Abstract
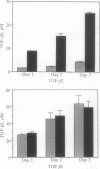
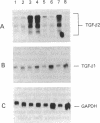
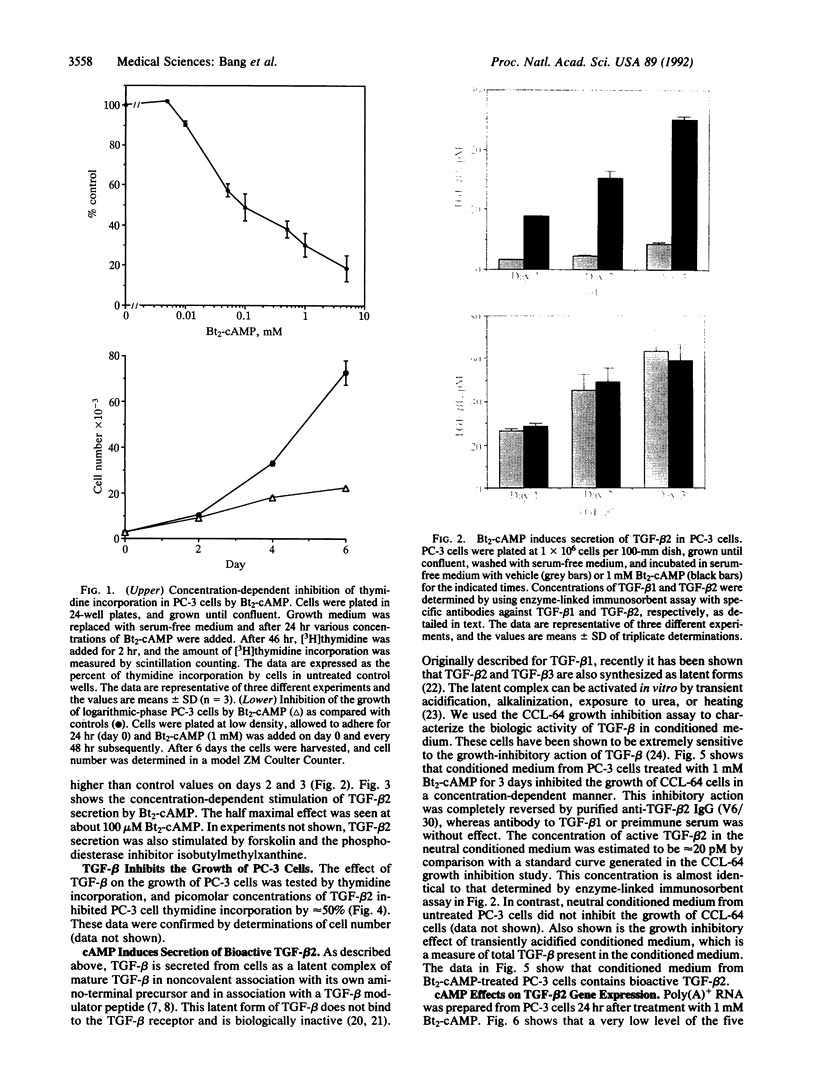
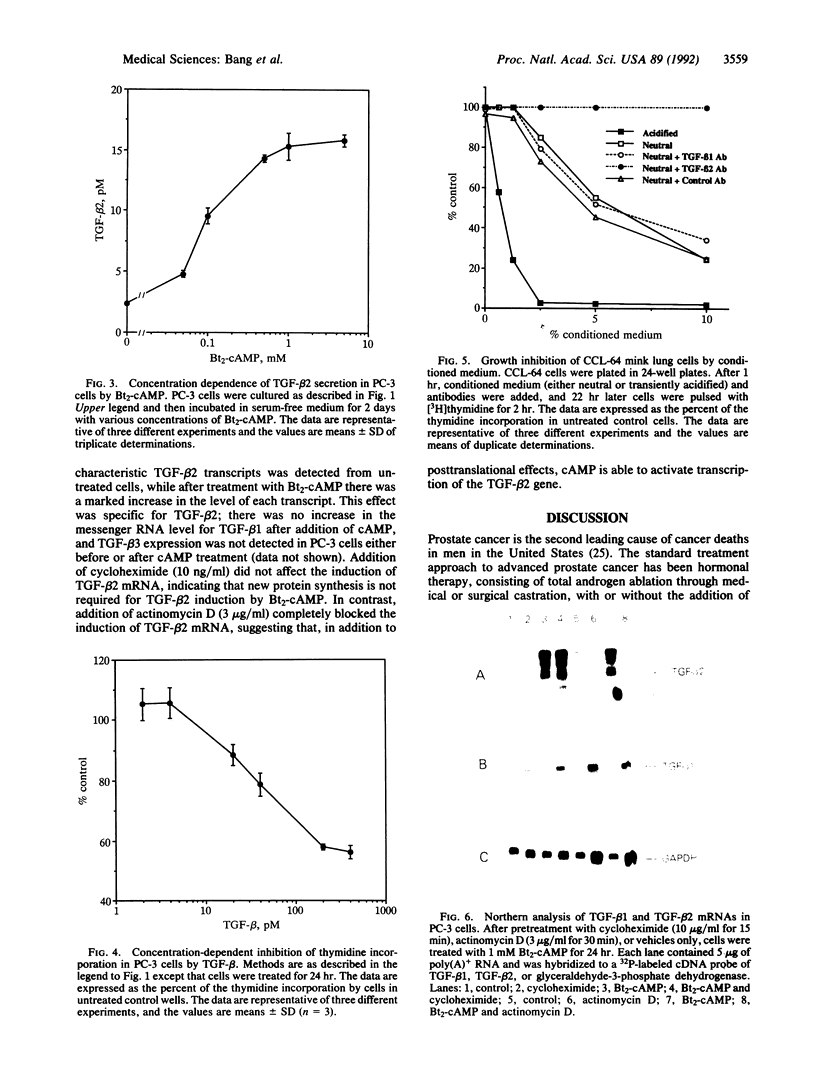
The standard therapy for advanced prostate cancer is androgen ablation. Despite transitory responses, hormonally treated patients ultimately relapse with androgen-independent disease that is resistant to further hormonal manipulation and cytotoxic chemotherapy. To develop an additional approach to the treatment of advanced prostate cancer, we have been studying the signal transductions controlling the growth of human androgen-independent prostate carcinoma cell lines. We report here that elevation of intracellular cAMP markedly inhibits the growth of the hormone-refractory cell line PC-3. To examine the mechanism of cAMP action in PC-3 cells, we tested the effect of the cAMP analog dibutyryl cAMP (Bt2-cAMP) on the regulation of the potent negative growth factor transforming growth factor beta (TGF-beta). Bt2-cAMP selectively induced the secretion of TGF-beta 2 and not TGF-beta 1 by PC-3 cells. This TGF-beta 2 was shown to be bioactive by using the CCL-64 mink lung cell assay. TGF-beta 1 was not activated despite being present at 3-fold higher concentrations than TGF-beta 2. Northern analysis showed that Bt2-cAMP induced an increase in the five characteristic TGF-beta 2 transcripts and had no effect on the level of TGF-beta 1 or TGF-beta 3 transcripts. TGF-beta 2 induction was only weakly enhanced by cycloheximide and was completely inhibited by actinomycin D. These data show that Bt2-cAMP induces the expression of active TGF-beta 2 by PC-3 prostate carcinoma cells, suggesting a new approach to the treatment of prostate cancer and a new molecular mechanism of cAMP action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashall F., Sullivan N., Puck T. T. Specificity of the cAMP-induced gene exposure reaction in CHO cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3908–3912. doi: 10.1073/pnas.85.