Abstract
Skeletal muscle strength and mass, major contributors to sprint/power athletic performance, are influenced by genetics. However, to date, only a handful of genetic variants have been associated with sprint/power performance. The ACVR1B A allele (rs rs2854464) has previously been associated with increased muscle-strength in non-athletic cohort. However, no follow-up and/or replications studies have since been conducted. Therefore, the aim of the present study was to compare the genotype distribution of ACVR1B rs2854464 between endurance athletes (E), sprint/power (S/P) athletes, mixed athletes (M), and non-athletic control participants in 1672 athletes (endurance athletes, n = 482; sprint/power athletes, n = 578; mixed athletes, n = 498) and 1089 controls (C) of both European Caucasians (Italian, Polish and Russians) and Brazilians. We have also compared the genotype distribution according to the athlete’s level of competition (elite vs. sub-elite). DNA extraction and genotyping were performed using various methods. Fisher's exact test (adjusted for multiple comparisons) was used to test whether the genotype distribution of rs2854464 (AA, AG and GG) differs between groups. The A allele was overrepresented in S/P athletes compared with C in the Caucasian sample (adjusted p = 0.048), whereas there were no differences in genotype distribution between E athletes and C, in neither the Brazilian nor the Caucasian samples (adjusted p > 0.05). When comparing all Caucasian athletes regardless of their sporting discipline to C, we found that the A allele was overrepresented in athletes compared to C (adjusted p = 0.024). This association was even more pronounced when only elite-level athletes were considered (adjusted p = 0.00017). In conclusion, in a relatively large cohort of athletes from Europe and South America we have shown that the ACVR1B rs2854464 A allele is associated with sprint/power performance in Caucasians but not in Brazilian athletes. This reinforces the notion that phenotype-genotype associations may be ethnicity-dependent.
Introduction
Skeletal muscle strength and mass are major contributors to elite athletic performance in a variety of sport disciplines, especially those where explosive muscle contractions are critical (e.g. sprint/power oriented disciplines) [1]. There is considerable variability in sprint, strength, and power performance within athletes of similar age, body composition, and training history. One possible explanation for this variability is the athletes’ genetic makeup [2]. The current estimated heritability for muscle strength and muscle mass ranges from 31% to 78%, with large differences between muscle groups, contraction velocities and muscle lengths [3,4].
To date, only a handful of genetic variants have been robustly associated with sprint/power performance. Two candidate gene variants associated with sprint/power performance with reasonable replication in different groups of elite sprint/power athletes are the ACTN3 R577X [5,6] and the ACE I/D [7,8]. We have also recently shown that variants in the EPAS1 [9] and the MCT1 genes [10] are associated with sprint/power performance in European athletes. However, these studies require replication and verification in larger cohorts, and the consensus shared between scientists is that there are many other undiscovered variants associated with sprint/power performance.
A gene variant (rs2854464) within the Activin A Receptor type 1b gene (ACVR1B) has previously been associated with muscle-strength phenotypes [11]. Initial studies using microsatellite markers and linkage analysis have identified linkage peaks associated with muscle strength in the 12q12-14 chromosomal region [12,13]. The same research group has then used different approaches to show that the ACVR1B rs2854464 A allele is associated with increased muscle strength in healthy, non-athletic individuals [11]. To date, no follow-up and/or replications studies have been conducted to confirm or refute these findings. Therefore, whether this gene variant influence athletic performance and/or gains in muscle mass and strength in either elite athletes or the general population remains unclear.
One of the major drawbacks in the field of Sports Genomics is the relatively low sample of elite athletes and collaborative effort is required to move the field forward, and enhance our understanding of the genes that influence athletic performance. The Athlome Project Consortium [14] has been therefore recently established, and one of its aims is to identify gene variants that contribute to elite performance in large-scale, collaborative efforts involving cohorts from different countries.
Therefore, the present study aimed to compare the genotype distribution of ACVR1B rs2854464 between endurance athletes, sprint/power athletes, and non-athletic control participants in a large cohort (n = 1672 athletes, and n = 1089 controls) of both European Caucasians (Italian, Polish and Russians) and Brazilians. Furthermore, the association between rs2854464 and athletic status (i.e., ‘elite’ and ‘sub-elite’ level) was also examined. In light of the previously observed association between the ACVR1B A allele and muscle strength, we hypothesized that the A allele would be associated with elite sprint/power performance.
