Abstract
Pre-transplant remission status in patients with acute myeloid leukemia (AML) is one of the most important factors determining their outcomes after allogeneic hematopoietic cell transplantation (allo-HCT). Most patients are in complete remission with full hematologic recovery (CR) prior to undergoing allo-HCT. However, some patients achieve CR without recovery of platelet count (CRp) or a morphologic leukemia-free state (MLFS), defined as meeting all CR criteria without recovery of both neutrophil and platelet counts. Currently, there is a paucity of data regarding transplant outcomes in AML patients achieving MLFS after chemotherapy. To address this question, we evaluated transplant outcomes in 270 AML patients who received 6/6 HLA-matched sibling or 10/10 HLA-matched unrelated donor transplantation at a single institution between 2006 and 2013. Of our 270 patients, 206 were in complete remission (CR), 45 were in complete remission with incomplete platelet count recovery (CRp), and 19 were in MLFS prior to allo-HCT. Patients in CR, CRp, or MLFS had similar 3-year overall survival (49%, 46%, and 47%, respectively; P = 0.88) and 3-year event-free survival (45%, 36%, and 40%, respectively; P = 0.53). However, the cumulative incidence of non-relapse mortality (NRM) was significantly higher in patients in MLFS compared to those in CR (58% vs. 22%, P =0.0004), while the cumulative incidence of relapse in patients in MLFS was significantly lower compared to those in CR (11% vs. 36%, P = 0.03). Our results suggest that survival outcomes in AML patients are not influenced by degree of hematologic recovery prior to allo-HCT.
Keywords: AML, transplantation, hematologic recovery, pre-transplantation remission status, morphologic leukemia-free state, complete remission
INTRODUCTION
Despite the advancements of biomedical knowledge and treatment of acute myeloid leukemia (AML), patients with AML continue to have poor survival outcomes with 5-year overall survival (OS) still close to only 25%. The prognosis is worse for patients older than 60 years, whose 5-year OS is only 5–10%1. With new knowledge on molecular and genomic abnormalities such as mutations in the FLT3, TP53, IDH1/2, TET2, and MLL genes, we also know that these AML-specific factors affect overall prognosis2–12. In addition to age and genetic mutations, remission status following chemotherapy is also important in determining prognosis. Achievement of complete remission (CR) following induction treatment has been shown to correlate with improved survival in AML patients13. Part of the definition of CR requires hematologic recovery including a platelet count greater than 100,000/uL and absolute neutrophil count greater than 1000/uL14. In contrast, studies have shown that complete remission with incomplete hematologic recovery (CRi) is associated with reduced overall survival and increased risk of relapse13,15–19. However, most of these studies focused predominantly on patients who did not receive allogeneic hematopoietic cell transplantation (allo-HCT). Furthermore, there is currently a paucity of data on outcomes in AML patients who achieve morphologic leukemia-free state (MLFS), defined as meeting all CR criteria except for hematologic recovery (platelet count < 100,000/uL and absolute neutrophil count < 1000/uL)14,20. It is currently a common practice to wait for complete hematologic recovery before proceeding with allo-HCT in most AML patients. However, this approach potentially increases the risk of infectious and non-infectious (bleeding, transfusion-related adverse events, etc.) complications, which could potentially make these patients ineligible for transplant and consequently jeopardize their chances of long-term survival. To address the question of whether achieving MLFS adversely affects survival and relapse in AML patients who undergo allo-HCT, we retrospectively analyzed the post-transplant outcomes of AML patients based on the extent of hematologic recovery following pre-transplant chemotherapy.
METHODS
Study population
The study included a total of 503 consecutive AML patients who underwent their first allo-HCT at Washington University Medical Center in St. Louis between 2006 and 2013. This study was approved by the Institutional Review Board of Washington University School of Medicine, St. Louis.
Patient, donor, and transplant characteristics were collected and retrospectively entered into the Washington University School of Medicine, Blood and Marrow transplant database. Of the 503 patients, data from 270 patients was analyzed based on the following eligibility criteria: (1) 6/6 match at HLA loci A, B, and DRB1 by low-resolution genotyping21 in related donor transplantation or 10/10 match at HLA loci A, B, C, DRB1, and DQB1 by high-resolution genotyping22 in unrelated donor transplantation; (2) use of unmodified stem cells/non-manipulated grafts; and (3) no evidence of active disease (bone marrow blasts < 5%) based on last bone marrow biopsy prior to transplant.
