Abstract
Several studies have demonstrated chronic opioid analgesic use is associated with increased risk of new onset depression. It is not known if patients with remitted depression are at increased risk of relapse following exposure to opioid analgesics. A retrospective cohort design using patient data from the Veterans Health Administration (VHA; n=5,400), and Baylor Scott & White Health (BSWH; n=842) was performed with an observation period 2002–2012 in VHA and 2003–2012 in BSWH. Eligible patients had a diagnosis of depression at baseline and experienced a period of remission. Risk of depression recurrence was modeled in patients that either started an opioid or remained without opioid prescriptions before or during remission. Cox-proportional hazard models measured the association between opioid use and depression recurrence controlling for pain, and other confounders. Patients exposed to an opioid compared to those unexposed had a significantly greater risk of depression recurrence in both patient populations (VHA: HR=2.17; 95% CI:2.01–2.34; BSWH: HR=1.77; 95% CI:1.42–2.21). These results suggest opioid use doubles the risk of depression recurrence even after controlling for pain, psychiatric disorders and opioid misuse. Further work is needed to determine if risk increases with duration of use. Repeated screening for depression after opioid initiation may be warranted.
Keywords: opioids, depression recurrence, pain, retrospective, epidemiology
INTRODUCTION
Pain and depression are highly comorbid, and this association is likely bi-directional.[5] A large community survey study found that persons with major depression, compared to those without, reported more severe and frequent pain and more pain sites.[15] In a prospective cohort study of remitted depression and anxiety, Gerrits and colleagues’[8] found neck, back, head, orofacial, chest, abdominal and joint pain, and number of pain conditions, measured during remission, were significantly associated with increased risk of depression recurrence. Because pain and depression have been shown to be closely linked and pain is a predictor of depression recurrence, evaluating the independent contribution of opioid analgesic use (OAU) to risk of depression recurrence must be done with rigorous control for confounding by pain.
Depression and opioid use are known to be associated. Patients with depression are more likely to use opioids even when functioning is good.[9] A well-established literature supports the conclusion that patients with chronic pain and comorbid depression are more likely to be prescribed opioids, receive higher doses, use for a longer duration and at higher doses, and misuse or abuse opioids compared to patients without depression.[1; 10; 11; 14; 26] A growing body of literature demonstrates the reverse pattern of association exists; patients with chronic non-cancer pain, prescribed opioids are more likely to develop depression. Specifically, we have previously reported that Veterans Health Administration (VHA) patients receiving opioids for >180 days were 51% more likely to have an incident depression diagnosis, compared to patients exposed for <90 days.[22] More recently, we found that the duration of opioid use, but not morphine equivalent dose (MED) was associated with a two-fold increased risk of incident depression. We found no association between maximum MED, modeled as 1–50 mg, 51–100 mg and >100 mg, and incident depression after controlling for duration of use and pain. We observed up to a two-fold increased risk of incident depression among patients who used for 90 days or longer compared to those who used for 1–30 days. This risk remained after controlling for MED and pain.[20] Our findings were consistent across three separate patient populations drawn from health care systems with large differences in demographics and comorbidity. Importantly our studies demonstrated chronic OAU leads to depression independent of pain, opioid misuse, and psychiatric and physical comorbidity. These studies were conducted with patients selected to be free of a depression diagnosis for two years prior to baseline. Therefore we do not know if opioid use among patients with a recent history of depression promotes depression recurrence. If opioid use is associated with increased risk of depression recurrence, the benefits vs. risks of opioids in chronic, non-cancer pain patients with recent depression will need to be carefully and frequently re-evaluated.
This current study, specifically sought to determine whether: 1) in a cohort of VHA patients in depression remission, initiation of an opioid prescription for non-cancer pain increases risk of recurrence after controlling for confounding from pain and other sources of confounding, and 2) results generalize to a patient cohort derived from a private-sector health care system.
METHODS
Patients
Patient data were obtained from VHA and Baylor Scott & White Health (BSWH) System electronic medical record files. BSWH data is part of the Health Care Systems Research Network (HCSRN; formerly HMORN) Virtual Data Warehouse.[18] VHA medical records included clinic encounters from January 1, 2000 through December 31, 2012, and BSWH data included encounters from January 1, 2003 through December 31, 2012. Patient data used in the present study included ICD-9-CM diagnostic codes, prescription records, vital signs and demographic data.
