Abstract
Background—
Maternal diabetes mellitus is associated with an increased risk of offspring congenital heart defects (CHD); however, the causal mechanism is poorly understood. We further investigated this association in a Danish nationwide cohort.
Methods and Results—
In a national cohort study, we identified 2 025 727 persons born from 1978 to 2011; among them were 7296 (0.36%) persons exposed to maternal pregestational diabetes mellitus. Pregestational diabetes mellitus was identified by using the National Patient Register and individual-level information on all prescriptions filled in Danish pharmacies. Persons with CHD (n=16 325) were assigned to embryologically related cardiac phenotypes. The CHD prevalence in the offspring of mothers with pregestational diabetes mellitus was 318 per 10 000 live births (n=232) in comparison with a baseline risk of 80 per 10 000; the adjusted relative risk for CHD was 4.00 (95% confidence interval, 3.51–4.53). The association was not modified by year of birth, maternal age at diabetes onset, or diabetes duration, and CHD risks associated with type 1 (insulin-dependent) and type 2 (insulin-independent) diabetes mellitus did not differ significantly. Persons born to women with previous acute diabetes complications had a higher CHD risk than those exposed to maternal diabetes mellitus without complications (relative risk, 7.62; 95% confidence interval, 5.23–10.6, and relative risk, 3.49; 95% confidence interval, 2.91–4.13, respectively; P=0.0004). All specific CHD phenotypes were associated with maternal pregestational diabetes mellitus (relative risk range, 2.74–13.8).
Conclusions—
The profoundly increased CHD risk conferred by maternal pregestational diabetes mellitus neither changed over time nor differed by diabetes subtype. The association with acute pregestational diabetes complications was particularly strong, suggesting a role for glucose in the causal pathway.
Keywords: congenital abnormalities; diabetes mellitus; heart defects, congenital; insulin; pregnancy
Women with diabetes mellitus have considerably higher risks of adverse pregnancy outcomes, including birth defects, than nondiabetic women,1–3 and pregestational maternal diabetes mellitus is the only relatively prevalent population risk factor for congenital heart defects (CHDs).4 Even though the association of pregestational diabetes mellitus with CHD has been known for decades, it is not clear if this knowledge has resulted in a substantive impact of the reduction of pregnancies complicated by diabetes mellitus or in the proportion of births with CHDs attributable to pregestational diabetes mellitus.
Editorial, see p 2219
Clinical Perspective on p 2253
Experimental studies suggest that hyperglycemia during early embryogenesis may alter gene expression in key cellular components of the developing heart, in particular, the embryonic heart’s outflow sections; however, the mechanism producing this altered gene expression is unclear.5–9 Maternal diabetes mellitus may be associated with specific types of CHD, yet epidemiological studies published to date have not focused specifically, finding the effect of diabetes mellitus to differ across sections of the embryonic heart corresponding to cardiac phenotypes.1,3,10–13 In addition, associations may differ for type 1 and type 2 diabetes mellitus, which would offer insight into possible teratogenic mechanisms.
In this Danish nationwide cohort study, we investigated the risk of CHDs in the offspring of women with pregestational diabetes mellitus. In addition, we investigated whether (1) the association has changed over time, (2) pregestational diabetes complications further increased the risk of CHD, (3) the association was linked to specific CHDs, and (4) the association varied by maternal diabetes type.
Methods
Data Sources
Since 1968, the Danish Civil Registration System has registered demographic, vital status, and kinship information on all persons in Denmark, using the unique personal identification number assigned to each Danish resident.14 The personal identification number permits accurate linkage of individual-level information from Denmark’s nationwide registers, including the National Patient Register (NPR),15 the Medical Birth Register,16 the Abortion Register,17 the Causes of Death Register, the Register of Medicinal Product Statistics,18 and the Danish Cytogenetic Central Register (postnatal diagnoses since 1968, prenatal diagnoses since 1978).
Study Cohort
Our study cohort included all singletons born alive in Denmark between 1978 and 2011, identified via the Civil Registration System. Persons with chromosomal aberrations (identified by using the Cytogenetic Central Register) or birth defects with recognizable syndromes or known causes (identified by using the NPR, International Classification of Diseases, 10th Revision (ICD-10) codes Q85, Q86, Q87, Q75.1, Q75.4, Q77.1, Q77.2, Q79.6, Q44.7B, Q61.9A), and those whose mother had a CHD, were excluded from the cohort.
Identification of Maternal Pregestational Diabetes Mellitus
We identified each cohort member’s mother using the Civil Registration System and determined which of these women had pregestational diabetes mellitus by using the NPR. We defined pregestational diabetes mellitus as pregestational registration of International Classification of Diseases, 8th Revision (ICD-8) codes 250 or 249, or ICD-10 codes E10.0 to E11.9.
Diabetes coding in the NPR has changed over time, with a single diabetes code (250) in the period 1977 to 1986, codes for insulin-dependent (249) and insulin-independent (250) diabetes mellitus from 1987 to 1993, and codes for type 1 (E10) and type 2 (E11) diabetes mellitus from 1994 onward. Preliminary analyses revealed that 39% of the mothers with pregestational diabetes mellitus had codes for both type 1/insulin-dependent and type 2/insulin-independent diabetes mellitus. We therefore defined type 1 and type 2 diabetes mellitus by using 2 approaches (timing of disease onset, first-time antidiabetic treatment). First, we used maternal age at diagnosis <30 years and ≥30 years as cut-offs for type 1 and type 2 diabetes mellitus, respectively.19,20 Second, for births, 1996 to 2011, we retrieved Register of Medicinal Product Statistics (established 1994) information, on first-time maternal prescriptions for insulin (Anatomic Therapeutic Chemical classification system code A10A) and oral antidiabetic medications (Anatomic Therapeutic Chemical code A10B) for mothers with NPR-defined pregestational diabetes mellitus.
Diabetic complications may reflect poor glycemic control. Therefore, we also used NPR to identify mothers with pregestational diabetes mellitus who had diabetic complications before pregnancy. We defined coma (ICD-8 codes 249.06, 249.07, 250.06, 250.07; ICD-10 codes E10.0, E11.0) and ketoacidosis (ICD-10 codes E10.1, E11.1) as acute complications, and nephropathy (ICD-8 codes 249.02, 250.02; ICD-10 codes E10.2, E11.2), retinopathy (ICD-8 codes 249.01, 250.01; ICD-10 codes E10.3, E11.3, H36.0–H36.9), neuropathy (ICD-8 codes 249.03, 250.03; ICD-10 codes E10.4, E11.4), vasculopathy/angiopathy (ICD-8 codes 249.04, 249.05, 250.04, 250.05; ICD-10 codes E10.5, E11.5), and other defined diabetic complications (ICD-8 codes 249.08, 250.08; ICD-10 codes E10.6, E11.6, E10.8, E11.8,) as late complications. Women were either classified as having a single complication or ≥2 complications, where the latter was defined as multiple complications (ICD-10 codes E10.7, E11.7) or registration of ≥2 of the above complications. In an alternate categorization scheme, women were classified as having ≥2 episodes of acute complications (coma, ketoacidosis), 1 episode of an acute complication, and late complications only.
