Stein et al. (15) and Wilhelmi et al. (18) added information. Stein et al. (15) used electron microscopy to study 33 porcine GBHVs, and identified occasional fibroblast-like cells in the valves, platelet deposition in 67%, and leukocyte infiltrates (mostly mononuclear cells) in 82%. The leukocytes appeared to have destroyed collagen fibers in the valve, and had crystalline material present on their surfaces, suggesting they may have been acting as a nidus for calcification.
Wilhelmi et al. (18) carried out an immunohistochemical analysis of (i) explanted GBHVs, (ii) explanted aortic valve allografts (homografts), and (iii) aortic valves of explanted chronically-rejected cardiac allografts, and compared them to normal aortic valves (from unused human donor hearts) as controls. The tissues were stained for selectins (e.g., ELAM-1), integrins (e.g., VLA-1), immunoglobulin supergene family members (e.g., ICAM-1 and -2, class I heavy chain proteins), complement adhesion molecules (e.g., CD34, CD44), and von Willebrand factor. ELAM-1, ICAM-1 and -2, CD34, CD44, and class I heavy chain proteins (all important for inflammatory processes) showed enhanced expression in the allogeneic and xenogeneic valves compared to the control valves or valves from chronically-rejected heart allografts. The explanted allogeneic and xenogeneic valves showed strong thrombogenicity, as evidenced by staining for von Willebrand factor, which was present on the surface of endothelial cells.
Animal studies have also suggested there is a humoral response to a GBHV. Human et al. (19) suggested a role for circulating graft-specific antibody in causing GBHV calcification. Dahm et al. (20), using enzyme-linked immunosorbent and lymphocyte proliferation assays, provided evidence that glutaraldehyde-treated bovine pericardial valves provoked cellular and humoral immunologic reactions.
Gabbay et al. (21) and Grabenwoger et al. (22) suggested that the histopathological findings seen represented an active immune process and not just a normal healing process. Gabbay et al. (21) implanted glutaraldehyde-fixed pericardial patch xenografts into the left atrial wall in dogs. These patches developed an intense fibrous reaction, calcification, and even bone formation on the inner surface in contact with the host's blood, with minimal reaction on the external surface of the patch (not in contact with the host's blood). Grabenwoger et al. (22) examined failed explanted heart valves from patients who had either had a GBHV (n=6) or a valve fashioned from autologous pericardium treated with glutaraldehyde (autogenic, n=8). The GBHVs demonstrated large areas of calcification and inflammatory infiltration with macrophages. In contrast, the autologous valves did not show any significant inflammatory infiltrate nor any calcification (as evaluated by radiographic analysis and microscopic methods), but had evidence of collagen fiber disruption by plasma proteins and erythrocytes. If the changes seen in this study were strictly related to surgical trauma, one would have expected the autologous valves (which were glutaraldehyde-treated) to show a similar extent of inflammation and calcification.
These combined studies suggest that the inflammatory/thrombogenic changes seen in GBHVs are indeed an immune response and not simply related to surgical healing. The platelet/thrombosis processes seen on these valves are likely part of the immune response and not a bystander effect as there is evidence in the literature suggesting that the hemostatic system does have an immune response role as well (23-25). Figure 2 (left hand side pictorially outlines some of the processes discussed above).
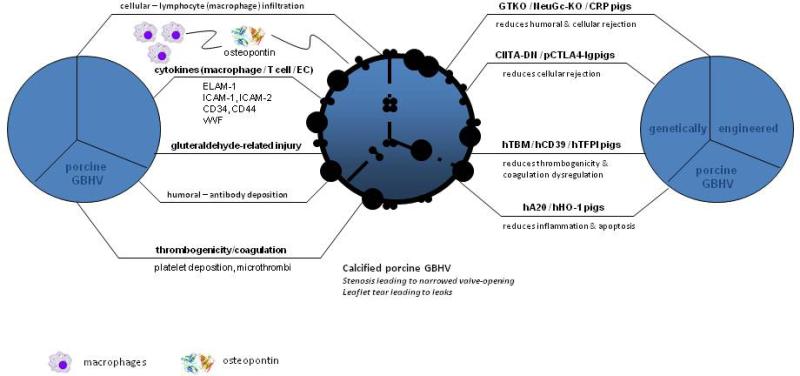
Figure 2. Immunological response to GBHV and potential ways a genetically-modified BHV may lessen or eliminate the immunological response.
