Abstract
Pancreatic acinar cells of adult mice (≥P60) are resistant to transformation by some of the most robust oncogenic insults including expression of K-Ras oncogenes and loss of p16Ink4a/p19Arf or Trp53 tumor suppressors. Yet, these acinar cells yield pancreatic intraepithelial neoplasias (mPanIN) and ductal adenocarcinomas (mPDAC) if exposed to limited bouts of non-acute pancreatitis, providing they harbor K-Ras oncogenes. Pancreatitis contributes to tumor progression by abrogating the senescence barrier characteristic of low-grade mPanINs. Attenuation of pancreatitis-induced inflammation also accelerates tissue repair and thwarts mPanIN expansion. Patients with chronic pancreatitis display senescent PanINs, if they have received anti-inflammatory drugs. These results put forward the concept that anti-inflammatory treatment of people diagnosed with pancreatitis may reduce their risk of developing PDAC.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the tumors with worst prognosis, with less than 5 percent of patients surviving five years after diagnosis. During the last few years there have been important advances in our understanding of the molecular events responsible for the development of PDAC (Jones et al., 2008; Hruban and Adsay, 2009). Yet, progress in prevention, early diagnosis and therapeutic treatment of PDAC has not experienced major advances (Hidalgo, 2010).
The development of genetically modified mouse tumor models of PDAC offers the possibility to replicate in experimental systems the multiple events that lead to this complex disease (Hingorani et al., 2003; Guerra et al., 2007). In these models, inducible expression of a resident K-Ras oncogene during embryonic development triggers preneoplastic pancreatic intraepithelial lesions (mPanINs), which can progress into invasive mPDAC. Addition of other mutations observed in human PDAC, including inactivation of the P16INK4A/P19ARF, TRP53 or SMAD4 tumor suppressors as well as activation of the HEDGEHOG signaling pathway, significantly accelerate tumor development leading to acquisition of a metastatic phenotype (Aguirre et al., 2003; Hingorani et al., 2005; Bardeesy et al., 2006; Ijichi et al., 2006; Pasca di Magliano et al., 2006).
Human PDAC is likely to originate from somatic mutations in K-RAS during adulthood rather than during embryonic development. We have previously shown that activation of the resident K-Ras oncogene in adult mice blocks its ability to induce mPanINs and mPDAC (Guerra et al., 2007). Recently, it has also been shown that rat adult acinar cells are also refractory to transformation by Ras oncogenes (Tanaka et al., 2010). The resistance of adult acinar cells to K-Ras oncogenes may stem from the exhaustion of permissive acinar progenitors or from intrinsic changes in the biology of acinar cells during adulthood.
In this study we have examined whether the resistance of adult acinar cells to malignant transformation by K-Ras oncogenes may result from the existence of a proliferative and/or senescence barrier mediated by p16Ink4a, a gene frequently inactivated in human PDAC, or by other tumor suppressors also implicated in PDAC development such as Trp53 (Collado et al., 2005). In addition, we have interrogated whether limited episodes of pancreatitis may cooperate with K-Ras oncogenes to induce mPDAC and have unveiled some of the mechanisms by which pancreatitis contributes to mPanIN development. Finally, we have extended these observations to human biopsies obtained from pancreatitis and PDAC patients.
Results
Adult acinar cells are resistant to multiple oncogenic insults
Expression of an endogenous K-Ras oncogene in acinar cells of K-Ras+/LSLG12Vgeo mice (designated from now on as K-Ras+/G12V) during late embryonic development (E16.5) leads to the generation of acinar-to-ductal metaplasias followed by the appearance of low and high-grade mPanINs that occasionally progress to mPDAC (Guerra et al., 2007). The neoplastic nature of these lesions has been validated by other investigators (Hruban et al., 2006). The percentage of K-RasG12V-expressing acinar cells susceptible of inducing mPanIN lesions become significantly reduced during early postnatal development (Figure S1A). In agreement with other laboratories (Friedlander et al., 2009, Morris et al., 2010), induction of K-RasG12V expression at three (P21) and six (P42) weeks of age resulted in progressive reduction in the number of mice that developed mPanINs and to a significant delay in the onset of mPDAC. Acinar cells become resistant to mPanIN development when mice become 2 month old (P60) (Figure S1). Other groups have described frequent mPanIN induction upon expression of K-Ras oncogenes in acinar cells of 6 week-old mice (De la O et al., 2008; Habbe et al., 2008). Although the bases for these variations are unknown, it is possible that genetic background may determine the precise timing at which postnatal acinar cells become resistant to transformation by K-Ras oncogenes.
Next, we examined whether this resistance could be overcome by inactivation of the oncogene-induced senescence (OIS) barrier likely to be mediated by tumor suppressors such as p16Ink4a/p19Arf or Trp53 (Collado and Serrano, 2010). Compound K-Ras+/G12V;Elas-tTA/tetO-Cre;p16Ink4a/p19Arflox/lox (n=13) and K-Ras+/G12V;Elas-tTA/tetO-Cre;Trp53lox/lox (n=7) mice were exposed to doxycycline from conception until P60 to prevent expression of the Elastase-driven Cre recombinase. Removal of doxycycline from the drinking water allowed K-RasG12V expression in adult acinar cells along with concomitant ablation of the p16Ink4a/p19Arflox and Trp53lox conditional alleles, respectively (Figure 1A,B). Mice were examined for the presence of pancreatic lesions six and twelve months after removal of doxycycline. Surprisingly, none of the pancreata presented detectable lesions including metaplasias or low-grade mPanINs in spite of serial analysis of the entire organ (Figure 1A,B). Excision of the conditional p16Ink4a/p19Arflox and Trp53lox alleles in K-RasG12V-expressing acinar cells was confirmed by PCR analysis of DNA extracted from β-galactosidase expressing cells, the surrogate marker for K-RasG12V expression (Guerra et al., 2007), with the help of a laser-capture microscope (Figure S1B,C). These observations indicate that adult acinar cells are extremely resistant to malignant transformation and can tolerate some of the most robust genetic insults responsible for neoplastic development.
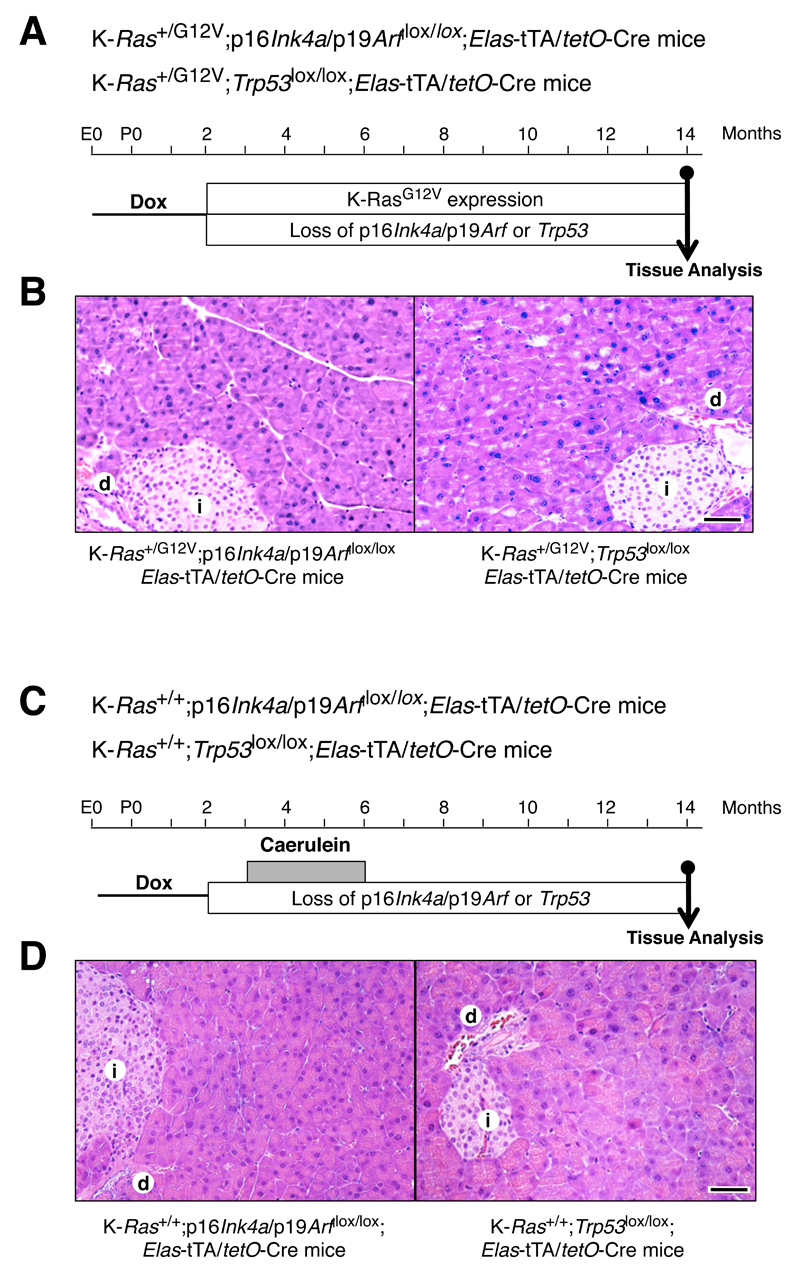
Figure 1. Loss of p16Ink4a/p19Arf or Trp53 tumor suppressors only contribute to mPanIN and mPDAC development in the presence of K-Ras oncogenes and pancreatitis.