Eligibility criteria
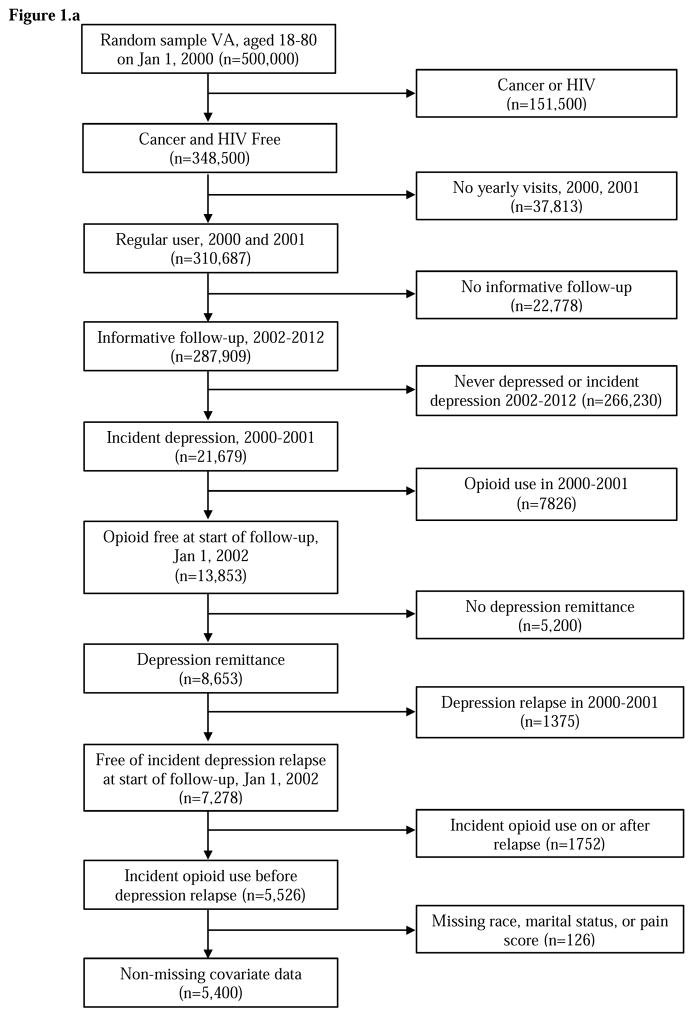
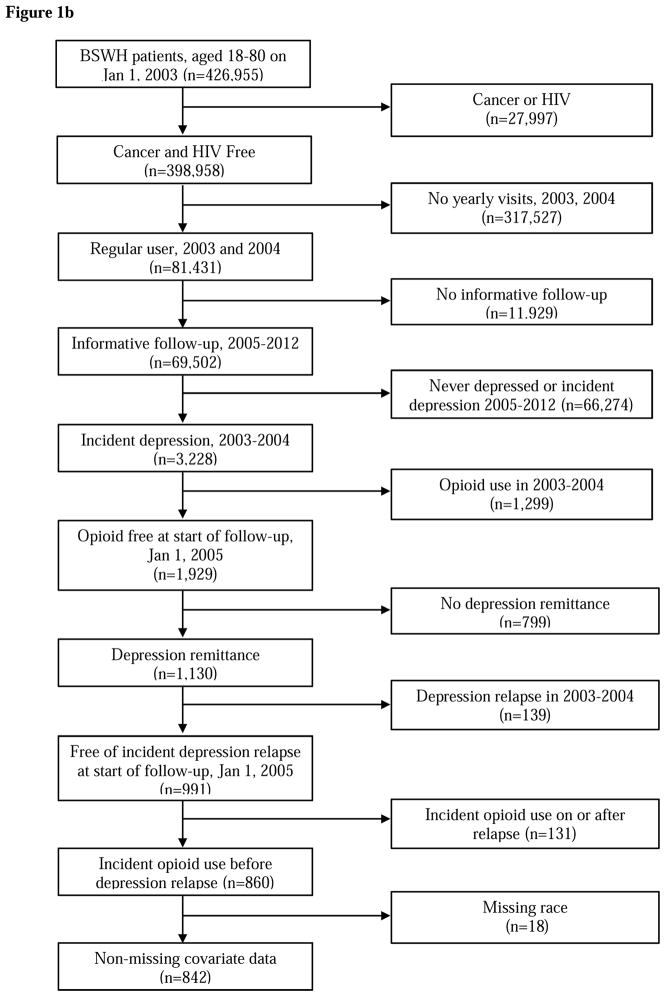
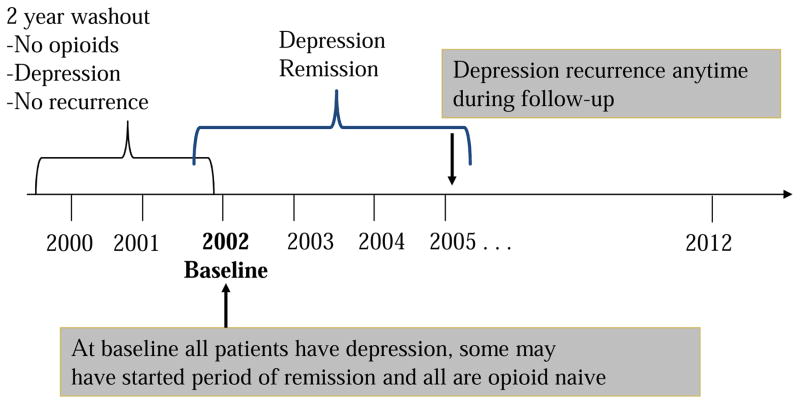
In both patient cohorts, we restricted the cohort to patients 18–80 years of age at baseline and free of a diagnosis for cancer or HIV. Baseline was January 1, 2002 in the VA data and January 1, 2005 in the Baylor Scott & White data. Further, as shown in Figure 1a and 1b, patients must have been regular users, i.e. have a visit at least once each year, of their healthcare system in the two years prior to baseline. These two years prior to baseline are called the “washout period” and were used to exclude patients with existing opioid use and those with remitted depression (e.g., diagnosed with depression in year 1 of washout and remitted in year 2 of washout). Last, patients initiating opioid use on or after the date of depression recurrence were excluded because they were not informative to the temporal order of the research question; that is, to determine if opioid use in remitted patients increases risk of depression recurrence. Eligibility criteria resulted in 5,400 VHA and 842 BSWH patients free of opioid use for at least 2 years and with a depression diagnosis. All patients must have experienced depression remission during follow-up. As shown in Figure 2, some patients could have experienced the beginning of a period of remission at the end of washout and others had yet to enter a period of remission.
Figure 1.
Figure 1a. VA patient eligibility
Figure 1b BSWH patient eligibility
Figure 2.
Opioid exposure variable
Patients were considered opioid-exposed if they received a prescription for any of the following medications: codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, oxycodone, oxymorphone, morphine and pentazocine, for any number of days and any daily dose. We created a binary variable indicating ever receiving an opioid vs. remaining opioid-free during follow-up. We did not have sufficient sample size to estimate the duration of exposure or groups defined by dose.
