Abstract
We examined the role that serotonin has in the modulation of sleep and wakefulness across a 12-h:12-h light-dark cycle and determined whether temperature and motor activity are directly responsible for potential disruptions to arousal state. Telemetry transmitters were implanted in 24 wild-type mice (Tph2+/+) and 24 mice with a null mutation for tryptophan hydroxylase 2 (Tph2−/−). After surgery, electroencephalography, core body temperature, and motor activity were recorded for 24 h. Temperature for a given arousal state (quiet and active wake, non-rapid eye movement, and paradoxical sleep) was similar in the Tph2+/+ and Tph2−/− mice across the light-dark cycle. The percentage of time spent in active wakefulness, along with motor activity, was decreased in the Tph2+/+ compared with the Tph2−/− mice at the start and end of the dark cycle. This difference persisted into the light cycle. In contrast, the time spent in a given arousal state was similar at the remaining time points. Despite this similarity, periods of non-rapid-eye-movement sleep and wakefulness were less consolidated in the Tph2+/+ compared with the Tph2−/− mice throughout the light-dark cycle. We conclude that the depletion of serotonin does not disrupt the diurnal variation in the sleep-wake cycle, motor activity, and temperature. However, serotonin may suppress photic and nonphotic inputs that manifest at light-dark transitions and serve to shorten the ultraradian duration of wakefulness and non-rapid-eye-movement sleep. Finally, alterations in the sleep-wake cycle following depletion of serotonin are unrelated to disruptions in the modulation of temperature.
Keywords: electroencephalography, electromyography, non-rapid-eye-movement sleep, paradoxical sleep, telemetry
Serotonin has a complex role in regulating sleep and wakefulness across the light-dark cycle. A number of experimental methods including pharmacological depletion of serotonin, serotonergic neuronal recordings or lesioning, and the administration of serotonin receptor agonists and antagonists have been employed to examine the effect of serotonin on the sleep-wake cycle (see Refs. 11, 29, and 45 for reviews of this topic). The results have led to important but equivocal findings. Early on, investigators surmised that serotonin promoted sleep, but more recent studies have indicated that serotonin promotes wakefulness and has a role in minimizing the transitional state between wake and sleep (21, 45). The equivocal nature of the findings in part represents the complexity of the serotonergic system, which is composed of at least 16 different receptor subtypes in the brain (6, 19, 36) and its interactions with other neuromodulators to regulate the sleep-wake cycle (39). Alternatively, methods that lack specificity have also contributed to the ambiguous findings.
Recently, transgenic mouse models that are linked to the life-time depletion of central nervous system serotonin have been developed (1, 15–18, 31, 43). These models could provide important information regarding the role of serotonin in the regulation of the sleep-wake cycle. However, to our knowledge only one study has used direct measures (i.e., electroencephalograms and electromyograms) of arousal state to examine sleep and wakefulness in transgenic mice with depleted serotonin. Buchanan and Richerson (8) reported that Lmx1bf/f/p mice spent more time awake and less time in non-rapid-eye-movement sleep compared with wild-type mice. Alterations in the sleep-wake cycle were reported to be a consequence of the inability to maintain core body temperature during sleep when atmospheric temperature was below 30°C (8). Consequently, Buchanan and Richerson surmised that the increased amount of wakefulness, accompanied by increased motor activity, occurred to maintain core body temperature at the expense of a disrupted sleep-wake cycle (8). Given the interpretation of these findings (see DISCUSSION for further comment), it remains unclear if alterations in the sleep-wake cycle occur independent of modifications in the regulation of core body temperature and motor activity in mice genetically depleted of brain serotonin. Likewise, it is uncertain if alterations in the sleep-wake cycle of the Lmx1bf/f/p model were a consequence of the elimination of serotonergic neurons or serotonin per se (18).
These uncertainties can be addressed using the tryptophan hydroxylase 2 (Tph2−/−) knockout model (1, 31, 43). Tryptophan hydroxylase 2 is the rate-limiting enzyme in the conversion of tryptophan to serotonin. In Tph2−/− mice central nervous system serotonin is depleted in the presence of intact serotonin neurons (1, 31, 43). Hickner et al. (15) and Alenina et al. (1, 31) reported that Tph2−/− mice are capable of regulating core body temperature under room air conditions and that these animals display increases in activity during wakefulness even when core body temperature is at or near-normal values (1, 31). Despite these findings, Alenina and colleagues (1) reported that Tph2−/− mice spent more time sleeping than awake. However, this finding had limited support since sleep and wakefulness were determined by the absence or presence of prolonged activity, respectively (1). Consequently, direct measures of sleep and wakefulness were not available to accurately characterize sleep architecture in Tph2−/− mice (e.g., quiet wake vs. non-rapid-eye-movement sleep, and non-rapid- eye-movement sleep vs. paradoxical sleep). Thus, in the present investigation, electroencephalography and electromyography were employed to compare the sleep-wake architecture of Tph2+/+ and Tph2−/− mice over a 12-h:12-h light-dark cycle. Core body temperature, standardized for arousal state, along with motor activity was measured to examine whether or not serotonergic regulation of these variables affected sleep-wake architecture. Based on the premise that serotonin promotes wakefulness, we hypothesized that Tph2−/− mice would experience hypersomnolence in the presence of an appropriately modulated core body temperature. Our hypotheses was supported in that core body temperature for a given arousal state was modulated in a similar fashion in Tph2+/+ and Tph2−/− mice. Contrary to our hypotheses, Tph2+/+ and Tph2−/− mice spent a similar percentage of time in a given state of arousal across the majority of time points over the 24-h cycle, although sleep was more consolidated in Tph2−/− mice. Moreover, at the onset of the light and dark cycle, and at the termination of the dark cycle, increases in active wakefulness and decreases in non-rapid-eye-movement sleep were evident in the Tph2−/− mice.
METHODS
Animals
All experiments were approved by Wayne State University Institutional Animal Care and Use Committee. The experiments were performed on young adult male C57BL/6-129Sv mice (24 Tph2+/+ and 24 Tph2−/−). The Tph2−/− mice were bred in our facility as described previously (2, 3, 43). Our published findings confirm that serotonin neurons in these transgenic mice are intact but serotonin is not detectable in the central nervous system (43). The mice were individually housed on a 12-h:12-h light-dark cycle (6 AM lights on and 6 PM lights off). The laboratory temperature was 23.8 ± 0.3°C and the humidity was 20.5 ± 3.0%. Food and water were available ad libitum.
