Abstract
Background
Takotsubo cardiomyopathy is an acute heart failure syndrome characterized by myocardial hypocontractility from the mid left ventricle to apex. It is precipitated by extreme stress and can be triggered by intravenous catecholamine administration, particularly epinephrine. Despite its grave presentation, Takotsubo cardiomyopathy is rapidly reversible with generally good prognosis. We hypothesised that this represents switching of epinephrine signalling through the pleiotropic β2-adrenoceptor (β2AR) from canonical Gs-activated cardiostimulant to Gi-activated cardiodepressant pathways.
Methods and Results
We describe an in vivo rat model in which a high intravenous epinephrine, but not norepinephrine, bolus produces the characteristic reversible apical depression of myocardial contraction coupled with basal hypercontractility. The effect is prevented via Gi inactivation by pertussis toxin pretreatment. β2AR number and functional responses were greater in isolated apical cardiomyocytes compared to basal cardiomyocytes, confirming higher apical sensitivity and response to circulating epinephrine. In vitro studies demonstrated high dose epinephrine can induce direct cardiomyocyte cardiodepression and cardioprotection in a β2AR-Gi dependent manner. Preventing epinephrine-Gi effects increased mortality in the Takotsubo model, while β-blockers which activate β2AR-Gi exacerbated the epinephrine-dependent negative inotropic effects without further deaths. In contrast levosimendan rescued the acute cardiac dysfunction without increased mortality.
Conclusions
We suggest that biased agonism of epinephrine for β2AR-Gs at low and Gi at high concentrations underpins the acute apical cardiodepression observed in Takotsubo cardiomyopathy, with an apical-basal gradient in β2ARs explaining the differential regional responses. We suggest this epinephrine-specific β2AR-Gi signalling may have evolved as a cardioprotective strategy to limit catecholamine-induced myocardial toxicity during acute stress.
Keywords: acute heart failure; catecholamines; receptors, adrenergic, beta; Tako-tsubo syndrome
Introduction
There has been a rapid increase in the recognition of a syndrome of acute and severe, but reversible, heart failure called Takotsubo or Stress cardiomyopathy,1–3 also known as ‘Broken Heart Syndrome’, which usually follows within hours of an identifiable emotional, psychological or physical stress. Takotsubo cardiomyopathy mimics symptoms of acute myocardial infarction (MI), but is distinguished by the lack of coronary occlusion and by characteristic regional wall motion abnormalities, classically a virtual apical ballooning appearance due to a hypercontractile base of the heart relative to hypo- or akinetic apical and mid left ventricular myocardium, the latter extending beyond a single coronary artery territory.1, 2 Initial recognition in earthquake survivors in Japan, plus the characteristic ventricular shape, led to the ‘Takotsubo’ (meaning octopus-pot) label.3, 4 It has become apparent that ~1-2% of all presentations with suspected acute coronary syndrome cases are finally diagnosed as Takotsubo cardiomyopathy.3
The pathophysiological mechanisms for this increasingly recognised syndrome are not known. Evidence points to epinephrine as the precipitating factor. Physical or psychological stress is a frequent precipitant, and serum catecholamine levels in Takotsubo patients 1-2 days after presentation are higher than those in patients with myocardial infarction with pulmonary oedema: epinephrine falls back to MI levels only after 7-9 days.1 Catecholamine storms, more associated with epinephrine-secreting phaeochromocytomas than norepinephrine- and dopamine-secreting phaeochromocytomas,5 can also precipitate Takotsubo cardiomyopathy.6 Particularly, the reproduction of the signs of Takotsubo by accidental administration of epinephrine (including single intramuscular 1mg doses from an ‘Epi-pen’) is most indicative of its central role.7 Although there is a significant mortality in the early period (1-1.5%) there is also a characteristic rapid (days to weeks) recovery of patients surviving the acute period of profound depression in left ventricular contractile function,1 with excellent prognosis and absent, or minimal, residual cardiac impairment. This striking difference from the normal prognosis of heart failure has led us to propose previously that the cardiodepression has elements derived from a beneficial physiological protective adaptation.8 Thus, the syndrome has interest for the cardiologist over and above the design of optimal treatment for the individual Takotsubo patient.
We have previously proposed a mechanism based upon two overarching principles for which there is prior evidence. Firstly the mammalian left ventricle contains apical-basal gradients of βARs and sympathetic innervation, with the apex characterised by highest βAR and lowest sympathetic nerve density.8 Rat, feline, rabbit and dog left ventricles show increased apical responses to global high dose isoproterenol challenges,9–12 with increased apical versus basal βAR levels measured directly in the dog ventricle.10 This pattern results in increased apical responsiveness to circulating catecholamines, predominantly epinephrine from the adrenal glands, as a compensatory mechanism for the sparse apical sympathetic innervation, to ensure optimal ventricular ejection during times of stress. Conversely the sympathetic innervation is highest in the basal myocardium, and lowest in the apex, and therefore cannot explain the localised apical dysfunction. This is also true of human left ventricle,13 whereas presence of a ventricular cardiomyocyte βAR gradient in the human heart remains to be determined.
Secondly epinephrine, at high levels, can act as a negative inotrope via ligand mediated trafficking of the β2AR from Gs to Gi subcellular signalling pathways. The β2AR is widely reported as pleiotropic, having the potential to couple through Gs-AC-cAMP (like the β1AR) but also though Giα, Gβγ and non-G-protein pathways.14, 15 β2AR-Gi-mediated depression of contraction was initially demonstrated using transgenic mice over expressing the β2AR (TGβ2).16,17 At high epinephrine concentrations, the β2AR switches its coupling from Gs protein to an inhibitory Gi protein,16 a process described as ligand or stimulus directed-trafficking or biased agonism. This switch would be favoured in conditions of high catecholamine stress, since it depends upon β2AR phosphorylation by both protein kinase A (PKA)18 and G-protein receptor-coupled kinases (GRKs).19 This is particularly relevant given the increased frequency of the L41Q GRK5 polymorphism, known to increase cardiac GRK5 activity and βAR phosphorylation, in recent study genotyping Takotsubo cardiomyopathy patients.20 The negative inotropic effect through Gi 21, 22 has contributions both from inhibition of Gs-cAMP production and through other pathways such as p38 mitogen-activated protein kinase (MAPK) alteration of myofilament sensitivity.23 No such role for β1ARs in this Gs-Gi trafficking switch has been documented, and the phenomenon is epinephrine specific. Norepinephrine has 20-fold lower affinity for the β2AR compared to the β1AR, and much weaker trafficking of β2AR stimulus trafficking to the Gi pathway.16 While this negative inotropy is detrimental from a mechanical perspective, the Gs to Gi switch is potentially both antiapoptotic and antiarrhythmic, 24, 25 and may represent a cardioprotective mechanism against β1AR-catecholamine cardiotoxicity. Both p38 MAPK and PI3K/Akt pathways have been implicated in β2AR-Gi mediated antiapoptotic effects in the adult cardiac myocytes,26, 27 and evidence for increased PI3K and Akt activation has been reported in myocardial biopsies from Takotsubo cardiomyopathy patients during the acute phase.28 Interestingly, direct negative inotropic effects of some β-blockers in human ventricular cardiomyocytes have been shown to depend on β2AR-Gi signalling,29 an observation which may have implications for their use in Takotsubo Cardiomyopathy.
In this study we have developed an epinephrine-induced in vivo model of Takotsubo cardiomyopathy which reproduces both the apically located negative inotropism and the reversible nature of this cardiodepression. We have used this to explore the role of β2AR apical-basal gradients; the involvement of Gi signalling and the cardioprotective nature of this condition. It has been supplemented by an in vitro model of acute epinephrine exposure to explore underlying cellular mechanisms. Potential pharmacological agents have been assessed in terms of treatment of the established Takotsubo cardiomyopathy, with the intention to mitigate the cardiodepression without disrupting any cardioprotective elements of the syndrome.