Outcome variable – depression recurrence
Patients were defined as having depression if they had two or more visits in the same 12-month period and/or 1 hospital discharge diagnosis with an ICD-9-CM code for depression (i.e., 296.2, 296.3 and 311). This algorithm, commonly used in studies of depression in administrative data, has excellent concordance when compared to written medical record[25] and self-report.[6]
The lack of consistently recorded depression severity (e.g. administrations of the PHQ-9) in administrative medical record data precludes defining the course of depression, including onset of remission, relapse and recurrence with the precision outlined in treatment trials.[13] Within the limits of our data we are able to determine when patients were no longer being treated for depression but less able to distinguish remission from recovery, and distinguish relapse, which occurs early in the process of recovery, from recurrence which occurs after many months of remission. In the present study, we acknowledge recurrence may better reflect relapse in some cases and remission may be partial or complete. With this caveat, and in the interest of using a consistent terminology, we defined periods of “remission” and “recurrence” using an algorithm previously reported in studies based on administrative medical record data.[7]
First, a period without depression was defined by at least 120 consecutive days with no record of a depression diagnosis including at least 90 days with no prescription for antidepressants. That is, patients were visiting the medical center but the medical record data indicated four months of care without a diagnosis and three months without pharmacotherapy for depression. Patients meeting this criteria were considered in remission. Depression recurrence was then defined as a new diagnosis for depression after a period of remission. The date of recurrence was the date of an inpatient diagnosis or the date of the first diagnosis when two diagnosis in the same 12-month period defined depression.
Covariates
Both patient data sets contained the same covariate measures with the exception of pain scores and marital status which were only available in VHA data. We selected covariates that might confound the association between opioid initiation and depression recurrence.[14; 16; 24; 26; 27] Importantly, the combination of covariates had to be a good predictor of opioid initiation to compute a propensity score that could be used to balance covariates between opioid users and never users as described in more detail below. Covariates included demographics; psychiatric and substance use disorders, the latter including opioid abuse/dependence; chronic physical conditions associated with depression; painful conditions; self-reported pain scores; and volume of health services use.
Demographics included age, race, gender, and marital status. A measure of insurance coverage was included to control for access to care outside of VHA, and in the private sector data, Medicare served as a proxy for low cost medication coverage. Psychiatric disorders, measured by ICD-9-CM codes, included posttraumatic stress disorder and a composite anxiety disorder variable which included panic disorder, generalized anxiety disorder, social phobia, obsessive-compulsive disorder and anxiety disorder not otherwise specified. Substance use disorder included ICD-9-CM codes for nicotine dependence or a code indicating any history of smoking, alcohol abuse/dependence and any drug abuse/dependence. Physical conditions identified by ICD-9-CM codes included type II diabetes, hypertension, cardiovascular and cerebrovascular disease, obesity, low testosterone and sleep apnea (the latter two variables may be confounders or may be in the causal pathway in that opioids lead to low testosterone and sleep apnea which in turn lead to depression recurrence). Volume of health services utilization defined by the quartile of mean clinic visits per month was used to adjust for detection bias which is the potential for finding a condition or other patient variable due to greater contact with healthcare providers.
Pain was measured by ICD-9-CM diagnosis for over 900 conditions for which an opioid may be prescribed.[23] These conditions were collapsed into five categories: arthritis, back pain, headache, musculoskeletal pain and neuropathic pain. Pain scores, available in VHA data, were measured on a 10-point scale with higher scores designating worse pain. We adjusted for the maximum pain score, i.e. worst pain, reported any time prior to end of follow-up.
Propensity scores (PS) were computed to control for confounding by indication, i.e. pain diagnoses, and to control for other confounders. In this study, the PS is a measure of the probability of a patient being prescribed an opioid. The PS was calculated by a multivariate logistic regression model containing covariates and interaction terms. The model selected for weighting data was determined by optimization of AIC with a c-statistic greater than 0.80. The PS was used to balance covariates in the opioid-treated and untreated groups via conventional Inverse Probability of Treatment Weighting (IPTW) methods.[2; 4; 12; 17] Patient data were weighted by the inverse probability of receiving opioid treatment. IPTW retains all patients and thus was chosen over matching techniques which often results in many lost observations. The model terms used to compute the PS and IPTW in VHA data were the same as those applied in BSWH data. In the VA data the mean weight was 0.98, SD=0.80, minimum=0.48 and maximum=9.59 and in BSWH data the mean weight was 1.01, SD=0.49, minimum=0.50 and maximum=9.34.
Analytic approach
Bivariate analysis included t-tests and chi-square tests computed to measure the associations among opioid use, covariates and depression recurrence. Bivariate associations were computed first in unweighted data and then in weighted data. Results of bivariate analysis using weighted data were evaluated to determine if the PS model and IPTW were successful in balancing potential confounders in opioid-exposed and unexposed patients. This was determined by showing covariates were similarly distributed and not significantly associated with opioid treatment after applying IPTW.
Using unweighted and weighted data, we computed Cox proportional hazard models to estimate the association between opioid treatment and time to depression recurrence. Cox models using weighted data controlled for confounding via weighting and we computed a final model that allowed for further adjustment for each pain condition and maximum pain score that could occur any-time prior to end of follow-up. This additional adjustment controlled for pain after initiation of opioid use. The unit of follow-up time was in months, and the end of follow-up was defined by depression recurrence, last patient encounter or end of the observation period. Cox proportional hazard models were computed using the PHREG procedure in SAS version 9.4 (SAS Institute, Cary, NC) with α set at 0.05. Two-tailed tests were used in all analysis. The proportional hazard assumption was violated in BSWH at the early part of follow-up, however use of time dependent covariates overcomes concerns about violating the assumption. This study was reviewed and approved by the Institutional Review Boards of participating institutions.