Protocol
To measure temperature, activity, and electroencephalography, one-lead biopotential telemetry transmitters (model TA11ETA-F10, Data Sciences International, St. Paul, MN) were surgically implanted into 34 mice (17 Tph2+/+ and 17 Tph2−/−). Two-lead biopotential telemetry transmitters (model TL11M2-F20-EET, Data Sciences International) were implanted in an additional subset of mice (7 Tph2+/+ and 7 Tph2−/−) to also measure electromyography. The weights of the one-lead and two-lead biopotential telemetry transmitters were 1.9 and 3.9 g, respectively. The mice were given at least 4 days of recovery or until the mice exhibited normal weight gain. Electroencephalography, electromyography, temperature, and activity were then measured for a 24-h period from 6 AM to 6 PM. On the following day activity was recorded for 3 h in the morning (7 AM to 10AM) and 3 h in the evening (7 PM to 10 PM) using Fusion Activity Cages (Accusan, Omnitech Electronics, Columbus, OH) in 18 Tph2+/+ and 17 Tph2−/− mice. Throughout the experiments the mice were unanesthetized and kept in their home cages to move freely with food and water ad libitum.
Surgical procedures
Sterile surgery was performed with the mice under anesthesia, using an intra-peritoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). One-fourth of the anesthetic dose was used as a supplement as needed throughout surgery. Bupivacaine was injected along the incision sites (2 mg/kg, divided among the incision sites). A temperature-controlled heating pad (HTP-1500 Heat Therapy Pump, Kent Scientific, Torrington, CT) was used to maintain normal body temperature during and after surgery.
The abdominal cavity of the mouse was exposed using a midline incision. A sterile telemetry transmitter was then inserted into the peritoneal cavity. The electrode leads were threaded through the abdominal wall and tunneled subcutaneously to the cranium of the mouse. The muscle of the abdomen was closed using 4-0 absorbable sutures (Ethicon, Cincinnati, OH), and wound clips were used to close the skin of the abdomen.
The skull was exposed using a midline incision, cleaned with hydrogen peroxide, and stabilized using a stereotaxic frame (model 51730, Stoelting, Wood Dale, IL). Two holes were drilled into the skull on each side 1–2 mm lateral to the midline and 1–2 mm anterior to the lambda fissure. Electrode wires were placed in the holes and secured using Vetbond (3M, St. Paul, MN). The electromyographic leads were threaded through the neck muscle and secured with Vetbond 2 mm apart from each other. The incision was closed using 4-0 nonabsorbable suture (Ethicon).
Lactated Ringer was administered postsurgery and throughout the recovery period, as needed. Buprenorphine (0.1 mg/kg) was administered subcutaneously every 12 h for at least 2 days and carprofen (0.5 mg/kg) once a day for at least 2 days, following surgery. The mice were weighed and monitored daily.
Data collection and analysis
The telemetry signals were detected by a receiver (model RPC-1, Data Science International) placed underneath each mouse cage. The signals were transmitted to a data exchange matrix (ACQ 7700, Data Sciences International) monitored using commercially available software (PONEMAH Physiology Platform; Data Sciences International). Core body temperature and electroencephalography from the one-lead biopotential telemeter was acquired at a rate of 200 Hz (model TA11ETA-F10, Data Sciences International), while electroencephalography and electromyography measures from the two-lead biopotential telemeter were sampled at 100 Hz (model TL11M2-F20-EET, Data Sciences International). Movement episodes were sampled at 16 Hz.
The acquired data were divided into 10-s epochs and analyzed using a commercially available software package (Neuroscore Version 3.0, Data Sciences International). Arousal state (i.e., quiet wake, active wake, non-rapid-eye-movement sleep, and paradoxical sleep) was scored visually using the electroencephalograms and electromyograms when available. If an epoch was scored as wakefulness and movement was detected by the implanted telemeter, the epoch was deemed active wake. In the additional subset of mice, active wakefulness was also confirmed using electromyograms. Corresponding periodograms of defined power bands, which included bandwidths from 0.5 to 4 Hz (δ), 6 to 9 Hz (θ), 9 to 12 Hz (α), 16 to 31 Hz (β), and 32 to 100 Hz (γ) (note that the γ periodogram was only acquired for the one-lead biopotential telemeter), along with measures of activity and temperature, were used to confirm visual identification of arousal state. Increases in the θ-to-δ power band ratio (θ/δ) in conjunction with the absence of activity counts and neck electromyographic activity, when available, were used to confirm paradoxical sleep. Additionally, increases in δ-to-γ (δ/γ) and δ-to-total power ratios (δ/total power) in conjunction with reduced electromyographic activity, decreases in temperature, and the absence of activity were used to confirm non-rapid-eye-movement sleep. When more than one state of arousal was observed in a single 10-s epoch, the dominant arousal state (>50%) was selected. After the arousal state was scored, the data were divided into 12 2-h bins. The percentage of total time spent in each state of arousal, along with corresponding measures of activity and temperature for each arousal state, was determined for each 2-h bin. Temperature measures corresponding to consolidated arousal states, which we defined as three consecutive epochs of a given state, were analyzed to avoid temperature measures associated with transitions between arousal states. The total number of non-rapid-eye-movement sleep bouts (defined as periods of uninterrupted sleep of 10 s or greater) and the average duration of each bout was determined for each 2-h bin. Additionally, the average δ/γ and δ/total power ratio in non-rapid-eye-movement sleep was determined for each 2-h bin.
Measures of activity were obtained every minute using Fusion Activity Cages (Accusan, Omnitech Electronics, Columbus, OH). Total distance, total number of counts, movement time (i.e., the length of time a mouse is engaged in either ambulatory or sterotypy activity), ambulatory activity counts (i.e., number of beam breaks related to ambulation), stereotypy activity counts (i.e., number of beam breaks related to stereotypy behavior), and movement episodes (i.e., total number of locomotor episodes defined as movement separated by rest periods of at least 1 s) were quantified. Stereotypy behavior was identified if the same beam or set of beams were repeatedly broken. This pattern is typically associated with grooming behaviors. Given that the differences between the Tph2+/+ vs. Tph2−/− mice were similar in the 7–10 AM and 7–10 PM recording sessions, the data were averaged across the morning and evening sessions.
