ABSTRACT
Chlamydia species are obligate intracellular pathogens that have a major impact on human health. The pathogen replicates within an intracellular niche called an inclusion and is thought to rely heavily on host-derived proteins and lipids, including ceramide. Sortilin is a transmembrane receptor implicated in the trafficking of acid sphingomyelinase, which is responsible for catalysing the breakdown of sphingomyelin to ceramide. In this study, we examined the role of sortilin in Chlamydia trachomatis L2 development. Western immunoblotting and immunocytochemistry analysis revealed that endogenous sortilin is not only associated with the inclusion, but that protein levels increase in infected cells. RNAi-mediated depletion of sortilin, however, had no detectable impact on ceramide delivery to the inclusion or the production of infectious progeny. This study demonstrates that whilst Chlamydia redirects sortilin trafficking to the chlamydial inclusion, RNAi knockdown of sortilin expression is insufficient to determine if this pathway is requisite for the development of the pathogen.
KEY WORDS: Chlamydia trachomatis, Ceramide, Sortilin, Acid sphingomyelinase
Summary: The acid sphingomyelinase trafficking protein sortilin is modulated by chlamydial infection. RNAi-mediated depletion of sortilin does not, however, perturb chlamydial infection.
INTRODUCTION
Chlamydia trachomatis is the most prevalent bacterial sexually transmitted infection amongst humans and the leading cause non-congenital blindness worldwide (Centers for Disease Control and Prevention, 2014; Belland et al., 2004). As an obligate intracellular pathogen, C. trachomatis passes its entire developmental cycle within an intracellular membrane-bound vacuole called the inclusion. The capacity to create and maintain such a specialised replicative niche is an important virulence property for many intracellular pathogens (Scott et al., 2002; Vergne et al., 2005; Dupont et al., 2009). To facilitate the growth of the inclusion and to ensure delivery of nutrients to the pathogen, Chlamydia manipulates the membrane trafficking pathways of the host cell (Elwell and Engel, 2012). Whilst there are indications that Chlamydia is capable of producing specific phospholipids autonomously (Yao et al., 2015), there is ample evidence to suggest that Chlamydia relies on host-derived lipids for survival such as sphingomyelin, phosphatidylcholine, phosphatidylinositol and cholesterol (Hatch and McClarty, 1998; Wylie et al., 1997; Hackstadt et al., 1995; Carabeo et al., 2003).
Using a short chain fluorescent analogue of ceramide (C6-NBD-ceramide), Hackstadt and co-workers demonstrated the conversion of ceramide to sphingomyelin in the Golgi and subsequent delivery and accumulation of the fluorescent lipid within the inclusion as early as 2 hours post-infection (hpi). (Hackstadt et al., 1996). Treatment with myriocin, a potent inhibitor of serine palmitoyltransferase, the initial enzyme in the biosynthesis of sphingomyelin (Hanada et al., 2000) perturbs homotypic fusion of chlamydial inclusions as well as inclusion membrane stability (Robertson et al., 2009). Concurrent treatment with dihydroceramide or sphingosine reversed this phenotype highlighting the requirement for ceramide and/or sphingomyelin in chlamydial biology (Robertson et al., 2009). Chlamydia are thought to acquire sphingomyelin by intercepting sphingomyelin-containing Golgi-derived exocytic vesicles destined for the plasma membrane (Hackstadt et al., 1996); fusion of multi-vesicular bodies with the inclusion (Beatty, 2008, 2006) or via non-vesicular means through the hijacking of the ceramide transfer protein (CERT) (Derre et al., 2011). With regards to the latter, Elwell and colleagues propose that C. trachomatis recruits CERT, its ER binding partner, VAP-A, and the sphingomyelin synthases, SMS1 and SMS2, to establish an ‘on-site sphingomyelin biosynthetic factory’ at or near the inclusion (Elwell et al., 2011). What is not clear is where the sphingomyelin precursor, ceramide, to support this system is sourced from.
The constitutive degradation of sphingolipids within the eukaryotic cell takes place in multi-vesicular bodies where sphingomyelin is converted to ceramide by acid sphingomyelinase (ASM) (Lansmann et al., 2003). ASM is demonstrated to play a central role in the cellular response to a variety of cellular stresses including environmental insults, apoptosis and pathogen infection (Smith and Schuchman, 2008). Sortilin is a transmembrane protein required for the trafficking of soluble ASM within the endosome/lysosome system (Willnow et al., 2008; Wahe et al., 2010). We speculated that sortilin-based trafficking of ASM may contribute to the supply of ceramide to the on-site sphingomyelin biosynthetic factory utilised by Chlamydia. We found that C. trachomatis L2 subverts the trafficking of sortilin to the inclusion leading to an increase in sortilin concentrations in infected cells. RNAi-mediated depletion of sortilin had no significant impact on chlamydial inclusion expansion or infectious progeny production.