Methods
All studies complied with the United Kingdom Home Office Regulation Governing the Care and use of Laboratory Animals and with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
In vivo Takotsubo cardiomyopathy model
Adult male Sprague-Dawley rats (250-350g) were anaesthetised and injected with 4.28x10-8mols.100g-1 epinephrine or 1.43x10-7moles.100g-1 norepinephrine via the right jugular vein as a bolus injection. Regional left ventricular responses were recorded using 2D echocardiography (Visualsonics Vevo 770) in the parasternal long axis. Baseline scans were performed before catecholamine administration. Preventative studies: a subgroup of animals were pretreated with the Gi protein inhibitor pertussis toxin (PTX) (25μg.Kg-1), the p38MAP kinase antagonist SB203580 (0.1-10mg.Kg-1) or the β2AR selective antagonist ICI 118,551 (1mg.Kg-1) followed by intravenous epinephrine bolus. A separate cohort of cases had continuous aortic blood pressure recording during the protocol using a 1.9F pressure-volume catheter (Scisense Inc, Ontario, Canada). Rescue strategies: a subgroup of animals were treated with intravenous propranolol (1.43x10-11 moles.100g-1), carvedilol (1.43x10-11 moles.100g-1), or levosimendan infusion (4.7μg/kg/min) fifteen minutes post epinephrine injection.
Rat cardiomyocyte isolation and β2AR overexpression studies
Myocytes were isolated from adult male Sprague-Dawley rats (Harlan, Bicester,UK; weight 250-350 grams) using the standard enzymatic technique as described previously.30 Isolated rat cardiomyocytes were plated in culture medium at a field density of 10,000 cells/well and infected with either Ad.β2AR.GFP, β2AR with mutations at the PKA phosphorylation sites 261, 262, 345, 346 S/A (β2AR-PKA-KO) (Ad.β2AR-PKA-KO) or Ad.GFP (control) at a multiplicity of infection (MOI) of 500 for 48 hours. For pertussis toxin (PTX) treatment, Ad.β2AR.GFP infected rat ventricular myocytes were cultured in the presence or absence of PTX (1.5 μg/ml) for 48 hours. Survival in culture was shown as a percentage of rod-shaped myocytes at the time off plating: >100 cells per well were counted, with triplicates for each condition. β2AR-specific contractile responses were measured on separately isolated apical and basal ventricular cardiomyocytes using isoproterenol (100nM) plus the β1AR selective antagonist CGP20712A (300nM) (see supplementary methods).21, 29
In vitro Takotsubo cardiomyopathy model
Freshly isolated rat ventricular cardiomyocytes were perfused with epinephrine (1µM) for20mins followed by washout (10min). A subgroup of cells was preincubated with PTX for 3h at 35°C (1.5 µg.ml-1).
βAR radioligand binding assay
Cell membranes, prepared from apical and basal-derived adult rat cardiomyocytes, were incubated for 2 hours at RT in assay buffer (50mM Tris, 5mM MgCl2) (pH 7.4), with 0.1-10nM of the non-selective β-AR radioligand, [125I]-cyanopindolol ([125I]-CYP) (Amersham, Freiburg, Germany), and increasing concentrations of the selective β2AR antagonist, ICI-118,551 (1x10-11M to 1x10-2M). Non-specific binding was determined in the presence of 10μM of the non-selective β-AR antagonist, propranolol.
FRET-mediated cAMP assay
FRET studies in EPAC-cAMPS expressing apical and basal ventricular cardiomyocytes were performed as previously described.31 Whole cell epinephrine-stimulated β2AR-mediated cAMP transients were recorded. A subgroup of cells was preincubated with PTX for 3h at 35°C (1.5 μg.ml-1).
Human tissue samples and cardiomyocyte isolation
Left or right ventricular tissues were obtained from failing human hearts at the time of heart transplantation; procedures for collecting human heart tissues conformed to the Ethics Committee requirements of the Royal Brompton and Harefield Hospital, UK. Written informed consent was provided by all patients. The investigation conforms to the principles outlined in the Declaration of Helsinki. Single human ventricular myocytes were isolated from explanted failing human hearts using a standard enzymatic technique as described before.32
Cardiomyocyte contractility studies
Statistical methods
Results are shown as mean ± SEM. Differences between cell responses for different treatments were determined using paired or unpaired Student t-tests for two, or one-way ANOVA for more than two, conditions. Concentration-response curves were compared using repeated measures ANOVA (RMANOVA). Comparison between responses to bolus epinephrine and norepinephrine, or ± pertussis toxin, beta-blockers or levosimendan, was performed using RMANOVA with time as a second factor. For individual time points after bolus, significant changes in fractional shortening compared to the pre-administration period are indicated: P values less significant than P<0.01 are not shown because of the multiple comparisons. Differences in mortality were analysed using the Chi-squared test, with Fisher’s exact test for small numbers. P values of 0.05 or below are taken as significant, NS=non-significant.
Results
High dose epinephrine injection recapitulates Takotsubo Cardiomyopathy
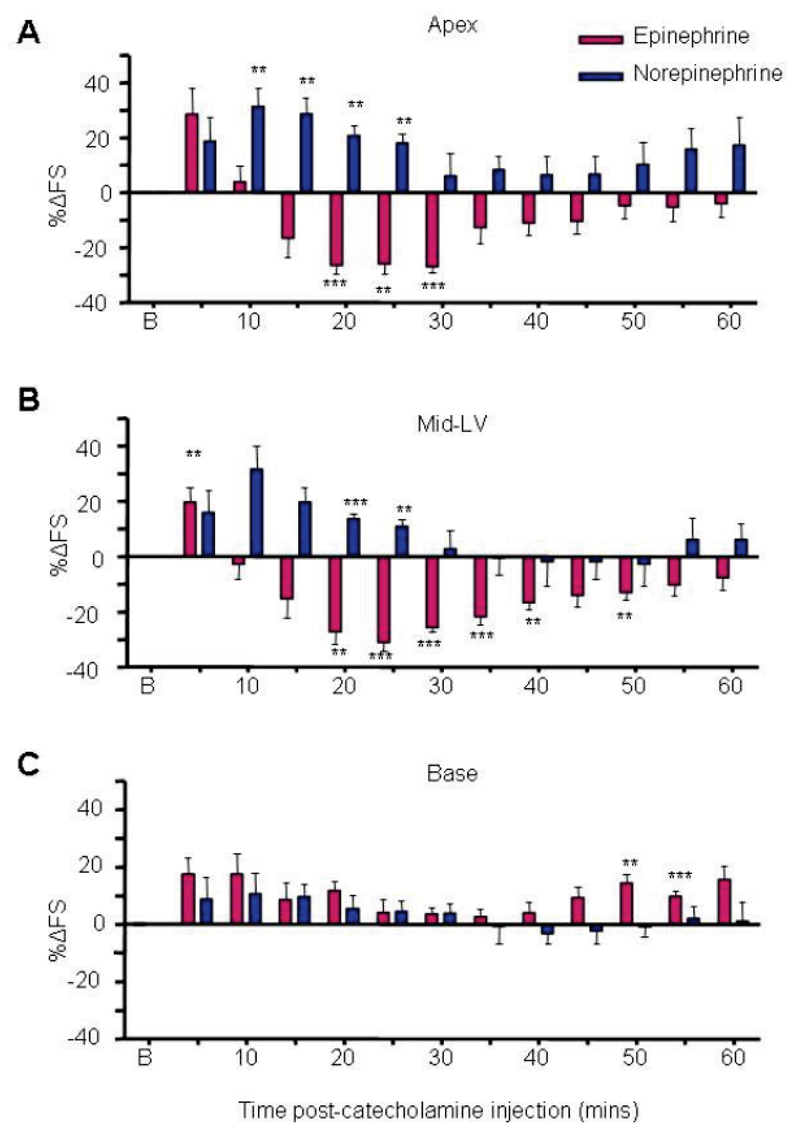
High serum epinephrine levels are a common feature in Takotsubo Cardiomyopathy patients suggesting a mechanistic link. We developed a model of Takotsubo Cardiomyopathy in which an anaesthetised rat receives an intravenous (jugular) bolus of 4.3x10-8moles.100g-1 epinephrine (equivalent to ~5mg in an adult human). Intravenous bolus delivery was selected to mimic the human physiological response to sudden high stress. Initial dose-response curves determined the highest catecholamine dose without excessive mortality (Figure S1). Epinephrine bolus triggered a rapid hypertensive response with reflex bradycardia within seconds of administration, which stabilised back to normotension after several minutes (Figure S2), and was associated with an initial global increase in left ventricular contractility (Figure 1). However, this dropped away to give a marked decrease in cardiac contraction, initiating at 15 min and reaching a nadir between 20 and 25 min. Contraction normalised within an hour. One defining characteristic of Takotsubo Cardiomyopathy is the apical and mid-ventricular localisation of dysfunction, and this was clearly reproduced in our model (Figure 1), and was confirmed by cardiac MRI (Figure S3 and Supplementary movie).
