The article is accompanied by the following Invited Commentary:
De Robertis E, Afshari A, Longrois D. The quest for the holy volume therapy. Eur J Anaesthesiol 2016; 33:483–487
Index
Table of contents 489
List of abbreviations 489
A. Rationale and goals 490
A1: Introduction 490
A2: Initial situation 490
A3: Guideline requirements 491
A4: Guideline objectives 491
A5: Target user group 491
B: Methodology 491
-
B1: Literature search and selection of evidence 491
Use of existing guidelines 491
Systematic literature search 491
Selection of literature identified 492
B2: Appraisal and extraction of evidence 493
B3: Formulation and consensus-forming for recommendations and statements 493
Formulation of recommendations and statements 493
Consensus building 494
Grades of Recommendation (GoR) 494
B4 Dissemination and implementation 494
B5: Quality indicators and evaluation 494
B6: Validity and revisions 494
B7: Funding and disclosure of possible conflicts of interest 494
Table of contents
Overall recommendations/statements: 495
Chapter 1: Diagnosis of hypovolaemia 496
Chapter 2: Therapy during the fasting phase 503
Chapter 3: Differences between peri-interventional and ICU patients 504
Chapter 4a: Differences between colloids and crystalloids in peri-interventional patients 504
Chapter 4b: Differences between colloids and crystalloids in ICU patients 505
Chapter 5a: Differences between colloids in peri-interventional patients 507
Chapter 5b: Differences between various colloids in ICU patients 508
Chapter 6a: Differences between the crystalloids in peri-interventional patients 509
Chapter 6b: Differences between crystalloids in ICU Patients 510
Chapter 7a: Management of volume therapy in peri-interventional patients 512
Chapter 7b: Management of volume therapy in ICU patients 515
References 28
List of Abbreviations
95% CI 95% confidence interval
AUC Area under the curve; usually this applies to the receiver operating characteristic curve.
AWMF Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (Association of the Scientific Medical Societies in Germany)
CVP Central venous pressure
DGAI Deutsche Gesellschaft für Anaesthesiologie und Intensivmedizin (German Society of Anaesthesiology and Intensive Care Medicine)
DO2 (I) Oxygen delivery index
DSG Deutsche Sepsis-Gesellschaft (German Sepsis Society)
EMA European Medicines Agency
FTc corrected aortic flow time
GEDV (I) Global end-diastolic volume (index)
GEF Global ejection fraction
GoR Grade of Recommendation
HES Hydroxyethyl starch
ITBV (I) Intra-thoracic blood volume (index)
ITTV Intra-thoracic thermal volume
IVC inferior vena cava
IQR Interquartile range
kgBW kilograms of body weight
LoE Level of Evidence
LVEDV (I) Left ventricular enddiastolic volume (index)
NaCl Sodium chloride
OR Odds ratio
PAOP Pulmonary artery occlusion pressure
PLR Passive leg raising
PPVar Pulse pressure variation
PTV Pulmonary thermal volume
ROC Receiver Operating Characteristic (Curve)
S3 S-Class of a Guideline. According to the AWMF, S-Classes categorise the scientific methodology of guidelines.
S2k S-Class of a Guideline. According to the AWMF, S-Classes categorise the scientific methodology of guidelines.
ScvO2 Central venous oxygen saturation
SPV Systolic pressure variation
SV Stroke volume
SVV Stroke volume variation
TEE Transoesophageal Echocardiography
TTE Transthoracic Echocardiography
VO2 (I) Oxygen consumption (index)
Rationale and goals
A1: Introduction
Medical guidelines present a systematically developed body of information and advice to assist diagnosis and treatment of specific health problems.1 They define standard practice for tackling a key health issue, and offer guidance to medical personnel and patients alike.2 Guidelines make an important contribution to rational and transparent decision making in healthcare provision, and their dissemination is intended to improve the quality of this provision.
The process used to develop guidelines must be systematic, independent and transparent.3 As defined by the Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF; Association of the Scientific Medical Societies in Germany), three ‘S-Classes’ are recognised within this process (Table 1). The S-Class of the present Guideline is S-Class 3.
Table 1.
Guideline development S-Classes as defined by the Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften
| S-Class 1: Expert group | An expert group formed of representative members of the specialist association(s) works in loose consensus on a recommendation that is adopted by the Board(s) of the specialist association(s) |
| S-Class 2: Formal evidence-based research or formal consensus building | Guideline development is based on the formal assessment of statements from the research literature (S2e) or guideline consultation and adoption is based on one of the following proven methods of achieving formal consensus: nominal group process, Delphi technique or consensus conference |
| S-Class 3: Guideline with all elements of systematic development | Guideline development comprises systematic literature research and assessment, the classification of case studies and recommendations according to the criteria of evidence-based medicine, and formal consensus building |
A2: Initial situation
Intravascular volume and fluid therapy is fundamental to the management of adult inpatients. The concept applies to all contexts and situations in which oral or enteral delivery of fluids is unable to meet fluid intake needs. The quantitative significance of intravascular volume therapy is justified merely by the fact that the vast majority of the 20 million patients approximately, treated in German hospitals every year (https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Gesundheit/Krankenhaeuser/Krankenhaeuser.html) require intravascular volume therapy at least on a temporary basis. This may be provided as a perioperative or peri-interventional measure whenever fasting is indicated for medical reasons, when the enteral fluid resorption rate falls below the necessary substitution rate (e.g. because of shock), in the event of high-fluid turnover rates during major surgery, or in cases of reduced enteral resorption because of sustained vomiting or severe diarrhoea.
Some recent multicentre studies have also triggered debate about the benefits and risks of previous therapeutic models based on pathophysiological findings. One result of this discussion has been the initiation of pharmacovigilance proceedings concerning the use of Hydroxyethyl starch (HES) by the European Medicines Agency (EMA) in November 2012, during the development of the present Guideline. The Pharmacovigilance Risk Assessment Committee advises against its use in patients suffering from sepsis and burns. (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Hydroxyethyl_starch-containing_solutions/human_referral_prac_000012.jsp&mid=WC0b01ac05805c516f) The recommendations of the present AWMF S3 Guideline are based on an analysis of the evidence and are to be viewed separately from the EMA recommendations.
Spurred on by the general importance of the subject and the uncertainties prevailing among its member physicians, the German Society of Anaesthesiology and Intensive Care Medicine has decided to initiate and coordinate the development of a Guideline to be issued by the AWMF as a contribution to evidence-based volume therapy.
A3: Guideline requirements
The Guideline must meet the following requirements:
Based on current findings from research as well as established practice, it provides support for decision making in specific situations.
It facilitates sound intravascular volume therapy for the vast majority of adult inpatients.
It is routinely assessed and updated to reflect current knowledge on a regular basis.
Through regular discussion with all stakeholders (physicians, nursing staff, patients and relatives) it achieves transparency for intravascular volume therapy goals and procedures.
It acknowledges that in light of the many situations in which intravascular volume therapy is required, a single treatment concept that meets all needs is impossible to achieve.
A4: Guideline objectives
Overall, the Guideline aims to improve the quality of volume therapy in peri-interventional or critically ill adult inpatients. The best volume therapy should include a correct indication (diagnosis of volume depletion), correct dosage (volume therapy management) and the selection of the infusion solution best suited to the patient. There are competing concepts for each of these three areas. By promoting effective, correctly dosed, efficient and evidence-based volume therapy with the best benefit to risk balance, the Guideline aims to secure the best treatment of volume depletion in adult patients treated in medical units throughout all levels of the healthcare system.
The Guideline's recommendations are intended to further improve the quality of hospitals’ structures and procedures, and help improve the quality of results. For this reason, the Guideline can and should be used in acute treatment situations, and in discussions about local protocols, in quality assurance and any other appropriate forum.
Since the Guideline authors wish to stimulate discussion about volume therapy, criticism and proposals for improvement are expressly requested. Ideally, recommended amendments should be concisely summarised, substantiated with references, and forwarded to the publisher.
The Guideline does not concern itself with the topic of infusion therapy for patients without volume depletion (as part of parenteral feeding and the correction of electrolyte or acid-base imbalances, or infusions used as carrier solutions for drug delivery). Nor does it concern itself with blood product therapy; here, the Guideline Group refers the reader to the appropriate cross-sectional Guideline from the German Medical Association.
A5: Target user group
The Guideline is addressed primarily to healthcare professionals who are familiar with one or more aspects of intravascular volume therapy for inpatients (diagnostics, choice of solution and therapy management). Such professionals will generally be physicians and nursing staff.
The Guideline also addresses individuals wishing to learn about evidence-based intravascular volume therapy for adult inpatients. This group includes members of other medical professions as well as patients and their families.
B: Methodology
The Guideline project was registered at the homepage of the AWMF (Association of the Scientific Medical Societies in Germany) on 7 October 2011 (http://www.awmf.org).
B1: Literature search and selection of evidence
Use of existing guidelines
At the start of the project, a systematic guideline search was conducted in guideline databases (National Guideline Clearinghouse, USA; Association of the Scientific Medical Societies, Germany; Guideline International Network, Scotland) and assessed for the potential reuse of material. As this process failed to identify any guidelines that satisfied the inclusion criteria in full, a decision was made to proceed with development of the Guideline de novo.
Systematic literature search
Taking the key issues as its starting-point, the Methods team from the Institut für Forschung in der Operativen Medizin (Institute for Research in Operative Medicine) worked closely with clinical experts to develop the strategies for a systematic literature search for the diagnosis and treatment of volume deficiency. Consensus was achieved for these strategies by the Guideline Group at the consensus conference of 17 April 2012, and they were used on 21 May 2012 (follow-up search: 14 June 2013) for a literature search in Medline (via PubMed), Embase and Cochrane Central Register of Controlled Trials (CENTRAL) databases. Alongside medical keywords (medical subject headings, MeSH) enhanced by a free-text search, study filters were applied to identify relevant systematic reviews and meta-analyses, (non) randomised controlled trials, prospective cohort studies and diagnostic studies (including cross-sectional studies). Publication languages were restricted to German and English, and within a publication time frame ranging from 1995 to the date of the literature search. A flowchart showing the numbers of retrieved, excluded, and included publications can be seen in Fig. 1.
Fig. 1.
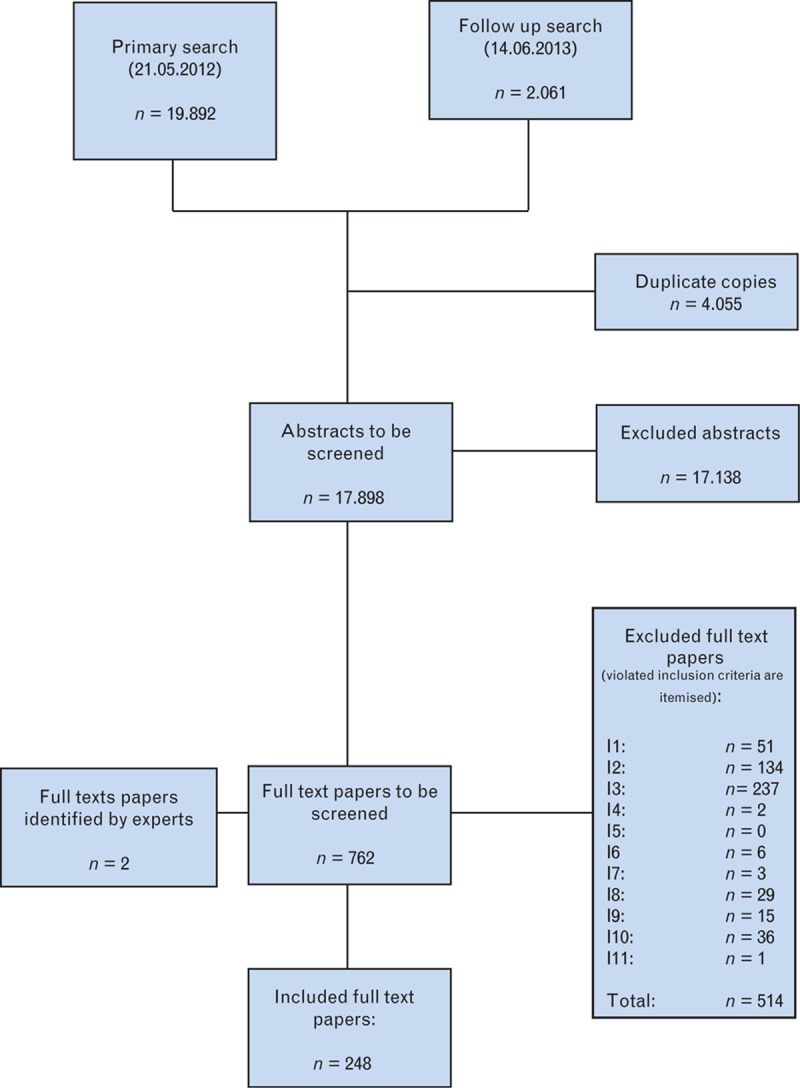
Flowchart of included references and abstracts and the screening process. Indicators of violated inclusion criteria (I1 to I11) are explained in Table 2. For each reference, only the first criterion violated was registered.
In addition, www.clinicaltrials.gov was searched on 14 June 2013 for completed but not yet published clinical trials.
Apart from this systematic search, each member of the Guideline Group could recommend further publications for inclusion in the evidence base. All publications, whether retrieved by systematic database research or by personal recommendation, passed through the screening, extraction and appraisal process described below.
Selection of literature identified
Two independent experts (one a methodologist, one a clinician) screened every retrieved publication for the prospectively defined inclusion criteria.2 The violation of any inclusion criterion resulted in the exclusion of the publication. Screening was performed at the level of the title and abstract for all of the publications identified by the search, and at the full-text level for studies still included after the title/abstract screening.
When both experts agreed in their judgement on a study, it was then included or excluded; where opinions differed, consensus was achieved by discussion between the experts. Where consensus was not achieved, the Coordination Group decided on inclusion/exclusion. The study selection flowchart is presented in Fig. 1.
B2: Appraisal and extraction of evidence
Each study included following the full-text screening was assigned to one or more key issues by the clinical expert. The methodological appraisal was conducted in accordance with the National Institute for Health and Care Excellence (UK) Methodology Checklist (http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b#close). The checklists can be requested from the corresponding author.
Methodological weaknesses were listed in the evidence tables. The checklists were also applied to determine the level of evidence (LoE) in accordance with the classification supplied by the Oxford Centre for Evidence-Based Medicine (version 2009). (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/) To simplify future updates and to improve comparability, the grading in accordance with version 2011 (http://www.cebm.net/index.aspx?o=5653) was used in parallel.
As a final step, evidence tables were created in extraction templates with a priori consensus: these contained all of the data from the respective publication relevant for the infusion therapy and study methodology, plus the authors’ conclusions and personal conclusions of the Methods team. These evidence tables, written in German, can be downloaded at http://www.awmf.org/leitlinien/detail/ll/001-020.html. The data extraction, the appraisal of the study methodology and the quality assurance of these steps were also completed by a clinical and methodological expert alternately, to ensure that all included studies were extensively analysed and appraised in accordance with both aspects. In the event of disagreement, a consensus-building discussion was envisaged identical to that used for the inclusion/exclusion screening.
B3: Formulation and consensus building for recommendations and statements
All Guideline authors were provided with the evidence tables, the National Institute for Health and Care Excellence (UK) checklists and the original publications, so as to provide the authors with sufficient opportunity to become familiar with the evidence base.
Formulation of recommendations and statements
The authors of the corresponding chapters reviewed and appraised the included studies assigned to their chapters, and prepared these for presentation during the consensus conferences. They also formulated preliminary recommendations, including the Grade of Recommendation (GoR), and statements. Recommendations offer guidance for action with a direct topical link to the core of the Guideline. Statements offer comment on or explanation of specific circumstances or key issues without a direct action being specified. Recommendations and statements are adopted in the course of the formal consensus procedure outlined below, and are based on the available scientific evidence and expert opinion. (http://leitlinienprogramm-onkologie.de/uploads/tx_sbdownloader/LL_OvCA_OL_Langversion.pdf, (http://www.krebsgesellschaft.de/download/s3-leitlinie-prostatakarzinom_2012.pdf).
The prepared evidence, preliminary recommendations, including GoR and preliminary statements, were presented by the coordinators of the respective chapters in the course of two consensus conferences (26–27 September 2013, 11–12 November 2013) and discussed with all of the delegates. Position statements and recommended alternative formulations were recorded by the conference chairs.
Consensus building
Using the Tele-Dialog system, an anonymous vote was held on every statement and every recommendation, including GoR. Where consensus could not be reached there was further discussion followed by a final vote. The classification of the degree of consensus is shown in Table 3. Each specialist association attending received one vote in the consensus process, regardless of the number of delegates.
Table 3.
Classification of degree of consensus
| >95% agreement among participants | Strong consensus |
| >75 to 95% agreement among participants | Consensus |
| >50 to 75% agreement among participants | Majority consensus |
| <50% agreement among participants | No consensus |
Grades of Recommendation
The Grades of Recommendation express the likelihood that a relevant positive effect can be expected or a negative effect can be avoided for the patient by following the recommended course of action. The GoR assigned by the Guideline authors takes into account the methodological quality of the underlying studies (LoE), the clinical relevance of the reported effectiveness criteria and observed effect sizes, the consistency of the study results, the transferability to the target population, the applicability in routine medical practice, ethical obligations and patient preferences. The relationship between the LoE and the GoR, as well as the modification of this mapping by the other factors mentioned, is indicated by the choice of terminology. In the wording used to formulate the recommendations, the terms ‘must’, ‘should’ and ‘may’ were associated with GoR A, B and 0, to ensure the recommendation accurately reflects the assigned GoR (Table 4).
Table 4.
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften wording schema to formulate recommendations based on the strength of the recommendation
| Grade of recommendation | Strength of recommendation | Wording |
| A | Strong recommendation | ‘must’ |
| B | Recommendation | ‘should’ |
| 0 | Open recommendation | ‘may’ |
B4 Dissemination and implementation
The Guideline was disseminated by publication to online media (AWMF website, links placed on websites of participating medical societies), by presentations at medical conferences [Deutsche Gesellschaft für Anaesthesiologie und Intensivmedizin (DGAI) Annual Congress 2014 (preliminary presentation) 8–10 May 2014, Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin Annual Congress 3–5 December 2014, DGAI Annual Congress 7–9 May 2015, Deutsche Gesellschaft für Innere Medizin Annual Congress 18–21 April 2015]. A manuscript summarising the key issues was submitted to the scientific part of the Deutsches Aerzteblatt (peer reviewed publication organ of the medical profession in Germany)
The effects of implementation will be measured by means of web-based questionnaire interviews using a longitudinal design. The questionnaire was circulated by the participating medical societies before the initial publication and will be repeatedly circulated at regular intervals thereafter.
A short version of the Guideline will also be published, presenting the recommendations in a concise format. Copies will be offered in A4 and A6 sizes – the latter for use in the production of medical pocket cards. There are also plans to develop smartphone applications, compatible with all of the popular operating systems.
B5: Quality indicators and evaluation
Volume therapy is used across an extraordinarily broad spectrum of applications and clinical situations. Because of this a change in treatment outcome because of the Guideline cannot be measured by ‘hard’ outcomes such as morbidity or mortality, as too many confounding variables are involved. Guideline efficacy can be measured meaningfully only in terms of process changes. These are monitored by the repeat surveys conducted during implementation research. The recommendations’ level of penetration into the knowledge of professional groups addressed – particularly physicians – is applied as a Guideline quality indicator. Review of implementation in practice is possible in the form of audits or in the course of peer reviews. No specific courses of action are planned here.
B6: Validity and revisions
The Guideline was last revised on 18 June 2014. This revision incorporated the comments made by the specialist associations.
The Guideline is valid until 31 July 2017.
The Management Board of the DGAI will again ask the participating specialist associations to appoint a delegate to help in the revision of the Guideline, 1 year before the validity period expires. The revision process will follow the standard procedure as described above.
B7: Funding and disclosure of possible conflicts of interest
Funding to cover the costs of methodological support, procurement of literature, organisation of the consensus conferences and materials has been provided by the DGAI. Attendee travel expenses were covered by delegates themselves or the specialist associations they represented.
At the beginning of each consensus conference, all participants disclosed any potential conflict of interest. In addition, all participants kept their conflicts of interest up-to-date until the completion of their Guideline work. A complete list of updated conflicts of interest statements from all participants is given in the guideline report (http://www.awmf.org/leitlinien/detail/ll/001-020.htmlpublished). When necessary, an anonymous vote was held among delegates of the specialist associations eligible to vote to decide on the relevance of existing conflicts of interest. As a result, no specialist association lost its vote in the consensus process because of a conflict of interest affecting its delegate.
Overall recommendations/statements:
Box 1.