(A) K-Ras+/G12V;p16Ink4a/p19Arf/lox/lox;Elas-tTA/tetO-Cre and K-Ras+/G12V;Trp53lox/lox;Elas-tTA/tetO-Cre mice were exposed to doxycycline (Dox, thin line) until P60 to allow expression of the K-RasG12V oncogene and to ablate the p16Ink4a/p19Arf and the Trp53 tumor suppressors in adult acinar cells.
(B) H&E staining of paraffin sections obtained from (left) K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice and (right) K-Ras+/G12V;Trp53lox/lox;Elas-tTA/tetO-Cre mice shows normal parenchyma with no mPanIN lesions one year after turning on K-RasG12V expression and ablating the p16Ink4a/p19Arf and the Trp53 tumor suppressors.
Scale bar represents 50 μm.
(C) K-Ras+/+;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre and K-Ras+/+;Trp53lox/lox;Elas-tTA/tetO-Cre mice were exposed to doxycycline (Dox, thin line) until P60 followed by caerulein treatment for three months (grey box) to ablate the p16Ink4a/p19Arf and the Trp53 tumor suppressors in adult acinar cells.
(D) H&E staining of paraffin sections obtained from (left) K-Ras+/+;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice and (right) K-Ras+/+;Trp53lox/lox;Elas-tTA/tetO-Cre mice shows normal parenchyma with no mPanIN lesions 8 months after finishing caerulein treatment.
(E) Sections obtained from control K-Ras+/G12V;Elas-tTA/tetO-Cre mice submitted to the same protocol display abundant mPanIN lesions (arrowheads) surrounded by areas of fibrosis with inflammatory infiltrates (asterisk).
(i) indicates islets; (d) indicates normal ducts. Scale bar represents 50 μm.
See also Figure S1.
Loss of p16Ink4a/p19Arf and Trp53 in adult acinar cells only contribute to mPanIN and mPDAC development in the presence of K-Ras oncogenes
In addition to K-RAS mutations, most human PanINs display inactivation of the P16INK4a/P14ARF locus. Thus, we examined whether pancreatitis may also cooperate with loss of these tumor suppressors to initiate mPanIN formation in the absence of K-Ras mutations. We induced pancreatitis by treating mice with daily doses of caerulein, a decapeptide analog of the pancreatic secretagogue cholecystokinin, as previously described (Guerra et al., 2007). K-Ras+/+;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice exposed to doxycycline until P60 were treated with caerulein and examined at 14 months of age. Systematic analysis of their pancreata (n=8) by serial sectioning failed to reveal any mPanINs (Figure 1C,D). Since TRP53 is also lost in many human PDACs, we examined whether pancreatitis cooperated with loss of this tumor suppressor to induce mPanIN formation in adult mice. K-Ras+/+;Trp53lox/lox;Elas-tTA/tetO-Cre mice (n=4) exposed to doxycycline until P60 and treated with caerulein also failed to display mPanINs in spite of serially sectioning of all pancreata (Figure 1C,D). As expected, control K-Ras+/G12V;Elas-tTA/tetO-Cre mice (8/8) submitted to the same treatments developed abundant low and high-grade mPanINs (Figure 1E). These observations indicate that activation of K-Ras oncogenes is an essential event to initiate mPanIN formation that cannot be replaced by loss of either p16Ink4a/p19Arf or Trp53 tumor suppressors.
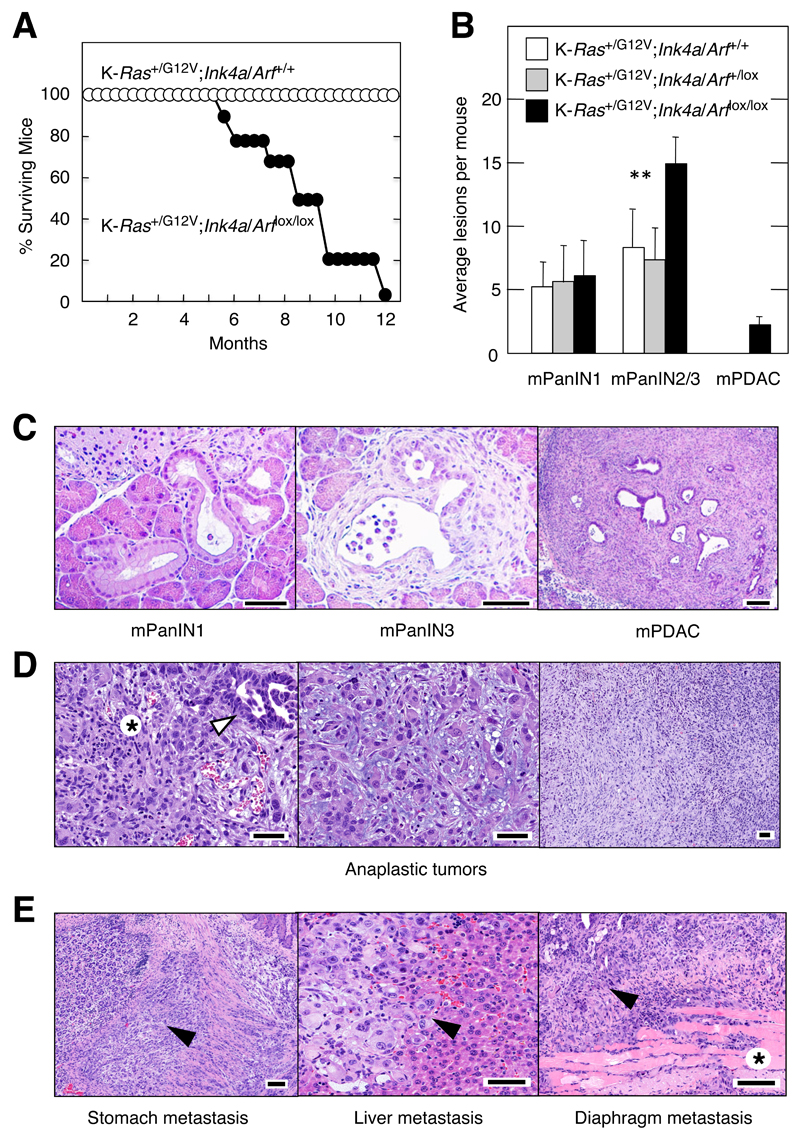
Loss of the p16Ink4a/p19Arf tumor suppressors in early pancreatic precursors during embryonic development efficiently cooperated with K-Ras oncogenes to induce invasive and metastatic mPDAC (Aguirre et al., 2003). Activation of K-Ras oncogenes along with concomitant loss of p16Ink4a/p19Arf in embryonic acinar cells also resulted in anaplastic carcinomas that metastasized to liver, stomach, diaphragm, lung, lymph nodes and spleen (Figure 2D,E). Thus, we examined whether loss of p16Ink4a/p19Arf could cooperate with K-RasG12V in adult mice. P90 K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice exposed to doxycycline until P60 were treated with caerulein to induce pancreatitis. As illustrated in Figure 2A, the lifespan of these mice was considerably shortened compared to mice that retained the p16Ink4a/p19Arf locus. Survival of heterozygous p16Ink4a/p19Arf+/lox mice was similar to that of wild type controls (data not shown). After three months of caerulein treatment, the number of low-grade mPanINs did not differ significantly between p16Ink4a/p19Arf wild-type, heterozygous or null mice (Figure 2B,C). However, the latter contained more high-grade lesions and developed mPDAC (Figure 2B,C and Figure S2A-H). Analysis of these tumors at humane end point revealed that many of these mPDAC (Figure S2I-L) had also metastasized to liver and diaphragm (Figure S2M-P). Moreover, we also observed an occasional sarcomatoid tumor when mice were sacrificed at humane end point (data not shown). These observations indicate that loss of p16Ink4a/p19Arf contributes to pancreatic cancer in adult mice, providing that the mice express K-Ras oncogenes and have suffered from pancreatitis.