Figure 1.
Takotsubo cardiomyopathy is epinephrine-specific. Effects of 4.28x10-8 moles.100g-1 epinephrine (dark red bars) and 1.43x10-7moles.100g-1 norepinephrine (dark blue bars) on apical (A), mid left-ventricular (B) and basal myocardium contractility (C). Values are expressed as the mean percentage change in LV fractional shortening (%∆FS) from baseline (untreated) levels ± SEM at each 5 min time point following injection. N=6 (epinephrine), n=6 (norepinephrine) (**p<0.01, ***p<0.001, ****p<0.0001 vs baseline FS = 0). Abbreviation: B (baseline). RM ANOVA: epinephrine vs norepinephrine: p<0.001 (apex), p<0.001 (MLV), p=NS (base); Time: P<0.001 (apex), P<0.001 (MLV), P<0.05 (base).
Apical hypokinesia is epinephrine-specific
We and others have previously reported that epinephrine or isoproterenol at high concentrations can switch the β2AR from positively inotropic Gs to negatively inotropic Gi coupling,17, 22, 33 while norepinephrine cannot.16 We found that equivalent high dose intravenous norepinephrine did not generate the negative effect observed after epinephrine bolus (Figure 1A-C), and concentration-response curves (Figure S1) confirmed that no concentration of norepinephrine was negatively inotropic. Changes in heart rate and systemic arterial blood pressure did not differ between epinephrine and norepinephrine, indicating appropriate matching of effective concentrations (Figure S2). Lack of negative effect of norepinephrine additionally eliminates either myocardial β1AR, or α1AR-mediated vasoconstriction as the principal mediator of the epinephrine-stimulated negative inotropic effect.
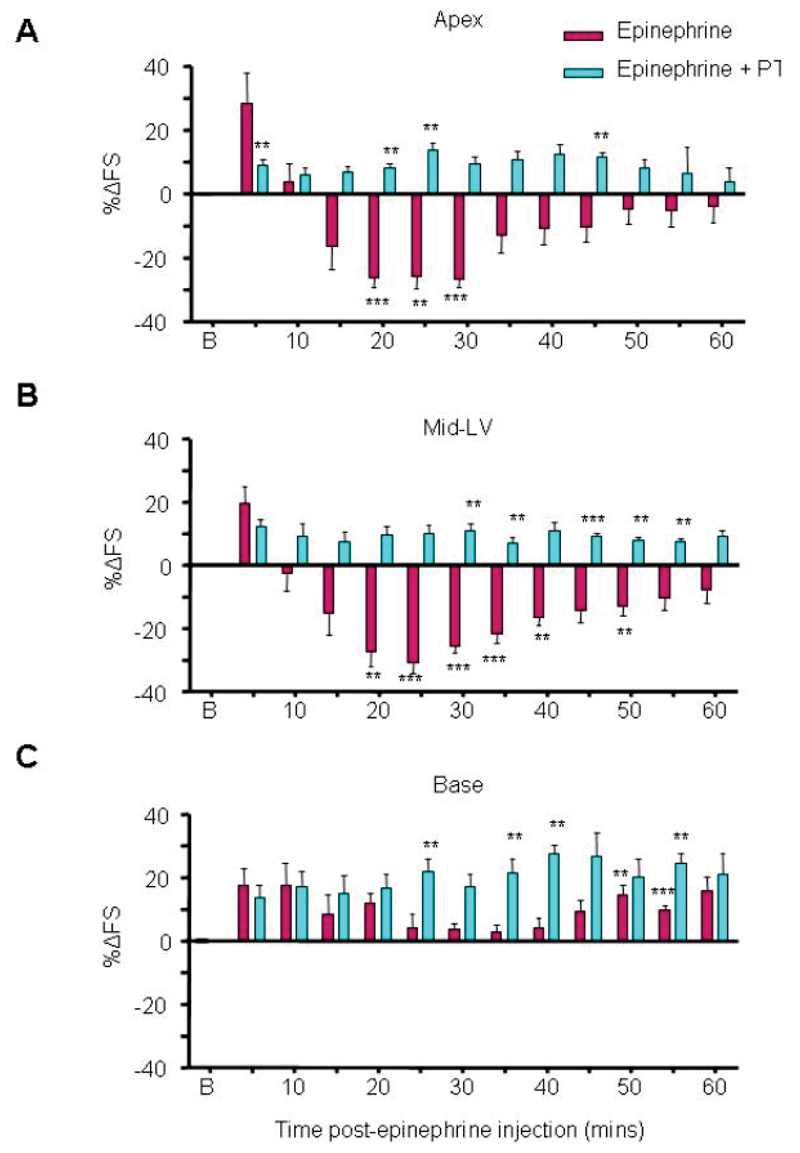
Epinephrine-induced apical hypokinesia is Gi dependent
We used pertussis toxin (PTX) to inhibit Gi by in vivo pre-treatment of the rats three days before the intravenous epinephrine challenge. In vitro challenge of isolated cardiomyocytes from these hearts with carbachol (after βAR stimulation) was used to verify inhibition of Gi effects (not shown). The negative effect of epinephrine was completely abolished by PTX (Figure 2A-C), providing strong evidence for a Gi-dependent mechanism of action. Apical and mid-LV switched completely to give an increase in contraction, and even basal hypercontractility was significantly enhanced. PTX pretreatment did not alter baseline function, the systemic arterial pressure response to epinephrine (Figure S3), or time-matched responses following control saline bolus (Figure S4-5). PTX pretreatment reduced the vagally-mediated reflex bradycardia during the first minutes after epinephrine injection (Figure S2E). However systemic vagal blockade with atropine pretreatment failed to prevent epinephrine-induced hypokinesia as observed with PTX pretreatment, and significantly increased mortality from cardiogenic shock (Figure S6). This excluded systemic vagal inhibition as the explanation for the PTX-mediated prevention of apical hypokinesia.
Figure 2.
In vivo Takotsubo Cardiomyopathy model and prevention by Pertussis toxin pretreatment. Contractile responses after an intravenous bolus injection of epinephrine (4.28x10-8 moles.100g-1-dark red bars) on left ventricular apical (A), mid left-ventricular (B) and basal myocardium (C). Values are expressed as the mean percentage change in LV fractional shortening (%∆FS) from baseline (untreated) levels ± SEM at each 5 minute time point following injection. Light blue bars show time-matched inotropic responses of the apical, mid-left ventricular and basal myocardium in PTX (25µg.Kg-1) pre-treated animals after equivalent i.v. epinephrine bolus, with loss of apical and MLV hypokinesis. N=6 (control epinephrine), n=5 (epinephrine+PTX) (**p<0.01, ***p<0.001 vs baseline FS = 0). Abbreviations: B (baseline). RM ANOVA (epinephrine vs epinephrine+PTX): p<0.001 (apex), p<0.01 (MLV), p<0.05 (base); Time: P<0.001 (apex), P<0.001 (MLV), P<0.001 (base).