Identification of Gestational Diabetes Mellitus
In women without pregestational diabetes mellitus, we identified women who developed gestational diabetes mellitus (ICD-8 code 634.74; ICD-10 code O24.4) in the second trimester (13–27 gestational weeks) or third trimester (≥28 gestational weeks).
Identification and Classification of Offspring CHDs
We identified persons with CHDs (ICD-8 codes 740–759, ICD-10 codes Q20–Q26) by using the NPR and the Causes of Death Register. CHDs were classified by using a previously published algorithm that uses a hierarchical approach to map CHDs into embryologically related defect phenotypes.13,21,22 These phenotypes were heterotaxia, conotruncal defect (truncus arteriosus, transposition of the great arteries, Tetralogy of Fallot, double-outlet right ventricle, other), atrioventricular septum defect, anomalous pulmonary venous return, left ventricular outflow tract obstruction (coarctation of the aorta, valvular aortic stenosis, other), right ventricular outflow tract obstruction (valvular pulmonary stenosis, other), atrial septum defect, ventricular septum defect, complex defect, valve defect, other specified defect, and unspecified defect. Isolated physiological heart conditions (persistent foramen ovale, pulmonary valve stenosis) present at birth but not mentioned at >6 weeks postnatal age, and persistent ductus arteriosus were not considered CHDs. ICD codes for severe CHDs have been validated against hospital records with very good agreement.23 In preliminary analyses, we distinguished between individuals with isolated CHDs and CHDs with extracardiac birth defects (ICD-10 codes Q00–Q19, Q27–Q99). Individuals with CHD and 1 minor defect24 were considered to have isolated CHD.
Identification of Offspring Noncardiac Birth Defects
Using the NPR, we also identified all individuals born in the period 1994 to 2011 with noncardiac birth defects (ICD-10 codes Q00–Q19, Q27–Q99). Individuals with isolated minor defects24 were not considered to have birth defects.
Statistical Analysis
Associations between maternal diabetes mellitus and offspring risk of CHD were estimated as risk ratios (RRs) with 95% confidence intervals (CIs) using log-linear binominal regression, with adjustment for year of delivery (1-year groups), maternal age in years (<20, 20–24, 25–29, 30–34, 35–39, ≥40), and birth order (first, second, or later; PROC GENMOD, SAS Institute, Inc., Cary, NC). Polytomous logistic regression (PROC NLMIXED in SAS) was used to test for differences between multiple outcomes. We addressed 2 specific hypotheses whether the effects of diabetes mellitus differ across sections of the embryonic heart corresponding to specific cardiac phenotypes,5–9 by testing whether the RRs were similar for the different outcomes of conotruncal versus nonconotruncal defects, and cardiac defects originating from the anterior secondary heart field versus those defects from the posterior heart field. All tests were likelihood ratio tests. In trend tests, the number of complications were entered as continuous variables. Analyses restricting to a woman’s first birth revealed that the potential correlation between sibling outcomes did not affect the observed RRs and CIs; consequently, we present results based on all offspring. Assuming a causal relationship between maternal pregestational diabetes mellitus and infant CHD, we calculated the attributable fraction among the exposed as (adjusted RR–1/adjusted RR)×100,25 interpreted as the proportion of CHDs among the offspring of diabetic mothers that could be attributed to maternal diabetes mellitus.
Medications used to treat diabetes mellitus might potentially have teratogenic effects. Therefore, we estimated RRs for CHD in the offspring of diabetic mothers by maternal use of antidiabetic medications (insulin, oral antidiabetic medications, oral antidiabetic medications and insulin, no antidiabetic treatment) between 14 and 56 days gestation, the presumed window of susceptibility for embryonic heart development. Antidiabetic medication use was determined by using information from the Register of Medicinal Product Statistics on dates relevant prescriptions were filled and the amount of medication dispensed, and the World Health Organization’s Defined Daily Dose.26
To evaluate whether there are unknown common factors for maternal diabetes mellitus and CHD, we also compared the postgestational risk of diabetes mellitus in women with and without offspring with CHDs. In this analysis, the study cohort included women with ≥1 births between 1994 and 2011, excluding mothers with diabetes mellitus before their first birth. We followed the women from their first birth after 1994 until diabetes mellitus (outcome), death, emigration, or end of follow-up (December 2011). The relative risk of diabetes mellitus after a CHD birth was estimated by rate ratios using log-linear Poisson regression, with history of CHD birth as a time-dependent variable and adjustment for maternal age at birth, birth order, and year of delivery.
Approvals
Statens Serum Institut has approval from the Danish Data Protection Agency to conduct register-based studies. The Danish Cytogenetic Central Register Board approved the study.
Results
There were 2 043 548 living singletons born in Denmark in the period 1978 to 2011. Of these, 6595 had a chromosomal aberration, 4942 had syndromic CHD, and 6750 had a mother with a CHD. These 17 821 persons were therefore excluded from the study population, leaving 2 025 727 (1 077 836 mothers) in the study cohort (Table 1). The proportion of offspring exposed to maternal pregestational diabetes mellitus was 0.36% (n=7296); this proportion increased with year of birth, increasing maternal age, and number of births. The overall prevalence of nonchromosomal CHDs was 81 per 10 000 births (n=16 325).
Table 1.