The type of conditioning regimen was classified based on consensus definition of conditioning regimen intensity23. Reduced intensity and non-myeloablative regimens were grouped together under the reduced intensity conditioning (RIC) cohort.
The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score was calculated for all patients and categorized into 3 risk groups: low risk defined as score of 0, intermediate risk defined as score of 1–2, and high risk defined as score of 3 or greater24.
Definitions
Based on hematologic recovery prior to initiating pre-transplant conditioning, patients were classified into 3 cohorts: (1) complete remission (CR); (2) complete remission with incomplete platelet count recovery (CRp); and (3) morphologic leukemia-free state (MLFS). CR was defined as follows: (1) bone marrow blasts less than 5%; (2) absolute neutrophil count greater than 1000/uL; (3) platelet count greater than 100,000/uL; (4) absence of blasts with Auer rods; (5) absence of extramedullary disease; and (6) independence of red cell transfusions, according to response criteria from the International Working Group14. CR was not further classified into cytogenetic CR or molecular CR. CRp was defined as meeting all CR criteria except for platelet count less than 100,000/uL. MLFS was defined as meeting all CR criteria except for a combination of absolute neutrophil count less than 1000/uL and platelet count less than 100,000/uL. The pre-transplant bone marrow was also evaluated for the persistence of cytogenetic (i.e., translocations, chromosomal deletions) and molecular (i.e., FLT3, NPM1, CEBPA mutations) abnormalities present at the time of original diagnosis.
Acute graft-versus-host disease (aGVHD) was diagnosed based on signs and symptoms and graded according to accepted criteria25. Chronic GVHD (cGVHD) was graded using National Institutes of Health consensus criteria26.
Etiology of AML was classified into (1) de novo AML or (2) secondary AML, defined as occurring from treatment (radiation, alkylating agents, topoisomerase inhibitors) and bone marrow disorders such as myeloproliferative neoplasm (MPN) or myelodysplastic syndrome (MDS)14,27. AML was classified into good, intermediate, and poor prognostic cohorts based on the European LeukemiaNet classification scheme for cytogenetic and molecular genetic data20.
Post-Transplantation Disease Monitoring
Engraftment of the donor cells was determined by PCR assay for short tandem repeats (STRs) or fluorescence in situ hybridization (FISH) from bone marrow samples and/or peripheral blood mononuclear cells28. Complete donor engraftment was defined as the presence of less than 5% of recipient cells at 30 days after transplant. Mixed chimerism was defined as presence of greater than 5% but ≤ 95% of recipient cells. Patients underwent bone marrow biopsies after allo-HCT at 30 days, 100 days, and then every 6 months or earlier if peripheral blood counts showed abnormal findings concerning for relapse. Disease in remission after transplant was defined as absence of excess blasts on bone marrow biopsy 30 days after transplant. Extramedullary disease or relapse was defined by presence of blasts in tissue biopsy or cerebrospinal fluid.
Study Endpoints and Statistical Analysis
The study end points included 3-year overall survival (OS); 3-year event-free survival (EFS); and cumulative incidences of relapse, non-relapse mortality (NRM), aGVHD, and cGHVD. OS was defined as the time from transplant to death from any cause or last follow-up. Those patients alive were censored at the last follow-up. EFS was defined as the time from transplant to relapse or death without relapse, whichever occurred first, while those patients alive and free of disease were censored at the last follow-up14. The distributions of demographic and clinical characteristics across the 3 cohorts (CR, CRp, and MLFS) were compared using Chi-square test, Kruskall-Wallis rank-sum test, or one-way ANOVA as appropriate. Survival curves by remission status were estimated using the Kaplan-Meier product-limit method, and the differences in OS or EFS at 3 years were compared using Klein's pseudo-value approach29. To assess whether remission status was an independent predictor of OS and EFS, propensity-score matching was used to adjust for potential confounding effects of patient characteristics30. The propensity scores for achieving complete remission were estimated using multivariate logistic regression, including age, donor-patient sex mismatch, disease etiology, disease status at transplant, disease classification by cytogenetics, conditioning regimen, transplant type, and anti-thymocyte globulin (ATG) regimen. A 3:1 matching (e.g., identifying 3 matched patients from CR cohort for every patients in MLFS cohort) was used for comparing CR versus MLFS cohorts, while a 1:1 matching was used for CR versus CRp cohorts. The cumulative incidences of NRM and relapse were calculated using Gray’s sub-distribution method to account for the presence of competing risks31. All analyses were two-sided, and significance was set at a P-value of 0.05. Statistical analyses were performed using statistical packages cmprsk (http://biowww.dfci.harvard.edu/~gray) for competing risk analysis and SAS 9.3 (SAS Institutes, Cary, NC) for all other analyses.