Statistical analysis
A three-way repeated measures analysis of variance (ANOVA) in conjunction with Student-Newman-Keuls post hoc test (GB-STAT 8.0, Dynamic Microsystems, Silver Spring, MD) was used to compare the percentage of time spent in a given state of arousal (i.e., wake, active wake, non-rapid-eye-movement and paradoxical sleep), as well as the corresponding temperature measured during each state. The factors in the ANOVA were mouse genotype (Tph2+/+ vs. Tph2−/−), time (6 × 2 h bins), and cycle (light vs. dark). A similar analysis was used to compare non-rapid-eye-movement sleep bouts, the mean duration of each bout and movement episodes during active wakefulness that was measured via telemetry. This analysis was also used to compare the δ/γ and δ/total power ratios between the Tph2+/+ and Tph2−/− mice. The factors in the ANOVA were mouse genotype (Tph2+/+ vs. Tph2−/−) and arousal state (non-rapid vs. paradoxical vs. wake vs. active wake). An unpaired t test was used to compare the age and weight of the Tph2+/+ and Tph2−/− mice. A similar analysis was used to compare the total distance, movement episodes, movement time, ambulatory activity, and stereotypy activity measured from the Tph2+/+ and Tph2−/− mice for the 3-h periods during the light and dark cycle. The data presented in the text and figures are means ± SE. P < 0.05 was considered statistically significant and 0.05 < P < 0.07 was considered to indicate a tendency towards statistical significance.
RESULTS
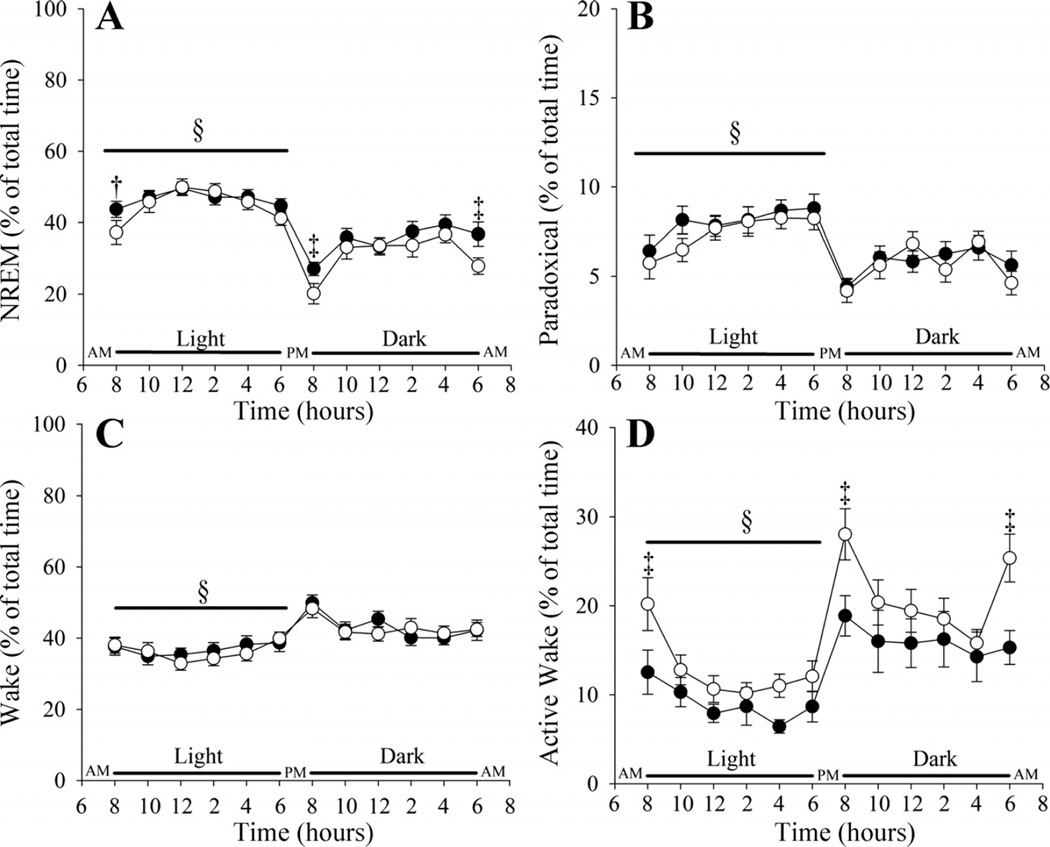
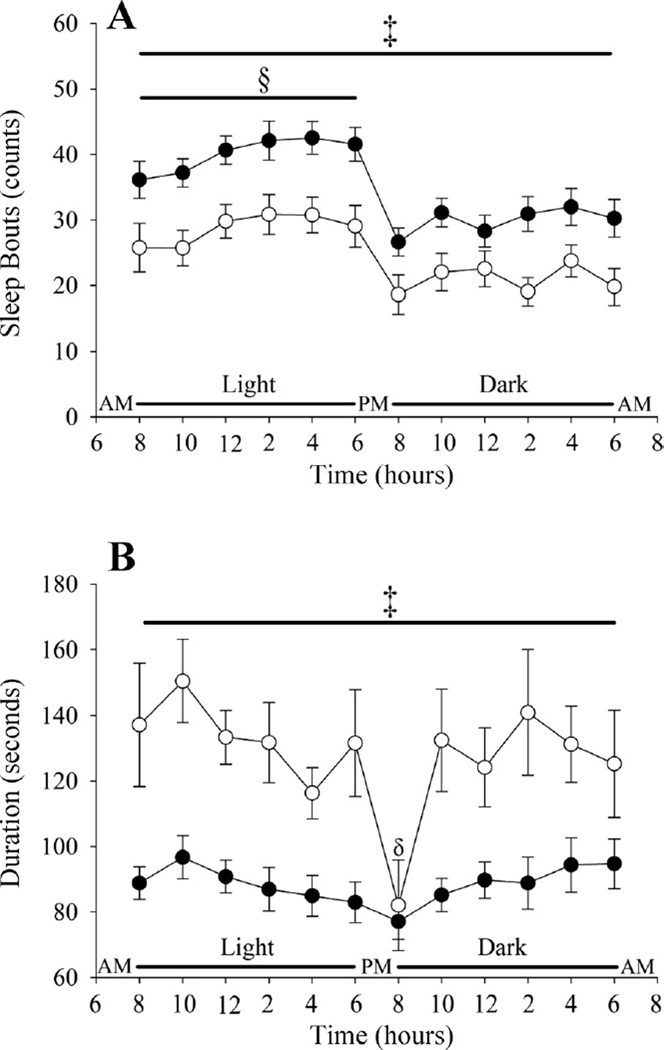
The ages of the Tph2+/+ and Tph2−/− mice were similar (17.12 ± 0.45 vs. 17.21 ± 0.42 wk), whereas the Tph2+/+ weighed less than the Tph2−/− mice (25.29 ± 0.52 vs. 27.84 ± 0.45 g; P < 0.001). As expected, the percentage of time spent in non-rapid-eye-movement and paradoxical sleep was greater during the light compared with the dark cycle (P < 0.0001) in both Tph2+/+ and Tph2−/− mice (Fig. 1, A and B). The percentage of non-rapid-eye-movement and paradoxical sleep at the onset of the light and dark cycle was less compared with values measured throughout the remainder of the cycle (P ≤ 0.0001) in both groups (Fig. 1, A and B). At the onset of the light and dark cycle and at the termination of the dark cycle (P ≤ 0.01), the percentage of time in non-rapid-eye-movement sleep was greater in the Tph2+/+ compared with the Tph2−/− mice (light onset: P < 0.07; dark onset: P < 0.05; last 2 h of dark: P ≤ 0.01) (Fig. 1A). Throughout the remaining time points in non-rapid-eye-movement sleep, and all through paradoxical sleep, the percentage of time spent in a given state was similar in the Tph2+/+ and Tph2−/− mice (Fig. 1, A and B). In addition, δ/γ (Tph2+/+ vs. Tph2−/−: 3.7 ± 0.47 vs. 4.1 ± 0.6) and δ/total power (Tph2+/+ vs. Tph2−/−: 0.26 ± 0.01 vs. 0.26 ± 0.01) during non-rapid-eye-movement sleep over the light-dark cycle was similar between groups. Despite these similarities, the average number of sleep bouts during non-rapid-eye-movement sleep was greater (P < 0.003) while the bout duration was reduced (P < 0.001) in the Tph2+/+ compared with the Tph2−/− mice (Fig. 2, A and B).