Prevalence of Maternal Pregestational Diabetes Mellitus* and CHDs† by Birth Characteristics in 2 025 727 Singleton Births,‡ Denmark, 1978 to 2011
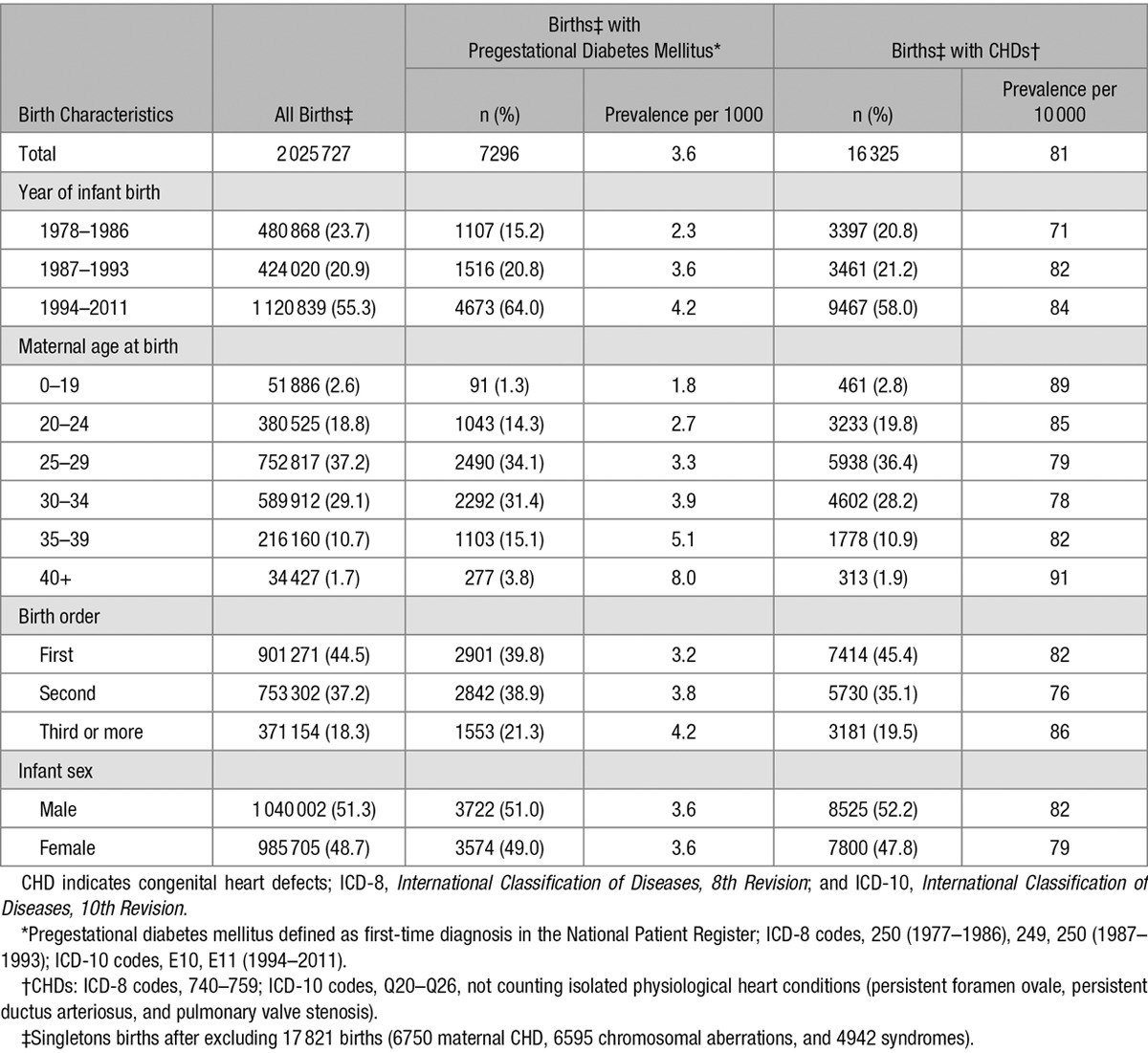
Offspring of women with pregestational diabetes mellitus were 4 times more likely to have CHDs than the offspring of mothers without diabetes mellitus (Table 2); (prevalence of CHD at birth 318 per 10 000 versus 80 per 10 000). RR magnitudes did not depend on maternal age at onset of diabetes mellitus or duration of diabetes mellitus. Before 1994, 92.8% of diabetic mothers were diagnosed before 30 years of age, whereas only 70.8% had an early diagnosis thereafter, but RR magnitudes did not depend on maternal age at diabetes onset in either period. Among the offspring of diabetic mothers, the proportions of CHDs attributable to maternal diabetes mellitus in 1978 to 1986, 1987 to 1993, and 1994 to 2011, were 79%, 76%, and 74%, respectively.
Table 2.
Relative Risk of Any Type of CHD,* Overall, and by Diagnosis Characteristics of Pregestational Diabetes Mellitus,† Pregestational Diabetes Complications, and Birth Characteristics in 2 025 727 Live Births,‡ Denmark, 1978 to 2011
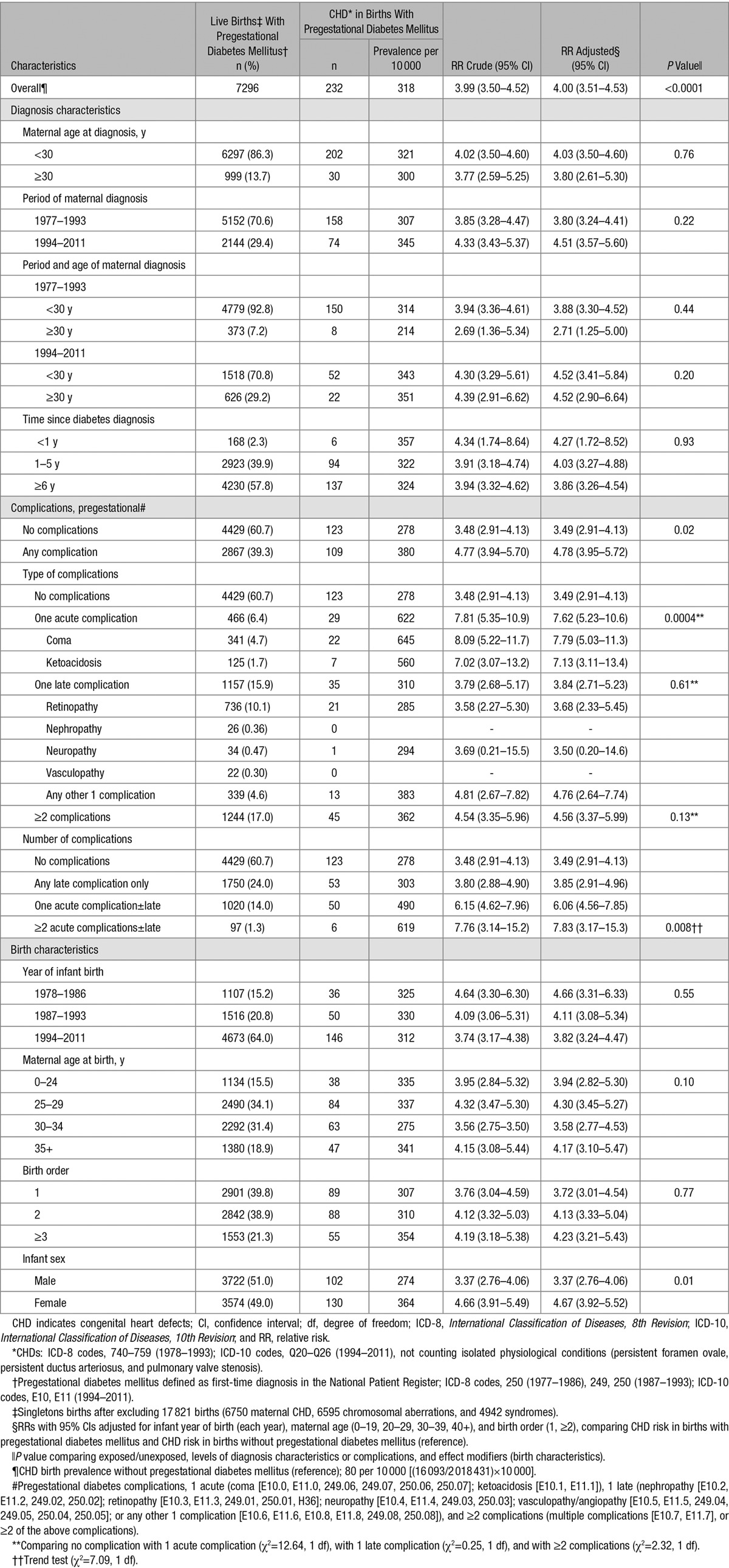
Among women with pregestational diabetes mellitus, 39.3% had pregestational diabetic complications. Offspring of women with single acute pregestational complications had a significantly higher CHD risk than the offspring of women without diabetes complications (P=0.0004), whereas the risk in offspring of women with single late complications was similar to that of the offspring of women without complications (P=0.61, Table 2). Using an alternative approach (dose-response), ≥2 episodes of acute complications (coma, ketoacidosis), 1 acute complication episode, and late complications only, the RRs were 7.83, 6.06, and 3.85, respectively, P(trend)=0.008.