RESULTS
Patient characteristics in the VHA and BSWH cohorts are shown in Table 1. On average, compared to BSWH, VHA patients were older (mean=50.4±12.4 vs 42.1±15.2) and predominantly male (86.8% vs. 23.9%). Both patient groups were mostly white (>79%).
Table 1.
Distribution of opioid exposure and covariates by healthcare organization
| Exposure/covariates, n(%) | VA (n=5,400) | BSWH (n=842) | p-value |
|---|---|---|---|
| Age, mean(sd) | 50.4 (±12.4) | 42.1 (±15.2) | <.0001 |
| Gender: male | 4687 (86.8) | 201 (23.9) | <.0001 |
| Race: White | 4292 (79.5) | 676 (80.3) | .591 |
| Insurance | |||
| VA only | 3860 (71.5) | -- | n/a |
| Medicare | -- | 104 (12.4) | |
| Marital status: Married | 2318 (42.9) | -- | n/a |
| Opioid use: Yes | 2809 (52.0) | 395 (46.9) | .006 |
| Psychiatric comorbidities # | |||
| PTSD | 1525 (28.2) | <6 (<1.0) | <.0001 |
| Other anxiety * | 1465 (27.1) | 131 (15.6) | <.0001 |
| Nicotine dependence/history of smoking | 2415 (44.7) | 72 (8.6) | <.0001 |
| Alcohol abuse/dependence | 1917 (35.5) | 36 (4.3) | <.0001 |
| Any illicit drug abuse/dependence | 1470 (27.2) | 19 (2.3) | <.0001 |
| Metabolic/Cardiovasc comorbidities # | |||
| Diabetes Type II | 1587 (29.4) | 122 (14.5) | <.0001 |
| Hypertension | 3530 (65.4) | 314 (37.3) | <.0001 |
| Cardiovascular disease@ | 3854 (71.4) | 424 (50.4) | <.0001 |
| Cerebrovascular disease | 726 (13.4) | 83 (9.9) | .004 |
| Obesity diagnosis | 1882 (34.8) | 164 (19.5) | <.0001 |
| Other comorbidities # | |||
| Low T | 137 (2.5) | 11 (1.3) | .029 |
| Sleep apnea | 440 (8.2) | 73 (8.7) | .608 |
| Painful conditions # | |||
| Arthritis | 3654 (67.7) | 442 (52.5) | <.0001 |
| Back pain | 3049 (56.5) | 419 (49.8) | <.001 |
| Headaches | 1311 (24.3) | 242 (28.7) | .005 |
| Musculoskeletal pain | 2729 (50.5) | 491 (58.3) | <.0001 |
| Neuropathic pain | 1323 (24.5) | 138 (16.4) | <.0001 |
| Maximum pain score, mean (sd) | 8.0 (±2.6) | -- | n/a |
| Healthcare utilization: Top 25th percentile | 1813 (33.6) | 239 (28.4) | .003 |
Opioid morphine equivalent dose (MED) at end of follow-up defined by incident depression, end or incident opioid prescription or last available observation,
Comorbidities occurring before incident depression
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
During the follow-up period, 52.0% of VHA and 46.9% of BSWH patients received at least one opioid prescription. Comorbid psychiatric disorders were more common among VHA patients as were physical health conditions with the exception of sleep apnea which was diagnosed in roughly 8% of both patient groups.
Over 50% of both patient groups had at least one painful condition. Arthritis, back pain and neuropathic pain were more common in VHA patients while headaches and musculoskeletal conditions more often diagnosed in BSWH patients. The average maximum pain score reported by VHA patients was 8.0±2.6, on a ten point scale.
The unweighted distributions of depression recurrence and covariates by opioid exposure are shown in Table 2. The cumulative incidence of depression in exposed and unexposed was similar (57.7% and 60.1%, respectively, p=.08). However, this is an artefact of not accounting for how fast depression onsets in the two groups.. For instance, when examining incidence rate in VA data, results showed that opioid exposed had a higher rate of depression recurrence (IR=130.1/1000 person-years, CI: 123.9–136.6) than non- exposed (IR=114.4/1000 person-years, CI: 108.8–120.2).
Table 2.