RESULTS
Sortilin is upregulated in C. trachomatis L2 infected cells and is associated with the chlamydial inclusion
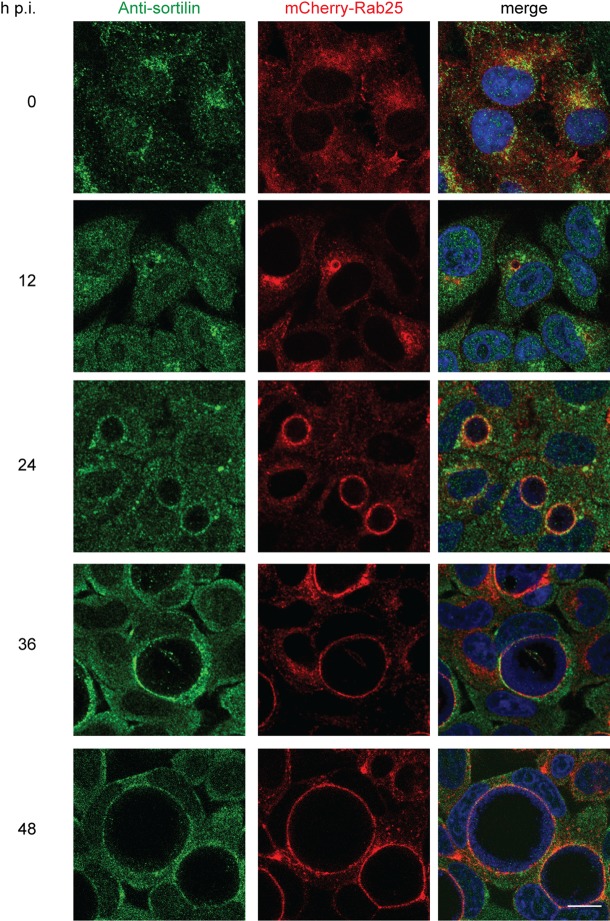
To investigate a role for sortilin in Chlamydia trachomatis L2 (CTL2) infection, Western immunoblotting was initially performed to monitor the levels of sortilin in infected cells. Western immunoblotting of HeLa cells infected with CTL2 at a multiplicity of infection (MOI) ∼0.5 revealed an increase in total cellular sortilin levels from 12 hpi, with an approximately two-fold increase observed by 48 hpi relative to uninfected cells cultured under the same conditions (Fig. 1A,B). To determine if sortilin is associated with the outer membrane of the chlamydial inclusion throughout the course of infection, indirect immunofluorescence was performed using anti-sortilin antibodies to examine the subcellular distribution of sortilin in infected cells. An unpublished screen of Rab-fusion proteins (data not shown) revealed that mCherry-Rab25 localises with high fidelity to the CTL2 inclusion throughout the infection (Fig. S1). As such, we employed mCherry-Rab25 stably expressing HeLa cells to monitor the limiting membrane of the chlamydial inclusion at 12 h intervals. The samples were immunolabeled with anti-sortilin antibodies, followed by a fluorescently-conjugated secondary antibody, counter-stained with DAPI and examined using confocal microscopy (Fig. 2A). Consistent with previously published studies, in uninfected cells the majority of the endogenous sortilin-labelling was observed to localise to the perinuclear region of the cell, within the Golgi and in vesicles dispersed throughout the cytoplasm, (Kwon and Christian, 2011; Ni and Morales, 2006) presenting little overlap with mCherry-Rab25. During a chlamydial infection, however, endogenous sortilin is localised to the mCherry-Rab25-positive inclusions from 12 hpi. As the infection progressed, sortilin remained associated with the inclusion. Therefore, sortilin is efficiently transported to and remains associated with the chlamydial inclusion throughout the infection.
Fig. 1.