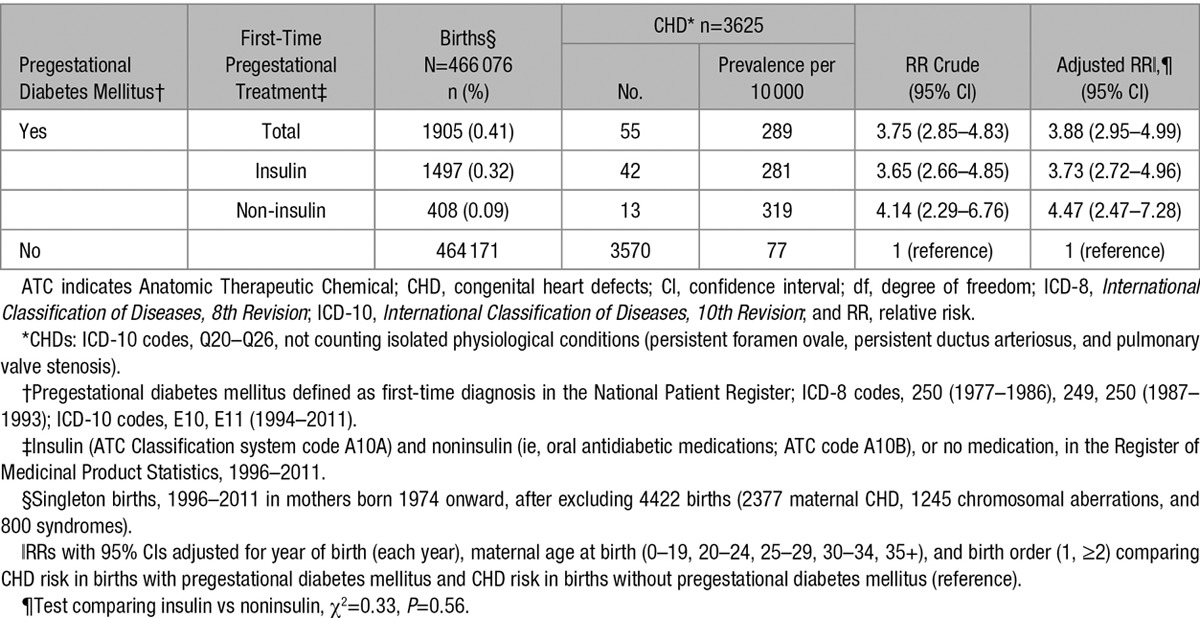
Information on initial antidiabetic treatment (a proxy for diabetes type) was available for women delivering from 1996 to 2011. RRs for CHD did not differ by type of treatment (insulin versus oral antidiabetic medications/no antidiabetic treatment (P=0.56, Table 3).
Table 3.
Relative Risk of Any Type of CHD* by Information of Pregestational Diabetes Mellitus,† and Pregestational First-Time Prescription‡ of Insulin or Noninsulin Treatment, in 466 076 Singleton Live Births,§ Denmark, 1996 to 2011
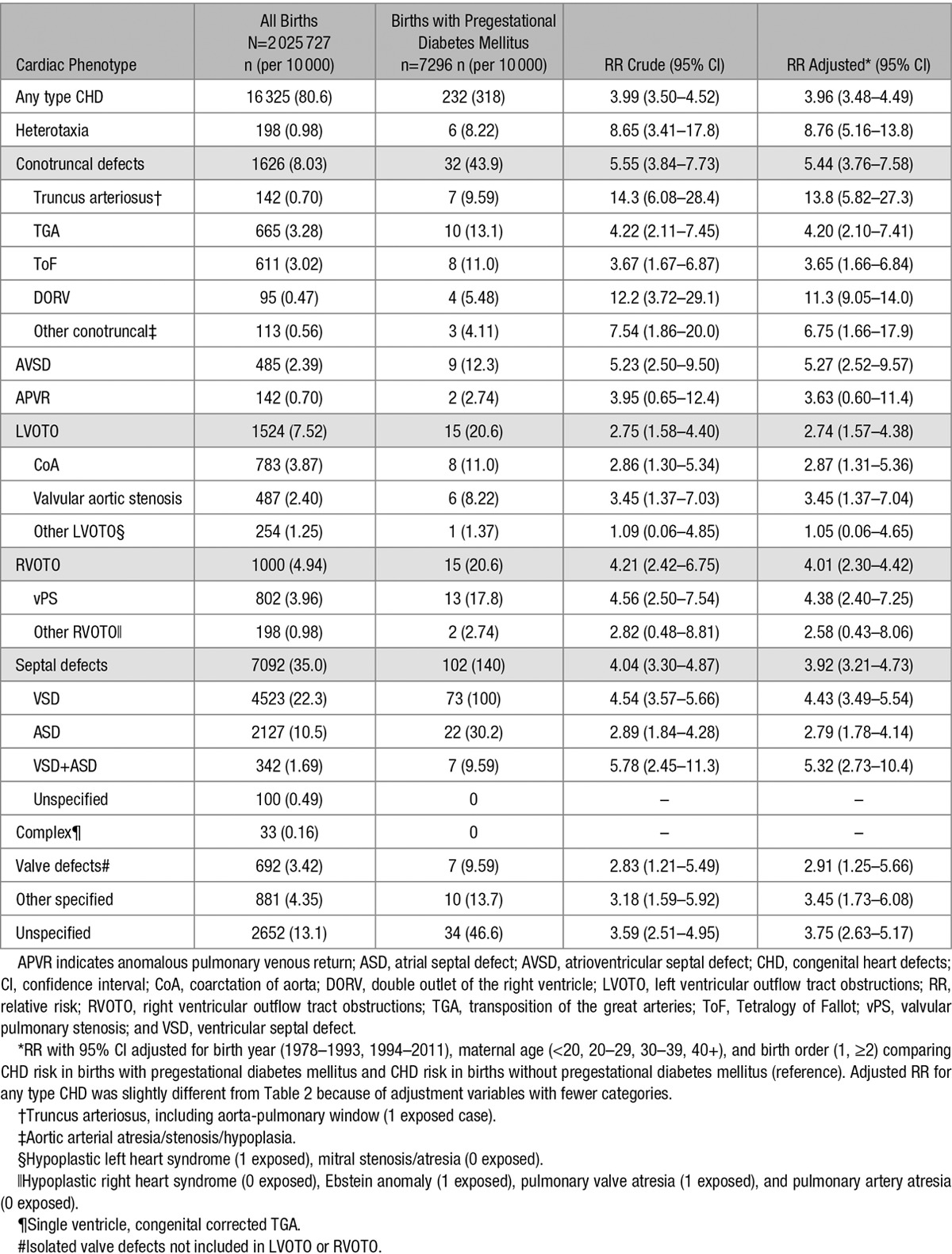
Maternal pregestational diabetes mellitus was associated with increased risks of most CHD phenotypes, with RRs ranging from 2.74 to 13.8 (Table 4). In 2 analyses chosen specifically, the RRs for conotruncal defects (RR, 5.44) and nonconotruncal defects (atrioventricular septal defects, anomalous pulmonary venous return, left or right ventricular outflow tract obstructions, ventricular septum defect, atrial septum defect, ventricular septum defect + atrial septum defect, isolated valve defects, other specified; RR, 3.78; 95% CI, 3.22–4.39) were significantly different from one another (P=0.05). Furthermore, the RR of cardiac defects originating from the anterior secondary heart field (truncus arteriosus, Tetralogy of Fallot, double-outlet right ventricle, left ventricular outflow tract obstruction, ventricular septum defect; RR, 4.31; 95% CI, 3.54–5.17) was significantly different from those defects from the posterior secondary heart field (anomalous pulmonary venous return, atrial septum defect; RR, 2.84; 95% CI, 1.84–4.13), the P value was 0.04.