Unweighted association of opioid use exposure (OAU) with outcome and covariates, by healthcare organization
| VA (n=5,400) | BSWH (n=842) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome/covariates, n(%) | No OAU (n=2591) | OAU (n=2809) | p-value | No OAU (n=447) | OAU (n=395) | p-value |
| Incident depression recurrence (yes) | 1557 (60.1) | 1621 (57.7) | .075 | 200 (44.7) | 152 (38.5) | 0.066 |
| Age, mean(sd) | 52.2 (±13.5) | 48.7 (±11.1) | <.0001 | 42.0 (±16.2) | 42.3 (±14.0) | 0.776 |
| Gender: male | 2275 (87.8) | 2412 (85.9) | .036 | 111 (24.8) | 90 (22.8) | 0.487 |
| Race: White | 2126 (82.1) | 2166 (77.7) | <.0001 | 355 (79.4) | 321 (81.3) | 0.501 |
| Insurance | ||||||
| VA only | 1773 (68.4) | 2087 (74.3) | <.0001 | -- | -- | -- |
| Medicare | -- | -- | -- | 65 (14.5) | 39 (9.9) | 0.040 |
| Marital status: Married | 1138 (43.9) | 1180 (42.0) | .156 | -- | -- | -- |
| Psychiatric comorbidities a | ||||||
| PTSD | 529 (20.4) | 996 (35.5) | <.0001 | < 5 | < 5 | 0.670 |
| Other anxiety b | 599 (23.1) | 866 (30.8) | <.0001 | 63 (14.1) | 68 (17.2) | 0.212 |
| Nicotine dependence/history of smoking | 967 (37.3) | 1448 (51.6) | <.0001 | 29 (6.5) | 43 (10.9) | 0.023 |
| Alcohol abuse/dependence | 806 (31.1) | 1111 (39.6) | <.0001 | 17 (3.8) | 19 (4.8) | 0.471 |
| Any illicit drug abuse/dependence | 568 (21.9) | 902 (32.1) | <.0001 | 7 (1.6) | 12 (3.0) | 0.151 |
| Metabolic/Cardiovasc comorbidities a | ||||||
| Diabetes Type II | 636 (24.6) | 951 (33.9) | <.0001 | 54 (12.1) | 68 (17.2) | 0.035 |
| Hypertension | 1541 (59.5) | 1989 (70.8) | <.0001 | 152 (34.0) | 162 (41.0) | 0.036 |
| Cardiovascular disease c | 1664 (64.2) | 2190 (78.0) | <.0001 | 205 (45.9) | 219 (55.4) | 0.006 |
| Cerebrovascular disease | 279 (10.8) | 447 (15.9) | <.0001 | 47 (10.5) | 36 (9.1) | 0.496 |
| Obesity diagnosis | 725 (28.0) | 1157 (41.2) | <.0001 | 69 (15.4) | 95 (24.1) | 0.002 |
| Other comorbidities a | ||||||
| Low T | 41 (1.6) | 96 (3.4) | <.0001 | < 5 | < 5 | 0.020 |
| Sleep apnea | 111 (4.3) | 329 (11.7) | <.0001 | 31 (6.9) | 42 (10.6) | 0.057 |
| Painful conditions a | ||||||
| Arthritis | 1357 (52.4) | 2297 (81.8) | <.0001 | 202 (45.2) | 240 (60.8) | <.0001 |
| Back pain | 1024 (39.5) | 2025 (72.1) | <.0001 | 186 (41.6) | 233 (59.0) | <.0001 |
| Headaches | 407 (15.7) | 904 (32.2) | <.0001 | 112 (25.1) | 130 (32.9) | 0.012 |
| Musculoskeletal pain | 833 (32.2) | 1896 (67.5) | <.0001 | 222 (49.7) | 269 (68.1) | <.0001 |
| Neuropathic pain | 396 (15.3) | 927 (33.0) | <.0001 | 54 (12.1) | 84 (21.3) | 0.0003 |
| Maximum pain score, mean (sd) | 6.9 (±3.0) | 9.0 (±1.5) | <.0001 | -- | -- | -- |
| Healthcare utilizationd: Top 25th percentile | 528 (20.4) | 1285 (45.8) | <.0001 | 164 (36.7) | 75 (19.0) | <.0001 |
Comorbidities occurring before incident depression relapse
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
top 25th percentile of the distribution of average health care encounters per month
In VHA patients, opioid exposure was significantly associated with younger age (p<0.0001), and significantly fewer opioid exposed patients were male and white (p<0.05 and p<0.0001, respectively). All psychiatric comorbidities were more common among opioid-exposed compared to non-exposed patients (p<0.0001). Physical health conditions, painful conditions, pain score and health services utilization were all more prevalent among patients receiving at least one opioid prescription compared to those who were not opioid-exposed (p<0.0001). Among BSWH patients, patients prescribed at least one opioid were significantly more likely to have a comorbid health diagnosis (p<0.5 to p<0.0001) with the exception of cerebrovascular disease and sleep apnea. The only psychiatric disorder significantly associated with opioid exposure was nicotine dependence/history of smoking (p<0.05). Last, contrary to the VHA patient sample, opioid-exposed BSWH patients had a significantly lower volume of health care utilization compared to non-exposed (p<0.0001).