Material and Methods
The study was conducted according to the Declaration of Helsinki. All participants have signed an informed consent form prior to the study. The study was approved by the ethics committees of the University of Sao Paulo, Brazil, the Pomeranian Medical University, Poland, the University of Cagliari, Italy and the Ural State University of Physical Culture, Russia. The full dataset used in this study can be found in S1 Table.
Participants
A total of 1672 athletes (endurance athletes, n = 482; sprint/power athletes, n = 578, mixed athletes, n = 498), and 1089 non-athletic controls volunteered to participate in this study. Athletes and controls were from four countries: Brazil (ncontrols = 257, nathletes = 474), Italy (ncontrols = 84, nathletes = 125), Poland (ncontrols = 500, nathletes = 350), and Russia (ncontrols = 248, nathletes = 723). Participants from the European countries (i.e., Italy, Poland, and Russia) were self-reported unrelated Caucasians for ≥ three generations, whereas the Brazilian athletes have been treated as a different group given the admixture in the Brazilian population (see below in ‘population stratification’ section). Athletes were classified as endurance (E), sprint/power (S/P) or mixed (M) athletes according to the characteristics of their sports disciplines (i.e., distance, duration and metabolic requirements) (Table 1). When categorization was not straightforward (e.g. if a runner, for example, was engaged in both the 800m (M) and 1500m (E) distances), we classified the athlete as uncertain (U). All athletes were in the top 10 national rank in their sports discipline and grouped as ‘elite level’ or ‘sub-elite (national) level’ according to individual’s best personal performance, as previously described [9,15]. Athletes in the elite group had participated in international competitions (e.g., World and Continental Championships, and/or Olympic Games), whilst those in the sub-elite group had only participated in national-level competitions.
Table 1. Classification of the athletes' disciplines.
| Endurance (E) | Sprint/power (S/P) | Mixed (M) | Uncertain (U) |
|---|---|---|---|
| Biathlon | Archery | Badminton | Running (800-1500m) |
| Canoeing marathon | Artistic gymnastics | Bandy | Speed skating (500-3000m) |
| Cross-country skiing | Wrestling | Boxing | Speed skating (500-5000m) |
| Cycling endurance | Canoeing speed | Canoeing (200-1000m) | Speed skating (500-10000m) |
| Marathon | Cycling (1000m) | Decathlon | Speed skating (1500-3000m) |
| Mountain cycling | Cycling (2000m) | Fencing | Speed skating (1500-5000m) |
| Racewalking (20000m) | Discus throw | Figure skating | Speed skating (3000-5000m) |
| Road cycling | Diving | Futsal | Swimming (100-200m) |
| Rowing (2000m) | Gymnastics | Handball | Swimming (200-400m) |
| Rowing (5000m) | Hammer throw | Heptathlon | |
| Rowing (2000-10000m) | High jump | Ice hockey | |
| Running (1500m) | Javelin throw | Judo | |
| Running (3000m) | Jump | Karate | |
| Running (1500-3000m) | Jump/Running (100-200m) | Kickboxing | |
| Running (5000m) | Long jump | Pentathlon | |
| Running (1500-5000m) | Mogul skiing | Rhythmic Gymnastics | |
| Running (5000-10000m) | Pole vault | Running (800m) | |
| Running (>10000m) | Powerlifting | Soccer (midfielder) | |
| Shooting | Rowing (200-500m) | Speed skating (3000m) | |
| Speed skating (5000m) | Rowing (200-1000m) | Swimming (200m) | |
| Speed skating (5000-10000m) | Rowing (500m) | Taekwondo | |
| Speed skating (10000m) | Rowing (1000m) | Volleyball | |
| Speed skating stayer | Running (100m) | Water polo | |
| Steeple-chase | Running (200m) | ||
| Swimming (400-800m) | Running (100-200m) | ||
| Swimming (800m) | Running (400m) | ||
| Swimming (800-1500m) | Running (100-400m) | ||
| Swimming (1500m) | Shot put | ||
| Swimming (>5000m) | Skating | ||
| Triathlon | Ski-cross | ||
| Walking | Ski jumping | ||
| Slalom skiing | |||
| Slopestyle | |||
| Snowboard-cross | |||
| Soccer (defender) | |||
| Speed skating (500m) | |||
| Speed skating (500-1000m) | |||
| Speed skating (500-1500m) | |||
| Speed skating (1000m) | |||
| Speed skating (1500m) | |||
| Speed skating (1000-1500m) | |||
| Swimming (50m) | |||
| Swimming (50-100m) | |||
| Swimming (100m) | |||
| Throw | |||
| Weightlifting |
Exclusion criteria
We have excluded from the analysis: athletes that had only participated in regional competitions (n = 7, all Brazilian); athletes whose genotype was undetermined (n = 2, all Polish); athletes that were of non-European origin in the Italian, Polish or Russian samples (n = 6, all Italian).