Fig. 1.
Line plots showing the percentage of time spent in non-rapid-eye-movement (NREM) sleep (A), paradoxical sleep (B), quiet wakefulness (C), and active wakefulness (D) over a 24-h period in Tph2+/+ (solid circles) and Tph2−/− (open circles) mice. Note that the Tph2+/+ compared with Tph2−/− mice experienced more NREM sleep and less active wakefulness at the start of the light and dark cycle and at the end of the dark cycle. Otherwise, the percentage of time spent in a given state of arousal was similar in the Tph2+/+ and Tph2−/− mice. §Significantly different from the dark cycle; ‡significantly different from Tph2−/− mice; †approaching statistical significance.
Fig. 2.
Line plots showing the average number of sleep bouts (A) and the average duration of bouts (B) during NREM sleep over a 24-h period in Tph2+/+ (solid circles) and Tph2−/− (open circles) mice. Note that the number of sleep bouts was greater and the duration of sleep bouts shorter in the Tph2+/+ compared with Tph2−/− mice. §Significantly different from the dark cycle; ‡significantly different from Tph2−/− mice; δsignificantly less compared with other bins in the dark cycle.
The percentage of time awake was less in the light compared with the dark cycle in both groups during quiet and active wake (P < 0.0001) (Fig. 1, C and D). The percentage of quiet and active wake at the onset of the light and dark cycle was greater compared with values measured throughout the remainder of the cycle (P ≤ 0.0001) (Fig. 1, C and D). At the onset of the light and dark cycle and at the termination of the dark cycle (P ≤ 0.01), the percentage of time in active wakefulness was less in the Tph2+/+ compared with the Tph2−/− mice (P < 0.03) (Fig. 1D).
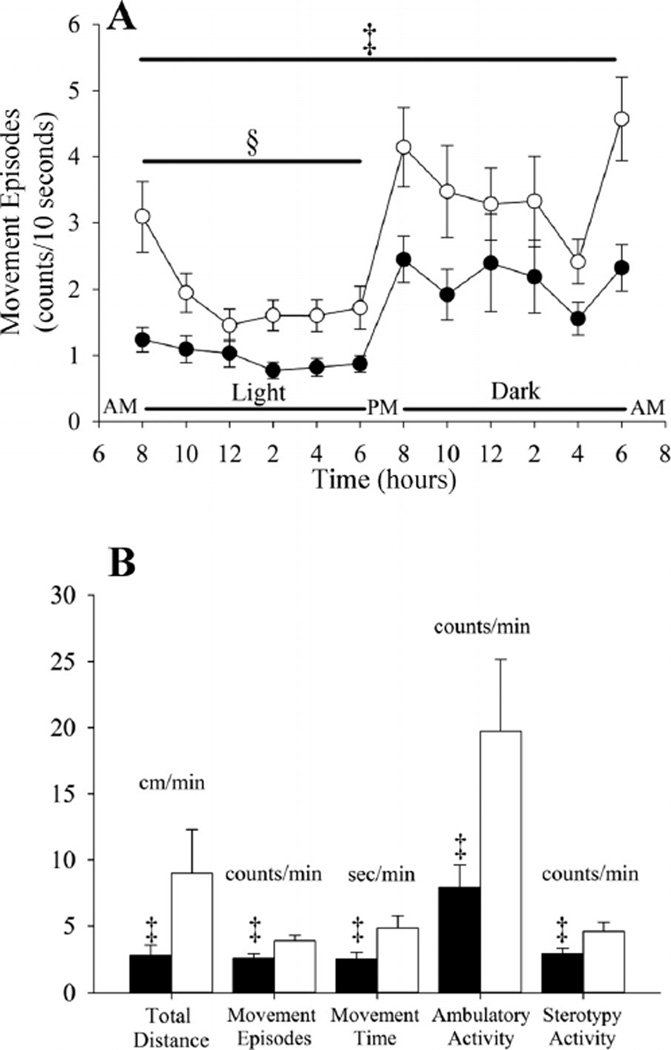
Activity was reduced in both groups during the light compared with the dark cycle (P < 0.0001) (Fig. 3A). The greatest activity was evident at the onset of the light and dark cycle and at the termination of the dark cycle (P ≤ 0.0001). However, the average number of movement episodes during wakefulness was less in the Tph2+/+ mice independent of the light or dark phase (P < 0.003) (Fig. 3A). This finding is supported by the activity cage data that showed that the total distance (P < 0.02), number of movement episodes (P < 0.02), movement time (P < 0.02), ambulatory activity counts (P < 0.01), and stereotypy activity (P < 0.05) counts were less in the Tph2+/+ compared with the Tph2−/− mice (light and dark phase combined) (Fig. 3B).
