Abstract
Based on an emerging neuroscience model of addiction, this study examines how an imbalance between two neurobehavioral systems (reward motivation and executive control) can distinguish between early adolescent progressive drug use and mere experimentation with drugs. Data from four annual assessments of a community cohort (N= 382) of 11–13 year olds were analyzed to model heterogeneity in patterns of early drug use. Baseline assessments of working memory (an indicator of the functional integrity of the executive control system) and three dimensions of impulsivity (characterizing the balance between reward-seeking and executive control systems) were used to predict heterogeneous latent classes of drug use trajectories from early-mid adolescence. Findings revealed that an imbalance resulting from weak executive control and heightened reward seeking was predictive of early progression in drug use, while heightened reward seeking balanced by a strong control system was predictive of occasional experimentation only. Implications of these results are discussed in terms of preventive interventions that can target underlying weaknesses in executive control during younger years, and potentially enable at-risk adolescents to exercise greater self-restraint in the context of rewarding drug-related cues.
Keywords: Drug use trajectories, impulsivity, sensation seeking, working memory, early adolescence
Many American adolescents initiate drug use before 15 years of age, with alcohol, tobacco, and marijuana being the most common drugs of onset (Swendsen et al., 2012). Although early initiation of drug use has consistently been linked to substance use disorders (SUD), it still remains unclear whether early drug use is a causal contributor to SUD (Grant & Dawson, 1997; Hawkins et al., 1997; Odgers et al., 2008) or simply a marker of underlying vulnerabilities (e.g., behavioral disinhibition) common to both early onset and subsequent SUD (Bobova, Finn, Rickert, & Lucas, 2009; Krueger et al., 2002; McGue, Iacono, Legrand, Malone, & Elkins, 2001; Prescott & Kendler, 1999). Much of the research supporting the causal role of early drug use in the development of SUD has been retrospective in nature, relying on adult recall of age at first use (Grant & Dawson, 1997). Besides issues related to recall bias, this line of research has overlooked the important distinction between different forms of early drug use, namely occasional experimentation with drugs versus the more persistent progression in use. Indeed, not all adolescents who experiment with drugs at a young age progress toward SUD; it is the adolescents who engage in consistent and progressive drug use following initial exposure who are at greater risk for subsequent SUD (Chassin, Presson, Pitts, & Sherman, 2000; Flory, Lynam, Milich, Leukefeld, & Clayton, 2004; Li, Duncan, & Hops, 2001).
The guiding premise of our research is that by disentangling the heterogeneity in early forms of drug use (Colder, Campbell, Ruel, Richardson, & Flay, 2002; Duncan, Duncan, & Strycker, 2006; Flory et al., 2004; Li et al., 2001) we can obtain critical information about the underlying vulnerabilities that predispose some adolescents to more dysfunctional forms of drug use following initial exposure. To that end, we test the utility of an emerging neurobehavioral model (Everitt & Robbins, 2005; Everitt et al., 2008) that makes clear predictions about the vulnerabilities associated with SUD and their role in drug use experimentation versus progression. Derived mainly from research with animals, we believe this model can provide important insights into why some adolescents engage in more dysfunctional forms of drug use, while others experiment with or use drugs only occasionally. Here we provide the first empirical test of this model in a human adolescent sample.
Neurobehavioral Systems of Drug Use
Research with both animal and human models increasingly points to the role of two interrelated neurobehavioral systems in the development of SUD - the reward motivation system, mediated by mesolimbic dopamine circuitry and the executive control system, centered in the prefrontal and parietal networks (Dalley, Everitt, & Robbins, 2011; de Wit & Richards, 2004; Jentsch & Taylor, 1999). The ‘neurobehavioral imbalance model’ attributes the development of SUD to predisposing vulnerabilities in each of these two systems, albeit at different stages of the addiction process (Everitt et al., 2008). For instance, high levels of reward motivation can increase propensity for experimentation with drugs (George & Koob, 2010), whereas weakness in executive control is associated with progressive (Tarter et al., 1999; Zucker, Heitzeg, & Nigg, 2011) and compulsive forms of drug use (Everitt & Robbins, 2005; Heitzeg, Nigg, Yau, Zucker, & Zubieta, 2010; Koob & Volkow, 2010). Crucially, and of relevance to the present research, this model posits that individual differences in the imbalance between the two neurobehavioral systems is what poses particular risk for the escalation of drug use (Dalley et al., 2011; Robbins, Gillan, Smith, de Wit, & Ersche, 2012). More specifically, this imbalance results from a hyper-active striatal reward system that is inadequately controlled by a hypo-active pre-frontally mediated executive control system.