Figure 2 depicts various immunological, coagulative/thrombogenic, and fixative (glutaraldehyde)-related injuries that can affect a porcine GBHV, leading to calcification and failure of the graft. Genetic engineering of pigs may reduce or prevent the above-mentioned injuries. Several groups have reported multiple genetic modifications of the pig, such as GTKO.hCD46.hCD55.hTBM, GTKO.hCD46.CIITA-DN, GTKO.NeuGC-KO, or GTKO.hCD55.hCD39.
A20 = tumor necrosis factor-alpha-induced protein 3; CIITA-DN = MHC class II transactivator-knockdown; CRP = complement regulatory proteins; EC = endothelial cell; ELAM-1 = endothelial-leukocyte adhesion molecule-1; GBHV = glutaraldehyde-fixed bioprosthetic heart valve; GTKO = α1,3-galactosyltransferase gene-knockout; h = human; HO-1 = hemeoxygenease-1; ICAM = intercellular adhesion molecule; NeuGc-KO = N-glycolylneuraminic acid gene-knockout; p = porcine TBM = thrombomodulin’; TFPI = tissue factor pathway inhibitor; vWF = von Willebrand factor.
Studies Suggesting a Link between the Immune Response and Calcification
A number of animal studies have suggested a link between inflammation/thrombogenicity/coagulation and calcification. Vincentelli et al. (26) provided data that suggested that glutaraldehyde was not responsible for graft calcification, but that the origin of the tissue (autologous versus heterologous, i.e., xenogeneic) was the primary factor in the development of calcification. The study of Human et al. (19), in which graft-specific antibody was identified, supported this conclusion. Figure 2 pictorially depicts immune response and calcification. A number of studies (18, 27, 28) have shown that experimental models in which the grafted tissue is not in contact with the blood (non-blood contact models, e.g., subcutaneous implantation of the graft) provide very different results from those in which the blood is in contact with the grafted tissue, suggesting that, to mimic the human situation, a biological model in which blood is in constant contact with the grafted tissue is important.
A study in a biological, xenogeneic model, with blood in contact with a glutaraldehyde-fixed valve, in young animals (to mimic clinical GBHV replacement in young patients) was conducted by Manji et al. (27). In this model, glutaraldehyde-fixed guinea pig aortic heart valves with segments of ascending aorta were transplanted into the abdominal aorta of young Lewis rats to create a xenogeneic transplantation model. Some rats were treated with corticosteroids to decrease inflammation/rejection. Compared to glutaraldehyde-fixed syngeneic rat controls, there was significant destruction of the transplanted valve and aortic tissue in the xenogeneic group (suggestive of what may occur when a porcine GBHV is implanted into a child or young adult). This inflammation/rejection was decreased if the rat recipients of the glutaraldehyde-fixed xenogeneic graft were treated with steroids (Figure 3). Compared to the glutaraldehyde-fixed syngeneic rat controls, there was significantly more macrophage and T cell infiltration into the xenograft tissue, with the pattern of cellular infiltrate being macrophage infiltration followed by T cell infiltration (which is also known to occur in live-tissue xenotransplantation (29)). Other mononuclear cells were also present in the xenografts, which the authors suggested may have been B cells and NK cells, though they did not specifically stain for these (27). There was evidence of microthrombi on the surface of the valves in the xenogeneic group, but not in the syngeneic group. There was also a rise in rat IgG in the recipients in the xenogeneic group compared to the syngeneic group, and this antibody response was attenuated if the recipient rats were treated with steroids. The differences between the syngeneic and xenogeneic groups were statistically significant. This study thus indicated that there were both cellular and humoral immune responses to the xenogeneic tissue, and that this was not simply related to glutaraldehyde-fixation or a foreign body reaction (27).
