Abstract
Purpose
Response to front-line treatment and subsequent clinical course for patients with chronic lymphocytic leukemia (CLL) are heterogeneous. Identifying pretreatment patient characteristics or prognostic factors associated with clinical outcomes is important for counseling patients, conducting clinical research, and evaluating trial results.
Patients and Methods
We evaluated the pretreatment characteristics of 595 previously untreated patients who had National Cancer Institute Working Group indications to initiate front-line therapy for predictors of complete response (CR), time to treatment failure (TTF), and overall survival (OS). Multivariable models were developed for all three end points.
Results
CR is an important treatment end point correlated with longer TTF and OS. In this retrospective analysis, front-line treatment regimen was a significant independent predictive factor for all three end points; chemoimmunotherapy was the superior treatment regimen. Considering front-line treatment regimen, other independent patient characteristics associated with CR included age and β2-microglobulin (β-2M). TTF was independently associated with age, β-2M, percent lymphocytes in bone marrow, and treatment regimen. Improved OS was independently associated with younger age, lower β-2M, and treatment regimen. Two weighted prognostic models or nomograms, one including and one excluding treatment regimen, were constructed using significant characteristics to predict 5- and 10-year survival probability and estimate median survival time.
Conclusion
Identifying pretreatment patient characteristics associated with CR, TTF, and OS establishes a baseline to compare and incorporate new prognostic factors. Treatment had an impact on the significance of these factors. Prognostic models may help patients and clinicians in decision making as well as facilitate clinical research through design and analyses of clinical trials.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common adult form of leukemia in the United States. There is clinical heterogeneity in stage and symptoms at diagnosis, time from diagnosis to therapy, response to treatment, remission duration, and survival. Most patients are diagnosed with early-stage disease and typically do not require immediate treatment. A minority of patients present with early-stage disease and symptoms such as fatigue or night sweats or with advanced-stage disease, requiring treatment.
Several important prognostic factors have been identified for patients with CLL. Correlations have been made with time from diagnosis to treatment, response to treatment, remission duration, and survival. Identifying prognostic factors and developing prognostic models for important clinical end points are critical in gaining insights into the biology of disease and may be very useful in stratifying patients in clinical trials and in forming the basis for comparing patients across clinical trials, assessing results of clinical trials, and evaluating new therapeutic modalities.
In this study, we identified a group of 595 previously untreated patients with CLL who presented to The University of Texas M. D. Anderson Cancer Center, had at presentation or developed an indication for treatment, were enrolled and treated on a front-line clinical trial, were evaluated for response, and were observed for time to treatment failure (TTF) and overall survival (OS). In this analysis, we evaluated patient characteristics at treatment initiation to identify relevant prognostic factors and developed models for important clinical end points. Finally, using the multivariable model for OS, nomograms were constructed to predict 5- and 10-year survival, as well as to estimate median survival time.
PATIENTS AND METHODS
Patients
We identified 595 previously untreated patients with CLL who presented to The M. D. Anderson Cancer Center (Houston, TX) from December 1985 to August 2004 for this analysis. Some patients had active disease at presentation, others developed active disease; all were enrolled onto front-line clinical trials on providing informed consent according to M. D. Anderson institutional review board guidelines. Patient characteristics were evaluated before treatment by history, physical examination, laboratory evaluation, and bone marrow examination, including age, sex, Rai and Binet stages, performance status, physical examination (including number of involved nodal sites, liver size, and spleen size), and laboratory evaluation, including complete blood cell count and measure of serum albumin (normal range, 3.5 to 4.7 gm/dL), alkaline phosphatase (ALP; normal range, 38 to 126 U/L), lactate dehydrogenase (LDH; normal range, 313 to 618 U/L), β2-microglobulin (β-2M; normal range, 0.6 to 2.0 mg/L), and quantitative immunoglobulin (Ig) levels (normal ranges: IgG, 624 to 1,680 mg/dL; IgA, 74 to 327 mg/dL; IgM, 29 to 214 mg/dL). Percent bone marrow cellularity, lymphocytes, and prolymphocytes were recorded. Diagnostic flow cytometry panel included at least CD5, CD19, CD23, light chain identification, and CD20. The median time from diagnosis to initiation of therapy was 19 months (range, 0 to 307 months).
All patients had active disease, with a National Cancer Institute Working Group (NCI-WG) indication for treatment.1 Patients were treated with one of three front-line regimens. These three regimens included fludarabine-based treatment, fludarabine combined with cyclophosphamide or anthracenedione (mitoxantrone; FC/FM), and chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR). All patients in the fludarabine group received fludarabine 25 mg/m2 daily for 5 days each 4-week course.2,3 There were 58 patients in this group who received fludarabine with prednisone. Prednisone had no significant impact of efficacy. Patients in this group received two courses beyond best response. The fludarabine group served as the reference group in multivariable analyses.