no caption available
Background to statement S-1
An intravascular volume deficit is characterised by haemodynamic instability, which occurs because of a critical depletion of circulating plasma volume with a concurrent drop in cardiac preload, reduction in cardiac output, and a decrease in microcirculation and tissue oxygenation. This is also accompanied by an interstitial fluid deficit. Accordingly, the provision of adequate fluid and volume therapy forms a very important part of the treatment of interstitial fluid and intravascular volume depletion. The objective of initial fluid and volume therapy is the restoration of normal blood volume and a sufficient volume of circulating plasma, so as to ensure adequate venous return to the heart and an appropriate cardiac output, increasing tissue oxygenation and tissue perfusion and achieving improved overall organ function.
In the extensive literature search conducted for this S3 Guideline on volume therapy in adult patients, with 17 898 abstracts screened for fulfilment of the inclusion criteria, 762 screened full-text articles and 248 full-text articles evaluated, no evidence could be found for the above-mentioned recommendation made on pathophysiological grounds, as the existing scientific evidence addresses itself to the problem of intravascular volume depletion.
For this reason, and following in-depth discussion of the relevant content, the Guideline group has decided to make the overall recommendation that the recommendations given for volume therapy are also applicable to fluid therapy in cases of isotonic dehydration.
Furthermore, specific forms of dehydration such as hypertonic dehydration are not dealt with, as these specific forms of fluid and volume depletion would significantly exceed the scope of the present Guideline.
Lastly, readers should also be aware that the EMA convened a pharmacovigilance risk assessment in 2013 concerning the use of HES. The Pharmacovigilance Risk Assessment Committee advises against use of HES in patients suffering from sepsis and burns. HES can continue to be used to treat hypovolaemia following acute blood loss. The recommendations of the present S3 Guideline are based on an analysis of the evidence and are to be considered separately from the EMA recommendations.
Box 2.

no caption available
Background to recommendation O-1
In the event of serious volume depletion combined with life-threatening hypotension, the rapid restoration of intravascular volume by forced volume substitution is always indicated. In terms of equipment, compressible containers for the required volume substitute offer a rapid means of implementing this essential requirement in a way that is decisive for survival. On the basis of this clinically relevant approach, consensus was achieved for the above recommendation despite the lack of scientific evidence for this recommendation.
Applying the ‘patient population, intervention, comparitor, outcome’ principle, each key question is formulated with consideration of the Patient population the guideline is made for, the Intervention and the respective Comparator and the Outcome considered as relevant. For this guideline, the intervention is defined by the substance used, although it is incompletely characterised without a description of the mode of delivery. Indeed, the importance of the mode of delivery in the context of volume therapy can easily be understood merely by considering the many clinical trials actively sought out and thoroughly evaluated in many meta-analyses;4–6 in some of these, volume substitutes were delivered over several days, based on a twice-daily dosage regimen.7
In light of the above, it appeared prudent to promote a rapid delivery option – especially in the event of a lack of large-lumen intravenous access routes – so as to be able to conduct volume therapy in the essential sense of the term. Nevertheless, a comment was raised during the consensus process to the effect that the acceptance of a temporarily low level of blood pressure (BP) may, in certain cases, be the advisable course of action (permissive hypotension).
Chapter 1: Diagnosis of hypovolaemia
Box 3.

no caption available
Background to recommendation 1-1
One of the most basic decisions for maintenance of the homeostasis of the human body is the choice of delivering external solutions. The objectives at the heart of such decision-making are the goals of volume substitution (intravascular) and fluid substitution (interstitial). In the majority of studies, the terms (intracellular) dehydration and (extracellular) volume depletion are used interchangeably, although defining two quite different entities. In addition, posture-related syncope or reflex tachycardia while standing (by over 30 beats min−1) is frequently indicative of a fluid volume deficit (hypovolaemia).77 In 1999, McGee et al. demonstrated that a dry axilla, in contrast to moist mucous membranes, would also increase the sensitivity for a diagnosis of hypovolaemia. Interestingly, capillary refill time and skin turgidity showed only very poor sensitivity, whereas presence of tachycardia when lying down had only a high specificity.77
As every substance delivered to the body interacts with the body's own fluids in accordance with its composition, stability and mode of delivery, predictions as to inertness or the exchange of substances (down to the level of the cell) are virtually impossible.
Total body water is held enclosed in various fluid spaces (compartments): two-thirds are found in cells (intracellular compartment), whereas the remaining water is held in the extracellular compartment and comprises interstitial fluid (31%), blood plasma (7%) and other transcellular fluid (cerebrospinal fluid, etc.). The body's own regulation of fluid balance is achieved by osmoregulation and volume regulation, and the thirst reflex. The goal is to maintain body water at a volume of approximately 42 l. As the volume of the intracellular space is generally co-controlled by the extracellular space (cell walls are water-permeable), any fluid delivered to the body can cause water displacement, down to the level of the cell. As the interstitial fluid forms part of the extracellular space and can be determined only indirectly as the difference between overall extracellular space and plasma volume, investigations of interstitial fluid deficit are virtually impossible – at least without additional instrument-based diagnostics. Accordingly, the substance-based substitution of the interstitial space is certainly neither advisable nor necessary. In the event of isotonic hyperhydration, for example extended intravascular volume delivery would result in an expansion of the extracellular space with an increased incidence of interstitial oedema because of diffusion.
The starting point for each further diagnostic step is a thorough physical examination that can reliably estimate the volume status while also estimating the response to volume loading. Options available include inspection of the tongue, jugular vein/dorsal venous network filling and the nail blanch test. Alongside pulmonary auscultation and percussion (for pleural effusion), abdominal palpation (ascites, distended intestinal loops) and inspection of the lower extremities for dependent oedema and skin turgidity are also necessary.
Saugel et al. in 2013, investigated the extent to which an increase in the cardiac index by volume loading (’volume responsiveness’, measured via transpulmonary thermodilution) could be predicted from a clinical examination performed by two independent practitioners. Apart from the fact that these practitioners were in agreement for about only half of the patients examined, the poor positive predictive value of 27.8% illustrates the inaccuracy of clinical assessment.59 In 2011, the same group achieved similarly poor results with another group of 71 internal medicine patients when applying the same clinical tests (poor inter-observer agreement, low sensitivity). Interestingly, hypovolaemia could almost always be excluded, with a negative predictive value of 70%.60 In summary, and in the context of differential diagnosis, we may conclude that the rapidity and simplicity of the physical examination means it must be performed on every patient suspected to be suffering from a volume deficit. Although the physical examination alone certainly fails to satisfy the requirements of comprehensiveness, it considerably improves diagnostic quality when combined with further tests.
Box 4.

no caption available
Background to recommendation 1-2
In recent years the collection of data on a host of laboratory variables, some redundant, has become established as a standard procedure on patient admittance. As a result, clinical assessment of the patient and the associated diagnostic expertise has been increasingly overshadowed, and is on occasion reduced to merely waiting for the laboratory results before even daring to make a decision or perform an examination. In the particular case of critical volume depletion shock, waiting for laboratory results is a pointless and often fatal exercise. Here, prompt clinical assessment is sufficient, combined with a medical history to identify the right treatment.
Notwithstanding the above, a handful of laboratory variables are important in the differential diagnosis of hypovolaemia, such as the determination of the urea-to-creatinine ratio to estimate volume loss, or hypernatraemia (in advanced dehydration), and should not be omitted.
Recently, attention has been increasingly directed toward the determination of the base excess following acid-base disturbances caused by tissue hypoxia or underperfusion following serious trauma, with volume depletion because of exsanguination or trauma-induced coagulopathy, as this facilitates an estimation of prognosis. In addition, lactate is a marker of anaerobic metabolism which is useful to assess ischemic tissue damage in extremities and vital organs. The determination of mixed venous (or simply central venous) oxygen saturation as a marker for the desaturation of erythrocytes and thus the systemic oxygen consumption (VO2) has now become established as the standard for intensive care medical services.
All laboratory variables can be used for monitoring, i.e. the measurements can be repeated at fixed intervals. This allows to detect improvement or worsening of the patient, or enables to estimate the prognosis, for example by considering lactate values in the treatment of sepsis.
In 2010, Futier et al.33 demonstrated that a decrease in central venous oxygen saturation (ScvO2) with restrictive volume therapy, seen in 70 visceral surgery patients intraoperatively, and confirmed in the multivariate analysis, was a predictor for postoperative sepsis or anastomotic insufficiency. In 2013, Saugel et al.59 recorded a negative predictive value and a specificity of just under 71% for ScvO2, with a threshold of under 70%, for the diagnosis of hypovolaemia in 38 internal medicine patients. This threshold of 70%, familiar from sepsis guidelines, was investigated by Monnet et al. as a surrogate marker for elevated oxygen consumption in 51 septic internal medicine patients. In contrast to lactate [area under the curve; usually this applies to the receiver operating characteristic (ROC) curve (AUC) 0.91 and a sensitivity of 93% with a cut-off of 2.77 mmol l−1], ScvO2 achieved an AUC value of only 0.68 and was therefore a poorer marker for the increase in VO2 (except for a value of under 50%). Interestingly, oxygen delivery decreased in the nonresponders, probably because of dilution and thus a reduced haemoglobin value. This, in turn, demonstrates that a ‘fluid challenge’ should be seen as a one-time attempt in the event of surrogate markers remaining unchanged.49
Box 5.

no caption available
Background to recommendation 1-3
For a long time, central venous pressure (CVP) formed part of basic routine monitoring for critically ill patients. Over the past few years, studies with various patient groups have shown repeatedly that the correlation of changes in CVP and pulmonary artery occlusion pressure with changes in cardiac output is at best very poor and at worst entirely absent, and that the measurement of the CVP has only minimal predictive power in determining the status of intravascular volume. The underlying reason for this is that CVP is dependent on a broad spectrum of factors, including intravascular volume, peripheral vascular tone, right ventricular compliance, pulmonary vascular resistance and intrathoracic pressure (in ventilated patients). Although low CVP may indeed indicate a volume deficit, elevated CVP is equally unable to exclude the presence of volume depletion. By way of illustration, an elevated CVP is possible in the event of right-sided heart failure, pulmonary embolism, cardiac tamponade, tension pneumothorax or hypervolaemia. In a much-cited study on early goal-oriented sepsis therapy, Rivers et al. were able to demonstrate a significant reduction in mortality by specifying a CVP target value between 8 and 12 mmHg. It should be noted that CVP in this study was utilised only as part of a pool of multiple haemodynamic target criteria. Accordingly, isolated evaluation of CVP in the context of this study is not possible.78
In a prospective observational study in patients with septic shock (n = 25), CVP, shock index and cardiac index were measured with transthoracic echocardiography immediately before and after a volume bolus.39 Haemodynamic response was defined as an increase in the cardiac index of at least 15%. Patients with CVP more than 8 mmHg and a lowered shock index did not benefit from volume loading. Yet with both lower CVP (<8 mmHg) and higher shock index it was still not possible to reliably predict volume responsiveness.
In a prospective study with 31 medical ICU patients, Saugel et al.59 investigated the extent to which clinical examination, CVP, passive leg raising, ScvO2 or transpulmonary thermodilution were able to predict volume responsiveness. All variables investigated, including that of CVP, demonstrated inadequate reliability in this study.
In a recent meta-analysis,79 CVP was investigated in terms of its capacity to predict volume responsiveness. A total of 43 studies were included in the analysis. Of these, 22 studies concerned intensive care patients, 20 analysed CVP within operative monitoring and one study was conducted with study participants. Volume responsiveness was equated with an increase in cardiac output or stroke volume following a defined volume loading procedure (volume challenge) or after passive leg raising (PLR). Most studies defined volume responsiveness as an increase in the cardiac index or stroke volume index by over 15%. The volume challenge constituted the intravenous delivery of 500 ml of fluid (usually HES). Data for the receiver operating characteristic curve were available in 20 studies. Overall, 57 ± 13% of patients were volume-responsive. Average CVP as initially measured was 8.2 ± 2.3 mmHg in the volume-responsive group and 9.5 ± 2.2 mmHg in nonvolume-responsive patients. The AUC value for the ROC curve was 0.56 [95% confidence interval (95% CI) 0.54 to 0.58]. No difference was seen between intensive care and operating theatre patients. The same results were also obtained for cardiac surgery and noncardiac surgery patients. In all groups, correlation of the initial CVP measurement with the change in cardiac index and stroke volume was poor.
In conclusion, the measured filling pressure cannot be used to draw reliable conclusions about a specific volume status. It is also subject to numerous factors that interfere with interpretation.
Existing guidelines:
The S3 guideline ‘Post-infarction cardiogenic shock: diagnosis, monitoring and therapy’ includes the statement that CVP should not be used as a guide for volume management. (http://www.awmf.org/leitlinien/detail/ll/019-013.html).
The S3 guideline ‘Provision of intensive care medicine to cardiosurgical patients: haemodynamic monitoring and cardiovascular therapy’ cites the limited usefulness of CVP in relation to volume management. The guideline nonetheless mentions the option of continuous measurement as a means of obtaining important information about acute changes in right ventricular compliance and/or volume status. (http://www.awmf.org/leitlinien/detail/ll/001-016.html).
The Surviving Sepsis Campaign ‘Guidelines for management of severe sepsis and septic shock’80 recommend CVP measurement in sepsis with signs of hypoperfusion with a target value of 8 to 12 mmHg in the first 6 h (grade 1C). The German S2k sepsis guideline81 also recommends a CVP goal of more than 8 and 12 mmHg with mechanical ventilation as part of a pool of haemodynamic target criteria for early haemodynamic stabilisation (grade of recommendation C).
Box 6.

no caption available
Common background to recommendations 1-4, 1-5, 1-6
With no major requirements in terms of equipment, PLR is an easily performed bedside examination for diagnosing volume depletion in addition to potential volume responsiveness. The manoeuvre results in a reversible autotransfusion of 300 to 450 ml. By increasing cardiac preload (in the case of a volume-depleted patient), the stroke volume can be increased (assuming peripheral resistance remains unchanged), and thus cardiac output, for the duration of the test. Use of the PLR manoeuvre can also avoid the often harmful excess volume resulting from frequent infusion boli with suspected hypovolaemia; rates of pulmonary oedema and perfusion disorders can also be reduced.
However, a standardised PLR manoeuvre in hospital presents considerable problems: the upper body may be inappropriately positioned, or an above-knee amputation may be present or there may be congestive heart failure.
In a prospective clinical trial with 39 intensive care patients receiving interdisciplinary treatment, Boulain et al.82 (2002) achieved a good correlation between increased stroke volume and a directly proportional rise in aortic pulse pressure, verifying the positive effects of simulated volume loading.
In 2002 meta-analysis that included a total of 9 studies and 353 participants (intensive care patients with shock because of a range of factors), Cavallaro et al. reported the reliability of the predictive power of PLR in relation to an increase in cardiac output and stroke volume (as determined via pulmonary catheter, transoesophageal echocardiography (TEE)/transthoracic echocardiography (TTE), transpulmonary thermodilution and uncalibrated pulse contour analysis). Despite variations in the performance of the PLR manoeuvre (from a supine or half-sitting position) and a range of definitions of responders, cumulated sensitivity was 89.4% with a specificity of 91.4% in relation to an increase in cardiac output (AUC 0.95). The measured rise in pulse pressure results in a sensitivity of only 59.5% with a specificity of 86.2% (AUC 0.76).12
Mandeville et al. performed a systematic review that considered eight studies using TTE to compare volume responsiveness, and showed that the PLR manoeuvre achieved a specificity of up to 99% with a maximum sensitivity of 100% in relation to stroke volume, stroke volume index and cardiac output. The positive predictive value fluctuated between 83 and 91%, with the volumes compared varying between 500 ml colloid and crystalloid. Included in this review was the cross-sectional study from Biais et al.,10 in which stroke volume was measured via TTE or the uncalibrated pulse contour analysis technique (software version 1.14) in 30 patients (19 intubated and spontaneously breathing). Here, too, the PLR manoeuvre (compared to 500 ml saline solution) was able to predict volume responsiveness in a reliable manner (compared to the uncalibrated pulse contour analysis, AUC 0.92). Better results were returned by the comparative TTE measurement (AUC 0.96).43
In the cross-sectional study from Lakhal et al. with 112 patients (ventilated, haemodynamically unstable, of which 21 were arrhythmic), volume responsiveness was investigated using noninvasive/invasive BP increases (systolic and mean pressures) induced by PLR (45°) compared to invasive cardiac output measurement as the reference measure. The change in SBP was also significant (AUC 0.75) even for the noninvasive BP measurement. With the application of an additional arbitrarily chosen CVP increase of at least 2 mmHg, the AUC rose to 0.94. Qualitatively, however, the data are very hard to interpret, as the specification of the reference test was nonuniform and specification of the CVP criterion was arbitrary.83
In 34 spontaneously breathing, hypotensive internal medicine patients, Maizel et al. validated the echocardiographically determined volume responsiveness of cardiac output and stroke volume via PLR or 500 ml saline solution (with noninvasive BP measurement). Nonetheless, the authors state in their final conclusions that, while the PLR manoeuvre has good predictive power, the poor quality of reporting and conflicting sets of results mean the statement must be treated with caution.42
Preau et al. used a different PLR manoeuvre for 34 spontaneously breathing septic patients. This passive leg-raising manoeuvre also involved lowering the upper body, thus simulating a larger volume bolus (of about 450 to 500 ml, in comparison to 500 ml 6% HES). Both the change in stroke volume and radial pulse pressure demonstrated good predictive power (AUC of 0.94 and 0.86, respectively).55
Dong et al. compared the change in indexed stroke volume with transpulmonary thermodilution measurement following the same PLR manoeuvre as Preau in 32 septic and ventilated internal medicine patients. Interestingly, with a cut-off of around 9%, the same sensitivity (72.7%) and specificity (80%) were seen as for a CVP change of at least 12.7%. The AUC was 0.882 for the change in stroke volume, whereas the CVP increase achieved an AUC of 0.805.25
In the course of performing the PLR manoeuvre in 65 septic internal medicine patients on continuous mandatory ventilation, Monnet et al. interestingly chose to use end-tidal carbon dioxide measurement as a surrogate marker for volume responsiveness rather than measuring cardiac ouput via transpulmonary thermodilution. Despite methodological deficiencies, AUC was shown to be 0.93 with a specificity of 100% (sensitivity 71%) for a cut-off of 5%. The AUC for measurement of cardiac output was 0.98. The authors conclude that if extended haemodynamic monitoring is not available, carbon dioxide measurement offers a suitable alternative.47
In conclusion, a positive PLR manoeuvre (rising arterial pulse pressure) can be used to provide a guideline estimate of the effect of volume loading on stroke volume or cardiac output, and thus be of assistance in resolving potential therapeutic conflicts.
In spontaneously breathing patients, however, one must remember the risk of aspiration, especially with visceral surgery patients. Nor should the manoeuvre be applied in the presence of cardiogenic shock, intracranial bleeding or elevated cerebral pressure.
No data are available for patients with elevated intra-abdominal pressure.
If extended haemodynamic monitoring is available (cardiac output/stroke volume measurement), then this should be used preferentially with a threshold between 8 and 15%. The correlation rises with the use of extended haemodynamic monitoring. Neither ventilation nor arrhythmias were relevant factors affecting the results.
In the case of basic monitoring, the (radial) pulse pressure (SBP − DBP) can be used – a positive prediction equates to an elevation of at least 9 to 12%.
Box 7.