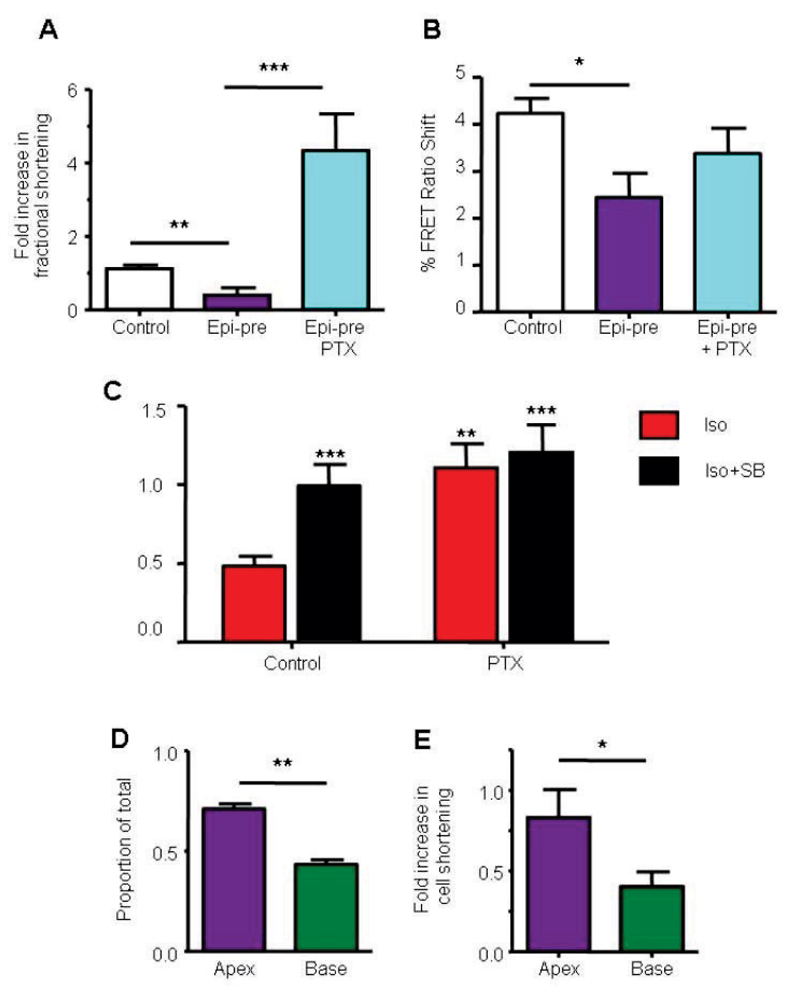
We also developed an in vitro model, in which isolated rat ventricular cardiomyocytes were treated for 20 min with epinephrine. These cells showed a decreased positive inotropic response to a subsequent β2AR challenge (Figures 3A and S7). Maximum responses to high calcium were unchanged, indicating that overall cellular and contractile function was not compromised (Figure S8). The depression of β2AR response after epinephrine pretreatment observed in this in vitro model was completely prevented (and the response became higher than control) after PTX treatment (Figures 3Aand S7). Notably, measurement of cAMP under the same conditions showed much less marked changes: PTX treatment increased contraction 11- fold without a significant increase in cAMP levels (Figure 3B). This implies a parallel negative inotropic pathway activated through Gi. Since p38 MAPK has been shown to be both Gi-dependent and negatively inotropic in rat ventricular myocytes we compared treatment with PTX and a p38 MAPK inhibitor (Figure 3C). Both were able to increase β2AR responses to a similar degree, and the effects of the two were not additive.
Figure 3.
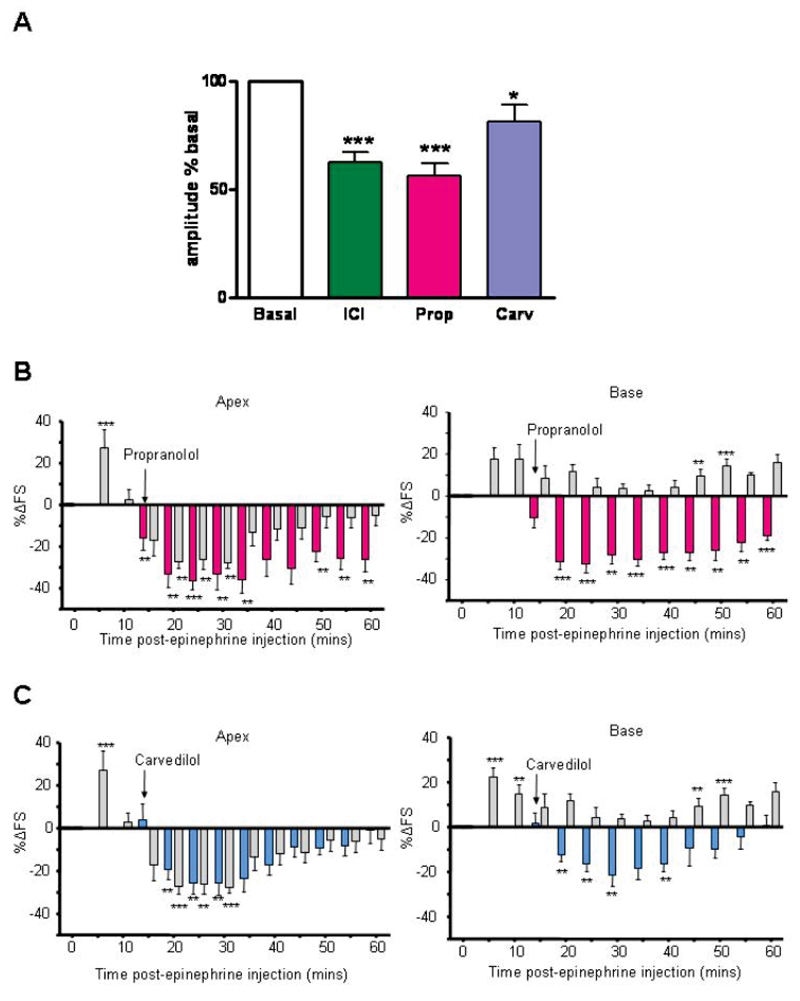
In vitro Takotsubo cardiomyopathy model induced by high dose epinephrine exposure. Effect of 20 min pretreatment with epinephrine (Epi-pre, 1µM), followed by 10 min wash, on subsequent β2AR contractile (A) and cAMP (B) responses with Gi (PTX-sensitive) component. A, Contraction amplitude in isolated rat ventricular myocytes: peak fold increase over basal: Control (n=15); Epi-pretreated alone (n=15); Epi-pretreated +PTX (n=7). B, Whole cell cyclic AMP levels, measured using an EPAC2-FRET sensor. Control (n=40); Epi-pretreated alone (n=10); Epi-pretreated +PTX (n=9). *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA Kruskal-Wallis test. C, β2AR-mediated inotropic response to 100nM isoproterenol in the presence of the β1AR blocker CGP20712A (300nM), peak fold increase over basal in control (n=13) or PTX-treated rat ventricular myocytes (n=13), in the presence and absence of SB20380 2.5μM. **P<0.01, ***P<0.001 vs control, unpaired t-test. D and E, Apically-derived cardiomyocytes demonstrate increased β2AR levels and responses. D, Proportion of β2ARs with respect to total βAR radioligand binding in ventricular myocytes from the apex and base of normal rat heart. N=4 preparations, **P<0.01 vs base, paired t-test. E, Apical cardiomyocytes (purple bars) show a larger increase in percentage cell shortening through the β2AR compared to basal cardiomyocytes (green bars). Fold increase in shortening with 100nM isoproterenol + 300nM CGP20712A. (*p<0.05 apex vs base, paired t-test. Base: n=13 cells; Apex: n=13 cells, n=13 animals).
Apical ventricular cardiomyocytes have higher β2AR density and β2AR-mediated contractile responses compared to basal cardiomyocytes
We have hypothesized that the increased apical sensitivity observed in Takotsubo cardiomyopathy patients and our model is due to a greater proportion of β2ARs relative to β1ARs in the apex,8 since the greater concentration of sympathetic innervation in the base of the heart34 is counterbalanced by increased apical βAR functional responses to circulating catecholamines.9–12 Using a radioligand binding-displacement assay to directly quantify the β2:β1AR ratio, we found that apical cardiomyocytes demonstrated an increased β2:β1AR ratio (Figure 3D). The functional consequences of a higher β2:β1AR ratio was studied and confirmed greater β2AR- specific contractile responses in apical ventricular cardiomyocytes compared to paired basal cardiomyocytes isolated from the same heart (Figure 3E). β2AR-dependent and maximal cAMP responses demonstrated no difference between apical and basal cardiomyocytes (Figure S9), and therefore could not explain the observed gradient and contractile response.