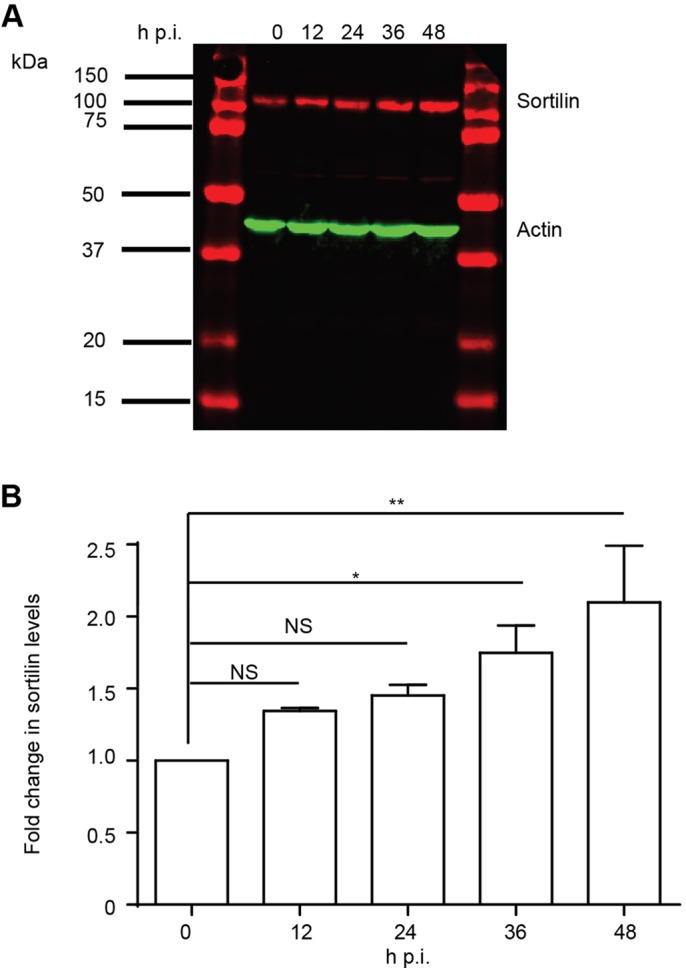
Chlamydia infection causes elevated levels of sortilin. (A) Whole cell lysates of C. trachomatis L2-infected HeLa cells were harvested at indicated time points. 20 μg of proteins were subjected to western blot using rabbit polyclonal anti-sortilin and mouse monoclonal anti-beta actin antibodies followed by IR dye-conjugated secondary antibodies. Fluorescent intensities were detected by Li-Cor Odyssey Infrared Imaging System and (B) quantified using FIJI demonstrating a stark increase sortilin levels during infection. Error bars denote mean±s.e.m., N=2; one-way ANOVA; *P<0.05, **P<0.005.
Fig. 2.
Sortilin is associated with the inclusion throughout its development. HeLa cells stably expressing mCherry-Rab25 were infected with CTL2 (MOI 0.5) for the indicated time points and fixed with 4% paraformaldehyde. Samples were then immunolabeled with anti-sortilin antibodies and appropriate secondary antibody and counter-stained with DAPI. Single optical sections were obtained using a Zeiss LSM710 meta confocal microscope with a 63× objective. Scale bar 20 µm.
Ceramide delivery to the inclusion is unaffected by depletion of sortilin
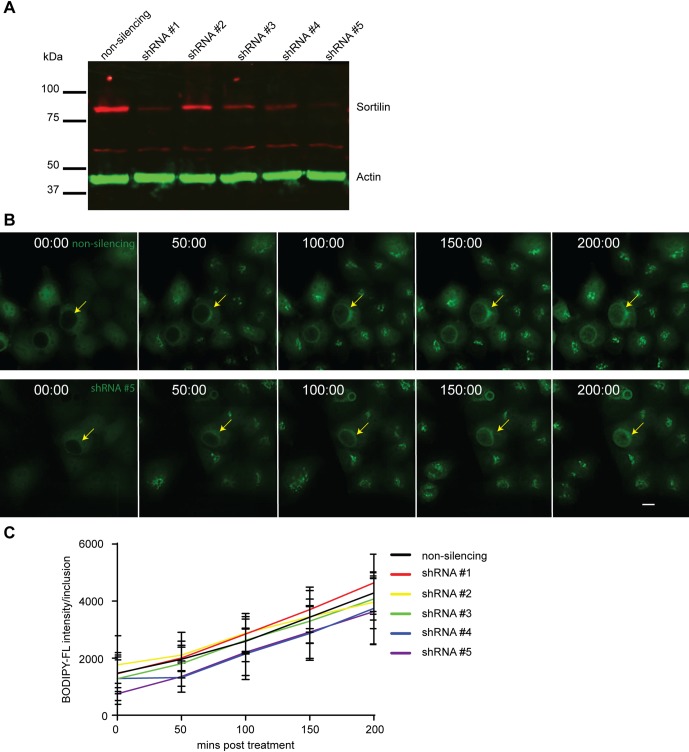
To determine if delivery of sortilin to the chlamydial inclusion is required for infection progression, five shRNA-sortilin knockdown HeLa cell lines were generated. Western immunoblotting confirmed a >70% decrease in endogenous sortilin in four of these lines when compared with non-silencing control cells (Fig. 3A). BODIPY-FL-ceramide was used to monitor delivery of ceramide and its metabolites, which include sphingomyelin and glucosylceramide, in living cells using time-lapse videomicroscopy of sortilin knockdown cells infected with CTL2 (MOI ∼0.5) for 23 h. Initially the inclusion is void of any BODIPY-FL-ceramide or TurboGFP fluorescence (shRNA reporter), highlighting the barrier function of the inclusion membrane. By following the fluorescent ceramide analogue, we observe the rapid transportation of the lipid, first to the Golgi, and the subsequent accumulation of ceramide within the inclusion (Fig. 3B). Quantification of the accumulated fluorescent intensity within the inclusion over a period of 4 h revealed no detectable difference between the control and sortilin knockdown cells (Fig. 3C) confirming that delivery of BODIPY-FL-ceramide and its metabolites from the Golgi to the inclusion still occurs efficiently in these cells and that CTL2 is readily able to replicate.