Population stratification
The Brazilian population is formed by extensive admixture between Amerindians, Europeans and Africans, and is one of the most variable populations in the world. Despite positive assortative mating by ancestry [16], self-reported ancestry remains an unreliable criteria for the Brazilian population [17–19]. Thus, we have treated the Brazilian cohort separately, regardless of their self-reported ethnicity.
Genotyping
Brazilian sample
Genomic DNA was isolated from buccal epithelium obtained from mouthwashes. DNA was then extracted using chloroform, precipitated using ethanol and resuspended with 1x Tris-EDTA (Invitrogen). DNA quantification and quality assessment were performed using spectrophotometer (NanoDrop 2000, Thermo Scientific). Genotyping of the ACVR1B rs2854464 polymorphism was performed by using a pre-designed specific TaqMan® SNP Genotyping Assays (ID: C__15826374_10, Applied Biosystems, Foster city, CA, USA), run and read performed in a Rotor Gene-Q real-time termocycler (Qiagen, Valencia, CA, USA), using 15 ng of the DNA samples and appropriate primers fluorescently labeled (FAM and VIC) MGB™ probes according to the manufacturer's instructions. A scatter plot was used to plot the final end data points and thus discriminating the alleles.
Italian sample
Genomic DNA was isolated from buccal swab, and extracted using Qiamp minikit according to the manufacturer's instructions. All samples were amplified with a classical PCR (Applied Biosystem) using the following primers: FORWARD-GCTTGCTGGTGCCTCTTTTC; REVERSE-CTTCACATTCCTCGGCCCTT. PCR products were sequenced by Macrogen through forward primers. For replication and genotype verification, 10% of samples were genotyped in duplicates.
Polish sample
Buccal epithelium was used to isolate genomic DNA with the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, Hamburg, Germany). In all the cohorts, genotypes were determined in duplicates using a Real-Time PCR-based allelic discrimination system (CFX96, Bio-Rad, USA) with Taqman probes. To discriminate ACVR1B rs2854464 alleles, TaqMan Pre-Designed SNP Genotyping Assays were used, similarly to those used in the Brazilian cohort.
Russian sample
Buccal epithelium or peripheral blood was used to isolate genomic DNA with the GeneJET™ Genomic DNA Purification Kit (Thermo Fisher Scientific Inc).
Genotyping of the ACVR1B rs2854464 polymorphism was performed with a TaqMan® SNP genotyping assay similar to the assay used in the Brazilian and Polish cohort. K562 DNA High Molecular Weight from Promega Corp. (Cat # DD2011, Madison, WI, USA) served as a positive control sample. The ACVR1B rs2854464 genotype of the K562 DNA was A/G.
Statistical analyses
Hardy—Weinberg equilibrium (HWE)
χ2 analysis was used to confirm whether the control group from each of the four samples met HWE expectations.
Distribution of rs2854464 genotypes between groups
Fisher's exact test was used to test whether the rs2854464 genotype distribution (AA, AG and GG) differs between groups. To eliminate the possibility of false positive results, p-values were adjusted for multiple comparisons as proposed by Benjamini and Hochberg [20]. Significance was set at p < 0.05.
Results
HWE and genotype distribution in the control sample
The genotype distribution of all control groups were in HWE (χ2 test, all p > 0.05). Moreover, the Italian, Polish and Russian controls had similar genotype distributions (Fisher's exact test, all p > 0.05, S1 Fig). Thus, to increase statistical power and to reduce the number of performed tests, we pooled the Italian, Polish and Russian samples together and referred to this sample as the "Caucasian sample". Genotype distributions of the individual cohorts are available in S1 Fig and Table 2.