RESULTS
Patient Characteristics
The distribution of patients among these cohorts was as follows: 206 in CR, 45 in CRp, and 19 in MLFS. Patient, disease, and transplant characteristics of these cohorts are summarized in Table 1. In our entire patient cohort, the median age was 54 years old (range, 17 to 74 years old). There was no significant difference in median age or time between last chemotherapy and transplant among the 3 individual cohorts. Likewise, there was no significant difference in distribution by gender, disease prognosis, disease etiology, and type of transplant. There were five significant differences between the cohorts. First, there were more female donor/male recipient transplants in the MLFS cohort than in the CR and CRp cohorts (P = 0.007). Second, there was a lower percentage of patients in the CRp cohort who underwent a myeloablative conditioning regimen than in the CR and MLFS cohorts (P = 0.024). Third, there was a higher percentage of patients in the CRp cohort with a high HCT-CI score (3 or greater) than in the CR and MLFS cohorts (P = 0.024). Fourth, there was a higher percentage of patients in the MLFS cohort with pre-transplant bone marrow cellularity of 10% or less than in the CR or CRp cohorts (P < 0.001). However, the range of bone marrow cellularity was wide in all cohorts, with a max cellularity of 80% in the MLFS cohort, 90% in the CR cohort, and 70% in the CRp cohort (data not shown). Fifth, there was a higher percentage of patients in the MLFS cohort who had persistent cytogenetic and/or molecular abnormalities in the pre-transplant bone marrow (P < 0.001).
TABLE 1.
Patient, Donor, and Transplant Characteristics
| All Patients | Patients in CR | Patients in CRp | Patients in MLFS | P value | |
|---|---|---|---|---|---|
| Number of patients (%) | 270 | 206 (76%) | 45 (17%) | 19 (7%) | |
| Median patient age, yr (range) |
54 (17–74) | 53 (17–72) | 58 (21–74) | 57 (24–73) | 0.085 |
| Patient sex (%) | |||||
| Female | 124 (46) | 101 (49) | 18 (40) | 5 (26) | 0.112 |
| Male | 146 (54) | 105 (51) | 27 (60) | 14 (74) | |
| Donor sex (%) | |||||
| Female | 93 (34) | 71 (34) | 13 (29) | 9 (47) | 0.364 |
| Male | 177 (66) | 135 (66) | 32 (71) | 10 (53) | |
| Donor-Patient sex (%) | |||||
| Female-Male | 44 (16) | 29 (14) | 7 (16) | 8 (42) | 0.007 |
| Other | 226 (84) | 177 (86) | 38 (84) | 11 (58) | |
| Disease etiology (%) | |||||
| De novo | 212 (79) | 166 (81) | 31 (69) | 15 (79) | 0.228 |
| Secondary | 37 (14) | 25 (12) | 8 (18) | 4 (21) | |
| Therapy related | 21 (7) | 15 (7) | 6 (13) | 0 (0) | |
| Disease classification by cytogenetics (%) |
|||||
| Favorable | 26 (10) | 21 (10) | 3 (7) | 2 (11) | 0.392 |
| Intermediate | 163 (60) | 123 (60) | 26 (58) | 14 (73) | |
| Poor | 77 (29) | 59 (29) | 16 (35) | 2 (11) | |
| Unknown | 4 (1) | 3 (1) | 0 (0) | 1 (5) | |
| Time from last chemotherapy to transplant, days (range) |
62 (16–366) | 61 (16–366) | 76 (66–198) | 61 (20–273) | 0.079 |