After weighting data, covariates were no longer significantly associated with opioid exposure in both VHA and BSWH patients (see Table 3). Mean pain scores among VHA opioid-exposed and unexposed patients were nearly identical (mean=8.0 non-exposed vs. mean=8.2 exposed). Overall, IPTW successfully balanced the distribution of covariates and, importantly, the percentage of painful conditions was nearly the same in opioid-exposed and non-exposed persons in both patient populations.
Table 3.
Weighted by inverse probability of opioid use exposure, association (%) of opioid use exposure (OAU) with covariates, by healthcare organization
| VA (n=5,400) | BSWH (n=842) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariates, (%) | No OAU (n=2591) | OAU (n=2809) | p-value | No OAU (n=447) | OAU (n=395) | p-value |
| Age, mean(sd) | 50.5 (±12.4) | 50.2 (±12.2) | .406 | 42.1 (±15.4) | 42.0 (±14.8) | 0.932 |
| Gender: male | 86.5 | 86.5 | .964 | 24.4 | 22.9 | 0.624 |
| Race: White | 79.3 | 78.6 | .531 | 78.9 | 79.7 | 0.764 |
| Insurance | ||||||
| VA only | 70.3 | 72.0 | .179 | -- | -- | -- |
| Medicare | -- | -- | -- | 12.1 | 12.6 | 0.805 |
| Marital status: Married | 42.7 | 51.6 | .429 | -- | -- | -- |
| Psychiatric comorbidities a | ||||||
| PTSD | 28.6 | 29.4 | .506 | < 5 | < 5 | 0.948 |
| Other anxiety b | 26.7 | 27.5 | .528 | 15.8 | 16.2 | 0.847 |
| Nicotine dependence/history of smoking | 44.4 | 45.8 | .324 | 8.3 | 8.9 | 0.779 |
| Alcohol abuse/dependence | 35.0 | 35.7 | .593 | 4.0 | 4.4 | 0.781 |
| Any illicit drug abuse/dependence | 26.7 | 27.2 | .697 | 1.7 | 2.1 | 0.669 |
| Metabolic/Cardiovasc comorbidities a | ||||||
| Diabetes Type II | 28.4 | 29.7 | .283 | 15.7 | 14.6 | 0.661 |
| Hypertension | 64.7 | 66.0 | .307 | 37.7 | 36.3 | 0.672 |
| Cardiovascular disease c | 72.1 | 73.7 | .199 | 51.2 | 50.3 | 0.800 |
| Cerebrovascular disease | 14.1 | 14.6 | .618 | 9.8 | 8.9 | 0.688 |
| Obesity diagnosis | 35.6 | 35.9 | .814 | 19.0 | 19.5 | 0.866 |
| Other comorbidities a | ||||||
| Low T | 2.7 | 2.6 | .760 | < 5 | < 5 | 0.110 |
| Sleep apnea | 7.8 | 8.3 | .508 | 7.2 | 7.9 | 0.724 |
| Painful conditions a | ||||||
| Arthritis | 67.6 | 69.8 | .089 | 53.7 | 53.7 | 0.995 |
| Back pain | 56.4 | 58.3 | .170 | 51.4 | 51.0 | 0.912 |
| Headaches | 24.4 | 25.8 | .274 | 29.0 | 28.8 | 0.931 |
| Musculoskeletal pain | 50.0 | 52.2 | .109 | 59.6 | 58.9 | 0.840 |
| Neuropathic pain | 23.8 | 24.9 | .347 | 17.2 | 16.2 | 0.709 |
| Maximum pain score, mean (sd) | 8.0 (2.6) | 8.2 (2.2) | .0003 | -- | -- | -- |
| Healthcare utilizationd: Top 25th percentile | 32.8 | 34.8 | .109 | 27.6 | 28.0 | 0.900 |
Comorbidities occurring before incident depression relapse
Other anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS
Cardiovascular disease = hyperlipidemia, ischemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction
top 25th percentile of the distribution of average health care encounters per month
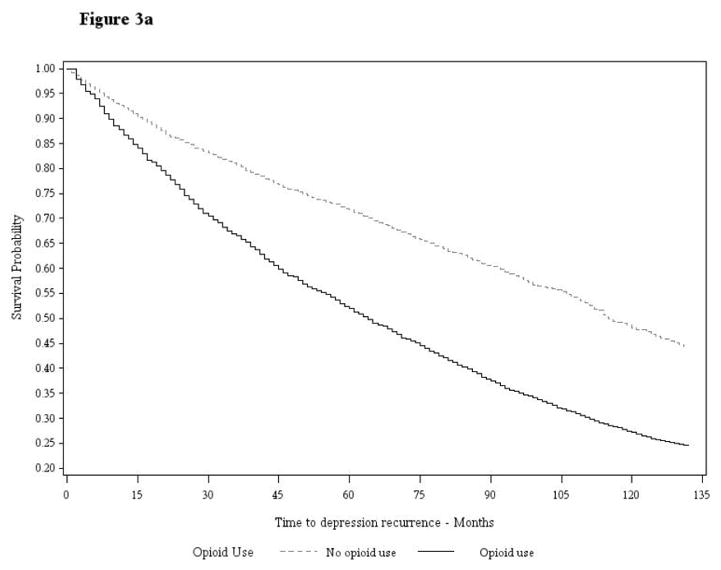
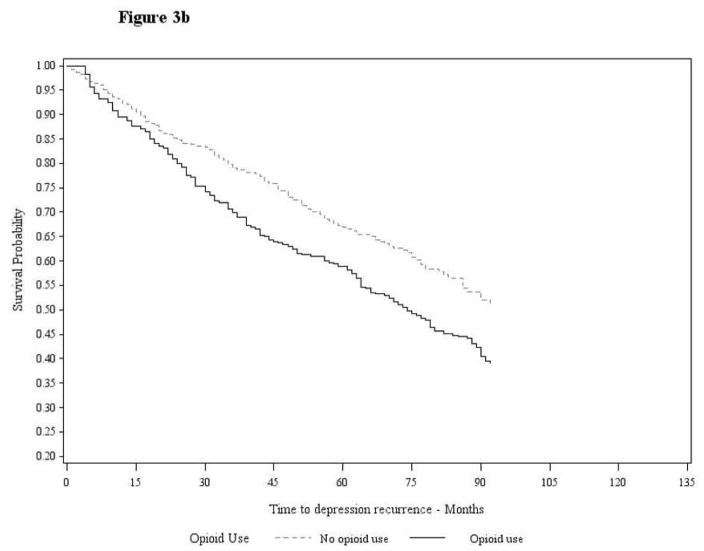
Results of Cox proportional hazard models are shown in Table 4. Prior to weighting data in Model 1, VHA and BSWH patients who initiated an opioid, compared to those that did not, were significantly more likely to have a depression recurrence (HR=1.87; 95% CI:1.74–2.02 and HR=1.50; 95% CI:1.19–1.88, respectively) in this univariate model. In Model 2, after correcting for confounding via data weighting, but without additional covariate adjustment, the association between opioid initiation and depression recurrence increased and remained significant in VHA and BSWH patient samples. The magnitude of association between opioid exposure and depression recurrence remained similar in Model 3 which included additional adjustment for painful conditions, and pain scores in VHA data, that may have occurred between initiation of an opioid and depression recurrence. In this model, we observed both VHA and BSWH opioid-exposed patients had up to a two-fold increased risk of depression recurrence relative to unexposed counterparts (HR=2.17; 95% CI:2.01–2.34 in VHA and HR=1.77; 95% CI:1.42–2.21 in BSWH). Survival curves illustrating the association between opioid exposure and time to depression recurrence are shown in Figure 3a (VA patients) and 3b (BSWH patients).
Table 4.