The second group received fludarabine 30 mg/m2 with cyclophosphamide 300 mg/m2, both daily for 3 days each 4-week course (FC).4,5 There were 39 patients who received granulocyte-macrophage colony-stimulating factor and 15 patients who received amifostine with FC. These additional agents had no significant impact on the efficacy of FC. Thirty-one patients in this group were treated with fludarabine 30 mg/m2 daily for 3 days with mitoxantrone 10 mg/m2 on day 1 only of each 4-week course. Patients were to receive six total courses.
The third group of patients received chemoimmunotherapy with combined fludarabine, cyclophosphamide, and rituximab.6 Fludarabine was given at 25 mg/m2 daily for 3 days, cyclophosphamide 25 mg/m2 daily for 3 days, and rituximab 375 to 500 mg/m2 for 1 day of each 4-week course. Patients were to receive six total courses.
All patients received their first course of treatment at M. D. Anderson; most patients received subsequent courses with their referring physician. All responses (NCI-WG criteria) were assessed at M. D. Anderson; evaluation included blood counts, chemistries, physical examination, bone marrow aspirate, and biopsy.1 All patients had follow-up at M. D. Anderson before course 4 and at least 2 months after completing therapy for response assessment. Patients were observed for progression and survival at 6-month intervals at M. D. Anderson for the first year and annually thereafter. Bone marrow aspirate and biopsy were performed at follow-up visits, in addition to blood counts, chemistries, and physical examination. If patients were unable to return to M. D. Anderson for follow-up after response assessment, their referring physician was contacted, and information regarding relapse and survival and confirmatory documents were faxed to M. D. Anderson.
Statistical Methods
Descriptive statistics, including median, range, and first and third quartiles, were used to summarize the patient characteristics. The difference between patient groups for each variable was assessed using the log-rank test.7 OS probability and TTF were estimated by the method of Kaplan and Meier.8 For OS, the time interval was measured from the day of registration on clinical trial until death or last follow-up. Death from all causes was included. For TTF, the time interval was measured from the day of registration on clinical trial until failure to achieve a response or progression of disease in responders.
Univariable and multivariable Cox proportional hazards models were fit to examine the relationship between survival time and TTF and patient characteristics.9 A final multivariable Cox model was obtained by performing a backward elimination with P value cutoff of .10, then allowing any variable previously deleted to enter the final model if its P value was less than .05.
Nomogram development began by identifying patient characteristics predictive for OS in the multivariable Cox model. The nomogram was constructed as described by Kattan et al.10 Patients without values were not included in the analysis. There were 574 patients included in the final multivariable model, and all had the specified characteristics measured at initial presentation. Log transformation was performed for β-2M and ALP to minimize skewing in distribution of values.
Validation of the nomogram consisted of discrimination and calibration. Discrimination was assessed by the concordance index, which is the probability that given two randomly drawn patients, the patient who dies first has the higher probability of death. This was calculated from 200 bootstrap samples each with a sample size of 574 patients, and it served as an unbiased measure of the ability of the nomogram to discriminate among patients. Calibration refers to how predictions from the nomogram compare to the observed outcomes. Plotting actuarial survival against predicted survival probabilities for patients stratified by predicted risk groups generated a calibration curve, and it is used to assess the prediction accuracy of the nomogram. All analyses were conducted with S-Plus 2000 Professional software (Insightful, Seattle, WA).
RESULTS
This analysis includes 595 previously untreated patients with CLL who were treated with front-line therapy. All patients had active disease, meeting criteria for treatment by the NCI-WG criteria.1 The initial purpose of this analysis was not to compare front-line regimens; however, in developing models for important clinical end points, treatment consistently was significant. Therefore, we present a summary of patient characteristics for each treatment group to give perspective to our analysis (Table 1), and multivariable models were constructed including and excluding treatment regimen.
Table 1.