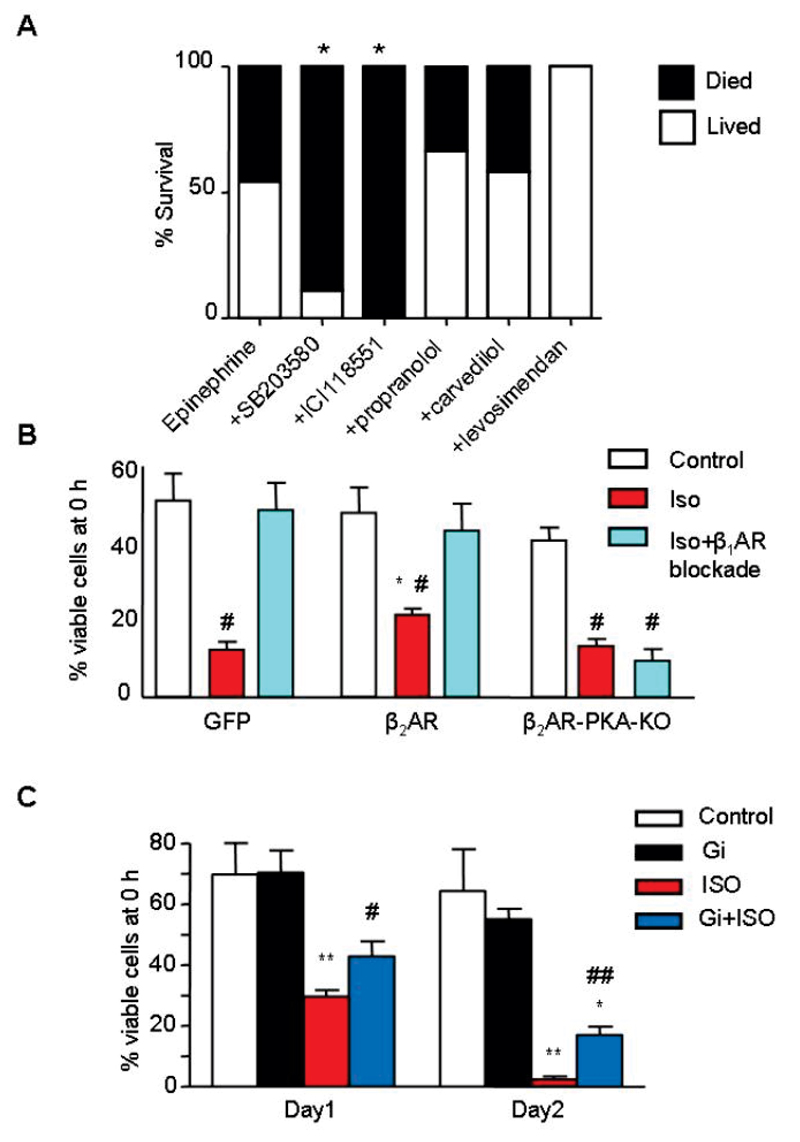
Epinephrine-induced β2AR-Gi signalling is cardioprotective
Since β2AR-Gi is widely reported to be antiapoptotic and cardioprotective,35–37 we hypothesized that blocking β2AR-Gi signalling might increase the cardiotoxic effects of high epinephrine levels via uninhibited β1AR-Gs and β2AR-Gs signalling. In the rat Takotsubo model in vivo, epinephrine-induced mortality was significantly increased by prior selective β2AR-blockade with ICI 118,551 (at concentrations insufficient to activate Gi) or p38MAPK inhibition with SB203580 (Figure 4A). Death often occurred within 5-10 min, and was due to cardiogenic shock and hypokinesia rather than primary ventricular fibrillation. In vitro, isoproterenol increased cell death in cultured myocytes, an effect largely inhibited by β1AR blockade (Figure 4B) while overexpression of the β2AR (Figure 4B) or Gi (Figure 4C) protected against catecholamine-induced cell death. β2AR switching from Gs to Gi coupling is thought to be enhanced after strong βAR-Gs activation, mediated by cAMP-dependent protein kinase (PKA).18 Overexpression of a β2AR construct in which PKA sites had been mutated to prevent phosphorylation, not only failed to protect but produced β1AR-independent cell death (Figure 4B). This mutant was also unable to support β2AR-dependent negative inotropism, in contrast to wild-type β2AR (Figure S10).
Figure 4.
Epinephrine-mediated β2AR-Gi signalling is cardioprotective. A, Mortality with in vivo bolus epinephrine (4.28x10-8 moles.100g-1) in the absence (n=14) or presence of 0.1-10mg.Kg-1 SB203580 (n=9), 1mg.Kg-1 ICI 118,551 (n=5), 1.43x10-11 moles.100g-1 propranolol (n=9), 1.43x10-11 moles.100g-1 carvedilol (n=12), 4.7 μg/kg/min levosimendan (n=5). *P<0.05 vs epinephrine alone, Fisher’s exact test. B, Survival of adult rat ventricular myocytes (% remaining at 48h compared to time 0) after exposure to 1μM isoproterenol (ISO) in the presence (light blue bars) and absence (red bars) of the β1AR blocker CGP20712A (300nM), compared to untreated controls (white bars). Myocytes were transduced using adenoviral vectors with GFP (control), the wild-type β2AR and β2AR with mutations at the PKA phosphorylation sites 261, 262, 345, 346 S/A (β2AR-PKA-KO) to prevent switching to Gi. N=6, # P<0.05 vs con/GFP, *p<0.05 vs GFP+ISO, One-way ANOVA. C, Effect of Gi expression upon ISO-induced myocyte toxicity over 48hrs in culture. Myocytes were transduced using adenoviral vectors with GFP (control), or Gi-GFP (Gi) at Day 0. N=6 preparations, *P<0.05, **P<0.01 vs respective control, #P<0.05, ##P<0.01 vs ISO alone, one-way ANOVA.
β-blockers which activate β2AR-Gi do not rescue, and may worsen, established apical hypokinesia.
In the previous section we note that pretreatment with a specific β2AR blocker before the epinephrine bolus did not appear to be a therapeutically useful manoeuvre. We also predicted that clinically used β-blockers which activate β2AR-Gi might exacerbate the epinephrine-induced negative inotropic effect. The Gi-dependent negative effect of β-blockers is most readily seen in myocytes from failing human hearts (where Gi is increased 29): we selected compounds that had either strong (propranolol) or modest (carvedilol) effects on these cells (Figure 5A). Figures 5B-C show the effect of the two blockers added 15 min after epinephrine in the in vivo model, when peak negative responses are developing. Propranolol, with higher β2AR-Gi agonism, significantly enhanced and prolonged the negative effects of epinephrine at both apex and base (Figure 5B), while carvedilol, with less pronounced β2AR-Gi agonism, had little effect on apex but converted the base from positive to significant negative responses (Figure 5C). In contrast, the β1AR-selective blocker bisoprolol reduced the positive effect of epinephrine at the base but did not convert it to significant negative response: there was no effect on the apical epinephrine response (Figure S11). These data support our hypothesis of synergistic effects of epinephrine with propranolol (and to a minor extent carvedilol) upon β2AR-Gi signalling. While the negative inotropic of epinephrine was enhanced, there was no increase in mortality with the addition of propranolol or carvedilol (Figure 4A).
Figure 5.
