Abstract
More than 100 recent collections of Valsaria sensu lato mostly from Europe were used to elucidate the species composition within the genus. Multigene phylogeny based on SSU, LSU, ITS, rpb2 and tef1 sequences revealed a monophyletic group of ten species within the Dothideomycetes, belonging to three morphologically similar genera. This group could not be accommodated in any known family and are thus classified in the new family Valsariaceae and the new order Valsariales. The genus Valsaria sensu stricto comprises V. insitiva, V. robiniae, V. rudis, V. spartii, V. lopadostomoides sp. nov. and V. neotropica sp. nov., which are phylogenetically well-defined, but morphologically nearly indistinguishable species. The new monotypic genus Bambusaria is introduced to accommodate Valsaria bambusae. Munkovalsaria rubra and Valsaria fulvopruinata are combined in Myrmaecium, a genus traditionally treated as a synonym of Valsaria, which comprises three species, with M. rubricosum as its generic type. This work is presented as a basis for additional species to be detected in future.
Keywords: Ascomycota, Dothideomycetes, Multigene phylogenetic analysis, Pyrenomycetes
Introduction
Little has been published about the genus Valsaria apart from the protologues of numerous species epithets and some collection records. Ju et al. (1996) examined numerous specimens and synonymized most names with the type species V. insitiva. They sketched the taxonomic history of the genus and accepted and provided a key to four species. Included were species of Myrmaecium Fuckel, which had been traditionally treated as a synonym of Valsaria. The taxonomic position of Valsaria had been controversial. The majority of fungal taxonomists agreed that the hamathecium consists of true, apically free paraphyses, a true ascomatal wall distinct from the surrounding pseudostroma and unitunicate asci (see e.g. Barr 1978, 1990; Glawe 1985; Huhndorf 1992), and Kirk et al. (2008) listed the genus as belonging in Diaporthales (Sordariomycetes). Among others Ju et al. (1996) recognized that the asci of Valsaria, although not obviously fissitunicate, are bitunicate and therefore considered Valsaria among the Dothideomycetes (as Loculoascomycetes). The combination of the above mentioned characters is however not known in any group of this class (Hyde et al. 2013).
Ju et al. (1996) described the asexual morph of V. bambusae. A detailed study of asexual morphs of a Valsaria from Gleditsia (identified as V. insitiva by the author but probably a different species as judged from the fabaceous host and the rather small ascospore size) was presented by Glawe (1985). He determined that Valsaria produces four asexual morphs, 1) yeast-like, conidia produced by budding of ascospores and conidia, 2) hyphomycetous, conidia produced from percurrently proliferating conidiogenous loci, 3) arthrospores, produced by disarticulation of hyphae and 4) pycnidial, with phialidic conidiogenesis. He compared the hyphomycetous state with genera such as Aureobasidium, Candida and Exophiala, while Ju et al. (1996) interpreted it as hormonema-like. Glawe (1985) confirmed Wehmeyer’s (1923) view that the pycnidial state, characterized by multiloculate conidiomata, phialidic conidiogenous cells and hyaline and oval conidia, can be ascribed to Cytosporella.
In this study, we investigated the species diversity of Valsaria sensu lato in Europe, augmented with isolates obtained from some specimens collected on other continents. Phylogenetic relationships of the three resulting genera are determined, confirming the taxonomic placement in the class Dothideomycetes as had been suggested by Ju et al. (1996). A new family and order are introduced to accommodate valsaria-like taxa which comprise fungi with a unique set of morphological characters.
Materials and methods
Isolates and specimens
Isolates used in this study originated from single ascospores, rarely conidia of fresh specimens or older specimens, which still contained living ascospores. Strain numbers and NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms starting with V are used here for both specimens and strains. Representative isolates have been deposited at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS) or the culture collection of Mae Fah Luang University (MFLUCC). Details of the specimens used for morphological studies are listed in the Taxonomy section under the respective descriptions. Herbarium or fungarium acronyms are according to Thiers (2014). Freshly collected specimens have been deposited in the Herbarium of the Institute of Botany, University of Vienna (WU) or the Mae Fah Luang University Herbarium (MFLU). Other specimens are housed in private fungaria; specimens labelled with JF in the fungarium of J. Fournier, those with JDR in the fungarium of J. D. Rogers; those with W.J. have been incorporated in WU.
Table 1.
Isolates and GenBank accession numbers used in the phylogenetic analyses. The KP-sequences were newly generated during this study
| GenBank accession numbers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Isolate No. | Code | Herbarium No.a | Substrate/Host | Country | SSU | ITS-LSU | rpb2 | tef1 |
| Bambusaria bambusae | MFLUCC 12-0851 | DDQ00253 | MFLU 15-0050 | Thyrsostachys siamensis | Thailand | – | KP687812 | KP687890 | KP687982 |
| Bambusaria bambusae | CBS 139763 | DDQ00254 | MFLU 15-0051 | Thyrsostachys siamensis | Thailand | KP687962 | KP687813 | KP687891 | KP687983 |
| Myrmaecium fulvopruinatum | CBS 139057 | VF | WU 33433 | Fagus sylvatica | Austria | KP687967 | KP687858 | KP687933 | KP688027 |
| Myrmaecium fulvopruinatum | VF1 | WU 33434 | Fraxinus excelsior, Alnus glutinosa | Austria | – | KP687859 | KP687934 | KP688028 | |
| Myrmaecium fulvopruinatum | VFB | WU 33436 | Betula pendula | Austria | – | KP687860 | KP687935 | KP688029 | |
| Myrmaecium fulvopruinatum | CBS 139058 | VFJ | NY (M.E.B.B. 6905) (E) | Fagus grandifolia | U.S.A. | KP687968 | KP687861 | KP687936 | KP688030 |
| Myrmaecium fulvopruinatum | VFJ1 | WU 33437 | unidentified corticated twigs | Taiwan | – | KP687862 | KP687937 | KP688031 | |
| Myrmaecium fulvopruinatum | CBS 139059 | VFQ | WU 33438 | Quercus cerris | Austria | KP687969 | KP687863 | KP687938 | KP688032 |
| Myrmaecium rubricosum | CBS 139067 | VRF | WU 33447 | unidentified bark | France | KP687977 | KP687881 | KP687955 | KP688049 |
| Myrmaecium rubricosum | VRJ | Quercus robur | France | – | KP687882 | KP687956 | KP688050 | ||
| Myrmaecium rubricosum | VRJ1 | WU 33448 | unidentified bark | U.S.A. | – | KP687883 | KP687957 | KP688051 | |
| Myrmaecium rubricosum | CBS 139069 | VRM | WU 33449 | Picea abies | Austria | KP687978 | KP687884 | – | KP688052 |
| Myrmaecium rubricosum | CBS 139068 | VRP | WU 33450 | Quercus pubescens | Croatia | KP687979 | KP687885 | KP687958 | KP688053 |
| Myrmaecium rubrum | CBS 109505 | Quercus sp. | Italy | GU456303 | GU456324b | GU456344 | GU456260 | ||
| Valsaria insitiva | CBS 139056 | VA | WU 33453 | Acer monspessulanum | Croatia | KP687965 | KP687847 | KP687922 | KP688016 |
| Valsaria insitiva | VAC | WU 33454 | Acer campestre | Austria | – | KP687849 | KP687924 | KP688018 | |
| Valsaria insitiva | VAF | WU 33455 | Ficus carica | France | – | KP687850 | KP687925 | KP688019 | |
| Valsaria insitiva | VCE | WU 33456 | Cercis siliquastrum | Greece | – | KP687854 | – | – | |
| Valsaria insitiva | CBS 139061 | VIJ | WU 33457 | unidentified corticated twigs | Taiwan | – | KP687866 | KP687941 | KP688035 |
| Valsaria insitiva | VIP | WU 33458 | Paliurus spina-christi | Greece | KP687971 | KP687867 | KP687942 | KP688036 | |
| Valsaria insitiva | VIR2 | WU 33459 | Robinia pseudacacia | Austria | – | KP687871 | KP687946 | KP688040 | |
| Valsaria insitiva | VJF | WU 33460 | Ficus carica | France | – | KP687873 | – | – | |
| Valsaria insitiva | VL | WU 33461 | unidentified corticated twigs | France | – | KP687875 | KP687949 | KP688043 | |
| Valsaria insitiva | CBS 127882 | VV | WU 33462 (E) | Vitis vinifera | Croatia | KP687980 | KP687886 | KP687959 | KP688054 |
| Valsaria insitiva | VV1 | WU 33463 | Vitis vinifera | Italy | KP687981 | KP687887 | KP687960 | KP688055 | |
| Valsaria insitiva | VV2 | WU 33464 | Vitis vinifera | Greece | – | KP687888 | – | – | |
| Valsaria insitiva | VW | WU 33465 | Wisteria sinensis | France | – | KP687889 | KP687961 | KP688056 | |
| Valsaria insitiva | V5 | WU 33466 | Cytisus scoparius | Italy | – | KP687842 | – | KP688012 | |
| Valsaria insitiva | V8 | WU 33467 | Spiraea sp. | Austria | – | KP687845 | – | – | |
| Valsaria insitiva | V35 | WU 33427 | Vitis vinifera | Austria | – | KP687839 | – | KP688009 | |
| Valsaria lopadostomoides | CBS 139062 | VIQ | WU 33470 (H) | Quercus ilex | Greece | KP687972 | KP687868 | KP687943 | KP688037 |
| Valsaria neotropica | CBS 139064 | VJM | WU 33471 (H) | unidentified corticated twig | France | KP687974 | KP687874 | KP687948 | KP688042 |
| Valsaria robiniae | CBS 121890 | VC | WU 33472 | Hippocrepis emerus | Slovenia | – | KP687851 | KP687926 | KP688020 |
| Valsaria robiniae | VC1 | WU 33473 | Hippocrepis emerus | Croatia | – | KP687852 | KP687927 | KP688021 | |
| Valsaria robiniae | CBS 128015 | VCA | WU 33474 | Caragana arborescens | Austria | – | KP687853 | KP687928 | KP688022 |
| Valsaria robiniae | CBS 125583 | VCol | WU 33475 | Colutea arborescens | Austria | KP687966 | KP687855 | KP687930 | KP688024 |
| Valsaria robiniae | VIR | WU 33477 | Robinia pseudacacia | Italy | – | KP687869 | KP687944 | KP688038 | |
| Valsaria robiniae | CBS 139063 | VIR1 | WU 33478 | Robinia pseudacacia | Italy | KP687973 | KP687870 | KP687945 | KP688039 |
| Valsaria robiniae | V29 | WU 33479 | Amorpha fruticosa | Hungary | – | KP687835 | KP687913 | KP688005 | |
| Valsaria robiniae | V30 | WU 33480 | Amorpha fruticosa | Hungary | – | KP687837 | KP687915 | KP688007 | |
| Valsaria rudis | VQC | WU 33483 | Quercus cerris | Croatia | – | KP687877 | KP687951 | KP688045 | |
| Valsaria rudis | CBS 139065 | VQM | WU 33484 | Quercus macrolepis | Greece | KP687975 | KP687878 | KP687952 | KP688046 |
| Valsaria rudis | CBS 139066 | VQP | WU 33485 (E) | Quercus pubescens | Austria | KP687976 | KP687879 | KP687953 | KP688047 |
| Valsaria rudis | V3 | WU 33486 | Quercus cerris | Italy | – | KP687836 | KP687914 | KP688006 | |
| Valsaria rudis | V7 | WU 33487 | Quercus petraea | Austria | – | KP687844 | KP687920 | KP688014 | |
| Valsaria rudis | V31 | WU 33488 | Quercus petraea | Austria | – | KP687838 | KP687916 | KP688008 | |
| Valsaria spartii | VA2 | WU 33493 | Acer sempervirens | Greece | – | KP687848 | KP687923 | KP688017 | |
| Valsaria spartii | CBS 121714 | VCBS | CBS H-19925 | Ceratonia siliqua | Greece | – | EU040213 | KP687929 | KP688023 |
| Valsaria spartii | CBS 125584 | VCS | WU 33494 | Cytisus scoparius | Italy | – | KP687856 | KP687931 | KP688025 |
| Valsaria spartii | VCV | WU 33495 | Calicotome villosa | Greece | – | KP687857 | KP687932 | KP688026 | |
| Valsaria spartii | VG | WU 33496 | Genista florida | Spain | – | KP687864 | KP687939 | KP688033 | |
| Valsaria spartii | CBS 139060 | VIC | WU 33497 | Ceratonia siliqua | Spain | KP687970 | KP687865 | KP687940 | KP688034 |
| Valsaria spartii | VIS | WU 33498 | Spiraea sp. | France | – | KP687872 | KP687947 | KP688041 | |
| Valsaria spartii | VMA | WU 33499 | Melia azedarach | Greece | – | KP687876 | KP687950 | KP688044 | |
| Valsaria spartii | CBS 128016 | VR | WU 33500 | Retama sphaerocarpa | Spain | – | KP687880 | KP687954 | KP688048 |
| Valsaria spartii | V1 | WU 33502 | Robinia pseudacacia | Italy | – | KP687814 | KP687892 | KP687984 | |
| Valsaria spartii | V2 | WU 33503 | Fraxinus ornus | Italy | – | KP687825 | KP687903 | KP687995 | |
| Valsaria spartii | V4 | WU 33504 | Cytisus sessilifolius | Italy | – | KP687840 | KP687917 | KP688010 | |
| Valsaria spartii | V4a | WU 33504 | Cytisus sessilifolius | Italy | – | KP687841 | KP687918 | KP688011 | |
| Valsaria spartii | CBS 139070 | V6 | WU 33505 (E) | Spartium junceum | Italy | KP687964 | KP687843 | KP687919 | KP688013 |
| Valsaria spartii | V9 | WU 33506 | Chamaecytisus proliferus | Spain | – | KP687846 | KP687921 | KP688015 | |
| Valsaria spartii | CBS 139071 | V10 | WU 33507 | Spartium junceum | Spain | KP687963 | KP687815 | KP687893 | KP687985 |
| Valsaria spartii | V11 | WU 33508 | Retama sphaerocarpa | Spain | – | KP687816 | KP687894 | KP687986 | |
| Valsaria spartii | V12 | WU 33509 | Acacia saligna | Spain | – | KP687817 | KP687895 | KP687987 | |
| Valsaria spartii | V13 | WU 33510 | Retama monosperma | Spain | – | KP687818 | KP687896 | KP687988 | |
| Valsaria spartii | V14 | WU 33511 | Calicotome villosa | Spain | – | KP687819 | KP687897 | KP687989 | |
| Valsaria spartii | V15 | WU 33512 | Retama sphaerocarpa | Spain | – | KP687820 | KP687898 | KP687990 | |
| Valsaria spartii | V16 | WU 33513 | Anagyris foetida | Spain | – | KP687821 | KP687899 | KP687991 | |
| Valsaria spartii | V17 | WU 33514 | Teline monspessulana | Spain | – | KP687822 | KP687900 | KP687992 | |
| Valsaria spartii | V18 | WU 33515 | Ulex parviflorus | Spain | – | KP687823 | KP687901 | KP687993 | |
| Valsaria spartii | V19 | WU 33516 | Teline linifolia | Spain | – | KP687824 | KP687902 | KP687994 | |
| Valsaria spartii | V20 | WU 33517 | Cytisus baeticus | Spain | – | KP687826 | KP687904 | KP687996 | |
| Valsaria spartii | V21 | WU 33518 | Retama monosperma | Spain | – | KP687827 | KP687905 | KP687997 | |
| Valsaria spartii | V22 | WU 33519 | Teline linifolia | Spain | – | KP687828 | KP687906 | KP687998 | |
| Valsaria spartii | V23 | WU 33520 | Acacia saligna | Spain | – | KP687829 | KP687907 | KP687999 | |
| Valsaria spartii | V24 | WU 33521 | Spartium junceum | Spain | – | KP687830 | KP687908 | KP688000 | |
| Valsaria spartii | V25 | WU 33522 | Cytisus striatus | Spain | – | KP687831 | KP687909 | KP688001 | |
| Valsaria spartii | V26 | WU 33523 | Spartium junceum | Spain | – | KP687832 | KP687910 | KP688002 | |
| Valsaria spartii | V27 | WU 33524 | Genista cinerea | Spain | – | KP687833 | KP687911 | KP688003 | |
| Valsaria spartii | V28 | WU 33525 | Ononis speciosa | Spain | – | KP687834 | KP687912 | KP688004 | |
(E) epitype, (H) holotype
Only LSU was available
Culture preparation, growth rate determination and phenotype analysis
Cultures were prepared and maintained as described previously (Jaklitsch et al. 2014; Chomnunti et al. 2014). For determination of growth rates and asexual morph morphology in culture, 90 mm diam Petri dishes containing MEA (2% w/v malt extract, 2 % w/v agar-agar; Merck, Darmstadt, Germany), were centrally inoculated and incubated at 22–25 °C under alternating 12 h cool daylight and 12 h darkness. For inoculations strains were reconstituted from −80 °C, thus fresh cultures may grow faster than given in the species descriptions. Microscopic observations were made in de-ionised water or 3% KOH, asexual morphic data generally determined in 3 % KOH; Congo Red and blue Waterman ink (Fig. 9k) were used for staining of ascal apical rings. Morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch 2009). Data were gathered using a Nikon Coolpix 995, Coolpix 4500, Nikon DS-U2 or Canon 550D digital camera and measured by using the NIS Elements D v. 3.0 software or the Tarosoft (R) Image Frame Work program. Methods of microscopy included stereomicroscopy using an Olympus SZ 60, Nikon SMZ 1500 or Zeiss Stereo Discovery V8 and Nomarski differential interference contrast (DIC) using the compound microscope Nikon Eclipse E600 or Nikon Eclipse 80i. For certain images of stromata the stacking software Zerene Stacker version 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses. The colour term rosy denotes a range of pale pinkish colours. Substellate and inversely stellate as used in the description of ectostromatic structures are deviations from star-shaped; inversely stellate is equivalent to the shape of a circular cake radially cut into slices, i.e. the slices become broader with distance from the centre.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch 2011; Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany), the Biospin Fungus Genomic DNA Extraction Kit (BioFlux®) or the modified CTAB method of Riethmüller et al. (2002). Five loci were amplified and sequenced: the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a ca. 0.9–1.3 kb fragment of the large subunit nuclear ribosomal DNA (nuLSU rDNA), amplified and sequenced as a single fragment with primers V9G (de Hoog and Gerrits van den Ende 1998) and LR5 (Vilgalys and Hester 1990), or as two separate fragments with primers ITS5 and ITS4 (White et al. 1990), and LROR (Vilgalys and Hester 1990) and LR5; a ca. 1.7–3.2 or 1.1 kb fragment of the small subunit nuclear ribosomal DNA (nSSU rDNA), amplified and sequenced with primers SL1 (Landvik et al. 1997) and NS24mod (Voglmayr and Jaklitsch 2011) or NS1 and NS4 (White et al. 1990), a ca. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999); and a ca. 1.3 kb fragment of the translation elongation factor 1-alpha (tef1) with primers EF1-728F (Carbone and Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr and Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, U.K.) with the same primers as in PCR and an automated DNA sequencer (3730xl Genetic Analyzer, Applied Biosystems); in addition, internal primers ITS4 and LR3 (Vilgalys and Hester 1990) were used for sequencing the partial nuSSU–complete ITS–partial nuLSU rDNA region, and nssu1088 and nssu1088R (Kauff and Lutzoni 2002) for sequencing of the nuSSU rDNA region.
Analysis of sequence data
All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit version v. 7.0.4.1 (Hall 1999). To investigate the phylogenetic relationships of Valsaria spp. within the Ascomycota, in particular the previously assumed affiliation with the Diaporthales, nuLSU rDNA sequences of all species sequenced in the present study were alignend with those of selected Sordariomycetes (from Calosphaeriales, Diaporthales and Xylariales) and Dothideomycetes (representatives from all orders recognised by Hyde et al. 2013 and Wijayawardene et al. 2014), resulting in a matrix of 1344 nucleotide positions. Subsequently, to reveal the phylogenetic position within Dothideomycetes, sequences of one representative isolate for each Valsariaceae species sequenced in the current study were aligned to the combined multigene (LSU, SSU, rpb2, tef1) matrix of Hyde et al. (2013). The resulting combined four-gene sequence matrix contained 424 taxa and 4645 alignment positions (1428, 1101, 1154 and 962 characters from LSU, SSU, rpb2 and tef1, respectively). Schismatomma decolorans (Roccellaceae, Arthoniomycetes) was selected as outgroup taxon as in Hyde et al. (2013). For the detailed investigation of phylogenetic relationships between the species of Bambusaria, Myrmaecium and Valsaria, a combined four-gene matrix of ITS, LSU, rpb2 and tef1 was produced and analysed. Bambusaria bambusae was selected as outgroup according to the results of the LSU tree. The resulting combined sequence matrix contained 4466 alignment positions (2018 from ITS-LSU, 1207 from rpb2 and 1241 from tef1).
Maximum parsimony (MP) analysis of the LSU matrix was performed with PAUP v. 4.0 b10 (Swofford 2002), using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. The COLLAPSE command was set to MINBRLEN. For the phylogenetic analysis of the combined matrix of Dothideomycetes and Valsariaceae, a parsimony ratchet approach was implemented. For this, ratchet nexus files were produced using PRAP 2.0b3 (Müller 2004), with the following settings: 1000 ratchet replicates, weight 2 and 25 % weighted characters. Subsequently, the matrices were analysed with PAUP. For the analysis of Valsariaceae, the resulting best trees were then reloaded into PAUP and used as starting trees for further tree search with TBR branch swapping and the same settings given above. For all three matrices, bootstrap analyses with 1000 replicates were performed in the same way, but using 5 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate; in addition, each replicate was limited to 1 million rearrangements.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro and Michalak 2012), using the ML+rapid bootstrap setting and the GTRGAMMA substitution model with 1000 bootstrap replicates. For the combined analyses, substitution model parameters were calculated separately for the different gene regions.