Patient Characteristics (N = 595)
| Characteristic | Treatment Regimen |
|||||
|---|---|---|---|---|---|---|
| Fludarabine-Based (n = 113) |
FC or FM (n = 137) |
FCR (n = 345) |
||||
| Median | Range | Median | Range | Median | Range | |
| Rai stage | ||||||
| 0 | 7 | 2 | 11 | |||
| I-II | 68 | 80 | 227 | |||
| III-IV | 35 | 55 | 106 | |||
| No. of nodal sites | ||||||
| 0 | 17 | 13 | 38 | |||
| 1 | 17 | 5 | 42 | |||
| 2 | 21 | 37 | 63 | |||
| 3 | 54 | 82 | 201 | |||
| Male sex | 71 | 92 | 245 | |||
| Age, years | 59 | 25-82 | 57 | 21-84 | 58 | 17-86 |
| WBC, 1,000/μL | 58.6 | 7-308 | 90 | 5-372 | 82 | 2-552 |
| ALC, 1,000/μL | 50.7 | 33-265 | 83 | 19-361 | 70 | 5-518 |
| ANC, 1,000/μL | 4.7 | 0-21.5 | 4.6 | 0-23.7 | 3.5 | 0-36.7 |
| HGB, g/dL | 12.9 | 5.7-17 | 12.3 | 7.7-16.2 | 12.6 | 6.1-18.7 |
| PLT, 1,000/μL | 169 | 26-450 | 140 | 24-414 | 156 | 8-419 |
| Creatinine, mg/dL | 1.0 | 0.5-1.8 | 1.1 | 0.7-7.1 | 1.1 | 0.5-9.2 |
| ALB, g/dL | 4.3 | 2.7-5.1 | 4.1 | 2.4-5.1 | 4.1 | 2.3-5.1 |
| Serum β-2M, mg/L | 2.9 | 1.4-12.5 | 3.3 | 1.2-11.8 | 3.7 | 1.5-16.4 |
| ALP, U/L | 87 | 40-418 | 81 | 40-362 | 81 | 18-223 |
| Uric acid, mg/dl | 6 | 2-9.8 | 5.6 | 0.6-10.8 | 5.8 | 1.0-12.6 |
| IgG, mg/dL | 848 | 188-4,650 | 736 | 45.0-3,160 | 740 | 89-5,000 |
| % BM prolymphocytes | 0 | 0-9 | 2.0 | 0.0-13.0 | 5 | 0-53 |
| % BM lymphocytes | 81 | 1.0-97 | 82 | 1-98 | 78 | 2-97 |
| Aspirate cellularity, % | 70 | 10.0-100 | 70 | 10-100 | 70 | 20-100 |
| BM biopsy cellularity, % | NA | 75 | 45-90 | 70 | 5-100 | |
| Spleen size, cm* | 0 | 0-22 | 0 | 0-20 | 1 | 0-22 |
| Liver size, cm* | 0 | 0-10 | 0 | 0-17 | 0 | 0-9 |
| Time from diagnosis to treatment, months | 7 | 0-145 | 17 | 0-307 | 24 | 0-156 |
| Follow-up all patients, months | 188 | 108 | 52 | |||
| Follow-up alive, months | 160 | 101 | 49 | |||
| Alive | ||||||
| No. | 26 | 58 | 284 | |||
| % | 23 | 43 | 82 | |||
Abbreviations: FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; HGB, hemoglobin; PLT, platelet; ALB, albumin; β-2M, β2 microglobulin; ALP, alkaline phosphatase; BM, bone marrow.
Measurement below costal margin.
Front-line fludarabine-based therapy was administered to 113 patients; 137 patients received FC/FM; 345 patients received chemoimmunotherapy with FCR. The characteristics for patients included in each treatment regimen are shown in Table 1. Notable differences between groups included the fact that patients in the FC/FM and FCR groups had higher absolute lymphocyte counts and higher β-2M, and time from diagnosis to treatment was shortest for the fludarabine group and longest for the FCR group.
The median follow-up time for all patients, the number of deaths, and the median follow-up time for living patients for each treatment group are shown in Table 1. The longest follow-up time is for the patients treated with fludarabine, followed by those treated with FC/FM, and shortest follow-up is available for the patients treated with FCR. To date, 227 patients have died (Appendix Table A1, online only). The most common cause of death was progressive CLL. Another relatively common cause of death was infection; the majority of these patients had active CLL at the time of death. Second malignancies were the third most common cause of death.