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1991. CA Cancer J Clin. 1991 Jan-Feb;41(1):19–36. doi: 10.3322/canjclin.41.1.19. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Wakefield L. M., Levinson A. D., Sporn M. B. Physicochemical activation of recombinant latent transforming growth factor-beta's 1, 2, and 3. Growth Factors. 1990;3(1):35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy. Cancer Res. 1990 Nov 15;50(22):7093–7100. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Kim K. Y., Dart L. L., Watanabe S., Roberts A. B., Sporn M. B. Sandwich enzyme-linked immunosorbent assays (SELISAs) quantitate and distinguish two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) in complex biological fluids. Growth Factors. 1989;2(1):61–71. doi: 10.3109/08977198909069082. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Klos D. J., Grant G. A. Purification and biological properties of type beta transforming growth factor from mouse transformed cells. Cancer Res. 1986 Oct;46(10):5153–5161. [PubMed] [Google Scholar]
- Gottesman M. M., Fleischmann R. D. The role of cAMP in regulating tumour cell growth. Cancer Surv. 1986;5(2):291–308. [PubMed] [Google Scholar]
- Hanks S. K., Armour R., Baldwin J. H., Maldonado F., Spiess J., Holley R. W. Amino acid sequence of the BSC-1 cell growth inhibitor (polyergin) deduced from the nucleotide sequence of the cDNA. Proc Natl Acad Sci U S A. 1988 Jan;85(1):79–82. doi: 10.1073/pnas.85.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Lioubin M. N., Marquardt H. Human transforming growth factor type beta 2: production by a prostatic adenocarcinoma cell line, purification, and initial characterization. Biochemistry. 1987 May 5;26(9):2406–2410. doi: 10.1021/bi00383a002. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990 Apr;10(4):1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Lafyatis R., Lechleider R., Kim S. J., Jakowlew S., Roberts A. B., Sporn M. B. Structural and functional characterization of the transforming growth factor beta 3 promoter. A cAMP-responsive