Table 4.
Birth Prevalence and Relative Risk of Type-Specific CHDs by Pregestational Diabetes in 2 025 727 Live Births, Denmark, 1978 to 2011
Pregestational Diabetes Mellitus and Offspring Risk of Noncardiac Birth Defects
Among 1 120 839 singletons born in 1994 to 2011, 4673 (0.42%) were born to women with pregestational diabetes mellitus and 28 780 (25.7 per 1000 births) had noncardiac birth defects. The risk of noncardiac birth defects was 66% greater in the offspring of women with pregestational diabetes mellitus than in the offspring of nondiabetic women (RR, 1.66; 95% CI, 1.44–1.90; n=195); this RR differed significantly (P<0.0001) from the corresponding RR for cardiac birth defects (RR, 3.82; 95% CI, 3.24–4.47).
Gestational Diabetes Mellitus and Offspring Risk of CHD
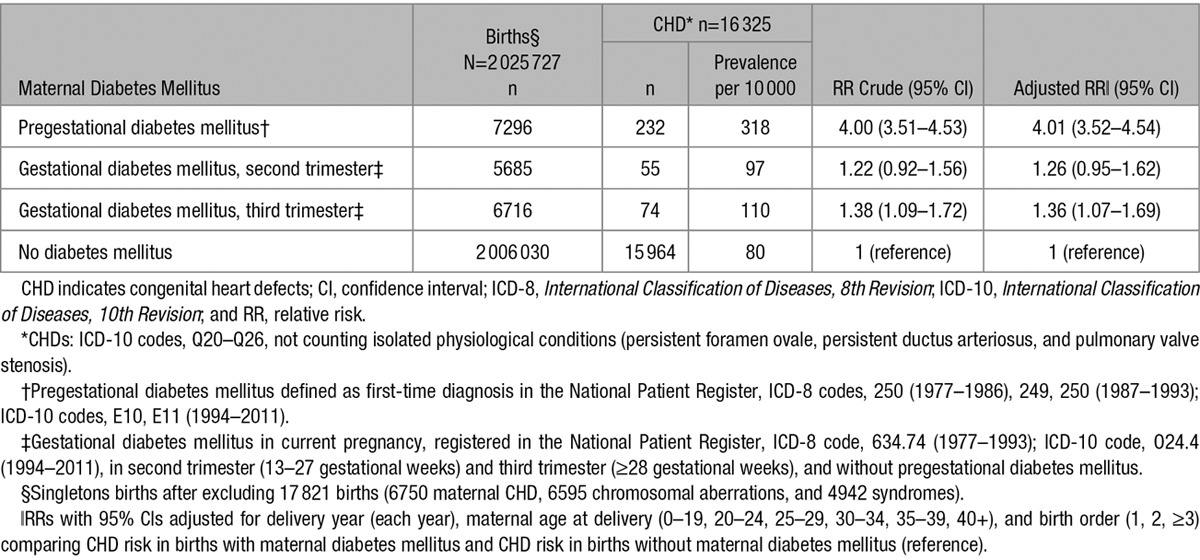
Among 2 018 431 singleton pregnancies without pregestational diabetes mellitus, gestational diabetes mellitus was diagnosed in the second or third trimester for 5685 and 6716 pregnancies, respectively (Table 5). RRs for offspring CHD were RRs 1.26; 95% CI, 0.95 to 1.62 and 1.36; 95% CI, 1.07 to 1.69, for gestational diabetes mellitus in the second and third trimesters, respectively. These RRs differed significantly (P=0.007) from the RR for CHD in offspring of women with pregestational diabetes mellitus.
Table 5.