Fig. 3.
Depletion of sortilin does not impact upon delivery of ceramide to the inclusion. (A) The protein levels of sortilin in stable cell lines expressing non-silencing shRNA or shRNA targeting sortilin were analysed by western immunoblotting. (B) Sortilin knockdown cells were infected with CTL2 (MOI 0.5). At 23 hpi, cells were treated with BODIPY-FL-ceramide and delivery of ceramide followed for 4 h via live cell microscopy. Arrows highlight chlamydial inclusion. Images were captured using a Nikon Ti-E deconvolution microscope with a 40× objective. (C) Line graph represent the shift in mean fluorescent intensity per inclusion analysed by ImageJ. At least 9 inclusions were monitored. Error bar denote mean±s.e.m., N=2. Scale bar 20 µm.
Chlamydia infection stabilises cellular sortilin concentrations
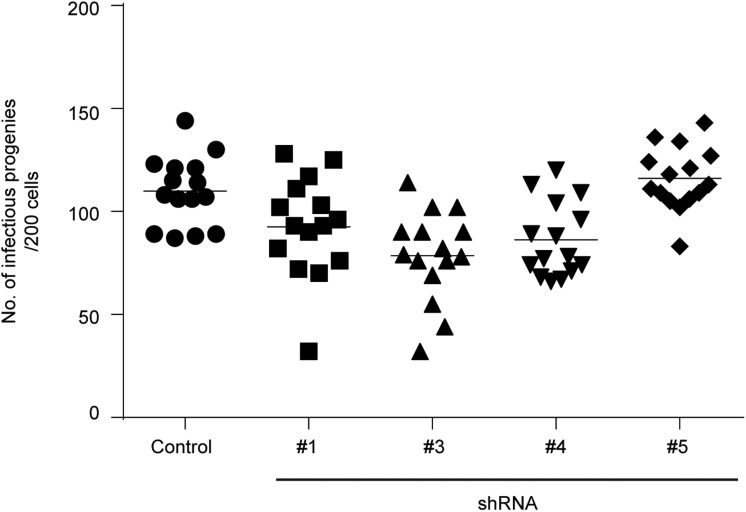
Sortilin is required for the efficient trafficking of soluble cargo like ASM from the Golgi to endosomes and in cells depleted for sortilin the intracellular trafficking of ASM is impaired (Wahe et al., 2010; Ni and Morales, 2006). Delivery of ASM to the chlamydial inclusion would be required for the bacteria to gain access to ceramide generated from the conversion of sphingomyelin, a lipid transported to the inclusion from the Golgi (Hackstadt et al., 1995; Elwell et al., 2011). An infectious progeny assay was performed to assess the impact of sortilin knockdown upon the intracellular development of CTL2 using the four most significantly depleted sortilin knockdown cell lines. Briefly, control and sortilin knockdown cells were infected with GFP-expressing CTL2 for 48 h before lysing the cells. The lysates were then subjected to serial dilution and the diluents used to infect a new population of mCherry-Rab25-expressing HeLa cells for 24 h before being fixed, DAPI labelled and imaged using a Nikon Deconvolution microscope. We observed no significant impact in the growth of the primary inclusions or upon the development of infectious progeny (Fig. 4), implying that sortilin-dependent protein trafficking is not requisite for the intracellular development of C. trachomatis L2.
Fig. 4.
Depletion of sortilin has no significant impact on the development of chlamydial infectious progeny. Control and sortilin knockdown cells were seeded onto 96 well plates and infected with GFP-CTL2 for 48 h. Quantitative infectious progeny assays were performed using a Nikon Ti-E deconvolution microscope with a 20× objective.