Table 2. Genotype distributions in the four studied samples.
| Brazilians | Brazilians elite | |||||
| AA | AG | GG | AA | AG | GG | |
| Control | 111 (43%) | 107 (42%) | 39 (15%) | 111(43%) | 107 (42%) | 39 (15%) |
| Endurance | 100 (44%) | 103 (46%) | 23 (10%) | 49 (43%) | 54 (48%) | 10 (9%) |
| Sprint/power | 57 (32%) | 97 (54%) | 26 (14%) | 41 (37%) | 56 (50%) | 15 (13%) |
| Mixed | 0 | 0 | 0 | 0 | 0 | 0 |
| Uncertain | 28 | 23 | 10 | 9 | 9 | 4 |
| All athletes | 185 (40%) | 223 (48%) | 59 (12%) | 99 (40%) | 119 (48%) | 29 (12%) |
| Italian Caucasian | Italian Caucasian elite | |||||
| AA | AG | GG | AA | AG | GG | |
| Control | 42 (50%) | 36 (43%) | 6 (7%) | 42 (50%) | 36 (43%) | 6 (7%) |
| Endurance | 14 (58%) | 8 (33%) | 2 (9%) | 11 (52%) | 8 (38%) | 2 (10%) |
| Sprint/power | 35 (49%) | 32 (45%) | 4 (6%) | 22 (51%) | 19 (44%) | 2 (5%) |
| Mixed | 11 (52%) | 9 (43%) | 1 (5%) | 9 (47%) | 9 (48%) | 1 (5%) |
| Uncertain | 0 | 1 | 0 | 0 | 0 | 0 |
| All athletes | 60 (51%) | 50 (43%) | 7 (6%) | 42 (51%) | 36 (43%) | 5 (6%) |
| Polish Caucasian | Polish Caucasian elite | |||||
| AA | AG | GG | AA | AG | GG | |
| Control | 263 (53%) | 187 (37%) | 50 (10%) | 263 (53%) | 187 (37%) | 50 (10%) |
| Endurance | 54 (56%) | 40 (41%) | 3 (3%) | 54 (56%) | 40 (41%) | 3 (3%) |
| Sprint/power | 55 (53%) | 42 (40%) | 7 (7%) | 55 (53%) | 42 (40%) | 7 (7%) |
| Mixed | 91 (62%) | 53 (36%) | 3 (2%) | 91 (62%) | 53 (36%) | 3 (2%) |
| Uncertain | 0 | 0 | 0 | 0 | 0 | 0 |
| All athletes | 200 (57%) | 135 (39%) | 13 (4%) | 200 (57%) | 135 (39%) | 13 (4%) |
| Russian Caucasian | Russian Caucasian elite | |||||
| AA | AG | GG | AA | AG | GG | |
| Control | 126 (51%) | 102 (41%) | 20 (8%) | 126 (51%) | 102 (41%) | 20 (8%) |
| Endurance | 72 (53%) | 50 (37%) | 13 (10%) | 19 (54%) | 14 (40%) | 2 (6%) |
| Sprint/power | 133 (59%) | 84 (38%) | 7 (3%) | 56 (62%) | 30 (33%) | 4 (5%) |
| Mixed | 188 (57%) | 119 (36%) | 23 (7%) | 75 (60%) | 48 (38%) | 3 (2%) |
| Uncertain | 21 | 11 | 2 | 11 | 5 | 0 |
| All athletes | 414 (57%) | 264 (37%) | 45 (6%) | 161 (60%) | 97 (36%) | 9 (4%) |
Endurance athletes vs. controls
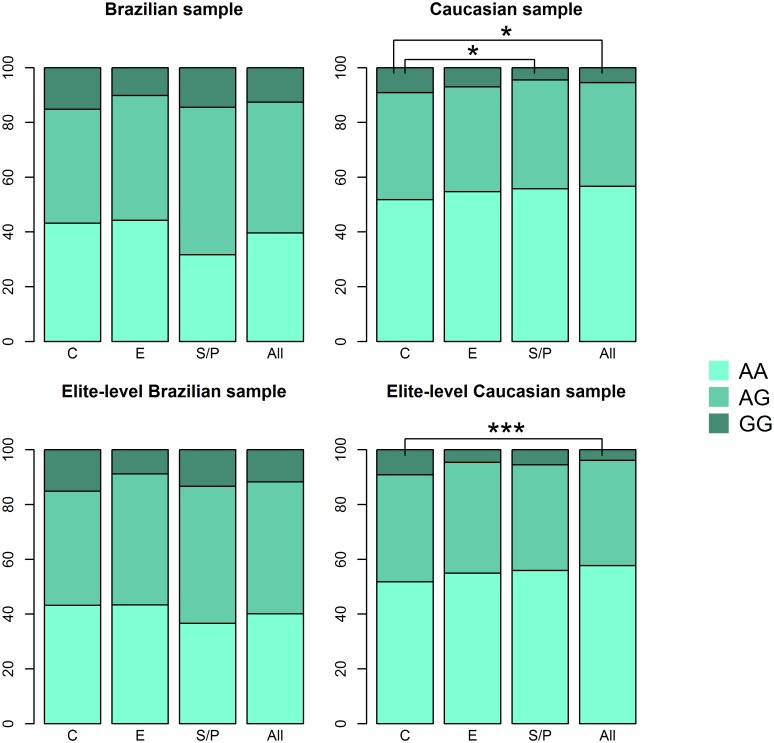
There were no differences in genotype distribution between E athletes and C, in neither the Brazilian nor the Caucasian samples (Fisher's exact test, all adjusted p > 0.05, Table 2, Fig 1).
Fig 1. Genotype distributions at rs2854464 in Brazilian and Caucasian samples.
C = controls; E = endurance athletes; S/P = sprint/power athletes; All = all athletes; *adjusted p-value < 0.05; **adjusted p-value < 0.01; ***adjusted p-value < 0.001.
Sprint/power athletes vs. controls
The A allele was overrepresented in S/P athletes compared with C in the Caucasian sample (Fisher's exact test, adjusted p = 0.048, Table 2, Fig 1). However, no difference was observed when comparing elite-level S/P athletes vs. C (Fisher's exact test, adjusted p = 0.17, Table 2, Fig 1).