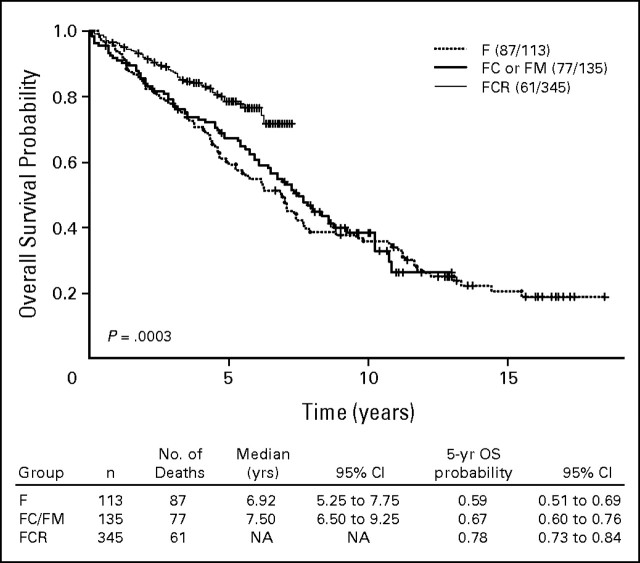
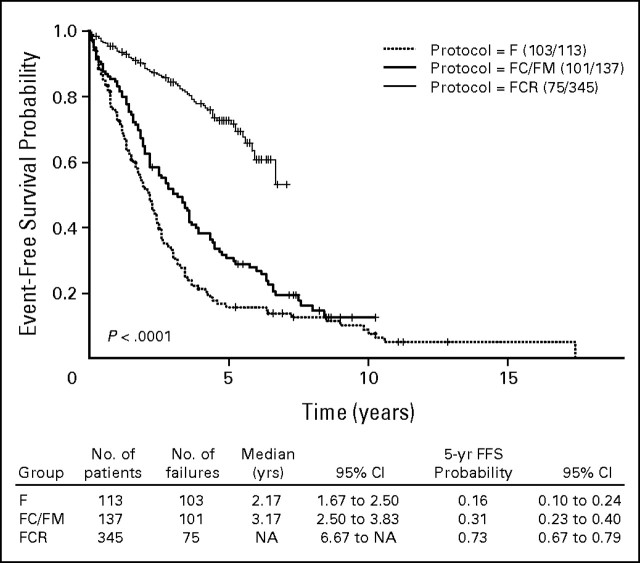
Response rates with treatment for each group are shown in Table 2. Among 595 patients in the three groups, 324 patients (54%) achieved complete remission (CR), 104 patients (17%) achieved nodular partial remission, 109 patients (18%) achieved partial remission, and 42 patients (7%) had no response to treatment. Ten patients were not assessable for response. Early death, defined as death before completing three courses of treatment, occurred in six patients. The highest CR rate was in patients treated with FCR. Among the 579 patients observed, 279 patients (48%) have experienced treatment failure or disease progression. The estimated TTFs by treatment group are shown in Appendix Figure A1 (online only). Significantly longer TTF is seen for the group treated with FCR. Among the 593 patients observed for OS, 225 patients (38%) have died. Overall, the median survival from treatment was 94 months (95% CI, 88 to 118 months). The estimates of OS by treatment regimen are shown in Figure 1.
Table 2.
Response by Treatment Regimen
| Treatment Regimen | No. of Patients | % of Patients |
|||
|---|---|---|---|---|---|
| CR | nPR | PR | NR | ||
| Fludarabine-based | 113 | 30 | 27 | 27 | 13 |
| FC/FM | 137 | 34 | 27 | 23 | 11 |
| FCR | 345 | 70 | 10 | 14 | 3 |
| Overall | 595 | 54 | 17 | 18 | 7 |
Abbreviations: CR, complete remission; nPR, nodular partial remission; PR, partial remission; NR, no response; FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab.
Fig 1.
Kaplan-Meier estimates of overall survival probability by treatment regimen. F, fludarabine; FC, fludarabine and cyclophosphamide; FM, fludarabine and mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; OS, overall survival.
Pretreatment patient characteristics were evaluated in univariable analyses to identify prognostic factors for CR, TTF, and OS (Appendix Table A2, online only). Appendix Table A2 provides P values from fitted univariable models for these three clinical end points. It is important to note that a significant characteristic may not be predictive for all three end points. Significant predictors for survival included age, Rai stage, hemoglobin (HGB), β-2M, ALP, albumin, and FCR versus fludarabine treatment. Age, Rai stage, HGB, β-2M, ALP, and treatment with FCR versus fludarabine were associated with both CR and OS.
The fitted multivariable logistic regression models for CR are shown in Appendix Table A3 (online only). Important independent covariates included age, β-2M, and treatment regimen, specifically treatment with chemoimmunotherapy (FCR). To more generally evaluate pretreatment characteristics, a multivariable model was developed that excluded treatment regimen. In this model age, β-2M, HGB, and percent bone marrow prolymphocytes were significant independent predictors for CR. These models were developed using characteristics as continuous variables.
The fitted multivariable Cox proportional hazards models for TTF are shown in Appendix Table A4 (online only). Important independent covariates in the first model included age, β-2M, percent bone marrow lymphocytes, and treatment regimen. Again, to evaluate factors excluding treatment regimen, a second multivariable model was developed. In this model for TTF, age, ALP, blood prolymphocytes, percent bone marrow lymphocytes, and time from diagnosis to treatment were significant independent covariates (Appendix Table A4).
Fitted multivariable Cox proportional hazards models were developed for OS (Table 3), and important independent covariates in the first model included age, β-2M, and treatment with FCR. In a multivariable model for OS, excluding treatment, the following were significant independent prognostic factors: age, β-2M, and ALP. Significantly improved OS was seen for patients treated with the FCR chemoimmunotherapy regimen (Appendix Table A2 and Fig 2). Rai stage was correlated with both CR and OS (Appendix Table A2). β-2M was a consistently important independent prognostic factor, predicting for CR and OS (Appendix Table A2 and Appendix Fig A2, online only).