no caption available
Background to recommendation 1-7
Volumetric preload indices can be determined using transpulmonary thermodilution. The cardiac ouput measured using thermodilution is multiplied together with the mean transit time and downslope time of the transpulmonary temperature-time curve to calculate the intra-thoracic thermal volume (ITTV) (ITTV = cardiac output × mean transit time) and the pulmonary thermal volume (PTV) (PTV = cardiac output × downslope time); the difference defines the global end-diastolic volume (GEDV = ITTV−PTV). The intra-thoracic blood volume (ITBV) is calculated from the GEDV using an empirically determined correction factor (ITBV = 1.25 × GEDV). The measurement requires the use of a specialised monitor and a cold fluid – generally 20 ml per indicator injection. To reduce the measurement's coefficient of variation, multiple indicator injections are typically performed at each time of measurement.84,85 Measurement can be performed in a largely standardised manner. Common errors such as inadequate injectate volume or excessively warm injectate are shown on the monitor, enabling the avoidance of typical causes of signal-to-noise ratios that are too low.
Multiple small cross-sectional studies were analysed to assess the value of volumetric variables as volume responsiveness predictors. The reference test used was the increase in thermodilution cardiac output or thermodilution stroke volume for defined volume loading, whereby separate threshold values were applied for the diagnosis of a volume deficit.
De Waal et al. investigated 22 patients during elective coronary bypass operations and defined volume responsiveness as a rise in stroke volume (thermodilution measurement) of at least 12% by an infusion of 6% HES 10 ml kg−1 BW. ROC analysis showed that indexed values of GEDV and ITBV were unable to predict volume responsiveness (AUC (95% CI) for GEDVI: 0.700 (0.460; 0.940); for ITBVI: 0.682 (0.441; 0.923), regardless of whether the thorax was opened (AUC value for ROC curve (95% CI): 0.756 (0.500; 1.011) for both) or closed.23
Hofer et al. investigated 40 patients during ‘off-pump’ coronary revascularisation, finding that GEDVI and ITBVI were unable to predict the volume responsiveness of stroke volume measured via thermodilution, with an AUC value for the ROC curve of 0.493 (95% CI: 0.292; 0.688). Of note, Hofer et al.35 used the unusually high threshold value of at least 25% as the diagnostic criterion for volume responsiveness.
In 32 ICU patients after major vascular interventions or coronary surgery, Trof et al. were able to show that the diagnostic quality of GEDVI is dependent on cardiac function. The global ejection fraction (GEF = 0.25 × stroke volume/GEDVI) was used for patient stratification. Where GEF at least 20%, volume responsiveness was predicted by considering the GEDVI. The AUC value was 0.72 (0.58; 0.83) or 0.89 (0.78; 0.95) depending on the threshold used for the rise in cardiac index (see below). Where GEF is less than 20%, however, the corresponding confidence intervals encompassed the value 0.5 for both threshold values. Cardiac index increases of at least 10 and 15% were analysed as threshold values for volume responsiveness. The AUC value was higher in both patient groups, however, when the higher threshold was applied. For patients with a GEF at least 20%, sensitivity, specificity and the positive and negative predictive values for the rise in cardiac index of at least 10% were 82, 56, 42 and 89%, respectively; the GEDVI diagnostic threshold for volume responsiveness was 890 ml m−2. For the cardiac index rise of at least 15%, the corresponding values were 71, 94, 63 and 93%, and the GEDVI threshold value was 623 ml m−2.65
Huang et al. investigated volume responsiveness in 22 patients experiencing early-stage ARDS. The AUC value for the ROC curve was the same for both GEDVI and ITBVI at 0.323. Accordingly, the suitability of both values for the diagnosis of volume depletion in ARDS patients could not be confirmed.36
Indirect indications of the suitability of ITBVI for diagnosing a volume deficit come from the findings of a study from Molnar et al. Using HES and gelatin infusion with the goal of boosting ITBVI to more than 900 ml m−2, they were able to significantly improve oxygen delivery index (DO2) independently of the colloid used.86
Szakmany et al. compared an ITBVI-based volume therapy with one that was CVP-based in patients during elective major visceral surgical intervention, and found a weak but significant correlation of the ITBVI with the changes in the stroke volume index. No data on diagnostic quality can be derived from this study, however.87
In summary, on the basis of the data as presented here, the suitability of volumetric measurements in the diagnosis of a volume deficit is limited. All of the studies cited involved small cohorts and exhibit a low level of methodological quality. Notwithstanding the above, the S3 guideline ‘Provision of intensive care medicine to cardiosurgical patients: haemodynamic monitoring and cardiovascular therapy’ considers that volumetric analysis is superior to CVP and pulmonary artery occlusion pressure (PAOP) in terms of estimating cardiac preload.88 The apparent discrepancy between these two guidelines can be traced to the different research strategies used. Further, it is clear that because disparity existed between the key issues considered by these two guidelines, the research that went into the ‘Provision of intensive care medicine to cardiosurgical patients’ guideline was more detailed than was achievable for the present Guideline. Nor did research into the present Guideline involve any background investigation limited to specific patient groups. The narrow scope of the evidence available to us does not permit endorsement in relation to the suitability of volumetric procedures for the diagnosis of volume depletion. For this reason, the Guideline Group recommends that volumetric variables are given the lowest grade of recommendation.
Box 8.

no caption available
Background to recommendation 1-8
The commonest motivation for delivering a fluid bolus to a patient is to increase stroke volume and cardiac output with a concomitant improvement in oxygen delivery – insofar as the patient is located on the ascending portion of the Frank-Starling curve. If not, the volume loading would have little beneficial influence on the cardiac output and actually produce an opposite and negative effect. In this light, establishing potential volume responsiveness is essential for patient care.
In recent years, a number of dynamic measurements based on changes to the pulse curve have emerged. Pulse pressure variation and systolic pressure variation are derived from the analysis of the arterial waveform, whereas stroke volume variation is taken from pulse contour analysis. Several methods of pulse contour analysis are available. One uses arterial pulse contour analysis and transpulmonary thermodilution to plot the proximally derived arterial pressure waveform against the stroke volume of the heart. Following calibration of the pulse contour analysis continuous estimates are made of a series of values, including stroke volume variation (SVV) and pulse pressure variation (PPVar) as additional indices of preload, and overall systemic resistance from stroke to stroke. Recalibration may be necessary every 4 to 8 h or after changes to individual vascular compliance.
The uncalibrated pulse contour analysis is based on the analysis of the arterial pressure waveform, combined with an individual calibration factor (χ), which depends on patient criteria (arterial compliance depends on age, sex and body surface area) and is dependent on the characteristics of the arterial waveform (slope and kurtosis, determined by peripheral resistance). Cardiac output and stroke volume are calculated using the formula
‘Pulsatility’ is the standard deviation of the arterial pressure waveform over a specified period.
The equation is based on a database consisting of arterial pressure waveforms and cardiac output reference values from thermodilution calculations. Initial versions of the software encountered major problems in achieving reliable calculation of cardiac output (because of a lack of human data). From version 1.10, however, the calibration factor χ is calculated from a larger database and updated every minute (second software generation). The third generation (from v. 3.0) of the uncalibrated procedure for pulse contour analysis compares the χ variable every minute with an even larger database, which contains more data from hyperdynamic patients with severe vasodilation, and is updated every 20 s.
Uncalibrated pulse contour analysis appears to be in broad agreement with the thermodilution method and cardiac output determined by echocardiographic measurement. Studies making direct comparisons between the methods are uncommon and are all from the field of cardiac surgery. Here, however, divergent results with occasionally low correlation coefficients are found. Accordingly, conclusive assessment as a sole reference value is difficult. Overall, transpulmonary thermodilution seems to achieve a better predictive value than uncalibrated pulse contour analysis.76
Physiologically, pulse contour analysis is based on the change in the pulse wave in monitored ventilation with intermittent fluctuations in biventricular preload, which responds differently to inspiration and expiration in accordance with the volume status. This leads to significant variation in systolic pressure variation (SPV); the greater the tidal volume, the better the potential evaluation (at least 8 ml kgBW−1). Spontaneous ventilation permits only very limited use of the calculated values.52
Unlike PPVar and SVV, however, SPV is often determined manually and this produces less reliable results than values calculated digitally in real time. The latter provide the typical characteristics of SPV.
Normally, the threshold value for volume responsiveness is located between 11 and 13% for all variables. Although slightly reduced cardiac output (ejection fraction <40%) would not adversely influence the soundness of the variables, arrhythmias, more serious valve defects and intracardiac shunts lessen the reliability of the measurements. High doses of vasopressors can also work to change arterial compliance.
Khwannimit et al. investigated 42 septic internal medicine patients on continuous mandatory ventilation using the third generation of the software (v. 3.01) for uncalibrated pulse contour analysis, to calculate SVV as a predictor for volume responsiveness. With a cut-off value of 10%, the AUC (0.92) was comparable with the calculated PPVar, a procedure based on mathematical analysis. With a cut-off value of 12%, PPVar achieved almost the same AUC (0.916) with P < 0.001.37 Cannesson et al. investigated 25 ventilated cardiosurgical patients to determine the predictive power of SVV, PPVar and cardiac index. Although a similar AUC value was found for PPVar and SVV (namely 0.871 and 0.857), values of only 0.298 and 0.533 were found for cardiac index and CVP. Interestingly, Cannesson took the view that, despite the different software algorithms used to determine SVV, the fluctuations nonetheless exhibited very good correlation with the volume status, regardless of the errors made in calculating stroke volume and cardiac output, as they were naturally related to the corresponding pulse contour analysis.11
In a systematic review of 568 patients, Zhang et al. measured an AUC of 0.84 for SVV with a sensitivity of 81% and a specificity of 80%, with the average cut-off value lying at approximately 10%. This review was adversely affected by pronounced heterogeneity of the studies included.76
Suehiro et al. compared two separate tidal volumes in 73 patients receiving one-lung ventilation to determine the predictive power (in relation to volume demand) of SVV using uncalibrated pulse contour analysis. With a tidal volume of 8 ml kgBW−1, the cut-off of 10% achieved an AUC of 0.776 with a sensitivity of 85.7% and a specificity of 66.7%. In the second group, with a tidal volume of 6 ml kgBW−1, an even lower cut-off of 8% was unable to achieve a sufficient level of statistical quality. A possible cause could be the smaller difference in transpulmonary or pleural pressure.64 In 2010, the same group also investigated 30 patients on one-lung ventilation, calculating a SVV of 10.5% with an AUC of 0.90 as an optimum predictive value. Yet the study data themselves were highly incoherent and inadequately validated.63
Yang et al. investigating 79 patients having elective surgery achieved a good correlation for PPVar compared with corrected aortic flow time measured with transoesophageal cardiography). The AUC value was 0.935 (cut-off 15%); the value for corrected aortic flow time (FTc) was 0.822. Conspicuous was the significantly (P = 0.014) improved predictive power of PPVar in the prone position (AUC 0.969) compared to FTc (AUC 0.846).73 Yazigi et al. investigated 60 cardiosurgical patients to compare PPVar with CVP and PAOP as a predictor of volume expansion with HES 7 ml kgBW−1. Neither CVP (0.43) nor PAOP (0.42) achieved the AUC value for PPVar (0.85, cut-off 11.5%), measured in terms of stroke volume change using pulmonary artery catheterisation.74
Shin et al. investigated 33 liver transplants during the anhepatic phase to determine the predictive power of CVP, PAOP and femoral SVV (uncalibrated pulse contour analysis). With a cut-off value of 8%, a sensitivity of 89% was achieved with a specificity of 80%; the value for AUC was 0.894 compared to 0.576 (CVP) and 0.67 (PAOP). The authors state that the cut-off of 8% represents a negligible difference to the calibrated pulse contour analysis with a cut-off of 9.5%, further noting that, especially in hypotonic patients, aortic pressure can be underestimated when BP is measured in the radial artery – although no relevant differences were found in either of the two measurements.61
The reduced predictive power of SVV (with calibrated pulse contour analysis) in 30 intubated but spontaneously breathing septic patients was made abundantly clear by Perner et al. Here, the multivariate analysis was unable to yield an adequate AUC value (0.52 to 0.64) for stroke volume variation. Probable options for improvement would be the extension of the 30-s SVV averaging or the calculation of SVV within a single respiratory cycle.52
Box 9.

no caption available
Background to recommendation 1-9
TTE has the advantages of being readily available and noninvasive. Its disadvantages are that the procedure is strongly investigator-dependent and cannot be deployed as a continuous procedure. In principle, the semi-quantitative filling state of the right and left ventricles can be used to draw conclusions about the volume status. However, probing inaccuracies translate to imprecision in the indices that determine hypovolaemia.89 A volume deficit can be safely assumed in the event of ventricles walls coming together (‘kissing ventricles’).
In a systematic review, Mandeville et al. identified eight studies that investigated TTE in relation to preload and volume responsiveness. The authors identified a good differentiation between volume responders and nonresponders using TTE. Unfortunately the pronounced heterogeneity of the studies (including the different TTE-based techniques) ruled out the performance of a meta-analysis. In addition, a majority of nonventilated patients were investigated with thermodilution techniques without comparison.43 A recent systematic review by Wetterslev et al.90 was unable to identify any study that compared TTE with cardiac output or stroke volume measured invasively via pulmonary arterial catheterisation or transpulmonary thermodilution, in the context of diagnosing a volume deficit.
Existing guidelines:
The American Heart Association classifies the deployment of TTE in assessing volume status for critically-ill patients as ‘Uncertain’, with a medium grade of recommendation (U5).91
Box 10.

no caption available
Background on recommendation 1-10
In patients exhibiting unclear haemodynamic instability, echocardiography can be used to distinguish between a wide range of differential diagnoses such as pericardial effusion and tamponade, acute right heart strain as an indicator of pulmonary arterial embolism, restricted pump function, valve defects and others. For cardiogenic shock in particular, TTE and TEE are an essential part of the diagnostic armoury.92
Existing guidelines:
The American Heart Association classifies the deployment of TTE in patients with hypotension or an unclear case of haemodynamic instability as ‘Appropriate’, with the highest grade of recommendation (A9).91
The S3 guideline ‘Treatment of multiple trauma/seriously-injured patients’ states that echocardiography should be performed in haemodynamically unstable patients with multiple traumas for the diagnosis of pericardial tamponade or rupture. The guideline recommends TTE as the method of choice. (http://www.awmf.org/leitlinien/detail/ll/012-019.html).
The S3 guideline ‘Postinfarction cardiogenic shock: diagnosis, monitoring and therapy’ states that TTE is essential for patients with post-infarction cardiogenic shock and should be performed as soon as possible following patient admission. Data obtained by echocardiography helps to assess the global and regional pump and valve function of the left and right ventricle, and to detect acute complications of a myocardial infarction, such as free wall rupture, ventricular septal defect or papillary muscle rupture. (http://www.awmf.org/leitlinien/detail/ll/019-013.html).
The S3 guideline ‘Provision of intensive care medicine to cardiosurgical patients: haemodynamic monitoring and cardiovascular therapy’ recommends echocardiography to confirm diagnosis in the perioperative period for patients exhibiting acute and sustained haemodynamic disorders who do not respond to initial treatment, and for those whose ventricular function and its determinants are unclear. The guideline recommends this as a prudent step that improves the clinical outcome (GoR B). (http://www.awmf.org/leitlinien/detail/ll/001-016.html).
Box 11.

no caption available
Background to recommendation 1-11
As a general rule, bedside determination of the size of the IVC directly underneath the diaphragm can help to diagnose hypervolaemia or hypovolaemia.8,21,28 The diameter of the IVC is influenced by the respiratory cycle, blood volume and right heart function. It must be remembered that sonographic determination of the size of the vena cava is an indirect indicator of CVP and is thus subject to the same limitations.
In a study investigating 20 septic ventilated ICU patients, responders and nonresponders were classified according to cardiac output increase following a volume bolus.20 In contrast to CVP, a vena cava distensibility index (respiratory change of IVC diameter/minimum diameter of IVC) of 18% was able to differentiate responders and nonresponders with a sensitivity and specificity of 90%.
Most of the older studies were conducted on ventilated patients with sepsis but a meta-analysis published in 2012 identified five prospective studies that investigated the diameter of the IVC in assessment of volume status in spontaneously breathing patients. For hypovolaemic patients, the maximum diameter of the vein was significantly smaller than in those who were euvolaemic (average difference; 95% CI 6.3 mm, 6.0 to 6.5 mm).93
Overall, there is a moderate body of evidence suggesting that the vein has a smaller diameter in hypovolaemia than with euvolaemia but there are no major multicentre studies that examine this key issue. Sonography of the vena cava is a simple procedure that can be performed rapidly and noninvasively. The examination can be combined with TTE. Although the present studies vary in terms of their diagnostic threshold values, the following values can be given as a general reference:
IVC diameter less than 10 mm = hypovolaemia likely
IVC diameter more than 22 mm = hypervolaemia likely (other causes also possible).
Hypovolaemia is also frequently indicated by strong variation in the vein diameter over the respiratory cycle.
Chapter 2: Therapy during the fasting phase
Box 12.

no caption available
Background to statement S-2
In recent years, interest in the fast-track model has greatly increased attention on volume therapy in the pre-interventional fasting phase. Despite this, the systematic literature search conducted for the present Guideline discovered only a few studies of its use in fast-track surgery. Accordingly, an evidence-based assessment of volume therapy in the fasting phase is not possible on the basis of the publications referenced here. Note that the above statement does not contradict the positive results obtained by the fast-track model. The scope of the fast-track model encompasses much more than preoperative fluid therapy, however, and the effects of fluid therapy have not been investigated separately. In the context of the present Guideline, therefore, the correct methodological approach is to derive no recommendations on preinterventional fluid therapy from these studies.
Box 13.

no caption available
Background to recommendation 2-1
As already discussed above, it is virtually impossible to derive evidence-based statements on preoperative fluid therapy from the systematic literature search for this Guideline. No direct evidence of the effects on mortality is available.52 Isolated data supporting a lower postoperative nausea and vomiting rate because of preoperative fluid substitution,94 a lesser degree of dehydration during preoperative bowel preparation,97 and assessments of liver blood flow and other haemodynamic variables95 are found in the literature but do not favour a strong recommendation.
However, it seems eminently reasonable to state that existing deficits should be rectified promptly; preinterventional fluid therapy is considered prudent in the event of preinterventional deficit. In this context one must consider that all studies on goal-oriented haemodynamic therapy, preload optimisation via PLR, or volume expansion, ultimately aim to compensate for a difference between the actual state and an optimum state – the rectification of a deficit. The generally positive results obtained by these studies and the absence of any information concerning the beneficial effects of delayed treatment have been evaluated by the Guideline group as strong indirect evidence for a prompt rectification of existing deficits.
Chapter 3: Differences between peri-interventional and ICU patients
Referenced literature:94,98,99
The key questions of Chapter 3 were withdrawn, as they were not clinically relevant in their present form. The relevant questions will be answered for their specific patient groups in the chapters below.
Chapter 4a: Differences between colloids and crystalloids in peri-interventional patients
Referenced literature:33,98,100–147
Box 14.

no caption available
Background to statement S-3
No evidence was found for renal insufficiency associated with peri-interventional administration of colloids, HES, albumin and gelatin as volume substitutes in contrast to crystalloids as a volume substitute. The consensus statement is based substantially on the most recent review available for this key issue: this review includes data on renal insufficiency from a total of 17 clinical trials.126
Box 15.

no caption available
Background to recommendation 4a-1:
The administration of 6% HES 130 and gelatin as colloids in contrast to crystalloids as a volume substitute was systematically reviewed in two meta-analyses. Gattas et al.98 analysed 1608 patients from 25 studies, and Martin et al.126 analysed 1230 patients from 17 studies. Both systematic reviews were able to show that there is currently no demonstrable association between the administration of HES 130/0.4 and gelatin and greater morbidity or mortality. In particular, no changes in serum creatinine and calculated creatinine clearance or in the incidence of acute renal failure were found to be present in the peri-interventional context. One limitation is that patients were followed up only for short periods in the present studies.
Box 16.

