Abstract
Background
Systematic evidence became available in the late 1990’s on efficacy of cholinesterase inhibitors (CHEIs) for patients with mild to moderate Alzheimer’s disease (AD) and they began to be used sporadically. Since January 2001 UK based guidelines indicated that one of three cholinesterase inhibitors (CHEIs) could be prescribed for these patients. Since then the cost of prescription in England and Wales has risen. There has been little investigation of uptake at the population level.
Objective
To estimate the population uptake of CHEIs in a population based study of dementia spanning this period.
Design
Using data from a ten year follow up and a later twelve year interview of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS), a UK population based longitudinal cohort study of people originally aged 65 years and above, we investigated who was taking CHEIs during the period 2001-2004. We sought information from respondents taking part in the study what medication they were taking on a regular basis.
Results
Only 12, of the 219 individuals who received a study diagnosis of dementia were prescribed CHEIs (5%, 95% Confidence interval 3%-9%) in 2001/2003 and none of the 28 individuals with a study diagnosis of dementia (0%, 95%CI 0-18%) in 2004 were prescribed CHEIs. Uptake was biased towards individuals with more education and higher social class.
Conclusions
These data suggest that any impact on AD progression at the population level will be negligible as prescription of CHEIs and uptake in the age group at highest risk is so limited. There is little evidence that this has changed over time.
Keywords: Alzheimer’s disease, cholinesterase inhibitors, NICE
Introduction
Clinical trials in the late 1990’s described the potential benefit of cholinesterase inhibitor (CHEI) usage in individuals with early stage Alzheimer’s disease. This was followed in 2001 by a review of the efficacy of the evidence followed by guidance from the National Institute of Clinical Excellence (NICE(National Institute for Clinical Excellence 2001)) as to when these drugs could be used across the whole of the NHS (and re-imbursed). Guidance stated that individuals residing in their own home could be prescribed short time periods (up to six months) one of three CHEIs when they have mild to moderate Alzheimer’s Disease (diagnosed by a specialist) where the Mini-Mental State Examination (MMSE) score is greater than 12. It was recommended that CHEI usage be discontinued if there was no evidence of improvement. Individuals were only recommended to continue if MMSE did not deteriorate, did not fall below 12 and where there was global improvement on behavioural and/or functional assessment and where these were at a level where the drug could have an effect. Individuals could be prescribed all three CHEIs in turn to seek these benefits.
The cost of prescription of these drugs has risen from £0 in 1998 to £48 million ($92million) in 2003/4 and would be expected to increase further(National Institute for Clinical Excellence 2005). A study of the change in uptake after the NICE guidelines suggested that uptake trebled by the end of 2001, suggesting a continuation of the change in behaviour(Sheldon et al. 2004). There was a mixed compliance with the guidelines of between 52-85% for mental health organisations and 21-46% for primary care, however these figures were inaccurate due to lack of knowledge on the fulfilment of the criteria for drug use and are not related to individual need(Sheldon et al. 2004). There has, however, been little assessment of population penetration in relation to dementia within community settings, where it is known whether individuals have dementia. In March 2005 NICE has issued a controversial recommendation that the drugs are no longer prescribed on the NHS due to lack of cost effectiveness(National Institute for Clinical Excellence 2005).
Methods
The MRC Cognitive Function and Ageing study (http://www.cfas.ac.uk) has followed 13,004 individuals aged 65 and above since 1991(MRC CFAS 1998). The ten-year follow-up on 3,145 individuals was undertaken between May 2001 and September 2003 and a further interview on 188 individuals was undertaken between May and September 2004 (data version 8.0). The study uses standardised interviews conducted by trained interviewers to measure the MMSE(Folstein et al. 1975) and collects information needed for a diagnosis of dementia using the AGECAT algorithm(Copeland et al. 1986). Subtypes of clinically based dementia or severity of diagnosis are not generated. The interview also includes cognitive testing including the widely used MMSE. Information on social class and education were obtained from respondents at the baseline interview. A subset of individuals also had informant information which can provide ICD10 diagnoses. All individuals were asked about all medication they took regularly including prescription and over the counter medication usage. Where possible information was abstracted from repeat prescription forms and from medication containers. Specific medication usage was extracted from the database including the terms – donepezil (Aricept), rivastigmine (Exelon), galantamine (Reminyl). Memantine (Exiba) was also searched in case individuals were already taking this from non-NHS sources, as it is not currently allowed within the NHS under the current NICE guidelines.