Relative Risk of Any Type of CHD* by Information of Pregestational Diabetes Mellitus,† Gestational Diabetes Mellitus (Second Trimester, Third Trimester),‡ in 2 025 727 Singleton Live Births,§ Denmark, 1978 to 2011
Maternal Antidiabetic Medication Use in Gestational Weeks 2 to 8 and Offspring Risk of CHD
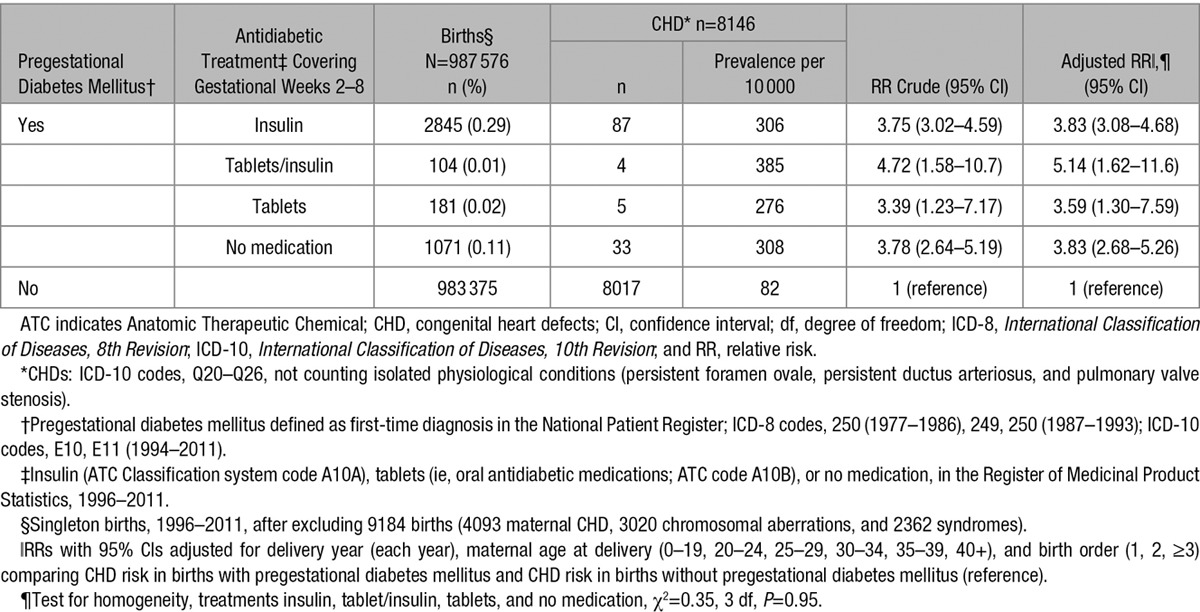
Information on antidiabetic treatment in very early pregnancy was available for women delivering between 1996 and 2011. RRs for CHD did not differ by type of treatment (P=0.95, Table 6).
Table 6.
Relative risk of any type of CHD* by Information of Pregestational Diabetes Mellitus† and Antidiabetic Medication‡ in Gestational Weeks 2 through 8, in 987 576 Singleton Live Births,§ Denmark, 1996 to 2011
Offspring CHD and Risk of Subsequent Maternal Diabetes Mellitus
Among 1 110 363 women with ≥1 births since 1977, 9219 had a child with CHD, and 17 888 developed diabetes mellitus after delivery; 321 (3.5%) women both had a child with a CHD and developed diabetes mellitus. The RR for later diabetes mellitus associated with CHD in the offspring was 1.42 (95% CI, 1.27–1.59).
Sensitivity Analysis; Potential Selection Bias Attributable to Pregnancy Termination
In the period 1994 to 2011, 1 120 839 live singletons were born, including 4673 (0.4%) born to women with pregestational diabetes mellitus. CHDs were identified in 146 (3.1%) children born to diabetic women and 9321 (0.8%) children born to nondiabetic women; the unadjusted RR was 3.74 (Table 2). In the same period, 3075 pregnancies, including 38 (1.2%) in diabetic women, were terminated because of fetal disease. In 2 extreme scenarios (including live births and terminated pregnancies), all 38 fetuses exposed to pregestational diabetes mellitus, and none of the 3037 unexposed fetuses, had CHD, yielding an unadjusted RR of 4.7; or none of the 38 exposed fetuses and all 3037 unexposed fetuses had CHD, yielding an unadjusted RR of 2.8.
Discussion
In this study of 2 million births over a 34-year period, the largest of its kind to date, maternal pregestational diabetes mellitus was associated with a 4-fold increase in offspring CHD risk. The increase in CHD risk was stable over time, and was similar for type 1 and type 2 diabetes mellitus. Pregestational acute diabetic complications conferred an almost 8-fold increase in CHD risk in comparison with nondiabetic women. Maternal pregestational diabetes mellitus was associated with an increased risk of all CHD subtypes, with a higher risk for cardiac outflow defects. Cardiac defects were significantly more prevalent than noncardiac defects among the offspring of diabetic women.
Our finding of the 4-fold increased CHD risk in offspring of mothers with pregestational diabetes mellitus, in comparison with offspring of nondiabetic mothers, is consistent with 3 published population-based studies of CHD risk in diabetic women (ie, in the United States,11 in Norway,3 and lately, in Canada13).
In recent years, prenatal care has improved and awareness of optimal glucose regulation before pregnancy has increased.27 However, our study results suggest that this improved care has not diminished the risk of offspring CHD for diabetic mothers. Indeed, the CHD risk associated with maternal diabetes mellitus changed very little over 34 years, and, among offspring born to diabetic mothers, 75% of CHD cases could be attributed to maternal diabetes mellitus. Other variables that changed over the study period (eg, increasing prevalence of obesity and type 2 diabetes mellitus, increasing maternal age) may have outweighed the effects of better prenatal care at the population level. Alternatively, pregestational diabetes mellitus may be associated with adverse fetal cardiac development independent of prenatal care, changing risk factors, and diabetes type. In our study, offspring CHD risk was not modified by timing of disease onset or duration of maternal diabetes mellitus.
The offspring of women whose initial pregestational diabetes treatment was insulin (most likely type 1 diabetes mellitus) had the same CHD risk as the offspring of women first treated with oral antidiabetic medications or no medication (most likely type 2 diabetes mellitus). Similar birth defect and CHD risks have been reported for type 1 and 2 diabetes mellitus in other studies.1,13 Because type 1 and type 2 diabetes mellitus have different etiologies, these results suggest that fetal heart development is susceptible to pathological processes shared by both diabetes types (eg, high glucose levels, reduced insulin sensitivity/increased insulin resistance, changes in triglycerides, oxidative stress, and inflammation). Interestingly, gestational diabetes mellitus was only weakly associated with offspring CHD risk, implying that high glucose levels very early in pregnancy may be key to abnormal heart development.5–9
The prevailing hypothesis explaining the observed association between maternal diabetes mellitus and offspring CHD is that excess glucose exerts a teratogenic effect on the developing heart.28 However, glucose itself is not a mutagen; instead, it may exert a teratogenic effect via a signaling pathway regulating insulin sensitivity. Insulin sensitivity is thought to be involved in the pathophysiology of both type 1 and type 2 diabetes mellitus,29 and insulin and related signaling pathways are also key mediators of embryogenesis and early development.29,30 Glucose may also affect gene expression in embryonic development via epigenetic changes (histone acetylation, microRNA expression).31 The alternative, that offspring CHD reflects maternally inherited genetic or epigenetic variations that confer risk of both diabetes mellitus and cardiac abnormalities, is less likely, because the risk of maternal diabetes mellitus subsequent to birth of a child with CHD was only modestly increased.