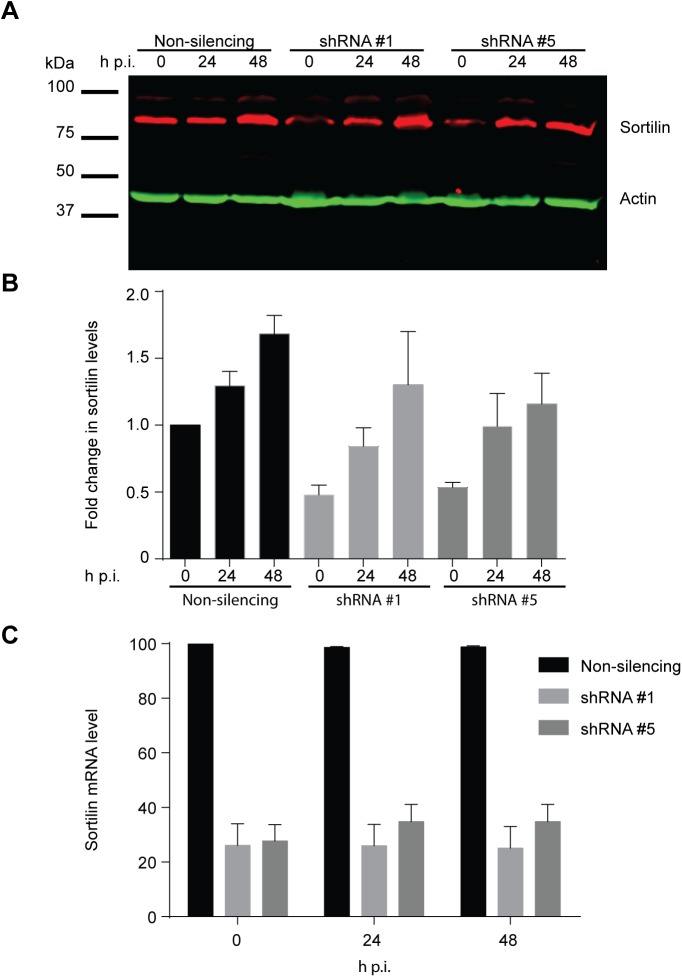
To further examine why RNAi-mediated depletion of sortilin had no impact on the intracellular growth and development of CTL2, we monitored the levels of sortilin in the two most significantly depleted knockdown lines throughout the course of infection. Surprisingly, at 24 hpi, sortilin levels in both knockdown lines had increased to levels comparable with control cells. This increase in sortilin levels was maintained throughout the infection (Fig. 5A,B). Analysis of sortilin mRNA levels by RT-PCR revealed no change during infection (Fig. 5C) suggesting that the observed increase in sortilin protein levels is a consequence of altered protein turnover rather than transcriptional regulation.
Fig. 5.
The level of sortilin protein within infected knockdown cells increases to that observed within control cells. (A) Non-silencing control and sortilin knockdown cells were infected and harvested at stipulated time points. 20 μg of proteins were subjected to western immunoblotting using rabbit polyclonal anti-sortilin and mouse monoclonal anti-beta actin antibodies followed by IR dye conjugated secondary antibodies. Fluorescent intensity captured by Li-Cor Odyssey Infrared Imaging System and (B) quantified using FIJI. (C) RNA from infected cells was extracted and prepared and quantified using Applied Science 7500 Real Time PCR Systems. Housekeeping gene GAPDH was used as an internal control. Error bars denote mean±s.e.m., N=2.
DISCUSSION
Whilst living within a membrane-bound vacuole provides protection from the host cell's innate immune system it also presents inherent challenges, including the need to remodel the host's membrane trafficking pathways to generate a replicative niche that will expand and provide essential nutrients to support replication. The earliest studies of lipid acquisition by Chlamydia demonstrated that it is capable of acquiring lipids from the Golgi (Hackstadt et al., 1996, 1995). However, when infected cells were treated with Brefeldin A, an inhibitor of protein trafficking from the endoplasmic reticulum to the Golgi, only expansion of the inclusion membrane was impaired but not chlamydial growth or development (Hackstadt et al., 1996). This suggests that Chlamydia may be capable of acquiring host lipids required for replication from multiple sources.
Sphingomyelin is important for chlamydial growth and development. When infected cells are treated with myricon, a potent inhibitor of serine palmitoyltransferase, the initial enzyme in the biosynthesis of sphingomyelin, multiple small inclusions are observed suggesting host cell sphingomyelin is required for the homotypic fusion of inclusions (Robertson et al., 2009). Here we reveal that sortilin, which is responsible for transporting ASM to endosomes, is associated with the chlamydial inclusion (Fig. 1) and that sortilin levels increase dramatically through the course of a C. trachomatis L2 infection. To dissect the role of sortilin in the context of a chlamydial infection, cells were depleted of endogenous sortilin using RNAi. When infected knockdown cells were treated with BODIPY-FL-ceramide, no discernible differences in its delivery to the inclusion compared to control cells were observed. Further, no impact on the development and production of infectious progeny was observed. These results suggest that the acquisition of ceramide, generated from sphingomyelin transported from the Golgi, by Chlamydia is independent of sortilin and the cargo proteins, including ASM, that it traffics. Indeed, ceramide acquisition by Chlamydia has been previously linked to CERT (Derre et al., 2011), Golgi fragmentation (Heuer et al., 2009) or interception of vesicles from the Golgi (Derre et al., 2011; Heuer et al., 2009; Hackstadt et al., 1996).
Further investigation revealed that when sortilin knockdown cells are infected with C. trachomatis L2, the protein levels of sortilin increased during the infection to levels similar to that of control cells. RT-PCR revealed no changes in sortilin mRNA levels, in either infected sortilin knockdown or control cells, indicating that sortilin transcriptional activity is not altered by the infection. Therefore, the increase in sortilin protein concentration observed in infected cells is likely a consequence of sequestration of sortilin within the inclusion. Sortilin sequestered within the chlamydial inclusion will not be exposed to degradative lysosomes preventing its normal turnover and newly synthesised sortilin will continue to be delivered to the inclusion from the Golgi. The resultant net increase in sortilin levels in spite of the introduced shRNAs indicates that RNAi-mediated depletion of sortilin is therefore unlikely to be an adequate tool for dissecting its possible role in chlamydial development. Normally sortilin is trafficked from the endosomal compartments to the Golgi via a retromer dependent retrograde trafficking pathway. While retromer was recently identified as enriched components on isolated C. trachomatis inclusions (Aeberhard et al., 2015), it remains unknown whether it forms functional transport carriers from the inclusion.