Medication usage data was obtained from the Office of National Statistics (http://www.ons.gov.uk).
Statistical analysis
Data are analysed as numbers and percentages. Impact of demographic factors on drug usage was tested using logistic regression. Projections of drug usage have been undertaken using drug cost information.
Ethics
The study has had local and multicentre ethical approval throughout the twelve years of follow-up.
Results
3,145 individuals aged 74 years and above (72% response rate) were seen at the 10-year interview. Information on medication taken regularly was available from 3053 (97%) respondents of whom 3037 (99%) had full MMSE score and dementia diagnosis (Table 1). Fifteen individuals in total were receiving a CHEI. Twelve (5%) of the total of 219 dementia cases were taking CHEIs. Two CHEI users had marked cognitive decline (MMSE of 20 with drops of at least 7 points) but had not met study criteria for dementia. The other individual had no evidence of dementia and had high cognitive readings (MMSE≥27 on all visits over 10 years). There is evidence to suggest that the individuals receiving CHEI are more likely to be of higher social class (p=0.02) and better educated (p=0.02), even after adjusting for age and sex differences.
Table 1.
Characteristics of individuals at interview (numbers and percentages of individuals without missing information)
| Demented MMSE<12 | Demented MMSE 12-30 | Not demented MMSE 12-30 | ||||
|---|---|---|---|---|---|---|
| CHEI | No CHEI | CHEI | No CHEI | CHEI | No CHEI | |
| Total | 2 | 48 | 10 | 159 | 3 | 2815 |
| Date of interview Before Jan 2002 | 0 (0%) | 4 (8%) | 1 (10%) | 18 (11%) | 0 (0%) | 367 (13%) |
| Jan 2002- | 1 (50%) | 11 (23%) | 1 (10%) | 39 (25%) | 0 (0%) | 752 (27%) |
| Jul 2002- | 1 (50%) | 14 (29%) | 5 (50%) | 50 (31%) | 3 (100%) | 769 (27%) |
| Jan 2003 onwards | 0 (9%) | 19 (40%) | 3 (30%) | 52 (53%) | 0 (0%) | 927 (33%) |
| Age <80 | 0 (0%) | 7 (15%) | 1 (10%) | 26 (16%) | 1 (33%) | 1194 (42%) |
| 80-84 | 0 (0%) | 12 (25%) | 6 (60%) | 37 (23%) | 2 (66%) | 939 (33%) |
| 85+ | 2 (100%) | 29 (60%) | 3 (30%) | 96 (60%) | 0 (0%) | 682 (24%) |
| Men | 1 (50%) | 10 (21%) | 3 (30%) | 49 (31%) | 1 (33%) | 1135 (40%) |
| Women | 1 (50%) | 38 (79%) | 7 (70%) | 110 (69%) | 2 (66%) | 1680 (60%) |
| Social class I/II | 1 (50%) | 10 (21%) | 5 (50%) | 43 (27%) | 2 (67%) | 1053 (37%) |
| III | 1 (50%) | 26 (54%) | 4 (40%) | 69 (43%) | 1 (33%) | 1268 (45%) |
| IV/V/Unclassifiable | 0 (0%) | 12 (25%) | 1 (10%) | 47 (30%) | 0 (0%) | 494 (18%) |
| Years of education 9 or less | 0 (0%) | 33 (71%) | 5 (50%) | 114 (72%) | 1 (33%) | 1516 (54%) |
| 10 or more | 2 (100%) | 13 (28%) | 5 (50%) | 45 (28%) | 2 (67%) | 1295 (46%) |
| MMSE <18 | 2 (100%) | 48 (100%) | 2 (20%) | 81 (53%) | 0 (0%) | 38 (1%) |
| 18-21 | 0 (0%) | 0 (0%) | 5 (50%) | 45 (29%) | 2 (66%) | 156 (6%) |
| 22-25 | 0 (0%) | 0 (0%) | 3 (30%) | 23 (15%) | 0 (0%) | 636 (23%) |
| 26-30 | 0 (0%) | 0 (0%) | 0 (0%) | 4 (3%) | 1 (33%) | 1969 (70%) |
| NICE exclusion† | 0 (0%) | 36 (75%) | 0 (0%) | 49 (31%) | 0 (0%) | 54 (2%) |
| Living in residential care | 0 (0%) | 34 (71%) | 0 (0%) | 43 (27%) | 0 (0%) | 54 (2%) |
| No disability | 0 (0%) | 0 (0%) | 1 (14%) | 26 (19%) | 2 (66%) | 1518 (55%) |
| IADL disability only | 0 (0%) | 0 (0%) | 2 (29%) | 18 (13%) | 1 (33%) | 722 (26%) |
| IADL and/or ADL disability | 1 (100%) | 44 (100%) | 4 (57%) | 91 (67%) | 0 (0%) | 526 (19%) |
NICE exclusion residential care or severe dementia diagnosis
Ten of the 120 demented individuals who would be eligible for CHEI treatment under NICE guidelines (MMSE>12, living at home and mild/moderate dementia) were prescribed them (8%). Only 12% of those with probable/possible Alzheimer’s disease were taking CHEIs (7/59). An additional 45 individuals with prescription data were too demented for subject interview, of whom four were prescribed CHEIs.