In contrast to what was observed in the Caucasian sample, there was a trend towards an underrepresentation of the A allele in S/P athletes compared to C in the Brazilian sample. (Fisher's exact test, adjusted p-value = 0.058, Table 2, Fig 1).
All athletes vs. controls
When comparing all Caucasian athletes regardless of their sporting discipline (E, S/P, M and U) to C, we found that the A allele was overrepresented in athletes compared to C (Fisher's exact test, adjusted p = 0.024, Table 2, Fig 1). This association was even more pronounced when only elite-level athletes were considered (Fisher's exact test, adjusted p = 0.00017, Table 2, Fig 1).
There were no differences in genotype distributions between all athletes and C in the Brazilians sample (Fisher's exact test, adjusted p = 0.35, Table 2, Fig 1).
Discussion
Sprint/power performance, as well as muscle strength and mass are influenced by genetics [5]; yet, only a few genetic variants associated with either elite sprint/power performance and/or muscle strength and muscle mass have been identified to date. The rs2854464 polymorphism within the Activin A Receptor type 1b gene was recently shown to be associated with muscle strength in a properly-designed study [11]. In the present study, we sought to further explore the relevance of this polymorphism to sprint/power performance. We found that the ACVR1B rs2854464 is differently associated with sprint/power performance in a relatively large cohort (n = 1,672) of Caucasian and Brazilian athletes. While the A allele was overrepresented in Caucasian athletes, and more specifically in sprint/power Caucasian athletes compared with controls, there was a trend towards an underrepresentation of the A allele (p = 0.058 after multiple testing correction) in sprint/power Brazilian athletes. Our results reinforce the hypothesis that an association between any genetic variant and athletic performance might be dependent on the population’s ethnic background.
In the present study we have addressed some of the limitations inherent in previous elite athlete case-control studies. Firstly, we have studied four cohorts of elite and sub-elite athletes, including three European Caucasian athletes and a Brazilian cohort. Consequently, the number of athletes (n = 1,672) is significantly higher compared to previous genetic association studies, and demonstrates the benefits of a collaborative approach that has been recommended in the field of exercise genomics [21,22]. Secondly, previous reports have grouped together sprint and power athletes from mixed sports disciplines and events. Here, we have embraced a more stringent approach and divided the athletes to four categories, based on the physiological demands of each specific event (Table 1). Thirdly, to avoid being exposed to false positive results and as recently suggested [23], all p-values were adjusted for multiple comparisons.