The possibility of functional redundancy in the trafficking of ASM also remains. Wahe et al. (2010) reported a significant reduction but not complete loss of prosaposin and ASM from phagosomes isolated from Sortilin−/− macrophages, which is consistent with an alternate pathway for trafficking of these proteins to the phagosomes. The mannose-6 phosphate receptor also binds ASM and delivers it to endosomes (Ni and Morales, 2006) and has not only been associated with the chlamydial inclusion but has been proposed to be required for the attachment and internalisation of the pathogen (Vanooij et al., 1997; Puolakkainen et al., 2005). Its presence on the chlamydial inclusion may explain why depleting sortilin alone had no impact on the infection. Targeted disruption of sortilin and/or mannose-6 phosphate receptor may be worth investigating in the future.
MATERIALS AND METHODS
Constructs and reagents
Rabbit polyclonal antibodies against sortilin (Abcam, ab16640, 1:1000), rabbit monoclonal antibody against chlamydial protease/chaperone protein, CtHtrA (Huston et al., 2008) and mouse monoclonal antibodies against beta-actin (Merck Millipore, MAB1501, 1:1000) were used. Secondary antibodies were purchased from Molecular Probes (Life Technologies) and Li-Cor Bioscience. mCherry-Rab25 was obtained by performing restriction digest using restriction enzymes BamHI and EcoRI on GFP-Rab25 (Casanova et al., 1999) to obtain the open reading frame of Rab25 and subcloned into mCherry-C1 following standard protocols. Stably expressing mCherry-Rab25 HeLa cells were generated by transient transfection of mCherry-Rab25 into HeLa cells using Lipofectamine 2000 as per manufacturer's instruction (Invitrogen). Transfected cells were selected using 400 µg/ml of G418 over a period of 14 days to generate stable cell lines expressing mCherry-Rab25. pGIPZ small hairpin RNA (shRNA) plasmids (Thermo Scientific) used included non-silencing control shRNA: RHS4346; human sortilin shRNAs (shRNA #1: V2LHS_31931, shRNA #2: V2LHS_31928, shRNA #3: V3LHS_359770, shRNA #4: V3LHS_359767, shRNA #5: V3LHS_359771) were supplied by the Institute for Molecular Bioscience Life Science Automation (LISA) Facility.
Cell culture, transfection and generation of sortilin knockdown lines
HeLa wild-type (WT) and mCherry-Rab25 stably expressing cells were maintained in DMEM supplemented with 10% (v/v) FCS and 2 mM L-glutamine (Invitrogen) in a humidified air/atmosphere (5% CO2) at 37°C. Cell lines were routinely tested for mycoplasma contamination. Cells were seeded into 6 cm dishes and grown to 70% confluency prior to transfection. pGIPZ plasmids were transfected into cells using Lipofectamine 2000 as per manufacturer's instruction (Invitrogen). Transfected cells were selected for using 1 µg/ml puromycin over a period of 14 days to generate stable cell lines expressing individual shRNAs. shRNA-expressing cells were subsequently cultured in growth media containing 0.5 µg/ml puromycin.
Quantitative RT-PCR
RNA from cells was extracted according to the manufacturer's instructions (Sigma-Aldrich). 1 µg of total RNA was used to produce cDNA using oligdT primers and Superscript III (Invitrogen). Quantitative RT-PCR was conducted using TaqMan gene expression assays for sortilin (SorCS1, Hs00364666_m1, Applied Biosciences) and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs99999905_m1, Applied Biosciences). GAPDH was used as an internal control to calculate the delta cycle threshold (ΔCT) for each sample.
Chlamydial infection
Chlamydia trachomatis L2 (ATCC VR-902B) and GFP-expressing Chlamydia trachomatis L2 were generated as described previously (Wang et al., 2011). Chlamydia was used to infect cells at the indicated multiplicity of infection (MOI). Cells were infected for 2 h in normal DMEM growth media supplemented with 10% FCS (v/v) and 2 mM L-glutamine at 37°C humidified with 5% CO2. After which the media was replaced with fresh growth media and grown to the stipulated time points.
Infectious progeny assay
Infectious progeny assay was performed as previously described (Gonzalez et al., 2014).
Indirect immunofluorescence
HeLa cells grown on coverslips were transiently transfected with mammalian constructs, fixed and stained with indicated antibodies as described previously (Yang et al., 2015). The slides were imaged using a confocal laser scanning microscope (LSM 710, Zeiss) under 63× magnification. Data was processed using FIJI (http://fiji.sc/Fiji) and assembled using Adobe Illustrator CS5.