no caption available
Background to recommendation 4a-2
The recommendation to use balanced solutions was substantially influenced by a series of endpoints classified as surrogate and supported in particular by the association – considered to be well substantiated – of unbalanced solutions and hyperchloraemia and acidosis, with adverse effects on mortality and other endpoints such as infection and renal insufficiency.143,148–150 These studies analysed the differential effects of balanced versus unbalanced solutions in general, for both crystalloid and colloid solutions.
The grade of recommendation (GoR B) reflects the fact that, in terms of the mortality endpoint, the studies and patient numbers available for analysis are exceptionally low. Accordingly, no adequately corroborated statements can be made in this context, and the recommendation for using balanced solutions is therefore based on the summary analyses of the other endpoints beyond that of mortality.
Box 17.

no caption available
Background to recommendation 4a-3
For this key issue, there are a large number of randomised controlled studies that use a variety of discrete study designs to investigate a small number of patients.62–65 Accordingly, no statement on patient-relevant endpoints can be derived from the studies. An additional limiting factor to be considered when making any comparison is that deciding on the most suitable ‘volume therapy’ – particularly when taking the ‘patient population, intervention, comparitor, outcome’ principle into account – cannot be considered within a properly defined goal. In light of the pronounced weakness of the available data, there are no grounds for assuming that the equivalence of the infusions is proven, despite the absence of a difference in mortality. Consequently the Guideline group achieved consensus on a statement that clarifies this circumstance and offers a ‘may’ recommendation (GoR 0) for the consideration of available synthetic colloids as equivalents for preloading before spinal anaesthesia to optimise intraoperative haemodynamic values.
Box 18.

no caption available
Background to recommendation 4a-4
Pregnant and breastfeeding women present a special situation in which potential benefits for the mother must be balanced against risks for the fetus. These considerations are properly taken into account by this recommendation, which operates on the basis that no data on the fetal transfer of synthetic or natural colloids are available, and a potential risk for the fetus cannot be safely ruled out. Studies are both feasible and necessary for this situation.
Specific indications – such as preemptive volume therapy during secondary caesarean section under epidural anaesthesia – were discussed at length by the Guideline group. No consensus was reached on a recommendation, however, because of a lack of evidence.
Chapter 4b: Differences between colloids and crystalloids in ICU patients
Referenced literature:4,98,111,151–170
NB: ICU patients within the meaning of this guideline are either critically ill with severe acute (or acute on chronic) organ dysfunction or organ failure, or are free from organ dysfunction but cannot be monitored outside an ICU for other reasons.
Box 19.

no caption available
Background to statement S-4
The administration of HES to critically ill ICU patients has been investigated by multiple randomised controlled trials.156,164,166 A significant correlation is seen between the administration of HES and the need to provide renal replacement therapy.111 In the 6S study, the administration of HES was associated with a negative outcome – in terms of mortality and renal insufficiency – compared to crystalloids.166 No difference in mortality was established by the CHEST study, which investigated 7000 patients.164 As a result, the EMA convened a pharmacovigilance risk assessment in 2013, which classified the use of HES in critically ill patients as contra-indicated.
The limitation of those studies that report an adverse outcome following treatment with HES is related solely to the method used for the administration of HES (see below) and not to the HES itself. There are also data from a randomised controlled multicentre trial,151 which demonstrated a benefit because of colloids, including HES, in terms of 90-day mortality. As the primary trial objective had been defined as a difference in 28-day mortality, the study returned a negative outcome. A key difference to earlier studies was that patients had been enrolled immediately without a declaration of consent being given by relatives or carers. Accordingly, this study was the first study capable of documenting the initial septic shock phase.
The trial protocol of the VISEP study156 permits no insights into the decisive early phase of treatment of patients with septic shock, because they could be enrolled up to 24 h after diagnosis on the regular ward and up to 12 h on the ICU. Within this period, the patients were given a maximum of 1000 ml of artificial colloids, including HES solutions. In the 12 h before study enrolment, 160/275 patients in the Ringer's lactate group and 155/262 patients in the HES group received median volumes of 700 ml (interquartile range: 500–1000) and 979 ml (interquartile range: 500–1000) of colloids respectively, including HES and gelatin, and in addition to crystalloid volume substitute. This resulted in over 80% of patients being haemodynamically stabilised before registration in the study (mean arterial pressure >65 mmHg, ScvO2 > 70%, CVP > 8 mmHg). In the two ‘clinical/pragmatic’ trials, 6S166 and CHEST,164 no protocol was provided for the indication, monitoring and management of volume therapy. Nor was there any algorithm for assessing the fluid response of patients (primarily clinical assessment, based on Surviving Sepsis Campaign criteria). In both studies, the contraindications relevant for patients with renal insufficiency, as listed in the Surviving Sepsis Campaign, were disregarded and, in addition, the maximum dose recommended for the HES solution was considerably exceeded by the VISEP study. Despite their methodological limitations, the results of these studies are nonetheless important. They highlight the fact that the cumulative dose of the colloid is clearly of great importance for patients suffering from severe sepsis and septic shock, and that the ‘pragmatic use’ of HES cannot be considered to be safe following initial stabilisation for septic patients not in shock.
A meta-analysis of 30 studies with 2700 patients revealed that the use of gelatin compared to crystalloids/HES/albumin is not associated with elevated risks of mortality or renal insufficiency.170
Whether or not the use of colloids in intensive care medicine is important for the safe and rapid haemodynamic stabilisation of critically ill patients in shock within the first 6 h cannot be established on the basis of the available data. It should be noted that the targets for the indication and management of volume therapy, for which a consensus was achieved in this Guideline, have not been considered by any study. The results of a randomised controlled pilot study suggest that, in patients with sepsis-induced hypoperfusion, the microcirculatory recruitment achieved by early goal-oriented treatment is improved by the use of HES compared to the use of 0.9% sodium chloride (NaCl).158
In light of the above, the Guideline group achieved consensus on a statement that clarifies this circumstance and advocates the performance of randomised studies that contrast colloid delivery (6% HES 130/gelatin/albumin) with crystalloid delivery in critically ill patients. The group calls for the consideration of immediate study enrolment and the application of the measures and targets agreed in this Guideline for the indication and management of volume therapy.
Box 20.

no caption available
Background to recommendations 4b-1, 4b-2, 4b-3
In several randomised controlled trials, crystalloids given to critically ill ICU patients were associated with improved survival (6S)111,166 and fewer instances of renal insufficiency (VISEP/CHEST)156,164 compared to HES.
Despite the methodological limitations of these studies, which are reflected in the GoR B, crystalloids were not associated with greater side effects. Accordingly, consensus was achieved by the Guideline group for this general recommendation.
The administration of HES to critically ill ICU patients has been investigated by multiple randomised controlled trials (VISEP, 6S and CHEST).156,164,166 A significant correlation is seen between the administration of HES and the need to provide renal replacement therapy.111 In the 6S166 study, the administration of HES was associated with a negative outcome in terms of mortality and renal insufficiency compared to crystalloids.
Accordingly, the use of HES in critically ill ICU patients is not recommended (high GoR).
In contrast to the above, one randomised controlled multicentre trial (CRISTAL)151 demonstrated a benefit from colloids, including HES, in terms of the 90-day mortality. As the primary trial objective had been defined as a difference in 28-day mortality, the study returned a negative outcome.
A meta-analysis of 30 studies with 2700 patients also revealed that the use of gelatins compared to crystalloids/HES/albumin is not associated with increased risks of mortality or renal insufficiency.170 In a randomised controlled trial, the administration of gelatin compared to 6% HES 200/0.62 was associated with lower renal insufficiency. In an Australian study investigating septic patients, 4% albumin was not significantly (P = 0.09) better than 0.9% NaCl.159
In intensive care medicine, the rapid haemodynamic stabilisation of shocked patients within the first 6 h is important to outcome. As the CRISTAL study demonstrated a significant benefit in outcome with the use of colloids after 90 days, the third recommendation supports supplementary colloidal volume substitution with gelatin or albumin for critically ill patients in intensive care medicine.
A randomised controlled pilot study investigating critically ill patients with abdominal compartment syndrome showed that colloidal solutions in volume therapy were associated with a positive influence on a series of endpoints classified as surrogate, and that their use could therefore be considered.157 However, the reported benefits have not been adequately validated by study data.
Box 21.

no caption available
Background to recommendation 4b-4
The recommendation to use balanced solutions was substantially influenced by a series of endpoints classified as surrogate that were supported by the well substantiated association between unbalanced solutions and hyperchloraemia and acidosis, with adverse effects on mortality, infection and renal insufficiency.143,148,150,168,171
These studies analysed in the intervention arms investigated the differential effects of balanced versus unbalanced crystalloid and colloid solutions in general.
The grade of recommendation (GoR B) reflects the fact that, in terms of the mortality endpoint, the studies and patient numbers available for analysis are exceptionally low. Accordingly, no adequately corroborated statements can be made in this context, and the recommendation for using balanced solutions is therefore based on the summary analyses of the other endpoints beyond that of mortality.
Chapter 5a: Differences between colloids in peri-interventional patients
Referenced literature:98–100,111,115,121,126,130,133,135,138,140,141,143,160,172–209
Box 22.

no caption available
Background to statement S-5 and recommendation 5a-1
No significant differences were found between the colloids investigated, HES, albumin and gelatin, in terms of the mortality associated with these volume substitution solutions. The conclusion reached is based substantially on the most comprehensive review available for this key issue, which includes data on mortality from a total of 57 clinical trials.179
In light of the pronounced weakness of the available data, there are no grounds for assuming the proven equivalence of the solutions, despite the absence of a difference in mortality. Consequently, the Guideline group achieved consensus on a statement that clarifies this circumstance without a grade of recommendation, offering a ‘may’ recommendation (GoR 0) for the consideration of available synthetic and natural colloids as equivalents, insofar as their use is indicated.
In a primarily perioperative context, and for the treatment of volume depletion, the data available to date offer no indication of a benefit for one of the above-mentioned colloidal solutions (group effect) in terms of efficacy or the potential for side-effects.
Another aspect requiring consideration is that the conclusions drawn are for the effects from all preparations within a group, making them potentially inadmissible for individual preparations. It is not surprising that considerably less data from randomised controlled trials are available to support comparisons of this kind. As differences in mortality are also generally absent, no reliable conclusions can be drawn.
Box 23.

no caption available
Background to recommendation 5a-2
No reliable conclusions can be drawn for the differential use of colloidal volume substitution in a perioperative context if the endpoint is mortality. Consensus has been achieved for a strong recommendation that additional endpoints such as allergenic potential should be used as a basis for decision-making about the solution for colloidal volume substitution, alongside logistical and financial aspects (storage, mode of delivery and documentation effort).
The recommendation, with a strong grade of recommendation, supports both general (for specific operative areas and care units) and case-by-case (individual patient management) decision-making for or against a specific group or a specific preparation for colloidal volume substitution in a perioperative context. Explicit reference is made to the fact that other regulatory frameworks need to be properly accounted for by such decision-making. These include the provisions of the German Transfusion Act, the ‘Guidelines for the Preparation of Blood and Blood Components and the Use of Blood Components (Haemotherapy)’ and recommendations made by the German Medical Association, such as ‘Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives’.
Aside from the price, which has strong regional variations and, in the authors’ opinion, should be assigned secondary importance at most when there are pertinent advantages for use (documentation effort and storage), the organisational and logistical aspects of handling different solutions have a strong bearing on convenience of use and ultimately choice in routine clinical practice. One example of this is the batch documentation necessary for specific preparations that can constitute a serious logistical disadvantage for certain operational units. A lack of compressibility in the container in which the volume substitution solution is provided can be counteracted by timely delivery for volume substitution, and thus the achievement of rapid haemodynamic stabilisation.
Box 24.
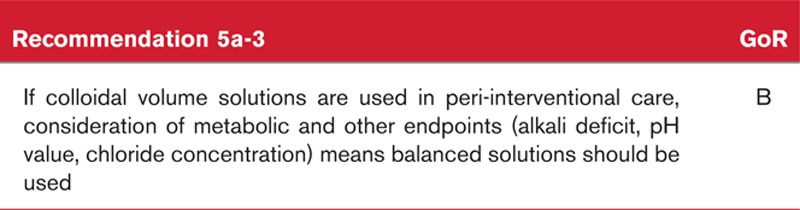
no caption available
Background to recommendation 5a-3
The conclusion reached is based substantially on the most comprehensive review available for this key issue. This review includes 14 publications from a total of 13 clinical trials.180
The analysis considered the differential effects of balanced versus unbalanced solutions in general, and therefore included both crystalloid and colloidal solutions in the intervention arms investigated. Notwithstanding this fact, the effects are nonetheless seen in the endpoints investigated, with the exclusion of the intervention studies in which a specific crystalloid or colloidal volume substitute was used.
The grade of recommendation (GoR B) reflects that, in terms of the mortality endpoint, the studies and patient numbers available for analysis are exceptionally low. Accordingly, no adequately corroborated statements can be made in this context, and the recommendation for using balanced solutions is therefore based on the summary analyses of other endpoints beyond that of mortality.
The recommendation to use balanced solutions was influenced substantially by a series of endpoints classified as surrogate and also supported in particular by the well substantiated association between unbalanced solutions and hyperchloraemia and acidosis, with adverse effects on mortality and other endpoints such as infection and renal insufficiency.148–150,171
Box 25.

no caption available
Background to recommendation 5a-4
Alongside legal, medical (transfusion), organisational, logistic and economic considerations, this recommendation also properly accounts for the differences between the various colloids of natural or synthetic origin in their potential to cause adverse side-effects. Although such considerations need not mean that one or other of these colloids is generally preferable in a peri-interventional context, their use in specific cases must nevertheless be properly accounted for. One example is HES, which is contraindicated in patients with limited renal function or where renal replacement therapy is required. In addition, the presence of an allergic disposition may be seen as a relative contraindication to the use of preparations containing gelatin.210 Secondary considerations such as the presence of hypoalbuminaemia in the context of a volume deficit can tip the scales in favour of the use of a specific volume substitute (such as albumin), even if the hypoalbuminaemia does not itself actually require compensation.
For a specific case, such information and recommendations can, because of a multitude of conceivable circumstances and a lack of evidence from clinical research for the specific situation, be considered as secondary decision-making criteria at best. For this reason the Guideline group necessarily and explicitly takes into account the fact that adequate study data will never be available to substantiate all of the circumstances possible, and hence that there are good reasons for an approach or methodology orientated toward the pathophysiology concerned.
Chapter 5b: Differences between various colloids in ICU patients
Referenced literature:4,6,7,86,98,99,111,152–154,163,170,171,179,209,211–213
Box 26.

no caption available
Background to recommendation 5b-1
For patients with a severe traumatic brain injury, volume therapy with 4% albumin was associated with significantly higher 24-month mortality (RR 1.88; 95% CI 1.31–2.70). The figures are derived from posthoc analysis of 460 patients from a subgroup analysis of the SAFE study.214 The point in question is the influence of hypo-osmolar albumin on the development of a vasogenic and/or cytotoxic cerebral oedema, and whether the effect is substance-specific or osmolar in nature cannot be determined from the data currently available. Nevertheless, there is a general consensus that the use of 4% albumin cannot be recommended at present for traumatic brain injury patients. Additional clinical studies are needed to enable further progress.
Chapter 6a: Differences between crystalloids in peri-interventional patients
Referenced literature:95,105,121,127,130,140,146,176,180,193,201,215–243
Box 27.

no caption available
Background to statement S-6 and recommendation 6a-1
Significant differences between various crystalloid solutions in relation to their associated mortality following peri-interventional administration can neither be proven nor safely excluded. The conclusion reached is based substantially on the most comprehensive review available for this key issue. It includes 14 publications from a total of 13 clinical trials.180
The analysis considered the differential effects of balanced versus unbalanced solutions in general, and therefore also included colloidal solutions in the intervention arms investigated. Nonetheless, the effects such as comparable outcome in terms of mortality are demonstrated in the endpoints investigated but with the exclusion of the intervention studies where a colloidal volume substitute was used. The conclusions drawn concerning the endpoints of mortality and morbidity properly take into account the fact that the numbers of studies or patients that are still available after applying this premise (exclusion of studies with colloidal solutions in the intervention arms) are vanishingly small. Accordingly, no sufficiently well founded statements can be made.
Despite this, the members of the consensus conference unanimously agreed (100% agreement with the recommendation) that a nonphysiological solution, namely isotonic saline, must not be used for planned peri-interventional volume substitution.
Box 28.

no caption available
Background to recommendation 6a-2
As noted above with regard to colloidal volume substitution, the recommendation to use balanced solutions was influenced substantially by a series of endpoints classified as surrogate and supported in particular by the well substantiated association between unbalanced solutions and hyperchloraemia, which is increasingly observed following the use of an isotonic saline, with adverse effects on mortality and other endpoints such as infection, renal insufficiency and acid-base balance.143,148,150,171
The available evidence supporting the use of balanced crystalloid solutions as a factor influencing acid-base balance is more comprehensive than for the endpoint of mortality. The data show that an unbalanced crystalloid solution (0.9% NaCl) produced a lower pH value, plus a larger alkali deficit and higher sodium and chloride values.
Despite this, it is too early to speak of an actual clinical superiority. Nevertheless the known negative effects of hyperchloraemic acidosis and its association with unfavourable outcome necessitate a recommendation in favour of the use of balanced solutions for volume substitution in a perioperative context. This also implies that, in the event of balanced solutions being unavailable, isotonic saline may exceptionally be given for volume substitution. The members of the consensus conference voiced a strong recommendation (80% agreement for GoR A) for the use and stockpiling of balanced solutions in operational units that are regularly involved in the provision of infusion therapy.
The recommendation to use ‘balanced solutions’ references the group effects found in the meta-analysis performed by Burdett et al. for all preparations within an entire group (’balanced crystalloid solution’ versus ‘unbalanced crystalloid solution’). Potentially, these recommendations may not fully reflect the actual effects of individual preparations.81 It is not surprising that little or no data at all (e.g. for use in distinguishing between mortality) are available to permit a reliable statement to be made on the superiority of individual solutions. However, it may be currently assumed that, in the absence of significant differences in clinical outcome (mortality) between balanced crystalloid solutions compared to unbalanced crystalloid solutions (first and foremost 0.9% NaCl), it is improbable that differences between specific balanced crystalloid solutions will be significant and hence can be considered negligible in the context of the data.
The recommendation does not take into account potential indications for the use of hypertonic NaCl solutions. Solutions of this kind are also used in a perioperative context, but in a limited manner (614 patients in 15 studies).82 Numbers are too small to permit comment on the incidence of mortality and harm, but hypertonic NaCl may have a place in the presence of an elevated serum sodium level, although overall perioperative demand is low. As a consequence the extent of the role of general or individual use of hypertonic solutions in peri-interventional volume therapy outside specialised indications (small volume resuscitation) must remain unresolved.
Box 29.

no caption available
Background to recommendation 6a-3
Because studies are few and the solutions used heterogeneous, no information is available to support the association of specific balanced infusion solutions with improved postoperative (surrogate) outcome.
Currently this applies in particular to balanced infusions containing anions able to enter the metabolic pool (acetate and malate). Despite the advantage of not inducing hyperchloraemia and in the absence of interference with lactate values when used as a diagnostic marker (surrogate endpoint), an effect on outcome is not guaranteed. Because these solutions are administered in a balanced form, a similar effect to balanced crystalloid infusions must be assumed. Conscious of the lack of clinical data on superiority, the consensus process resulted in a weak recommendation to ‘consider’ these solutions (GoR 0), with strong level of agreement.
Chapter 6b: Differences between crystalloids in ICU Patients
Referenced literature:162,168,171,244–248
Box 30.
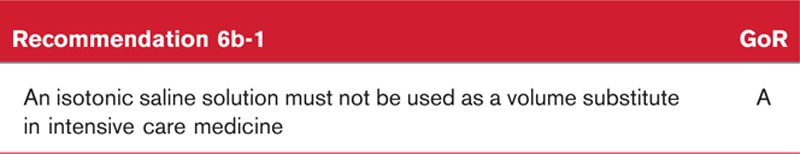
no caption available
Background to recommendation 6b-1
In comparison to isotonic saline, the use of balanced solutions is associated with a significantly lower incidence of hyperchloraemic acidosis within the first 48 h following the start of treatment (hazard ratio 0.28; 95% CI 0.11, 0.70; P = 0.006).168 It has been postulated that hyperchloraemic acidosis influences haemostasis and also gastrointestinal and cognitive function. A prospective open-label pilot study investigating 760 patients who were treated either with chloride-rich or chloride-poor solutions (balanced solutions or chloride-poor 20% albumin) was able to show that treatment with chloride-rich solutions was associated with a significantly higher serum creatinine level (P = 0.03), an increase in the incidence of renal insufficiency (P < 0.001) and a greater need for renal replacement therapy (P = 0.004).171 Based on the available data, isotonic saline solutions must no longer be used for volume therapy in intensive care medicine. Instead, balanced isotonic electrolyte solutions must be used as a volume substitute for critically ill ICU patients.
Box 31.