Results
Molecular phylogeny
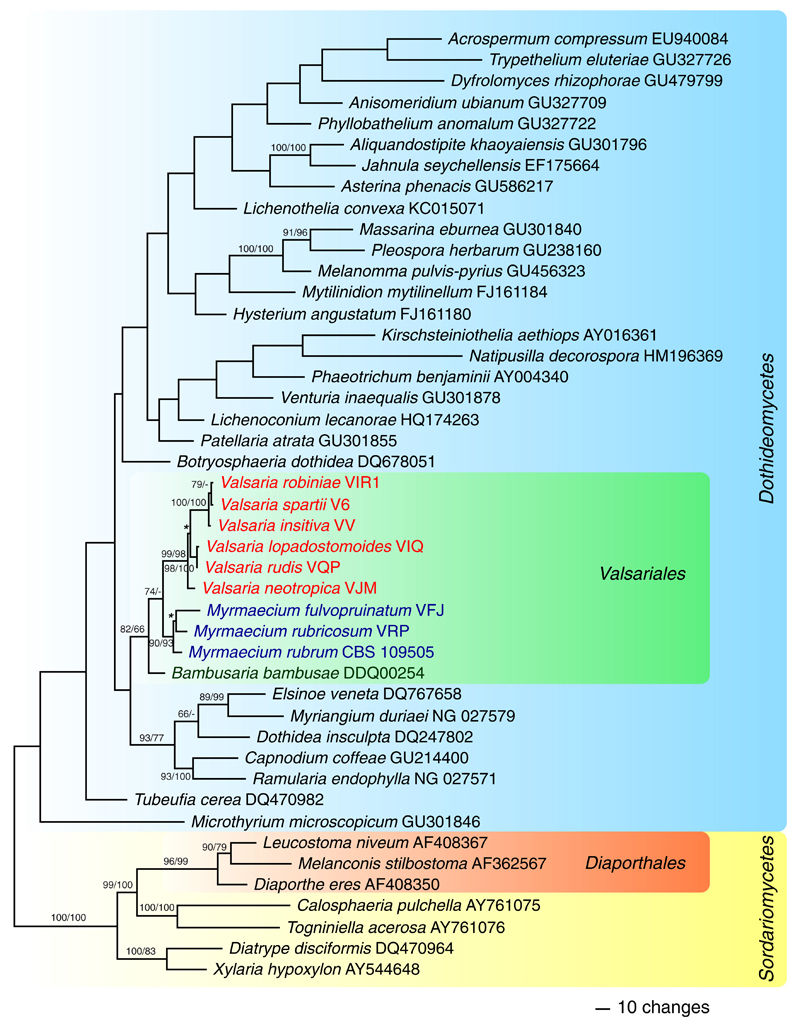
All phylogenetic analyses support the Valsariales as a monophylum within Dothideomycetes, but the closest relatives of the order could not be revealed due to lack of backbone support. Highly supported is also the split of Valsaria into three distinct genera (Bambusaria, Myrmaecium and Valsaria), as well as the placement of Munkovalsaria rubra within Myrmaecium.
Of the 1344 characters of the LSU matrix, 424 were parsimony informative. MP analyses revealed three MP trees with a score of 2484, one of which is shown as Fig. 1. The three MP trees differed slightly in the positions of Myrmaecium rubrum and Valsaria neotropica. The phylogenetic analyses placed Valsariaceae within Dothideomycetes with maximum support. Within Valsariaceae three genera, Bambusaria, Myrmaecium and Valsaria, are highly supported, but the sister group relationship of Bambusaria to the other two genera receives only medium (MP) or no (ML) bootstrap support.
Fig. 1.
Phylogram showing one of three MP trees 2484 steps long revealed by PAUP from an analysis of the nuLSU rDNA matrix of selected Sordariomycetes and representatives of all accepted orders of Dothideomycetes, showing the phylogenetic position of Bambusaria, Myrmaecium and Valsaria (Valsariaceae, Valsariales) within Dothideomycetes. MP and ML bootstrap support above 60 % are given above or below the branches. GenBank accession numbers or strain/culture designations are given following the taxon names. Nodes marked by an asterisk (*) collapsed in the strict consensus of the three MP trees
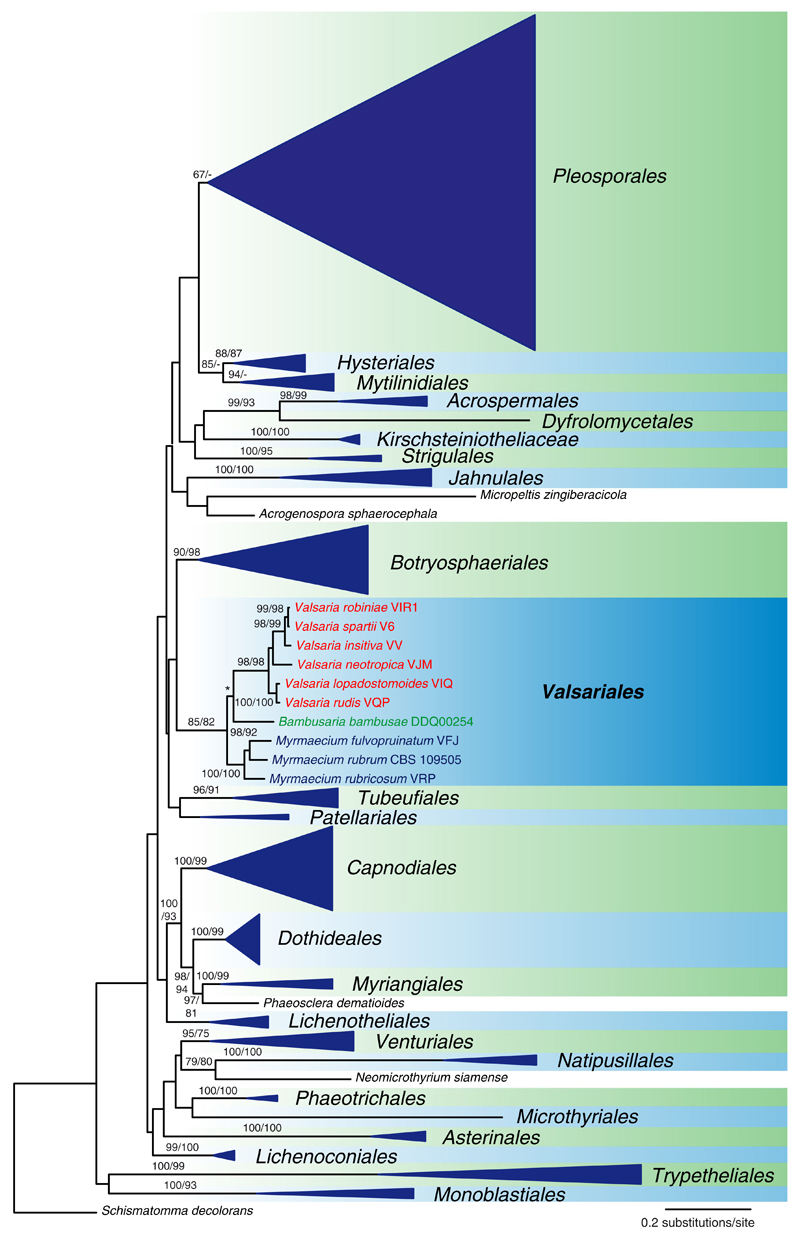
Of the 4645 characters included in the combined four-gene matrix of Dothideomycetes, 2345 were parsimony informative. The parsimony ratchet revealed 82 trees of score 40833 (not shown). Figure 2 shows the best tree (lnL = –180369.8567) revealed by RAxML; to enable an easier overview, all lineages except Valsariales were collapsed to ordinal level; for details on the various orders see Hyde et al. (2013). The status of Valsariales as a distinct order is highly supported. Within the Valsariaceae clade, the three genera Bambusaria, Myrmaecium and Valsaria are again highly supported, but Bambusaria does not occupy a basal position. However, in the MP trees obtained by the parsimony ratchet, Bambusaria is placed in a basal position as in the LSU analysis with 76 % MP bootstrap support (data not shown).
Fig. 2.
Simplified phylogram of the best maximum likelihood tree (lnL= –180369.8567) revealed by RAxML from an analysis of the combined multigene (LSU, SSU, rpb2, tef1) matrix of Hyde et al. (2013), containing 414 taxa of Dothideomycetes and the sequences of all species of Bambusaria, Myrmaecium and Valsaria (Valsariaceae, Valsariales) sequenced in the present study. Except for Valsariales, all lineages were collapsed to ordinal level. ML and MP bootstrap support above 70 % are given above or below the branches. The asterisk (*) denotes the node in conflict with the MP tree, where Bambusaria has a basal position in Valsariales with 76 % MP bootstrap support. The tree was rooted with Schismatomma decolorans according to Hyde et al. (2013)
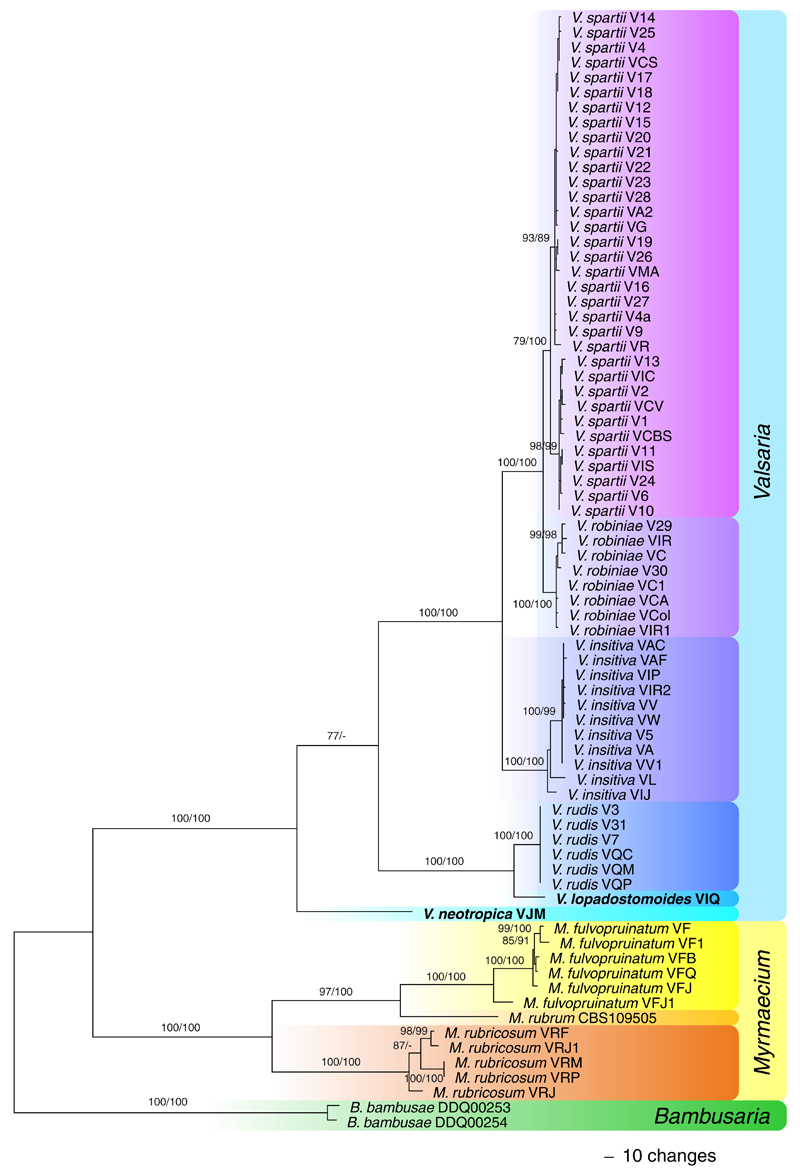
Of the 4466 characters included in the combined matrix of Valsariaceae, 1110 were parsimony informative. MP heuristic search on the 96 best trees obtained in the parsimony ratchet analysis revealed 1022 MP trees with a score of 2362, one of which is shown as Fig. 3. All MP trees were identical except for minor topological differences within the same species (data not shown). The three genera Bambusaria, Myrmaecium and Valsaria as well as the various species received maximum support, except for V. spartii which receives maximum support in ML but medium support (79 %) in MP analyses. Within Valsaria, six genetically distinct species were revealed, which all would have previously been classified as V. insitiva. Remarkably, within V. spartii two highly supported subclades were present. It is notable that in all accessions of V. lopadostomoides, V. robiniae and V. spartii sequenced, except one (V26) of V. spartii, a homologous species-specific insertion of ca. 400 bp was present at the 3’ end of the LSU fragment. In V. spartii two distinct alleles of this insertion were present according to the two subclades of the phylogenetic analyses (Fig. 3).
Fig. 3.
Phylogram showing one of 1022 MP trees 2362 steps long revealed by PAUP from an analysis of the combined four-gene (ITS, LSU, rpb2, tef1) matrix of Bambusaria, Myrmaecium and Valsaria. MP and ML bootstrap support above 70 % are given above or below the branches. Strain/culture designations are given following the taxon names; new species are marked in bold
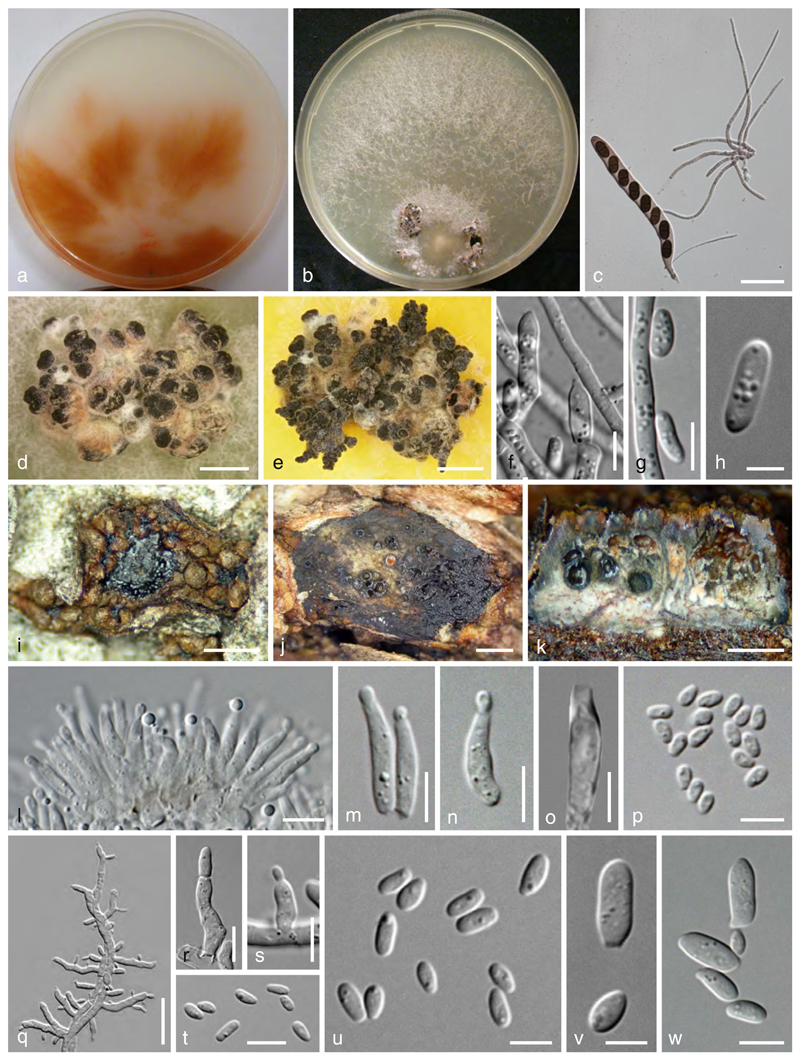
Phenotype, cultures
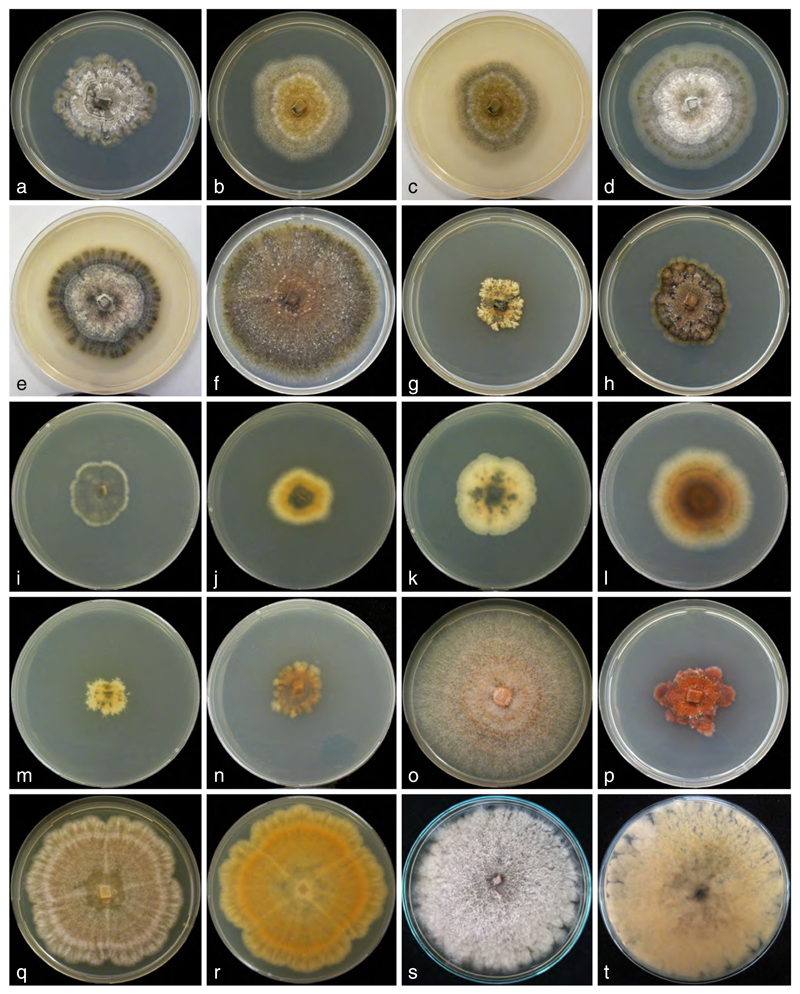
Detailed descriptions of sexual morphs, cultures and asexual morphs are given for each genus and species in the Taxonomy section. Besides molecular data there are hardly any useful morphological characters for species distinction within Valsaria, but macroscopic appearance of cultures (Fig. 4) and growth rates are however more or less diagnostic, although in some instances predominantly conidia are formed by budding of ascospores, with little hyphal growth, which ceases quickly. Differences in culture appearance involve colour, abundance and organization of aerial hyphae and zonation, but also secondary changes due to the formation of pycnidia. In species of Myrmaecium the mycelium covers a centrally inoculated 90 mm Petri dish at room temperature within a week, Bambusaria grows only slightly slower. In contrast, growth in Valsaria is considerably slower, ranging from 3 mm colony radius in V. rudis to 20 mm in V. robiniae and V. neotropica after a week. The most closely related species can also be distinguished by the amount of arthroconidia formed, being inconspicuous in V. robiniae and abundant in V. spartii.
Fig. 4.
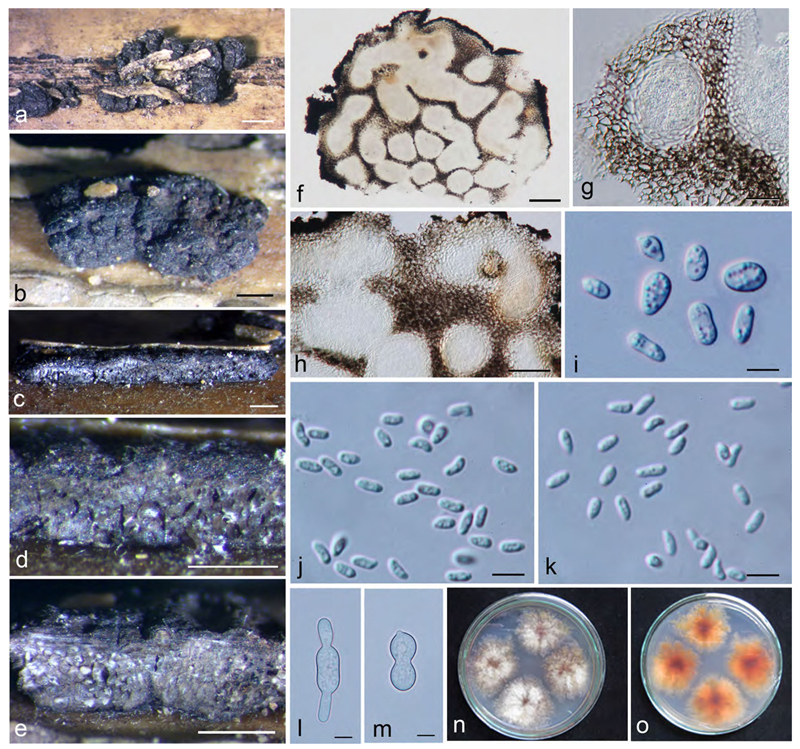
Cultures of Valsariales on MEA at 22–25 °C. a, i Valsaria insitiva VV b, c, j V. lopadostomoides VIQ. d, e, k. V. neotropica VJM. f, l V. robiniae VIR1. g, m V. rudis VQP. h, n V. spartii V6. o, p. Myrmaecium rubricosum VRP. (p on PDA). q, r M. fulvopruinatum VF. s, t Bambusaria bambusae DDQ00253. a–h, o, p, q, s. Surface view. i–n, r, t. Reverse. a–h. After 17 days. i–n. After 10 days. o, q, r. After 7 days. p, s, t. After 14 days
Ecology and distribution
Stromata of Valsariaceae generally occur in little to moderately decayed bark. In Europe, the only continent where we collected specimens extensively, species of Valsaria occur in warmer regions, predominantly in Southern Europe, particularly the (Sub-)Mediterranean. The dominant and common species of the Mediterranean is V. spartii; other species such as V. insitiva, V. lopadostomoides, V. robiniae and V. rudis are uncommon or rare in this region. In other European regions the genus occurs in areas favoured by a warm climate such as wine-growing areas or regions influenced by the Gulf Stream, as 10 records (possibly including Myrmaecium) are evidenced in the Checklist of the British Mycological Society (FRDBI 2015). Although infrequently, it occurs also in southern Sweden (Eriksson 2014). The genus is absent or rare in areas with severe winters. This may be extrapolated to other continents: Schweinitz (1822) already described taxa of Valsaria in North America, and Ellis and Everhart (1892) listed 23 species. Barr et al. (1996) gave an account of taxa described by J. B. Ellis and detected additional names that belong to Valsaria or Myrmaecium. Valsaria appears to be uncommon or understudied in tropical regions. Bambusaria bambusae, as described below, is host-specific for Bambusoideae and is presently only known from India and Thailand. Members of the genus Myrmaecium have a worldwide distribution and typically occur on sun-exposed, corticated logs and branches of coniferous and broadleaf trees.