Agonist-independent negative inotropic effect of betablockers and potentiation of Takotsubo cardiomyopathy. A, Negative inotropic effect of βAR blockers on contraction of ventricular myocytes from failing human heart. Contraction amplitude relative to basal (open bar) for ICI 118,551 (3μM, n=21), propranolol (Prop, 5μM, n=9) and carvedilol (Carv, 3μM, n=24), *P<0.05, ***P<0.001 vs 100%, one-way ANOVA. B and C, The β-blockers propranolol (B) and carvedilol (C) (both 1.43x10-11 moles.100g-1 (i.v.)) either enhance or fail to prevent the negative inotropic effects of epinephrine (4.28x10-8 moles.100g-1 (i.v.)) at the apex and also reverse the positive effects of epinephrine at the base, in the in vivo rat model. Values are expressed as the mean percentage change in LV FS from baseline (untreated) levels ± SEM at each 5 min point following intravenous injection. N=6 (epi), n=6 (epi+propranolol), n=7 (epi+carvedilol). (**p<0.01, ***p<0.001, ****p<0.0001 vs baseline FS = 0). RM ANOVA epi vs epi+Propranolol: apex, P=0.05; base P<0.01: time, apex P<0.001; base P<0.001. Epi vs epi+carvedilol: apex P=NS; base P<0.001. Time: apex P<0.001; base P<0.001.
Levosimendan reverses epinephrine-induced apical dysfunction without increased mortality
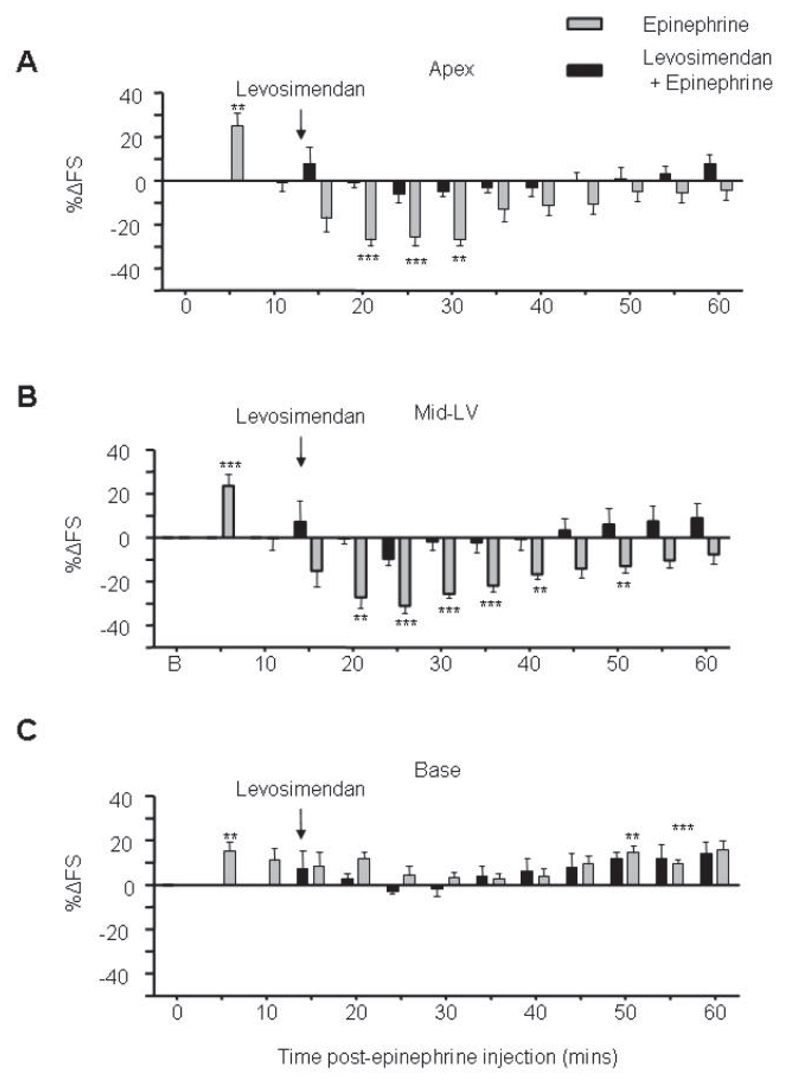
Levosimendan was selected for comparison as it is an inotrope with a cAMP-independent mechanism of action, increasing myofilament calcium sensitivity.38 Global cardiac contraction in untreated hearts was increased with infusion of this compound (not shown). In contrast to other agents, application of levosimendan at the point where epinephrine negative effects were beginning, was effective in preventing further decline in cardiac function (Figure 6). This contractile benefit and rescue occurred with no deaths in the epinephrine-treated group (Figure 4A).
Figure 6.
Levosimendan rescues the Takotsubo cardiomyopathy model. Effects of 0.28mg/kg/h (4.7 μg/kg/min) levosimendan infusion (i.v.) (black bars) on the inotropic responses of the apical (A), mid left-ventricular (B) and basal myocardium contractility (C) after 4.26x10-8 moles.100g-1 epinephrine (i.v.), compared to epinephrine alone (grey bars). Values are expressed as the mean percentage change in LV FS from baseline ± SEM at each 5 min time point following injection. N=6 (epinephrine), n=5 (levosimendan + epinephrine) (**p<0.01, ***p<0.001, ****p<0.0001 vs baseline FS = 0). RM ANOVA Epi vs epi+levosimendan: P<0.01 (apex), P<0.01 (MLV), P=NS (base). Time: P<0.001 (apex); P<0.001 (MLV); P<0.001 (base).
Discussion
Takotsubo cardiomyopathy is an increasingly recognised acute cardiac syndrome in the modern era of early access to diagnostic coronary angiography.1–3 As a cardiac response to extreme stress levels it carries a relatively good prognosis, but has the intriguing feature of regional (apical) hypokinesia, which is counterintuitive in relation to the systemic nature of the trigger and the evolutionary drive for increased cardiac output during ‘flight or fight’ responses. We have developed a rat model mimicking the clinical features with acute, reversible apical and mid-ventricular myocardial hypokinesia, but preserved or enhanced basal contractility (Figure 1). Rapid high dose intravenous epinephrine bolus, designed to mimic the serum catecholamine response to acute stress compared with the traditional infusion protocols, recapitulated the classical clinical findings, whereas the equivalent norepinephrine bolus did not (Figure 1). This implied the mechanism is epinephrine-specific, and confirms the observation that dysfunction is not typically observed in the region with the highest density of norepinephrine-releasing sympathetic nerve terminals.13
We have further investigated this concept of apical-basal gradients of catecholamine responsiveness to βAR subtype, and demonstrate that apical ventricular cardiomyocytes have a higher β2AR density and a greater β2AR-induced sensitivity compared with basal cardiomyocytes isolated from the same heart (Figure 3D-E). The inability of norepinephrine at equivalent (and higher) doses to initiate acute apical dysfunction excludes coronary vasospasm or β1AR-mediated signalling as a primary effector (Figure 1). This agrees with clinical observations that the apical dysfunction in Takotsubo cardiomyopathy extends beyond the territory of a single coronary bed.1–3, 8 Supporting the predominance of a cardiomyocyte-based explanation rather than a vascular one is also the ability of our in vitro cardiomyocyte model used here to reproduce a number of the key in vivo observations (Figure 3), as well as the matched responses of heart rate and blood pressure between epinephrine and norepinephrine cohorts.
Norepinephrine also differs in that it does not couple β1ARs or β2ARs to Gi signalling, while epinephrine at high concentrations produces a β2AR-Gs to Gi switch. β2AR-Gi coupling has been reported in a number of experimental models including β2AR and Gi overexpression, and importantly in chronic heart failure, where Gi levels are increased.29 β2AR-Gi coupling occurs via a process termed stimulus/ligand-directed trafficking or biased agonism. Other agonists such as high dose isoproterenol also produce this switch, and we note a study in which isoproterenol infusion over 2 weeks also produced a specific apical contraction defect.9 The key role of Gi in the cardiodepression was shown by the ability of PTX to convert apical responses to epinephrine from negative to positive (Figure 2). It should be noted that the response of basal myocardium was also increased, implying that β2AR-Gi was operational even in this region despite the β1AR predominance. Non-classical examples of Takotsubo Cardiomyopathy have been observed where base or mid-LV is affected,39 and this may reflect individual patterns of β2AR expression. The in vivo observations were supported by those in isolated cells. In untreated apical myocytes, positive inotropic responses to β2AR stimulation were enhanced by PTX (Figures 3C and S7, and as previously reported40). In myocytes pretreated with epinephrine, PTX was able to rescue and further enhance the depressed β2AR-mediated positive responses (Figure 3A and S7). cAMP responses were decreased modestly in pretreated myocytes (Figure 3B), though less affected than contraction. However, PTX was able to rescue contractile responses with no significant effect on cAMP (Figure 3B), implying the existence of a separate negatively inotropic Gi-dependent pathway. Inhibition of p38 MAPK produced similar and non-additive effects to PTX, consistent with the suggested role for this pathway as a Gi-dependent negatively inotropic modulator.