element regulates basal and induced transcription. J Biol Chem. 1990 Nov 5;265(31):19128–19136. [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Noma T., Glick A. B., Geiser A. G., O'Reilly M. A., Miller J., Roberts A. B., Sporn M. B. Molecular cloning and structure of the human transforming growth factor-beta 2 gene promoter. Growth Factors. 1991;4(4):247–255. doi: 10.3109/08977199109043910. [DOI] [PubMed] [Google Scholar]
- Pircher R., Lawrence D. A., Jullien P. Latent beta-transforming growth factor in nontransformed and Kirsten sarcoma virus-transformed normal rat kidney cells, clone 49F. Cancer Res. 1984 Dec;44(12 Pt 1):5538–5543. [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Casscells W., Roberts A. B., Sporn M. B. A second messenger RNA species of transforming growth factor beta 1 in infarcted rat heart. Cell Regul. 1991 Mar;2(3):241–249. doi: 10.1091/mbc.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Shacter E., Stadtman E. R., Jurgensen S. R., Chock P. B. Role of cAMP in cyclic cascade regulation. Methods Enzymol. 1988;159:3–19. doi: 10.1016/0076-6879(88)59003-1. [DOI] [PubMed] [Google Scholar]
- Slungaard A., Confer D. L., Schubach W. H. Rapid transcriptional down-regulation of c-myc expression during cyclic adenosine monophosphate-promoted differentiation of leukemic cells. J Clin Invest. 1987 May;79(5):1542–1547. doi: 10.1172/JCI112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J. B., Colamonici O. R., Kelly K., Schwab G., Watt R. A., Sausville E. A., Jaffe E. S., Neckers L. M. Transcriptional inactivation of c-myc and the transferrin receptor in dibutyryl cyclic AMP-treated HL-60 cells. Mol Cell Biol. 1987 Jul;7(7):2644–2648. doi: 10.1128/mcb.7.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Wilding G., Zugmeier G., Knabbe C., Flanders K., Gelmann E. Differential effects of transforming growth factor beta on human prostate cancer cells in vitro. Mol Cell Endocrinol. 1989 Mar;62(1):79–87. doi: 10.1016/0303-7207(89)90115-9. [DOI] [PubMed] [Google Scholar]