There is a biochemical/cellular rationale to suggest that common variants within ACVR1B would be associated with sprint/power and/or strength performance. ACVR1B encodes the Activin A receptor type 1b protein, which is part of the TGF-β (Transforming Growth Factor-β) superfamily, a set of growth factors that regulates the expression level of several genes implicated in controlling muscle growth [24]. Myostatin is perhaps one of the most important members of the TGF-β; it down-regulates muscle mass during both pre- and post-natal stages [24]. Activin receptor type 2b (ACVR2B) is the primary type 2 receptor for myostatin. However, the type 1 receptor is important for the muscle signalling cascade following the interaction between myostatin and ACVR2B, being essential for the signal propagation through the plasma membrane. After binding of myostatin to ACVR2B, ACVR1B is recruited and contributes to the formation of a heteromeric active receptor complex [25]. According to Windelinckx et al. [11], “the rs2854464 polymorphism is located in a putative miR-24-binding site in the 3' untranslated region (UTR) of the ACVR1B mRNA”. There is evidence showing that miR-24 may decrease gene and protein expression of ACVR1B [26] and play a role in myoblast differentiation, inhibiting the skeletal muscle differentiation induced by TGF- β [27]. A potential explanation for the downstream association between rs2854464 A allele and sprint/power performance, is that it might provide a better affinity between the 3' UTR of ACVR1B mRNA and miR-24, leading to a more effective translational inhibition and decay of ACVR1B mRNA. It has been shown that pharmacological blockade of the activin A signalling pathway by using soluble activin type II receptors (ligand level), or antibody to ActRII (receptor level) increases muscle and bone mass, correct anaemia or protect against diet-induced obesity [28,29].
Using knock-out (KO) mice model, and multiple human association studies, the biochemical/cellular rationale that common genetic variants in muscle-related genes may influence sprint/power performance has been well demonstrated in the case of the ACTN3 R577X variant, currently the most promising candidate gene to influence sprint/power performance [30,31]. However, this variant can explain only ~1.5% of the variance in elite sprint/power performance [6] and many other gene variants are still to be identified. Therefore, with the limited knowledge we currently hold, a recent consensus has stated that genetic tests are not yet valid for talent identification or for individualizing training prescription to optimise performance [32].
Here, we have identified for the first time an association between the ACVR1B rs2854464 and elite sprint/power athletic status in Caucasian athletes. We note that the next step required is to replicate these findings in other cohorts of elite athletes with different geographical backgrounds (similarly to ACTN3 R577X). We also stress that to further confirm our findings, future studies should examine functional outcomes (i.e., causation) related to the effects of ACVR1B on muscle physiology. The recently launched Gene SMART (Skeletal Muscle Adaptive Response to Training) study, that aims to identify gene variants that predict skeletal muscle responses to High-Intensity Interval Training, might be useful to further confirm whether ACVR1B is indeed important to performance.
Conclusion
In conclusion, in a relatively large cohort of athletes from Europe and South America, we have shown that the ACVR1B rs2854464 A allele is associated with sprint/power performance in Caucasians but not in Brazilians athletes. This reinforces the notion that phenotype-genotype associations may be ethnicity-dependent. We acknowledge that elite athletic performance is a polygenic trait [33]; therefore, more genetic variants influencing sprint/power performance are yet to be discovered.
Supporting Information
C = controls; E = endurance athletes; S/P = sprint/power athletes; All = all athletes.
(TIF)
(XLSX)
Acknowledgments
The study was partly funded by FAPESP (grant #12/22516-6).