no caption available
Background to recommendation 6b-2
As the use of balanced isotonic electrolyte solutions is recommended both for hospitalised adults receiving peri-interventional treatment and for critically ill ICU patients, we refer to the background information provided for the consensus recommendation 6a-2.
Box 32.

no caption available
Background to recommendation 6b-3
Please refer to the background information for the 3rd consensus recommendation 6a-3.
Chapter 7a: Management of volume therapy in peri-interventional patients
Referenced literature:8,11,13–24,30,32–35,38,40,50,53,56,58,59,61,63–65,68–71,73–76,87,101,123,249–263
Box 33.
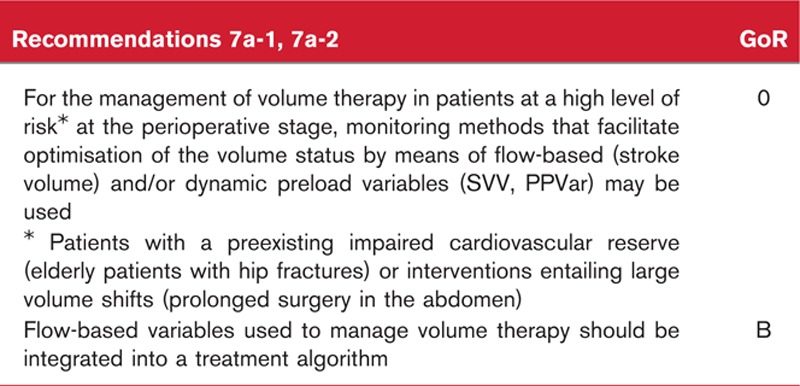
no caption available
Background to recommendations 7a-1 and 7a-2
Although study quality is dubious, the available data on the use of flow-based (stroke volume and cardiac output) or dynamic preload variables (SVV and PPVar) for perioperative improvement of volume status through increasing DO2 to the periphery, suggest that a favourable effect on (combined endpoints for) morbidity is possible. It is difficult to issue a statement on the value of volume status optimisation in isolation because the impact of preload changes (in accordance with the Frank–Starling mechanism) is confounded by the administration of inotropic agents. Mortality in the context of the (elective) procedures investigated is low overall and statements addressing the impact on mortality are not possible. Peri-interventional optimisation of the volume status with the aim of raising oxygen availability is unlikely to have an adverse influence on clinical endpoints.
On the basis of available data, no statement can be made on the application of surrogate markers of haemodynamics to fluid therapy.
A recently conducted review of the perioperative optimisation of blood flow and oxygen transport included 31 studies with a total of 5292 participants.264 The period of study started 24 h before and ended 6 h after surgery. Haemodynamic targets included cardiac output, the cardiac index, DO2 and DO2I, VO2 and VO2I, the stroke volume and stroke volume index, ScvO2, oxygen extraction rate and lactate. The review did not consider the importance and validity of the chosen haemodynamic targets. Treatment with vasoactive inotropic agents or volume did not achieve any reduction in mortality. The length of hospital stay was reduced by 1 day, however.
The optimisation of fluid delivery according to SVV was investigated in a randomised study looking at elective abdominal surgery.250 When SVV exceeded 10%, 3 ml kgBW−1 of a colloidal volume substitute was administered. Dobutamine was given where the cardiac index was less than 2.5 l min−1 m−2, so as to place it in a target range between 2.5 and 4 l min−1 m−2. Patients in the SVV group (n = 60) received a greater intra-operative volume than the control group (n = 60). In the SVV group fewer hypotensive events were registered; the lactate level was also lower toward the end of the operation. Complications occurred significantly more often in the control group (58.3 vs. 30%, P = 0.0033).
In female patients with severe eclampsia, PLR with simultaneous determination of the velocity time integral of subaortic blood flow via Doppler echocardiography permitted a reliable estimate to be made of volume responsiveness.253
Intra-operative use of oesophageal Doppler measurement of aortic blood flow was investigated by systematic review249 which included five studies with 420 patients receiving a major abdominal surgical intervention. FTc in the descending aorta was used to optimise volume with a target value of more than 350 ms. The intervention group had a shorter hospital stay, fewer complications and fewer ICU admissions.
Patients monitored with oesophageal Doppler receiving major abdominal surgery, with volume therapy managed by means of FTc in the descending aorta, had shorter hospital stays and a more rapid restoration of bowel function.257
Total 90 patients were monitored during surgery with oesophageal Doppler;34 respiratory SVV proved to be an excellent tool for predicting volume responsiveness (AUC value for the ROC curve 0.91) but FTc did not permit any reliable estimates to be made (AUC value for the ROC curve 0.49).
In all 40 patients (with preserved left ventricular pump function) were investigated during a bypass operation (off-pump).35 SVV and PPVar were good predictors of volume responsiveness (AUC value for the ROC curve 0.823 and 0.808) but CVP, PAOP, the left ventricular end-diastolic area index (determined using echocardiography) and GEDVI did not permit any reliable estimates to be made.
Use of PPVar in surgical high-risk patients to estimate volume responsiveness (goal of volume therapy = PPVar <10%) reduced the length of hospital stay and the number of complications in the intervention group.123
A systematic Cochrane Review analysed the significance of volume therapy optimisation in patients receiving surgery following a proximal femoral fracture265 but only three studies with a total of 200 patients were admissible. Studies that failed to monitor haemodynamic status of the intervention group with transoesophageal Doppler or pulse contour analysis were excluded. No relevant benefits for protocol-based haemodynamic optimisation of fluid therapy were found, but the numbers studied may have been too small for a definitive conclusion.
A systematic review investigated the significance of perioperative goal-oriented haemodynamic optimisation through volume loading during surgical procedures. A total of 24 studies were included in the review. The patient groups, the monitoring procedure used and even the target variables for the respective protocols reveal a high degree of variation overall.262 Despite this there was a reduction in postoperative renal damage [Odds ratio (OR) 0.59].
We may draw the following conclusions:
The patient groups investigated are highly heterogeneous.
The protocols deployed and the target variables used to estimate volume responsiveness are also highly heterogeneous.
Pressure-based static variables such as CVP and/or PAOP are not suitable for estimation of volume responsiveness.
SVV and PPVar are suitable for the estimation of volume responsiveness.
Oesophageal Doppler offers an option for the continuous monitoring of the volume status.
Currently there are no binding protocols available for volume therapy management. However, perioperative volume therapy should be guided by treatment algorithms that primarily use flow-based target variables for haemodynamic optimisation.
Chapter 7b: Management of volume therapy in ICU patients
Referenced literature:9,10,25–29,31,36,37,39,41–49,51,52,54,55,57,62,66,67,72,75,76,266,267
Box 34.

no caption available
Background to recommendation 7b-1
Clinical examination attempts
To determine whether the symptoms are being caused by hypovolaemia;
And if so, to determine the degree of hypovolaemia.77
Examination of skin turgidity assesses the elastic recoil of the skin, which is pulled up between finger and thumb. The protein elastin is primarily responsible for this recoil and is generally determined by the moisture content of the skin.268 A moisture loss of only 3.4% (by weight) prolongs the recoil of the skin after being pinched by a factor of 40. Elastin degradation also occurs with advanced age. Skin turgidity therefore decreases with age. Normal skin turgidity has never been scientifically assessed; nor are there any studies that describe the exact technique that should be used to determine it.77
Cellular dehydration, interstitial fluid depletion and poor perfusion are responsible for a multitude of other clinically accepted signs of hypovolaemia. These signs include a dry tongue, a dry axilla and soft eyeballs. Only a handful of studies are available that describe the precision of these clinical signs as evidence of hypovolaemia. The finding of a dry axilla increases the likelihood of hypovolaemia (positive OR 2.8; 95% CI 1.4 to 5.4), even though sensitivity is only 50%,269 and the finding of a moist axilla weakens this likelihood but only minimally (negative OR 0.6; 95% CI 0.4 to 1.0).
In a study investigating 55 older patients with suspected hypovolaemia, seven clinical signs (confusion, weakness, slurred speech, dry mucous membranes, dry tongue, furrowed tongue and sunken eyeballs) correlated best with serum sodium and the serum urea to creatinine ratio.270 The absence of dry mucous membranes, sunken eyeballs and a furrowed tongue are the best indicators for the absence of hypovolaemia.
Despite a lack of high-quality evidence, a thorough physical examination should always be performed, and the findings obtained should be critically cross-checked with the results of instrument-based examination and invasive haemodynamic monitoring.
Box 35.

no caption available
Background to recommendations 7b-2, 7b-3, 7b-4
Despite the lack of scientifically acceptable evidence, CVP continues to be deployed in the management of volume therapy. As recently as 2008, a meta-analysis demonstrated the inadequate agreement of CVP with volume status. Changes in CVP did not correlate with changes in stroke volume following defined volume loading (volume challenge).271 The AUC value for the ROC curve was 0.56. Recently, the significance of CVP in a broad range of clinical scenarios was once again investigated by a meta-analysis of 43 studies.79 Of these, 22 studies concerned intensive care patients, 20 studies analysed the use of CVP within operative monitoring and one study was conducted with study participants. To satisfy the inclusion criteria, a study must have investigated CVP as a predictor of volume responsiveness, which was defined as an increase in cardiac output and stroke volume following a volume challenge or after PLR. Most studies defined volume responsiveness as an increase in cardiac index or the stroke volume index by at least 15%. The volume challenge consisted of 500 ml of intravenous fluid (typically HES). Data for the ROC were available in 20 studies. Overall, 57% ± 13% of patients were volume-responsive. The average CVP when measured initially was 8.2 ± 2.3 mmHg in the volume-responsive group and 9.5 ± 2.2 mmHg in non-volume responders. The AUC value for the ROC curve was 0.56 (95% CI 0.54; 0.58). No difference was seen between intensive care and operating theatre patients. The same results were also obtained for cardiac surgery and noncardiac surgery patients. In all groups, there was no correlation of the initial CVP measurement with the change in cardiac index/stroke volume. Neither CVP nor PAOP correlate with actual cardiac preload, nor was there any linear relationship. CVP is typically measured as the difference between the intravascular space and atmospheric pressure. For ventricular filling, however, transmural pressure – the difference between intra-ventricular and pericardial pressure – is the more relevant index. Intrapericardial pressure is not available under clinical conditions, however. Accordingly, the true transmural pressure remains unknown and the pressure value measured against atmospheric pressure is routinely used as a surrogate for the filling pressure. In the special case of ventilated patients receiving either intermittent positive pressure ventilation or positive endexpiratory pressure, CVP will actually increase without any concomitant increase in end-diastolic ventricular volume.272
CVP also depends on the intravascular volume and peripheral vascular tone, right ventricular compliance and pulmonary vascular resistance. Misleadingly high values can occur during treatment with vasopressors. The use of vasopressor agents can result in a substantial increase in CVP without any change occurring in the intravascular volume. Clinically relevant tricuspid insufficiency can also interfere with measured values to a significant degree.272
A retrospective study investigated 96 ventilated patients with severe sepsis and septic shock.273 All patients were monitored by means of a pulmonary arterial catheter. CVP and PAOP were measured at the end of expiration. In addition, care was taken to ensure that the catheters were placed in West's zone 3. The indication for a volume challenge was defined as a clinical sign of acute circulatory collapse or signs of hypoperfusion. This required all patients to receive an infusion of 500 ml 6% HES over 20 min. An increase in the cardiac index of at least 15% was graded as a positive volume response (’responder’). Pre-infusion CVP was not significantly different in responders compared to nonresponders (8 ± 4 mmHg vs. 9 ± 4 mmHg), whereas pre-infusion PAOP was significantly lower in the responders (10 ± 4 mmHg vs. 11 ± 4 mmHg, P <0.05). A CVP of less than 8 mmHg had a positive predictive value of only 51%, whereas for a PAOP less than 11 mmHg it was only 54%. The combination of CVP and PAOP did not improve the prediction. Even in patients with a low stroke volume index of less than 30 ml m−2, the predictive power of CVP and PAOP for a positive volume response remained inadequate.
In healthy study participants, changes in CVP and PAOP before and after the administration of 3 l of 0.9% NaCl over 3 h did not correlate with changes in the left ventricular enddiastolic volume (index).98 Normal static pressure measurements of CVP and PAOP were entirely unable to reflect adequate filling of either the right or the left ventricle. A CVP of 9 mmHg corresponded to a right ventricular end-diastolic volume index between 50 and 90 ml m−2. Similar variability was exhibited by PAOP. Here, an left ventricular end-diastolic volume (index) between 50 and 80 ml m−2 was found for a PAOP of 11 mmHg.274 These data in healthy study participants and also in seriously ill patients and those with cardiovascular disorders in particular, demonstrate an unhelpful relationship between static preload variables and end-diastolic volume.
In 22 ventilated patients with adult respiratory distress syndrome receiving lung-protective ventilation therapy (low tidal volume and high positive end-expiratory pressure), various haemodynamic monitoring variables were investigated for their ability to predict volume responsiveness.28 Here, PPVar provided the best AUC value at 0.768 for the ROC curve (CVP = 0.429, PAOP = 0.187, GEDVI = 0.323, ITBVI = 0.323, SVV = 0.606).
In 42 patients with septic shock, the SVV was compared with PPVar.37 Both methods were comparatively well suited to predicting volume responsiveness. The AUC value for the ROC curve was 0.92 for SVV and 0.916 for PPVar. The optimum cut-off for SVV for predicting volume responsiveness was 10%; the value was 12% for PPVar.
In spontaneously breathing ICU patients, Doppler echocardiography measurements of cardiac output and stroke volume following PLR can be reliable indicators of hypovolaemia.42 An increase of cardiac output or stroke volume by more than 12% following PLR gave a good AUC value for the ROC curve of 0.89 and 0.90 in predicting volume responsiveness.
A systematic review investigated the significance of TTE for estimating volume responsiveness in critically ill patients.43 Included in this analysis were studies considering PLR and the determination of transaortic SVV, the impact of respiration on transaortic SVV, and the impact of respiration on IVC diameter. In all the studies included, the AUC value for the ROC curve was over 0.90: accordingly, we may assume that echocardiography measurement techniques offer an excellent means of predicting volume responsiveness.
In 40 ventilated patients with septic circulatory collapse, PPVar and SPV were well suited for use as predictors of volume responsiveness (AUC value for the ROC curve 0.98 and 0.91).44 CVP and PAOP were entirely unsuitable for use as predictors of volume responsiveness (AUC value for the ROC curve 0.51 and 0.40).
Box 36.

no caption available
Background to recommendation 7b-5
A systematic review43 investigated the ability of TTE to estimate volume responsiveness. This review excluded TEE studies. Out of 3183 possible studies, only eight studies were ultimately included in the systematic review. Of these, five studies investigated Doppler echocardiography measurement of transaortic SVV for estimating volume responsiveness following PLR. All studies demonstrated good sensitivity (77 to 100%) and specificity (88 to 99%) in predicting an increase in stroke volume or cardiac output by 10 to 15%. One study275 investigated volume responsiveness by measuring transaortic stroke volume variation as a value dependent on the inspiration and expiration of ventilated patients. Here, too, the sensitivity and specificity of a stroke volume variation of 9% were excellent (100 and 88%) for its use as a cut-off for predicting volume responsiveness.
Two further studies investigated the respiratory-based variation of the IVC diameter in ventilated patients. Maxima and minima for the respiratory-dependent diameter of the IVC were measured directly underneath its confluence with the hepatic vein. IVC distensibility was measured as a percentage index. The study from Barbier et al.8 chose a distensibility index of 18%, whereas 12% was chosen by Feissel et al.28 In the first study, sensitivity and specificity were 90%. In the second study, a variation in the diameter of the IVC of over 12% permitted the differentiation of volume-responsive patients with a positive predictive value of 93% and a negative predictive value of 92%.28
One limitation of the studies was the low number of enrolled patients. Also there was variation between the studies in terms of the quantity, nature and rapidity of infusion of the intravenous fluids.
In critically ill patients, methodological limitations also arise from unfavourable ultrasound conditions and inadequate expertise or experience on the part of the investigators.
Box 37.

no caption available
Background to recommendation 7b-6
No prospective studies are available that investigate the significance of detecting extravasations (e.g. pleura, abdomen, bowel or interstitium) in the course of evaluating a volume deficit or volume overload.
Box 38.
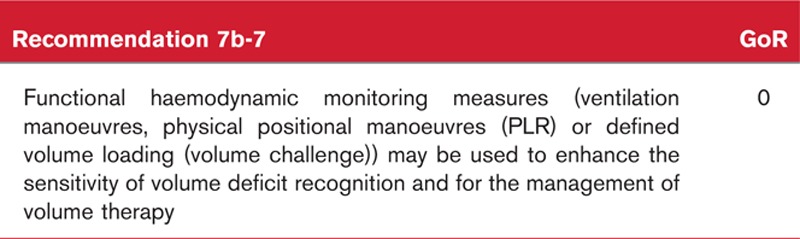
no caption available
Background to recommendation 7b-7
As a rule, the functional haemodynamic measures in the studies were supplemented by a volume challenge of 500 ml HES 6% over 30 min. Patients were defined as volume-responsive if stroke volume or the cardiac output (index) increased by 10 to 15%.
Changes in mean arterial pressure do not show good agreement with changes in the cardiac index after a defined volume challenge and are therefore unsuited for use as estimators of volume responsiveness.54
By using PLR with simultaneous measurement of the stroke volume with Doppler echocardiography, volume responsiveness in spontaneously breathing patients can be reliably predicted by an increase in stroke volume of over 12% during the physical body positional manoeuvre. The AUC value for the ROC is 0.90 ± 0.06 for the determination of stroke volume.42
Stroke volume, radial pulse pressure and the maximum velocity of blood flow in the femoral artery were measured in spontaneously breathing patients (n = 34) during a physical positional manoeuvre involving PLR.25 Volume responsiveness was predicted by a SVV of at least 10% with a sensitivity of 86% and a specificity of 90%, and by a PPVar of at least 9% with a sensitivity of 79% and a specificity of 85%, and by maximum blood flow in the femoral artery with a sensitivity of 86% and a specificity of 80%.
PPVar (threshold 13%) is a useful predictor of volume responsiveness in septic ventilated patients (n = 40) with circulatory collapse.44 Patients with a cardiac index of at least 15% compared to the initial value following a volume challenge were classified as responders. Measurement of haemodynamic variables took place immediately before and 30 min. after volume expansion with 500 ml HES 6%. Pulse pressure variation revealed excellent differentiation with an AUC value for the ROC curve of 0.98 ± 0.03. Sensitivity was 94% and specificity was 96% in predicting volume responsiveness. In this study, patients were ventilated with a tidal volume of 8 ml kgBW−1.
During ventilation with a lower tidal volume (6 to 7 ml kg−1 ideal body weight) respiratory pulse pressure variation is limited in its ability to differentiate when used as a predictor for volume responsiveness.51 A PPVar of at least 10% still shows reliable differentiation, with an AUC value for the ROC curve of 0.74 (95% CI: 0.56 to 0.90). In a further study investigating ventilated septic patients given lung-protective ventilation, pulse pressure variation was shown to be a reliable predictor of volume responsiveness. A cut-off of 6.5% had a sensitivity of 0.89 and a specificity of 0.90.31
In spontaneously breathing patients, PPVar and SVV have limited sensitivity, and are thus less able to differentiate when used as predictors of volume responsiveness.62 If the initial value is very high, however, and without simultaneous right ventricular dysfunction, volume responsiveness can also be predicted for spontaneously breathing patients.
Box 39.