Table 3.
Fitted Multivariable Cox Proportional Hazards Model for Overall Survival
| Variable | Coefficient | SE | Relative Risk | P |
|---|---|---|---|---|
| All variables included, n = 574 | ||||
| Age | 0.03 | 0.007 | 1.04 | < .001 |
| Ln(β-2M) | 0.85 | 0.16 | 2.35 | < .001 |
| Regimen = FC/FM (v fludarabine-based) | −0.13 | 0.17 | 0.88 | .45 |
| Regimen = FCR (v fludarabine-based) | −0.87 | 0.19 | 0.42 | < .001 |
| Excluding treatment regimen, n = 561 | ||||
| Age | 0.04 | 0.007 | 1.04 | < .001 |
| Ln(β-2M) | 0.59 | 0.17 | 1.81 | .001 |
| Ln(ALP) | 0.59 | 0.23 | 1.81 | .01 |
Abbreviations: Ln, natural log; β-2M, β2 microglobulin; FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; ALP, alkaline phosphatase.
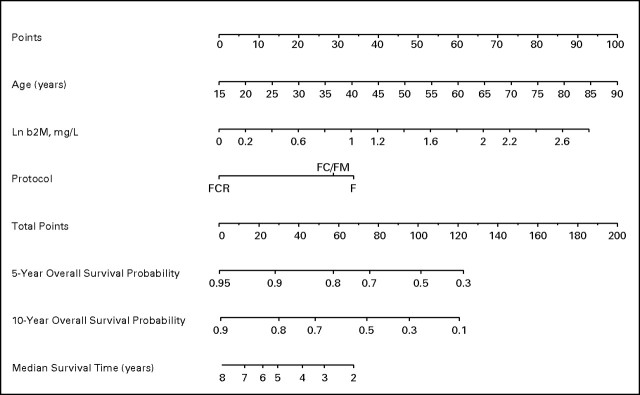
Fig 2.
Nomogram for survival of untreated patients with chronic lymphocytic leukemia. Ln, natural log; b2M, β2-microglobulin; F, fludarabine; FC, fludarabine and cyclophosphamide; FM, fludarabine and mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab.
Based on the Cox proportional hazards model for OS, a nomogram was developed incorporating treatment regimen (Fig 2). This nomogram is used to predict 5-year and 10-year OS and estimate median survival. The nomogram is used by totaling the point score for age, Ln(β-2M), and treatment regimen, and using this total point to reference the probability. The formula to calculate each patient's total point score is as follows:
 |
where I is an indicator function that is equal to 1 if the condition is met, and 0 otherwise. The total point scores ranged from 34.4 to 187.6, with a median of 110.5. The concordance index for the calibration curve validating this nomogram was 0.81 (data not shown).
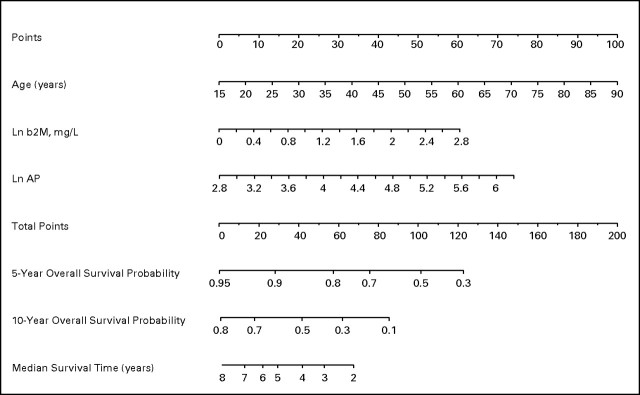
Based on the Cox proportional hazards model for OS, excluding treatment regimen (Table 3), a second nomogram for OS was developed, excluding treatment regimen (Fig 3). This nomogram included age, β-2M, and ALP. The formula to calculate each patient's total point score is as follows:
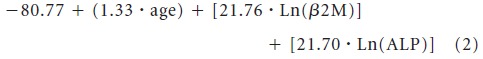 |
The median total point score was 82.9 (range, 31.5 to 187.1).
Fig 3.
Nomogram for survival of untreated patients with chronic lymphocytic leukemia, excluding treatment regimen. Ln, natural log; b2M, β2-microglobulin; AP, alkaline phosphatase.
DISCUSSION
Identifying prognostic factors and developing models that predict for clinical end points are of great importance for providing information to patients and understanding disease. Prognostic models can be of significant clinical utility in identifying high-risk patients for clinical trials and understanding the biology of CLL. These models are a step toward understanding the heterogeneity in patients with this disease.