Taxonomy
Valsariales Jaklitsch, K.D. Hyde & Voglmayr, ord. nov.
MycoBank MB 811900
Type family: Valsariaceae Jaklitsch, K.D. Hyde & Voglmayr
Saprobic in bark or on culms of bamboo. Sexual morph: Ascomata perithecioid, immersed in eu- or pseudostromata. Ostiole periphysate. Hamathecium of true paraphyses. Asci bitunicate, usually without obvious fissitunicate dehiscence. Ascospores dark brown, bicellular, budding in artificial culture. Asexual morphs in nature coelomycetous, in culture hyphomycetous and/or coelomycetous.
Valsariaceae Jaklitsch, K.D. Hyde & Voglmayr, fam. nov.
MycoBank MB 811901
Type genus: Valsaria Ces. & De Not.
Saprobic in bark or on culms of bamboo. Sexual morph: Ascomata perithecioid, clustered, immersed in immersed, erumpent to superficial eu- or pseudostromata, usually monostichous in valsoid or diatrypoid configuration, upright or oblique, with several ostiolar necks fusing into one. Ostiole periphysate. Hamathecium of true, apically free paraphyses. Asci cylindrical, bitunicate, usually without obvious fissitunicate dehiscence, (4–)6–8-spored, with short pedicel and an inconspicuous non-amyloid apical ring often staining in Congo Red, persistently attached to the ascogenous hyphae at maturity. Ascospores ellipsoid to subfusiform, dark brown, 2-celled, with a dark, usually non-constricted or scarcely constricted septum, budding in artificial culture, with surface ornamentation. Asexual morphs in nature coelomycetous, in culture hyphomycetous or coelomycetous. Conidia produced on phialides, minute pegs or by budding of ascospores and conidia, 1-celled, hyaline, smooth.
Valsaria Ces. & De Not., Comm. Soc. crittog. Ital. 1: 205 (1863).
Type species: Valsaria insitiva (Tode : Fr.) Ces. & De Not.
Sexual morph: Stromata pseudostromatic, immersed-erumpent from bark to superficial on wood, scattered or gregarious to coalescing into variable clusters; pustular, broadly conical or subglobose; enclosed on top and/or at the sides by a black pseudoparenchymatous crust spreading around the base and blackening the wood surface between adjacent stromata. Stroma surface usually irregularly tubercular, sometimes cerebriform. Ectostroma frequently forming substellate or inversely stellate structures around the inconspicuous ostiolar openings, consisting of 3–5 greyish, olivaceous brown to dull black, sharply delimited tubercular segments with a depressed centre; sometimes reduced or absent and replaced by a smooth cupulate surface partly roughened by the ostiolar papillae; the tissue just beneath the black crust pseudoparenchymatous, a soft greyish to brown textura angularis of small and thin-walled cells; the tissue between ostiolar necks and at the stromatal base prosenchymatous, grey, soft, forming a textura intricata of hyaline to subhyaline, 2–4 µm wide hyphae, often mixed with bark cells; tissue between ascomata typically absent. Ascomata monostichously arranged in valsoid configuration, 4–12(–20) per individual cluster, vertically to obliquely arranged, subglobose to flask-shaped, typically laterally collapsed when dry; peridium 14–25 µm thick, consisting of pale brown flattened cells, turning darker towards the outside. Ostiolar necks long, cylindrical, converging and often fusing, i.e. ostiolar opening at the surface eventually containing necks of 1–3(–5) ascomata; ostiolar wall of pale brown, small, thin to moderately thick-walled angular cells; interior densely periphysate. Ostiolar openings usually inconspicuous at the surface, less commonly necks arising as stout but fragile, conical, more or less sulcate, shiny black columns. Paraphyses numerous, simple, unbranched, tapering upwards, apically free, 1.5–6 µm wide. Asci bitunicate but without obvious fissitunicate dehiscence, cylindrical, containing (4–)6–8 uniseriate ascospores; with a short pedicel and a thick apex containing an ocular chamber and a more or less pulvinate ring staining in Congo Red. Ascospores ellipsoid, dark brown, 2-celled, with a dark central, non-constricted to distinctly constricted septum thicker than the wall; with finely tuberculate, dotted or reticulate surface ornamentation.
Asexual morph on natural substrates: when present, interpretable as multiloculate pycnidia.
Locules present at upper levels of young sexual stromata, or stromata exclusively containing irregularly arranged locules or meandering paths. Walls of locules lined by dense palisades of phialides in variable whorls on short cylindrical, few-celled hyaline conidiophores. Phialides lageniform to cylindrical, straight to sinuous, often inequilateral; conidia also formed on aphanophialides (lateral pegs on or below phialides producing conidia). Conidia numerous, oblong, allantoid, ellipsoid or bullet-shaped, rarely subglobose, 1-celled, hyaline, smooth, with inconspicuous guttules and indistinct or truncate scar.
Cultures and asexual morphs: Ascospores germinating within 24 h with germ tubes and budding, producing conidia. Growth on MEA slow, agar of a centrally inoculated 90 mm Petri dish becoming entirely covered after (2–)3–4 weeks or growth ceasing before this period. Conidiation effuse. Conidia forming by budding of ascospores and conidia, and on minute pegs, more rarely solitary phialides produced by fertile, cylindrical or botryose, sparingly branched hyphae not differentiated as well-defined conidiophores. Conidia very variable in size and shape, 1-celled, hyaline, often truncate at the lower end, smooth, with minute guttules, budding. Arthroconidia when formed, produced by disarticulation of more or less botryose hyphae, cylindrical to subglobose, 1–3-celled, smooth, hyaline, with numerous guttules. Pycnidia when present usually formed after ca. 2 weeks, more or less immersed in the agar to erumpent, solitary or grouped, globose or conical, variably covered by white or brownish mycelium, black, firm, multiloculate, with a pseudoparenchymatous peridium of large, thick-walled, more or less globose, olive cells. Phialides lining the inner side of the pycnidial wall, densely packed in variable whorls in palisades on short cylindrical, slightly inflated, 1–3 celled, hyaline conidiophores and on peridial cells. Phialides lageniform, cylindrical or conical, straight, curved or sigmoid, hyaline, often with a broad collarette. Conidia 1-celled, emitted in hyaline, pale pinkish, pale brownish or olive drops, minute, oblong, bullet-shaped to ellipsoid, sometimes subglobose, hyaline, smooth, often with truncate scar and small guttules.
Notes: The inversely stellate ectostromatic structure seems to be specific for Valsaria and other relatives in the family Valsariaceae. In Valsaria the ascomata are more or less basal and usually little hypostroma is present below them. Asci are usually 6–8 spored, 4-spored asci occur in incompletely developed ascomata. The ascal apical ring is usually invisible in KOH, and in Congo Red after KOH-treatment it often does not become pigmented or it becomes pigmented but often also strongly enlarged vertically. Ascospores may often become distorted and swollen in 3 % KOH. In some isolates predominantly budding conidia are formed and hyphal growth is much reduced and soon ceases.
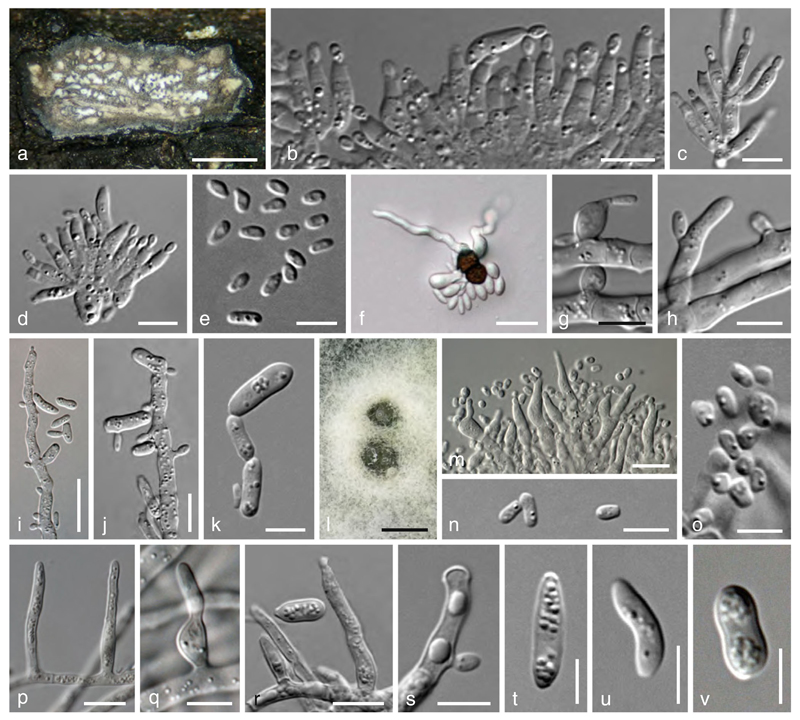
Valsaria insitiva (Tode : Fr.) Ces. & De Not., Comm. Soc. crittog. Ital. 1: 205 (1863). (Figs. 4 a, i, 5, 6 a–o)
Fig. 5.
Valsaria insitiva. a Ectostroma in face view. b Projecting ectostromatic structures. c Transverse section at the ostiolar level. d, e Transverse section at the ascomatal level (d immature). f–i. Vertical stroma sections (f compound stroma; h showing fused ostioles; i showing peridium and adjacent prosenchymatous stroma). j–l Asci. m–p Ascospores (o, p. showing surface ornamentation). q Apical rings and apically free paraphyses in Congo red. Sources: a–d, h. VV; e. VW; f, l. VA; g, i. VCE; j. VV2; k, m, q. V8; n, o. VV1; p. V5. Scale bars: a, b, e, f=0.5 mm. c, g=0.3 mm. d=0.7 mm. h=0.2 mm. i=30 µm. j–l =15 µm. m, o–q=7 µm. n= 10 µm
Fig. 6.
a–o Valsaria insitiva. a–e Asexual morph on natural host. a Stroma with locules in horizontal section. b–d Phialides. e Conidia. f Ascospore germination (CMD, after 18 h; note conidia and germ hyphae). g–k Effuse conidiation (MEA, 4–5 d). g Phialide. h Conidium formed on denticle. l–o Pycnidial conidiation (MEA, 15–18 d). i, j Fertile hyphae with denticles and pegs and conidia (partly budding). k Conidia. p–v V. lopadostomoides (VIQ). Effuse conidiation (MEA, 3–14 d). p Right-angled hyphal branching. q, r Phialides. s Conidium formed on denticle. t–v Conidia. Sources: a–e. VA. f. V35. g–o. VV. Scale bars: a=1 mm. l=0.5 mm. b–d, g, h, k, n, q, s=7 µm. e, o, t–v=5 µm. f, i= 20 µm. j, m, p, r=10 µm
Basionym: Sphaeria insitiva Tode , Fung. Mecklenb. sel. 2: 36 (1791); Tode : Fr., Syst. Mycol. II: 366 (1823)
Synonyms: see Discussion.
Facesoffungi number: 00607
Sexual morph: Stromata pseudostromatic, immersed-erumpent, mostly gregarious to coalescing into clusters ranging from narrowly elongate and up to 13×2.5 mm to irregularly shaped and up to 15×8 mm; pustular, lenticular to broadly conical or subglobose with flattened base, (0.3–)0.7–1.5(–1.8) mm high, 0.8–1.7mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust, blackening the wood surface between adjacent stromata. Ectostroma forming 0.4–1.3 mm broad and 0.2–1 mm high sub- or inversely stellate structures of 3–5 grey, brown to black segments around the ostiolar openings; tissue beneath the black crust pseudoparenchymatous; basal pseudostromatic tissue prosenchymatous, grey, of hyaline, 2–4 µm wide hyphae, mixed with bark cells. Ostiolar openings inconspicuous, less commonly necks arising as conical, sulcate, 0.4–0.7 mm high, black papillae. Ascomata 0.25–0.45 mm high, 0.18–0.4 mm diam, monostichously arranged in valsoid configuration, 5–8(–12) per individual cluster, vertical to oblique, subglobose to flask-shaped, laterally collapsed when dry; peridium 14–25 µm thick, pseudoparenchymatous, brown. Ostiolar necks long, cylindrical, converging and often fusing, i.e. the ostiolar opening containing 1–3(–5) necks; interior periphysate. Paraphyses numerous, simple, unbranched, tapering upwards, apically free, 1.5–5 µm wide. Asci (96–)106–143(–158)×(10–)11–14(–18.5) µm (n=30), bitunicate but without obvious fissitunicate dehiscence, cylindrical, containing 6–8 uniseriate ascospores; stipe short, truncate; apex containing an ocular chamber and a pulvinate ring (3.8–)4.5–6.0(–6.3)×(2.0–)2.5–3.5(–3.8) µm (n=30), staining in Congo Red. Ascospores (12–)15–20(–22)×(6.5–)7.5–9.8(–11.7) µm, l/w=(1.6–)1.8–2.2(–2.4) (n=130), ellipsoid, dark brown, 2-celled, with a dark central, not or hardly constricted septum thicker than the wall; surface finely tuberculate.
Asexual morph on natural substrates: Upper levels of young stromata containing irregular locules filled with hyaline tissue. Walls of locules lined by dense palisades of phialides in variable whorls on short cylindrical, few-celled hyaline conidiophores. Phialides (5.8–)7.3–10.0(–12.0)×(1.8–)2.0–3.0(–3.8) µm, l/w (2.4–)2.9–3.9(–4.6) (n=37), lageniform. Conidia (2.2–)2.7–3.7(–4.3)×(1.0–)1.3–1.7(–2.0) µm, l/w (1.5–)1.7–2.4(–2.9) (n=45), oblong to cylindrical, rarely subglobose, 1-celled, hyaline, smooth, with few guttules; also formed on aphanophialides.
Cultures and asexual morphs: Ascospores germinating within 24 h with conidia or with conidia and germ hyphae mixed (Fig. 6f). Hyphal growth slow, on CMD colony radius 18–24 mm after 15–17 days at 22 °C; colony flat, often irregularly lobate, first white, turning dilute grey-brown after 2 weeks; odour indistinct, sometimes slightly musty or fruity. Conidia spreading in masses from the centre, oblong, allantoid or ellipsoid, 1-celled, hyaline, smooth. Growth on PDA (Merck) as on MEA or slightly faster. On MEA colony radius ca. 4–5 mm after 3 days, 9–14 mm after 7 days, 20 mm after 14 days, 30 mm after 21 day, 35 mm after 28 days; plate often (nearly) covered after 1 month. Colony often with irregular outline, lobate, first white, soon turning greyish, dark brown to black, slightly floccose by whitish aerial hyphae; reverse dark grey to nearly black; odour indistinct. Mycelium often ceasing growth before covering the plate; sometimes brown diffusing pigment present, with crystals in the agar. Conidia formed within 24 h after inoculation by budding and on 3–7(–9.5) µm wide, more or less botryose hyaline hyphae on minute pegs, rarely on solitary terminal ellipsoid phialides ca. 7–10×3.5–5 µm, with short-cylindrical apex, arising laterally on hyphae. No differentiated conidiophores formed. Conidia (3.5–)5.3–11.3(–17.2)×(1.7–)2.0–4.0(–6.3) µm, l/w (1.7–)2.3–3.6(–4.2) (n=55), cylindrical, allantoid or ellipsoid, hyaline, smooth, with minute guttules; budding. After ca. 2 weeks black multiloculate pycnidia formed. Pycnidia 0.3–0.7 mm diam, globose, variably surrounded by white mycelium; wall pseudoparenchymatous, of large, thick-walled, globose to clavate, olive cells, giving rise to short, cylindrical to globose hyaline cells. Phialides (6.6–)7.5–11.0(–12.7)×(2.2–)2.5–3.5(–5.0) µm, l/w (2.1–)2.6–3.8(–4.6) (n=30), produced by the latter in variable whorls, more or less parallel, densely packed in palisades, narrow, lageniform, cylindrical or conical, with broad collarette. Conidia (2.4–)2.7–4.0(–5.6)×(1.3–)1.5–2.0(–3.0) µm, l/w (1.2–)1.6–2.3(–2.9) (n=50), ejected from pycnidia in hyaline, pale pinkish to pale brownish drops, minute, oblong, to bullet shaped to ellipsoid, sometimes subglobose, 1-celled, hyaline, smooth, lower end often truncate.
Ecology and distribution: Valsaria insitiva typically occurs on Vitis, but also on several unrelated hosts, in Europe with a primarily submediterranean distribution.
Types: Neotype: ITALY, vines of Vitis, 1837, De Notaris (RO, neotype of Sphaeria insitiva, designated by Ju et al. 1996 (as „epitype“)).
Epitype: CROATIA, Istria, 2 km south-west of Buje, on Vitis vinifera, 16 May 2010, W. Jaklitsch & H. Voglmayr (WU 33462, epitype of Sphaeria insitiva here designated; ex-epitype culture CBS 127882, VV; ex-epitype sequences KP687980 (SSU), KP687886 (ITS-LSU), KP687959 (rpb2), KP688054 (tef1)).
Background: Tode’s (1791) Sphaeria insitiva from Vitis resembles a species of Diatrypaceae, judging from his description and illustration, but in absence of microscopic data this is uncertain. As no material of Tode is extant, Ju et al. (1996) selected a specimen of De Notaris from Vitis (RO) as epitype of Sphaeria insitiva, in order to rescue both the generic name Valsaria and the epithet insitiva. However, according to Eriksson and Hawksworth (1997) this is a neotype. The epitypification of Sphaeria insitiva and thus of Valsaria insitiva here is necessary to fix the name of the type species, which is member of a morphologically difficult complex, with a specimen, a preserved culture and molecular data.
Other material examined: AUSTRIA, Niederösterreich, Hippersdorf, on Vitis vinifera, 15 November 2014, W. Jaklitsch (WU 33427; culture V35); Mühlleiten, on Acer campestre, soc. Thyronectria rhodochlora, 23 March 2013, H. Voglmayr (WU 33454; culture VAC); Vienna, 19th district, Oberer Reisenbergweg, on Fallopia baldschuanica, 7 October 2001, W. Jaklitsch W.J. 1836 (WU 33469); 21st district, Ignaz Köck-Strasse, on Robinia pseudoacacia, 15 September 2013, W. Jaklitsch (WU 33459; culture VIR2); 22nd district, Donaupark, on Spiraea sp., 30 November 2013, W. Jaklitsch (WU 33467; culture V8). CROATIA, Istria, Golaš, on Acer monspessulanum, 18 October 2010, W. Jaklitsch (WU 33453; culture CBS 139056=VA). FRANCE, Ariege, Le Mas d’Azil, on Ficus carica, 8 August 2013, A. Gardiennet AG13153 (WU 33455; culture VAF); Rimont, on Ficus carica, 22 December 2010, J. Fournier 10185 (WU 33460; culture VJF); Rimont, Las Muros, on Cornus sanguinea, 22 January 1996, J. Fournier 96007 (WU 33468; culture V33); Saint Girons, in a private garden, on Wisteria sinensis, 17 March 2012, J. Fournier 12034 (WU 33465; culture VW); Guadeloupe, Gourbeyre, Marina Riviére Sens, track to Houelmont, on corticated twigs, 9 August 2011, C. Lechat CLLGUAD 11014 (WU 33461; culture VL). GREECE, Corfu, Dassia, at the hotel Fiori, on Cercis siliquastrum, 24 April 2012, H. Voglmayr & W. Jaklitsch (WU 33456; culture VCE); Gouvia, Danilia, on Paliurus spina-christi, 20 April 2012, W. Jaklitsch & H. Voglmayr (WU 33458; culture VIP); Crete, Kakopetros, on Vitis vinifera, 25 November 2011, W. Jaklitsch (WU 33464; culture VV2). ITALY, Lazio, Province of Viterbo, Norchia, on Cytisus scoparius, 14 October 2013, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33466; culture V5); Veneto, Fontanafredda, on Vitis vinifera, 23 October 2011, W. Jaklitsch & H. Voglmayr (WU 33463; culture VV1). TAIW AN, Tainan City, Ssu-tsao, behind Ta-chung Temple, on unidentified twigs, 24 February 2001, Y.-M. Ju & H.-M. Hsieh 90022401 (WU 33457; part in HAST; culture CBS 139061= VIJ).
