Abstract
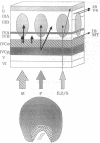
Visual information reaching striate cortex comes from parallel pathways, and the information is organized, or processed, by the layers and columns of striate cortex. To better understand how this is accomplished anatomically, we asked whether parallel pathways originating in the lateral geniculate nucleus (LGN), and terminating separately in layer IV, remain separate in layer III of macaque monkeys. Layer III is of interest since it may play a special role in color and form vision but not in analysis of visual motion. The chief finding was that cells in "blobs" of layer III that stain densely for cytochrome oxidase receive indirect input, via layer IVC, from both LGN magnocellular (M) and parvocellular (P) cells. This is important because the P and M pathways may represent color/form and motion-processing channels, respectively. Interblob cells receive indirect input, via layers IVC and IVA, from the LGN P cells. Also, as suggested by others, our data demonstrate that layer III can be subdivided. The bottom tier, layer IIIB, receives direct projections from all cortical layers. Output from layer IIIB appears to remain intrinsic to striate cortex. In contrast, the top tier, layer IIIA, receives projections from layer IIIB as well as from layers IVA, IVB (blobs only), and V, but it receives no direct projections from LGN recipient layers IVC and VI. Unlike layer IIIB, the output of layer IIIA reaches extrastriate areas. Thus, impulses arriving from parallel LGN pathways may be recombined through serial stages in striate cortex to produce a set of parallel pathways that are qualitatively different from the original LGN set.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988 May;11(5):219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Diamond I. T., Conley M., Itoh K., Fitzpatrick D. Laminar organization of geniculocortical projections in Galago senegalensis and Aotus trivirgatus. J Comp Neurol. 1985 Dec 22;242(4):584–610. doi: 10.1002/cne.902420408. [DOI] [PubMed] [Google Scholar]
- Eskin T. A., Merigan W. H. Selective acrylamide-induced degeneration of color opponent ganglion cells in macaques. Brain Res. 1986 Jul 23;378(2):379–384. doi: 10.1016/0006-8993(86)90941-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Itoh K., Diamond I. T. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus). J Neurosci. 1983 Apr;3(4):673–702. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D., Lund J. S., Blasdel G. G. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J Neurosci. 1985 Dec;5(12):3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Livingstone M. S. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987 Nov;7(11):3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvarday Z. F., Cowey A., Smith A. D., Somogyi P. Interlaminar and lateral excitatory amino acid connections in the striate cortex of monkey. J Neurosci. 1989 Feb;9(2):667–682. doi: 10.1523/JNEUROSCI.09-02-00667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica E. A., Casagrande V. A. Development of primate retinogeniculate axon arbors. Vis Neurosci. 1988;1(1):103–123. doi: 10.1017/s095252380000105x. [DOI] [PubMed] [Google Scholar]
- Lachica E. A., Mavity-Hudson J. A., Casagrande V. A. Morphological details of primate axons and dendrites revealed by extracellular injection of biocytin: an economic and reliable alternative to PHA-L. Brain Res. 1991 Nov 8;564(1):1–11. doi: 10.1016/0006-8993(91)91344-z. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Rodieck R. W., Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981 Sep 4;213(4512):1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987 Nov;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6098–6101. doi: 10.1073/pnas.79.19.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Local circuit neurons of macaque monkey striate cortex: I. Neurons of laminae 4C and 5A. J Comp Neurol. 1987 Mar 1;257(1):60–92. doi: 10.1002/cne.902570106. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Malpeli J. G., Schiller P. H., Colby C. L. Response properties of single cells in monkey striate cortex during reversible inactivation of individual lateral geniculate laminae. J Neurophysiol. 1981 Nov;46(5):1102–1119. doi: 10.1152/jn.1981.46.5.1102. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., Newsome W. T. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- Norton T. T., Casagrande V. A., Irvin G. E., Sesma M. A., Petry H. M. Contrast-sensitivity functions of W-, X-, and Y-like relay cells in the lateral geniculate nucleus of bush baby, Galago crassicaudatus. J Neurophysiol. 1988 Jun;59(6):1639–1656. doi: 10.1152/jn.1988.59.6.1639. [DOI] [PubMed] [Google Scholar]
- Norton T. T., Casagrande V. A. Laminar organization of receptive-field properties in lateral geniculate nucleus of bush baby (Galago crassicaudatus). J Neurophysiol. 1982 Apr;47(4):715–741. doi: 10.1152/jn.1982.47.4.715. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Pandya D. N. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979 Dec 21;179(1):3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Logothetis N. K., Charles E. R. Role of the color-opponent and broad-band channels in vision. Vis Neurosci. 1990 Oct;5(4):321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., Hamilton S. L., Switkes E., De Valois R. L. Functional anatomy of macaque striate cortex. V. Spatial frequency. J Neurosci. 1988 May;8(5):1610–1624. doi: 10.1523/JNEUROSCI.08-05-01610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. T., Huerta M. F., Kaas J. H., Harting J. K. The projections of the lateral geniculate nucleus of the squirrel monkey: studies of the interlaminar zones and the S layers. J Comp Neurol. 1983 Jan 10;213(2):135–145. doi: 10.1002/cne.902130203. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Yukie M., Iwai E. Laminar origin of direct projection from cortex area V1 to V4 in the rhesus monkey. Brain Res. 1985 Nov 4;346(2):383–386. doi: 10.1016/0006-8993(85)90875-3. [DOI] [PubMed] [Google Scholar]
- Zeki S., Shipp S. The functional logic of cortical connections. Nature. 1988 Sep 22;335(6188):311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]