The epinephrine-dependent β2AR-Gi mediated negative inotropism requires a preceding high β1AR-Gs activation to initiate cAMP-dependent PKA- and GRK-phosphorylation of the β2AR.18, 41 This implies that, while norepinephrine does not directly couple receptors to Gi, the rise in cAMP it produces will predispose the β2AR to traffic to Gi upon subsequent epinephrine binding. Here we demonstrate that PKA-mediated β2AR phosphorylation is critical for Gi coupling as deleting the phosphorylation sites prevented both negative inotropism and cardioprotection attributable to β2AR-Gi coupling (Figures 4B and S10). This also explains the reversibility of the Takotsubo cardiomyopathy syndrome. As the serum epinephrine levels fall β2AR dephosphorylation, or internalisation and replacement with de novo unphosphorylated β2ARs, would reduced the β2AR-Gi stimulus trafficking and restore normal contractile function in the surviving cardiomyocytes. Studies in model cell systems overexpressing fluorescently labelled β2AR demonstrate the dependence of both PKA- and GRK-mediated β2AR phosphorylation for β2AR internalisation from the surface membrane and recycling to different surface microdomains.19 Interestingly they also demonstrate the epinephrine-specific dependence of this trafficking.41 This is relevant to the Takotsubo cardiomyopathy patients as to date there has been failure to identify any associated polymorphisms in the α1ARs, β1AR or β2ARs,42 but one study, albeit with small patient numbers, found an increased prevalence of the GRK polymorphism L41Q in the Takotsubo cardiomyopathy patient cohort compared to healthy matched controls.20 This is a ‘gain-of-function’ mutation, previously referred to as genetic betablockade,43 confers reduced responsiveness to βAR agonists, and improved prognosis in the population carrying this polymorphism,43 both conceivably consistent with enhanced myocardial β2AR-Gi coupling.
Although the final outcome for the Takotsubo patient is generally good, they have been through an acute cardiac event requiring hospitalisation, and there is a significant incidence of cardiogenic shock (~4%), malignant ventricular arrhythmias (1-2%) and death (1-1.5%). It therefore seemed reasonable to try to block the depression of contraction with either a specific β2AR antagonist or a p38 MAPK inhibitor. However the marked increase in mortality produced by this manoeuvre gave a clear indication that this was a counterproductive strategy (Figure 4A). The rapidity of the death, usually within 5-10 and always within 45 min, made it unlikely that apoptosis was the underlying mechanism. The β2AR and/or Gi have been implicated in suppression of arrhythmias,44 and β2AR variants with sudden cardiac death.45 β2AR knockout mice went into cardiogenic shock following doxorubicin, through a β1AR-related mechanism46 and β2AR/Gi mechanisms have also been implicated in post-ischemic stunning.47 All these are potential mechanisms which could underlie the acute mortality. We suggest that the enhanced β2AR-Gi coupling initiated by high epinephrine levels is protective to dampen the effects of toxic βAR-Gs coupling, which is left unchecked would be fatal
Few β-blockers are pure neutral antagonists, with most having some other effect such as partial agonism (intrinsic sympathomimetic activity), inverse agonism (reduction in activity of constitutively active receptors) or biased agonism (ligand directed trafficking to other pathways). It has been amply demonstrated that blockers of the β2AR can activate other signalling pathways, both G-protein and non-G-protein-dependent.48 We were first alerted to the possibility that the cardiodepressant effects in Takotsubo Cardiomyopathy were β2AR-Gi dependent by their similarity to the β2AR-Gi-mediated effect of β-blockers on myocytes from failing human heart.29 We therefore hypothesised that β-blockers with strong β2AR-Gi agonism would synergise with the negative inotropic effect of epinephrine. Propranolol, a particularly cardiodepressant agent, markedly enhanced and prolonged the negative phase when given after the epinephrine bolus in our in vivo model (Figure 5B). In support of the hypothesis of additive negative inotropic effects of propranolol and epinephrine, we note a recent report that an acute dilated cardiomyopathy was precipitated in a patient with phaeochromocytoma upon taking propranolol for migraine.49 Carvedilol had a more modest effect, reversing the basal hypercontractility whilst having a neutral effect upon the apical hypokinesia (Figure 5C). Carvedilol (and propranolol) are also able to produce biased agonism through Gβγ mechanisms,50 which would be PTX sensitive. Although possibly exacerbating the syndrome, carvedilol could be useful in treatment in the minority of Takotsubo cardiomyopathy patients with severe left ventricular outflow tract obstruction secondary to the basal hypercontractility. It should be noted that neither blocker had a deleterious effect on mortality in the Takotsubo Cardiomyopathy model. Bisoprolol, which is predominantly β1AR selective, did not reproduce these effects to synergise with epinephrine.
We considered the implications for treating Takotsubo cardiomyopathy, and for heart failure therapy more generally. For the Takotsubo patient, strategies to raise cAMP (catecholaminergic inotropes or phosphodiesterase inhibitors) would clearly be contraindicated. Indeed dobutamine administered for stress echocardiography testing has precipitated Takotsubo Cardiomyopathy.7 A cAMP-independent inotrope, levosimendan, was effective in reversing the negative inotropic effect of epinephrine and rescue occurred without increased (and a trend to decreased) mortality (Figures 4A and 6). We suggest that this is likely to be a safe supporting and bridging strategy for the sickest patients with cardiogenic shock until spontaneous recovery occurs, and preliminary clinical reports support this view.51, 52 It should be noted that at the higher doses levosimendan can inhibit phosphodiesterases,38 and increase cAMP, and thus we only would recommend the lower (non-vasodilatory) doses. The value of non-selective β- blockers, which may also act as agonists at β2AR-Gi, is more difficult to predict, since they may amplify both the negative inotropic and the protective effects of epinephrine. It could further be suggested that the beneficial effects of β-blockers in heart failure has taken serendipitous advantage of cardioprotective β2AR-Gi biased agonism. If those two effects could be modulated separately, this might point the way for an improved design of future β-blockers by selecting for cardioprotection through β2AR-Gi biased agonism.
Supplementary Material
Acknowledgements:
We thank Orion Pharma for the gift of levosimendan, Drs Menick, Charleston and Wang, University of California, San Diego for the p38DN vector, and Professor Walter J. Koch, Center for Translational Medicine, Philadelphia for the β2AR-PKA-KO vector. We dedicate this paper to the memories of Sir James Black (Nobel laureate, 1924-2010) and Professor Philip Poole-Wilson (1943-2009).