Notes: Valsaria insitiva, the type species of Valsaria, is primarily defined by its occurrence on Vitis, and DNA data from four isolates of specimens collected on this host in Austria, Croatia, Greece and Italy are identical. The species however occurs also on several other unrelated hosts, sometimes also on Fabaceae, e.g. Cercis, Cytisus, Robinia and Wisteria, a family on which Valsaria robiniae and V. spartii are much more common.
Valsaria lopadostomoides Jaklitsch & Voglmayr, sp. nov.
MycoBank MB 811902, Facesoffungi number: FoF 00608, Figs. 4 b, c, j, 6 p–v, 7
Fig. 7.
Valsaria lopadostomoides VIQ. a–c Ectostroma in face view. d Transverse section at the ascomatal level. e Vertical stroma section. f–h Asci. i, l–n. Ascospores (i. immature; i, n. showing surface ornamentation). j, k Ascus apices (showing ring in j. water and k. Congo red). o Apically free paraphyses in Congo red. Scale bars: a= 1 mm. b, d=0.7 mm. c=0.4 mm. e=0.5 µm. f–h= 15 µm. i=5 µm. j, m, n=7 µm. k, l, o=10 µm
Etymology: referring to the resemblance of its stroma with species of Lopadostoma.
Stromata pseudostromatic, erumpent from bark, scattered or coalescing into clusters up to 5×4 mm; pustular to subglobose with flattened base, 1.2–1.4 mm high, 1.7–2.5 mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust. Ectostroma typically forming 0.5–0.9 mm broad and 0.15–0.25 mm high sub- or inversely stellate structures consisting of 3–5 greyish, brown to black segments; the tissue beneath the black crust pseudoparenchymatous; the tissue at the stromatal base prosenchymatous, grey, of hyaline hyphae mixed with bark cells. Ostiolar openings inconspicuous. Ascomata 0.25–0.45 mm high, 0.18–0.4 mm diam, arranged in valsoid configuration, 8–12 per individual cluster, vertical to oblique, subglobose or flask-shaped, typically laterally collapsed when dry; peridium of brown flattened cells. Ostiolar necks long, cylindrical, converging and often fusing; interior periphysate. Paraphyses unbranched, tapering upwards, apically free, 1.5–5.5 µm wide. Asci (112–)115–128(–136)×(10.2–)11.0–13.7(–15.5) µm (n=25), cylindrical, bitunicate, containing 6–8 uniseriate ascospores; stipe short, truncate, without a crozier; apex containing a small ocular chamber and a pulvinate ring (4.5–)4.8–5.8(–6.3) µm wide, (1.7–)2.3–3.2(–3.5) µm high (n=15), slightly refractive in water, in Congo Red pulvinate, broadly conical or cap-like, staining red. Ascospores (13.0–)14.5–16.7(–18.6)×(8.0–)8.5–9.3(–9.8) µm, l/w (1.4–)1.6–1.9(–2.2) (n=51), ellipsoid, 2-celled, dark brown, with a dark central, not or hardly constricted septum thicker than the wall; surface ornamentation variable, distinctly warted, reticulate or spotted, with 1 large guttule per cell. No asexual morph observed on the natural substrate.
Cultures and asexual morph: On MEA colony radius ca. 4 mm after 3 days, 10 mm after 7 days, 21 mm after 14 days, 32 mm after 21 days, 40 mm after 28 days; plate (nearly) entirely covered after 1 month. Colony circular, with a whitish mat of aerial mycelium, partly sulphur yellow hyphae with yellow exudates spreading from the plug, surface turning dull yellow to olive, reverse yellowish, turning grey-green to black; odour indistinct to slightly unpleasant. Conidiation effuse; no pycnidia formed within a month. Conidia accumulating in large hyaline to yellow masses and small hyaline to greyish drops, formed by budding and on minute pegs, rarely on solitary phialides ca. 8–16×3–5 µm on 2–5 µm wide hyphae with branches often at right angles. Conidia (3.4–)5.0–9.0(–12.7)×(1.5–)2.0–3.7(–5.7) µm, l/w (1.5–)1.9–3.3(–4.2) (n=64; after 10–14 days), very variable, cylindrical, allantoid or ellipsoid, 1-celled, smooth, budding. Arthroconidia uncommon, e.g. 11.5×4.7 µm. On PDA virtually not growing, yellow diffusing pigment produced.
Ecology and distribution: In bark of Quercus ilex; only known from the type locality in Corfu, Greece.
Type: GREECE, Corfu, Kanakades, on Quercus ilex, soc. Cryptovalsa cf. protracta, 20 April 2012, H. Voglmayr & W. Jaklitsch (WU 33470, holotype; ex-holotype culture CBS 139062=VIQ; ex-holotype sequences KP687972 (SSU), KP687868 (ITS-LSU), KP687943 (rpb2), KP688037 (tef1)).
Notes: This taxon is based on a single, mostly overmature specimen collected as a species of Lopadostoma. The species seems to be rare and may be specific for Quercus ilex and related evergreen oaks.
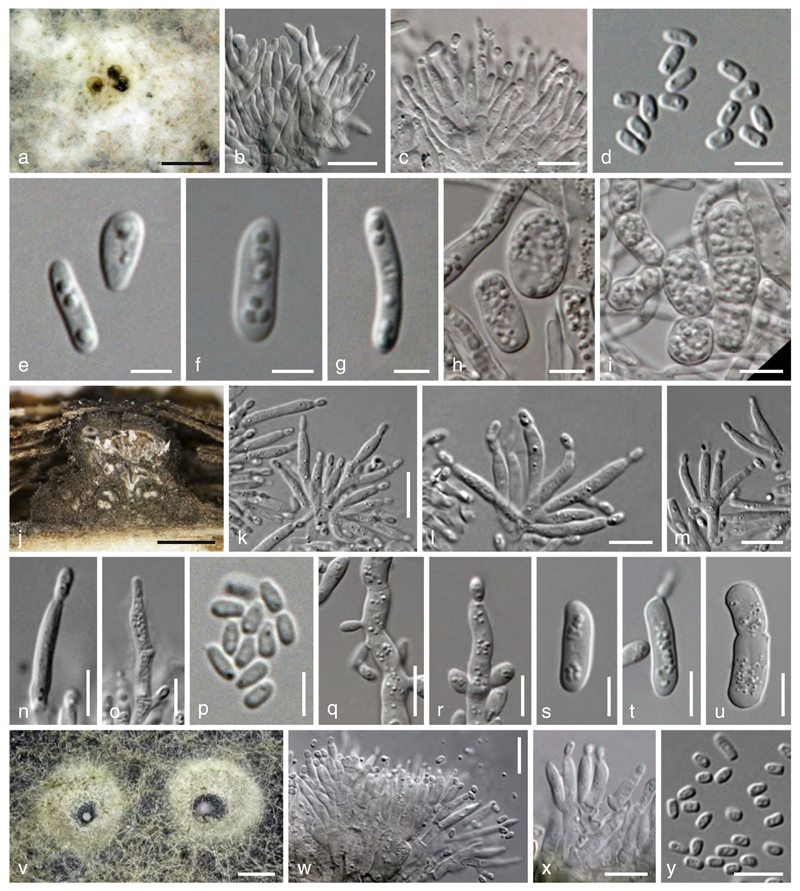
Valsaria neotropica Jaklitsch, J. Fourn. & Voglmayr, sp. nov.
MycoBank MB 811903, Facesoffungi number: FoF 00609, Figs. 4 d, e, k, 8, 10 a–i
Fig. 8.
Valsaria neotropica VJM. a, b Ectostromata in face view. c, d Transverse section at the ostiolar and ascomatal levels. e, f Vertical stroma sections. g Apical rings in Congo red. h, i Apically free paraphyses. j–l Asci. m–r Ascospores (m in Congo red, mostly immature; m, q showing surface ornamentation). Scale bars: a, b=0.2 mm. c=0.4 mm. d–f= 0.5 mm. g=7 µm. h=5 µm. i, m–r=10 µm. k, j, l=15 µm
Fig. 10.
Asexual morphs. a–i Valsaria neotropica VJM. a–d Pycnidial conidiation (MEA, 17 d). a Pycnidia. b, c Phialides. d Conidia. e–i Effuse conidiation. e–g Conidia (MEA, 4–6 d). h, i Arthroconidia (3-celled in i; MEA, 14 d). j–y V. robiniae VIR1. j–p On natural host. j Stroma with locules in vertical section. k–o Phialides. p Conidia. q–u Effuse conidiation (MEA, 4 d). q Conidia formed on denticles and pegs. r Phialide. s, t Conidia (t. budding). u 2-celled arthroconidium. v–y Pycnidial conidiation (MEA, 17 d). v Pycnidia. w, x Phialides. y Conidia. Scale bars: a=1 mm. b, c, k, m, q, u, w=10 µm. d, h, n–p, s= 5 µm. e–g=3 µm. i, l, r, t, x, y=7 µm. j, v=0.5 mm
Etymology: referring to its detection on the neotropical island Martinique.
Stromata pseudostromatic, erumpent from bark, scattered, rarely aggegating to groups of 2–3; pustular, conical to subglobose with flattened base, 0.75–1 mm high, 0.8–1.7 mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust. Ectostroma often forming 0.4–0.6 mm broad and 0.1–0.2 mm high sub- or inversely stellate structures of 3–5 greyish, brown to black, tubercular segments; tissue beneath the crust pseudoparenchymatous; tissue between the ostiolar necks and at the stromatal base prosenchymatous, grey, soft, mixed with bark cells. Ostiolar openings inconspicuous at the surface or indistinctly papillate. Ascomata arranged in valsoid configuration, 5–8(–12) per individual cluster, monostichous, subglobose to flask-shaped, 0.25–0.45 mm high, 0.18–0.4 mm diam, typically laterally collapsed when dry; peridium 14–25 µm thick, of pale brown compressed cells. Ostiolar necks long, cylindrical, converging and often fusing; interior periphysate. Paraphyses numerous, unbranched, tapering upwards, apically free, 1.5–6 µm wide. Asci (102–)108–148(–175)×10.5–14(–16) µm (n = 24), bitunicate but without obvious fissituncate dehiscence, cylindrical, containing 6–8 uniseriate ascospores; apex containing an ocular chamber and a short-cylindrical to barrel-shaped ring (4.2–)4.7–5.8(–6.2) µm wide, (1.8–)2.3–3.8(–4.2) µm high (n=15), staining in Congo Red. Ascospores (17.7–)19.0–22.5(–27.8)×(7.7–)8.3–10.7(–13.7) µm, l/w (1.6–)2.0–2.4(–2.8) (n=138), ellipsoid, 2-celled, dark brown, with a dark, thick, central, not or hardly constricted septum; surface finely tuberculate. No asexual morph observed on the natural substrate.
Cultures and asexual morphs: On MEA colony radius ca. 6 mm after 3 days, 12–20 mm after 7 days, 27 mm after 14 days, 38 mm after 21 day, >40 mm after 28 days; plate (nearly) entirely covered after 3–4 weeks. Colony whitish, pale brownish to greyish, pinkish in the centre, covered by a mat of white, condensed aerial hyphae. Reverse first yellowish, turning grey to black in spots or zones. Odour indistinct to slightly unpleasant. Conidiation first effuse; conidial masses colourless, yellowish to pinkish in the centre, spreading. Fertile (aerial) hyphae slightly narrower than vegetative hyphae, 2–5(–7.5) µm wide, sparingly branched, often at right angles. Conidia (3.5–)4.5–10.2(–16.7)×(1.5–)1.8–2.7(–3.3) µm, l/w (1.9–)2.4–4.1(–5.9) (n=60), formed by budding and on minute pegs, cylindrical to slightly allantoid, less commonly ellipsoid, 1-celled, hyaline, smooth, with minute guttules. After ca. 2 weeks arthroconidia numerous, cylindrical to subglobose, (7.3–)9.5–20.0(–28.5)×(3.7–)5.0–8.5(–9.8) µm, l/w (1.0–)1.3–3.5(–6.0) (n=25), 1–3-celled, smooth, hyaline, with numerous guttules. Pycnidia appearing after 2–3 weeks, immersed in the agar, covered by aerial hyphae, solitary or incorporated in dense groups of up to 5 with 0.6–1.8 mm diam, black, firm, with olive peridium. Phialides (7.7–)9.0–13.0(–15.0)×(1.7–)2.0–2.8(–3.2) µm, l/w (2.9–)3.6–5.7(–7) (n=26), lageniform to cylindrical, straight, curved or sigmoid, hyaline, lining pycnidial locules in palisades on short cylindrical, slightly inflated, hyaline to olive cells. Conidia (2.3–)2.5–3.5(–3.8)×(1.3–)1.5–1.7(–1.8) µm, l/w (1.4–)1.6–2.1(–2.4) (n=35), emitted in olive drops, ellipsoid-oblong, 1-celled, hyaline, smooth, with truncate scar and 1–2 guttules. On PDA growth faster than on MEA, plate entirely covered after 2–3 weeks, colony dull brown.
Ecology and distribution: host identity unknown; only known from the type locality in Martinique.
Type: FRANCE, Martinique, Case Pilote, trail to Morne Venté, on unidentified twig, 25 August 2010, J. Fournier (WU 33471, holotype; ex-holotype culture CBS 139064= VJM, ex-holotype sequences KP687974 (SSU), KP687874 (ITS-LSU), KP687948 (rpb2), KP688042 (tef1)).
Notes: This species is based on a single specimen, for the most part comprising immature small stromata that are rarely coalescing, with a well-defined ectostroma and non-papillate ostiolar necks; many asci are 4-spored. DNA data set V. neotropica clearly apart from all other species of Valsaria. The growth rate of V. neotropica nearly approaches that of V. robiniae. Aerial hyphae are much denser than in other species. Arthroconidia are nearly as common as in V. spartii.
Valsaria robiniae (Schwein.) Cooke, Grevillea 13: 37 (1884).
Basionym: Sphaeria robiniae Schwein., Schr. naturf. Ges. Leipzig 1: 33 (1822); Schwein. : Fr., Syst. Mycol. II: 352 (1823). Facesoffungi number: FoF 00610, Figs. 4 f, l, 9, 10 j–y
Fig. 9.
Valsaria robiniae. a–d Ectostromata in face view. e Transverse section at the ascomatal level. f–i Vertical stroma sections. k Apically free paraphysis and apex of immature ascus. k, l. Apical rings (k. in blue ink; l. in Congo red). m–o Asci. p–t Ascospores (p, s showing surface ornamentation). Sources: a, d. VC; b. VCA; c, e, i, k. V30; f, j, m. VIR1; g, h, l, r. VCol; n–p, s, t. V29; q. isolectotype BPI 800813. Scale bars: a, b=1 mm. c, d, g–i=0.3 mm. e,f=0.5 mm. j–l, p, q, s, t=7 µm. m–o=15 µm. r=10 µm
Stromata pseudostromatic, erumpent from bark, scattered or mostly coalescing into linear rows or clusters up to 4.5×2.5 mm; pustular, lenticular to broadly conical or subglobose with flattened base, (0.5–)0.7–1 (–1.3) mm high, 0.7–1.2(–1.7) mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust, blackening the wood surface between adjacent stromata. Ectostroma forming 0.3–1 mm broad and 0.2–0.3 mm high sub- or inversely stellate structures of 3–5 greyish, brown to black segments with a depressed centre; tissue beneath the black crust pseudoparenchymatous; tissue at the stromatal base prosenchymatous, grey, soft, often mixed with bark cells. Ostiolar openings inconspicuous at the surface, less commonly ostiolar necks arising as conical, sulcate, 0.2–0.7 mm high, black papillae. Ascomata 0.25–0.45 mm high, 0.2–0.4 mm diam, monostichously arranged in valsoid configuration, 4–10 per individual cluster, vertical to oblique, subglobose to flask-shaped, typically laterally collapsed when dry; peridium 14–25 µm thick, of pale brown flattened cells. Ostiolar necks long, cylindrical, converging and often fusing; interior periphysate. Paraphyses simple, apically free, 1.5–4.5 µm wide, tapering upwards to 1.5–2 µm. Asci (116–)127–144(–155)×(10.3–)11.7–14.5(–16.0) µm (n=33), bitunicate but without obvious fissituncate dehiscence, cylindrical, containing (4–)6–8 uniseriate ascospores; base with croziers; apex with an ocular chamber and a pulvinate to barrel-shaped ring (4.8–)5.0–6.2(–7.5) µm wide, (2.3–)2.7–3.7 µm high (n=15), staining in Congo Red. Ascospores (15.3–)17.5–21.0(–25.5)×(8.0–)8.8–10.5(–11.7) µm, l/w (1.6–)1.8–2.2(–2.6) (n=130), ellipsoid, 2-celled, dark brown, with a dark, thick, central, non-constricted to distinctly constricted septum; ends broadly rounded; surface warted to reticulate.
Asexual morph on natural substrates: Upper levels of young stromata above ascomata or the entire stroma containing irregular locules. Phialides densely arranged in palisades lining the walls of the locules on short cylindrical conidiophores arising from thin-walled, olive to black stroma cells <10 µm diam. Phialides (6.7–)8.3–11.5(–14.0)×(2.0–)2.2–2.8(–3.2) µm, l/w (2.2–)3.2–4.9(–5.9) (n=33), lageniform to cylindrical; 2–4 µm long phanerophialides common. Conidia (2.5–)2.7–3.7(–4.5)×(1.3–)1.4–1.7(–2.0) µm, l/w (1.5–)1.8–2.3(–2.7) (n=35), oblong, ellipsoid or bullet-shaped, 1-celled, hyaline, smooth, with inconspicuous guttules and indistinct or truncate scar.
Cultures and asexual morphs: On MEA colony radius ca. 6 mm after 3 days, 17 mm after 7 days, 35 mm after 14 days, >40 mm after 21 days; plate entirely covered after 2–3 weeks. Colony irregular, whitish, greyish to dull brown, zonate, with whitish margin and whitish, slightly floccose surface; sometimes brown pigment diffusing into the agar. Reverse dull brown, often with reddish or yellowish tint. Odour indistinct to yeast-like. In some isolates predominantly budding conidia formed, hyphal growth much reduced and soon ceasing. Conidiation first effuse; colourless to brown conidial masses forming around the plug. Conidia mostly formed on pegs and by budding, rarely by lageniform phialides ca. 8–10×3–5 µm, arising from hyphae sparingly branched at right angles. Conidia (4.3–)5.2–10.0(–14.8)×(1.8–)2.0–3.5(–4.2) µm, l/w (1.9–)2.0–3.6(–5.6) (n=35), variable in size and shape, cylindrical, oblong to ellipsoid, 1-celled, hyaline, smooth. Arthroconidia ca. 8–14(–28)×4–11 µm, formed after ca. 10 days on slightly botryose hyphae, inconspicuous, 1–2 celled. Pycnidia formed after ca. 2 weeks, often in radial rows, immersed in agar, ca. 0.8–1.2 mm diam, conical, multiloculate, black, surface whitish to greyish due to aerial hyphae, apex black, ca. 0.3–0.5 mm diam. Conidia ejected in hyaline drops. Short cylindrical, 1–3 celled, hyaline conidiophores arising from large olive cells on walls of the locules. Phialides (5.3–)7.0–10.8(–13.5)×(2.0–)2.3–3.0(–3.2) µm, l/w (2.1–)2.6–4.2(–5.5) (n=38), lageniform, formed in palisades on the conidiophores. Conidia (2.2–)2.5–3.5(–4.3)×(1.4–)1.5–1.7(–2.0) µm, l/w (1.2–)1.5–2.2(–2.7) (n=35), minute, oblong to subglobose, 1-celled, hyaline, smooth, with truncate lower end. On PDA growth as on MEA or slightly faster; also pycnidia formed, immersed in agar.
Ecology and distribution: In bark of Robinia and related fabaceous hosts; known from Europe (Austria, Croatia, Hungary, Italy, Slovenia) and North America (North Carolina, Pennsylvania) in temperate to submediterranean climates.
Types: USA, Pennsylvania, Bethlehem and North Carolina, Salem, Syn. 1233, decorticated wood (PH00077157, lectotype of Sphaeria robiniae, here designated); Schweinitz Collection 1233, no place and date given (BPI 800813, isolectotype; from the Shear Study Collection Types & Rarities Series I).
Reference specimen: ITALY, Galzignano, Turri, on Robinia pseudoacacia, 23 October 2011, W. Jaklitsch & H. Voglmayr (WU 33478; culture CBS 139063=VIR1), reference sequences KP687973 (SSU) KP687870 (ITS-LSU), KP687945 (rpb2), KP688039 (tef1).