Fig. 3.
A: line plot showing the average number of movement episodes during active wakefulness over a 24-h period in Tph2+/+ (solid circles) and Tph2−/− (open circles) mice. B: histograms showing a number of activity cage measures obtained from the Tph2+/+ (solid bars) and Tph2−/− (open bars) mice. Note that the number of movement episodes was less in the Tph2+/+ compared with Tph2−/− mice throughout the 24-h period. Likewise, all activity cage measures were less in the Tph2+/+ compared with Tph2−/− mice. §Significantly different from the dark cycle; ‡significantly different from Tph2−/− mice.
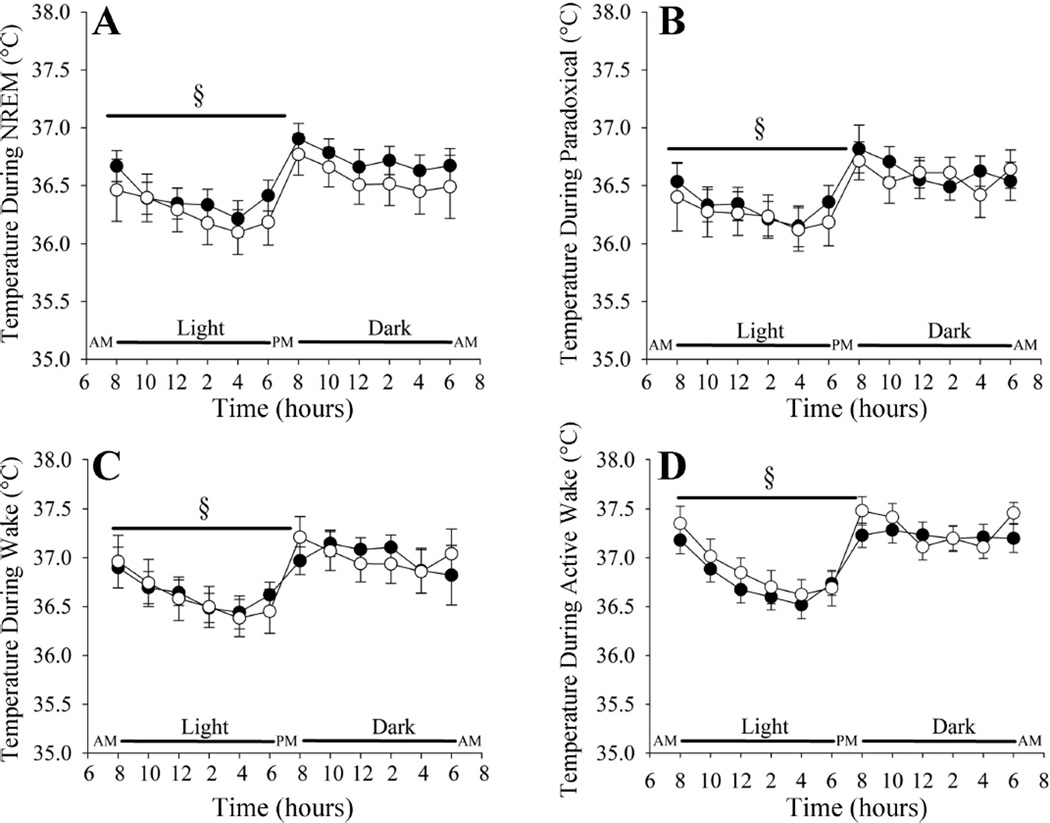
The temperature during non-rapid-eye-movement sleep, paradoxical sleep, quiet wakefulness, and active wakefulness was modulated in a similar manner in the Tph2+/+ and Tph2−/− mice over the 24-h period (Fig. 4). In other words, for all arousal states temperature was lowest during the light phase and highest during the dark phase in both the Tph2+/+ and Tph2−/− mice (P < 0.001) (Fig. 4). Within a given phase, the temperature during sleep or wake was similar in the Tph2+/+ and Tph2−/− mice (Fig. 4).
Fig. 4.
Line plots showing the temperature in NREM sleep (A), paradoxical sleep (B), quiet wakefulness (C), and active wakefulness (D) over a 24-h period in Tph2+/+ (solid circles) and Tph2−/− (open circles) mice. Note that the temperature in the Tph2+/+ and Tph2−/− mice was modulated in a similar manner in each arousal state throughout the 24-h period. §Significantly different from the dark cycle.
DISCUSSION
Our results showed that a diurnal variation in arousal state, motor activity, and temperature was evident despite the genetic depletion of central nervous system serotonin. However, at the onset of the light and dark cycle and at the termination of the dark cycle, a reduction in non-rapid-eye-movement sleep coupled to an increase in active wakefulness and motor activity was evident in the Tph2−/− mice. Likewise, throughout the 12-h:12-h light-dark period, sleep and wakefulness were more consolidated (i.e., sleep and wake bouts were more prolonged) in Tph2−/− mice. We also established that temperature for a given arousal state was appropriately modulated throughout the light-dark cycle despite depletion of serotonin in the central nervous system.
Diurnal modulation of arousal state, temperature, and motor activity
As expected, a diurnal variation in temperature, arousal state, and motor activity was evident in the Tph2+/+ mice in the present investigation. Temperature, wakefulness, and motor activity were greater, while sleep was reduced, in the dark compared with the light phase. Despite the depletion of serotonin, diurnal oscillations in temperature, arousal state, and motor activity were also evident in the Tph2−/− mice. Although diurnal variations in temperature have not been extensively documented in serotonin-depleted transgenic mice, our results are in agreement with studies that reported diurnal alterations in arousal state in Tph2−/− (1) and Lmx1bf/f/p (8) mice and motor activity in PET1−/− mice (10, 34). Our findings are also in agreement with some studies that have reported diurnal variations in locomotor activity following pharmacological depletion of serotonin (7, 22, 30), but are in contrast to other studies that indicated that this perturbation eliminates the diurnal nature of the sleep-wake cycle (20, 28, 33) and locomotor activity (28, 33). The maintenance of a diurnal pattern in the sleep-wake cycle, motor activity, and temperature suggests that the role of the suprachiasmatic nucleus in modulating the physiological variables across the 12-h:12-h light-dark cycle is preserved despite the depletion of serotonin. This speculation is supported by the recent findings of Miyamoto and colleagues (28) who reported that diurnal neuronal activity within the suprachiasmatic was maintained despite the pharmacological depletion of serotonin.