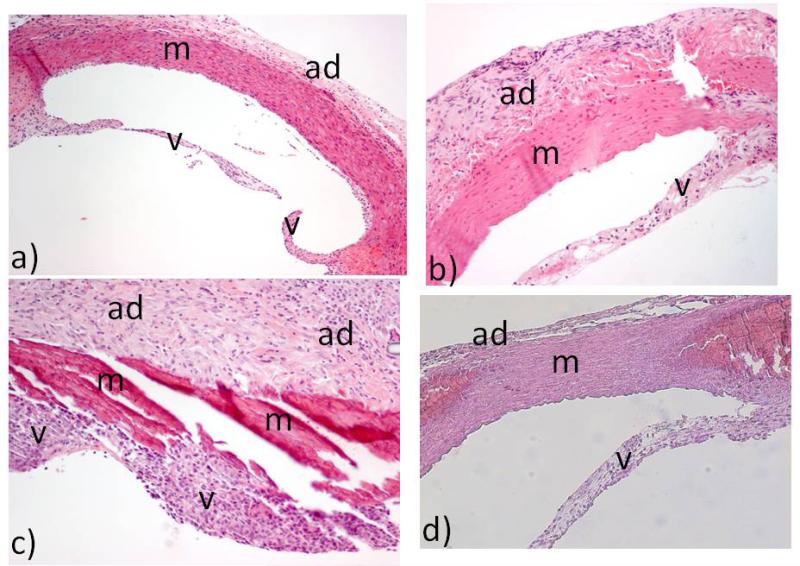
Figure 3. Histopathology following aortic valve and ascending aorta transplantation in rodent models [27].
Figure 3a demonstrates a syngeneic rat-to-rat aortic valve and ascending aorta transplant without any glutaraldehyde fixation (i.e., surgical negative control). Figure 3b demonstrates a glutaraldehyde-fixed syngeneic (rat-to-rat) transplant showing mild inflammation. Figures 3c demonstrates a glutaraldehyde-fixed xenogeneic (guinea pig-to-rat) transplant showing severe inflammation. Figure 3d demonstrates the reduction in inflammation when the rat recipient of the glutaraldehyde-fixed xenogeneic (guinea pig-to-rat) transplant is treated with steroids. V = aortic valve; m = media of aortic wall; ad = adventitia of aortic wall. (H&E x100 for 3a and x200 for 3b, 3c, 3d).
More importantly, this study also demonstrated a linear relationship between inflammation and calcification (27). The extent of calcification correlated with the intensity of the cellular infiltrate (especially the macrophage infiltrate). Treating the xenograft group with steroids significantly decreased the inflammatory infiltrate, the number of microthrombi, and the extent of calcification. The potential mechanism linking inflammation and calcification may be related to the cytokine, osteopontin, made by macrophages and T cells (30), which is important in the calcification process. Therefore, in a model with relevance to clinical GBHV implantation, strong evidence was provided linking the inflammatory/immune process to calcification.
Live Xenotransplantation Studies and GBHV Failure
In 1992, it was reported that the most important pig antigen to which humans and certain nonhuman primates have naturally-occurring antibodies (that initiate hyperacute rejection of pig organs) is galactose-α1,3-galactose (Gal) (31, 32). Anti-Gal antibodies are present in mammals that do not express Gal (humans, apes and Old World monkeys), but not in mammals in which Gal is expressed (e.g., pigs and cows) (33). It is generally believed that so-called ‘natural’ or ‘preformed’ antibodies, such as anti-Gal and anti-N-glycolylneuraminic acid (NeuGc), develop as a protective response following colonization of the gastrointestinal tract during infancy (34). All lower order mammalian species express Gal antigens (and therefore do not make anti-Gal antibodies). The production of α1,3-galactosyltransferase gene-knockout (GTKO) pigs (which do not express Gal) was a major step forward in overcoming the barriers to successful xenotransplantation (35, 36). The first report using GTKO pig hearts in baboons was published in 2005 (37). GTKO pig hearts rarely undergo hyperacute rejection.
The Gal antigen is expressed on pig endothelial cells, with ~106 to 107 epitopes per cell (33), and, like A and B blood group antigens, is probably important for functions such as cell signalling and cell-cell interactions (33), though it is clearly not essential. In humans, anti-Gal antibodies are produced by approximately 1% of circulating plasma cells (33). Anti-Gal IgM comprises 4-8% of total IgM, and anti-Gal IgG about 1% of total IgG (33). The physiological purpose of anti-Gal antibody is believed to be protection against pathogens and malignant cells, and the removal of senescent or abnormal red blood cells (as these are known to express Gal antigens when they become old or diseased) (33). Anti-Gal antibody may also play a role in human autoimmune disease because in some of these (e.g., Grave's disease) Gal is expressed on some tissues (33).