Western immunoblotting
Cell monolayers were lysed directly with SDS lysis buffer (100 mM Tris/HCl, pH 6.8, 4% SDS, 20% glycerol, 0.02% bromophenol blue, 200 nM dithhiothreitol). Cell lysates were boiled at 95°C for 10 min. Equal amounts of protein were loaded and proteins resolved on 10% SDS-polyacrylamide gels and transferred onto Immobilon-FL PVDF membranes (Millipore, USA) according to the manufacturer's instructions. Western immunoblotting using ECL and Odyssey infrared imaging system (Li-Cor Biosciences) were performed as described previously (Yang et al., 2015). Fluorescence intensities were detected by Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences). Protein analysis and quantification was performed using FIJI.
BODIPY-FL-C5-ceramide labelling
Cells were seeded onto glass bottom 96 well dishes (MatTek Corporation) and infected with C. trachomatis L2 (MOI ∼0.5). At 23 hpi, the infected cells were treated with 1.25 µg/ml BODIPY-FL-C5-ceramide (Molecular Probes, B22650, 1:4000) as previously described (Pagano et al., 1991), and time-lapse videomicroscopy performed using an interval of 5 min on an inverted deconvolution microscope (Nikon Ti-E deconvolution, Nikon) under 40× magnification for 4 h.
Statistical analysis
All data are presented as mean±s.e.m. and analysed with GraphPad Prism (GraphPad Software). For analysis of western blots, FIJI was used to quantify protein intensity and analysed with GraphPad Prism. To measure the delivery of BODIPY-FL-C5-ceramide to the chlamydial inclusion, nine inclusions were measured for each replicate. For infectious progeny assays, five images were captured on a Nikon Ti-E deconvolution microscope at 10× magnification and the number of inclusions quantified using FIJI. Final data represented the cumulative result from two independent experiments.
Acknowledgements
Microscopy was carried out at the Australian Cancer Research Foundation (ACRF)/Institute for Molecular Bioscience (IMB) Dynamic Imaging Facility for Cancer Biology.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
All authors contributed to the conception, design, interpretation of the findings, drafting and preparation of the manuscript. W.X.T. and M.C.K. executed the experiments.
Funding
This work was supported by funding from the National Health and Medical Research Council (NHMRC) of Australia [606788] and Australian Research Council [DP150100364]. M.C.K. is supported by an Australian Research Council Discovering Early Career Researcher Award [DE120102321]. R.D.T. is supported by NHMRC Senior Research Fellowship [APP1041929].
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/suppl/doi:10.1242/bio.016485/-/DC1
References
- Aeberhard L., Banhart S., Fischer M., Jehmlich N., Rose L., Koch S., Laue M., Renard B. Y., Schmidt F. and Heuer D. (2015). The proteome of the isolated Chlamydia trachomatis containing vacuole reveals a complex trafficking platform enriched for retromer components. PLoS Pathog. 11, e1004883 10.1371/journal.ppat.1004883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W. L. (2006). Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 119, 350-359. 10.1242/jcs.02733 [DOI] [PubMed] [Google Scholar]
- Beatty W. L. (2008). Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63. Infect. Immun. 76, 2872-2881. 10.1128/IAI.00129-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland R., Ojcius D. M. and Byrne G. I. (2004). Focus - Chlamydia. Nat. Rev. Microbiol. 2, 530-531. 10.1038/nrmicro931 [DOI] [PubMed] [Google Scholar]
- Carabeo R. A., Mead D. J. and Hackstadt T. (2003). Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc. Natl. Acad. Sci. USA 100, 6771-6776. 10.1073/pnas.1131289100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Wang X., Kumar R., Bhartur S. G., Navarre J., Woodrum J. E., Altschuler Y., Ray G. S. and Goldenring J. R. (1999). Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47-61. 10.1091/mbc.10.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). Sexually Transmitted Disease Surveillance 2013. Atlanta: USA: Department of Health and Human Services. [Google Scholar]
- Derre I., Swiss R. and Agaisse H. (2011). The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog. 7, e1002092 10.1371/journal.ppat.1002092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N., Lacas-Gervais S., Bertout J., Paz I., Freche B., Van Nhieu G. T., van der Goot F. G., Sansonetti P. J. and Lafont F. (2009). Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6, 137-149. 10.1016/j.chom.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Elwell C. A. and Engel J. N. (2012). Lipid acquisition by intracellular Chlamydiae. Cell. Microbiol. 14, 1010-1018. 10.1111/j.1462-5822.2012.01794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell C. A., Jiang S. B., Kim J. H., Lee A., Wittmann T., Hanada K., Melancon P. and Engel J. N. (2011). Chlamydia trachomatis co-opts GBF1 and CERT to acquire host Sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 7, e1002198 10.1371/journal.ppat.1002198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E., Rother M., Kerr M. C., Al-Zeer M. A., Abu-Lubad M., Kessler M., Brinkmann V., Loewer A. and Meyer T. F. (2014). Chlamydia infection depends on a functional MDM2-p53 axis. Nat. Commun. 