Although a diurnal pattern in the measured physiological variables was evident in the Tph2+/+ and Tph2−/− mice, some differences were clearly apparent at the onset of the light and dark cycle and at the termination of the dark cycle. Active wakefulness in tandem with motor activity was greater, and non-rapid eye movement sleep was less at the onset of the light and dark periods and at the termination of the dark phase in the Tph2−/− mice, while the modulation of temperature was not disrupted. These alterations could be indicative of disruption in the diurnal modulation of arousal state and motor activity that are a consequence of the life-long depletion of central nervous system serotonin. Alternatively, it could be argued that the observed adaptations are simply unique to the inbred strain(s) of mice used in our investigation, as previously reported (4, 12, 42). However, examination of the Tph2+/+ mice in our study indicates that the arousal state and motor activity response during the dark phase was not bimodal; supporting the premise that the modifications observed in the Tph2−/− mice were a consequence of the genetic depletion of serotonin.
The differences in arousal state and motor activity that we observed at the onset of the light and dark cycle, and at the termination of the dark cycle, have not been previously reported in Tph2−/− and Lmx1bf/f/p mice (1, 8). In contrast, differences in motor activity at the onset of the light and dark period and at the terminal end of the dark period have been documented in other species following pharmacological depletion of serotonin (22, 26) and in PET1−/− mice that are characterized by an 80% reduction in serotonergic neurons accompanied by a similar reduction in serotonin (10, 34). In contrast to our findings, the increase in activity at the onset of the dark period was greater in PET1+/+ compared with PET1−/− mice (10, 34). The increase in activity was followed by a pronounced decline in motor activity throughout most of the dark phase so that motor activity in the PET1−/− mice eventually exceeded wild-type measures (10, 34). Our results are similar to the findings in the PET1−/− mouse in that motor activity abruptly increased at the terminal end of the dark phase, whereas in the wild-type animals this increase was either not evident or the amplitude of the increase was significantly less. Our results were also similar to the elevated level of activity at the onset of the light phase previously reported in PET1−/− mice (10).
The mechanisms responsible for the disruption in active wakefulness and motor activity at the onset of the light and dark cycle and at the termination of the dark cycle were not determined in our investigation. However, given that serotonin is known to suppress glutamatergic inputs from the retina to the hypothalamus in response to light (26, 37, 40, 47), we speculate that this mechanism contributed to the maintenance of elevated levels of active wakefulness and motor activity at the onset of the light phase in the Tph2−/− mice. Likewise, it is apparent that a similar serotonergic modulatory suppression of neurotransmitters that contribute to arousal and motor activity might occur at the start and end of the dark phase. There are multiple neurotransmitters that affect arousal state and motor activity in the dark phase whose release is modulated (i.e., suppressed) by serotonin, including orexin (44), norepinephrine (13, 25, 35), glutamate (40, 41), ghrelin (23), and nitric oxide (whose release is dependent on glutamatergic receptor activation in the suprachiasmatic nucleus) (27). Moreover, although serotonin could be interacting with separate neurotransmitters at the start and end of the dark cycle, it is interesting to note that some of the neurotransmitters listed are characterized by increased levels at the start and end of the dark phase (27, 32). However, whether or not the depletion of serotonin eliminates the suppression of neuronal inputs that release one of the listed neurotransmitters and results in a time-specific disruption in the sleep-wake cycle and motor activity remains to be determined.
Modulation of arousal state, temperature, and motor activity within the light and dark phase
Although increases in active wakefulness and decreases in non-rapid-eye-movement sleep were evident in Tph2−/− mice at the onset of the light and dark cycle and at the termination of the dark cycle, the duration of time spent in each arousal state was appropriately modulated at the remaining time points in the Tph2−/− mice. Likewise, temperature was correctly modulated during sleep, as well as the other arousal states, under conditions of normal room temperature. Further studies are necessary to confirm that the appropriate modulation of temperature, recorded from the abdominal cavity in our study, is evident in brain and brown adipose tissue. Interestingly, an increase in motor activity was evident in the Tph2−/− mice even though temperature and arousal state was appropriately modulated in the Tph2−/− mice. This latter finding is similar to previous studies which reported that pharmacological (14, 46) or transgenic (1) depletion of serotonin is accompanied by hyperactivity. Moreover, our results revealed that increased levels of activity during wakefulness are not necessarily linked to declines in core body temperature during sleep in mice depleted of central nervous system serotonin. This finding is in contrast to previous results which indicated that non-rapid-eye-movement sleep was reduced and wakefulness increased in Lmx1bf/f/p mice compared with wild-type mice when room temperature was 23°C or 30°C (8). Buchanan and Richerson (8) surmised that the inability to maintain core body temperature was responsible for sleep disruption in Lmx1bf/f/p mice. More specifically, these authors stated, but did not extensively document, that temperature decreased precipitously during sleep over the 24-h period in Lmx1bf/f/p compared with the wild-type mice, leading to arousal from sleep and increased activity during wakefulness to maintain core body temperature (8). The reason that core body temperature was appropriately modulated during sleep in our serotonin-depleted mice but not the Lmx1bf/f/p mice at normal room temperature is unknown but may be related to differences between transgenic models (18). Serotonergic neurons are absent or reduced in number in Lmx1bf/f/p mice but remain intact in Tph2−/− mice. Nevertheless, Buchanan and Richerson (8) hypothesized that their findings might explain earlier discoveries which indicated that serotonin is sleep promoting; since the insomnia observed following the pharmacological depletion of serotonin could have been due to the inability to maintain core body temperature rather than a consequence of serotonin depletion per se. However, the increase in active wakefulness that we observed at the onset of the light and dark cycle and at the termination of the dark cycle (see Diurnal modulation of arousal state, temperature, and motor activity), despite the correct modulation of temperature, would suggest otherwise. Despite the differences observed at normal room temperature, our results obtained in the Tph2−/− mice reflect those obtained in the Lmx1bf/f/p mice when core body temperature was presumably maintained at an appropriate level during sleep when room temperature was 33°C (8). Consequently, serotonin might have little influence on the time spent in wakefulness and sleep throughout most of the light-dark cycle.