Other material examined: AUSTRIA, Niederösterreich, Marchfeldkanalweg, near Gerasdorf, on Colutea arborescens, 5 September 2009, W. Jaklitsch & O. Sükösd VCol1 (WU 33476); Vienna, 21st district, Marchfeldkanalweg, near Jedlersdorferstraße, on Colutea arborescens, 5 September 2009, W. Jaklitsch & O. Sükösd (WU 33475; culture CBS 125583= VCol); Marchfeldkanalweg near Stammersdorf, on Caragana arborescens, 5 June 2010, W. Jaklitsch & O. Sükösd (WU 33474; culture CBS 128015 = VCA); Stammersdorf, at the chuch, grid square, on Colutea arborescens, 6 May 2001, W. Jaklitsch W.J. 1752 (WU 33481). CROATIA, Istria, SE Rovinj, coastal area, on Hippocrepis emerus, 24 June 2012, W. Jaklitsch & O. Sükösd (WU 33482); Opatija, at Ičići, on Hippocrepis emerus, 15 May 2010, W. Jaklitsch (WU 33473; culture VC1). HUNG ARY, between Besenyötelek and Kömló, 47°41’16.6” N, 20°25’51” E, 100 m, on Amorpha fruticosa, 31 May 2014, W. Jaklitsch & H. Voglmayr (WU 33480; culture V30); south of Eger, 47°51’15’ N, 20°22’0’ E, 235 m, on Amorpha fruticosa, 30 May 2014, W. Jaklitsch & H. Voglmayr (WU 33479; culture V29). ITALY, Veneto, between Bastia and Rovolon, on Robinia pseudoacacia, 22 October 2011, W. Jaklitsch & H. Voglmayr (WU 33477; culture VIR). SLOVENIA, Podnanos, Nanos, mountain road, on Hippocrepis emerus, 4 July 2007, W. Jaklitsch (WU 33472; culture CBS 121890=VC).
Notes: Valsaria robiniae was described from the eastern USA by Schweinitz. Lectotypification was necessary, because there are two collections of Sphaeria robiniae in PH. We have no recent material of this species from North America, therefore it is not certain, whether European material corresponds definitely to American material. For this reason we do not epitypify V. robiniae, but designate our collection WU 33478 from Italy as a reference specimen of V. robiniae until it can be recollected in North America. In Europe the species is predominantly found on Robinia and related hosts in temperate and submediterranean regions. Growth of V. robiniae is the fastest of all Valsaria species treated here. Furthermore, colony zonation seems to be diagnostic.
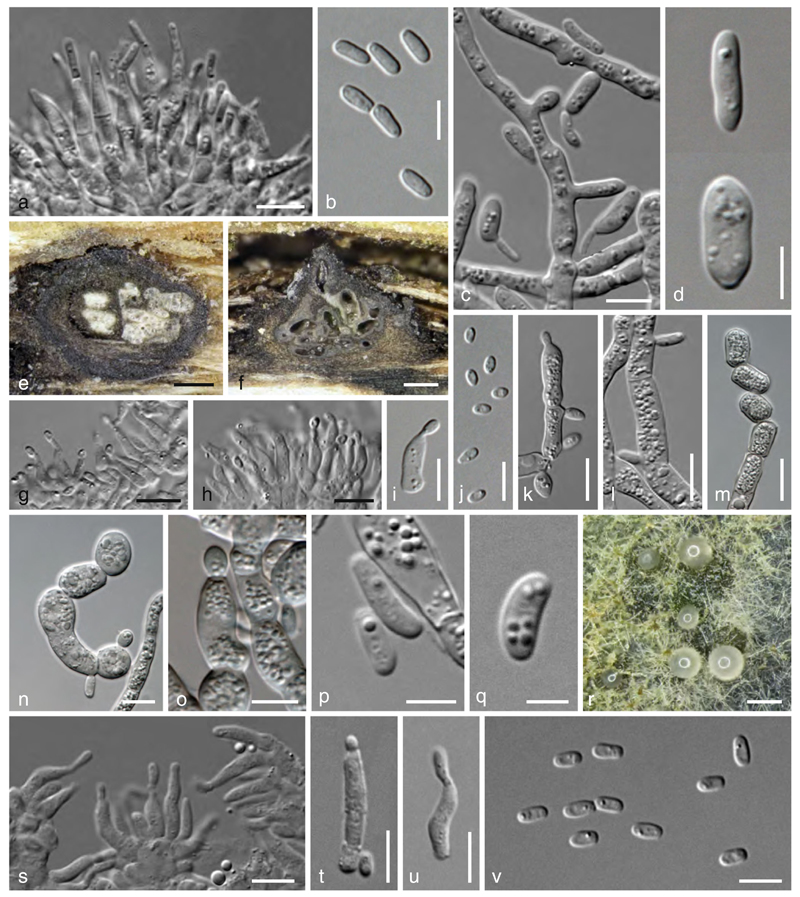
Valsaria rudis (P. Karst. & Har.) Theiss. & Syd., Ann. mycol. 12: 273 (1914).
Basionym: Dothidea rudis P. Karst. & Har., J. Bot., Paris 3: 206 (1889); Facesoffungi number: FoF 00611, Figs. 4 g, m, 11, 13 a–d
Fig. 11.
Valsaria rudis. a–f Ectostromata in face view. g, h Transverse section at the ascomatal level. i, j Vertical stroma sections. k Apically free paraphysis. l, m Apical rings in Congo red (m. in face view). n–p Asci. q–t Ascospores (q, r showing surface ornamentation). Sources: a, f, i, n, q, r. VQM; b, p. lectotype; c, g, t. W.J. 1819; d, e, h. V7; l, s. VQC; j, k, m, o. VQP. Scale bars: a=0.7 mm. b, d, f=0.3 mm. c, g–j=0.5 mm. e=1 mm. k, s, t=10 µm. l=5 µm. m=3 µm. n–p=15 µm. q, r=7 µm
Fig. 13.
Asexual morphs. a–d Valsaria rudis. a, b From natural host. a Phialides. b Conidia. c, d Effuse conidiation (MEA, 11 d). c Fertile hyphae and budding conidia. d Conidia. e–v V. spartii. e–j On natural host. e Stroma with locules in horizontal section. f Stroma with locules in vertical section. g–i Phialides. j. Conidia. k–q Effuse conidiation (MEA, 4–5 d). k, l, o Conidia formed on phialides and pegs (note phialides in k and o). m, n Arthroconidia. p, q Conidia. r–v Pycnidial conidiation (MEA, 18 d). r Immersed pycnidia with conidial drops. s–u Phialides. v Conidia. Sources: a, b. VQC. c, d. VQP. e, f, i–v. V6. g, h. VIC. Scale bars: a, c, h–j, o, s–u=7 b, d,p, v=5 μm. e, f, r=0.2 mm. g, k, l, n=10 μm. m=15 μm. q=3 μm
Stromata pseudostromatic, erumpent from bark, scattered or gregarious to coalescing into variable clusters 3–6×2.5–3.5 mm; pustular, broadly conical or subglobose with flattened base, 1.2–1.7 mm high, 1.2–2.5 mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust blackening the wood surface between adjacent stromata. Ectostroma forming (0.2–)0.6–1.2(–1.6) mm broad and 0.15–0.4 mm high inversely stellate structures of 3–5 greyish, olivaceous to black tubercular segments; often highly reduced or absent and replaced by a smooth cupulate surface partly roughened by the ostiolar papillae; the tissue beneath the black crust pseudoparenchymatous; tissue at the stromatal base prosenchymatous, grey, of narrow hyaline hyphae mixed with bark cells. Ostiolar openings inconspicuous at the surface, less commonly necks arising as low and cruciform or conical, sulcate, 0.12–0.4 mm high, black papillae. Ascomata 0.3–0.6 mm high, 0.2–0.5 mm diam, monostichously arranged in valsoid configuration, 5–12(–20) per individual cluster, vertical to oblique, subglobose to flask-shaped, typically laterally collapsed when dry; peridium of pale brown flattened cells. Ostiolar necks long, cylindrical, converging or fusing; interior periphysate. Paraphyses simple, apically free, 1.5–4.5 µm wide, tapering upwards. Asci (100–)112–132(–146)×(9.8–)11.0–14.2(–16.0) µm (n= 40), cylindrical, containing 6–8 uniseriate ascospores, bitunicate; fissitunicate dehiscence sometimes apparent in the lower third; apex containing an ocular chamber and a pulvinate apical ring (4.3–)4.8–6.5(–7.5) µm wide, (1.4–)2.0–3.6(–4.6) µm high (n=41), staining in Congo Red, sometimes slightly refractive in water. Ascospores (13.5–)15.8–18.0(–19.5)×(6.8–)8.0–9.2(–10.0) µm, l/w (1.5–)1.8–2.1(–2.7) (n=100), ellipsoid, ellipsoid, 2-celled, ellipsoid, with a dark, thick, central, not to distinctly constricted septum; surface finely warted to reticulate.
Asexual morph on natural substrates: Ostiolar levels of young stromata sometimes containing irregular locules. Phialides densely arranged in palisades lining the walls of the locules on short cylindrical conidiophores. Phialides (7.0–)7.3–9.8(–11.7)×2.3–2.7(–3.0) µm, l/w 2.8–3.9(–4.4) (n=8), lageniform. Conidia (3.8–)4.5–5.2(–5.7)×(1.1–)1.5–2.2(–2.2) µm, l/w 2.1–3.3(–4.4) (n=27), cylindrical, oblong, rarely allantoid, 1-celled, hyaline, smooth, with inconspicuous guttules; scar indistinct.
Cultures and asexual morphs: Growth slow on all media, on CMD colony often remaining white for several weeks, turning diffusely olive-greenish, conidial masses colourless to yellowish. On MEA colony radius 1–2 mm after 3 days, 3–7 mm after 7 days, 7–12 mm after 14 days, 13–15 mm after 21 days. Colony irregular, lobate, usually remaining whitish to yellowish up to 2 weeks, turning grey with yellow tones, brown or dull yellow with grey spots. Yellow spots caused by conidial masses spreading from the centre. At 15 °C colony turning dark green and forming dull yellow pigment in the agar. Reverse yellowish grey to black. Odour indistinct to slightly yeast-like. Conidiation on MEA effuse. Minute pegs on short erect, filiform to slightly botryose hyphae forming variable, oblong, cylindrical, allantoid or ellipsoid conidia (4.5–)5.5–8.5(–10.5)×(1.5–)2.0–3.5(–4.7) µm, l/w (1.9–)2.2–3.2(–4.1) (n=35), 1-celled, hyaline, smooth, budding. On PDA nearly not growing, colony radius 3–4 mm after 10 days.
Ecology and distribution: In bark of deciduous Quercus spp.; known from Europe (Austria, Croatia, France, Greece, Italy, Switzerland).
Types: Lectotype: FRANCE, on Quercus sp. (Q. petraea or Q. robur), date unknown, (H, herb. Karsten 3713, lectotype of Dothidea rudis here designated).
Epitype: AUSTRIA, Niederösterreich, Pfaffstätten, on Quercus pubescens, 15 April 2012, H. Voglmayr (WU 33485, epitype of Dothidea rudis here designated; MBT200958; ex-epitype culture CBS 139066=VQP; exepitype sequences KP687976 (SSU), KP687879 (ITS-LSU), KP687953 (rpb2), KP688047 (tef1)).
Other material examined: AUSTRIA, Burgenland, Mattersburg, Starenbühl/Rosaliengebirge, grid square 8264/3, on Quercus petraea, 1 October 2001, W. Jaklitsch W.J. 1819 (WU 33490); Niederösterreich, Krems, Egelsee, on Quercus petraea, soc. Valsa sp., 27 October 2013, W. Jaklitsch & H. Voglmayr (WU 33487; culture V7). Steiermark, Hartberg, Buchberg, near animal park Herberstein, 15°48’27”E, 47°13’08”N, grid square 8760/4, on Quercus petraea, 2 March 2013, G. Friebes (WU 33488; culture V31); Wundschuh, Kaiserwald, at the Seerestaurant, grid square 9058/4, on Quercus petraea, 10 September 2002, W. Jaklitsch W.J. 1998 (WU 33491); Vienna, 22nd district, Lobau, Panozzalacke, grid square 7865/1, on Quercus robur, 5 April 2003, W. Jaklitsch W.J. 2073 (WU 33492). CROATIA, Istria, Rovinj, ca. 3 km south-east of Kukoletovica, on Quercus cerris, 18 May 2012, H. Voglmayr (WU 33483; culture VQC). GREECE, Corfu, Aharavi, opposite the Hydropolis Water Park, on Quercus macrolepis, 24 April 2012, W. Jaklitsch & H. Voglmayr (WU 33484; culture CBS 139065=VQM). ITALY, Lazio, Bolsena Lake, Lungolago di Gradoli, San Magno, from the restaurant Il Purgatorio to the road to road SS312, on Quercus cerris, 13 October 2013, H. Voglmayr, W. Jaklitsch & W. Gams (WU 33486; culture V3). SWITZERLAND, Valais, Les Follateyres, on Quercus sp., 31.9.1982, F. Rappaz (WU 33489).
Notes: Designation of a lectotype was necessary, as several parts of the original collection are present in several herbaria, and the material in H is labelled as syntype. Ascospore size in the lectotype is smaller than given in the protologue (Karsten and Hariot 1889), which was already noted by Petrak and Sydow (1923), but it fits perfectly to all specimens recently collected from Quercus spp. Valsaria rudis is apparently specific for deciduous species of Quercus. An ectostroma is often lacking, leaving cupulate ostiolar discs on the stroma surface. Growth of V. rudis is the slowest of all studied species of Valsaria. As in the other Quercus-inhabiting species, V. lopadostomoides, no pycnidial asexual morph was detected in cultures of V. rudis. We designate an epitype to stabilize the name, because the species is regarded as cryptic and cannot be reliably identified by morphology alone; it cannot be ruled out that the plurivorous V. insitiva might occur on Quercus.
Valsaria spartii Maubl., Bull. Soc. mycol. Fr. 21: 88 (1905).
= Valsaria ceratoniae Crous & M.J. Wingf., Fungal Planet, no. 11–21: 15: [2] (2007)
Facesoffungi number: FoF 00612, Figs. 4 h, n, 12, 13 e–v
Fig. 12.
Valsaria spartii. a–f Ectostromata in face view. g Transverse section at the ostiolar level. h, i Transverse section at the ascomatal level. j–l Vertical stroma sections. m, n Apical rings in Congo red. o–q Asci. r–v Ascospores (u, v showing surface ornamentation; u immature; v note free paraphysis apex). Sources: a, l. V2; b, c, k, m, o, t. V6; d, g, i. V22; e. V15; f, j, n. VA2; h. V1; p, r. Kreta; q, u. VIC; s, v. holotype PC0167077. Scale bars: a, f, h, i=0.5 mm. b, c, j=1 mm. d, e, g, k= 0.3 mm. l=0.15 mm. m, n, r=10 μm. o–q=15 μm. s–v=7 μm
Stromata pseudostromatic, erumpent from bark, mostly gregarious or coalescing into clusters ranging from narrowly elongate up to 4.7×1.5 mm to irregularly shaped, sometimes superficial, massive and up to 11×6.5 mm; pustular, broadly conical to subglobose with flattened base, (0.4–)0.6–1.0(–2.0) mm high, with individual ascomatal clusters 0.7–1.2(–1.5) mm diam, enclosed on top and/or at the sides by a black, 20–50 µm thick pseudoparenchymatous crust blackening the wood surface between adjacent stromata. Ectostroma typically irregularly tubercular or amorphous or forming 0.3–1 mm wide and 0.15–0.3 mm high, sub-or inversely stellate structures of 3–5 greyish, brown to dull black segments; tissue beneath the black crust pseudoparenchymatous; tissue at the stromatal base prosenchymatous, grey, soft, often mixed with bark cells. Ostiolar openings inconspicuous at the surface, less commonly necks arising as conical, more or less sulcate, 0.1–0.8 mm high, black necks. Ascomata 0.2–0.8 mm high, 0.2–0.5 mm diam, monostichously arranged in valsoid configuration, 5–10(–15) per individual cluster, vertical to oblique, subglobose to flask-shaped, typically laterally collapsed when dry; peridium of pale brown flattened cells. Ostiolar necks long, cylindrical, converging and often fusing; interior periphysate. Paraphyses numerous, unbranched, apically free, 1.5–4.5 µm wide from top to base. Asci (105–)113–138(–146)×(10.8–)11.5–13.5(–15.0) µm(n=25), cylindrical, containing 6–8 uniseriate ascospores, bitunicate without obvious fissituncate dehiscence; apex with ocular chamber and a pulvinate apical ring (3.8–)4.5–6.3(–7.3) µm wide, (1.7–)2.3–3.5(–4.3) µm high (n = 20), staining in Congo Red. Ascospores (14.3–)16.3–20.2(–23.7)×(7.7–)8.5–10(–11) µm, l/w (1.6–)1.8–2.2(–2.6) (n=100), ellipsoid, 2-celled, dark brown, very variable, plump with constricted septum or slender and inequilateral, with a dark, thick, central, nonconstricted septum; surface finely warted or reticulate; ends acute or broadly rounded.
Asexual morph on natural substrates: Locules forming at ostiolar levels of stromata that contain immature ascomata, or stromata containing irregularly arranged locules exclusively. Interior of locules white when dry, glassy to gelatinous when wet; walls lined by dense palisades of short cylindrical, hyaline conidiophores with densely packed, terminal whorls of phialides. Phialides (6.8–)7.7–11.0(–12.5)×(1.8–)2.0–3.0(–3.7) µm, l/w (2.5–)2.9–4.3(–5.5) (n=36), lageniform, straight to sinuous, often inequilateral, with narrow or broad collarette; also aphanophialides producing conidia. Conidia (2.5–)3.0–3.7(–4.0)×(1.2–)1.5–2.0(–2.2) µm, l/w (1.4–)1.6–2.2(–3.1) (n=50), oblong to ellipsoid, 1-celled, hyaline, smooth; 1 end often truncate.
Cultures and asexual morphs: On CMD colony entirely covering a 90 mm plate after 3–4 weeks, typically homogeneous, round, often containing hyperbranching mycelium, surface turning (olivaceous-) brown to black. Odour indistinct. Conidiation effuse and after several weeks in immersed to superficial pycnidia. On MEA colony radius 4–5 mm after 3 days, 8–16 mm after 7 days, 18–26 mm after 14 days, 28–39 mm after 21 days; plate entirely covered after 3–4 weeks. Colony irregular, often lobate, not zonate, surface whitish to greyish or light brownish, sometimes with yellow tones, with whitish, slightly floccose mat of aerial hyphae and whitish margin, darkening to dull brown. Reverse brown, with whitish to yellowish margin. Odour indistinct, yeast- or bakery-like. In some isolates growth soon ceasing; mycelium releasing brown pigment into the agar. Conidiation first effuse. Conidial masses spreading from the centre, forming pale drops. Conidia formed on minute pegs, rarely on lageniform phialides (e.g. 14×6.5 μm) produced on filiform aerial hyphae with scarce, often right-angled branching. Conidia (4.3–)5.0– 8.3(–10.4)×(1.8–)2.2–3.5(–4.5) μm, l/w (1.3–)1.7–3.1(–4.1) (n=35), oblong to reniform, 1-celled, hyaline, often truncate at the lower end, smooth. After few days hyphae becoming botryose and chain-like and frequently disarticulating into thick-walled, subglobose 1–2-celled arthroconidia 6–10(–18)×4–9 μm; the latter sometimes producing conidia. Pycnidia usually appearing within 2 weeks, mostly 0.3–0.5 mm diam, black, multiloculate, for the most part remaining immersed in the agar and covered by whitish to brownish aerial hyphae, releasing conidia as large hyaline to pinkish, later pale brown drops. Locules containing typical palisades of short cylindrical, hyaline, smooth or warted conidiophores with phialides in variable clusters. Phialides (4.5–)7.3–11.5(–13.3)×(1.5–)2.0–3.0(–4.0) μm, l/w (2.4–)2.9–4.5(–5.8) (n=50), cylindrical or lageniform, straight, curved or sigmoid; also formed in whorls on large olive, warted globose wall cells; also aphanophialides producing conidia. Conidia (2.3–)2.8–4.3(–6.4)×(1.3–)1.6–1.9(–2.1) μm, l/w (1.4–)1.7–2.5(–3.6) (n= 50), oblong, 1-celled, hyaline, smooth. On PDA growth and appearance as on MEA.
Ecology and distribution: In bark of Robinia and related fabaceous hosts; known from Europe (France, Greece, Italy, Spain), common in the Mediterranean.
Types: Holotype: FRANCE, Loire-Inferieure, Pornic, on Spartium junceum, A. Maublanc, April 1904 (PC0167077!, holotype of Valsaria spartii).
Epitype: ITALY, Lazio, Province of Viterbo, Montalto di Castro, Vulci, on Spartium junceum, 15 October 2013, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33505, epitype of Valsaria spartii, here designated; MBT200959; ex-epitype culture CBS 139070=V6; ex-epitype sequences KP687964 (SSU), KP687843 (ITS-LSU), KP687919 (rpb2), KP688013 (tef1)).