There are landmark time points in the course of CLL that are critical in identifying prognostic factors and developing prognostic models. These time points include diagnosis, initiation of front-line treatment, and initiation of salvage treatments. Significant prognostic factors depend on timing in the course of the disease and on the clinical end point being predicted (eg, CR v TTF v OS). For example, some prognostic factors may be significant at initial presentation but become less important as disease progresses and patients require therapy or for patients receiving salvage treatment who have received multiple different prior treatments. We chose response (CR), TTF, and OS in this analysis because these were data collected for patients treated on M. D. Anderson front-line trials. Time to first salvage therapy is a potentially meaningful end point; however, this data was not routinely collected. In addition, there is subjectivity introduced when deciding to initiate salvage therapy.
We previously reported an analysis of prognostic factors and developed a prognostic model for OS in previously untreated patients at initial presentation to M. D. Anderson.11 In the current analysis, we evaluated characteristics in patients who were initiated on therapy.
To date, a similar analysis has not been performed on a patient population of this size. This analysis includes a broad cross-section of patients at initial treatment, with all Rai stages represented, and includes patients treated with different front-line treatment regimens. In our analysis, treatment had a significant impact on all three end points, including OS. Importantly, treatment regimen was a significant predictor for all clinical end points. The model including treatment was validated internally with concordance index of 0.81. A second model was developed that excluded the front-line treatment regimen. In this model, additional pretreatment characteristics were important. We also developed a nomogram from this model. These results indicate that future analyses of prognostic factors must not only consider the patient population, but also the treatment as well as the clinical end point.
In all the multivariable models that incorporated the treatment regimen, important independent covariates included age and β-2M. Younger age and lower β-2M are associated with better prognosis. The patients treated with FCR had a shorter follow-up time; nevertheless, the FCR regimen was associated with improved CR rate, longer TTF, and longer OS compared with fludarabine alone. In comparing fludarabine with FC/FM, although FC/FM was associated with higher CR rate and longer TTF, there was no significant difference in OS. Consistent with this, randomized trials have not shown a survival advantage with FC/FM versus fludarabine front-line treatment.
In our prior analysis of previously untreated patients at presentation to M. D. Anderson, regardless of the presence of an indication for treatment, multivariable analysis identified β-2M, age, LDH, absolute lymphocyte count, Rai stage, and number of involved nodal groups as significant independent covariates predictive for survival.11 In the model for untreated patients at initial treatment, only age, β-2M, and treatment regimen independently predicted for survival. In the model excluding treatment, age and β-2M remain significant and ALP comes into the model. ALP and LDH have previously been shown to have prognostic significance in patients with CLL.12 We hypothesize that the initial treatment with chemoimmunotherapy has a significant impact on OS and covariates that predict for OS. Therefore, prognostic factors for clinical end points need to be evaluated in the context of specific treatment regimens.
There are some limitations to this analysis. This is a single-institution analysis done at a large referral center. Therefore, patients included in this study may not fully represent patients seen in general community practice. Notably, the median age for these patients is significantly younger than the median age for patients in the community. Also, patients included in this analysis received treatment on single-institution, single-arm, phase II clinical trials. It does not incorporate patients treated with other treatment regimens that may be commonly used, such as fludarabine combined with rituximab.13 In addition, these are not contemporaneous treatment regimens, and therefore, follow-up time is different for each treatment regimen (Table 3). Patients treated with fludarabine-based therapy had the longest follow-up; follow-up was shorter for patients treated with FC/FM and FCR. A long-term follow-up of the front-line FCR patients was recently reported.14 Shorter follow-up and censoring affect the final multivariable model and nomograms.
This analysis does not include the more recently identified prognostic factors, including Ig heavy-chain variable gene mutation analysis, ZAP70 or CD38 expression, or fluorescent in situ hybridization analysis for chromosome abnormalities. These data are currently being collected for patients in clinical trials, and future work will identify which of these prognostic factors may be important and useful in this type of model. Models will need to be developed based on treatment regimens.
Finally, this is an analysis of patients treated with front-line chemotherapy at M. D. Anderson. The typical clinical course for patients with this disease includes subsequent relapse and repeated treatment regimens. There is no standard salvage treatment regimen and salvage treatments change with time; this modeling does not incorporate information regarding subsequent salvage therapy. Subsequent treatment may affect survival and may affect the multivariable analysis and nomogram for patients included in this analysis.
Appendix
Fig A1.
Kaplan-Meier estimates of time to treatment failure by treatment regimen. F, fludarabine; FC, fludarabine and cyclophosphamide; FM, fludarabine and mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; FFS, failure-free survival.
Fig A2.