Although the time spent in a given arousal state remained unaltered in the Tph2−/− mice at most points in the sleep-wake cycle, the architecture of the cycle was affected by serotonin depletion, since the duration increased and the number of sleep bouts decreased in the Tph2−/− mice during non-rapid-eye-movement sleep. Similar alterations in sleep-wake architecture were also reported previously in Tph2−/− (1) and Lmx1bf/f/p (8) mice under room temperature conditions of 23°C or 30°C but not 33°C. Although our observations are similar to previous findings in Tph2−/− mice (1), our interpretation of the outcome differs because electroencephalograms were used to identify arousal state in our investigation while sleep was documented indirectly (i.e., activity levels) previously (1). Based on this indirect method, Alenina and colleagues (1) hypothesized that the prolonged sleep bouts in Tph2−/− mice was indicative of an increase in the percentage of time spent in non-rapid-eye-movement sleep, supporting the role of serotonin as a wake-promoting neuromodulator (1). However, as indicated by our electroencephalogram findings, it is probable that the prolonged sleep bouts did not reflect an increase in the percentage of time spent in non-rapid-eye-movement sleep.
Instead, alterations in the composition of the sleep-wake cycle in the Tph2−/− mice (1) and Lmx1bf/f/p mice (8) suggest that the arousal threshold might be increased in mice with depleted central nervous system serotonin. Given these findings, we were interested in determining whether the higher arousal threshold was simply a reflection of differences in the depth of sleep. However, the δ/γ and δ/total power ratios during non-rapid-eye-movement sleep were similar in the Tph2+/+ and the Tph2−/− mice. Thus modifications in arousal threshold leading to prolonged sleep bouts occurs independent of the depth of sleep following depletion of serotonin in the central nervous system. It is of interest to note that potential alterations in arousal threshold that may have been responsible for the alterations in sleep architecture in our Tph2−/− mice were independent of the control of temperature during sleep. In contrast, differences in bout length between wild-type and Lmx1bf/f/p mice were eliminated once room temperature was increased from 23°C and maintained at 33°C (8). The increase in sleep bout length that presumably reflected an increased arousal threshold at 23°C in the Lmx1bf/f/p is perplexing, given the proposed hypothesis that increases in active wakefulness were necessary to elevate core body temperature following significant reductions during sleep in Lmx1bf/f/p mice compared with wild-type mice (8). From a survival standpoint, if activity was necessary to maintain core body temperature following depletion of serotonin in the central nervous system, it might be anticipated that the number of sleep bouts would be increased and the duration decreased to reflect lowering of the arousal threshold to prevent a precipitous decline in core body temperature during sleep. Given that this did not occur, it is possible that the decreases in core body temperature that were reported to occur during sleep in Lmx1bf/f/p mice were not a consequence of alterations in the modulation of temperature per se but rather was a consequence of the prolonged bouts of sleep, given that temperature decreases during sleep and its decline is linked to the duration and depth of sleep. Indeed, the length of sleep bouts in the Lmx1bf/f/p were double the length measured in our Tph2−/− mice, and the increase compared with wild-type controls was greater in the Lmx1bf/f/p mice than our Tph2−/− mice (8). Thus alterations in the arousal threshold could account for the greater decrease in temperature that was reported. Nonetheless, overall our results indicate that the depletion of serotonin per se may be responsible for alterations in arousal threshold leading to disruption in sleep-wake architecture. Further studies are required to identify if alterations in the arousal threshold are linked to specific stimuli in Tph2−/− mice.
In conclusion, the maintenance of a diurnal pattern in the sleep-wake cycle, motor activity, and temperature in our investigation suggests that the role of the suprachiasmatic nucleus in modulating the physiological variables across the 12:12 light-dark cycle is preserved despite the life-long depletion of serotonin. Whether or not our results reflect the effect of acute disruption in serotonergic neuronal activation and serotonin release on sleep and/or thermoregulation requires further investigation, since our findings could be a sign of central nervous system compensation for the loss of serotonin. Nevertheless, despite the maintenance of a diurnal periodicity, disruption in the timing (i.e., at the onset of the light and dark cycle, and at the termination of the dark cycle), and composition of the sleep-wake cycle (i.e., across the 24-h period) was evident. These results suggest that serotonin has a role in suppressing arousal inputs [i.e., photic and non-photic inputs (7, 37, 40, 47)] that serve to shorten the ultra-radian duration of wakefulness and non-rapid-eye-movement sleep at the start of the light and dark cycle and at the termination of the dark cycle.
Perspectives and Significance
Based on our summarized findings (see conclusions), we speculate that brain regions that control the sleep-wake cycle and motor activity downstream from the suprachiasmatic nucleus might be disrupted by the depletion of serotonin in the central nervous system. Indeed, Miyamoto and colleagues (28) showed that depletion of serotonin disrupted neuronal activity in the basal forebrain and preoptic area (i.e., brain regions that contribute to the control of the sleep wake cycle), as well as, the ventral subparaventricular zone, which relays information from the suprachiasmatic nucleus to the basal forebrain and preoptic area. This response occurred even though a diurnal variation in neuronal activity in the suprachiasmatic nucleus was preserved (28). Moreover, despite the increase in active wakefulness and motor activity at specific points of the light-dark cycle and alterations in composition of the sleep-wake cycle in our study, no disruption in quiet wakefulness, paradoxical sleep, or temperature occurred at any point across the light-dark cycle, as previously reported in rats and mice (28). Thus separate neuronal pathways and brain regions may be preferentially impacted by the depletion of serotonin. In support of this suggestion, Miyamoto and colleagues (28) reported that the diurnal rhythmicity of paradoxical sleep was maintained even though the rhythmicity of non-rapid-eye-movement sleep was abolished following the administration of ritanserin at the onset of the dark cycle in mice. Moreover, unique to the neuronal pathway responsible for controlling arousal state and motor activity, the dorsal subparaventricular zone is critical for controlling diurnal rhythms of body temperature through its projections to the medial preoptic region, which includes the median preoptic and ventromedial preoptic nuclei (38). Indeed, lesions of the ventral subparaventricular zone or dorsal medial hypothalamus cause a disruption of the diurnal sleep-wake rhythm and reduction of locomotor activity in rats, while brain temperature rhythm is not affected (5, 9, 24).
Acknowledgments
GRANTS
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (I21RX001412-JHM and I01RX000458-DMK).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z.S., M.A.-P., D.M.K., and J.H.M. conception and design of research; J.Z.S., M.A.-P., D.M.K., and J.H.M. performed experiments; J.Z.S. and J.H.M. analyzed data; J.Z.S. and J.H.M. interpreted results of experiments; J.Z.S. and J.H.M. prepared figures; J.Z.S. and J.H.M. drafted manuscript; J.Z.S., M.A.-P., D.M.K., and J.H.M. edited and revised manuscript; J.Z.S., M.A.-P., D.M.K., and J.H.M. approved final version of manuscript.