Figure 2. Loss of p16Ink4a/p19Arf tumor suppressors accelerates mPanIN and mPDAC development and induces anaplastic sarcomatoid tumors.
(A) Survival of (solid circles) K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre and (open circles) K-Ras+/G12V;p16Ink4a/p19Arf+/+;Elas-tTA/tetO-Cre mice exposed to doxycycline until P60 to activate the mutations during adulthood and subsequently treated with caerulein.
(B) Average number of mPanINl, mPanIN2/3 and mPDAC lesions displayed at 6 months of age by (solid bars) K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre (n=5), (grey bars) K-Ras+/G12V;p16Ink4a/p19Arf+/lox;Elas-tTA/tetO-Cre (n=4) and (open bars) K-Ras+/G12V;p16Ink4a/p19Arf+/+;Elas-tTA/tetO-Cre (n=5) mice exposed to doxycycline until P60 to activate the mutations during adulthood and subsequently treated with caerulein. Data shown represent mean ± SD. **p < 0.01.
(C) H&E stained paraffin sections depicting representative (left) low-grade mPanINl, (middle) high-grade mPanIN3 and (right) mPDAC lesions observed in the K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice described in (B).
The scale bars represent (left and centre) 20 μm and (right) 50μm.
(D) H&E staining of anaplastic tumors displayed by K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice not exposed to doxycycline that underwent K-RasG12V expression and loss of p16Ink4a/p19Arf tumor suppressors in acinar cells during embryonic development. (Left) Anaplastic tumor with scarce glandular component. Anaplastic (asterisk) and glandular (arrowhead) areas are indicated. (Center) Anaplastic carcinoma with large, bizarre cells lying in a loose, mixoid stroma. (Right) Panoramic view of an anaplastic carcinoma. Note the absence of ductular structures. Mice were sacrificed at humane end point (15-20 weeks of age).
(E) H&E staining of metastasis displayed by the K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice described in (D). (Left) Stomach tissue. Tumoral cells are infiltrating the muscular layer between forestomach and glandular stomach (arrowhead). (Center) Undifferentiated cells metastasizing to the liver (arrowheads). (Right) Metastasis to the diaphragm (arrowhead). Some eosinophilic muscular fascicles can be recognized (asterisk). Mice were sacrificed at humane end point (15-20 weeks of age). The scale bar represents 50 μm.
See also Figure S2.
Episodic pancreatitis is sufficient to induce mPanINs and mPDAC in K-RasG12V expressing adult acinar cells
Chronic pancreatitis is one of the highest risk factors for the development of PDAC in humans (Lowenfels et al., 1993; Malka et al., 2002). In adult mice (P≥60), chronic pancreatitis is essential to induce mPanIN lesions and mPDAC in acinar cells expressing a resident K-RasG12V oncogene (Guerra et al., 2007). Yet, it is not known whether short episodes of pancreatitis may also represent a risk for PDAC development. To explore this possibility, we exposed adult (P90) K-Ras+/G12V;Elas-tTA/tetO-Cre mice to caerulein for three months, 30 days after turning on K-RasG12V expression. This treatment had no detectable consequences on the overall health of the animals. In spite of its asymptomatic nature, this treatment led to significant atrophy in the parenchyma including mucinous metaplasia, edema and fibrosis regardless of whether the mice expressed a K-Ras oncogene (Figure S3A). In addition, all animals exhibited an inflammatory response throughout the entire parenchyma, primarily made up of macrophages and T lymphocytes (data not shown). At eight months of age, two months after cessation of the caerulein treatment, all K-Ras+/G12V;Elas-tTA/tetO-Cre mice (n=15), but not control K-Ras+/+;Elas-tTA/tetO-Cre animals (n=7), displayed low and high-grade mPanIN lesions (Figure 3A). At this time, the pancreata of control mice showed limited improvement of atrophic areas and decreased numbers of inflammatory cells. Mutant mice, however, displayed increased atrophy and fibrosis, whose levels were directly related to the number and extent of the mPanIN lesions (data not shown). In these mutant mice, the inflammatory cells, mainly macrophages and T lymphocytes, were abundant and closely associated with mPanIN lesions (Figure 4B).
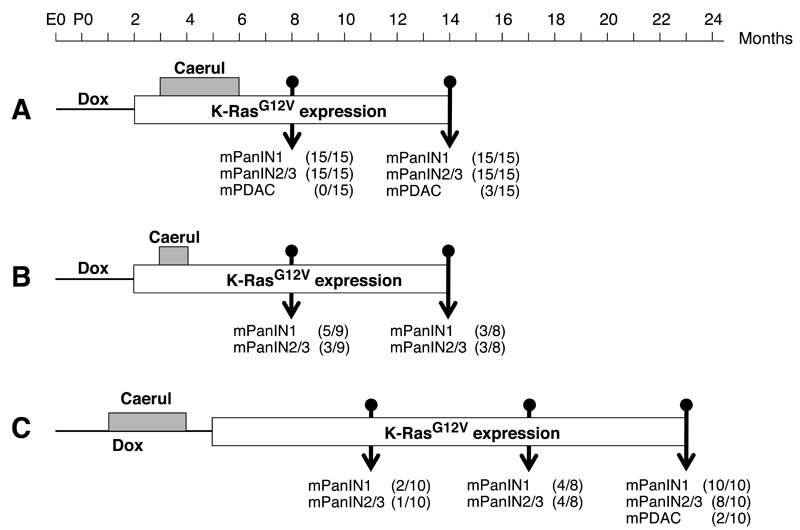
Figure 3. Episodic pancreatitis induces mPanINs and mPDAC in K-Ras+/G12V;Elas-tTA/tetO-Cre mice.
K-Ras+/G12V;Elas-tTA/tetO-Cre mice were exposed to doxycycline (thin line) and caerulein (grey box) for the indicated periods of time. The open box indicates the time of K-RasG12V expression. The number of animals positive for low-grade mPanINl, high-grade mPanIN2/3 and mPDAC is indicated for each protocol and time point.
(A) Mice exposed to doxycycline (Dox) from conception (E0) to P60 were treated with caerulein (Caerul) for three months, from P90 to P180. Mice were sacrificed at 8 and 14 months of age, that is 6 and 12 months after turning on K-RasG12V expression.
(B) Mice exposed to doxycycline from conception (E0) to P60 were treated with caerulein for one month, from P90 to P120. Mice were sacrificed at 8 and 14 months of age, that is 6 and 12 months after turning on K-RasG12V expression.
(C) Mice exposed to doxycycline from conception (E0) to P150 were treated with caerulein for three months, from P30 to P120. Mice were sacrificed at 11, 17 and 23 months of age, that is, 6, 12 and 18 months after turning on K-RasG12V expression.
See also Figure S3.
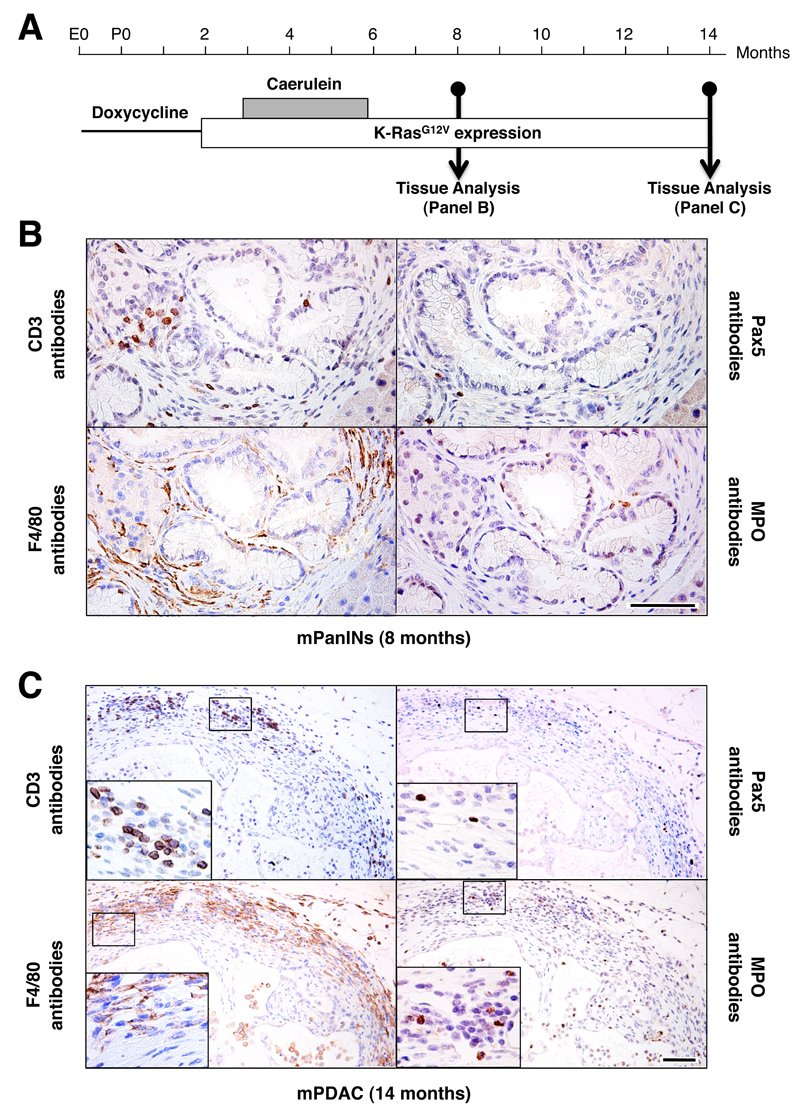
Figure 4. Inflammatory infiltrates in mPanIN lesions and mPDAC in K-RasG12V expressing adult mice treated with caerulein for three months.