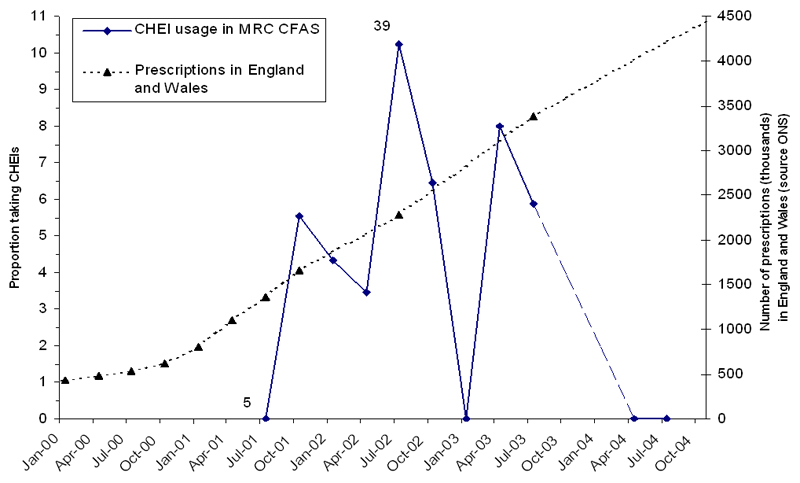
None of the individuals in a small substudy (n=188, 19 with dementia) interviewed during May-September 2004 were prescribed CHEIs; this proportion is consistent with the proportion found in the earlier phase (0%, 95% confidence interval 0%-18%). The proportion of individuals with dementia who were prescribed CHEIs changed little over time, from 5% in early 2001 to 8% in late 2003, with 0% estimated during 2004 (Figure 1). The amount of CHEI prescribed nationally during the study period increased dramatically, however including all time periods from 2001-2004 no similar increase in the population uptake of CHEI use was demonstrated.
Figure 1.
CHEI usage in the CFAS population together with prescription costs for NHS CHEI prescriptions
Discussion
This study agrees with anecdotal reports of a lack of penetration of CHEIs to the current NICE guideline eligible population(Alzheimer's Scotland 2003;Alzheimer's Society 2004;Jones et al. 2003). It shows for the first time in a population based study that, despite NICE guidelines on the use of CHEIs within England and Wales, these are not prescribed to most of the population that were thought to potentially benefit from them. The persistent lack of penetration throughout the whole time period from May 2001 to September 2004 would not have been expected either from the cost data or the audit of the take-up of the guidelines(National Institute for Clinical Excellence 2005;Sheldon et al. 2004). The majority of the interviews have been undertaken in late 2002 – September 2003 therefore giving plenty of time for detection of change in prescription procedures.