We did not have information on maternal pregestational glucose levels. However, we investigated offspring CHD risk by number and type of maternal diabetic complications (a proxy for the degree to which maternal glucose levels were uncontrolled). Almost 40% of women with pregestational diabetes mellitus had experienced diabetic complications, but only women with acute complications or multiple complications had higher offspring CHD risks than women with no diabetic complications. A recent British study also reported higher risks of congenital anomalies in offspring of diabetic mothers with high pregestational glucose levels or other clinical correlates of hyperglycemia.12 However, this study also found an increased risk of congenital anomalies associated with late diabetic complications.12 The lack of excess CHD risk associated with single late complications in our study could be related to coding practices; serious late complications may have been coded as multiple complications or unspecified. Because the degree of insulin sensitivity is highly correlated with diabetic complications,32 these findings support the hypothesis that insulin sensitivity may be mechanistically involved in the association between CHD and pregestational diabetes mellitus.
Conotruncal defect risk increased in the offspring of diabetic women, consistent with experimental study findings that hyperglycemia in early pregnancy affects regulatory gene expression in the embryo, leading to cardiac neural crest cell death and increased CHD risk, particularly for conotruncal and outflow tract abnormalities.5–7 When we grouped the outflow tract malformations and the inflow tract malformations to correspond to the anterior and posterior second heart fields in early embryonic development,8,9 we found that the association with maternal diabetes mellitus was much stronger for anterior second heart field defects than posterior second heart field defects, suggesting that the most sensitive cell populations are located in the anterior second heart field.8 However, more detailed mechanistic studies will be required to define the role of glucose sensitivity in cells from the neural crest and anterior second heart field during cardiac development. Maternal diabetes mellitus was also associated with the entire spectrum of CHD phenotypes. The nonspecific nature of the association suggests that hyperglycemia in early pregnancy may not only influence specific sequences in cardiac development, but affects cardiac development in general, or exerts its detrimental effect before formation of the primitive heart tube, with subsequent early and late consequences for fetal cardiac development.
Pregestational diabetes mellitus has also been associated with nonspecific embryopathy, because increased risks of noncardiac defects have also been reported.11 In our study, however, associations with maternal pregestational diabetes mellitus were considerably stronger for cardiac defects than for noncardiac defects, implying that the developing heart could be more vulnerable to hyperglycemia than the other organs.
The use of information on initial antidiabetic treatment and timing of disease onset helped to correct misclassification of type 1 and type 2 diabetes mellitus based on ICD codes from the National Patient Register. Our cohort included only pregnancies ending in a live birth, which could potentially have induced selection bias if diabetic women were more likely than nondiabetic women to receive fetal echocardiography and terminate a pregnancy if the fetus had a CHD. Both pregestational diabetes mellitus and CHDs also increase the risk of stillbirth.2 However, sensitivity analyses suggested that missing information on CHD in terminated pregnancies did not produce substantial selection bias. Adjustment of the association between CHD and diabetes mellitus for known potential confounders had little effect, and investigating unknown common factors for maternal diabetes mellitus and CHD (inverse examination of CHD and later onset of diabetes mellitus) suggested the absence of strong unknown confounders. Finally, the higher CHD risk in offspring of women with pregestational diabetes mellitus could not be explained by the use of antidiabetic medications in very early pregnancy.
Conclusions
Maternal pregestational diabetes mellitus (type 1 and type 2) was associated with a 4-fold increased offspring CHD risk, and this increased risk was stable over 34 years. Single maternal episodes of acute diabetic complications pregestation were associated with even higher risks of offspring CHD, suggesting a role for glucose in the causal pathway. The offspring of diabetic women had similar risks of most types of CHDs, suggesting that maternal diabetes mellitus impacts general cardiac development very early in embryogenesis. The increased risk of CHDs greatly exceeded the increased risk of noncardiac defects associated with maternal diabetes mellitus.
Acknowledgments
Tatiana Fomina, PhD, Department of Global Health and Primary Care, University of Bergen, Norway, programmed the algorithm that maps congenital heart defects into embryologically related defect phenotypes.
Sources of Funding
Dr Øyen was funded by grants from Research Council Norway (190858/V50), Beckett foundation, Copenhagen, Denmark (28213/28215), Western Norway Regional Health Authorities (911734), and University of Bergen, Norway. Dr Leirgul was funded by Research Council Norway, and Meltzer Foundation, University of Bergen, Norway. Dr Priest was funded from Pediatric Scientist Development Program, NIH-NICHD (K12-HD000850).
Disclosures
None.
CLINICAL PERSPECTIVE
In this cohort of 2 million births over a 34-year period, maternal pregestational diabetes mellitus (both types 1 and 2) was associated with a 4-fold increase in offspring risk of congenital heart defects (CHDs), whereas gestational diabetes mellitus was only weakly associated with offspring CHD risk. In recent years, prenatal care has improved and awareness of optimal glucose regulation before pregnancy has increased. However, our study results suggest that this improved care has not diminished the risk of offspring CHD for diabetic mothers. The CHD risk associated with maternal diabetes mellitus changed very little over 34 years, and, among the offspring of diabetic mothers, 75% of CHD cases could be attributed to maternal diabetes mellitus. Maternal pregestational diabetes mellitus was associated with an increased risk of all CHD subtypes, with a higher risk for cardiac outflow defects. Almost 40% of women with pregestational diabetes mellitus had experienced diabetic complications, but only women with acute complications or multiple complications had higher offspring CHD risks than women without diabetic complications. Women with pregestational acute diabetic complications (coma, ketoacidosis) had an almost 8-fold increase in CHD risk in comparison with nondiabetic women. The higher CHD risk in offspring of women with pregestational diabetes mellitus could not be explained by the use antidiabetic medications very early in pregnancy. Our findings suggest that high glucose levels very early in pregnancy may be involved in abnormal fetal heart development. Optimal glucose regulation before pregnancy does not appear to eliminate the baseline risk of cardiac defects in infants whose mothers have pregestational diabetes mellitus.