Association (HR (95% CI))1 between opioid use and incident depression relapse, unweighted and weighted by inverse probability of opioid use exposure
| VA patients (n=5,400) | BSWH patients (n=842) | |||||
|---|---|---|---|---|---|---|
| Model 1 Unweighted data | Model 2 Weighted data | Model 3 Weighted data + pain | Model 1 Unweighted data | Model 2 Weighted data | Model 3 Weighted data + pain | |
| Opioid use: yes | 1.87 (1.74–2.02) | 2.20 (2.04–2.37) | 2.17 (2.01–2.34) | 1.50 (1.19–1.88) | 1.81 (1.46–2.26) | 1.77 (1.42–2.21) |
| Arthritis | -- | -- | 0.95 (0.88–1.04) | -- | -- | 1.01 (0.80–1.29) |
| Back pain | -- | -- | 1.30 (1.20–1.41) | -- | -- | 1.09 (0.87–1.36) |
| Headache | -- | -- | 1.09 (1.00–1.18) | -- | -- | 1.44 (1.14–1.80) |
| Musculoskeletal pain | -- | -- | 1.12 (0.92–1.11) | -- | -- | 1.16 (0.92–1.48) |
| Neuropathy | -- | -- | 1.01 (0.92–1.11) | -- | -- | 1.04 (0.75–1.44) |
| Pain Score | -- | -- | 0.98 (0.97–1.00) | -- | -- | -- |
HR=hazard ratio; CI=confidence interval
Figure 3.
Figure 3a. Survival Curve Veterans Health Administration Patient Data
Figure 3b. Survival Curve Baylor Scott & White Patient Data
Supplemental analysis
We computed post-hoc analysis in the VHA patient cohort to determine if risk of recurrence differed in patients who began opioids before entering a period of remission versus initiating while in a period of remission. Among 2,809 opioid starts in VHA patients, 30.8% occurred before remission (n=865) and 69.2% occurred during the remission period (n=1,944). We computed an unadjusted survival model comparing risk of depression recurrence among patients who started opioids before remission versus those who started while in remission. Results indicated starting opioids while depressed was associated with a significant risk of depression recurrence compared to starting opioids during remission (HR=1.87; 95% CI:1.70–2.07). This finding is preliminary as confounders were not balanced between these two groups. Although sample size prohibited computing survival models with an exposure variable for the duration of opioid use, we observed that the distribution of VHA patients who used for 1–30 days, 31–90 days and >90 days was similar in those who did and did not experience recurrence. Among the VHA patients who experienced a depression recurrence, 75.5% used opioids for 1–30 days, 12.7% used for 31–90 days and 11.8% used opioids for >90 days. Among VHA patients that did not experience a depression recurrence, 75.6% used opioids for 1–30 days, 13.6% for 31–90 days and 10.8% for > 90 days. We also found morphine equivalent dose was similarly distributed in patients with and without depression recurrence.
Last, to overcome immortal time bias, we computed the association between duration of opioid use in VA data and risk of recurrence. by restricting opioid use to start at least one year after remission. In balanced data, compared to patients who used for 1–30 days, we found 31–90 days of continuous use and >90 days of continuous opioid use were not significantly associated with increased risk of recurrence but point estimates were in the direction of increasing risk with longer duration of use (HR=1.04; 95%CI:0.80–1.35 and HR=1.17; 95%CI:0.88–1.58).
DISCUSSION
In two large patient cohorts with large differences in demographics and comorbidity burden, we observed that patients who were in a period of depression remission had an approximately two-fold greater risk of depression recurrence if they initiated an opioid medication compared to patients who remained unexposed to opioids. While a growing body of evidence supports the association between longer duration of opioid use and increasing risk of new-onset depression[21; 22], to our knowledge, this study is novel in demonstrating that opioid therapy increases the risk of depression recurrence among patients with remitted depression.
Additional research is necessary to determine the mechanisms underlying our findings. However, one potential explanation is that opioid exposure acts to prevent full remission and then opioids worsen partly remitted depression which leads to recurrence. Patients who start opioid medication prior to a period of remission may experience partial remission and be more vulnerable to worsening depression and recurrence. While limited, supplemental analysis indicated opioid initiation while depressed compared to when in remission was associated with increased risk of recurrence (HR=1.87). This provides limited evidence that risk of recurrence may be driven by opioid use leading to incomplete remission which in turn places patients at risk for recurrence.
In this study, we did not find evidence for an association between longer duration or higher dose of opioids and increased risk of depression recurrence. This contrasts with our studies of incident depression in which longer duration was associated with increasing risk [19; 20]. We speculate that the role of opioid dose and duration in risk of incident depression may not apply to risk of depression recurrence for which risk may not be increased as a function of dose or longer duration. As suggested by supplementary data analysis, there is very limited evidence that duration is associated with risk of recurrence.
Limitations
Opioid exposure was based on prescription fills and it is impossible to know whether patients took their medications as prescribed. If patients did not use their prescription, and were misclassified as opioid exposed, the error would bias results to the null and our hazard ratios may actually under-estimate risk of recurrence.
The algorithms for remission and recurrence are imperfect indicators of the course of disease. If consistently available for all patients, repeated, monthly assessments of depression symptoms would have allowed for more precise measures of remission and recurrence; however, such data are rarely available in retrospective medical record databases. In the VA patient sample, 638 had PHQ-9 scores during remission and recurrence. Of these, 259 were not exposed to opioids and 379 did initiate an opioid. On average PHQ-9 scores among opioid free patients were 10.9±7.6 during remission and 10.9±7.6 at recurrence compared to 11.5±7.1 during remission and 11.4±7.3 during recurrence in opioid exposed patients. A PHQ-9 score of 10 is the cut-point for moderate depression and these values may point to re-initiating treatment for those who did not experience full remission. These data are limited to a small subsample of the VHA patients and do not include pre-remission depression severity measures. While these PHQ-9 scores raise the possibility that opioid exposure is not associated with limited remission, they could also indicate that patients with more persistent symptoms are also the patients receiving multiple PHQ-9 measures. In sum, these data were insufficient to determine if opioids limit depression remission. Further research with prospective data collection is needed to understand how opioid use impacts the course of depression.