| Pre-transplant bone marrow cellularity ≤ 10% (%) |
37 (14) | 18 (8.7) | 10 (22) | 9 (47) | <0.001 |
| Persistent cytogenetic and/or molecular abnormalities on pre- transplant bone marrow (%) |
37/244 (15) | 24/183 (13) | 7/42 (17) | 6/19 (32) | <0.001 |
| HCT-CI score (%) | |||||
| Low risk | 28 (11) | 26 (13) | 1 (3) | 1 (5) | 0.024 |
| Intermediate risk | 47 (17) | 37 (18) | 6 (13) | 4 (21) | |
| High risk | 195 (72) | 143 (69) | 38 (84) | 14 (74) | |
| Conditioning regimen (%) | |||||
| MA | 193 (72) | 155 (76) | 25 (56) | 13 (68) | 0.024 |
| RIC | 76 (28) | 50 (24) | 20 (44) | 6 (32) | |
| Unknown | 1 (0) | 1 (0) | 0 (0) | 0 (0) | |
| ATG regimen (%) | |||||
| No | 206 (76) | 160 (78) | 31 (69) | 15 (79) | 0.437 |
| Yes | 64 (24) | 46 (22) | 14 (31) | 4 (21) | |
| Transplant type (%) | |||||
| MRD | 104 (39) | 78 (38) | 18 (40) | 8 (42) | 0.913 |
| MUD | 166 (61) | 128 (62) | 27 (60) | 11 (58) | |
| Stem cell source (%) | |||||
| Peripheral blood | 254 (94) | 191 (93) | 45 (100) | 18 (95) | 0.195 |
| Bone marrow | 15 (6) | 14 (7) | 0 (0) | 1 (5) | |
| Immune prophylaxis (%) | |||||
| MTX, MMF, tacrolimus | 33 (12) | 23 (11) | 8 (18) | 2 (11) | 0.801 |
| MTX, tacrolimus | 213 (79) | 165 (80) | 33 (73) | 15 (78) | |
| Other1 | 24 (9) | 18 (9) | 4 (9) | 2 (11) | |
| CMV Reactivation (%) | |||||
| Yes | 134 (50) | 97 (47) | 24 (53) | 13 (68) | 0.118 |
| No | 136 (50) | 109 (53) | 21 (47) | 6 (32) | |
MRD, matched related donor; MUD, matched unrelated donor; MA, myeloablative; RIC, reduced intensity and non-myeloablative; ATG, anti-thymocyte globulin; MTX, methotrexate; MMF, mycophenolate mofetil.
Other includes cyclosporine and sirolimus.
The types of chemotherapy regimens immediately prior to transplant were relatively similar in distribution for the 3 cohorts, except that high-dose cytarabine was less commonly used in the MLFS cohort than in the CR and CRp cohorts, while CLAG- or FLAG-based regimens were less commonly used in the CR cohort than in the MLFS and CRp cohorts. These results are summarized in Table 2.
TABLE 2.
Last chemotherapy prior to transplant
| Patients in CR, number (%) |
Patients in CRp, number (%) |
Patients in MLFS (%) |
P value | |
|---|---|---|---|---|
| MEC1 | 27 (13%) | 5 (11%) | 2 (11%) | <0.001 |
| HiDAC2 | 92 (45%) | 16 (36%) | 1 (5%) | |
| CLAG or CLAG-M3 | 4 (2%) | 4 (9%) | 1 (5%) | |
| FLAG, FLAG-I, FLAG-IM4 |
11 (5%) | 7 (16%) | 3 (16%) | |
| Decitabine | 11 (5%) | 2 (4%) | 2 (11%) | |
| Other5 | 61 (30%) | 11 (24%) | 10 (52%) |
MEC = Mitoxantrone, etoposide, cytarabine
HiDAC = High-dose cytarabine
CLAG = Cladribine, cytarabine, filgrastim; CLAG-M = CLAG plus mitoxantrone
FLAG = Fludarabine, cytarabine, filgrastim; FLAG-I = FLAG plus idarubicin; FLAG-IM = FLAG-I plus gemtuzumab ozogamicin
Includes clofarabine, all-trans retinoic acid, sorafenib, plerixafor, 5+2, 7+3, gemtuzumab ozogamicin alone, mitomycin, imatinib, and azacitidine.