After overcoming the barrier of hyperacute rejection by addressing Gal, other forms of antibody-mediated rejection (associated with the presence of anti-nonGal antibodies), innate immune system responses (NK cells and macrophages), cell-mediated responses (T-cell responses), and coagulation disorders have surfaced as barriers (38, 39).
A non-Gal antigen that is almost certain to be important in clinical xenotransplantation and in the implantation of porcine GBHVs is N-glycolylneuraminic acid (NeuGc). This oligosaccharide is expressed in all mammals with the exception of humans (40), and therefore its importance in the immune response to a pig graft has been impossible to assess in pig-tononhuman primate organ transplantation models. Nevertheless, in vitro studies suggest it may play an equivalent role to Gal. In the absence of expression of NeuGc on their tissues, humans produce anti-NeuGc antibodies (41, 42), another ‘natural’ antibody (34). Expression of NeuGc on porcine and bovine tissues, therefore, may well be an important factor in the destruction of GBHVs in clinical practise. Very recently, pigs that express neither Gal nor NeuGc have been produced (43). These pigs will almost certainly play a significant role in future clinical trials of pig organ and cell transplantation.
An increasing number of other genetically-engineered pigs are also becoming established (reviewed in (39, 44)) to address the remaining barriers to xenotransplantation, including pigs transgenic for human complement-regulatory, coagulation-regulatory, and/or anti-inflammatory proteins (Table 1).
TABLE 1.
Genetically-modified pigs produced for xenotransplantation research*
| Complement regulation by human complement-regulatory gene expression: |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Antigen ‘masking’ or deletion: |
| human H-transferase gene expression (expression of blood type O antigen) |
| endo-beta-galactosidase C (reduction of Gal antigen expression) |
| α1,3-galactosyltransferase gene-knockout (GTKO) |
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene-knockout (NeuGc-KO) |
| Suppression of cellular immune response by gene expression or downregulation |
| CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| human FAS ligand (CD95L) |
| human GnT-III (N-acetylglucosaminyltransferase III) gene |
| porcine CTLA4-Ig (Cytotoxic T-Lymphocyte Antigen 4 or CD152) |
| human TRAIL (tumor necrosis factor-alpha-related apoptosis-inducing ligand) |
| Anticoagulation and anti-inflammatory gene expression or deletion |
| von Willebrand factor (vWF)-deficient (natural mutant) |
| human tissue factor pathway inhibitor (TFPI) |
| human thrombomodulin |
| human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| human A20 (tumor necrosis factor-alpha-induced protein 3) |
| human heme oxygenase-1 (HO-1) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| PERV siRNA |
Combinations of GTKO and expression of transgenes are available (e.g., GTKO.hCD46.hCD55)
Modified from Ekser B, et al [39]
As the Gal antigen is so important in live porcine xenotransplantation (with expression of the antigen on pig tissues and high levels of anti-Gal antibody in humans and certain nonhuman primates), it seemed reasonable to question whether the Gal antigen/anti-Gal antibody barrier could be involved in the failure of GBHVs. Though GBHVs are not live tissue and thus cannot undergo intrinsic endothelial cell activation (and its consequent detrimental sequelae), if prepared from wild-type (i.e., genetically-unmodified) pigs, surface antigens, e.g., Gal, even if only expressed on collagen and other structural components of the valve, incite an immune response, as do other decellularized tissues from these pigs (45).
Several studies have demonstrated the expression of Gal antigens on pig valves and on commercially-available GBHVs (46), as well as on other stromal structures (44, 47, 49). In fact, Naso et al. (48) have quantified the expression of Gal epitopes on current GBHVs. A Gal-specific antibody response has also been detected in patients with porcine GBHVs (46). Gal antigens can be largely removed by the enzyme, α-galactosidase (49), reducing injury to the valve by the human immune system. Konakci et al. (50) found that immunogenic Gal epitopes were present on fibrocytes interspersed in the connective tissue of porcine valves. Furthermore, patients receiving porcine GBHVs develop a significant increase in anti-Gal IgM compared to patients with a MHV or those undergoing coronary artery bypass grafting. These antibodies were shown to be cytotoxic to Gal-bearing PK-15 cells (a porcine kidney cell line). Treating the sera with soluble Gal antigen to adsorb the antibodies decreased the cytotoxicity. Jin et al. (51) demonstrated that human monocytes recognize the Gal antigen on porcine aortic endothelial cells in culture through the galectin-3 receptor on the monocyte. It is not clear if this occurs with GBHVs, but mononuclear cells and macrophages are the primary cellular infiltrate in GBHVs. Thus, the galectin-3 (human monocyte)-to-Gal (pig cell) interaction may be important in the inflammatory response seen in GBHVs. Furthermore, there is evidence that the absence of Gal expression on the vascular endothelium decreases the cellular immune response as well as the humoral response (52).