Funding Sources: This work was supported by grants from the Biotechnology and Biological Sciences Research Council (HP, SEH), Academy of Medical Sciences/Wellcome Trust Clinical Lecturer Start up grant (ARL), the British Heart Foundation (ARL (FS/11/67/28954), JG/SEH (NH/10/3/28574)) and the Wellcome Trust (SEH, JG (090594/Z/09/Z). ARL is supported by the National Institute for Health Research-funded Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A. Apical Ballooning Syndrome: An Important Differential Diagnosis of Acute Myocardial Infarction. Circulation. 2007;115:56–59. doi: 10.1161/CIRCULATIONAHA.106.669341. [DOI] [PubMed] [Google Scholar]
- 3.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Fujita S, Saito A, Ikeda Y, Kitazawa H, Takahashi M, Ishiguro J, Okabe M, Nakamura Y, Nagai T, Watanabe H, et al. Increased incidence of transient left ventricular apical ballooning (so-called 'Takotsubo' cardiomyopathy) after the mid-Niigata Prefecture earthquake. Circ J. 2006;70:947–953. doi: 10.1253/circj.70.947. [DOI] [PubMed] [Google Scholar]
- 5.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 6.Zielen P, Klisiewicz A, Januszewicz A, Prejbisz A, Kabat M, Peczkowska M, Stepinska J, Hoffman P. Pheochromocytoma-related 'classic' takotsubo cardiomyopathy. J Hum Hypertens. 2010;24:363–366. doi: 10.1038/jhh.2009.115. [DOI] [PubMed] [Google Scholar]
- 7.Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009;53:1320–1325. doi: 10.1016/j.jacc.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 9.Heather LC, Catchpole AF, Stuckey DJ, Cole MA, Carr CA, Clarke K. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol. 2009;60:31–39. [PubMed] [Google Scholar]
- 10.Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–198. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- 11.Lathers CM, Levin RM, Spivey WH. Regional distribution of myocardial beta-adrenoceptors in the cat. Eur J Pharmacol. 1986;130:111–117. doi: 10.1016/0014-2999(86)90189-5. [DOI] [PubMed] [Google Scholar]
- 12.Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic Nerve Stimulation Reverses Ventricular Repolarization Sequence in Rabbit Hearts. Circ Res. 2007;100:e72–e80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 14.Evans BA, Sato M, Sarwar M, Hutchinson DS, Summers RJ. Ligand-directed signalling at beta-adrenoceptors. Br J Pharmacol. 2010;159:1022–1038. doi: 10.1111/j.1476-5381.2009.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heubach JF, Ravens U, Kaumann AJ. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65:1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- 17.Hasseldine AR, Harper EA, Black JW. Cardiac-specific overexpression of human beta(2) adrenoceptors in mice exposes coupling to both G(s) and G(i) proteins. Br J Pharmacol. 2003;138:1358–1366. doi: 10.1038/sj.bjp.0705191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Ramani B, Soto D, De A V, Xiang Y. Agonist dose-dependent phosphorylation by protein kinase A and G protein-coupled receptor kinase regulates beta2 adrenoceptor coupling to G(i) proteins in cardiomyocytes. J Biol Chem. 2009;284:32279–32287. doi: 10.1074/jbc.M109.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinelli L, Trimarco V, Di MS, Marino M, Iaccarino G, Trimarco B. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail. 2010;12:13–16. doi: 10.1093/eurjhf/hfp173. [DOI] [PubMed] [Google Scholar]
- 21.Gong H, Adamson DL, Ranu HK, Koch WJ, Heubach JF, Ravens U, Zolk O, Harding SE. The effect of Gi-protein inactivation on basal, b1- and b2AR-stimulated contraction of myocytes from trangenic mice overexpressing the b2-adrenoceptor. Br J Pharmacol. 2000;131:594–600. doi: 10.1038/sj.bjp.0703591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heubach JF, Blaschke M, Harding SE, Ravens U, Kaumann AJ. Cardiostimulant and cardiodepressant effects through overexpressed human b2-adrenoceptors in murine heart. Naunyn-Schmiedebergs Arch Pharmacol. 2003;367:380–390. doi: 10.1007/s00210-002-0681-4. [DOI] [PubMed] [Google Scholar]
- 23.Liao P, Wang SQ, Wang S, Zheng M, Zhang SJ, Cheng H, Wang Y, Xiao RP. p38 Mitogenactivated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res. 2001;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 25.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Communal C, Colucci WS, Singh K. P38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta -adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- 28.Nef HM, Mollmann H, Hilpert P, Troidl C, Voss S, Rolf A, Behrens CB, Weber M, Hamm CW, Elsasser A. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur J Heart Fail. 2009;11:758–764. doi: 10.1093/eurjhf/hfp076. [DOI] [PubMed] [Google Scholar]
- 29.Gong H, Sun H, Koch WJ, Rau T, Eschenhagen T, Ravens U, Heubach JF, Adamson DL, Harding SE. The specific b2AR blocker, ICI 118,551, actively decreases contraction through a Gi-coupled form of the b2AR in myocytes from failing human heart. Circulation. 2002;105:2497–2503. doi: 10.1161/01.cir.0000017187.61348.95. [DOI] [PubMed] [Google Scholar]
- 30.Sato M, O'Gara P, Harding SE, Fuller SJ. Enhancement of adenoviral gene transfer to adult rat cardiomyocytes in vivo by immobilization and ultrasound treatment of the heart. Gene Therapy. 2005;12:936–941. doi: 10.1038/sj.gt.3302476. [DOI] [PubMed] [Google Scholar]
- 31.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 32.Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation. 1995;92:2540–2549. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- 33.Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG, Cheng H. Enhanced G(i) signaling selectively negates beta2-adrenergic receptor (AR)--but not beta1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- 34.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 35.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–1048. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- 36.Tong H, Bernstein D, Murphy E, Steenbergen C. The role of beta-adrenergic receptor signaling in cardioprotection. FASEB J. 2005;19:983–985. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Xiang J, Wang X, Liu H, Hu B, Feng M, Fu Q. Beta(2)-adrenoceptor agonist clenbuterol reduces infarct size and myocardial apoptosis after myocardial ischaemia/reperfusion in anaesthetized rats. Br J Pharmacol. 2010;160:1561–1572. doi: 10.1111/j.1476-5381.2010.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, Solaro RJ. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Recalde A, Costero O, Oliver JM, Iborra C, Ruiz E, Sobrino JA. Images in cardiovascular medicine. Pheochromocytoma-related cardiomyopathy: inverted Takotsubo contractile pattern. Circulation. 2006;113:e738–e739. doi: 10.1161/CIRCULATIONAHA.105.581108. [DOI] [PubMed] [Google Scholar]
- 40.Xiao RP, Ji X, Lakatta EG. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- 41.Wang Y, De A V, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–1807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- 42.Sharkey SW, Maron BJ, Nelson P, Parpart M, Maron MS, Bristow MR. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol. 2009;53:53–57. doi: 10.1016/j.jjcc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rau T, Nose M, Remmers U, Weil J, Weissmuller A, Davia K, Harding SE, Peppel K, Koch WJ, Eschenhagen T. Overexpression of wild-type Galpha(i)-2 suppresses beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;17:523–525. doi: 10.1096/fj.02-0660fje. [DOI] [PubMed] [Google Scholar]
- 45.Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, Lemaitre RN, Rea TD, Durda JP, Chang JM, Lumley TS, et al. Beta2-Adrenergic Receptor Genetic Variants and Risk of Sudden Cardiac Death. Circulation. 2006;113:1842–1848. doi: 10.1161/CIRCULATIONAHA.105.582833. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, Kobilka BK. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 47.Vittone L, Said M, Mattiazzi A. Beta2-Adrenergic stimulation is involved in the contractile dysfunction of the stunned heart. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:60–70. doi: 10.1007/s00210-006-0045-6. [DOI] [PubMed] [Google Scholar]
- 48.Baker JG, Hill SJ, Summers RJ. Evolution of beta-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci. 2011;32:227–234. doi: 10.1016/j.tips.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krasnow MR, Coyle D, Meyer M. Severe dilated cardiomyopathy after propranolol treatment in an undiagnosed adrenal pheochromocytoma. Circ Heart Fail. 2011;4:e10–e12. doi: 10.1161/CIRCHEARTFAILURE.111.961508. [DOI] [PubMed] [Google Scholar]
- 50.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De S V, Vitale D, Tritapepe L, Greco C, Pietropaoli P. Use of levosimendan for cardiogenic shock in a patient with the apical ballooning syndrome. Ann Intern Med. 2008;149:365–367. doi: 10.7326/0003-4819-149-5-200809020-00028. [DOI] [PubMed] [Google Scholar]
- 52.Karvouniaris M, Papanikolaou J, Makris D, Zakynthinos E. Sepsis-associated takotsubo cardiomyopathy can be reversed with levosimendan. Am J Emerg Med. 2012;30:832–837. doi: 10.1016/j.ajem.2011.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.