Other material examined: FRANCE, Dép. Aude, Saint Papoul, on Spiraea sp., 23 February 2011, J. Fournier (WU 33498; culture VIS); Haute Garonne, Avignonet, Le Mares, on Spartium junceum, 1 May 2003, J. Fournier JF 03060 (WU 33527). GREECE, Corfu, Castellani, on Calicotome villosa, 21 April 2012, W. Jaklitsch & H. Voglmayr (WU 33495; culture VCV); Episkepsis, on Melia azedarach, 24 April 2012, W. Jaklitsch & H. Voglmayr (WU 33499; culture VMA); Crete, near Askifou, on Acer sempervirens, 26 November 2011, W. Jaklitsch (WU 33493; culture VA2); near Voleones, east from the Amari Dam Reservoir, on Spartium junceum, 14 October 2014, W. Jaklitsch (WU 33526); Rhodes (old town castle), on Ceratonia siliqua, 1 June 2006, P.W. Crous & M.J. Wingfield CPC 13245 (CBS H-19925, holotype of V. ceratoniae; ex-type CBS 121714=VCBS; only culture sequenced). ITALY, Lazio, Bolsena Lake, Lungolago di Gradoli, San Magno, between the restaurant Il Purgatorio and the road SS312, on Cytisus sessilifolius, 13 October 2013, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33504; cultures V4, V4a); Bolsena Lake, Monte Bisenzio, on Fraxinus ornus, 13 October 2013, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33503; culture V2); ibidem, on Robinia pseudoacacia, 13 October 2013, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33502; culture V1); Riserva Monte Casoli di Bomarzo, on Cytisus scoparius, 30 July 2009, W. Jaklitsch, H. Voglmayr & W. Gams (WU 33494; culture CBS 125584=VCS). SPAIN, Andalucia, Cádiz, Alcalá de los Gazules, on Acacia saligna, 1 April 2014, W. Jaklitsch (WU 33509; culture V12); south of Alcalá de los Gazules, at the service road parallel to the motorway A381, on Teline linifolia, 6 April 2014, W. Jaklitsch (WU 33519; culture V22), ibidem, on Acacia saligna, 6 April 2014, W. Jaklitsch (WU 33520; culture V23); at the A381 exit to Benalup, on Spartium junceum, 6 April 2014, W. Jaklitsch (WU 33521; culture V24); Castaño del Robledo, on Cytisus striatus, 8 April 2014, W. Jaklitsch (WU 33522; culture V25); north of Castellar de la Frontera, on Cytisus baeticus, 5 April 2014, W. Jaklitsch (WU 33517; culture V20); Puerto Galis, on Retama monosperma, 2 April 2014, W. Jaklitsch (WU 33510; culture V13); La Cañada Real Tesoro, on Retama sphaerocarpa, 3 April 2014, W. Jaklitsch (WU 33512; culture V15); Los Pescadores (between San Pablo de Buceite and Gaucín), on Anagyris foetida, 3 April 2014, W. Jaklitsch (WU 33513; culture V16); ibidem, on Retama sphaerocarpa, 22 March 2011, W. Jaklitsch & H. Voglmayr VR1 (WU 33501); Santuario de la Señora de los Santos, on Retama sphaerocarpa, 1 April 2014, W. Jaklitsch (WU 33508; culture V11); La Sauceda, lower part of the walking path to Aljibe, on Teline linifolia, 4 April 2014, W. Jaklitsch (WU 33516; culture V19); at km 26 between La Sauceda and Puerto Galis, on Calicotome villosa, 2 April 2014, W. Jaklitsch (WU 33511; culture V14); ibidem, on Ulex parviflorus, 4 April 2014, W. Jaklitsch (WU 33515; culture V18); Montes de Jimena, on Teline monspessulana, 4 April 2014, W. Jaklitsch (WU 33514; culture V17); Punta Palomas, on Retama monosperma, 5 April 2014, W. Jaklitsch (WU 33518; culture V21); Granada, Guájar Alto, 36°51′13.7″ N, 3°37′50.3″ W, 540 m, on Ononis speciosa, 16 May 2014, S. Tello & W. Jaklitsch (WU 33525; culture V28); southwest of Montefrío, 37°17′24″ N, 4°4′46″ W, elev. 660 m, soc. Diaporthe sp., on Spartium junceum, 11 May 2014, W. Jaklitsch (WU 33523; culture V26); Jaén, Castillo de Locubin, 37°30′50.6″ N, 3°58′3″ W, elev. 790 m, on Genista cinerea, 11 May 2014, W. Jaklitsch (WU 33524; culture V27); Málaga, Cortes de la Frontera, on Retama sphaerocarpa, 22 June 2010, W. Jaklitsch & O. Sükösd (WU 33500; culture CBS 128016=VR); Asturias, above Villar de Vildas, on Genista florida, 6 June 2013, W. Jaklitsch & H. Voglmayr (WU 33496; culture VG); Canarias, La Palma, near El Paso, on Chamaecytisus proliferus, 30 December 2003, P. Karasch (WU 33528); Tenerife, Teno Alto, on Chamaecytisus proliferus, 15 December 2013, W. Jaklitsch (WU 33506; culture V9); La Esperanza, on Spartium junceum, 19 December 2013, W. Jaklitsch (WU 33507; culture CBS 139071 =V10); Islas Baleares, Mallorca, Algaida, Cas Brau, on Ceratonia siliqua, soc. Cryptovalsa sp., 21 November 2010, W. Jaklitsch (WU 33497; culture CBS 139060=VIC).
Notes: The epithet spartii is the oldest for a Valsaria occurring on Spartium. We stabilize the name by epitypification with a specimen from Spartium Valsaria spartii is the most common species of Valsaria, especially in the Mediterranean and occurs besides Spartium on a very wide range of Fabaceae but also other, unrelated hosts. Valsaria spartii has often very well-developed pseudostromatic tissues, which results in a striking similarity with species of Diaporthe (Diaporthales). In cultures of this species arthroconidia are most commonly and prominently formed, whereas fertile hyphae of V. insitiva are much narrower and do not produce arthroconidia. Formation of arthroconidia by the closely related V. robiniae is inconspicuous.
Myrmaecium Nitschke ex Fuckel, Jb. Nassau. Ver. Naturk. 23–24: 227 (1870); non Myrmaecium Sacc., Michelia 2: 138 (1880).
= Phaeocreopsis Sacc. & Syd. apud Lindau in Engl. & Prant., Nat. Pflanzenfam. Nachtr., p 541 (1900)
= Hypoxylonopsis Henn., Hedwigia 43: 256 (1904)
Type species: Myrmaecium rubricosum Fuckel, Jb. nassau. Ver. Naturk. 23–24: 227 (1870) [1869–70]
Stromata eu- or pseudostromatic, immersed, erumpent to superficial on bark, rarely decorticated wood, scattered or aggregated, pulvinate, labiate or ring-like, or conical to subglobose, 0.5–4 mm diam, or fusing to compound stromata up to 15×8 mm, 0.5–8 mm high. Ectostroma inconspicuous and continuous or conspicuous and slightly to strongly emerging above the bark surface; surface flat, concave or convex, continuous to coarsely cracked around the slightly projecting black ostiolar necks, reddish, brick-coloured, yellow-brown, dull olive brown or reddish to cinnamon brown, or dark reddish to black, sometimes covered by bright yellow to orange flakes or scurf, sometimes forming substellate or inversely stellate structures. Pigments usually extractable with KOH, purple or pink in KOH, vinaceous, yellow to greenish in lactic acid. Ecto- and entostroma above ascomata pseudoparenchymatous. Entostroma at lower levels soft-textured, greyish brown, yellowish brown, tan or off-white, prosenchymatous or plectenchymatous, of loose or densely intertwined hyaline to reddish brown or red thin-walled hyphae. Black marginal zones lacking. Rarely a cottony hyphal mat present at the base of stromata, developing between the bark and the wood, pale brown or purplish, sometimes staining wood or bark purple to violaceous. Ascomata (0.1–)0.2–0.5 mm diam, arranged monostichously at near basal position or in the stroma middle, sometimes immediately below the outer crust, in valsoid or diatrypoid configuration, flaskshaped to subglobose, with 2-layered peridium of flattened brown (external layer) and hyaline (internal layer) cells. Ostiolar necks short and discrete or up to ca. 1 mm long and convergent to fusing, black, interior periphysate. Paraphyses numerous, filiform, unbranched, apically free, 1.5–5.5 μm wide, attenuated towards the apex, ends sometimes slightly swollen. Asci 74–141×10–19 μm, numerous, cylindrical, oblong to subclavate, bitunicate, with or without obvious fissitunicate opening, containing 4–8 (often obliquely) uniseriate, sometimes partly biseriate ascospores; with a short stipe, a small ocular chamber, a thin or thick apex with or without a thin, inconspicuous, 2.5–7 μm wide, 1.2–4.5 μm high apical ring, weakly refractive in water, staining weakly in Congo Red, esp. after KOH pretreatment. Ascospores 12–20×6–10, ellipsoid, 2-celled, dark brown, symmetrical or inequilateral, septum usually thicker than the wall, not or slightly constricted at the septum, with reticulate or labyrinthine surface ornamentation.
Asexual morph on natural substrates lacking or formed in stromata as locules at ostiolar levels above immature ascomata. Phialides densely packed, lageniform to cylindrical, with broad collarette. Conidia oblong or bullet-shaped, 1-celled, hyaline, orange to orange-brown in mass.
Culture characterists: Growth on MEA fast, centrally inoculated 90 mm plates usually entirely covered by mycelium within 1 week. Odour of colonies indistinct or strongly chemical, cresol-like. Conidiation effuse. Conidia 3–16×1–5 μm, ellipsoid, oblong to allantoid, 1-celled, hyaline, budding; formed by budding, on minute pegs and scant lageniform phialides. Sometimes fertile sexual stromata or solitary ascomata produced in culture.
Myrmaecium rubricosum (Fr. : Fr.) Fuckel, Jb. Nassau. Ver. Naturk. 23–24: 227 (1870).
Basionym: Sphaeria rubricosa Fr., Elench. fung. 2: 63 (1828)
≡ Hypoxylon rubricosum (Fr. : Fr.) Fr., Summa Veg. Scand. 2: 384 (1849)
≡ Melogramma rubricosum (Fr. : Fr.) Tul. & C. Tul., Sel. Fung. Carp. 2: 84 (1863)
= Hypoxylon walterianum Berk. & Ravenel, in Ravenel, Fung. Carol. Exs. Fasc. IV, no. 35. (1855)
= Myrmaecium durissimum Fuckel, Jb. Nassau. Ver. Naturk. 23–24: 228 (1870)
≡ Valsaria durissima (Fuckel) Sacc., Syll. Fung. I: 748 (1882)
≡ Pseudovalsa durissima (Fuckel) Cooke, Grevillea 14: 55 (1885)
= Hypoxylon gemmatum Berk. & Ravenel, in Berk., Grevillea 4: 50 (1875)
≡ Melogramma gemmata (Berk. & Ravenel) Cooke. Grevillea 13: 103 (1885)
≡ Valsaria gemmata (Berk. & Ravenel) Ellis & Everh., N. Amer. Pyren., p. 562 (1892)
= Melogramma cinnamomi Ces., Atti Accad. Sci. Fis. 8: 19 (1879)
≡ Valsaria cinnamomi (Ces.) Sacc., Syll. Fung. I: 748 (1882)
≡ Valsonectria cinnamomi (Ces.) Huhndorf, Mycologia 84: 642 (1992)
= Melogramma eucalypti Kalchbr. & Cooke, Grevillea 9: 31 (1880)
≡ Valsaria eucalypti (Kalchbr. & Cooke) Sacc., Syll. Fung. I: 746 (1882)
= Valsaria purpurea Peck, Bull. Torrey Bot. Club 11:28 (1884)
= Valsaria hypoxyloides Ellis & Everh., J. Mycol. 7: 131 (1892)
≡ Valsonectria hypoxyloides (Ellis & Everh.) M.E. Barr, Mycotaxon 39: 137 (1990)
= Valsaria pseudohypoxylon Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 6 : 276 (1898)
= Hypocreopsis hypoxyloides Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 6: 291 (1898)
≡ Phaeocreopsis hypoxyloides (Speg.) Sacc. & Syd., Syll. Fung. XVI: 592 (1902)
= Myrmaecium hypoxyloides Rehm, Hedwigia 40: 148 (1901)
= Hypoxylonopsis hurae Henn., Hedwigia 43: 256 (1904)
≡ Valsaria hurae (Henn.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss. Wien, Math.-Nalurwiss. Cl., Abt. 1, 119: 924 (1910)
= Valsaria hypoxyloides Rehm, in Theiss., Ann. Mycol. 10: 12 (1912)
= Valsaria rehmiana Teng, Sinensia 4: 391 (1934)
= Hypoxylonopsis rehmiana (Teng) Teng, Chung-kuo Ti Chenchun [Fungi of China], p. 761 (1963); as Hypoxylopsis
= Valsonectria reticulata Loeffler & E. Müll., Ber. Schweiz. Bot. Ges. 71: 413 (1961)
= Valsaria mundkurina Muderji & Kapoor, Ceská Mykol. 23: 258 (1969)
= Valsaria reticulata Kar & Maity, Indian Phytopathol. 32: 425 (1979)
(synonyms taken from Ju et al. 1996).
Facesoffungi number: FoF 00613, Figs. 4 o, p, 14, 16 a-h
Fig. 14.
Myrmaecium rubricosum. a–d, i Ectostromata in face view (d, i. showing ostioles). e, f, j Vertical stroma sections. g Pink pigment dissolved in KOH. h Vertical section of a perithecium. k Prosenchymatous stroma tissue. l Subiculum. m–o Apical ascal rings in Congo red (note free ends of paraphyses in n and o). p–s Asci. t–v Ascospores (u, v showing surface ornamentation). Sources: a, e, j. MP133; b, g. WJ940; c. VRF; d. TH; f, m, o, t, v. VRM; h, k, n, r, s. VRP; i, l. VRJ1; p, u. isolectotype; q. WJ1247. Scale bars: a, b, j=2 mm. c, l =1 mm. d, e=0.5 mm. f=0.3 mm. h=30 μm. i=0.2 mm. k=20 μm. m, n, t–v=7 μm. o–s =10 μm
Fig. 16.
a–h Myrmaecium rubricosum. a, b Cultures at 22 °C after 21 days (a On CMD; b On MEA, with sexual morph stromata in the centre). c Ascus and paraphyses from stroma formed in MEA culture (after 70 days). d, e. Stroma on MEA (d. after 21 days; e. after 71 days). f–h Effuse conidiation (MEA, 3 d). f Phialides. g, h Conidia. i–w Myrmaecium fulvopruinatum asexual morphs. i–p On natural host. i Asexual stroma in face view. j Stroma with locules in horizontal section. k Stroma with locules and ascomata in vertical section. l–o Phialides. p Conidia. q–w Effuse conidiation (MEA, 3 d). q Conidiophore. r, s Phialides. t–w Conidia. Sources: a–e. VRM. f–h. VRP. i–k. PWB. l–p. VF. q–w. VFJ. Scale bars: c, q=30 μm. d, e=2.5 mm. f, g, l, s, w=7 μm. h=3 μm. i–k=0.6 mm. m–p, u, v=5 μm. r, t=10 μm
Stromata eustromatic, erumpent-superficial on bark, rarely decorticated wood, scattered or aggregated in groups, pulvinate with flattened to slightly convex surface, 0.7–4×0.5–1.4 mm, or coalescent forming compound stromata up to 15 × 8 mm long, 0.7–8.3 mm high, with variable, sometimes constricted and more or less cylindrical base; upper fertile part emerging above the ruptured periderm, often with slightly revolute margins, surface and sides brick red when dry, soft textured, smooth, becoming cracked, roughened by the more or less projecting black ostiolar necks; surface of lower parts in bark tissue or wood gradually turning blackish downward. Uppermost stromatic layer 40–80 μm thick, pseudoparenchymatous, of small thin-walled cells with pigmented contents releasing pink to purple pigments in 3–10 % KOH and ammonia, vinaceous in lactic acid. Entostroma below this layer and the ascomata soft-textured, grey to tan, solid to loosely fibrous, plectenchymatous, composed of densely intertwined, hyaline to reddish brown, thin-walled, 4–11 μm wide hyphae; cottony pale brown or purplish to violaceous subiculum sometimes present at the base of stromata between bark and wood; sometimes bark or wood also stained purple. Ascomata 0.2–0.4 mm high×0.15–0.35 mm diam, immersed beneath the upper outer crust in a single layer in diatrypoid configuration, flask-shaped to subglobose; peridium 15–25 μm thick, composed of flattened cells, dark brown in an outer layer, hyaline in an inner layer. Ostiolar necks usually separate, not fusing, 70–580 μm long, black, smooth, round-ended, often unevenly distributed on the stromatal surface, 80–170 μm wide including walls, flush or projecting to 40–170 μm above the surface, periphysate. Paraphyses 1.5–5.0 μm wide, unbranched, apically free. Asci (74–)83–102(–118)×(10.3–)11.5–14.5(–16.2) μm (n=30), usually plump, oblong to cylindrical, bitunicate, containing 4–8 (often obliquely) uniseriate, sometimes partly biseriate ascospores; without obvious fissitunicate dehiscence but sometimes exotunica breaking in the lower third, with a short stipe and crozier and a thin, inconspicuous apical ring (2.5–)3.5–5.8(–6.8) μm wide, (1.3–)1.7–3.2(–4.5) μm high (n=21), weakly refractive in water, staining weakly in Congo Red, slightly more intensely after KOH pretreatment. Ascospores (12.2–)14.0–16.3(–18.0)×(6.8–)7.5–8.5(–9.0) μm, l/w (1.5–)1.7–2.1(–2.4) (n=80), ellipsoid, 2-celled, medium to dark brown, with a central, not distinctly thickened and nonconstricted septum, with distinct reticulate surface ornamentation. No asexual morph detected on natural substrates.
Cultures, asexual morph and sexual morph in culture: On MEA colony radius 15–19 mm after 3 days, 32–34 mm after 5 days; centrally inoculated 90 mm plates entirely or nearly entirely covered by mycelium within 1 week. Colony circular, pale pinkish, orange or reddish, slightly floccose or fluffy by white aerial hyphae (turning partly violaceous in 3 % KOH), not zonate, without radial texture. Reverse rosy with yellowish tint and brown dots. Bright yellow pigment diffusing into the agar in aged cultures. Odour indistinct. Effuse conidiation only present around the inoculation plug in initial cultures. Conidia formed by budding and on minute pegs and scant lageniform phialides ca. 7–15×3.5–5 μm arising from little branched, long filiform hyphae. Conidia (4.5–)4.7–10.7(–16.0)×(1.8–)2.0–3.0(–3.7) μm, l/w (2.0–)2.3–3.4(–4.3) (n=23), oblong to allantoid, rarely ellipsoid, 1-celled, hyaline, smooth, with minute guttules, budding. Stromata appearing after ca. 10 days as black dots in the colony centre, growing and becoming fertile after ca. 2 months; up to 11×8 mm and 3 mm high, irregular, consisting of variously shaped, more or less black segments and partly covered by whitish to reddish-brownish mycelium. Stromatic tissue pseudoparenchymatous, dark red, releasing red pigment in 3 % KOH. Ascomata with long black cylindrical ostiolar necks, apically free paraphyses and fissitunicate, cylindrical asci with 8 uniseriate ascospores 13–16×7.5–9 μm.
On PDA growth slow (colony radius e.g. 18 mm after 14 days); colony irregular, velvety, dark reddish brown, thick and dense; ascomata formed; odour pleasant, bakery-like.
Ecology and distribution: on sun-exposed, corticated logs and branches of coniferous and broadleaf trees; worldwide, but uncommon. Sometimes co-occurring with M. fulvopruinatum.
Types: Lectotype: FRANCE, Angers, date unknown, J.P. Guépin (L9118162 (L), lectotype of Sphaeria rubricosa, designated by Ju et al. (1996); PC0167074!, isolectotype). Guépin was cited by Fries 1828, Elench. Fung. II: 63.
Other material examined: AUSTRIA, Kärnten, Obermieger, roadside, grid square 9452/1, on corticated logs of Picea abies, 4 January 2004, W. Jaklitsch W.J. 2494 (WU 33449; culture CBS 139069=VRM); St. Margareten im Rosental, Schwarzgupf, grid square 9452/4, on corticated logs of Abies alba, 24 October 1998, W. Jaklitsch W.J. 1247 (WU 33452); Steiermark, Eibiswald, near Radlpaß, grid square 9357/1, on logs of Picea abies, 16 September 1996, W. Jaklitsch W.J. 940 (WU 33451). CHILE, locality and date not indicated (PC0167073; as Hypoxylon rubricosum). CROATIA, Istria, ca. 1,5 km south of Bale, on sun-exposed cut branches of Quercus pubescens, 18 May 2012, H. Voglmayr (WU 33450, culture CBS 139068 =VRP). FRANCE, Dept. Landes, Tartas, Souprosse, on Quercus robur, 8 April 1996, F. Candoussau 4869-E434, as Phaeocreopsis hypoxyloides (JDR; culture VRJ); Vienne, on Populus sp., date unknown (PC0167075; as Hypoxylon rubricosum); Martinique, Saint Esprit, edge of Bois La Charles, on bark, 20 August 2013, J. Fournier MJF 13328 (WU 33447; culture CBS 139067 = VRF); French Guyana, locality and date not indicated, F.R. Leprieur 237 (PC0167072; as Hypoxylon rubricosum); Mayotte, Saziley, 12°57,98′ S 45°11,07′ E, on corticated wood, 5 April 2014, M. Pélissier, MP 2014–133 (JF). THAILAND, Chiang Mai Province, Mae Teang District, Bahn Pha Deng, Mushroom Research Centre, 19°01′615″ N, 98°41′884″ E, 900 m, on bark of Lithocarpus sp., 12 June 2005, JF-TH 12–01 (JF). USA, Hawaiian Islands, Maui, Hana Highway, on twigs, 5 August 2005, Y.-M. Ju & H.-M. Hsieh 94080504 (WU 33448; culture VRJ1; part in HAST).