The link between Gal antigen and calcification of GBHVs was further suggested by studies reported by McGregor et al. (53) and Lila et al. (54) who investigated pericardium from GTKO pigs as a potential source for GBHVs. In these experiments, GTKO pig pericardium and wild-type pericardium were pre-labelled with human anti-Gal antibody and implanted subcutaneously in rodents. The GTKO pig pericardium calcified less than did pericardium from wild-type pigs (expressing Gal antigens). The authors concluded that anti-Gal antibodies interacting with Gal antigens on currently-used porcine GBHVs may accelerate the calcification process, and suggested that GTKO pigs may be a source for future GBHVs. The issue with the experiments done above is that they were subcutaneous implant models which have their limitations, as discussed previously (i.e., do not mimic the human situation with blood flowing past the valve).
Several of the other genetic modifications that have been made in pigs for purposes of organ xenotransplantation (Table 1) may prove beneficial to the outcome of a GBHV implanted clinically. The NeuGc-knockout pig has already been mentioned, but pigs expressing complement- and/or coagulation-regulatory proteins and/or anti-inflammatory genes are also likely to protect from the host immune response, and thus reduce the speed and/or extent of calcification. The pig-to-nonhuman primate model would be the optimal model to test GBHVs from these pigs (except for NeuGc-knockout pig grafts, as NeuGc is also expressed in all nonhuman primates).
Genetically-Engineered Pigs and Future GBHVs
There is evidence to suggest that there is rejection of a GBHV and that pig antigens are involved in inciting the immune response. McGregor et al. (53) and Lila et al. (54) have provided initial evidence that genetically-modified pigs may provide improved GBHVs in the future. It may soon be possible to breed pigs that might provide valves that are ‘ideal’ for implantation into humans. These valves may not deteriorate structurally or calcify, or at least these processes may be greatly slowed. Since the valves would be protected, at least to some extent, from the human immune response, glutaraldehyde-treatment may become unnecessary (or less necessary). It is likely that glutaraldehyde-fixation will inhibit strong function of at least some of these transgenic ‘protective’ proteins, though the extent of this inhibition is currently unknown. It is likely that, if sufficient protection to the valve is provided by transgenesis, glutaraldehyde-fixation may no longer be required, and ‘fresh’ porcine valves could be transplanted. However, if genetically-engineered ‘fresh’ pig valves were to be implanted, they would be considered ‘xenografts’ and the breeding, housing, and testing of the source pigs would be subject to the rigorous regulations of the regulatory authorities, which would increase the cost of the valves significantly. It would therefore be hoped that this could be avoided by retaining the glutaraldehyde-fixation process.
The genetic manipulations that might prove particularly beneficial are those in which human complement-regulatory genes (e.g., CD46, CD55, CD59), coagulation-regulatory genes (e.g., thrombomodulin, endothelial protein C receptor, tissue factor pathway inhibitor, CD39), and anti-inflammatory genes (e.g., heme oxygenase-1, A20) are introduced (Table 1). In addition, however, manipulations aimed at protecting the graft from the human T cell response may also improve long-term graft survival; in this respect, the introduction of a human mutant MHC class II transactivator that results in inhibition of expression of swine leukocyte antigen class II, would be valuable, as might local expression of an immunosuppressive gene, e.g., CTLA4-Ig.
The valves could be implanted into children and young adults, as well as in older patients, thereby reducing the need for MHVs and their associated problems. Figure 2 diagrammatically shows the presumed mechanisms thought to cause failure of a GBHV and ways that genetically-modified pigs may resolve the problems. It should be noted though that if glutaraldehyde fixation is still needed, then the chemical processes associated with glutaraldehyde-related injury (left-hand side of Figure 2) may still occur and will need to be addressed (by the anti-calcification treatments currently being undertaken). There may also be new processes that may surface as problems following immune-mediated injury are addressed.