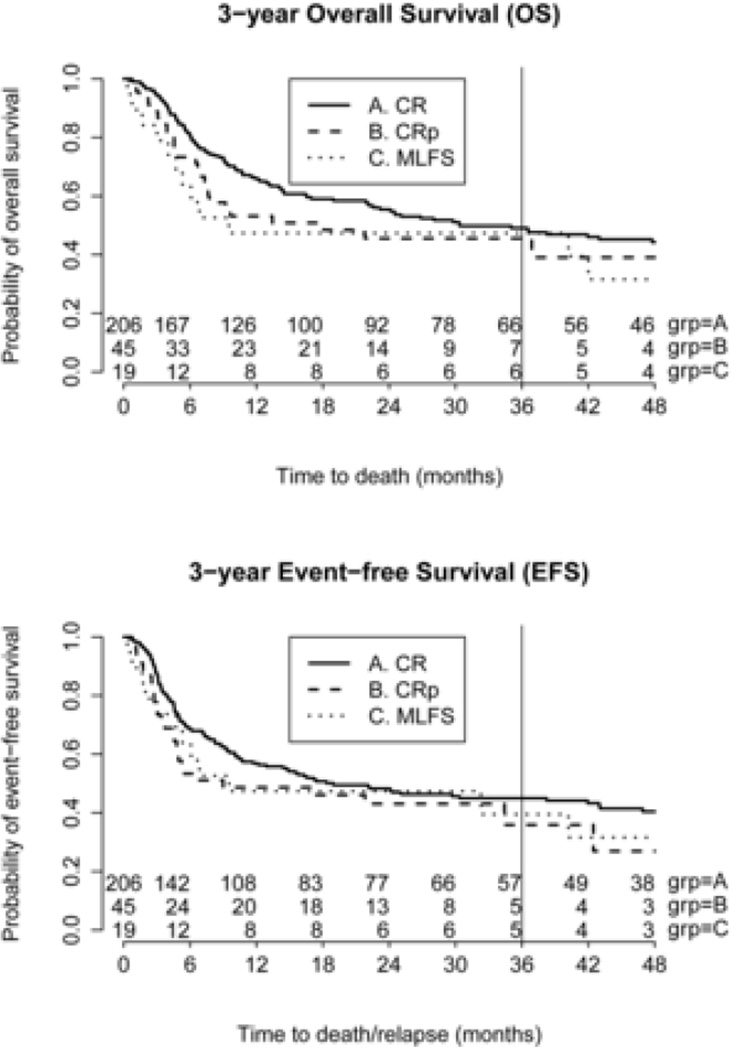
3-year Overall Survival and Event-Free Survival
Median follow-up for the CR, CRp, and MLFS cohorts were 524, 410, and 290 days, respectively. The upper panel of Figure 1 described the differences in OS using data from all patients, and the 3-year OS for the CR, CRp, and MLFS cohorts were 49%, 46%, and 47%, respectively (p=0.88). After adjusting for the effects of other demographic and clinical characteristics using propensity-score matching, we did not find any significant difference in 3- year OS between the MLFS and CR cohorts (P = 0.73). Similarly, we did not find any significant difference between the CRp and CR cohorts (P = 0.62).
Figure 1.
OS and EFS stratified for the 3 cohorts.
The lower panel of Figure 1 described the differences in EFS using data from all patients, and the 3-year EFS for the CR, CRp, and MLFS cohorts were 45%, 36%, and 40%, respectively (p=0.53). After propensity-score matching, we did not find any significant difference in EFS between the MLFS and CR cohorts (P = 0.84). Similarly, we did not find any significant difference between the CRp and CR cohorts (P = 0.40).