Notes: Myrmaecium rubricosum is a distinctive species and in the order Valsariales being the only one which produces massive superficial eustromata. Myrmaecium fulvopruinatum differs in many respects from M. rubricosum and forms smaller stromata and is more variable in stromatal and ascomatal characters. Asci of M. rubricosum are rather plump and oblong, shorter than in Valsaria spp. and remarkably stable in water. The isolectotype contains several typical stromata to 4 mm long and 2.5 mm high, glued on paper. In several specimens from the tropics (e.g. specimens from Hawaii, Martinique and Mayotte) the bark is stained purple by the fungus. Contrary to M. fulvopruinatum and M. rubrum no cresol is formed in MEA cultures of M. rubricosum.
Myrmaecium fulvopruinatum (Berk.) Jaklitsch & Voglmayr, comb nov.
Basionym: Sphaeria fulvopruinata Berk., London J. Bot. (Hooker) 4: 312 (1845)
≡ Valsaria fulvopruinata (Berk.) Sacc., Syll. fung. 1: 747 (1882)
= Valsaria decorticans (Kunze ex Ces. & De Not.) Bagl., Ces. & De Not., Erb. Crittog. Ital., ser. l. fasc. 26, no. 1280. (1865); non (Fr.: Fr.) Ces. & De Not. (1863)
= Valsaria kunzeana De Not., Comm. Soc. Crittog. Ital. 2: 482 (1867)
= Valsaria exasperans (W.R. Gerard) Sacc., Syll. Fung. 2: 55 (1883)
= Myrmaecium quadratum (Schwein. ex Berk.) Rehm, Ascomyc. no. 325 (1876)
= Valsaria quadrata (Schwein. ex Berk.) Sacc., Syll. Fung. 1: 745. (1882)
= Myrmaecium abietinum Niessl, Hedwigia 13: 42 (1874) See Ju et al. (1996) for additional synonyms.
MycoBankMB 811904, Facesoffungi number: FoF 00614, Figs. 4 q, r, 15, 16 i–w
Fig. 15.
Myrmaecium fulvopruinatum. a–f Ectostromata in face view (f showing yellow pigment particles).g Stroma side view (surrounded by bark at upper level). h Transverse section at the ascomatal level. i Vertical stroma section. j Subiculum. k Purple pigment dissolved in KOH. l Yellow-green pigment dissolved in lactic acid. m, n Entostroma (m upper level; n below ascomata). k Peridium. p Section through stromatic zone. q Paraphysis. r, s Fissitunicate ascus dehiscence. t, u Apical ascal rings in Congo red. v–x. Asci. y–a4. Ascospores (y, a1. showing surface ornamentation). Sources: a, e, m–q. PWB=Picea; b, f, j. VFQ; c, v, al, a3. VFJ; d, t, u, x, a4. VFB; g, i, k, l. VF; h. VFJ1; r. WJ870; s, w. WJ1190; y. isolectotype L0819035; z, a2. VFA. Scale bars: a, d, e, h, j=0.5 mm. b, c, g, i=1 mm. f=0.3 mm. m–p=20 μm. q–s, v–z, a2-a4=10 μm. t, u, a1 = 5 μm
Stromata eustromatic, immersed-erumpent, causing small bumps in bark, subpulvinate, labiate or ring-like, often longish, with often concave or flattened, less commonly slightly convex surface, separate, 0.8–4×0.2–2.5 mm, aggregated in linear rows or coalescent forming compound stromata up to 7 mm long and 1–2.6 mm high. Ectostroma often partly covered by bright yellow to orange flakes or scurf composed of amorphous granules or acicular crystals, scarcely emerging above the ruptured periderm, yellow-brown, dull olive brown or reddish- to cinnamon brown, typically coarsely cracked around the ostiolar necks and frequently forming sub- or inversely stellate structures; stroma base flattened, sometimes fringed by yellow or pale purple mycelium. Uppermost stromatic layer 40–90 μm thick, pseudoparenchymatous, of small thin-walled cells with pigmented contents releasing pink to purple pigments in 3–10 % KOH and ammonia, yellow to greenish yellow in lactic acid, replaced at sides and the base by a blackish layer 30–45 μm thick composed of plectenchymatous to pseudoparenchymatous cells almost opaque externally, gradually less pigmented inwardly. Entostroma below this layer soft-textured, greyish brown, dull brown or tan, darker around ascomata and just beneath the surface, frequently tan to off-white below the ascomata, solid to loosely fibrous, plectenchymatous, composed of densely intertwined, hyaline to reddish brown, thin-walled, minutely incrusted, 4.5–8 μm wide hyphae; pale brown or purplish cottony subiculum sometimes present at the base of stromata between bark and wood. Ascomata 0.2–0.5 mm high, 0.2–0.4 mm diam, arranged monostichously in valsoid groups of 5–10 at near basal position or in the stroma middle, less commonly in diatrypoid configuration just below the stroma surface, flask-shaped to subglobose; peridium 18–28 μm thick, composed of a thin outer layer of flattened dark brown cells and an inner layer of flattened hyaline cells. Ostiolar necks converging into a common neck, less commonly separate, 250–700(–1100) μm long, (90–)170–230 μm diam, black, smooth; apices at the surface usually with distinct circular outline, flush with the stroma surface or projecting to ca. 120 μm; periphysate. Paraphyses filiform, unbranched, apically free, to ca. 150 μm long, 1.5–4.5(–5.5) μm wide, attenuated upwards, often slightly enlarged at the tip. Asci (97–)107–130(–141)×(11.5–)13.0–16.5(–19.0) (n=35), numerous, cylindrical to subclavate, containing (4–)6–8 (often obliquely) uniseriate or partly biseriate ascospores, unstable in water, more stable in KOH, bitunicate, fissitunicate (mostly in the middle), with croziers, small ocular chamber, thick apex; apical ring absent or thin, inversely trapezoid and faintly reddish in Congo Red after KOH pretreatment, 3.0–3.5(–3.7) μm wide, (1.2–)1.5–2.5(–2.8) μm high (n = 8). Ascospores (14.2–)16.5–19.2(–20.5)×(7.8–)8.5–9.7(–10.5) μm, l/w (1.7–)1.8–2.1(–2.3) (n=101), ellipsoid, 2-celled, dark brown to black, plump or attenuated towards apices with 1 large drop per cell and densely reticulate surface ornamentation.
Asexual morph on natural substrates: Immature stromata often with a dark and gelatinous flat central surface, containing conidiomata as irregular, labyrinthine locules at ostiolar levels above immature ascomata; interior of locules hyaline to orange or brown, walls lined by palisades of densely clustered, lageniform to cylindrical, often basally curved phialides (6.8–)8.5–12.0(– 15.5)×(1.9–)2.4–3.0(–3.3) μm, l/w (2.8–)3.1–4.7(–6.5) (n=78), with broad collarette, originating from large brown incrusted cells 8–15 μm diam and hyaline cylindrical cells. Conidia (2.5–)2.8–3.7(–5.0)×(1.5–)1.7–2.0(–2.2) μm, l/w (1.2–)1.5–2.0(–3.0) (n=71), oblong to bullet-shaped or subglobose, 1-celled, hyaline, orange-brown in mass, with rounded upper and truncate lower end.
Cultures and asexual morph: On MEA colony radius 14–19 mm after 3 days, 26–33 mm after 5 days; centrally inoculated 90 mm plates entirely or nearly entirely covered by mycelium within 1 week; growth sometimes ceasing earlier. Colony zonate, surface and aerial hyphae with distinct macroscopically visible radial arrangement; aerial hyphae forming white radial streaks; first white, soon turning yellowish to pale orange or rosy to yellow-brown. Odour pungent, cresol-like. Conidiation effuse; yellowish to rosy conidial masses of spreading from the plug. Conidia formed by budding and on minute pegs and solitary, terminal, lageniform, oblong or ellipsoid to saccate, straight, curved or sigmoid phialides (6.5–)7.0–13.3(–21.0)×(2.5–)3.3–4.8(–5.8) μm, l/w (1.5–)1.9–3.1(–3.7) (n=30), arising from hyaline, short, erect conidiophores spreading from the centre. Conidiophores simple, filiform, with infrequent paired or unpaired branching; branches often at right angles, 1- to several-celled, rarely with secondary branching, 2.5–6.5 μm wide. Conidia (3.0–)4.3–8.0(–10.8)×(1.5–)2.0–3.5(–4.8) μm, l/w (1.5–)1.8–2.8(–4.1) (n = 65), very variable, ellipsoid, oblong, less commonly allantoid, 1-celled, hyaline, smooth, with indistinct or truncate scar; budding. No pycnidia or sexual stromata formed. On PDA as on MEA, but growth slower and colony without distinct radial structure and zonation. On CMD growth slower than on MEA, but more conidia formed.
Ecology and distribution: on sun-exposed, corticated logs and branches of coniferous and broadleaf trees; worldwide, but uncommon. Sometimes co-occurring with M. rubricosum.
Type: Holotype: USA, Ohio, in bark of Platanus occidentalis, T.G. Lea, ex herb. M.J. Berkeley (K(M) 193954!, holotype of Sphaeria fulvopruinata).
Epitype: USA, Massachusetts, Conway, Baptist Hill, on Fagus grandifolia, 5 December 1982, M.E. Barr 6905 (as Valsaria exasperans) (NY!, epitype of Sphaeria fulvopruinata here designated; ex-epitype culture CBS 139058=VFJ; ex-epitype sequences KP687968 (SSU), KP687861 (ITS-LSU), KP687936 (rpb2), KP688030 (tefl)).
Other material examined: AUSTRIA, Burgenland, Oberpullendorf, on Quercus cerris, 2 October 2010, W. Jaklitsch & O. Sükösd (WU 33438; culture CBS 139059= VFQ); Kärnten, St. Margareten im Rosental, Wograda, grid square 9452/3, on Picea abies, 17 May 1996, W. Jaklitsch (WU 33440; culture WJ 870); St. Margareten im Rosental, Schwarzgupf, grid square 9452/4, on logs of Fagus sylvatica and Abies alba, 24 October 1998, W. Jaklitsch W.J. 1246 (WU 33443); Niederösterreich, Weidlingbach, on logs of Fagus sylvatica, 21 August 2010, W. Jaklitsch (WU 33433; culture CBS 139057=VF); ibidem, on logs of Picea abies, 31 August 2014, W. Jaklitsch PWB (WU 33439); Berndorf, Steinhof, Großer Geyergraben, on logs of Fagus sylvatica, 25 October 2014, H. Voglmayr & I. Greilhuber (WU); Oberösterreich, St. Willibald, Aichet, on Fraxinus excelsior and Alnus glutinosa, 11 December 2011, H. Voglmayr (WU 33434; culture VF1); Steiermark, Graz-Umgebung, St. Oswald bei Plankenwarth, opposite Schloss Plankenwarth, on Fagus sylvatica, 16 January 2015, I. Wendelin, comm. G. Friebes (GJO 73406); Hartberg, on Betula pendula, 2 October 2010, H. Voglmayr (WU 33436; culture VFB); Voitsberg, at the railway station, on a fence made of corticated trunks of Abies alba, Rabenhorst Fungi Europaei 1718 (L0819039!, lectotype of Myrmaecium abietinum, here designated); Vienna, 19th district, Hermannskogel, near Fischerhaus, grid square 7763/2, on Ulmus sp., 13 October 1996, W. Jaklitsch W.J. 984 (WU 33441); Kahlenberg, grid square 7763/2, on Fagus sylvatica, 30 August 1998, W. Jaklitsch W.J. 1190 (WU 33442); ibidem, on Fagus sylvatica, 8 November 1998, W. Jaklitsch W.J. 1276 (WU 33444); ibidem, on Aesculus hippocastaneum, 19 October 2014, W. Jaklitsch & H. Voglmayr (WU 33432; culture VFA); 23rd district, Maurer Wald, grid square 7863/1, on Carpinus betulus, 4 November 2000, W. Jaklitsch W.J. 1692 (WU 33445). CZECH REPUBLIC, Hranice, Höllenschlucht at Podhorn, on Fagus sylvatica, 20 August 1912, F. Petrak, Flora Bohemiae et Moraviae Exsiccata 47 (L0819031!, as Valsaria rubricosa f. fagi); Hranice, on Fagus sylvatica, September 1912, F. Petrak (L0819033! and L0819034!, as Valsaria rubricosa); Hranice, Hrabuvka, on hardwood trunks (possibly Carpinus betulus or Fagus sylvatica), August 1940, F. Petrak, Reliquiae Petrakianae 1694 (L0819035!, as V. rubricosa). FRANCE, Rhóne, Sain Bel, in bark of Alnus glutinosa, November 1881, J. Therry 6514, C. Roumeguere. Fungi Gallici Exsiccati 2190 (L0819036!, as Valsaria rubricosa f. alni-glutinosi); Rhóne, Lyon, in bark of Prunus cerasus, ?spring 1881, J. Therry, de Thuemen, Mycotheca universalis 2261 (L0819037!, as Valsaria rubricosa). GERM any, Bayern, Knetzgau, close to Mariaburghausen, left roadside heading from Knetzgau to Haßfurt, grid square 5929/3, on logs of Fagus sylvatica, 29 August 2006, W. Jaklitsch & H. Voglmayr W.J. 2964 (WU 33446). SPAIN, Basque Country, Álava, Ziorraga, on logs of Fagus sylvatica, 1 November 2010, W. Jaklitsch VF2 (WU 33435). TAIWAN, Taichung County, Ho-ping, Pi-lu Creek, on unidentified twigs, 20 August 2006, J.-R. Guu 95082003 (HAST; part as WU 33437; culture VFJ1).
Notes: Myrmaecium fulvopruinatum was originally described from the USA. The residual material of the holotype is however immature, therefore we epitypify Sphaeria fulvopruinata with a specimen from the USA. According to Ju et al. (1996) this taxon is common in the eastern USA, but according to our experience it is also common in Europe. Remarkably most European mycologists misidentified M. fulvopruinatum as M. rubricosum and most European varieties and forms of V. rubricosa are M. fulvopruinatum. Myrmaecium abietinum is a synonym of M. fulvopruinatum, not of M. rubricosum as previously assumed (e.g. with a ? by Ju et al. 1996). Myrmaecium fulvopruinatum is the only species of Valsariales, where fissitunicate opening of asci can be seen rather regularly, and it is the only species forming rather well-defined conidiophores. Apart from surface colours and the substellate structures M. fulvopruinatum is also more variable in the stromata, which do not become superficial, with ascomata usually in valsoid but rarely in diatrypoid configuration. Ascospores are slightly wider than in M. rubricosum, and asci are unstable in water contrary to M. rubricosum. Myrmaecium fulvopruinatum shares the ability to produce cresol in cultures with M. rubrum. In the strain we studied on MEA we only observed the effuse hyphomycetous asexual morph, in contrast to Ju et al. (1996), who detected a pycnidial asexual morph in a different strain on oatmeal agar.
Myrmaecium rubrum (Aptroot et al.) Jaklitsch & Voglmayr, comb nov.
Basionym: Munkovalsaria rubra Aptroot, Aa & Petrini, in Aptroot, Nova Hedw. 60: 349 (1995)
MycoBank MB 811905, Facesoffungi number: FoF 00615, Fig. 17
Fig. 17.
Myrmaecium rubrum (holotype). a, b Ectostromata in face view. c Stroma in side view. d Stroma tissue in KOH. e Stroma in MEA culture. f, g Free ends of paraphyses. h Ascus apex in Congo Red. i–k Asci (k. in Congo Red). l–p Ascospores (l, m showing surface ornamentation). Scale bars: a, b=0.1 mm. c=0.2mm. d, i–k=15 μm. e=1 mm. f, g, n=7 μm. h, l, m, o, p=5 μm
Stromata pseudostromatic, immersed, conical to subglobose, up to ca. 1 mm high and wide at the base, appearing at the host surface as dark reddish-violaceous to black, smooth or irregularly tubercular dots 0.2–0.8(–1.2) mm diam, containing 1 or few inconspicuous to slightly papillate black ostioles. Stromatic tissue above ascomata dark red to black, purple in 3 % KOH, pseudoparenchymatous; cell walls red and brown, inhomogenously pigmented; pseudostromatic tissue at ascomatal levels prosenchymatous, yellow-brown, of hyaline hyphae, but red in the immediate vicinity of ascomata. No black marginal zone apparent. Ascomata 0.13–0.3 mm high and 0.1–0.2 mm wide, basal, monostichous, up to ca. 10 densely clustered in valsoid configuration, globose to pyriform; peridium brown, 2-layered, pseudoparenchymatous. Ostiolar necks long, convergent or fusing. Paraphyses numerous, unbranched, apically free, 2–5 μm wide, ends sometimes slightly widened. Asci (83–)96–115×(10.5–)10.7–12.7(–13.2) μm (n = 8), cylindrical, bitunicate without obvious fissitunicate dehiscence, containing 8 (obliquely) uniseriate ascospores; apex thin, no ring visible in Congo Red. Ascospores (11.7–)12.8–15.2(–18.7)×(6.0–)6.7–8.2(–9.5) μm, l/w (1.6–)1.7–2.1(–2.6) (n=81), ellipsoid, 2-celled, dark brown to black, symmetrical or inequilateral, septum much thicker than the wall, not or slightly constricted at the septum, with delicately labyrinthine ornamentation.
Culture, asexual morph and sexual morph in culture (based on a dry culture contained in the holotype and information from the protologue (Aptroot 1995): Culture on CMA with orange-red aerial mycelium and red diffusing pigment in the medium, with a strong odour of m-cresol. Conidiophores irregularly branched, 3–5 μm wide, with mostly intercalary phialides. Conidia 4–5×1–2 μm, long ellipsoid, 1-celled, hyaline, with a broad hilum and a rounded tip. Sexual stromata or solitary ascomata forming on Lupinus stalks on CMA. Stromata up to 6 mm long, compact, hard, black inside; surface covered by yellow- to orange-brown hyphae; ascomata basal. Stroma cortex red, pseudoparenchymatous, reddish brown to purple in 3 % KOH, but no pigment dissolved; residual stromatic tissue of hyaline hyphae in gel matrix. Ascomata containing paraphyses and mature asci with ascospores as in stromata from natural substrates.
Ecology and distribution: in bark of Ulmus minor, also reported from Citrus, Quercus and, according to Aptroot (1995), also isolated from lichens in South Africa.
Type: Italy, near Bari, on bark of Ulmus minor, S. Frisullo, 1986 (as Didymosphaeria sp.; CBS H-5584, holotype!; CBS 345.86, ex-type culture).
Notes: The description above is based on the somewhat depauperate holotype, which may not exhibit the whole natural variation of the species. Microscopic features in stromata from nature and artificial culture are identical, and measurements were thus combined. Basically the fungus produces reduced Valsaria-like stromata with partly red stromatic tissues. Myrmaecium rubrum shares red colours and stroma formation in culture with M. rubricosum, but production of cresol in culture with M. fulvopruinatum. Ascospores of M. rubrum are larger than given in the protologue and there is no statistical difference in this respect between stromata formed in culture and those formed in nature. The asexual morph description is reproduced from Aptroot (1995). According to the author growth of the fungus is fast and its asexual morph resembles Lecythophora. The GenBank sequences of M. rubrum used here are from strain CBS 109505 (isolated from Quercus sp.). ITS and LSU sequences of this strain are identical with those of the ex-type strain CBS 345.86 (G. Verkley, pers. comm.).
Bambusaria Jaklitsch, D.Q. Dai, K.D. Hyde & Voglmayr, gen. nov.
MycoBank MB 811906
Etymology: For a Valsaria occurring on Bambusoideae.
Type species: Bambusaria bambusae (J.N. Kapoor & H.S. Gill) Jaklitsch, D.Q. Dai, K.D. Hyde & Voglmayr
Stromata eustromatic, immersed, erumpent to superficial, globose to irregular, black, with rugose surface, KOH–, internally dark brown, pseudoparenchymatous. Ascomata immersed in the stromata in valsoid configuration, monostichous, globose to pyriform; peridium pseudoparenchymatous, of subhyaline to pale brownish compressed cells. Ostioles periphysate, necks separately emerging or fusing. Paraphyses unbranched, tapering upwards, apically free. Asci cylindrical, bitunicate without obvious fissitunicate dehiscence, with a short stipe, containing 8 uniseriate ascospores; apex containing an ocular chamber and a pulvinate ring staining in Congo Red. Ascospores ellipsoid to broadly fusiform or biconoid, brown, 1-septate, not or slightly constricted at the septum, longitudinally ribbed.
Asexual morph on natural substrates: Multiloculate conidiomata resembling stromata, immersed-erumpent, globose to irregular, black, with rugose surface, pseudoparenchymatous. Locules with a dark brown, pseudoparenchymatous wall, internally lined by dense palisades of lageniform phialides. Conidia oblong, 1-celled, hyaline, smooth.
Asexual morphs in culture: Conidiation on MEA effuse, hyphomycetous; on OA also in pycnidia.
Ecology and distribution: the monotypic genus is only known from Bambusoideae in India and Thailand.
Bambusaria bambusae (J.N. Kapoor & H.S. Gill) Jaklitsch, D.Q. Dai, K.D. Hyde & Voglmayr, comb nov.
Basionym: Valsaria bambusae J.N. Kapoor & H.S. Gill, Indian Phytopath. 14: 152 (1962) [1961]
MycoBankMB811907, Facesoffungi number: FoF 00616, Figs. 4 s, t, 18, 19
Fig. 18.
Bambusaria bambusae. a–c Stromata on host surface. d, e, h Stromata in vertical section. f, g Stromata in transverse section at the ascomatal level. i Ascoma in vertical section. j, k Ascus apices (j Congo red, k cotton blue). l Germinating ascospore. m, s–u Ascospores (m, t, u showing surface ornamentation). n–q Asci with ascospores. r Paraphyses. v, w Culture on PDA (w. reverse). a–l. MFLU 15–0050; m. isotype IMl 180328. Scale bars: a–c=1mm. d–g=0.5 mm. h=100 μm. i, l=50 μm. j, k, m–u=10 μm
Fig. 19.