Vladimir P Pushkarev would like to acknowledge the Polish co-authors (Pawel Cieszczyk and Marek Sawczuk) for donating the TaqMan® SNP Genotyping Assay which was used in the present study. The Russian group would also like to acknowledge Dmitry Adamov (USUPC) for his comprehensive technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was partly funded by FAPESP (grant #12/22516-6). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Slater G, Phillips SM. Nutrition guidelines for strength sports: sprinting, weightlifting, throwing events, and bodybuilding. J Sports Sci. England; 2011;29 Suppl 1: S67–77. 10.1080/02640414.2011.574722 [DOI] [PubMed] [Google Scholar]
- 2.Pitsiladis Y, Wang G, Wolfarth B, Scott R, Fuku N, Mikami E, et al. Genomics of elite sporting performance: what little we know and necessary advances. Br J Sports Med. 2013; bjsports–2013–092400–. 10.1136/bjsports-2013-092400 [DOI] [PubMed] [Google Scholar]
- 3.Costa AM, Breitenfeld L, Silva AJ, Pereira A, Izquierdo M, Marques MC. Genetic inheritance effects on endurance and muscle strength: an update. Sports Med. New Zealand; 2012;42: 449–458. 10.2165/11650560-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Peeters MW, Thomis MAI, Beunen GP, Malina RM. Genetics and sports: an overview of the pre-molecular biology era. Med Sport Sci. Switzerland; 2009;54: 28–42. 10.1159/000235695 [DOI] [PubMed] [Google Scholar]
- 5.Eynon N, Hanson E, Lucia A, Houweling P, Garton F, North K, et al. Genes for Elite Power and Sprint Performance: ACTN3 Leads the Way. Sport Med. Springer International Publishing AG; 2013; 1–15. 10.1007/s40279-013-0059-4 [DOI] [PubMed] [Google Scholar]
- 6.Papadimitriou ID, Lucia A, Pitsiladis YP, Pushkarev VP, Dyatlov DA, Orekhov EF, et al. ACTN3 R577X and ACE I/D gene variants influence performance in elite sprinters: a multi-cohort study. BMC Genomics. England; 2016;17: 285 10.1186/s12864-016-2462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puthucheary Z, Skipworth JRA, Rawal J, Loosemore M, Van Someren K, Montgomery HE. The ACE gene and human performance: 12 years on. Sport Med. ADIS INT LTD; 2011;41: 433–448. Available: http://discovery.ucl.ac.uk/1309731/ [DOI] [PubMed] [Google Scholar]
- 8.Eynon N, Alves AJ, Yamin C, Sagiv M, Duarte JA, Oliveira J, et al. Is there an ACE ID—ACTN3 R577X polymorphisms interaction that influences sprint performance? Int J Sports Med. 2009;30: 888–891. 10.1055/s-0029-1238291 [DOI] [PubMed] [Google Scholar]
- 9.Voisin S, Cieszczyk P, Pushkarev VP, Dyatlov D a, Vashlyayev BF, Shumaylov V a, et al. EPAS1 gene variants are associated with sprint/power athletic performance in two cohorts of European athletes. BMC Genomics. 2014;15: 382 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4035083&tool=pmcentrez&rendertype=abstract 10.1186/1471-2164-15-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawczuk M, Banting LK, Cieszczyk P, Maciejewska-Karlowska A, Zarebska A, Leonska-Duniec A, et al. MCT1 A1470T: a novel polymorphism for sprint performance? J Sci Med Sport. Australia; 2015;18: 114–118. 10.1016/j.jsams.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 11.Windelinckx A, De Mars G, Huygens W, Peeters MW, Vincent B, Wijmenga C, et al. Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1B (ACVR1B) as a muscle strength gene. Eur J Hum Genet. England; 2011;19: 208–215. 10.1038/ejhg.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huygens W, Thomis MA, Peeters MW, Aerssens J, Janssen R, Vlietinck RF, et al. Linkage of myostatin pathway genes with knee strength in humans. Physiol Genomics. United States; 2004;17: 264–270. 10.1152/physiolgenomics.00224.2003 [DOI] [PubMed] [Google Scholar]
- 13.Huygens W, Thomis MAI, Peeters MW, Aerssens J, Vlietinck R, Beunen GP. Quantitative trait loci for human muscle strength: linkage analysis of myostatin pathway genes. Physiol Genomics. United States; 2005;22: 390–397. 10.1152/physiolgenomics.00010.2005 [DOI] [PubMed] [Google Scholar]
- 14.Pitsiladis YP, Tanaka M, Eynon N, Bouchard C, North KN, Williams AG, et al. The Athlome Project Consortium: A Concerted Effort to Discover Genomic and other “OMIC” Markers of Athletic Performance. Physiol Genomics. 2015; physiolgenomics.00105.2015. 10.1152/physiolgenomics.00105.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massidda M, Eynon N, Bachis V, Corrias L, Culigioni C, Cugia P, et al. The Association between MCT1 A1470T Polymorphism and Fat-Free Mass in Well-Trained Young Soccer Players. J strength Cond Res / Natl Strength Cond Assoc. 2015; 10.1519/JSC.0000000000001176 [DOI] [PubMed] [Google Scholar]