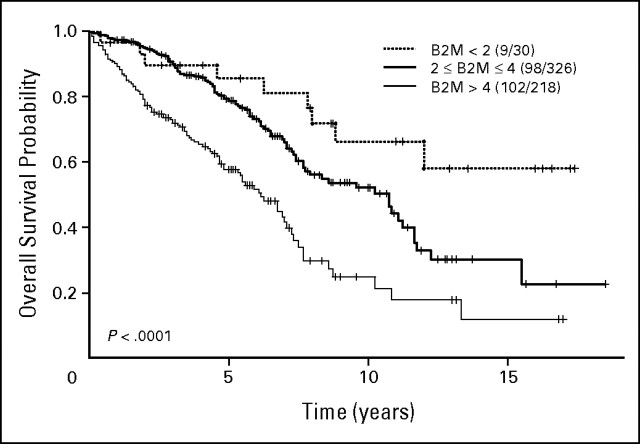
Kaplan-Meier estimates of overall survival probability by β2-microglobulin (B2M).
Table A1.
Causes of Death
| Cause of Death | % of Deaths |
|||
|---|---|---|---|---|
| All (n = 227) | Fludarabine-Based (n = 87) | FC or FM (n = 79) | FCR (n = 61) | |
| Progressive CLL | 49 | 41 | 48 | 62 |
| Infection | 22 | 29 | 19 | 18 |
| Second malignancy | 11 | 9 | 12 | 11 |
| General non-CLL | 4 | 5 | 5 | 2 |
| Cardiac | 4 | 7 | 4 | 2 |
| AIHA | 1 | 0 | 3 | 0 |
| Unknown | 8 | 9 | 9 | 5 |
Abbreviations: FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; CLL, chronic lymphocytic leukemia; AIHA, autoimmune hemolytic anemia.
Table A2.
P Values From Fitted Univariable Models for Complete Remission, Time to Treatment Failure, and Overall Survival
| Characteristic |
P |
||
|---|---|---|---|
| Complete Remission | TTF | Overall Survival | |
| Age | .001 | 0.21 | < .0001 |
| Age2 | NA | .007 | NA |
| Rai Stage = III-IV (v 0-II) | .005 | .15 | .02 |
| Ln(WBC) | .73 | .24 | .26 |
| Ln(ALC) | .64 | .15 | .31 |
| HGB | .003 | .25 | .0002 |
| PLT | .02 | .48 | .23 |
| Ln(β-2M) | .005 | .24 | < .0001 |
| Ln(ALP) | .03 | .0002 | < .0001 |
| Ln(creatinine) | .12 | .78 | .16 |
| Ln(ALB) | .65 | .67 | .002 |
| Ln(IgG) | .50 | .23 | .41 |
| Ln(IgA) | .19 | .57 | .29 |
| Ln(IgM) | .02 | .11 | .51 |
| Ln(blood prolymphocytes) | < .0001 | < .0001 | .93 |
| BM lymphocytes | .34 | .007 | .75 |
| BM aspirate cellularity | .16 | .05 | .56 |
| BM biopsy cellularity | .26 | .09 | .20 |
| No. of nodal sites | .66 | .35 | .90 |
| 1 (v 0) | |||
| 2 (v 0) | .45 | .55 | .91 |
| 3 (v 0) | .53 | .009 | .94 |
| Ln(spleen size) | .10 | .06 | .50 |
| Ln(liver size) | .22 | .39 | .26 |
| Regimen | |||
| FC/FM (v fludarabine) | .002 | .01 | .74 |
| FCR (v fludarabine) | < .0001 | < .0001 | .0001 |
| Ln(time: diagnosis to treatment) | .35 | .007 | .43 |
Abbreviations: TTF, time to treatment failure; Age2, age x age; Ln, natural log; ALC, absolute lymphocyte count; HGB, hemoglobin; PLT, platelet; β-2M, β2 microglobulin; ALP, alkaline phosphatase; ALB, albumin; BM, bone marrow; FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab.
Table A3.
Fitted Multivariable Logistic Regression Model for Complete Remission
| Variable | Coefficient | SE | Odds Ratio | P |
|---|---|---|---|---|
| All variables included, n = 567 | ||||
| (Intercept) | 1.26 | 0.53 | — | .02 |
| Age | −0.02 | 0.01 | 0.98 | .03 |
| Ln(β-2M) | −0.89 | 0.25 | 0.41 | < .001 |
| Regimen = FC/FM (v fludarabine) | 0.34 | 0.30 | 1.40 | .26 |
| Regimen = FCR (v fludarabine) | 2.04 | 0.27 | 7.71 | < .001 |
| Excluding treatment regimen, n = 471 | ||||
| Intercept | 0.03 | 1.02 | — | .98 |
| Age | −0.02 | 0.01 | 0.98 | .05 |
| Ln(β-2M) | −0.67 | 0.27 | 0.51 | .02 |
| HGB | 0.12 | 0.06 | 1.12 | .04 |
| Ln(BM prolymphocytes) | 0.63 | 0.12 | 1.88 | < .001 |
Abbreviations: Ln, natural log; β-2M, β2 microglobulin; FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; HGB, hemoglobin; BM, bone marrow.