This analysis is based on survivors from the original population and those who continue to agree to take part. Research and clinical diagnoses is subject to misclassification, which may have some impact on the findings, but this is likely to be limited. Even assuming that 10% are incorrectly diagnosed and 60% would have received a diagnosis of AD, 88% of individuals would not be receiving CHEIs. Individuals may be classified as not taking the drugs if they have found them ineffective. Assuming that 50% only take the drugs for six months the estimate of the prevalence would only increase to 8% overall. It is unlikely that continued participation in the study is biased in respect of this analysis – an analysis has shown sex, age and poor cognitive ability are independently related to dropout(Matthews et al. 2004). These are unlikely to lead to increased or reduced participation of individuals on CHEIs. Individuals defined as demented in this study are not necessarily known to services and, it is likely that only around a third are in contact with services (e.g. O’Connor (O'Connor et al. 1988)). The population in the study older than those in trials, it could be that individuals are not receiving treatments as they have other impairments seen as exclusions for CHEI usage under the NICE guidelines. However, a large proportion of the population were still living at home with only mild disability at most. Barriers to being prescribed these medicines therefore include both receiving preliminary dementia diagnoses from general practitioners and referral to specialist centres for assessment and treatment. There is some evidence from this study that treatment is biased towards the better educated and higher social classes.
Conclusion
Despite widespread press coverage of the NICE guidelines rapidly rising costs of prescriptions and public awareness of CHEIs(Moise et al. 2004) they do not appear to have reached the individuals recommended to receive them. When these medications were originally promoted at the population level it was hoped they might reduce costs related to institutionalisation. With the limited penetration of the eligible population found by this study, such effects are unlikely to be achieved. For these and new treatments to be effective at a population level more attention would need to be paid to improving services to detect and treat people with dementia in the UK, regardless of their social circumstances.
Key Points.
A large proportion of the eligible population are not prescribed cholinesterase inhibitors.
Uptake of treatment may be biased towards those individuals with higher education and social class.
The barriers to successful dementia treatment are more complex than currently assumed.
Acknowledgements
The Medical Research Council Cognitive Function and Ageing study has received funding from the Medical Research Council and Department of Health. The study continues to be indebted to the respondents, their families and general practitioners.
Funding source:
The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) is funded by a Co-operative grant from the UK Medical Research Council.
Footnotes
Conflict of Interest
There are no conflicts of interest. The funding bodies approved the study design, but have had not input on the analysis or decision to publish. JB acted as social science consultant to Pfizer on raising awareness of dementia in Europe (including awareness of the range of potential treatments available).
Contributions
Fiona Matthews undertook the analysis and is the guarantor, all authors contributed to writing the paper. MRC CFAS collaborators have been involved in the study design, interviewing and are responsible for the study integrity (see website http://www.cfas.ac.uk) for details.
References
- National Institute for Clinical Excellence. National Institute for Clinical Excellence: Technology Appraisal Guidance. 2001. Guidance on the Use of Donepezil, Rivastigmine and Galantamine for the treatment of Alzheimer’s disease. [Google Scholar]
- National Institute for Clinical Excellence. Appraisal Consultation Document: Alzheimer’s disease - donepezil, rivastigmine, galantamine and memantine (review) 2005. [Google Scholar]
- Sheldon TA, Cullum N, Dawson D, Lankshear A, Lowson K, Watt I, West P, Wright D, Wright J. What’s the evidence that NICE guidance has been implemented? Results from a national evaluation using time series analysis, audit of patients’ notes, and interviews. BMJ. 2004;329(7473):999. doi: 10.1136/bmj.329.7473.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC CFAS. Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Psychol Med. 1998;28(2):319–335. doi: 10.1017/s0033291797006272. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;313:1419–1420. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16(1):89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Scotland. Postcode prescribing persists. 2003 http://www.alzscot.org/policy/ppp.html
- Jones M, Irvine B CIVITAS. NICE or NASTY: Has NICE eliminated the ‘Postcode lottery’ in the NHS. 2003 http://www.civitas.org.uk/pdf/NICE.pdf
- Alzheimer’s Society. Drugs for the treatment of Alzheimer’s disease. 2004 http://www.alzheimers.org.uk//News_and_campaigns/Campaigning/PDF/NICE2004/Alzheimers Society submssion to Nice review of drugs 2004.pdf.
- Matthews FE, Chatfield M, Freeman C, McCracken C, Brayne C. Attrition and bias in the MRC cognitive function and ageing study: an epidemiological investigation. BMC Public Health. 2004;4(1):12. doi: 10.1186/1471-2458-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DW, Pollitt PA, Hyde JB, Brook CP, Reiss BB, Roth M. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297(6656):1107–1110. doi: 10.1136/bmj.297.6656.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise P, Schwarzinger M, Um M-Y, Dementia experts group Dementia Care in 9 OECD countries: a comparative analysis. 2004 http://www.oecd.org/dataoecd/10/52/33661491.pdf