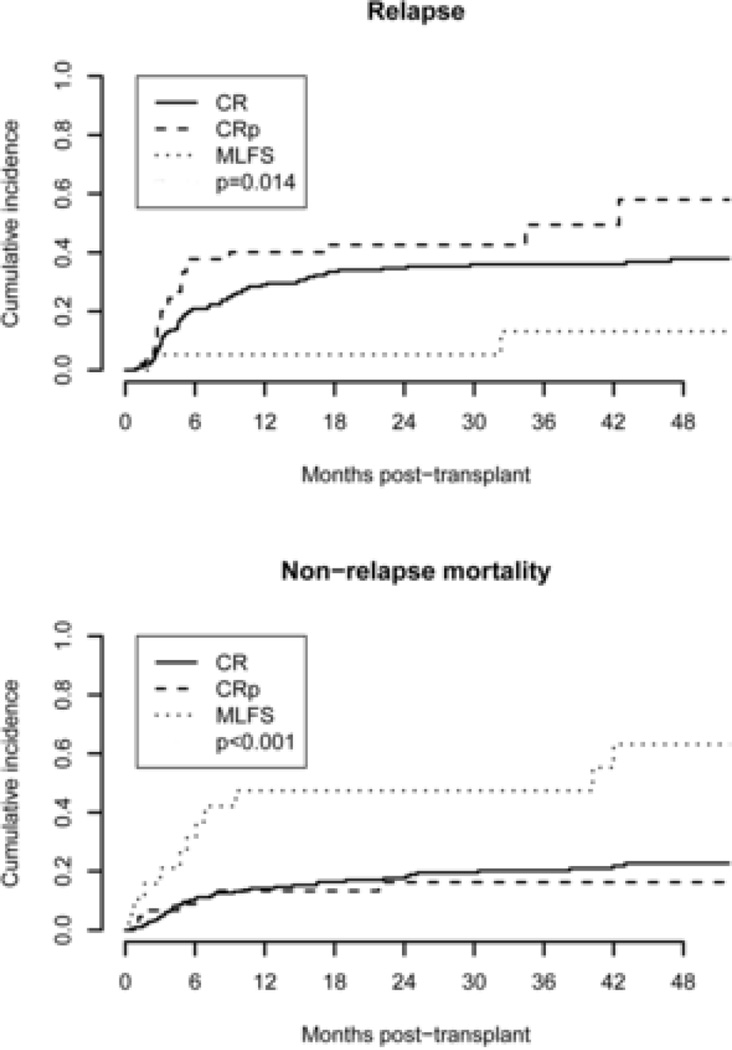
NRM, Relapse and GVHD
The cumulative incidence of relapse in the MLFS cohort was 11%, significantly lower than 36% (P = 0.03) for those in CR and 47% (P = 0.004) for those in CRp (Fig. 2). However, the cumulative incidence of NRM in the MLFS cohort was 58%, significantly higher than 22% (P = 0.0004) for those in CR and 16% (P = 0.002) for those in CRp (Fig. 2). Within the MLFS cohort, 8/13 (62%) patients who received myeloablative conditioning experienced NRM compared to 3/6 (50%) patients who received reduced intensity conditioning (P > 0.99). The causes of death included infection, sepsis, recurrence or progression of leukemia, graft failure, and acute and chronic GVHD. There was a higher percentage of patients in the MLFS cohort who died from causes other than GVHD, infection, sepsis, graft failure, and relapse or progression of leukemia. Those deaths included 2 from multiple organ failure, 1 from accidental death, 1 from sinusoidal obstruction syndrome, 1 from cardiac arrest, and 1 from unknown cause. Two of these deaths (1 cardiac arrest, 1 sinusoidal obstruction syndrome) occurred before remission and engraftment were documented. The results are summarized in Table 3.
Figure 2.
Cumulative incidences of relapse and NRM for the 3 cohorts.
TABLE 3.
Cause of Death
| Cause of Death | Patients in CR, number (%) |
Patients in CRp, number (%) |
Patients in MLFS, number (%) |
P value |
|---|---|---|---|---|
| Acute GVHD | 13 (12) | 0 (0) | 3 (24) | 0.012 |
| Chronic GVHD | 6 (5) | 3 (12) | 0 (0) | |
| Infection or sepsis | 17 (15) | 3 (12) | 2 (15) | |
| Relapse or progression of disease |
59 (53) | 17 (65) | 2 (15) | |
| Graft failure | 1 (1) | 1 (4) | 0 (0) | |
| Other1 | 15 (14) | 2 (7) | 6 (46) | |
| Total | 111 | 26 | 13 |
Other includes multiple organ failure, sinusoidal obstruction syndrome, cardiac arrest, intracranial hemorrhage, and unknown.