Table A4.
Fitted Multivariable Cox Proportional Hazards Model for Time to Treatment Failure
| Variable | Coefficient | SE | Relative Risk | P |
|---|---|---|---|---|
| All variables, n = 539 | ||||
| Age | 0.003 | 0.01 | 1.00 | .58 |
| Age2 | 0.001 | 0.0003 | 1.00 | .05 |
| Ln(β-2M) | 0.33 | 0.16 | 1.39 | .03 |
| BM lymphocytes | 0.01 | 0.004 | 1.01 | .01 |
| Regimen = FC/FM (v fludarabine) | −0.37 | 0.15 | 0.69 | .02 |
| Regimen = FCR (v fludarabine) | −1.65 | 0.17 | 0.19 | < .001 |
| Excluding treatment regimen, n = 461 | ||||
| Age | 0.01 | 0.01 | 1.01 | .28 |
| Age2 | 0.001 | 0.0004 | 1.00 | .02 |
| Ln(ALP) | 1.26 | 0.23 | 3.52 | < .001 |
| Ln(blood prolymphocytes) | −0.39 | 0.08 | 0.68 | < .001 |
| BM lymphocytes | 0.01 | 0.005 | 1.01 | .01 |
| Ln(time: diagnosis to treatment) | −0.15 | 0.05 | 0.86 | .01 |
Abbreviations: Age2, age x age; Ln, natural log; β-2M, β2 microglobulin; BM, bone marrow; FC, fludarabine with cyclophosphamide; FM, fludarabine with mitoxantrone; FCR, fludarabine, cyclophosphamide, and rituximab; ALP, alkaline phosphatase.
Footnotes
W.G.W. is a Leukemia and Lymphoma Society Clinical Scholar.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: William G. Wierda, Genentech (C), Biogen Idec (C), Bayer HealthCare Pharmaceuticals (C); Susan O'Brien, Biogen Idec (C); Michael Keating, Genentech (C), Biogen Idec (C), Bayer HealthCare Pharmaceuticals (C); Stefan Faderl, Bayer HealthCare Pharmaceuticals (C); Alessandra Ferrajoli, Genentech (C), Bayer HealthCare Pharmaceuticals (C) Stock Ownership: None Honoraria: None Research Funding: William G. Wierda, Biogen Idec, Bayer HealthCare Pharmaceuticals; Susan O'Brien, Genentech, Biogen Idec, Bayer HealthCare Pharmaceuticals; Michael Keating, Bayer HealthCare Pharmaceuticals, Genzyme; Stefan Faderl, Genzyme; Alessandra Ferrajoli, Bayer HealthCare Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: William G. Wierda
Provision of study materials or patients: William G. Wierda, Susan O'Brien, Stefan Faderl, Alessandra Ferrajoli, Guillermo Garcia-Manero, Jorge Cortes, Deborah Thomas, Charles Koller, Jan Burger, Hagop Kantarjian, Michael Keating
Collection and assembly of data: William G. Wierda, Susan Lerner
Data analysis and interpretation: William G. Wierda, Xuemei Wang, Kim-Anh Do
Manuscript writing: William G. Wierda
Final approval of manuscript: William G. Wierda, Susan O'Brien, Michael Keating
REFERENCES
- 1.Cheson BD, Bennett JM, Rai KR, et al. Guidelines for clinical protocols for chronic lymphocytic leukemia: Recommendations of the National Cancer Institute-sponsored working group. Am J Hematol. 1988;29:152–163. doi: 10.1002/ajh.2830290307. [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, O'Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 3.O'Brien S, Kantarjian H, Beran M, et al. Results of fludarabine and prednisone therapy in 264 patients with chronic lymphocytic leukemia with multivariate analysis-derived prognostic model for response to treatment. Blood. 1993;82:1695–1700. [PubMed] [Google Scholar]
- 4.O'Brien SM, Kantarjian HM, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:1414–1420. doi: 10.1200/JCO.2001.19.5.1414. [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou AM, Keating MJ, Giles FJ, et al. Fludarabine and mitoxantrone for patients with chronic lymphocytic leukemia. Cancer. 2004;100:2583–2591. doi: 10.1002/cncr.20264. [DOI] [PubMed] [Google Scholar]
- 6.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Cox DR, Snell EJ. Analysis of Binary Data. ed 2. London, United Kingdom: Chapman and Hall; 1989. [Google Scholar]
- 10.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 11.Wierda WG, O'Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Dixon DO, Kantarjian HM, et al. Prognosis of chronic lymphocytic leukemia: A multivariate regression analysis of 325 untreated patients. Blood. 1987;69:929–936. [PubMed] [Google Scholar]
- 13.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 14.Tam CS, O'Brien S, Wierda W, et al. Long term results of the fludarabine, cyclophosphamide & rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]