no caption available
Background to recommendation 7b-8
This is an assessment made by the experts, and the assessment is not supported by evidence from relevant studies (inclusion criteria for the studies are given in Table 2).
Table 2.
Inclusion criteria for the retrieved references
| Indicator | Description |
| I1 | ≥80% of included patients were adults with intravascular (hypovolaemia) or interstitial (dehydration) fluid deficit in the context of any operative or interventional procedure or an intensive care treatment. They did not have chronic renal failure requiring dialysis. |
| I2 | Therapeutic intervention: intravascular fluid therapy with colloids (no dextrans) or crystalloids compared against each other or against placebo with regard to elimination or course of the fluid deficit and to patient-relevant endpoints1) or diagnostic intervention: diagnoses of hypovolaemia or dehydration by the use of specified criteria or diagnostic procedures1) |
| 1)Mortality/survival: ICU, hospital, specified period and risk adjusted. Morbidity: hypervolemia, organ failure, acid base disturbance, allergic reactions, coagulopathy, abdominal compartment, sepsis, pruritus and impaired wound healing. Quality of life. Surrogates: fluid balance; ventilator or vasopressor-free days; vasopressor dosage; volume, pressure, or flow-based variables; dynamic methods (SVV, passive leg raising, etc.); clinical signs of hypoperfusion; metabolic variables (ScvO2, lactate, etc.); microcirculatory parameters | |
| I3 | Article describes a randomized controlled trial, a quasi-randomised controlled trial, a clinical controlled trial, a prospective cohort study, a cross-sectional study or a systematic review/meta-analysis including the study types mentioned |
| I4 | Sample size: n ≥ 20 (n < 20 possible for clinical studies focussing on adverse events) |
| I5 | The reference was published between 1995 and the day of the literature search |
| I6 | The article is written in English or German |
| I7 | The reference is not an additional publication without additional infomation |
| I8 | The full-text study can be acquired |
| I9 | The infusion used is approved for use in Germany (including off label use) |
| I10 | The article was not retracted and the verisimilitude is not doubted. In systematic reviews, retracted or dubious studies can be separated |
| I11 | The article allows comparison of the study with others with regard to methodology, reporting, and internal validity |
A reference that violated a single criterion was excluded.
ScvO2, central venous oxygen saturation; SVV, stroke volume variation.
Acknowledgements related to this article
Assistance with the guidelines: These Guidelines were written in German by the guidelines’ authors and translated into English by KERN AG Sprachdienste, branch office Nuremberg. Published under the auspices of the German Society of Anaesthesiology and Intensive Care Medicine (leading member) with the participation of the following societies:
DGAV – Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie (German Society for General and Visceral Surgery)
DGAI – Deutsche Gesellschaft für Anaesthesiologie und Intensivmedizin (German Society of Anaesthesiology and Intensive Care Medicine)
DGCH – Deutsche Gesellschaft für Chirurgie (German Society of Surgery)
DGGG – Deutsche Gesellschaft für Gynaekologie und Geburtshilfe (German Society of Gynaecology and Obstetrics)
DGIM – Deutsche Gesellschaft für Innere Medizin (German Society of Internal Medicine)
DGIIN – Deutsche Gesellschaft für Internistische Intensivmedizin und Notfallmedizin (German Society of Medical Intensive Care and Emergency Medicine)
DGK – Deutsche Gesellschaft für Kardiologie (German Society of Cardiology)
DGNI – Deutsche Gesellschaft für Neurointensiv- und Notfallmedizin (German Society of Neuro-Intensive Care and Emergency Medicine)
DGOU – Deutsche Gesellschaft für Orthopädie und Unfallchirurgie (German Society for Orthopaedics and Trauma)
DGP – Deutsche Gesellschaft für Pflegewissenschaften (German Society for Nursing Science)
DGTHG – Deutsche Gesellschaft für Thorax-, Herz-, und Gefäßchirurgie (German Society for Thoracic and Cardiovascular Surgery)
DGU – Deutsche Gesellschaft für Urologie (German Society of Urology)
DIVI – Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (German Interdisciplinary Association of Intensive Care and Emergency Medicine)
DSG – Deutsche Sepsis-Gesellschaft (German Sepsis Society)
The authors are grateful to Professor Edoardo De Robertis, acting chairman of the guidelines committee of the European Society of Anaesthesiology (ESA), and all members of the ESA guideline committee, for valuable comments.
Financial support and sponsorship: The Deutsche Gesellschaft für Anaesthesiologie und Intensivmedizin (German Society of Anaesthesiology and Intensive Care Medicine, DGAI) financed all external resources needed for methodological guidance, for meetings and translation of the manuscript.
Conflicts of interest: Conflicts of interests are declared in Appendix X, pages 63 to 67 of the Guideline Report (‘Leitlinienreport’) (http://www.awmf.org/leitlinien/detail/ll/001-020.htmlpublished). Comment from the Editor: The ESA was not involved in the development of these guidelines. PK is an Associate Editor of the European Journal of Anaesthesiology.
References
- 1.Field MJ and K.N. L, eds. Comittee to advise the public health service on clinical practice guidelines, directions of a new program. Washington, DC: National Academy Press, 1990. [Google Scholar]
- 2.Bollschweiler E. Lauterbach KW, Schrappe M. Nationale und klinikinterne Leitlinien: Definition und Problemlage. Gesundheitsökonomie, Qualitätsmanagement und Evidence-based Medicine. Stuttgart, Germany: Schattauer; 2003. 493–500. [Google Scholar]
- 3.Europa-Rat. Developing a Methodology for drawing up Guidelines on Best Medical Practices: Recommendation Rec(2001)13 adopted by the Committee of Ministers of the Council of Europe on 10 October 2001 and explanatory memorandum. In: Strasbourg Cedex: Council of Europe, 2001. [Google Scholar]
- 4.Haase N, Perner A, Hennings LI, et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ 2013; 346:f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, Waheed U, Brett SJ. Randomised trials of 6% tetrastarch (hydroxyethyl starch 130/0.4 or 0.42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Med 2013; 39:811–822. [DOI] [PubMed] [Google Scholar]
- 6.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013; 309:678–688. [DOI] [PubMed] [Google Scholar]
- 7.Dolecek M, Svoboda P, Kantorova I, et al. Therapeutic influence of 20% albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: a randomized controlled trial. Hepatogastroenterology 2009; 56:1622–1628. [PubMed] [Google Scholar]
- 8.Barbier C, Loubieres Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004; 30:1740–1746. [DOI] [PubMed] [Google Scholar]
- 9.Biais M, Cottenceau V, Stecken L, et al. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med 2012; 40:1186–1191. [DOI] [PubMed] [Google Scholar]
- 10.Biais M, Vidil L, Sarrabay P, et al. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: comparison between echocardiography and Vigileo/FloTrac device. Crit Care (London, England) 2009; 13:R195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannesson M, Musard H, Desebbe O, et al. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg 2009; 108:513–517. [DOI] [PubMed] [Google Scholar]
- 12.Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med 2010; 36:1475–1483. [DOI] [PubMed] [Google Scholar]
- 13.Cecconi M, Fasano N, Langiano N, et al. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care 2011; 15:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecconi M, Monti G, Hamilton MA, et al. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol 2012; 78:527–533. [PubMed] [Google Scholar]
- 15.Challand C, Struthers R, Sneyd JR, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 2012; 108:53–62. [DOI] [PubMed] [Google Scholar]
- 16.Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg 2006; 102:1511–1517. [DOI] [PubMed] [Google Scholar]
- 17.Chytra I, Pradl R, Bosman R, et al. Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Crit Care (London, England) 2007; 11:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn SM, Pearl RG, Acosta SM, et al. A prospective randomized pilot study of near-infrared spectroscopy-directed restricted fluid therapy versus standard fluid therapy in patients undergoing elective colorectal surgery. Am Surg 2010; 76:1384–1392. [PubMed] [Google Scholar]
- 19.Conway DH, Hussain OA, Gall I. A comparison of noninvasive bioreactance with oesophageal Doppler estimation of stroke volume during open abdominal surgery: an observational study. Eur J Anaesthesiol 2013; 30:501–508. [DOI] [PubMed] [Google Scholar]
- 20.Conway DH, Mayall R, Abdul-Latif MS, et al. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 2002; 57:845–849. [DOI] [PubMed] [Google Scholar]
- 21.Corl K, Napoli AM, Gardiner F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg Med Australas 2012; 24:534–539. [DOI] [PubMed] [Google Scholar]
- 22.Dalibon N, Guenoun T, Journois D, et al. The clinical relevance of systolic pressure variations in anesthetized nonhypotensive patients. J Cardiothorac Vasc Anesth 2003; 17:188–192. [DOI] [PubMed] [Google Scholar]
- 23.De Waal EEC, Rex S, Kruitwagen CLJJ, et al. Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med 2009; 37:510–515. [DOI] [PubMed] [Google Scholar]
- 24.Deflandre E, Bonhomme V, Hans P. Delta down compared with delta pulse pressure as an indicator of volaemia during intracranial surgery. Br J Anaesth 2008; 100:245–250. [DOI] [PubMed] [Google Scholar]
- 25.Dong ZZ, Fang Q, Zheng X, et al. Passive leg raising as an indicator of fluid responsiveness in patients with severe sepsis. World J Emerg Med 2012; 3:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feissel M, Badie J, Merlani PG, et al. Preejection period variations predict the fluid responsiveness of septic ventilated patients. Crit Care Med 2005; 33:E2534.1–E2534.7. [DOI] [PubMed] [Google Scholar]
- 27.Feissel M, Kalakhy R, Banwarth P, et al. Plethysmographic variation index predicts fluid responsiveness in ventilated patients in the early phase of septic shock in the emergency department: a pilot study. J Crit Care 2013; 28:634–639. [DOI] [PubMed] [Google Scholar]
- 28.Feissel M, Michard F, Faller JP, et al. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 2004; 30:1834–1837. [DOI] [PubMed] [Google Scholar]
- 29.Feissel M, Teboul JL, Merlani P, et al. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med 2007; 33:993–999. [DOI] [PubMed] [Google Scholar]
- 30.Fellahi JL, Fischer MO, Rebet O, et al. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth 2012; 26:217–222. [DOI] [PubMed] [Google Scholar]
- 31.Freitas FG, Bafi AT, Nascente AP, et al. Predictive value of pulse pressure variation for fluid responsiveness in septic patients using lung-protective ventilation strategies. Br J Anaesth 2013; 110:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Q, Mi W, Zhang H. Stroke volume variation and pleth variability index to predict fluid responsiveness during resection of primary retroperitoneal tumors in Hans Chinese. Biosci Trends 2012; 6:38–43. [DOI] [PubMed] [Google Scholar]
- 33.Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145:1193–1200. [DOI] [PubMed] [Google Scholar]
- 34.Guinot PG, De Broca B, Abou Arab O, et al. Ability of stroke volume variation measured by oesophageal Doppler monitoring to predict fluid responsiveness during surgery. Br J Anaesth 2013; 110:28–33. [DOI] [PubMed] [Google Scholar]
- 35.Hofer CK, Muller SM, Furrer L, et al. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest 2005; 128:848–854. [DOI] [PubMed] [Google Scholar]
- 36.Huang CC, Fu JY, Hu HC, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med 2008; 36:2810–2816. [DOI] [PubMed] [Google Scholar]
- 37.Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol 2012; 29:64–69. [DOI] [PubMed] [Google Scholar]
- 38.Lansdorp B, Lemson J, van Putten MJ, et al. Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth 2012; 108:395–401. [DOI] [PubMed] [Google Scholar]
- 39.Lanspa MJ, Brown SM, Hirshberg EL, et al. Central venous pressure and shock index predict lack of hemodynamic response to volume expansion in septic shock: a prospective, observational study. J Crit Care 2012; 27:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Jeon Y, Bahk JH, et al. Pulse pressure variation as a predictor of fluid responsiveness during one-lung ventilation for lung surgery using thoracotomy: randomised controlled study. Eur J Anaesthesiol 2011; 28:39–44. [DOI] [PubMed] [Google Scholar]
- 41.Loupec T, Nanadoumgar H, Frasca D, et al. Pleth variability index predicts fluid responsiveness in critically ill patients. Crit Care Med 2011; 39:294–299. [DOI] [PubMed] [Google Scholar]
- 42.Maizel J, Airapetian N, Lorne E, et al. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 2007; 33:1133–1138. [DOI] [PubMed] [Google Scholar]
- 43.Mandeville JC, Colebourn CL. Can transthoracic echocardiography be used to predict fluid responsiveness in the critically ill patient? A systematic review. Crit Care Res Pract 2012; 2012:513480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000; 162:134–138. [DOI] [PubMed] [Google Scholar]
- 45.Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Brachial artery peak velocity variation to predict fluid responsiveness in mechanically ventilated patients. Crit Care (London, England) 2009; 13:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Arterial pressure changes during the Valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med 2009; 35:77–84. [DOI] [PubMed] [Google Scholar]
- 47.Monnet X, Bataille A, Magalhaes E, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med 2013; 39:93–100. [DOI] [PubMed] [Google Scholar]
- 48.Monnet X, Guerin L, Jozwiak M, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth 2013; 110:207–213. [DOI] [PubMed] [Google Scholar]
- 49.Monnet X, Julien F, Ait-Hamou N, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med 2013; 41:1412–1420. [DOI] [PubMed] [Google Scholar]
- 50.Nordstrom J, Hallsjo-Sander C, Shore R, et al. Stroke volume optimization in elective bowel surgery: a comparison between pulse power wave analysis (LiDCOrapid) and oesophageal Doppler (CardioQ). Br J Anaesth 2013; 110:374–380. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira-Costa CD, Friedman G, Vieira SR, et al. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics (Sao Paulo) 2012; 67:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand 2006; 50:1068–1073. [DOI] [PubMed] [Google Scholar]
- 53.Phan TD, Ismail H, Heriot AG, et al. Improving perioperative outcomes: fluid optimization with the esophageal Doppler monitor, a metaanalysis and review. J Am Coll Surg 2008; 207:935–941. [DOI] [PubMed] [Google Scholar]
- 54.Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 2012; 38:422–428. [DOI] [PubMed] [Google Scholar]
- 55.Preau S, Saulnier F, Dewavrin F, et al. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med 2010; 38:819–825. [DOI] [PubMed] [Google Scholar]
- 56.Price JD, Sear JW, Venn RM. Perioperative fluid volume optimization following proximal femoral fracture. Cochrane Database Syst Rev 2004. CD003004.DOI: 10.1002/14651858.CD003004.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Raue W, Swierzy M, Koplin G, et al. Comparison of electrical velocimetry and transthoracic thermodilution technique for cardiac output assessment in critically ill patients. Eur J Anaesthesiol 2009; 26:1067–1071. [DOI] [PubMed] [Google Scholar]
- 58.Sandroni C, Cavallaro F, Marano C, et al. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis (Provisional abstract). In: Database of abstracts of reviews of effects 2012: 1429–1437. [DOI] [PubMed] [Google Scholar]
- 59.Saugel B, Kirsche SV, Hapfelmeier A, et al. Prediction of fluid responsiveness in patients admitted to the medical intensive care unit. J Crit Care 2013; 28:537. [DOI] [PubMed] [Google Scholar]
- 60.Saugel B, Ringmaier S, Holzapfel K, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care 2011; 26:402–410. [DOI] [PubMed] [Google Scholar]
- 61.Shin YH, Ko JS, Gwak MS, et al. Utility of uncalibrated femoral stroke volume variation as a predictor of fluid responsiveness during the anhepatic phase of liver transplantation. Liver Transplant 2011; 17:53–59. [DOI] [PubMed] [Google Scholar]
- 62.Soubrier S, Saulnier F, Hubert H, et al. Can dynamic indicators help the prediction of fluid responsiveness in spontaneously breathing critically ill patients? Intensive Care Med 2007; 33:1117–1124. [DOI] [PubMed] [Google Scholar]
- 63.Suehiro K, Okutani R. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing one-lung ventilation. J Cardiothorac Vasc Anesth 2010; 24:772–775. [DOI] [PubMed] [Google Scholar]
- 64.Suehiro K, Okutani R. Influence of tidal volume for stroke volume variation to predict fluid responsiveness in patients undergoing one-lung ventilation. J Anesth 2011; 25:777–780. [DOI] [PubMed] [Google Scholar]
- 65.Trof RJ, Danad I, Reilingh MW, et al. Cardiac filling volumes versus pressures for predicting fluid responsiveness after cardiovascular surgery: the role of systolic cardiac function. Crit Care (London, England) 2011; 15:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallee F, Fourcade O, De Soyres O, et al. Stroke output variations calculated by esophageal Doppler is a reliable predictor of fluid response. Intensive Care Med 2005; 31:1388–1393. [DOI] [PubMed] [Google Scholar]
- 67.Vallee F, Richard JC, Mari A, et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med 2009; 35:1004–1010. [DOI] [PubMed] [Google Scholar]
- 68.Vistisen ST, Struijk JJ, Larsson A. Automated preejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand 2009; 53:534–542. [DOI] [PubMed] [Google Scholar]
- 69.Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642. [DOI] [PubMed] [Google Scholar]
- 70.Walsh SR, Tang T, Bass S, et al. Doppler-guided intra-operative fluid management during major abdominal surgery: systematic review and meta-analysis. Int J Clin Pract 2008; 62:466–470. [DOI] [PubMed] [Google Scholar]
- 71.Westphal GA, Silva E, Caldeira Filho M, et al. Variation in amplitude of central venous pressure curve induced by respiration is a useful tool to reveal fluid responsiveness in postcardiac surgery patients. Shock 2006; 26:140–145. [DOI] [PubMed] [Google Scholar]
- 72.Wyffels PAH, Durnez PJ, Helderweirt J, et al. Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg 2007; 105:448–452. [DOI] [PubMed] [Google Scholar]
- 73.Yang SY, Shim JK, Song Y, et al. Validation of pulse pressure variation and corrected flow time as predictors of fluid responsiveness in patients in the prone position. Br J Anaesth 2013; 110:713–720. [DOI] [PubMed] [Google Scholar]
- 74.Yazigi A, Khoury E, Hlais S, et al. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2012; 26:387–390. [DOI] [PubMed] [Google Scholar]
- 75.Yin JY, Ho KM. Use of plethysmographic variability index derived from the Massimo(registered trademark) pulse oximeter to predict fluid or preload responsiveness: a systematic review and meta-analysis. Anaesthesia 2012; 67:777–783. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z, Lu B, Sheng X, et al. Accuracy of stroke volume variation in predicting fluid responsiveness: A systematic review and meta-analysis. J Anesth 2011; 25:904–916. [DOI] [PubMed] [Google Scholar]
- 77.McGee S, Abernethy WB, Simel DL. Is this patient hypovolemic? J Am Med Assoc 1999; 281:1022–1029. [DOI] [PubMed] [Google Scholar]