REFERENCES
- 1.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angoa-Perez M, Kane MJ, Briggs DI, Herrera-Mundo N, Sykes CE, Francescutti DM, Kuhn DM. Mice genetically depleted of brain serotonin do not display a depression-like behavioral phenotype. ACS Chem Neurosci. 2014 doi: 10.1021/cn500096g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angoa-Perez M, Kane MJ, Sykes CE, Perrine SA, Church MW, Kuhn DM. Brain serotonin determines maternal behavior and offspring survival. Genes Brain Behav. 2014;13:579–591. doi: 10.1111/gbb.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arraj M, Lemmer B. Circadian rhythms in heart rate, motility, and body temperature of wild-type C57 and eNOS knock-out mice under light-dark, free-run, and after time zone transition. Chronobiol Int. 2006;23:795–812. doi: 10.1080/07420520600827111. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 6.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Bobrzynska KJ, Vrang N, Mrosovsky N. Persistence of nonphotic phase shifts in hamsters after serotonin depletion in the suprachiasmatic nucleus. Brain Res. 1996;741:205–214. doi: 10.1016/s0006-8993(96)00913-4. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciarleglio CM, Resuehr HE, Axley JC, Deneris ES, McMahon DG. Pet-1 deficiency alters the circadian clock and its temporal organization of behavior. PLos One. 2014;9:e97412. doi: 10.1371/journal.pone.0097412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Pontes ALB, Engelberth RCGJ, da Silva Nascimento J, Cavalcante JC, de Oliveira Costa MSM, Pinato L, de Toledo AB, de Souza Cavalcante J. Serotonin and circadian rhythms. Psychology Neurosci. 2011;3:217–228. [Google Scholar]
- 12.de VL, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006;5:458–466. doi: 10.1111/j.1601-183X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorea E, Adrien J. Serotonergic regulation of noradrenergic coerulean neurons: electrophysiological evidence for the involvement of 5-HT2 receptors. Eur J Pharmacol. 1988;154:285–291. doi: 10.1016/0014-2999(88)90203-8. [DOI] [PubMed] [Google Scholar]
- 14.Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3,4-methylenedioxymethamphetamine: association with serotonin depletion. Eur J Pharmacol. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- 15.Hickner S, Hussain N, Angoa-Perez M, Francescutti DM, Kuhn DM, Mateika JH. Ventilatory long-term facilitation is evident after initial and repeated exposure to intermittent hypoxia in mice genetically depleted of brain serotonin. J Appl Physiol (1985) 2014;116:240–250. doi: 10.1152/japplphysiol.01197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–140. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008;164:350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol (1985) 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 20.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 21.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 22.Kawai K, Yokota N, Yamawaki S. Effect of chronic tryptophan depletion on the circadian rhythm of wheel-running activity in rats. Physiol Behav. 1994;55:1005–1013. doi: 10.1016/0031-9384(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 23.Kirsz K, Zieba DA. A review on the effect of the photoperiod and melatonin on interactions between ghrelin and serotonin. Gen Comp Endocrinol. 2012;179:248–253. doi: 10.1016/j.ygcen.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Yoshioka M, Togashi H, Tochihara M, Ikeda T, Saito H. Modulation of norepinephrine release by serotonergic receptors in the rat hippocampus as measured by in vivo microdialysis. J Pharmacol Exp Ther. 1995;272:1044–1051. [PubMed] [Google Scholar]
- 26.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitome M, Shirakawa T, Oshima S, Nakamura W, Oguchi H. Circadian rhythm of nitric oxide production in the dorsal region of the suprachiasmatic nucleus in rats. Neurosci Lett. 2001;303:161–164. doi: 10.1016/s0304-3940(01)01744-x. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto H, Nakamaru-Ogiso E, Hamada K, Hensch TK. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. J Neurosci. 2012;32:14794–14803. doi: 10.1523/JNEUROSCI.0793-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Morin LP, Blanchard J. Depletion of brain serotonin by 5,7-DHT modifies hamster circadian rhythm response to light. Brain Res. 1991;566:173–185. doi: 10.1016/0006-8993(91)91696-x. [DOI] [PubMed] [Google Scholar]
- 31.Mosienko V, Beis D, Pasqualetti M, Waider J, Matthes S, Qadri F, Bader M, Alenina N. Life without brain serotonin: reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 33.Nakamaru-Ogiso E, Miyamoto H, Hamada K, Tsukada K, Takai K. Novel biochemical manipulation of brain serotonin reveals a role of serotonin in the circadian rhythm of sleep-wake cycles. Eur J Neurosci. 2012;35:1762–1770. doi: 10.1111/j.1460-9568.2012.08077.x. [DOI] [PubMed] [Google Scholar]
- 34.Paulus EV, Mintz EM. Developmental disruption of the serotonin system alters circadian rhythms. Physiol Behav. 2012;105:257–263. doi: 10.1016/j.physbeh.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen K, Aghajanian GK. Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res. 1986;385:395–400. doi: 10.1016/0006-8993(86)91090-5. [DOI] [PubMed] [Google Scholar]
- 36.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 37.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6:367–378. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibition of light-induced fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Res. 1993;621:181–188. doi: 10.1016/0006-8993(93)90105-v. [DOI] [PubMed] [Google Scholar]
- 41.Srkalovic G, Selim M, Rea MA, Glass JD. Serotonergic inhibition of extracellular glutamate in the suprachiasmatic nuclear region assessed using in vivo brain microdialysis. Brain Res. 1994;656:302–308. doi: 10.1016/0006-8993(94)91474-5. [DOI] [PubMed] [Google Scholar]
- 42.Tankersley CG, Irizarry R, Flanders S, Rabold R. Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. J Appl Physiol (1985) 2002;92:870–877. doi: 10.1152/japplphysiol.00904.2001. [DOI] [PubMed] [Google Scholar]
- 43.Thomas DM, Angoa Perrez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 45.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 46.Williams JH, Azmitia EC. Hippocampal serotonin re-uptake and nocturnal locomotor activity after microinjections of 5,7-DHT in the fornixfimbria. Brain Res. 1981;207:95–107. doi: 10.1016/0006-8993(81)90681-8. [DOI] [PubMed] [Google Scholar]
- 47.Yannielli P, Harrington ME. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog Neurobiol. 2004;74:59–76. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]