(A) K-Ras+/G12V;Elas-tTA/tetO-Cre mice were exposed to doxycycline (thin line) and caerulein (grey box) for the indicated periods of time. Expression of the K-RasG12V oncogene (open box) is indicated. Mice were sacrificed at 8 and 14 month of age.
(B,C) Immunostaining of inflammatory cells surrounding (B) mPanIN lesions in 8 month old mice (2 months after cessation of caerulein) and (C) mPDAC in 14 month old mice (8 months after cessation of caerulein treatment) using antibodies against T lymphocytes (CD3 antibodies), B lymphocytes (Pax5 antibodies), macrophages (F4/80 antibodies) and neutrophils (MPO antibodies). Insets show detailed areas containing the corresponding immune cells. Scale bars represent 50 μm.
When control K-Ras+/+;Elas-tTA/tetO-Cre mice (n=15) were examined six months later, their pancreata had recovered almost completely and we only observed few areas of inflammatory cells. K-RasG12V-expressing pancreata had also recovered except for areas of atrophy intimately associated with the mPanIN lesions. The levels of inflammatory cells had also subsided except for those closely associated with the mPanIN lesions (data not shown). At this time, all mice displayed low and high-grade mPanIN lesions (Figure 3A and Figure S3B). Moreover, three of the fifteen K-Ras+/G12V;Elas-tTA/tetO-Cre animals had developed mPDAC, of which two showed invasion of the proximal parenchyma and one displayed metastasis in liver and lung (Figure 3A and Figure S3C-I). These mPDACs displayed high levels of stroma similar to those observed in human patients (Figure S3C,D). In these lesions, inflammatory cells persisted but were located in the periphery of the stroma that surrounded the glandular structures of the tumor (Figure 4C and Figure S3F).
Next, we reduced the time of caerulein exposure to one month (Figure 3B). This shorter treatment also induced parenchyma atrophy and recruitment of inflammatory cells, although the extent of atrophic areas and inflammatory foci were significantly reduced (data not shown). When mice were analyzed at eight months of age, four months after cessation of caerulein exposure, more than half of the mice (5/9) had developed low-grade mPanINs and three of them displayed high-grade lesions (Figure 3B and Figure S3J). At this time, the parenchyma was well preserved with few mucinous metaplasia and small focal areas of inflammatory cells, mostly associated with mPanIN lesions (data not shown). Analysis of K-Ras+/G12V;Elas-tTA/tetO-Cre mice at 14 months of age revealed a similar percentage of animals with high-grade mPanINs (3/8) (Figure 3B and Figure S3J). However, none of them had developed mPDAC at this time. When we allowed these mice (n=11) to age (1.5 and 3 years of age) we observed mPanIN lesions in most of them (10.2±5.1 low-grade and 4.8±2.6 high-grade mPanINs/mouse, respectively) with only one mouse failing to show any lesions. Yet, only two mice developed mPDAC (Figure S3K-N). These observations indicate that development of high-grade mPanIN and mPDAC in adult mice depends on the extent of tissue damage and the inflammatory response.
We also interrogated whether PDAC development required the presence of K-Ras oncogenes at the time of pancreatic damage. To this end, K-Ras+/G12V;Elas-tTA/tetO-Cre mice were maintained in doxycycline until P150 while they were treated with caerulein from P30 to P120. Thus, mice had one month to recover from caerulein treatment before expressing their resident K-RasG12V oncogene (Figure 3C). Analysis of ten pancreata at six months after turning on K-RasG12V expression (11 months of age) revealed the presence of mPanINs in few mice (Figure 3C). Six month later (17 months of age) the percentage of mice depicting mPanINs had increased to half (4/8) (Figure 3C). All animals (n=10) displayed mPanINs upon 18 months of continuous K-RasG12V expression (23 months of age) (Figure 3C and Figure S3O), and two of them had mPDACs that invaded the adjacent parenchyma (Figure 3C and Figure S3P). No lesions were observed when K-RasG12V expression was activated at P150 in mice not exposed to caerulein (Figure S1A). These observations indicate that K-Ras oncogenes can initiate mPanIN and mPDAC development in adult mice providing that there is a preexisting inflammatory response.
Pancreatitis induces expression of genes characteristic of progenitor cells such as Pdx1 (Jensen et al., 2005; Fendrich et al., 2008) and Sox9 (Seymour et al., 2007; Yoshida et al. 2008). Hence, raising the possibility that the susceptibility to K-Ras oncogenic signaling might be due to the induction of a less mature differentiated state. As illustrated in Figure S3Q, caerulein treatment induces high levels of Pdx1 and Sox9 in acini as well as in metaplasias. However, Pdx1 expression faded away from the acinar cells within 30 days after caerulein withdrawal and was not expressed (except in very few cells) at the time (P150) when K-RasG12V expression was turned on (Figure S3Q). As previously reported, Pdx1 becomes re-expressed in low-grade mPanINs (Hingorani et al., 2003; Guerra et al., 2007; Morris et al., 2010). Instead, Sox9 expression was maintained throughout the transformation process including metaplasias (Figure S3Q) and mPanINs (Morris et al., 2010). As expected, the expression pattern of acinar cell markers such as chymotrypsin and elastase was retained at the time of K-RasG12V expression as well as after a month of continuous oncogene expression (Figure S3Q).
The inflammatory response observed immediately after cessation of caerulein exposure (P120) (Figure S3R) significantly subsided 30 days later in both in wild type and mutant mice (Figure S3S) at a time, P150, in which induction of K-RasG12V expression has not yet taken place (Figure 3C and S3Q). At this time, we could only observe the presence of T lymphocytes (Figure S3S). Occasionally, isolated B lymphocytes, macrophages and neutrophils were also observed (Figure S3S). At P180, that is 30 days after turning on K-RasG12V expression, the inflammatory response remained at similar levels and no significant differences were observed between wild type and mutant mice (Figure S3T). No mPanIN lesions were observed at either P150 or P180 (Figure S3S,T). Whether the presence of these inflammatory cells play a key role in the subsequent development of mPanIN lesions remains to be determined.
The senescence program present in low-grade mPanIN is abrogated during progression to high-grade mPanIN lesions
Metaplasias and low-grade mPanINs display senescence markers similar to those present in preneoplastic stages of other tumor types (Collado and Serrano, 2010). However, OIS is not an immediate consequence of K-RasG12V expression since morphologically normal acinar cells expressing this oncogene do not display senescence markers (Figure S4A). Thus, induction of OIS must require additional changes likely to be involved in the acinar-ductal transdifferentiation process required to generate low-grade mPanIN lesions. Low-grade mPanINs developing in K-Ras+/G12V;p16Ink4a/p19Arflox/lox;Elas-tTA/tetO-Cre mice lacking the p16Ink4a/p19Arf tumor suppressors did not display senescence markers (Figure S4B). Thus, indicating that these tumor suppressors play a key role in the induction of senescence in low-grade mPanIN lesions.