- 78.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 79.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013; 41:1774–1781. [DOI] [PubMed] [Google Scholar]
- 80.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 81.Reinhart K, Brunkhorst FM, Bone HG, et al. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinare Vereinigung fur Intensiv- und Notfallmedizin (DIVI)). Ger Med Sci 2010; 8:Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boulain T, Achard JM, Teboul JL, et al. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 2002; 121:1245–1252. [DOI] [PubMed] [Google Scholar]
- 83.Lakhal K, Ehrmann S, Benzekri-Lefevre D, et al. Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim 2012; 31:e67–e72. [DOI] [PubMed] [Google Scholar]
- 84.Chiolero R, Mavrocordatos P, Bracco D, et al. O2 consumption by the Fick method: methodologic factors. Am J Respir Crit Care Med 1994; 149:1118–1122. [DOI] [PubMed] [Google Scholar]
- 85.Heerdt PM, Pond CG, Blessios GA, et al. Comparison of cardiac output measured by intrapulmonary artery Doppler, thermodilution, and electromagnetometry. Ann Thorac Surg 1992; 54:959–966. [DOI] [PubMed] [Google Scholar]
- 86.Molnar Z, Mikor A, Leiner T, et al. Fluid resuscitation with colloids of different molecular weight in septic shock. Intensive Care Med 2004; 30:1356–1360. [DOI] [PubMed] [Google Scholar]
- 87.Szakmany T, Toth I, Kovacs Z, et al. Effects of volumetric vs. pressure-guided fluid therapy on postoperative inflammatory response: a prospective, randomized clinical trial. Intensive Care Med 2005; 31:656–663. [DOI] [PubMed] [Google Scholar]
- 88.Carl M, Alms A, Braun J, et al. [S3 Guideline:Provision of Intensive care medicine to cardiosurgical patients: haemodynamic monitoring and cardiovascular therapy] (AWMF Register 001/016). AWMF 2011; UIRL:http://www.awmf.org/leitlinien/detail/ll/001-016.html. [Google Scholar]
- 89.Campo dell’ Orto M, Hamm C, Rolf A, et al. [Echocardiography as a signpost in the peri-resuscitation phase: an Advanced Life Support-compliant model.]. Kardiologe 2010; 4:407–424. [Google Scholar]
- 90.Wetterslev M, Haase N, Johansen RR, et al. Predicting fluid responsiveness with transthoracic echocardiography is not yet evidence based. Acta Anaesthesiol Scand 2013; 57:692–697. [DOI] [PubMed] [Google Scholar]
- 91.American College of Cardiology Foundation Appropriate Use Criteria Task F, American Society of E, American Heart A et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol 2011; 57:1126–1166. [DOI] [PubMed] [Google Scholar]
- 92.DIVI Workgroup “Shock”. [Diagnosis und treatment of shock: part 1.]. Anästhesiol Intensivmed 2005; 46:63–69. [Google Scholar]
- 93.Dipti A, Soucy Z, Surana A, et al. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am J Emerg Med 2012; 30:1414–1419.e1411. [DOI] [PubMed] [Google Scholar]
- 94.Adanir T, Aksun M, Ozgurbuz U, et al. Does preoperative hydration affect postoperative nausea and vomiting? A randomized, controlled trial. J Laparoendosc Adv Surg Tech A 2008; 18:1–4. [DOI] [PubMed] [Google Scholar]
- 95.Klein TF, Osmer C, Muller M, et al. The effect of a preoperative infusion of Ringer's solution on splanchnic perfusion in patients undergoing coronary artery bypass grafting. Anaesthesia 2002; 57:756–760. [DOI] [PubMed] [Google Scholar]
- 96.Raue W, Haase O, Langelotz C, et al. Influence of preoperative fluid infusion on volume status during oesophageal resection: a prospective trial. Acta Anaesthesiol Scand 2008; 52:1218–1225. [DOI] [PubMed] [Google Scholar]
- 97.Sanders G, Mercer SJ, Saeb-Parsey K, et al. Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. Br J Surg 2001; 88:1363–1365. [DOI] [PubMed] [Google Scholar]
- 98.Gattas DJ, Dan A, Myburgh J, et al. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely Ill patients: an updated systematic review and meta-analysis. Anesth Analg 2012; 114:159–169. [DOI] [PubMed] [Google Scholar]
- 99.Mukhtar A, Aboulfetouh F, Obayah G, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg 2009; 109:924–930. [DOI] [PubMed] [Google Scholar]
- 100.Abraham M, Sharma M, Srivastava A, et al. Comparison of preloading with normal saline, 6% hydroxyethyl starch and 5% albumin on cardiovascular parameters during sitting neurosurgical operations. J Anaesthesiol Clin Pharmacol 2005; 21:31–37. [Google Scholar]
- 101.Alsatli RA. Assessment of the hemodynamic changes following fluid preloading in cardiac surgery. Saudi J Anaesth 2012; 6:56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ando Y, Terao Y, Fukusaki M, et al. Influence of low-molecular-weight hydroxyethyl starch on microvascular permeability in patients undergoing abdominal surgery: comparison with crystalloid. J Anesth 2008; 22:391–396. [DOI] [PubMed] [Google Scholar]
- 103.Buggy D, Higgins P, Moran C, et al. Prevention of spinal anesthesia-induced hypotension in the elderly: comparison between preanesthetic administration of crystalloids, colloids, and no prehydration. Anesth Analg 1997; 84:106–110. [DOI] [PubMed] [Google Scholar]
- 104.Capel Cardoso MMS, Bliacheriene S, Freitas CRC, et al. Preload during spinal anesthesia for cesarean section: comparison between crystalloid and colloid solutions. Rev Bras Anestesiol 2004; 54:781–787. [DOI] [PubMed] [Google Scholar]
- 105.Chaudhary S, Sethi AK, Motiani P, et al. Preoperative intravenous fluid therapy with crystalloids or colloids on postoperative nausea and vomiting. Indian J Med Res 2008; 127:577–581. [PubMed] [Google Scholar]
- 106.Dureja GP, Jha AM. Comparative evaluation of ringer lactate, low molecular dextran and 3.5% polygeline as spinal preloading fluid. J Anaesthesiol Clin Pharmacol 2002; 18:53–56. [Google Scholar]
- 107.Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in cardiac surgical patients. Crit Care Med 2001; 29:2299–2302. [DOI] [PubMed] [Google Scholar]
- 108.Ewaldsson CA, Hahn RG. Bolus injection of Ringer's solution and dextran 1 kDa during induction of spinal anesthesia. Acta Anaesthesiol Scand 2005; 49:152–159. [DOI] [PubMed] [Google Scholar]
- 109.Feldheiser A, Pavlova V, Bonomo T, et al. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br J Anaesth 2013; 110:231–240. [DOI] [PubMed] [Google Scholar]
- 110.French GWG, White JB, Howell SJ, et al. Comparison of pentastarch and Hartmann's solution for volume preloading in spinal anaesthesia for elective Caesarean section. Br J Anaesth 1999; 83:475–477. [DOI] [PubMed] [Google Scholar]
- 111.Gattas DJ, Dan A, Myburgh J, et al. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med 2013; 39:558–568. [DOI] [PubMed] [Google Scholar]
- 112.Gunusen I, Karaman S, Ertugrul V, et al. Effects of fluid preload (crystalloid or colloid) compared with crystalloid co-load plus ephedrine infusion on hypotension and neonatal outcome during spinal anaesthesia for caesarean delivery. Anaesth Intensive Care 2010; 38:647–653. [DOI] [PubMed] [Google Scholar]
- 113.Haentjens LL, Ghoundiwal D, Touhiri K, et al. Does infusion of colloid influence the occurrence of postoperative nausea and vomiting after elective surgery in women? Anesth Analg 2009; 108:1788–1793. [DOI] [PubMed] [Google Scholar]
- 114.Hasan AB, Mondal MK, Badruddoza NM, et al. Comparison of three fluid regimens for preloading in elective caesarean section under spinal anaesthesia. Mymensingh Med J 2012; 21:533–540. [PubMed] [Google Scholar]
- 115.Himpe D. Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: a meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg 2003; 54:207–215. [PubMed] [Google Scholar]
- 116.Jabalameli M, Soltani HA, Hashemi J, et al. A randomized comparative trial of combinational methods for preventing postspinal hypotension at elective cesarean delivery. J Res Med Sci 2011; 16:1129–1138. [PMC free article] [PubMed] [Google Scholar]
- 117.James MFM, Michell WL, Joubert IA, et al. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth 2011; 107:693–702. [DOI] [PubMed] [Google Scholar]
- 118.Karinen J, Rasanen J, Alahuhta S, et al. Effect of crystalloid and colloid preloading on uteroplacental and maternal haemodynamic state during spinal anaesthesia for Caesarean section. Br J Anaesth 1995; 75:531–535. [DOI] [PubMed] [Google Scholar]
- 119.Kaya S, Karaman H, Erdogan H, et al. Combined use of low-dose bupivacaine, colloid preload and wrapping of the legs for preventing hypotension in spinal anaesthesia for caesarean section. J Int Med Rese 2007; 35:615–625. [DOI] [PubMed] [Google Scholar]
- 120.Ko JS, Kim CS, Cho HS, et al. A randomized trial of crystalloid versus colloid solution for prevention of hypotension during spinal or low-dose combined spinal-epidural anesthesia for elective cesarean delivery. Int J Obstet Anesth 2007; 16:8–12. [DOI] [PubMed] [Google Scholar]
- 121.Kumar M, Saxena N, Saxena AK. The effect of a colloid or crystalloid preload on hypotension caused by induction of anaesthesia with propofol and fentanyl. J Anaesthesiol Clin Pharmacol 2008; 24:409–412. [Google Scholar]
- 122.Lindroos AC, Niiya T, Silvasti-Lundell M, et al. Stroke volume-directed administration of hydroxyethyl starch or Ringer's acetate in sitting position during craniotomy. Acta Anaesthesiol Scand 2013; 57:729–736. [DOI] [PubMed] [Google Scholar]
- 123.Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care (London, England) 2007; 11:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Madi-Jebara S, Ghosn A, Sleilaty G, et al. Prevention of hypotension after spinal anesthesia for cesarean section 6% hydroxyethyl starch 130/0.4 (Voluven(registered trademark)) versus lactated Ringer's solution. J Med Liban 2008; 56:203–207. [PubMed] [Google Scholar]
- 125.Marhofer P, Faryniak B, Oismuller C, et al. Cardiovascular effects of 6% hetastarch and lactated Ringer's solution during spinal anesthesia. Reg Anesth Pain Med 1999; 24:399–404. [DOI] [PubMed] [Google Scholar]
- 126.Martin C, Jacob M, Vicaut E, et al. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology 2013; 118:387–394. [DOI] [PubMed] [Google Scholar]
- 127.Morgan PJ, Halpern SH, Tarshis J. The effects of an increase of central blood volume before spinal anesthesia for cesarean delivery: a qualitative systematic review. Anesth Analg 2001; 92:997–1005. [DOI] [PubMed] [Google Scholar]
- 128.Potter BJ, Deverenne B, Doucette S, et al. Cardiac output responses in a flow-driven protocol of resuscitation following cardiac surgery. J Crit Care 2013; 28:265–269. [DOI] [PubMed] [Google Scholar]
- 129.Pouta AM, Karinen J, Vuolteenaho OJ, et al. Effect of intravenous fluid preload on vasoactive peptide secretion during Caesarean section under spinal anaesthesia. Anaesthesia 1996; 51:128–132. [DOI] [PubMed] [Google Scholar]
- 130.Pratap M, Chhabra B, Nakra D. Prevention of spinal anaesthesia induced hypotension in elderly: preloading versus no preloading. J Anaesthesiol Clin Pharmacol 2006; 22:53–58. [Google Scholar]
- 131.Riley ET, Cohen SE, Rubenstein AJ, et al. Prevention of hypotension after spinal anesthesia for cesarean section: six percentage hetastarch versus lactated Ringer's solution. Anesth Analg 1995; 81:838–842. [DOI] [PubMed] [Google Scholar]
- 132.Salinas FV, Liu SS, Sueda LA, et al. Concurrent expansion of plasma volume and left ventricular end-diastolic volume in patients after rapid infusion of 5% albumin and lactated Ringer's solution. J Clin Anesth 2006; 18:510–514. [DOI] [PubMed] [Google Scholar]
- 133.Saxena N, Chauhan S, Ramesh GS. A comparison of Hetastarch, albumin and Ringer lactate for volume replacement in coronary artery bypass surgery. J Anaesthesiol Clin Pharmacol 1997; 13:117–120. [Google Scholar]
- 134.Siddik SM, Aouad MT, Kai GE, et al. Hydroxyethylstarch 10% is superior to Ringer's solution for preloading before spinal anesthesia for Cesarean section. Can J Anaesth 2000; 47:616–621. [DOI] [PubMed] [Google Scholar]
- 135.Singh U, Saha U. Prevention of hypotension following spinal anaesthesia for caesarean section-comparison of volume preloading with ringer lactate & 6% hydroxyethyl starch (HES 130/0.4). J Anaesthesiol Clin Pharmacol 2009; 25:54–58. [Google Scholar]
- 136.Sirvinskas E, Sneider E, Svagzdiene M, et al. Hypertonic hydroxyethyl starch solution for hypovolaemia correction following heart surgery. Perfusion 2007; 22:121–127. [DOI] [PubMed] [Google Scholar]
- 137.Soares RR, Ferber L, Lorentz MN, Soldati MT. Intraoperative volume replacement: crystalloids versus colloids in surgical myocardial revascularization without cardiopulmonary bypass. Rev Bras Anestesiol 2009; 59:439–451. [DOI] [PubMed] [Google Scholar]
- 138.Tamilselvan P, Fernando R, Bray J, et al. The effects of crystalloid and colloid preload on cardiac output in the parturient undergoing planned cesarean delivery under spinal anesthesia: a randomized trial. Anesth Analg 2009; 109:1916–1921. [DOI] [PubMed] [Google Scholar]
- 139.Ueyama H, He YL, Tanigami H, et al. Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective cesarean section. Anesthesiology 1999; 91:1571–1576. [DOI] [PubMed] [Google Scholar]
- 140.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69:488–498. [DOI] [PubMed] [Google Scholar]
- 141.Verheij J, van Lingen A, Beishuizen A, et al. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med 2006; 32:1030–1038. [DOI] [PubMed] [Google Scholar]
- 142.Verheij J, van Lingen A, Raijmakers PGHM, et al. Effect of fluid loading with saline or colloids on pulmonary permeability, oedema and lung injury score after cardiac and major vascular surgery. Br J Anaesth 2006; 96:21–30. [DOI] [PubMed] [Google Scholar]
- 143.Wilkes NJ, Woolf R, Mutch M, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 2001; 93:811–816. [DOI] [PubMed] [Google Scholar]
- 144.Wu JJK, Huang MS, Tang GJ, et al. Hemodynamic response of modified fluid gelatin compared with lactated ringer's solution for volume expansion in emergency resuscitation of hypovolemic shock patients: preliminary report of a prospective, randomized trial. World J Surg 2001; 25:598–602. [DOI] [PubMed] [Google Scholar]
- 145.Xie R, Wang L, Bao H. Crystalloid and colloid preload for maintaining cardiac output in elderly patients undergoing total hip replacement under spinal anesthesia. J Biomed Res 2011; 25:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, Qiao H, He Z, et al. Intraoperative fluid management in open gastrointestinal surgery: goal-directed versus restrictive. Clinics (Sao Paulo) 2012; 67:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zorko N, Kamenik M, Starc V. The effect of trendelenburg position, lactated ringer's solution and 6% hydroxyethyl starch solution on cardiac output after spinal anesthesia. Anesth Analg 2009; 108:655–659. [DOI] [PubMed] [Google Scholar]
- 148.Boniatti MM, Cardoso PR, Castilho RK, et al. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care 2011; 26:175–179. [DOI] [PubMed] [Google Scholar]
- 149.McCluskey SA, Karkouti K, Wijeysundera D, et al. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 2013; 117:412–421. [DOI] [PubMed] [Google Scholar]
- 150.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to plasma-lyte. Ann Surg 2012; 255:821–829. [DOI] [PubMed] [Google Scholar]
- 151.Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817. [DOI] [PubMed] [Google Scholar]
- 152.Base EM, Standl T, Lassnigg A, et al. Efficacy and safety of hydroxyethyl starch 6% 130/0.4 in a balanced electrolyte solution (volulyte) during cardiac surgery. J Cardiothorac Vasc Anesth 2011; 25:407–414. [DOI] [PubMed] [Google Scholar]
- 153.Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med 2012; 40:2543–2551. [DOI] [PubMed] [Google Scholar]
- 154.Bayer O, Reinhart K, Sakr Y, et al. Renal effects of synthetic colloids and crystalloids in patients with severe sepsis: a prospective sequential comparison. Crit Care Med 2011; 39:1335–1342. [DOI] [PubMed] [Google Scholar]
- 155.Bellomo R, Morimatsu H, French C, et al. The effects of saline or albumin resuscitation on acid-base status and serum electrolytes. Crit Care Med 2006; 34:2891–2897. [DOI] [PubMed] [Google Scholar]
- 156.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139. [DOI] [PubMed] [Google Scholar]
- 157.Du XJ, Hu WM, Xia Q, et al. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas 2011; 40:1220–1225. [DOI] [PubMed] [Google Scholar]
- 158.Dubin A, Pozo MO, Casabella CA, et al. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care 2010; 25:659.e1–659.e8. [DOI] [PubMed] [Google Scholar]
- 159.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256. [DOI] [PubMed] [Google Scholar]
- 160.Gondos T, Marjanek Z, Ulakcsai Z, et al. Short-term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. Eur J Anaesthesiol 2010; 27:794–800. [DOI] [PubMed] [Google Scholar]
- 161.Guidet B, Martinet O, Boulain T, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 versus 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jarvela K, Koskinen M, Kaukinen S, et al. Effects of hypertonic saline (7.5%) on extracellular fluid volumes compared with normal saline (0.9%) and 6% hydroxyethyl starch after aortocoronary bypass graft surgery. J Cardiothorac Vasc Anesth 2001; 15:210–215. [DOI] [PubMed] [Google Scholar]
- 163.Leitch A, Craig G, Sadler P. Human albumin solution resuscitation in severe sepsis and septic shock 1A02, 2C04, 3C00. J Intensive Care Soc 2013; 14:45–52. [Google Scholar]
- 164.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 165.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. In: Cochrane Database of Systematic Reviews: John Wiley & Sons, Ltd, 2011. [DOI] [PubMed] [Google Scholar]
- 166.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012; 367:124–134. [DOI] [PubMed] [Google Scholar]
- 167.Roberts I. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Br Med J 1998; 317:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roquilly A, Loutrel O, Cinotti R, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care 2013; 17:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Finfer S, McEvoy S, Bellomo R. SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011; 37:86–96. [DOI] [PubMed] [Google Scholar]
- 170.Saw MM, Chandler B, Ho KM. Benefits and risks of using gelatin solution as a plasma expander for perioperative and critically ill patients: a meta-analysis (Provisional abstract). Anaesth Intensive Care 2012; 40:17–32. [DOI] [PubMed] [Google Scholar]
- 171.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012; 308:1566–1572. [DOI] [PubMed] [Google Scholar]
- 172.Abdel-Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab J Gastroenterol 2010; 11:24–29. [Google Scholar]
- 173.Alessandria C, Elia C, Mezzabotta L, et al. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis 2011; 43:881–886. [DOI] [PubMed] [Google Scholar]
- 174.Al-Ghamdi A. Hydroxyethylstarch 6% preload does not prevent the hypotension following induction with propofol and fentanyl. Middle East J Anesthesiol 2004; 17:959–968. [PubMed] [Google Scholar]
- 175.Awad S, Dharmavaram S, Wearn CS, et al. Effects of an intraoperative infusion of 4% succinylated gelatine (Gelofusine(R)) and 6% hydroxyethyl starch (Voluven(R)) on blood volume. Br J Anaesth 2012; 109:168–176. [DOI] [PubMed] [Google Scholar]
- 176.Banerjee A, Stocche RM, Angle P, et al. Preload or coload for spinal anesthesia for elective Cesarean delivery: a meta-analysis. Can J Anesth 2010; 57:24–31. [DOI] [PubMed] [Google Scholar]
- 177.Beyer R, Harmening U, Rittmeyer O, et al. Use of modified fluid gelatin and hydroxyethyl starch for colloidal volume replacement in major orthopaedic surgery. Br J Anaesth 1997; 78:44–50. [DOI] [PubMed] [Google Scholar]
- 178.Brock H, Rapf B, Necek S, et al. Volume replacement after cardiac surgery. A comparison of 'small-volume resuscitation’ and two different colloid solutions. Anaesthesist 1995; 44:486–492.7661335 [Google Scholar]