There are, of course, a potentially large number of pig antigens against which humans could produce antibodies. Clearly, with present technology, not all of these could be deleted from the pig. However, if the two known key antigens, Gal and NeuGc, are deleted, then there is evidence that the expression of human complement- and coagulation-regulatory transgenes can protect against the innate immune response to ‘minor’ pig antigens, although exogenous immunosuppressive therapy is required to suppress an adaptive immune response. It would be detrimental to the care of patients with BHVs for exogenous immunosuppressive therapy to be necessary, and so other means of enabling graft survival would need to be sought, e.g., genetic-engineering to introduce the human MHC class II transactivator mutation. Nevertheless, if BHV survival could be significantly prolonged (if not indefinite), this would be hugely beneficial to young patients in need of valve replacement.
In addition to the above benefits, physicians are undertaking an increasing number of procedures carried out percutaneously. Percutaneous techniques (that avoid full open surgery) are desirable as the patients experience less pain, and postoperative recovery is much quicker. Recently, percutaneous transcatheter valve replacements are being offered to elderly patients with aortic stenosis felt to be at too high risk for open surgery (55). The percutaneous valves are GBHVs. As experience with this technique improves, and less invasive procedures are demanded by more younger patients, genetically-modified bioprosthetic heart valves could be implanted by these techniques, allowing earlier return to work.
An important hurdle that will need to be overcome will relate to the costs of the use of genetically-modified pigs. At present, the raw materials required to fashion a GBHV (i.e., a pig valve or bovine pericardium from wild-type animals) can be obtained from slaughterhouses at manageable, if not minimal, cost. In view of the development costs associated with genetic engineering, valves from genetically-modified pigs would be significantly more expensive, but the costs are likely to decrease significantly with expansion of breeding herds. These pigs are originally produced by nuclear transfer/cloning technology, but are then bred naturally, and so expansion of the herds could be achieved rapidly and inexpensively. The reduced need for early GBHV replacement, including hospitalization (and, in the case of a MHV, of long-term anticoagulation, with its associated monitoring) could offset the increased cost of a genetically-engineered pig, though this may not have a direct financial benefit to the manufacturers of either form of prosthesis.
Conclusions
This review has provided evidence to suggest that there is indeed an immune response to a GBHV, and that the rapidity and extent of calcification of the valve (which is a very important component of valve failure) is correlated with the immune response. Pig antigens that are known to be important in graft survival in live pig-to-nonhuman primate xenotransplantation are almost certainly playing a role in the failure of a GBHV (though GBHVs are not living tissue). Within the foreseeable future, genetically-modified pigs are likely to provide the ‘ideal’ heart valve for implantation into humans (especially children and young adults). If such valves could be fashioned to provide prolonged survival in young patients and in patients in whom long-term anticoagulation is contraindicated, there would be a world-wide paradigm shift in valve replacement. Although there is an urgent medical need for such valves – and a huge potential ‘market’ – the costs of genetically-engineered pigs have to be considered.
Acknowledgements
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants # U19 AI090959, # U01 AI068642 and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. Burcin Ekser MD is a recipient of a NIH NIAID T32 AI 074490 Training Grant.
Abbreviations
- Gal
galactose-α1,3-galactose
- GBHV
glutaraldehyde-fixed bioprosthetic heart valve
- GTKO
α1,3-galactosyltransferase gene-knockout
- MHV
mechanical heart valve
- NeuGc
N-glycolylneuraminic acid
- SVD
structural valve deterioration
Footnotes
Conflict of Interest
The authors have no financial or other conflict of interest.