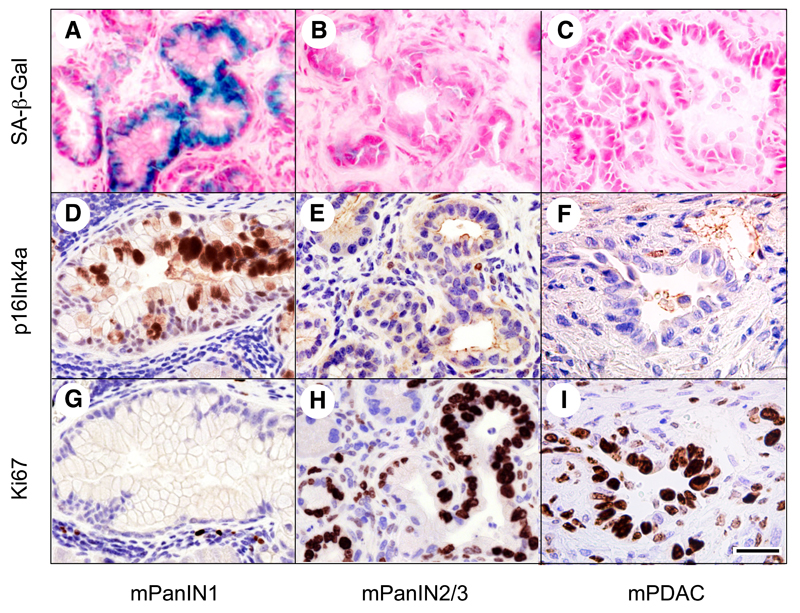
The senescence phenotype of low-grade mPanINs disappeared during tumor progression. As illustrated in Figure 5, high-grade mPanIN2/3 lesions as well as mPDAC were negative for senescence markers including endogenous senescence-associated β-galactosidase (SA-β-Gal) and expression of the p16Ink4a tumor suppressor. Expression of these senescence markers inversely correlated with the appearance of proliferative markers such as Ki67. Whereas Ki67 was expressed in less than 10% of the cells in SA-β-Gal positive mPanIN1 lesions, it was detected in more than 50% of the cells of high-grade mPanINs and mPDAC (Figure 5).
Figure 5. Senescence markers are a feature of low-grade mPanINs, but disappear in high-grade mPanIN2/3 and mPDAC.
(A-C) Senescence associated β-galactosidase (SA-β-Gal) staining (blue) in (A) low-grade mPanIN1 but not in (B) high-grade mPanIN2/3 or (C) mPDAC.
(D-F) p16Ink4a immunostaining (brown) in (D) low-grade mPanIN1 but not in (E) high-grade mPanIN2/3 or (F) mPDAC.
(G-I) Ki67 immunostaining (brown) inversely correlates with the expression of the above senescence markers. Ki67 is detected in a low percentage of cells of low-grade mPanIN1 (G) and in a high percentage of cells of high-grade mPanIN2/3 (H) and mPDAC (I).
Note that panels D-G, E-H and F-I correspond to serial sections. Scale bar represents 50 μm.
See also Figure S4.
Pancreatitis-induced inflammation contributes to mPanIN development by inhibiting OIS
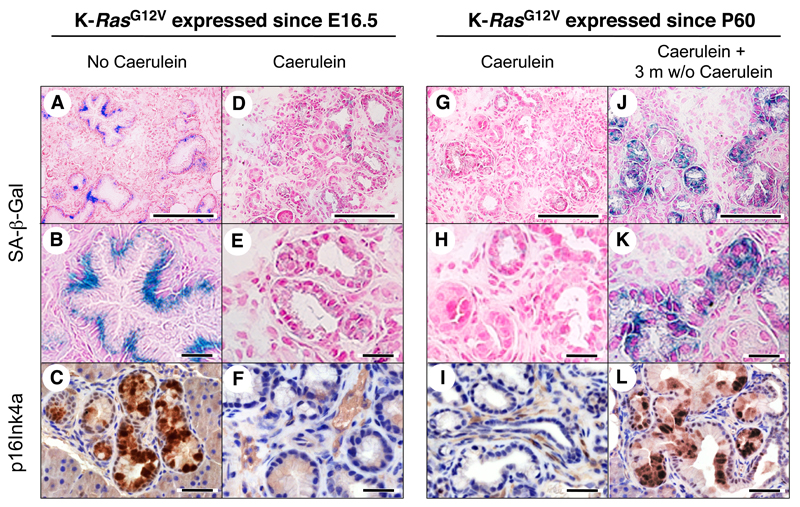
Surprisingly, senescence markers were not present in low-grade mPanINs induced by K-RasG12V expression in adult mice, a process that requires exposure to caerulein. Thus, we decided to ascertain whether senescence was a property of lesions initiated in the embryonic pancreas or was inhibited by caerulein-induced pancreatitis. K-Ras+/G12V;Elas-tTA/tetO-Cre mice expressing K-RasG12V since late embryonic development were either allowed to develop mPanINs by themselves or were treated with caerulein for three months. Whereas the low-grade mPanINs present in control mice (not treated with caerulein) contained senescent cells (Figure 6A-C), none of those mPanINs present in animals exposed to caerulein displayed senescence markers, including SA-β-Gal and p16Ink4a (Figure 6D-F).
Figure 6. Senescent low-grade mPanINs reappear upon partial recovery from pancreatitis injury.
(LEFT) K-Ras+/G12V;Elas-tTA/tetO-Cre mice not exposed to doxycycline, thus expressing the endogenous K-RasG12V oncogene since embryonic development (E16.5). (A-C) Pancreatic sections showing low-grade mPanIN1 lesions. Sections were stained for (A,B) SA-β-Gal (blue) or (C) p16Ink4a (brown) expression. Panel B is an amplified version of panel A to better illustrate SA-β-Gal expression in mPanIN1 lesions.
(D-F) Pancreatic sections of mice treated with caerulein for three months (P60 to P150) depicting low-grade mPanIN1 lesions. Sections were stained for (D,E) SA-β-Gal (blue) or (F) p16Ink4a (brown) expression. Panel E is an amplified version of panel D to better illustrate the absence of SA-β-Gal expression in mPanIN1 lesions.
(RIGHT) K-Ras+/G12V;Elas-tTA/tetO-Cre mice exposed to doxycycline from conception to P60, thus expressing the endogenous K-RasG12V oncogene since P60.
(G-I) Pancreatic sections of mice treated with caerulein for three months (P90 to P180) showing low-grade mPanIN1 lesions. Sections were stained for (G,H) SA-β-Gal (blue) and (I) p16Ink4a (brown) expression. Panel H is an amplified version of panel G to better illustrate the absence of SA-β-Gal expression in mPanIN1 lesions.
(J-L) Pancreatic sections of mice treated with caerulein for three months (P90 to P180) and allowed to recover for three additional months showing low-grade mPanIN1 lesions. Sections were stained for (J,K) SA-β-Gal (blue) and (L) p16Ink4a (brown) expression. Panel K is an amplified version of panel J to better illustrate the reappearance of SA-β-Gal expression in mPanINl lesions. Scale bars represent 50 μm (A, D, G, J) and 20 μm (B,C,E,F,H,I,K,L).
See also Figure S5.
As described above, the pathological features characteristic of pancreatitis disappeared upon withdraw of caerulein. Thus, we examined whether progressive disappearance of the inflammatory response had any effect on senescence. Adult K-Ras+/G12V;Elas-tTA/tetO-Cre mice starting K-RasG12V expression at P60 were treated with caerulein for three months and either immediately sacrificed or allowed to recover for three additional months. Mice sacrificed at the end of the caerulein treatment displayed low-grade mPanINs devoid of senescence markers (Figure 6G-I). In contrast, those that were allowed to recover for three months had low-grade mPanINs positive for SA-β-Gal staining and p16Ink4a (Figure 6J-L) as well as for PAI-1 and Sprouty-4 expression (Figure S5A). Indeed, senescence reappeared as shortly as one month after the cessation of caerulein exposure, a time when the inflammatory response had not completely subsided (Figure S5B). These observations indicate that OIS can be inhibited by limited episodes of pancreatitis but can reappear after the pancreatitis-induced damage has partially subsided.