Bambusaria bambusae (MFLU 15–0051). a–h, j–m Asexual morph on the natural host. a, b. Conidiomata. c–g. Vertical sections of multiloculate conidiomata and locules. i–m. Conidia (i from MEA, 10 d, 24 °C; l, m germinating and budding). n, o Culture from conidia on PDA (o. reverse). Scale bars: a=3 mm. b–e=0.5 mm. f=100 μm. g, h=50 μm. i–k=5 μm. l, m=10 μm
Stromata immersed, erumpent to superficial, globose, subglobose or irregular, 0.5–2 mm diam, 0.4–1.5 mm high, solitary to gregarious, often confluent into linear rows or irregular clusters, externally surrounded by a black warted, ca. 10–80 μm thick crust; ectostroma often sub- or inversely stellate; internally dark brown, entirely pseudoparenchymatous, of nearly black, thin- to moderately thick-walled cells to 17 μm diam; without KOH-extractable pigments. Ascomata immersed in the stromata in valsoid configuration, monostichous, globose to flask-shaped, 0.2–0.45 mm wide, 0.3–0.8 μm high. Peridium 9–15 μm wide, composed of subhyaline to pale brown compressed cells. Ostiolar necks 150–350 μm long and 100–250 μm diam at the top, separately emerging or several fusing into one, often slightly projecting above the stroma surface and sulcate, black; interior periphysate. Paraphyses unbranched, tapering upwards, apically free, 1.5–9 μm wide. Asci cylindrical, (126–)134–156×13.5–16.8 μm (n = 25), bitunicate without obvious fissitunicate dehiscence, with a short stipe, containing 8 uniseriate ascospores; apex containing an ocular chamber and a pulvinate ring 2.0–8.5×(3.0–)4.0–5.5(–6.2) μm (n=20), staining in Congo Red. Ascospores ellipsoid to broadly fusiform or double rhomboid, (15.5–)18.5–22.7(–28.5)×(7.7–)9.0–11.0(–12.2) μm, l/w (1.5–)1.8–2.3(–3.1) (n=185), medium to dark brown, 1-septate, not or slightly constricted at the septum, rounded or acute at both ends, with sinuous longitudinal ribs partially anastomosing and forming labyrinthine to coarsely reticulate ornamentation.
Asexual morph on natural substrates: Stromata resembling sexual stromata, globose, conical to irregular, black, soft, with a black warted superficial crust, pseudoparenchymatous entostroma, multiloculate. Locules with a pale brown, 9–20 μm wide wall of compressed cells, internally lined by dense palisades of lageniform phialides. Conidia oblong, (2.5–)3.0–3.8(–4.0)×(1.0–)1.3–1.7(–2.0) μm, l/w (1.8–)2.3–3.0(–3.2) (n=40), 1-celled, straight, hyaline, obtuse at both ends, smooth.
Cultures and asexual morph: On MEA colony radius 9–11 mm after 3 days, 25–32 mm after 5 days, 35–40 mm after 7 days; centrally inoculated 90 mm plates entirely covered by mycelium after 10–14 days. Colony circular with irregular margin, covered by a white mat of aerial hyphae; reverse turning yellowish from the centre after 5–7 days. Odour indistinct. Conidiation effuse, conidia appearing after ca. 2 weeks, formed by budding and on minute pegs on filiform, little branched fertile hyphae, (5.6–)6.5–9.5(–10.8)×(2.5–)3.0–5.5(–6.7) μm, l/w (1.2–)1.5–2.6(–3.8) (n=100), 1-celled, ellipsoid to subglobose, hyaline, smooth, straight or curved, with minute guttules. On PDA colony radius ca. 22 mm after 2 weeks, with irregular margin, flat, white, cottony; reverse turning orange to orange-brown from the centre. Conidiation effuse.
Types: INDIA, Bombay, Dharwar, on culms of Bambusa sp., May 1915, G.S. Kulkami, (HCIO 26844, holotype!; microscope slides IMl 180328 in K: isotype).
Reference specimen: THAILAND, Chiang Rai Prov., Mae Kon subdistrict, Horticulture Research Centre, on dead culms of Thyrsostachys siamensis, 5 October 2012, D.Q. Dai DDQ00253 (MFLU 15–0050; culture from ascospores: MFLUCC 12–0851); reference sequences KP687812 (ITS-LSU), KP687890 (rpb2), KP687982 (tef1).
Other material examined: INDIA, Assam, Research centre Burnihat, on culms of bamboo, 30 April 1979, H.K. Baisya 45 (IMI 238159; K); Maharashtra, Dhule Dist., Ranipur, on culms of Dendrocalamus strictus, 30 November 1988. M.R. Ujjainkar & S.D. Deoray (IMI 333847 at K; culture studied by Ju et al. 1996); no locality given, on culms of bamboo, 25 October 1974, K.S. Panwar (IMI 189339, IMI 189341 at K). All as Valsaria bambusae. THAILAND, Chiang Rai Prov., Mae Kon subdistrict, Horticulture Research Centre, on dead culms of Thyrsostachys siamensis, asexual morph, 5 October 2012, D.Q. Dai DDQ00254 (MFLU 15–0051; culture from conidia: MFLUCC 15–0001 =CBS 139763).
Notes: The material in the holotype is depauperate and effete, but the isotype contains sections comprising stromatic tissue, ascomata and typical striate ascospores, and also an asexual morphic stroma. Ju et al. (1996) reported that ascospore ornamentation varies from longitudinally ribbed to reticulate. This is correct, but even when reticulate, the aspect of the ascospores is longitudinally ribbed or striate, with striae appearing sinuous. This aspect and the occurrence on bamboos are considered sufficient for identification of the fungus. As our sources of molecular data are from a different country (Thailand, versus India) and a different host genus (Thyrsostachys versus Bambusa) than the type material, we presently cannot exclude that the cited specimens represent a species complex. We designate the specimen MFLU 15–0050 as reference specimen until fresh material from India becomes available. The pycnidial asexual morph reported by Ju et al. (1996) was not seen in the strains studied here. This may be a consequence of a different culture medium used.
Key to genera and species:
Note: Species of Valsaria can only be determined with certainty using gene sequences, except for V. lopadostomoides and V. rudis, which are regarded as host-specific for Quercus.
-
1.
Stromata with shades of red or yellow-brown to cinnamon, often showing ionomidotic reaction (KOH-extractable pigments), growth in culture fast Myrmaecium 2
-
1.
Stromata black, without bright colours or ionomidotic reaction, growth in culture slow 4
-
2.
Stromata conspicuous, eustromatic, releasing purple pigment in KOH 3
-
2.
Stromata inconspicuous, pseudostromatic, on Citrus, Quercus or Ulmus, rare M. rubrum
-
3.
Stroma erumpent-superficial, large, surface red, producing sexual stromata in culture M. rubricosum
-
3.
Stromata immersed-erumpent, yellow-brown to cinnamon, often with yellow scurf, not producing sexual stromata in culture M. fulvopruinatum
-
4.
Eustromatic, ascospores longitudinally ribbed, on bamboos Bambusaria bambusae
-
4.
Pseudostromatic, ascospores with verruculose or delicately reticulate ornamentation, in bark, not on bamboos Valsaria 5
-
5.
On Quercus spp. 6
-
5.
On other hosts 7
-
6.
On evergreen oaks, e.g. Quercus ilex V. lopadostomoides
-
6.
On deciduous oaks, e.g. Quercus cerris, Q. petraea V. rudis
-
7.
On unknown hosts in the Neotropics V. neotropica
-
7.
Known from warmer regions of Europe (and North America) 8
-
8.
On Vitis and predominantly non-fabaceous hosts V. insitiva
-
8.
Predominantly on fabaceous hosts 9
-
9.
On Amorpha, Caragana, Colutea, Hippocrepis and Robinia in Central Europe and submediterranean regions, and the eastern USA; arthroconidia in culture inconspicuous V. robiniae
-
9.
On various species of woody Fabaceae, less commonly on other hosts such as Acer sempervirens, Spiraea, Melia, Fraxinus; arthroconidia in culture conspicuous and abundant; common in the (Sub-)Mediterranean V. spartii
Discussion
We studied the genus Valsaria as formerly conceived. The morphology of sexual morphs, the coelomycetous asexual morph in nature, the hormonema-like hyphomycetous and the cytosporella-like pycnidial asexual morphs in culture as described by Glawe (1985) and Ju et al. (1996) have been confirmed in this study. We did not find an asexual morph in nature in Valsaria lopadostomoides and V. neotropica, but both species are based on a single specimen. In cultures of Myrmaecium fulvopruinatum and Bambusaria bambusae we did not detect a pycnidial asexual morph, although they were described by Ju et al. (1996), but this may depend on the different medium used or perhaps the strain studied. Also the quercicolous species V. lopadostomoides and V. rudis did not form pycnidia in culture. Colonies of the latter species propagate very slowly and become covered by large masses of conidia.
Ascospores of Valsaria and even more those of Myrmaecium are extremely durable, as Ju et al. (1996) obtained a culture from a 12 year old specimen (VFJ=CBS 139058) of M. fulvopruinatum and we obtained a culture from a nearly 11 year old specimen of M. rubricosum (VRM). These findings correlate with the occurrence of Myrmaecium spp. on sunexposed logs and of other representatives of the Valsariales in warm climates with extended periods of drought. In times of global warming it seems justified to expect that these fungi will enlarge their distribution area. An indication in this direction may be the observation that species of Valsaria have become more common in warmer parts of Austria, esp. around Vienna, although this may also be a consequence of increased planting of ornamental shrubs belonging to the Fabaceae.
Apart from the combination of true paraphyses and bitunicate asci budding of ascospores is remarkable. This property is shared with several genera from different unrelated taxonomic groups. Examples are Oevstedalia Ertz & Diederich (Pezizomycotina, uncertain position), Rhamphoria (uncertain position [Annulatascaceae s.l.], Sordariomycetes), Thyronectria (Nectriaceae, Hypocreales; see Jaklitsch and Voglmayr 2014), Tympanis (Tympanidaceae, Phacidiales) or Thyridium. The latter genus (as discussed by Ju et al. 1996) has much in common with Valsaria, as it has ascomata arranged in valsoid fashion in pseudostromata, ascopores that germinate by budding off conidia or producing germ hyphae and coelo- and hyphomycetous asexual morphs. Asci of Thyridium vestitum look bitunicate, therefore the fungus was classified in Fenestella (e.g. by Munk 1957), but phylogenetically the genus belongs to a distinct family, Thyridiaceae, in the Sordariomycetes (Jaklitsch and Voglmayr 2014).
Our phylogenetic study has shown that a widely conceived concept of Valsaria cannot be upheld, and Myrmaecium represents a distinct genus as well as V. bambusae, which we transfer to the new genus Bambusaria. The hitherto morphologically conceived Valsaria insitiva is split into six distinct species, which barely differ in morphological traits. Infraspecific genetic variation in the Valsariaceae is relatively low, and isolates of M. rubricosum from specimens collected in Europe, Hawaii and Martinique clearly grouped into a monophylum, European and North American isolates of M. fulvopruinatum are clearly one species and strains of V. insitiva from specimens collected in Guadeloupe and Taiwan clearly formed a clade with those from Europe.
However, the detection of V. neotropica from Martinique as a distinct species but with a morphology that is scarcely different from V. insitiva, but also the recognition of V. robiniae, V. rudis and V. spartii, which had been synonymized by Ju et al. (1996) with V. insitiva, as distinct species, question the synonymy of other epithets. After removal of a few incorrect names 182 names of Myrmaecium and Valsaria can be found in Index Fungorum (http://www.indexfungorum.org/Names/Names.asp). Most fungi described in Myrmaecium have hyaline ascospores and thus do not belong to the genus, few are dubious or probable synonyms. Owing to distinct morphology and genetic stability recognition of synonyms in Myrmaecium is much easier than in Valsaria, and 19 epithets of Valsaria have been relegated to Myrmaecium by Ju et al. (1996) under V. rubricosa and V. fulvopruinata. Many epithets of Valsaria have been recognized as synonyms of other fungi, most notably Pseudovalsaria (Spooner 1986; Untereiner et al. 2013), Endoxylina (Ju et al. 1996; Untereiner et al. 2013) or Rousso7#x00EB;lla (Ju et al. 1996), and several other pyrenomycetous genera. Ju et al. (1996) studied all type specimens and other herbarium material available to them and synonymized numerous names with V. insitiva. The recognition of V. robiniae, V. rudis and V. spartii as distinct species, however, makes it difficult to determine, which names are synonyms of which species, as they cannot be distinguished by morphology, complicated by the fact that none of the species detected here have sharply defined host preferences, except for the two Quercus-specific ones. Twenty-three described names are forms or varieties and thus not relevant at the species level. Others are dubious and in effect 30–40 epithets require re-assessment, the vast majority of which were described from Africa, Asia and North and South America. Additional species may be recognized based on molecular phylogeny, and already published epithets may apply to some, and this can only be ascertained via re-collection in the original regions on the original hosts.
Some species may deserve comments, as they were treated in more recent times. Barr (1989) combined Didymosphaeria sphaerophora Ellis & Everh. in Valsaria. Her concept of Valsaria was a genus encompassing taxa with unitunicate asci. Whether or not this fungus, which is only known from the type specimen collected on Agave in Mexico and has striate ascospores, is a member of Valsariaceae remains to be assessed. Spooner (1986) combined Pseudothyridaria insitiva Petr. in Valsaria. Ju et al. (1996), however, accepted Pseudothyridaria because of the presence of pseudoparaphyses. This fungus, described from Aesculus, has to be recollected for determination of DNA data. Spooner (1986) also recognized that V. foedans does not belong to Valsaria and described the new genus Pseudovalsaria for it, a genus of the Boliniales; see Untereiner et al. (2013) for a recent treatment, phylogenetic placement and synonyms. Kale (1970) summarized Valsaria in India. He listed four species including Bambusaria bambusae (as V. bambusae); V. indica was considered to be a synonym of V. insitiva.
Species of Myrmaecium are known as sources of secondary metabolites. Volatile compounds are formed in culture by M. fulvopruinatum and M. rubrum, and for the latter the source of strong odour was identified as m-cresol (Aptroot 1995 and refs. therein). Pigments of M. rubricosum were identified as the two anthraquinones valsarin and 7-dichloroemodin by Bohman (1969) and Briggs et al. (1972), and Lam et al. (1972) synthesized these compounds.
Acknowledgments
We sincerely thank F. Candoussau, G. Friebes, A. Gardiennet, J.-R. Guu, H.-M. Hsieh, P. Karasch, C. Lechat, M. Pélissier, F. Rappaz and J.D. Rogers for providing specimens, Y.-M. Ju for specimens, cultures and helpful comments on the manuscript, W. Gams for excursion support in Italy, F. Balao, J. Herrera and S. Tello for support and help with determination of fabaceous hosts in Spain; the fungarium curators S. Dominick and E. Roark (BPI), P. Salo (H), B. Aguirre-Hudson (K), N. Sol and G. Thijsse (L), E. Bloch (NY), B. Buyck (PC), A. Freire-Fierro and N. Phillips (PH) for loans and information of specimens, W. Till at WU for sending and managing collections; T. Merkx (CBS) for managing our cultures, G. Verkley (CBS) for providing cultures and information about strains of M. rubrum; and W. Dämon and I. Greilhuber for insertion of specimens into WU. The financial support by the Austrian Science Fund (FWF; project P25870-B16) is gratefully acknowledged. K.D. Hyde and D.Q. Dai thank the Mae Fah Luang University for the grant “Taxonomy and Phylogeny of selected families of Dothideomycetes (Grant number: 56101020032). D.Q. Dai is grateful to the Mushroom Research Foundation, Bandoo, Chiang Rai Province, Thailand, for providing postgraduate scholarship support and thanks the Mae Fah Luang University for the GMS grant.
References
- Aptroot A. Redisposition of some species excluded from Didymosphaeria (Ascomycotina) Nova Hedwigia. 1995;60:325–379. [Google Scholar]
- Barr ME. The Diaporthales in North America, with emphasis on Gnomonia and its segregates. Mycol Mem. 1978;7:1–232. [Google Scholar]
- Barr ME. Some unitunicate taxa excluded from Didymosphaeria. Stud Mycol. 1989;31:23–27. [Google Scholar]
- Barr ME. Podromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon. 1990;39:43–184. [Google Scholar]
- Barr ME, Huhndorf SM, Rogerson CT. The pyrenomycetes described by J.B. Ellis. Mem New York Bot Gdn. 1996;79:1–137. [Google Scholar]
- Bohman G. Chemical studies on lichens 22. Anthraquinones from the Lichen Lasallia papulosa var. rubiginosa and the fungus Valsaria rubricosa. Acta Chem Scand. 1969;23:2241–2244. doi: 10.3891/acta.chem.scand.23-2241. [DOI] [PubMed] [Google Scholar]
- Briggs LH, Castaing DR, Denyer AN, Orgias EF, Small CW. Chemistry of fungi. 8. Constituents of Valsaria rubricosa and the identification of papulosin with valsarin. J Chem Soc Perkin Trans. 1972;1:1464–1466. doi: 10.1039/p19720001464. [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alis AS, Xu J, Liu X, Stadler M, Hyde KD. The sooty moulds. Fungal Divers. 2014;66:1–36. [Google Scholar]
- de Hoog GS, Gerrits van den Ende AHG. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Ellis JB, Everhart BM. The North American pyrenomycetes. Newfield, New Jersey: 1892. p. 793. Published by the authors. [Google Scholar]
- Eriksson OE. Checklist of the non-lichenized ascomycetes of Sweden. Acta Univ Upsal Symb Bot Upsal. 2014;36(2):7–499. [Google Scholar]
- Eriksson OE, Hawksworth DL. Notes on ascomycete systematics - Nos. 2140–2255. Syst Ascomyc. 1997;15:139–173. [Google Scholar]
- Glawe DA. The pleomorphic asexual state of Valsaria insitiva in artificial culture. Mycologia. 1985;77:62–71. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis. program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Huhndorf SM. Neotropical ascomycetes 1. Valsonectria cinnamomi in artificial culture. Mycologia. 1992;84:642–649. [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, et al. Families of Dothideomycetes. Fungal Divers. 2013;63:1–313. [Google Scholar]
- Jaklitsch WM. European species of Hypocrea - part I. Stud Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Fournier J, Rogers JD, Voglmayr H. Phylogenetic and taxonomic revision of Lopadostoma. Persoonia. 2014;32:52–82. doi: 10.3767/003158514X679272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-M, Rogers JD, Huhndorf SM. Valsaria and notes on Endoxylina, Pseudothyridaria, Pseudovalsaria, and Roussoella. Mycotaxon. 1996;58:419–481. [Google Scholar]
- Kale SB. The genus Valsaria in India. Sydowia. 1970;23:194–197. [Google Scholar]
- Karsten PA, Hariot P. Fungi nonnulli gallici. J Bot (Paris) 1889;3:206. [Google Scholar]
- Kauff F, Lutzoni F. Phylogeny of Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol Phylogenet Evol. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. Ainsworth & Bisby’s dictionary of the fungi. 10th. CABI; Wallingford: 2008. [Google Scholar]
- Lam JKK, Sargent MV, Elix JA, Smith DON. Synthesis of valsarin and 5,7-dichloroemodin. J Chem Soc Perkin Trans. 1972;1:1466–1470. doi: 10.1039/p19720001466. [DOI] [PubMed] [Google Scholar]
- Landvik S, Egger K, Schumacher T. Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nord J Bot. 1997;17:403–418. [Google Scholar]
- Liu YL, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Müller K. PRAP - calculation of Bremer support for large data sets. Mol Phylogenet Evol. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Munk A. Danish pyrenomycetes. Dansk Bot Arkiv. 1957;17:1–491. [Google Scholar]
- Petrak F, Sydow H. Kritisch-systematische originaluntersuchungen über Pyrenomyzeten, Sphaeropsideen und Melanconieen. Ann Mycol. 1923;21:349–384. [Google Scholar]
- Riethmüller A, Voglmayr H, Göker M, Weiß M, Oberwinkler F. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia. 2002;94:834–849. doi: 10.1080/15572536.2003.11833177. [DOI] [PubMed] [Google Scholar]
- Schweinitz LD. Synopsis fungorum carolinae superioris. Schr Naturf Ges Leipzig. 1822;1:1–105. [Google Scholar]
- Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–337. [Google Scholar]
- Spooner BM. New or rare British microfungi from Esher Common, Surrey. Trans Br Mycol Soc. 1986;86:401–408. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Thiers B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. 2014 http://sweetgum.nybg.org/ih/
- Tode HJ. Fungi Mecklenburgenses Selecti. 1791;2:1–64. [Google Scholar]
- Untereiner WA, Bogale M, Carter A, Platt HWB, Hanson S-Å et al. Molecular phylogeny of Boliniales (Sordariomycetes) with an assessment of the systematics of Apiorhynchostoma, Endoxyla and Pseudovalsaria. Mycologia. 2013;105:564–588. doi: 10.3852/12-326. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Prosthecium species with Stegonsporium anamorphs on Acer. Mycol Res. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Divers. 2011;46:133–170. [Google Scholar]
- Wehmeyer LE. The imperfect stage of some higher pyrenomycetes obtained in culture. Pap Michigan Acad Sci. 1923;3:245–266. [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. Academic; San Diego: 1990. pp. 315–322. [Google Scholar]
- Wijayawardene NN, Crous PW, Kirk PM, et al. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]