- 179.Bunn F, Trivedi D. Colloid solutions for fluid resuscitation. In: Cochrane Database of Systematic Reviews: John Wiley & Sons, Ltd, 2012. [DOI] [PubMed] [Google Scholar]
- 180.Burdett E, Dushianthan A, Bennett-Guerrero E, et al. Perioperative buffered versus nonbuffered fluid administration for surgery in adults. Cochrane Database Syst Rev 2012; 12:CD004089. [DOI] [PubMed] [Google Scholar]
- 181.Carvalho B, Mercier FJ, Riley ET, et al. Hetastarch co-loading is as effective as preloading for the prevention of hypotension following spinal anesthesia for cesarean delivery. Int J Obstet Anesth 2009; 18:150–155. [DOI] [PubMed] [Google Scholar]
- 182.Coppejans HC, Temurziev S, Jacquemyn Y, et al. A comparison of hes 6% 130/0.42 versus hes 6% 130/0.4 before spinal anaesthesia in caesarean section patients: the effects on haemodynamics, haemoglobin, liver tests and thromboelastometry. Rom J Anaesth Intensive Care 2012; 19:21–27. [Google Scholar]
- 183.Davies P, French GWG. A randomised trial comparing 5 mL/kg and 10 mL/kg of pentastarch as a volume preload before spinal anaesthesia for elective caesarean section. Int J Obstet Anesth 15D 2006:279–283. [DOI] [PubMed] [Google Scholar]
- 184.Donati A, Scarcella M, Nardella R, et al. Fluid challenge in patients submitted to spinal block. Minerva Anestesiol 2007; 73:213–218. [PubMed] [Google Scholar]
- 185.Dytkowska B, Karwacki Z, Suchorzewska J, et al. Comparative assessment of 200/0.5 HAES 6% and Gelafundin in the treatment of hypovolaemia in postcoronary bypass patients. Med Sci Monit 1998; 4:1000–1003. [Google Scholar]
- 186.Ellinger K, Fahnle M, Schroth M, et al. Optimal preoperative titrated dosage of hypertonic-hyperoncotic solutions in cardiac risk patients. Shock (Augusta, Ga) 1995; 3:167–172. [DOI] [PubMed] [Google Scholar]
- 187.Friedman G, Araujo DT, Neves FS, et al. Central venous-arterial carbon dioxide difference: an additional marker of perfusion in shock? Intensive Care Med 2009; 35:S80. [Google Scholar]
- 188.Gan TJ, Bennett-Guerrero E, Phillips-Bute B, et al. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Hextend Study Group. Anesth Analg 1999; 88:992–998. [DOI] [PubMed] [Google Scholar]
- 189.Gandhi SD, Weiskopf RB, Jungheinrich C, et al. Volume replacement therapy during major orthopedic surgery using Voluven(registered trademark) (Hydroxyethyl starch 130/0.4) or hetastarch. Anesthesiology 2007; 106:1120–1127. [DOI] [PubMed] [Google Scholar]
- 190.Godet G, Lehot JJ, Janvier G, et al. Safety of HES 130/0.4 (Voluven(registered trademark)) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial. Eur J Anaesthesiol 2008; 25:986–994. [DOI] [PubMed] [Google Scholar]
- 191.Goertz AW, Mehl T, Lindner KH, et al. Effect of 7.2% hypertonic saline/6% hetastarch on left ventricular contractility in anesthetized humans. Anesthesiology 1995; 82:1389–1395. [DOI] [PubMed] [Google Scholar]
- 192.Kuitunen A, Suojaranta-Ylinen R, Kukkonen S, et al. A comparison of the haemodynamic effects of 4% succinylated gelatin, 6% hydroxyethyl starch (200/0.5) and 4% human albumin after cardiac surgery. Scand J Surg 2007; 96:72–78. [DOI] [PubMed] [Google Scholar]
- 193.Kulla M, Weidhase R, Lampl L. Hydroxyethyl starch 6% 130/0.42 in acetate-buffered Ringer's solution as a part of a balanced-volume resuscitation in abdominal surgery. Anasthesiol Intensivmed 2008; 49:7–18. [Google Scholar]
- 194.Mehta Y, Dhar A, Sujatha, et al. Comparison of new HES (130/0.4) and HES (200/0.5) in OPCAB surgery. J Anaesthesiol Clin Pharmacol 2007; 23:273–278. [Google Scholar]
- 195.Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg 2012; 144:223–230.e225. [DOI] [PubMed] [Google Scholar]
- 196.Ngan Kee WD, Khaw KS, Lee BB, et al. Randomized controlled study of colloid preload before spinal anaesthesia for Caesarean section. Br J Anaesth 2001; 87:772–774. [DOI] [PubMed] [Google Scholar]
- 197.Niemi T, Schramko A, Kuitunen A, et al. Haemodynamics and acid-base equilibrium after cardiac surgery: comparison of rapidly degradable hydroxyethyl starch solutions and albumin. Scand J Surg 2008; 97:259–265. [DOI] [PubMed] [Google Scholar]
- 198.Nishikawa K, Yokoyama N, Saito S, et al. Comparison of effects of rapid colloid loading before and after spinal anesthesia on maternal hemodynamics and neonatal outcomes in cesarean section. J Clin Monit Comput 2007; 21:125–129. [DOI] [PubMed] [Google Scholar]
- 199.Ragaller M, Muller M, Bleyl JU, et al. Hemodynamic effects of hypertonic hydroxyethyl starch 6% solution and isotonic hydroxyethyl starch 6% solution after declamping during abdominal aortic aneurysm repair. Shock (Augusta, Ga) 2000; 13:367–373. [DOI] [PubMed] [Google Scholar]
- 200.Senagore AJ, Emery T, Luchtefeld M, et al. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum 2009; 52:1935–1940. [DOI] [PubMed] [Google Scholar]
- 201.Shao L, Wang B, Wang S, et al. Comparison of 7.2% hypertonic saline: 6% hydroxyethyl starch solution and 6% hydroxyethyl starch solution after the induction of anesthesia in patients undergoing elective neurosurgical procedures. Clinics (Sao Paulo) 2013; 68:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shroff PP, Kulkarni S, Lele S, et al. Randomized, comparative study between polygeline and hydroxyethyl starch (130/0.4) as volume preload before spinal anaesthesia. J Anaesthesiol Clin Pharmacol 2007; 23:269–272. [Google Scholar]
- 203.Siddik-Sayyid SM, Nasr VG, Taha SK, et al. A randomized trial comparing colloid preload to coload during spinal anesthesia for elective cesarean delivery. Anesth Analg 2009; 109:1219–1224. [DOI] [PubMed] [Google Scholar]
- 204.Sneyd RJ, Dean BC, Dwyer C. Safety and tolerability of volplex(registered trademark) in elective surgery. Clin Drug Investig 2004; 24:73–79. [DOI] [PubMed] [Google Scholar]
- 205.Teoh WHL, Sia ATH. Colloid preload versus coload for spinal anesthesia for cesarean delivery: the effects on maternal cardiac output. Anesth Analg 2009; 108:1592–1598. [DOI] [PubMed] [Google Scholar]
- 206.Tercanli S, Schneider M, Visca E, et al. Influence of volume preloading on uteroplacental and fetal circulation during spinal anaesthesia for caesarean section in uncomplicated singleton pregnancies. Fetal Diagn Ther 2002; 17:142–146. [DOI] [PubMed] [Google Scholar]
- 207.Turker G, Yilmazlar T, Basagan Mogol E, et al. The effects of colloid preloading on thromboelastography prior to caesarean delivery: hydroxyethyl starch 130/0.4 versus succinylated gelatine. J Int Med Res 2011; 39:143–149. [DOI] [PubMed] [Google Scholar]
- 208.Wang SS, Chu SH, Lin HM, et al. Clinical use of pentastarch in cardiac surgery without homologous blood transfusion. J Formos Med Assoc 1995; 94:153–158. [PubMed] [Google Scholar]
- 209.Wiedermann CJ, Dunzendorfer S, Gaioni LU, et al. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care 2010; 14:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Laxenaire MC, Charpentier C, Feldman L. Anaphylactoid reactions to colloid plasma substitutes: incidence, risk factors, mechanisms. A French multicenter prospective study. Ann Fr Anesth Reanim 1994; 13:301–310. [DOI] [PubMed] [Google Scholar]
- 211.Asfar P, Kerkeni N, Labadie F, et al. Assessment of hemodynamic and gastric mucosal acidosis with modified fluid gelatin versus 6% hydroxyethyl starch: a prospective, randomized study. Intensive Care Med 2000; 26:1282–1287. [DOI] [PubMed] [Google Scholar]
- 212.Inal MT, Memis D, Karamanlioglu B, et al. Effects of polygeline and hydroxyethyl starch solutions on liver functions assessed with LIMON in hypovolemic patients. J Crit Care 2010; 25:361.e1–361.e5. [DOI] [PubMed] [Google Scholar]
- 213.Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet 2001; 357:911–916. [DOI] [PubMed] [Google Scholar]
- 214.Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 2007; 357:874–884. [DOI] [PubMed] [Google Scholar]
- 215.Apfel CC, Meyer A, Orhan-Sungur M, et al. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: quantitative review. Br J Anaesth 2012; 108:893–902. [DOI] [PubMed] [Google Scholar]
- 216.Arndt JO, Bomer W, Krauth J, et al. Incidence and time course of cardiovascular side effects during spinal anesthesia after prophylactic administration of intravenous fluids or vasoconstrictors. Anesth Analg 1998; 87:347–354. [DOI] [PubMed] [Google Scholar]
- 217.Bose M, Kini G, Krishna HM. Comparison of crystalloid preloading versus crystalloid coloading to prevent hypotension and bradycardia following spinal anaesthesia. J Anaesthesiol Clin Pharmacol 2008; 24:53–56. [Google Scholar]
- 218.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cardoso MM, Santos MM, Yamaguchi ET, et al. Fluid preload in obstetric patients. How to do it? Rev Bras Anestesiol 2004; 54:13–19. [DOI] [PubMed] [Google Scholar]
- 220.Casati A, Fanelli G, Berti M, et al. Cardiac performance during unilateral lumbar spinal block after crystalloid preload. Can J Anaesth 1997; 44:623–628. [DOI] [PubMed] [Google Scholar]
- 221.De Aguilar-Nascimento JE, Diniz BN, Do Carmo AV, et al. Clinical benefits after the implementation of a protocol of restricted perioperative intravenous crystalloid fluids in major abdominal operations. World J Surg 2009; 33:925–930. [DOI] [PubMed] [Google Scholar]
- 222.Dyer RA, Farina Z, Joubert IA, et al. Crystalloid preload versus rapid crystalloid administration after induction of spinal anaesthesia (coload) for elective caesarean section. Anaesth Intensive Care 2004; 32:351–357. [DOI] [PubMed] [Google Scholar]
- 223.Ghafari MH, Majid Moosavizadeh SA, Moharari RS, et al. Hypertonic saline 5% vs. lactated ringer for resuscitating patients in hemorrhagic shock. Middle East J Anesthesiol 2008; 19:1337–1347. [PubMed] [Google Scholar]
- 224.Kabon B, Akca O, Taguchi A, et al. Supplemental intravenous crystalloid administration does not reduce the risk of surgical wound infection. Anesth Analg 2005; 101:1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kamenik M, Paver-Erzen V. The effects of lactated Ringer's solution infusion on cardiac output changes after spinal anesthesia. Anesth Analg 2001; 92:710–714. [DOI] [PubMed] [Google Scholar]
- 226.Kato S, Goto F. Hypertonic saline for intraoperative fluid therapy in transurethral resection of the prostate. J Anesth 1996; 10:170–175. [DOI] [PubMed] [Google Scholar]
- 227.Kinsella SM, Pirlet M, Mills MS, et al. Randomized study of intravenous fluid preload before epidural analgesia during labour. Br J Anaesth 2000; 85:311–313. [DOI] [PubMed] [Google Scholar]
- 228.Kolsen-Petersen JA, Nielsen JOD, Bendtzen K, et al. Infusion of hypertonic saline (7.5% NaCl) causes minor immunological changes in normovolaemic women. Acta Anaesthesiol Scand 2004; 48:224–233. [DOI] [PubMed] [Google Scholar]
- 229.Kubli M, Shennan AH, Seed PT, et al. A randomised controlled trial of fluid preloading before low dose epidural analgesia for labour. Int J Obstet Anesth 2003; 12:256–260. [DOI] [PubMed] [Google Scholar]
- 230.McAlister V, Burns KE, Znajda T, et al. Hypertonic saline for peri-operative fluid management. Cochrane Database Syst Rev 2010. CD005576.DOI: 10.1002/14651858.CD005576.pub2. [DOI] [PubMed] [Google Scholar]
- 231.McArdle GT, McAuley DF, McKinley A, et al. Preliminary results of a prospective randomized trial of restrictive versus standard fluid regime in elective open abdominal aortic aneurysm repair. Ann Surg 2009; 250:28–34. [DOI] [PubMed] [Google Scholar]
- 232.Ouerghi S, Bougacha MA, Frikha N, et al. Combined use of crystalloid preload and low dose spinal anesthesia for preventing hypotension in spinal anesthesia for cesarean delivery: a randomized controlled trial. Middle East J Anesth 2010; 20:667–672. [PubMed] [Google Scholar]
- 233.Park GE, Hauch MA, Curlin F, et al. The effects of varying volumes of crystalloid administration before cesarean delivery on maternal hemodynamics and colloid osmotic pressure. Anesth Analg 1996; 83:299–303. [DOI] [PubMed] [Google Scholar]
- 234.Peng X, Liu H, Xi L, et al. Effects of colloid preload on placenta stereology and cord blood S100beta protein during cesarean section under spinal anesthesia. Nan Fang Yi Ke Da Xue Xue Bao 2013; 33:161–165. [PubMed] [Google Scholar]
- 235.Rabadi D. Effect of normal saline administration on circulation stability during general anesthesia induction with propofol in gynecological procedures: randomised-controlled study. Rev Bras Anestesiol 2013; 63:258–261. [DOI] [PubMed] [Google Scholar]
- 236.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999; 90:1265–1270. [DOI] [PubMed] [Google Scholar]
- 237.Shannon MT, Ramanathan S. An intravenous fluid bolus is not necessary before administration of intrathecal fentanyl for labor analgesia. J Clin Anesth 1998; 10:452–456. [DOI] [PubMed] [Google Scholar]
- 238.Sharma SK, Gajraj NM, Sidawi JE. Prevention of hypotension during spinal anesthesia: a comparison of intravascular administration of hetastarch versus lactated Ringer's solution. Anesth Analg 1997; 84:111–114. [DOI] [PubMed] [Google Scholar]
- 239.Singh J, Ranjit S, Shrestha S, et al. Effect of preloading on hemodynamic of the patient undergoing surgery under spinal anaesthesia. Kathmandu Univ Med J 2010; 8:216–221. [DOI] [PubMed] [Google Scholar]
- 240.Wang BW, Chiou YH, Chen WB, et al. Intravenous pretreatment of hypertonic saline can prevent systemic hypotension induced by spinal anesthesia. Acta Anaesthesiol Sin 1997; 35:85–90. [PubMed] [Google Scholar]
- 241.Williamson W, Burks D, Pipkin J, et al. Effect of timing of fluid bolus on reduction of spinal-induced hypotension in patients undergoing elective cesarean delivery. AANA J 2009; 77:130–136. [PubMed] [Google Scholar]
- 242.Young JB, Utter GH, Schermer CR, et al. Saline versus plasma-lyte a in initial resuscitation of trauma patients: a randomized trial. Ann Surg 2013; 259:255–262. [DOI] [PubMed] [Google Scholar]
- 243.Yousefshahi F, Bashirzadeh M, Abdollahi M, et al. Effect of hypertonic saline infusion versus normal saline on serum NGAL and cystatin C levels in patients undergoing coronary artery bypass graft. J Tehran Heart Cent 2013; 8:21–27. [PMC free article] [PubMed] [Google Scholar]
- 244.Fang ZX, Li YF, Zhou XQ, et al. Effects of resuscitation with crystalloid fluids on cardiac function in patients with severe sepsis. BMC Infect Dis 2008; 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jarvela K, Kaukinen S. Hypertonic saline (7.5%) after coronary artery bypass grafting. Eur J Anaesthesiol 2001; 18:100–107. [DOI] [PubMed] [Google Scholar]
- 246.Jarvela K, Kaukinen S. Hypertonic saline (7.5%) decreases perioperative weight gain following cardiac surgery. J Cardiothorac Vasc Anesth 2002; 16:43–46. [DOI] [PubMed] [Google Scholar]
- 247.Shao YS, Zhang YT, Peng KQ, et al. Effects of 7.5% hypertonic saline on fluid balance after radical surgery for gastrointestinal carcinoma. World J Gastroenterol 2005; 11:1577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van Haren FM, Sleigh J, Cursons R, et al. The effects of hypertonic fluid administration on the gene expression of inflammatory mediators in circulating leucocytes in patients with septic shock: a preliminary study. Ann Intensive Care 2011; 1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia 2008; 63:44–51. [DOI] [PubMed] [Google Scholar]
- 250.Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care (London, England) 2010; 14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Benomar B, Ouattara A, Estagnasie P, et al. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med 2010; 36:1875–1881. [DOI] [PubMed] [Google Scholar]
- 252.Brandstrup B, Svendsen PE, Rasmussen M, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: Near-maximal stroke volume or zero fluid balance? Br J Anaesth 2012; 109:191–199. [DOI] [PubMed] [Google Scholar]
- 253.Brun C, Zieleskiewicz L, Textoris J, et al. Prediction of fluid responsiveness in severe preeclamptic patients with oliguria. Intensive Care Med 2013; 39:593–600. [DOI] [PubMed] [Google Scholar]
- 254.Bundgaard-Nielsen M, Jans O, Muller RG, et al. Does goal-directed fluid therapy affect postoperative orthostatic intolerance? A randomized trial. Anesthesiology 2013; 119:813–823. [DOI] [PubMed] [Google Scholar]
- 255.Forget P, Lois F, De Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg 2010; 111:910–914. [DOI] [PubMed] [Google Scholar]
- 256.Forget P, Lois F, Kartheuser A, et al. The concept of titration can be transposed to fluid management. but does is change the volumes? Randomised trial on pleth variability index during fast-track colonic surgery. Curr Clin Pharmacol 2013; 8:110–114. [DOI] [PubMed] [Google Scholar]
- 257.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97:820–826. [DOI] [PubMed] [Google Scholar]
- 258.Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care (London, England) 2010; 14:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lee JH, Jeon Y, Bahk JH, et al. Pulse-pressure variation predicts fluid responsiveness during heart displacement for off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2011; 25:1056–1062. [DOI] [PubMed] [Google Scholar]
- 260.Lee JY, Park HY, Jung WS, et al. Comparative study of pressure- and volume-controlled ventilation on stroke volume variation as a predictor of fluid responsiveness in patients undergoing major abdominal surgery. J Crit Care 2012; 27:531.e9–531.e14. [DOI] [PubMed] [Google Scholar]
- 261.Lobo SM, Ronchi LS, Oliveira NE, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care (London, England) 2011; 15:R226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Prowle JR, Chua HR, Bagshaw SM, et al. Clinical review: volume of fluid resuscitation and the incidence of acute kidney injury: a systematic review. Crit Care 2012; 16:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scheeren TWL, Wiesenack C, Gerlach H, et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput 2013; 27:225–233. [DOI] [PubMed] [Google Scholar]
- 264.Grocott MP, Dushianthan A, Hamilton MA, et al. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 2012; 11:CD004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Brammar A, Nicholson A, Trivella M, et al. Perioperative fluid volume optimization following proximal femoral fracture. Cochrane Database Syst Rev 2013; 9:CD003004. [DOI] [PubMed] [Google Scholar]
- 266.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010; 303:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Natalini G, Rosano A, Taranto M, et al. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg 2006; 103:1478–1484. [DOI] [PubMed] [Google Scholar]
- 268.Dorrington KL. Skin turgor: do we understand the clinical sign? Lancet 1981; 1:264–266. [DOI] [PubMed] [Google Scholar]
- 269.Eaton D, Bannister P, Mulley GP, et al. Axillary sweating in clinical assessment of dehydration in ill elderly patients. BMJ 1994; 308:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gross CR, Lindquist RD, Woolley AC, et al. Clinical indicators of dehydration severity in elderly patients. J Emerg Med 1992; 10:267–274. [DOI] [PubMed] [Google Scholar]
- 271.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? Chest 2008; 134:172–178. [DOI] [PubMed] [Google Scholar]
- 272.Weyland A, Grune F. Cardiac preload and central venous pressure. Anaesthesist 2009; 58:506–512. [DOI] [PubMed] [Google Scholar]
- 273.Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35:64–68. [DOI] [PubMed] [Google Scholar]
- 274.Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 2004; 32:691–699. [DOI] [PubMed] [Google Scholar]
- 275.Biais M, Nouette-Gaulain K, Roullet S, et al. A comparison of stroke volume variation measured by Vigileo/FloTrac system and aortic Doppler echocardiography. Anesth Analg 2009; 109:466–469. [DOI] [PubMed] [Google Scholar]