The cumulative incidence of grade 2–4 aGVHD in the CR, CRp, and MLFS cohorts were 38%, 38%, and 42%, respectively (P = 0.93). Likewise, the cumulative incidence of cGVHD in patients in the CR, CRp, and MLFS cohorts were 34%, 38%, and 16%, respectively (P = 0.22).
DISCUSSION
Our results suggest that AML patients in CRp or MLFS prior to allo-HCT have similar overall and event-free survival compared to patients in CR, suggesting that hematologic recovery following pre-transplant chemotherapy does not significantly influence survival outcomes. Previous studies have shown that incomplete hematologic recovery (CRi) is associated with reduced overall survival and increased risk of relapse13,15–19. However, these studies did not include outcomes in AML patients with MLFS in which neither absolute neutrophil count nor platelet count has recovered. Our results seem to suggest that allo-HCT overcomes the negative impact that poor hematologic recovery might have on survival in AML patients. In fact, a study by Sievers et al. showed similar overall survival in gemtuzumab-treated patients who received allo-HCT, whether or not they were in CR or CRp32. It is not clear why there was a higher incidence of NRM in our MLFS cohort. One possibility is the type of conditioning regimen prior to allo-HCT as there seems to be a higher rate of NRM in patients who received myeloablative conditioning compared to those who received non-myeloablative or reduced intensity conditioning, though this did not reach statistical significance possibly due to the small cohort size. There was also a higher percentage of female donor-to-male recipient (FM) transplants in our MLFS cohort which could have also contributed to high NRM in this cohort consistent with a recent published study33. Furthermore, differences in HCT-CI or performance status were unlikely to have contributed to higher NRM in MLFS patients as we did not find any significant differences in these measures among the three cohorts. Similarly, there is no obvious explanation for the lower relapse rates seen in this cohort, though it is possible that use of myeloablative conditioning regimens and female donor-to-male recipient transplants also contributed to lower relapse rates in these patients.
There are possible explanations why some patients have poor hematologic recovery after chemotherapy. These include the number of pre-existing comorbidities, type of chemotherapy used, and the persistence of cytogenetic and/or molecular abnormalities on the pre-transplant bone marrow. Our CR cohort had the lowest percentage of patients with an HCT-CI score of 3 or greater. There was also a significant difference in the distribution of types of chemotherapy regimens used in our three cohorts. Some chemotherapy regimens might be more myelosuppressive than others. In fact, Martin et al. showed that the addition of gemtuzumab ozogamicin to FLAG-I chemotherapy was associated with more thrombocytopenia with a CRp rate of 27% compared to 13% in patients who underwent FLAG-I by itself17. Furthermore, failure to recover counts might be a harbinger of residual leukemia as suggested by a higher percentage of patients in our MLFS cohort with persistent cytogenetic and/or molecular abnormalities. Recently, several studies have shown the negative impact of minimal residual disease (MRD) on post-transplant outcomes in AML patients34–36. We did not assess the pre-transplant MRD status in any of the patient cohorts, which could have potentially influenced our results.
In summary, our results suggest that in AML patients undergoing allo-HCT, degree of hematologic recovery after pre-transplant chemotherapy has no significant effect on survival outcomes. Thus, it might not be necessary to wait for a prolonged period of time for complete hematologic recovery prior to transplant, thereby mitigating the potential risk of infectious and non-infectious complications which are relatively common in patients with prolonged cytopenias. Furthermore, MLFS in AML patients could indicate persistence of cytogenetic and/or molecular abnormalities. Lower relapse rates observed in our MLFS cohort are encouraging, however the higher incidence of NRM (> 50%) suggests that allo-HCT might best be reserved for MLFS patients who still have good performance status and low co-morbidity scores.
-
-
Allogeneic hematopoietic cell transplantation provides acceptable long-term survival in AML patients with CR, CRp and MLFS.
-
-
There is a higher persistence of cytogenetic and molecular abnormalities in AML patient with MLFS.