Anti-inflammatory treatment reverts tissue damage and delays progression of pancreatitis-induced mPanIN lesions
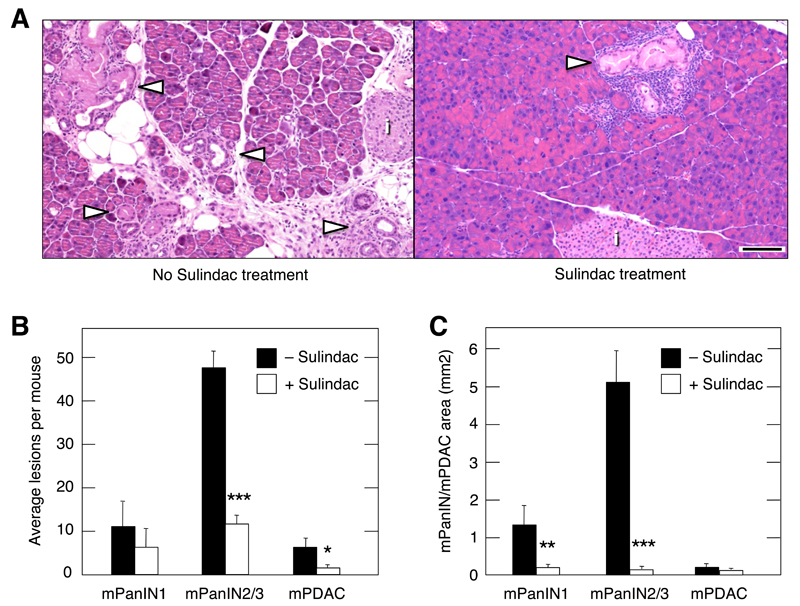
To evaluate the overall contribution of inflammation to tumor progression, we studied the effect of Sulindac on mPanIN/mPDAC development. Sulindac is a non-steroidal anti-inflammatory drug thought to act on COX-1 and COX-2 enzymes (Smith et al., 1994). K-Ras+/G12V;Elas-tTA/tetO-Cre animals raised in the presence of doxycycline until P60 were treated with caerulein at P90 for three months. At the end of the treatment, mice were allowed to recover for three additional months either without further treatment or treated with Sulindac. Mice not exposed to Sulindac displayed the typical lesions induced by pancreatitis such as parenchyma atrophy, edema and infiltration of inflammatory cells (Figure 7A and Figure S6A). Moreover, their pancreata exhibited multiple diffuse mPanIN lesions, ranging from low-grade mPanIN1A to invasive mPDAC, although most of them were high-grade mPanIN2/3 lesions (Figure 7A,B). In contrast, mice treated with Sulindac had well-preserved pancreata with few areas of parenchyma atrophy and limited infiltration of inflammatory cells (Figure 7A and Figure S6B). Moreover, we observed a dramatic reduction (75%) in the number of high-grade lesions (Figures 7A,B and Figure S6B). Perhaps more importantly, those lesions present in the Sulindac treated animals were considerably smaller (Figure 7A,C and Figure S6B). Whereas the average size of the high-grade mPanINs in control mice was 5.21 mm2, those present in Sulindac-treated animals was only 0.14 mm2, a dramatic 95% reduction (Figure 7C). These results strongly implicate inflammation as a key contributor to the effect of pancreatitis not only in promoting mPanIN formation, but also in inducing progression to mPDAC.
Figure 7. Inhibition of the inflammatory response by Sulindac reverts tissue damage and delays progression of mPanIN lesions.
K-Ras+/G12V;Elas-tTA/tetO-Cre mice, exposed to doxycycline until P60 to achieve expression of K-RasG12V in the adult pancreas were treated with caerulein for three months (P90-P180). Half the mice were allowed to recover for three months without further treatment whereas the other half was treated with Sulindac.
(A) H&E-stained paraffin sections of representative pancreata of mice (Left) not treated or (Right) treated with Sulindac. Note that the pancreata of the untreated mice displayed high levels of edema, fibrosis, parenchyma atrophy and abundant large mPanIN lesions. In contrast, mice that underwent Sulindac treatment showed a well-preserved parenchyma and contained few small mPanINs. Arrowheads point to mPanIN lesions, (i) indicates an islet. Scale bar represents 50 μm.
(B) Average lesions per mouse. Solid bars indicate mice not treated with Sulindac (n=3). Open bars correspond to mice treated with Sulindac (n=3). Data shown represent mean ± SD. ***p < 0.00017, *p < 0.036.
(C) Area of mPanIN1, mPanIN2/3 and mPDAC lesions observed in serial pancreata sections. Solid bars indicate mice not treated with Sulindac (n=3). Open bars correspond to mice treated with Sulindac (n=3). Data shown represent mean ± SD. **p < 0.0037, ***p < 0.00012.
See also Figure S6.
Pancreatitis-induced inflammation blocks senescence in human PanINs
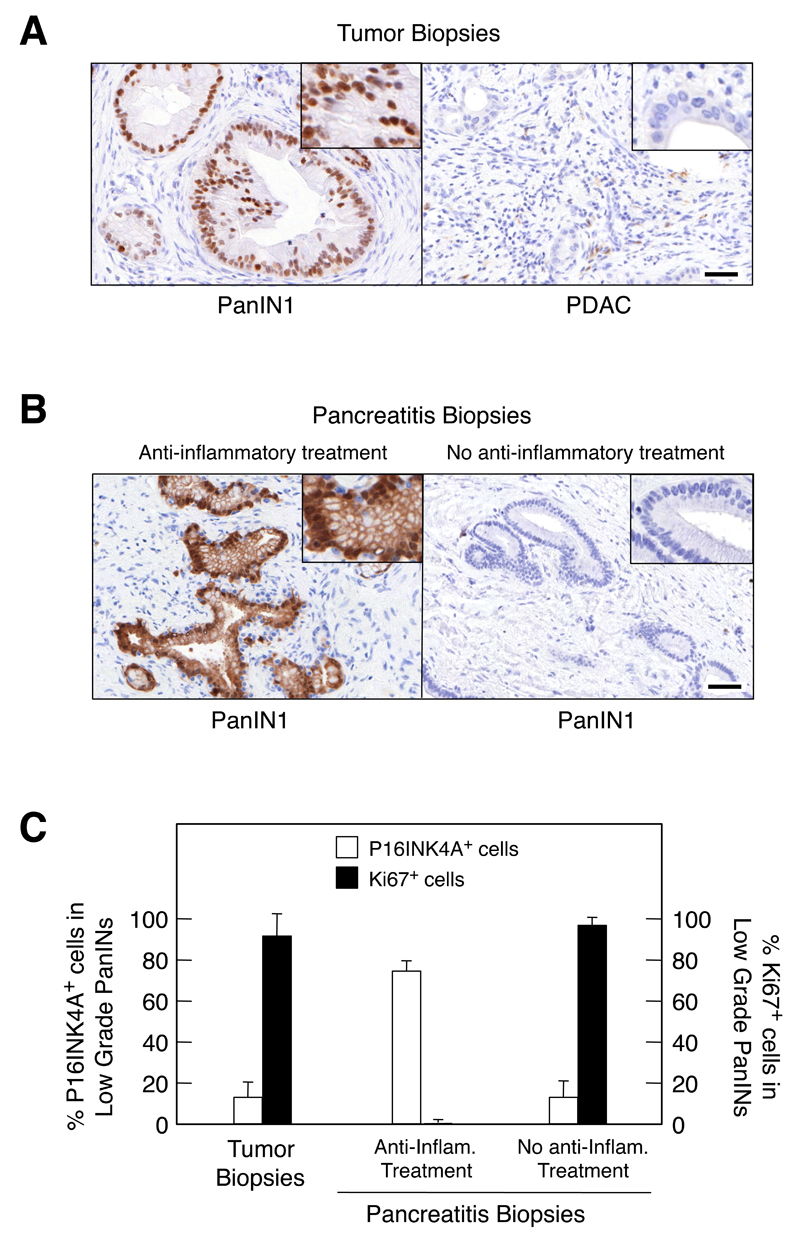
Finally, we examined whether some of these observations could be extended to human patients. To this end, we examined the expression of P16INK4a as a marker of senescence in samples obtained from pancreata of six patients with PDAC that also contained low-grade PanINs. Whereas none of the cells within the PDAC expressed detectable levels of this tumor suppressor, all six low-grade PanINs presented a very strong nuclear signal when probed with anti P16INK4a antibodies (Figure 8A). Parallel analysis of Ki67 staining, a marker for proliferation, revealed an inverse correlation with P16INK4a immunostaining (Figure 8C), an observation highly reminiscent of the results obtained in mice. These findings indicate that human low-grade PanINs also contain senescent cells.
Figure 8. Senescence markers in human low-grade PanINs.
(A) Low-grade PanINs present in biopsies from PDAC patients display senescence markers. (Left) Sections showing low-grade PanIN1s are positive for P16INK4a immunostaining. (Right) Sections depicting PDAC lesions are negative for P16INK4a immunostaining. Insets show amplified images to illustrate nuclear staining in PanIN1 lesions. Scale bar represents 50 μm.
(B,C) Anti-inflammatory treatment restores senescence in low-grade PanIN of chronic pancreatitis patients.
(B) P16INK4a immunostaining of biopsies obtained from patients suffering from chronic pancreatitis. (Left) Representative section displaying low-grade PanIN1s positive for P16INK4a staining present in a biopsy obtained from a patient treated with anti-inflammatory drugs. (Right) Representative section displaying low-grade PanIN1s negative for P16INK4a staining present in a biopsy obtained from a patient not-treated with anti-inflammatory drugs. Insets show amplified images to illustrate nuclear staining in PanIN1 lesions. Scale bar represents 50 μm.
(C) Quantification of the percentage of cells present in low-grade PanIN1 lesions positive for (open bars) P16INK4a immunostaining or (solid bars) Ki67 staining. Samples were obtained from (left) PDAC patients, (center) chronic pancreatitis patients treated with anti-inflammatory drugs and (right) chronic pancreatitis patients not treated with anti-inflammatory drugs.