5, 5201 10.1038/ncomms6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Scidmore M. A. and Rockey D. D. (1995). Lipid metabolism in chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92, 4877-4881. 10.1073/pnas.92.11.4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Rockey D. D., Heinzen R. A. and Scidmore M. A. (1996). Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 15, 964-977. [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Nishijima M., Fujita T. and Kobayashi S. (2000). Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells: a novel evaluation system using an SPT-defective mammalian cell mutant. Biochem. Pharmacol. 59, 1211-1216. 10.1016/S0006-2952(00)00251-3 [DOI] [PubMed] [Google Scholar]
- Hatch G. M. and Mcclarty G. (1998). Phospholipid composition of purified Chlamydia trachomatis mimics that of the eucaryotic host cell. Infect. Immun. 66, 3727-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer D., Lipinski A. R., Machuy N., Karlas A., Wehrens A., Siedler F., Brinkmann V. and Meyer T. F. (2009). Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature 457, 731-735. 10.1038/nature07578 [DOI] [PubMed] [Google Scholar]
- Huston W. M., Theodoropoulos C., Mathews S. A. and Timms P. (2008). Chlamydia trachomatis responds to heat shock, penicillin induced persistence, and IFN-gamma persistence by altering levels of the extracytoplasmic stress response protease HtrA. BMC Microbiol. 8, 190 10.1186/1471-2180-8-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. and Christian J. L. (2011). Sortilin associates with transforming growth factor-beta family proteins to enhance lysosome-mediated degradation. J. Biol. Chem. 286, 21876-21885. 10.1074/jbc.M111.228262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansmann S., Schuette C. G., Bartelsen O., Hoernschemeyer J., Linke T., Weisgerber J. and Sandhoff K. (2003). Human acid sphingomyelinase. Assignment of the disulfide bond pattern. Eur. J. Biochem. 270, 1076-1088. 10.1046/j.1432-1033.2003.03435.x [DOI] [PubMed] [Google Scholar]
- Ni X. and Morales C. R. (2006). The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic 7, 889-902. 10.1111/j.1600-0854.2006.00429.x [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C., Kang H. C. and Haugland R. P. (1991). A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J. Cell Biol. 113, 1267-1279. 10.1083/jcb.113.6.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakkainen M., Kuo C.-C. and Campbell L. A. (2005). Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect. Immun. 73, 4620-4625. 10.1128/IAI.73.8.4620-4625.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. K., Gu L., Rowe R. K. and Beatty W. L. (2009). Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog. 5, e1000664 10.1371/journal.ppat.1000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. C., Cuellar-Mata P., Matsuo T., Davidson H. W. and Grinstein S. (2002). Role of 3-phosphoinositides in the maturation of Salmonella-containing vacuoles within host cells. J. Biol. Chem. 277, 12770-12776. 10.1074/jbc.M110399200 [DOI] [PubMed] [Google Scholar]
- Smith E. L. and Schuchman E. H. (2008). The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 22, 3419-3431. 10.1096/fj.08-108043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanooij C., Apodaca G. and Engel J. (1997). Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect. Immun. 65, 758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Chua J., Lee H.-H., Lucas M., Belisle J. and Deretic V. (2005). Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 102, 4033-4038. 10.1073/pnas.0409716102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahe A., Kasmapour B., Schmaderer C., Liebl D., Sandhoff K., Nykjaer A., Griffiths G. and Gutierrez M. G. (2010). Golgi-to-phagosome transport of acid sphingomyelinase and prosaposin is mediated by sortilin. J. Cell Sci. 123, 2502-2511. 10.1242/jcs.067686 [DOI] [PubMed] [Google Scholar]
- Wang Y., Kahane S., Cutcliffe L. T., Skilton R. J., Lambden P. R. and Clarke I. N. (2011). Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 7, e1002258 10.1371/journal.ppat.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T. E., Petersen C. M. and Nykjaer A. (2008). VPS10P-domain receptors - regulators of neuronal viability and function. Nat. Rev. Neurosci. 9, 899-909. [DOI] [PubMed] [Google Scholar]
- Wylie J. L., Hatch G. M. and Mcclarty G. (1997). Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 179, 7233-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Soderholm A., Lung T. W. F., Giogha C., Hill M. M., Brown N. F., Hartland E. and Teasdale R. D. (2015). SseK3 is a Salmonella effector that binds TRIM32 and modulates the host's NF-kappaB signalling activity. PLoS ONE 10, e0138529 10.1371/journal.pone.0138529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Dodson V. J., Frank M. W. and Rock C. O. (2015). Chlamydia trachomatis scavenges host fatty acids for phospholipid synthesis via an acyl-acyl carrier protein synthetase. J. Biol. Chem. 290, 22163-22173. 10.1074/jbc.M115.671008 [DOI] [PMC free article] [PubMed] [Google Scholar]