- 16.Kehdy FSG, Gouveia MH, Machado M, Magalhaes WCS, Horimoto AR, Horta BL, et al. Origin and dynamics of admixture in Brazilians and its effect on the pattern of deleterious mutations. Proc Natl Acad Sci U S A. United States; 2015;112: 8696–8701. 10.1073/pnas.1504447112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pimenta JR, Zuccherato LW, Debes AA, Maselli L, Soares RP, Moura-Neto RS, et al. Color and genomic ancestry in Brazilians: a study with forensic microsatellites. Hum Hered. Switzerland; 2006;62: 190–195. 10.1159/000096872 [DOI] [PubMed] [Google Scholar]
- 18.Lins TC, Vieira RG, Abreu BS, Gentil P, Moreno-Lima R, Oliveira RJ, et al. Genetic heterogeneity of self-reported ancestry groups in an admixed Brazilian population. J Epidemiol. Japan; 2011;21: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardena MMSG, Ribeiro-Dos-Santos A, Santos S, Mansur AJ, Pereira AC, Fridman C. Assessment of the relationship between self-declared ethnicity, mitochondrial haplogroups and genomic ancestry in Brazilian individuals. PLoS One. United States; 2013;8: e62005 10.1371/journal.pone.0062005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. JSTOR; 1995;57: 289–300. [Google Scholar]
- 21.Eynon N, Ruiz JR, Oliveira J, Duarte JA, Birk R, Lucia A. Genes and elite athletes: a roadmap for future research. J Physiol. 2011;589: 3063–3070. 10.1113/jphysiol.2011.207035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eynon N, Ruiz JR, Femia P, Pushkarev VP, Cieszczyk P, Maciejewska-Karlowska A, et al. The ACTN3 R577X Polymorphism across Three Groups of Elite Male European Athletes. PLoS One. 2012;7: e43132 10.1371/journal.pone.0043132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Z-H, Hu Y, Li Y-C, Gong L-J, Cieszczyk P, Maciejewska-Karlowska A, et al. PGC-related gene variants and elite endurance athletic status in a Chinese cohort: A functional study. Scand J Med Sci Sports.: in press. 10.1111/sms.12188 [DOI] [PubMed] [Google Scholar]
- 24.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol. United States; 2008;104: 579–587. 10.1152/japplphysiol.01091.2007 [DOI] [PubMed] [Google Scholar]
- 25.Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). England; 2012;205: 324–340. 10.1111/j.1748-1716.2012.02423.x [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Huang Z, Xue H, Jin C, Ju X-L, Han J-DJ, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. United States; 2008;111: 588–595. 10.1182/blood-2007-05-092718 [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Zhang Y, Yang G, Chen X, Zhang Y, Cao G, et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. England; 2008;36: 2690–2699. 10.1093/nar/gkn032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JL, Walton KL, Al-Musawi SL, Kelly EK, Qian H, La M, et al. Development of novel activin-targeted therapeutics. Mol Ther. United States; 2015;23: 434–444. 10.1038/mt.2014.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. United States; 2014;34: 606–618. 10.1128/MCB.01307-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, et al. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. 2003;73: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratzliff AD, Soltesz I. Differential immunoreactivity for alpha-actinin-2, an N-methyl-D-aspartate-receptor/actin binding protein, in hippocampal interneurons. Neuroscience. United States; 2001;103: 337–349. [DOI] [PubMed] [Google Scholar]
- 32.Webborn N, Williams A, McNamee M, Bouchard C, Pitsiladis Y, Ahmetov I, et al. Direct-to-consumer genetic testing for predicting sports performance and talent identification: Consensus statement. Br J Sports Med. England; 2015;49: 1486–1491. 10.1136/bjsports-2015-095343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz JR, Arteta D, Buxens A, Artieda M, Gómez-Gallego F, Santiago C, et al. Can we identify a power-oriented polygenic profile? J Appl Physiol. 2010;108: 561–566. 10.1152/japplphysiol.01242.2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C = controls; E = endurance athletes; S/P = sprint/power athletes; All = all athletes.
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.