Next, we examined the presence of senescent cells in PanINs present in surgically removed samples from nine patients suffering from chronic pancreatitis. The clinical histories of these patients are summarized in Table S1. To our surprise, low-grade PanINs were strongly positive for P16INK4a expression only in four of the nine samples (Figure 8B). Examination of the clinical history of these patients revealed that the four patients whose samples displayed senescence markers had received anti-inflammatory treatments. Whereas two of them had been treated with prednisolone, the other two had received NSAIDs. In contrast, none of the five patients that lacked of P16INK4a expression had received anti-inflammatory therapy (Figure 8B and Figure S7). These pancreatitis-derived biopsies also displayed significant differences when they were analyzed for Ki67 expression. As illustrated in Figure 8C, PanINs present in biopsies derived from pancreatitis patients that have received anti-inflammatory treatment were essentially negative for Ki67. In contrast, those PanINs present in biopsies derived from untreated patients contained high levels of Ki67. These results strongly suggest that inhibition of the inflammatory response induced by pancreatitis helped to maintain senescence, possibly contributing to the clinical benefit provided by anti-inflammatory drugs.
Discussion
Most cancers arise from somatic mutations during adulthood. Thus, proper understanding of how tumors are initiated requires modeling cancer in adult animals. In pancreas, the susceptibility to transformation by a resident K-Ras oncogene displayed by embryonic pancreatic precursors is lost in adult mice (P≥60) (Guerra et al., 2007). Indeed, the resistance of the adult pancreas to malignant transformation extends beyond K-Ras oncogenic signaling. As illustrated here, adult acinar cells are also refractory to transformation by concomitant expression of a resident K-Ras oncogene and loss of the p16Ink4a/p19Arf or Trp53 tumor suppressors, some of the most robust mutational events known in cancer.
In spite of their resistance to mutational insults, adult acinar cells retain their capacity to proliferate in response to certain pathological insults and to acquire tumorigenic properties (Slater et al., 1998; Jura et al., 2005). Exposure of adult mice to caerulein-induced pancreatitis induces proliferation of their acinar cells in order to repair tissue damage but does not result in formation of mPanINs or other preneoplastic lesions (Strobel et al., 2007). However, caerulein treatment restores permissiveness of adult acinar cells to malignant transformation by K-Ras oncogenes (Guerra et al., 2007; Friedlander et al., 2009; Morris et al., 2010). Additional loss of the p16Ink4a/p19Arf or Trp53 (unpublished results) tumor suppressors exacerbates the transformation process. Interestingly, pancreatitis does not cooperate with loss of either of these tumor suppressors. Thus, indicating that K-Ras oncogenes are essential for initiation of mPDAC.
mPDACs generated by a combination of oncogenic insults including K-Ras oncogenes, loss of p16Ink4a/p19Arf or Trp53 and caerulein-induced pancreatitis often display invasive and metastatic properties, albeit those induced in adult animals require longer latencies (Aguirre et al., 2003; Hingorani et al., 2005; Guerra et al., 2007). Moreover, a combination of K-Ras oncogenes and loss of p16Ink4a/p19Arf in early pancreatic precursors (Aguirre et al., 2003) or in embryonic acinar cells (this study) frequently result in anaplastic carcinomas, an aggressive tumor type seldom observed when these mutations are induced in adult animals. These results suggest that tumors that originate in the embryo develop more aggressively than those initiated during adulthood. Moreover, the more frequent occurrence of anaplastic carcinomas suggest that these oncogenic insults may engage pathways in embryonic acinar cells that are downregulated in their adult counterparts.
In humans, chronic pancreatitis represents one of the highest risk factors for developing PDAC (Lowenfels et al., 1993). However, most PDAC patients do not have a history of chronic pancreatitis. Thus, it is important to determine whether less aggressive forms of pancreatitis, including sporadic or even asymptomatic pancreatitis, may also increase the risk of developing PDAC. As illustrated here, limited bouts of mild pancreatitis lasting as little as one month were sufficient to elicit mPanIN formation, providing that the injured acinar cells expressed a K-Ras oncogene. Longer pancreatitis events resulted in increased incidence of mPanIN formation along with the appearance of high-grade mPanIN lesions and mPDAC with reduced latencies. The number and size of the lesions observed in these two protocols were considerably lower than those observed in mice suffering from chronic pancreatitis (Guerra et al., 2007). Finally, mPDAC can also develop if pancreatitis occurs before activation of K-Ras oncogenes, providing that the inflammatory response has not subsided. These observations raise the possibility that PDAC development in humans may also stem from limited bouts of pancreatitis providing that the inflammatory response co-exists with sporadic K-Ras mutations.
Pancreatitis is not only essential for initiation of mPanIN lesions in adult mice. As illustrated here, pancreatitis, most likely through its inflammatory component, contributes to mPanIN progression by abrogating OIS, a natural defense mechanism against tumor development (Collado and Serrano, 2010). Post-mortem analysis of pancreatic tissue obtained from autopsies of healthy individuals has revealed the presence of PanIN lesions carrying K-Ras oncogenes that, presumably had not progressed to PDAC due to an active senescence program (Terhune et al., 1998; Lüttges et al., 1999; Löhr et al., 2005). Low-grade mPanIN lesions induced in the embryonic pancreas do not display senescence markers if the mice suffered from pancreatitis. In adult animals, which require pancreatitis for mPanIN development, senescence markers can only be detected once the inflammatory response has subsided. These observations suggest that one of the mechanisms by which pancreatitis-induced inflammation contributes to the progression of mPDAC is by eliminating the senescence barrier that prevents progression of low-grade mPanINs into more aggressive lesions.
In addition, the inflammatory response delays repair of the non-transformed pancreata and stimulate expansion of mPanIN lesions. Whether the latter effect is solely mediated by inhibiting senescence, remains to be determined. Animals treated with the dual COX-1/2 inhibitor Sulindac for three months after exposure to caerulein displayed almost normal pancreata with only few areas of atrophy and limited numbers of inflammatory cells. Whereas Sulindac had limited effect on the number of low-grade lesions, it caused a significant reduction (up to 75%) in the number of high-grade mPanINs and mPDAC. Yet, the most dramatic effect of this anti-inflammatory treatment was observed on the extent of the area occupied by mPanIN lesions, which displayed a dramatic 95% reduction. These observations suggest that, in addition to eliminating the senescence barrier, the inflammatory response induced by episodic pancreatitis may contribute to mPDAC development by additional mechanisms.
How relevant are these observations to human patients?. As illustrated in this study, low-grade PanINs present in samples surgically removed from PDAC patients displayed senescence markers. No such markers were observed in pancreatic tumor cells. Yet, the most informative observations correlating PanIN development with senescence came from the analysis of samples surgically removed from chronic pancreatitis patients. Although the number of samples analyzed is still small, we found an intriguing correlation between the occurrence of senescence in low-grade PanINs and prior exposure of the patients to anti-inflammatory agents. The results were independent of whether the anti-inflammatory treatment was based on steroids or on NSAIDs. These observations suggest that pancreatitis patients may benefit from anti-inflammatory treatments by retaining the senescent phenotype in their low-grade PanIN lesions, possibly preventing their progression into high-grade lesions.
K-RAS mutations have been identified in the pancreas of healthy individuals (Terhune et al., 1998; Lüttges et al., 1999; Löhr et al., 2005). In addition, patients with chronic pancreatitis have over 10 fold increased risk of developing PDAC (Lowenfels et al., 1993; Malka et al., 2002). As illustrated in this study, mPDAC develop in adult mice expressing a resident K-Ras oncogene if they undergo episodic events of pancreatitis, even under asymptomatic conditions. These observations raise the possibility that in humans, at least some PDACs may originate from mild bouts of pancreatitis in individuals that unknowingly carry K-RAS mutations. Since the identification of K-RAS oncogene containing cells within the pancreas of healthy individuals is not possible, it will be important to monitor people that have been diagnosed with pancreatitis for the development of pancreatic lesions, that if unattended may lead to PDAC. Likewise, it will be important to develop biomarkers that can identify individuals that have undergone from asymptomatic pancreatitis, at least in high-risk populations.
The correlation between inflammation and loss of OIS also has important implications for the prevention of PDAC development. Treatment with antiinflammatory drugs maintained senescence markers in low-grade PanINs present in chronic pancreatitis patients. Interestingly, recent epidemiological studies have suggested a beneficial correlation between NSAIDs and pancreatic cancer risk (Bonifazi et al., 2010; Bradley et al., 2010; Rothwell et al., 2011). Our results suggest that antiinflammatory treatments might decrease the risk of PDAC development by maintaining the senescence phenotype of early PanIN lesions. Now, it will be necessary to determine whether inhibition of the inflammatory response will have a beneficial response in patients already carrying fully developed PDAC. The mouse models utilized here should contribute to address this question.