-
-
MLFS in AML patients is associated with high NRM and therefore transplant conditioning regimens should be carefully chosen in these patients.
Acknowledgments
The authors also wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: K.V., S.M., and R.R., planned the study design and interpreted data. K.V. and R.R. prepared the dataset and drafted the manuscript. F.G performed the statistical analysis. K.V., S.M., J.F.D., F.G., K.E.S-G., G.L.U., P.W., R.V., M.A.S., C.N.A., A.F.C., T.A.F., N.A.B., and R.R interpreted data and critically reviewed the manuscript. All authors approved the final manuscript.
REFERENCES
- 1.Mrózek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J. Clin. Oncol. 2012;30(36):4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2014 doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyas P, Appelbaum FR, Craddock C. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2015;21(1):8–15. doi: 10.1016/j.bbmt.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Marcucci G, Yan P, Maharry K, et al. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J. Clin. Oncol. 2014;32(6):548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley TJ, Mardis ER, Ding L, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010;363(25):2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaidzik V, Döhner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin. Oncol. 2008;35(4):346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Niederwieser C, Kohlschmidt J, Volinia S, et al. Prognostic and biologic significance of DNMT3B expression in older patients with cytogenetically normal primary acute myeloid leukemia. Leukemia. 2014 doi: 10.1038/leu.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamadani M, Awan FT, Copelan EA. Hematopoietic stem cell transplantation in adults with acute myeloid leukemia. Biol. Blood Marrow Transplant. 2008;14(5):556–567. doi: 10.1016/j.bbmt.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol. Blood Marrow Transplant. 2007;13(6):655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Greef GE, van Putten WLJ, Boogaerts M, et al. Criteria for defining a complete remission in acute myeloid leukaemia revisited. An analysis of patients treated in HOVON-SAKK co-operative group studies. Br. J. Haematol. 2005;128(2):184–191. doi: 10.1111/j.1365-2141.2004.05285.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J. Clin. Oncol. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 16.Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104(7):1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 17.Martin MG, Augustin KM, Uy GL, et al. Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am. J. Hematol. 2009;84(11):733–737. doi: 10.1002/ajh.21545. [DOI] [PubMed] [Google Scholar]
- 18.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M.D. Anderson Cancer Center Study. J. Clin. Oncol. 2010;28(10):1766–1771. doi: 10.1200/JCO.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alatrash G, Pelosini M, Saliba RM, et al. Platelet recovery before allogeneic stem cell transplantation predicts posttransplantation outcomes in patients with acute myelogenous leukemia and myelodysplastic syndrome. Biol. Blood Marrow Transplant. 2011;17(12):1841–1845. doi: 10.1016/j.bbmt.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 21.Ottinger HD, Ferencik S, Beelen DW, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA26 mismatched related donors, and HLA-matched unrelated donors. Blood. 2003;102(3):1131–1137. doi: 10.1182/blood-2002-09-2866. [DOI] [PubMed] [Google Scholar]
- 22.Speiser DE, Tiercy JM, Rufer N, et al. High resolution HLA matching associated with decreased mortality after unrelated bone marrow transplantation. Blood. 1996;87(10):4455–4462. [PubMed] [Google Scholar]
- 23.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol. Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli G, Trabetti E, Farabegoli P, et al. Early detection of bone marrow engraftment by amplification of hypervariable DNA regions. Haematologica. 82(2):156–160. [PubMed] [Google Scholar]
- 29.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat. Med. 2007;26(24):4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 31.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999;94(446):496–509. [Google Scholar]
- 32.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 2001;19(13):3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 33.Kongtim P, Di Stasi A, Rondon G, et al. Can a Female Donor for a Male Recipient Decrease the Relapse Rate for Patients with Acute Myeloid Leukemia Treated with Allogeneic Hematopoietic Stem Cell Transplantation? Biol. Blood Marrow Transplant. 2015;21(4):713–719. doi: 10.1016/j.bbmt.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137–144. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J. Clin. Oncol. 2011;29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oran B, Popat U, Rondon G, et al. Significance of persistent cytogenetic abnormalities on myeloablative allogeneic stem cell transplantation in first complete remission. Biol. Blood Marrow Transplant. 2013;19(2):214–220. doi: 10.1016/j.bbmt.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]