References
- 1.SIDDIQUI RF, ABRAHAM JR, BUTANY J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 2.CARPENTIER A, LEMAIGRE G, ROBERT L, CARPENTIER S, DUBOST C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58:467–483. [PubMed] [Google Scholar]
- 3.MILLER DC, STINSON EB, OYER PE, et al. The durability of porcine xenograft valves and conduits in children. Circulation. 1982;66:I172–185. [PubMed] [Google Scholar]
- 4.RUEL M, CHAN V, BEDARD P, et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation. 2007;116:I294–300. doi: 10.1161/CIRCULATIONAHA.106.681429. [DOI] [PubMed] [Google Scholar]
- 5.VAN DE WERF F, BRUECKMANN M, CONNOLLY SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163:931–937. e931. doi: 10.1016/j.ahj.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 6.STEWART RA, ASTELL H, YOUNG L, WHITE HD. Thrombosis on a Mechanical Aortic Valve whilst Anti-coagulated With Dabigatran. Heart Lung Circ. 2012;21:53–55. doi: 10.1016/j.hlc.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 7.DOHERTY TM, ASOTRA K, FITZPATRICK LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BASARABA RJ, BIELEFELDT-OHMANN H, ESCHELBACH EK, et al. Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis (Edinb) 2008;88:69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CHO HJ, KIM HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11:206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 10.SCATENA M, LIAW L, GIACHELLI CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 11.SIMIONESCU DT. Prevention of calcification in bioprosthetic heart valves: challenges and perspectives. Expert Opin Biol Ther. 1971-1985;20044 doi: 10.1517/14712598.4.12.1971. [DOI] [PubMed] [Google Scholar]
- 12.DIAZ-JAUANEN E, STRICKLAND RG, WILLIAMS RC. Studies of human lymphocytes in the newborn and the aged. Am J Med. 1975;58:620–628. doi: 10.1016/0002-9343(75)90497-0. [DOI] [PubMed] [Google Scholar]
- 13.TALBERT WM, JR., WRIGHT P. Acute aortic stenosis of a porcine valve heterograft apparently caused by graft rejection: case report with discussion of immune mediated host response. Tex Heart Inst J. 1982;9:225–229. [PMC free article] [PubMed] [Google Scholar]
- 14.PHILLIPS HR, SPRAY TL, LOWE JE, MORRIS KG, WECHSLER AS. Subvalvular thrombotic obstruction of an aortic porcine heterograft. Chest. 1982;81:756–758. doi: 10.1378/chest.81.6.756. [DOI] [PubMed] [Google Scholar]
- 15.STEIN PD, WANG CH, RIDDLE JM, MAGILLIGAN DJ, JR Leukocytes, platelets, and surface microstructure of spontaneously degenerated porcine bioprosthetic valves. J Card Surg. 1988;3:253–261. doi: 10.1111/j.1540-8191.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 16.RIDDLE JM, MAGILLIGAN DJ, JR., STEIN PD. Surface morphology of degenerated porcine bioprosthetic valves four to seven years following implantation. J Thorac Cardiovasc Surg. 1981;81:279–287. [PubMed] [Google Scholar]
- 17.BUTANY J, ZHOU T, LEONG SW, et al. Inflammation and infection in nine surgically explanted Medtronic Freestyle stentless aortic valves. Cardiovasc Pathol. 2007;16:258–267. doi: 10.1016/j.carpath.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 18.WILHELMI MH, MERTSCHING H, WILHELMI M, LEYH R, HAVERICH A. Role of inflammation in allogeneic and xenogeneic heart valve degeneration: immunohistochemical evaluation of inflammatory endothelial cell activation. J Heart Valve Dis. 2003;12:520–526. [PubMed] [Google Scholar]
- 19.HUMAN P, ZILLA P. Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann Thorac Surg. 2001;71:S385–388. doi: 10.1016/s0003-4975(01)02492-4. [DOI] [PubMed] [Google Scholar]
- 20.DAHM M, LYMAN WD, SCHWELL AB, FACTOR SM, FRATER RW. Immunogenicity of glutaraldehyde-tanned bovine pericardium. J Thorac Cardiovasc Surg. 1990;99:1082–1090. [PubMed] [Google Scholar]
- 21.GABBAY S, BORTOLOTTI U, FACTOR S, SHORE DF, FRATER RW. Calcification of implanted xenograft pericardium. Influence of site and function. J Thorac Cardiovasc Surg. 1984;87:782–787. [PubMed] [Google Scholar]
- 22.GRABENWOGER M, FITZAL F, GROSS C, et al. Different modes of degeneration in autologous and heterologous heart valve prostheses. J Heart Valve Dis. 2000;9:104–109. discussion 110-101. [PubMed] [Google Scholar]
- 23.HUANG HS, CHANG HH. Platelets in inflammation and immune modulations: functions beyond hemostasis. Arch Immunol Ther Exp (Warsz) 2012;60:443–451. doi: 10.1007/s00005-012-0193-y. [DOI] [PubMed] [Google Scholar]