Experimental Procedures
Mice
K-Ras+/LSLG12Vgeo;Elas-tTA/tetO-Cre (Guerra et al., 2007), p16Ink4a/p19Arflox/lox (Krimpenfort et al., 2001), and Trp53lox/lox (Jonkers et al., 2001) strains have been described. All experiments were approved by the CNIO Ethical Committee and performed in accordance with the guidelines for Ethical Conduct in the Care and Use of Animals as stated in The International Guiding Principles for Biomedical Research involving Animals, developed by the Council for International Organizations of Medical Sciences (CIOMS).
Human Samples
Human samples were obtained from the Histopathology files of University College Hospital from patients who had undergone pancreatoduodectomy, distal pancreatectomy or total pancreatectomy with a primary diagnosis of chronic pancreatitis or ductal adenocarcinoma from 2004 to 2008 after approval by the institutional Research Ethics Committee (REC 3, Reference 06/Q0512/106).
Histopathology and immunohistochemistry
Specimens were fixed in 10% buffered formalin and embedded in paraffin. For histopathological analysis, pancreata were serially sectioned (3 μm) and every 10 sections stained with hematoxylin and eosin (H&E). Remaining sections were kept for immunohistochemical studies. Antibodies used for mouse samples include CD3 (1/250, goat polyclonal, Santa Cruz 1127), Cytokeratin19 (rat monoclonal, Troma III, Developmental Studies Hybridoma Bank), Chymotrypsin (1/50, mouse monoclonal 4E1, ABD Serotec, 2100-0657), Elastase (1/50, rabbit polyclonal, ABCAM, ab21590), F4/80 (1/25, rat monoclonal BM8, BMA Biomedicals), Ki67 (prediluted rabbit monoclonal SP6, Master Diagnóstica 0003110QD), MPO (1/1250, rabbit polyclonal, DAKO A0398), PAI-1 (1/50, rabbit polyclonal, Santa Cruz sc-8979), Pax5 (1/500, goat polyclonal, Santa Cruz 1974), phospho-Histone H3-Ser10 (1/200, rabbit polyclonal, MILLIPORE 06-570), prosurfactant protein C (SPC) (1/50, rabbit polyclonal, MILLIPORE AB3786), p16Ink4a (1/75, rabbit polyclonal, Santa Cruz 1207), Sox9 (1/800, rabbit polyclonal, MILLIPORE AB5535) and Sprouty-4 (1/50, goat polyclonal, Santa Cruz 18607) antibodies. Antibodies against Pdx1 were kindly provided by A. Skoudy. Positive cells were visualized using 3,3-diaminobenzidine tetrahydrochloride plus (DAB+) as a chromogen. For human samples, P16INK4a immunolabeling was performed according to the manufacturer’s protocol (CINtecR Histology Kit, mtmlabs, Heidelberg, Germany). Ki67 staining was performed with mouse monoclonal Anti-Human Ki-67 (Dako).
Supplementary Material
Significance.
Pancreatic ductal adenocarcinoma (PDAC) is one of the tumor types with worst prognosis. Efforts to understand its etiology and early development may help to save lives. Here we report that brief bouts of asymptomatic pancreatitis in adult mice lead to mPDAC as long as the acinar cells express K-Ras oncogenes. K-Ras mutations occurring after pancreatitis also induce mPDAC providing that the inflammatory response has not subsided. Inflammation contributes to mPDAC by eliminating the senescence program characteristic of benign lesions. Samples obtained from pancreatitis patients also display senescence markers but only if they have received anti-inflammatory therapy. Thus, anti-inflammatory treatment of patients diagnosed with even mild bouts of pancreatitis may prevent, or at least reduce the risk of developing PDAC.
Highlights.
Adult acinar cells are resistant to multiple oncogenic insults
Limited bouts of pancreatitis cooperate with K-Ras to induce mPDAC in adult mice
Pancreatitis abrogates senescence in low-grade PanINs
Pancreatitis patients have senescent PanINs if treated with anti-inflammatory drugs
Acknowledgements
We thank I. Aragón, M. Lamparero, M. Lozano, E. Martínez, M. San Román and R. Villar for excellent technical assistance. We also value the excellent support provided by V. Alvarez, E. Gil, M. Gómez, P. González, and N. Matesanz with histopathology. We thank A. Skoudy (IMIM, Barcelona) for providing the Pdx1 antibody, J.I. Gordon (Washington University School of Medicine, St. Louis, MO) for providing the tetO-PhCMV-Cre mice and Paul Grippo (Northwestern University, Chicago. IL) and Eric Sandgren (University of Wisconsin, Madison, WI) for providing the Elastase-tTA strain. Work in the laboratory of M.B. was supported by grants from the EU-Framework Programme (LSHG-CT-2007-037665), European Research Council (ERC-AG/250297-RAS AHEAD), Spanish Ministry of Science and Innovation (MICINN) (SAF2006-11773 and CSD2007-00017), Autonomous Community of Madrid (GR/SAL/0587/2004 and S2006/BIO-0232) and Fundación de la Mutua Madrileña del Automovil to M.B and by grants from Fondo de Investigación Sanitaria (PI042124) and Autonomous Community of Madrid (GR/SAL/0349/2004) to C.G. Work in the laboratory of M.S. was funded by grants from the EU-Framework Programme (PROTEOMAGE), European Research Council (ERC-AG/233270), MICINN (SAF2008-02959 and CSD2007-00017), Autonomous Community of Madrid (GsSTEM) and the Marcelino Botin Foundation. M.R.-J. is supported by the UCLH/UCL Comprehensive Biomedical Research Centre (London, UK). M.Collado is the recipient of a "Ramon y Cajal" contract from the MCINN.
References
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi M, Gallus S, Bosetti C, Polesel J, Serraino D, Talamini R, Negri E, La Vecchia C. Aspirin use and pancreatic cancer risk. Eur J Cancer Prev. 2010;19:352–354. doi: 10.1097/CEJ.0b013e32833b48a4. [DOI] [PubMed] [Google Scholar]
- Bradley MC, Hughes CM, Cantwell MM, Napolitano G, Murray LJ. Nonsteroidal anti-inflammatory drugs and pancreatic cancer risk: a nested case – control study. British Journal of Cancer. 2010;102:1415–1421. doi: 10.1038/sj.bjc.6605636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, et al. Tumour biology: senescence inpremalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La O JP, Emersonb LL, Goodmana JL, Froebea SC, Illuma BE, Curtisa AB, Murtaugh LCh. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, Maitra A. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson D. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40:612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15:72–77. doi: 10.1038/sj.cr.7290269. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature. 2001;413:83–86. doi: 10.1038/35092584. [DOI] [PubMed] [Google Scholar]
- Löhr M, Klöppel G, Maisonneuve P, Lowenfels AB, Lüttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- Lüttges J, Schlehe B, Menke MA, Vogel I, Henne-Bruns D, Klöppel G. The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer. 1999;85:1703–1710. [PubMed] [Google Scholar]
- Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, 4th, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow ChP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. The Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. PNAS. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater SD, Williamson RC, Foster CS. Proliferation of parenchymal epithelial cells enhanced in chronic pancreatitis. J Pathol. 1998;186:104–108. doi: 10.1002/(SICI)1096-9896(199809)186:1<104::AID-PATH141>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Smith WL, Meade EA, Hewitt DL. Pharmacology of prostaglandin endoperoxide synthase isozymes-1 and -2. Ann N Y Acad Sci. 1994;714:136–142. doi: 10.1111/j.1749-6632.1994.tb12037.x. [DOI] [PubMed] [Google Scholar]
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Fernández–Del Castillo C, Warshaw AL, Thayer SP. In Vivo Lineage Tracing Defines the Role of Acinar-to-Ductal Transdifferentiation in Inflammatory Ductal Metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Fukamachi K, Futakuchi M, Alexander DB, Long N, Tamamushi S, Kohtaro M, Susumu S, Hirotaka O, Takashi J, Hiroyuki T. Mature acinar cells are refractory to carcinoma development by targeted activation of Ras oncogene in rats. Cancer Sci. 2010;101:341–346. doi: 10.1111/j.1349-7006.2009.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhune PG, Phifer DM, Tosteson TD, Longnecker DS. K-ras mutations in focal proliferative lesions of human pancreas. Cancer Epidemiol Biomarkers Prev. 1998;7:515–521. [PubMed] [Google Scholar]
- Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, Yeo CJ, Kern SE, Hruban RH. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998;58:4740–4744. [PubMed] [Google Scholar]
- Yoshida T, Nobuaki Shiraki N, Baba H, Goto M, Fujiwara S, Kume K, Kume S. Expression patterns of epiplakin in pancreas, pancreatic cancer and regenerating pancreas. Genes Cells. 2008;13:667–678. doi: 10.1111/j.1365-2443.2008.01196.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.