Conclusions
Prescription opioid use among patients with a recent history of depression increases the chance of recurrence and this effect is independent of pain diagnoses and pain intensity scores. In addition to monitoring pain patients for new onset depression, clinicians should be aware and discuss the probability of depression recurrence with patients considering opioid therapy.
As of 2013, in the developed world, back pain and depression were ranked number 1 and 2 in the top ten causes of years lived with disability.[3] The burden of disease due to these conditions may be increased if opioid treatment for chronic pain, especially back pain, leads to persistent depression. The present findings highlight a particularly challenging situation faced by clinicians and pain patients and point to the need for careful consideration of depression in the assessment of benefit and risk. To improve clinical outcomes and for public health, the current study highlights the need to develop effective non-opioid pain medication and non-pharmacological therapies for chronic pain.
Perspective.
In two large patient cohorts with large differences in demographics and comorbidity, patients with remitted depression, exposed to opioid analgesics were 77% to 117% more likely to experience a recurrence of depression than those who remained opioid free. Routine, not just at initiation of treatment, screening for depression is warranted.
Acknowledgments
Funding: This study was supported by the National Institute of Mental Health, Prescription Opioid Analgesics and Risk of Depression, R21MH101389.
Footnotes
Conflict of interest: none
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. General hospital psychiatry. 2009;31:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GBoDS. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 doi: 10.1016/S0140-6736(15)60692-4. published on-line June, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analysis with observational databases. Medical Care. 2007;45:S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 5.Fishbain D, Cutler R, Rosomoff H, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. The Clinical journal of pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Frayne SM, Miller DR, Sharkansky EJ, Jackson VW, Wang F, Halanych JH, Berlowitz DR, Kader B, Rosen CS, Keane TM. Using adminstrative data to identify mental illness: What approach is best? American Journal of Medical Quality. 2010;25:42–50. doi: 10.1177/1062860609346347. [DOI] [PubMed] [Google Scholar]
- 7.Garfield LD, Scherrer JF, Chrusciel T, Nurutdinova D, Lustman PJ, Fu Q, Burroughs TE. Factors associated with receipt of adequate antidepressant pharmacotherapy in a population of VA patients with recurrent depression. Psychiatric Services. 2011;62:381–388. doi: 10.1176/ps.62.4.pss6204_0381. [DOI] [PubMed] [Google Scholar]
- 8.Gerrits MMJG, van Oppen P, Leone SS, van Marwijk HWJ, van der Horst HE, Pennix BW. Pain, not chronic disease, is associated with the recurrence of depression and anxiety disorders. BMC Psychiatry. 2014;14:187. doi: 10.1186/1471-244X-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goesling J, Henry MJ, Moser SE, Rastogi M, Hassett AL, Clauw DJ, Brummett CM. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. The Journal of Pain. 2015;16:844–851. doi: 10.1016/j.jpain.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Annals of family medicine. 2012;10:304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. General hospital psychiatry. doi: 10.1016/j.genhosppsych.2013.10.003. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick RDGD, Brookhart MA, Polley E, Rothman KJ, Bradbury BD. Exploring large weight deletion and the ability to balance confounders when using inverse probability of treatment weighting in the presence of rate treatment decisions. Pharmacoepidemiology and drug safety. 2013;22:111–121. doi: 10.1002/pds.3297. [DOI] [PubMed] [Google Scholar]
- 13.Kupfer DJ. Long-term treatment of depression. Journal of Clinical Psychiatry. 1991;52(5 suppl):28–34. [PubMed] [Google Scholar]
- 14.Merrill JO, Von Korff M, Banta-Green CJ, Sullivan MD, Saunders KW, Campbell CI, Weisner C. Prescribed opioid difficulties, depression and opioid dose among chronic opioid therapy patients. General hospital psychiatry. 2012;34(6):581–587. doi: 10.1016/j.genhosppsych.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohayon MM, Schatzberg AF. Chronic pain and major depressive disorder in the general population. Journal of Psychiatric Research. 2010;44:454–461. doi: 10.1016/j.jpsychires.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clinical Journal of Pain. 2005;21:422–431. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika Trust. 1983;70:41–55. [Google Scholar]
- 18.Ross TR, Ng D, Brown JS, Pardee R. The HMO Research Network Virtual Data Warehouse: A public data model to support collaboration. eGEMS (Generating Evidence and Methods to improve patient outcomes. 2014;2:1–8. doi: 10.13063/2327-9214.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, Lawler EV, PJL Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29:491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherrer JF, Salas J, Copeland LA, Stock EM, Ahmedani BK, Sullivan M, Burroughs T, Schneider FD, Bucholz KK, Lustman PJ. Prescription opioid duration, not dose, is associated with increased risk of depression in three large patient populations. Ann Fam Med. doi: 10.1370/afm.1885. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherrer JF, Salas J, Lustman PJ, Burge S, Schneider FD Texas RRNo. Change in opioid dose and change in depression in a longitudinal primary care patient cohort. Pain. 2015;156:348–355. doi: 10.1097/01.j.pain.0000460316.58110.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, Lawler EV, Lustman PJ. Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29:491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US Veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 24.Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD) Pain Physician. 2012;15:ES145–ES156. [PubMed] [Google Scholar]
- 25.Solberg LI, Engebretson KI, Sperl-Hillen JM, Hroscikoski MC, O’Connor PG. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease and depression. American Journal of Medical Quality. 2006;21:238–245. doi: 10.1177/1062860606288243. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 27.Tsuang MT, Tohen M. Textbook in Psychiatric Epidemiology. 2. New York, NY: Wiley-Liss; 2002. [Google Scholar]