- 24.ENGELMANN B, MASSBERG S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2012;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 25.TRZECIAK-RYCZEK A, TOKARZ-DEPTULA B, DEPTULA W. Platelets--an important element of the immune system. Pol J Vet Sci. 2013;16:407–413. doi: 10.2478/pjvs-2013-0058. [DOI] [PubMed] [Google Scholar]
- 26.VINCENTELLI A, LATREMOUILLE C, ZEGDI R, et al. Does glutaraldehyde induce calcification of bioprosthetic tissues? Ann Thorac Surg. 1998;66:S255–258. doi: 10.1016/s0003-4975(98)01098-4. [DOI] [PubMed] [Google Scholar]
- 27.MANJI RA, ZHU LF, NIJJAR NK, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 28.OZAKI S, HERIJGERS P, FLAMENG W. Influence of blood contact on the calcification of glutaraldehyde-pretreated porcine aortic valves. Ann Thorac Cardiovasc Surg. 2003;9:245–252. [PubMed] [Google Scholar]
- 29.KIRCHHOF N, SHIBATA S, WIJKSTROM M, et al. Reversal of diabetes in nonimmunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11:396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 30.NAU GJ, GUILFOILE P, CHUPP GL, et al. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci U S A. 1997;94:6414–6419. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GOOD AH, COOPER DK, MALCOLM AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 32.COOPER DK, GOOD AH, KOREN E, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 33.KOBAYASHI T, COOPER DKC. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. In: Avila UGaJL., editor. Alpha-Gal and Anti-Gal: alpha-1,3-Galactosyltransferase, alpha-Gal epitopes, and the Natural Anti-Gal Antibody. Kluwer Academic/Plenum; New York, London: 1999. [Google Scholar]
- 34.GALILI U, MANDRELL RE, HAMADEH RM, SHOHET SB, GRIFFISS JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COOPER DK, KOREN E, ORIOL R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 36.PHELPS CJ, KOIKE C, VAUGHT TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.KUWAKI K, TSENG YL, DOR FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 38.EZZELARAB M, GARCIA B, AZIMZADEH A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EKSER B, EZZELARAB M, HARA H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 40.PADLER-KARAVANI V, VARKI A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 181-5 doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ZHU A. Binding of human natural antibodies to nonalphaGal xenoantigens on porcine erythrocytes. Transplantation. 2000;69:2422–2428. doi: 10.1097/00007890-200006150-00036. [DOI] [PubMed] [Google Scholar]
- 42.ZHU A, HURST R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 43.LUTZ AJ, LI P, ESTRADA JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 44.COOPER DK, EKSER B, BURLAK C, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DALY KA, STEWART-AKERS AM, HARA H, et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A. 2009;15:3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 46.PARK CS, PARK SS, CHOI SY, YOON SH, KIM WH, KIM YJ. Anti alpha-gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis. 2010;19:124–130. [PubMed] [Google Scholar]
- 47.MCPHERSON TB, LIANG H, RECORD RD, BADYLAK SF. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000;6:233–239. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 48.NASO F, GANDAGLIA A, BOTTIO T, et al. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013 doi: 10.1111/xen.12044. [DOI] [PubMed] [Google Scholar]
- 49.KASIMIR MT, RIEDER E, SEEBACHER G, WOLNER E, WEIGEL G, SIMON P. Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng. 2005;11:1274–1280. doi: 10.1089/ten.2005.11.1274. [DOI] [PubMed] [Google Scholar]
- 50.KONAKCI KZ, BOHLE B, BLUMER R, et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 51.JIN R, GREENWALD A, PETERSON MD, WADDELL TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha-GAL. J Immunol. 2006;177:1289–1295. doi: 10.4049/jimmunol.177.2.1289. [DOI] [PubMed] [Google Scholar]
- 52.WILHITE T, EZZELARAB C, HARA H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 1956-63 doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MCGREGOR CG, CARPENTIER A, LILA N, LOGAN JS, BYRNE GW. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2010;141:269–275. doi: 10.1016/j.jtcvs.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 54.LILA N, MCGREGOR CG, CARPENTIER S, RANCIC J, BYRNE GW, CARPENTIER A. Gal knockout pig pericardium: new source of material for heart valve bioprostheses. J Heart Lung Transplant. 2009;29:538–543. doi: 10.1016/j.healun.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 55.LEON MB, SMITH CR, MACK M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]