References
- 1.Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, Golightly S, Miller A. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333:177. doi: 10.1136/bmj.38856.692986.AE. doi: 10.1136/bmj.38856.692986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eidem I, Vangen S, Hanssen KF, Vollset SE, Henriksen T, Joner G, Stene LC. Perinatal and infant mortality in term and preterm births among women with type 1 diabetes. Diabetologia. 2011;54:2771–2778. doi: 10.1007/s00125-011-2281-7. doi: 10.1007/s00125-011-2281-7. [DOI] [PubMed] [Google Scholar]
- 3.Eidem I, Stene LC, Henriksen T, Hanssen KF, Vangen S, Vollset SE, Joner G. Congenital anomalies in newborns of women with type 1 diabetes: nationwide population-based study in Norway, 1999-2004. Acta Obstet Gynecol Scand. 2010;89:1403–1411. doi: 10.3109/00016349.2010.518594. doi: 10.3109/00016349.2010.518594. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL American Heart Association Council on Cardiovascular Disease in the Young. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SD, Dheen ST, Tay SS. Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc Diabetol. 2007;6:34. doi: 10.1186/1475-2840-6-34. doi: 10.1186/1475-2840-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roest PA, van Iperen L, Vis S, Wisse LJ, Poelmann RE, Steegers-Theunissen RP, Molin DG, Eriksson UJ, Gittenberger-De Groot AC. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res A Clin Mol Teratol. 2007;79:231–235. doi: 10.1002/bdra.20341. doi: 10.1002/bdra.20341. [DOI] [PubMed] [Google Scholar]
- 7.Morgan SC, Relaix F, Sandell LL, Loeken MR. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol. 2008;82:453–463. doi: 10.1002/bdra.20457. doi: 10.1002/bdra.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gittenberger-de Groot A, Calkoen E, Poelmann R, Bartelings M, Jongbloed M. Morphogenesis and molecular considerations on congenital cardiac septal defects. Ann Med. 2014;46:640–652. doi: 10.3109/07853890.2014.959557. [DOI] [PubMed] [Google Scholar]
- 9.Moazzen H, Lu X, Ma NL, Velenosi TJ, Urquhart BL, Wisse LJ, Gittenberger-de Groot AC, Feng Q. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc Diabetol. 2014;13:46. doi: 10.1186/1475-2840-13-46. doi: 10.1186/1475-2840-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferencz C, Rubin JD, McCarter RJ, Clark EB. Maternal diabetes and cardiovascular malformations: predominance of double outlet right ventricle and truncus arteriosus. Teratology. 1990;41:319–326. doi: 10.1002/tera.1420410309. doi: 10.1002/tera.1420410309. [DOI] [PubMed] [Google Scholar]
- 11.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–237.e9. doi: 10.1016/j.ajog.2008.06.028. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell R, Glinianaia S, Tennant P, Bilous R, Rankin J. Peri-conception hyperglycemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012;55:936–947. doi: 10.1007/s00125-012-2455-y. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS Canadian Perinatal Surveillance System (Public Health Agency of Canada) Association between maternal chronic conditions and congenital heart defects: a population-based cohort study. Circulation. 2013;128:583–589. doi: 10.1161/CIRCULATIONAHA.112.001054. doi: 10.1161/CIRCULATIONAHA.112.001054. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 15.Lynge E, Sandegaard J, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 17.Lohse SR, Farkas DK, Lohse N, Skouby SO, Nielsen FE, Lash TL, Ehrenstein V. Validation of spontaneous abortion diagnoses in the Danish National Registry of Patients. Clin Epidemiol. 2010;2:247–250. doi: 10.2147/CLEP.S13815. doi: 10.2147/CLEP.S13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 suppl):38–41. doi: 10.1177/1403494810394717. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 19.Feltbower RG, McKinney PA, Campbell FM, Stephenson CR, Bodansky HJ. Type 2 and other forms of diabetes in 0-30 year olds: a hospital based study in Leeds, UK. Arch Dis Child. 2003;88:676–679. doi: 10.1136/adc.88.8.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloyn AL, Ellard S. Defining the genetic aetiology of monogenic diabetes can improve treatment. Expert Opin Pharmacother. 2006;7:1759–1767. doi: 10.1517/14656566.7.13.1759. doi: 10.1517/14656566.7.13.1759. [DOI] [PubMed] [Google Scholar]
- 21.Øyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. National time trends in congenital heart defects, Denmark, 1977-2005. Am Heart J. 2009;157:467–473.e1. doi: 10.1016/j.ahj.2008.10.017. doi: 10.1016/j.ahj.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Leirgul E, Fomina T, Brodwall K, Greve G, Holmstrøm H, Vollset SE, Tell GS, Øyen N. Birth prevalence of congenital heart defects in Norway 1994-2009–a nationwide study. Am Heart J. 2014;168:956–964. doi: 10.1016/j.ahj.2014.07.030. doi: 10.1016/j.ahj.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Agergaard P, Hebert A, Bjerre J, Sørensen KM, Olesen C, Ostergaard JR. Children diagnosed with congenital cardiac malformations at the national university departments of pediatric cardiology: positive predictive values of data in the Danish National Patient Registry. Clin Epidemiol. 2011;3:61–66. doi: 10.2147/CLEP.S15627. doi: 10.2147/CLEP.S15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EUROCAT Guide 1.3 - Instructions for the Registration and Surveillance of Congenital Anomalies. 2005. Sep 30, http://www.eurocat-network.eu/ Accessed June 2014.
- 25.Benichou J. Attributable risk. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2nd ed. Vol 1. Chichester, West Sussex, England: Wiley; 2005. pp. 249–262. [Google Scholar]
- 26.WHO Collaborating Centre for Drug Statistics Methodology. 2015. May 5, http://www.whocc.no/ Accessed June 1, 2015.
- 27.Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94:435–444. doi: 10.1093/qjmed/94.8.435. [DOI] [PubMed] [Google Scholar]
- 28.Cousin L. Etiology and prevention of congenital anomalies among 65 infants of overt diabetic women. Clin Obstet Gynecol. 1991;34:481–493. [PubMed] [Google Scholar]
- 29.Wilkin TJ. Is autoimmunity or insulin resistance the primary driver of type 1 diabetes? Curr Diab Rep. 2013;13:651–656. doi: 10.1007/s11892-013-0407-7. doi: 10.1007/s11892-013-0407-7. [DOI] [PubMed] [Google Scholar]
- 30.Bowman CJ, Streck RD, Chapin RE. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Res B Dev Reprod Toxicol. 2010;89:339–349. doi: 10.1002/bdrb.20249. doi: 10.1002/bdrb.20249. [DOI] [PubMed] [Google Scholar]
- 31.Salbaum JM, Kappen C. Diabetic embryopathy: a role for the epigenome? Birth Defects Res A Clin Mol Teratol. 2011;91:770–780. doi: 10.1002/bdra.20807. doi: 10.1002/bdra.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–712. doi: 10.2337/dc06-1982. doi: 10.2337/dc06-1982. [DOI] [PubMed] [Google Scholar]