An important prediction of this model observed in animals (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Dalley et al., 2007; Everitt et al., 2008), but not yet tested in humans, is that certain forms of impulsivity characterized by strong reward-seeking tendencies and weakness in executive control are more strongly related to the escalation in drug use, than other dimensions like sensation-seeking (SS) that do not necessarily represent a failure of the top-down regulatory control. Impulsivity is broadly characterized as a tendency to act prematurely, with little forethought for the consequences of one’s behavior (Eysenck & Eysenck, 1980). Although impulsivity is commonly implicated as a core deficit in SUD (Giancola & Tarter, 1999; Nigg et al., 2006), it is a multifaceted construct that can include several different dimensions (Whiteside & Lynam, 2001), each of which can contribute differentially and at different stages of the addiction process (Dalley et al., 2011; Winstanley, Olausson, Taylor, & Jentsch, 2010).
Impulsivity is usually assessed in two interrelated domains. First, in its most classic form as an inability to inhibit pre-potent behavior, namely acting-without-thinking (AWT) (Barratt, 1985), and second, as an inability to delay the gratification of a small immediate reward in favor of a larger deferred reward, i.e. delay discounting (DD) (Madden & Bickel, 2010). Although impulsivity is reflective of a hypo-functioning executive control system (Bechara, 2005), it also shares characteristics with SS by virtue of its association with the mesolimbic dopamine reward system (Buckholtz et al., 2010; Pattij & Vanderschuren, 2008; Zald et al., 2008). This link between different dimensions of impulsivity and sensation seeking is further established by neuroimaging research which has found overlapping activation patterns in ventral striatal reward circuitry for all three personality dimensions – DD (Ballard & Knutson, 2009; Hariri et al., 2006; McClure, Laibson, Loewenstein, & Cohen, 2004), AWT (Buckholtz et al., 2010), and SS (Zald et al., 2008).
In comparison, what most saliently distinguishes SS from AWT and DD is the level of functioning of the executive control system, especially as indicated by working memory (WM) performance. WM is a higher-order cognitive ability that facilitates flexible, goal-directed behavior (Cools, Sheridan, Jacobs, & D’Esposito, 2007; Miller & Cohen, 2001; Miyake & Shah, 1999). Executive attention, an important component of WM (Kane, Conway, Hanbrick, & Engle, 2007), enables individuals with stronger WM to exert inhibitory control over impulses and to consider alternatives that may be more adaptive, making them less prone to engaging in impulsive and potentially risky behaviors (Romer et al., 2011; Shamosh et al., 2008). Since youth high in SS do not share weakness in executive control (Dalley et al., 2011), they may be better able to control their drug use urges (Gullo & Dawe, 2008). This is confirmed by research that finds that although sensation-seekers may be attracted to novel and exciting experiences (like drug use) due to their reward-seeking tendencies (Cyders, Flory, Rainer, & Smith, 2009; Magid, MacLean, & Colder, 2007), they are not likely to exhibit signs of progression or loss of control over the use of drugs, leading to symptoms of SUD (Dick et al., 2010; Smith et al., 2007). Furthermore, evidence from cross-sectional research with human drug-abusing populations reveals weaknesses in executive control and greater impulsive (but not SS) tendencies (Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010; Rogers & Robbins, 2001). The strength of the executive control system commonly reported among sensation seekers (Raine, Reynolds, Venables, & Mednick, 2002; Spear, 2010) suggests that adolescents high in SS would be able to learn from their experiences, especially the negative consequences associated with drug use (Romer, Duckworth, Sznitman, & Park, 2010) and not engage in repetitive patterns of dysfunctional behaviors.
Developmental Changes in Reward vs. Control Systems
Longitudinal research focusing on individual differences in the developmental trajectories of SS and impulsivity in relation to drug use has found a slower developmental decline in impulsivity (indicative of an imbalance between the two systems) to be associated with a more rapid increase in alcohol, marijuana and tobacco use from ages 15–26 (Quinn & Harden, 2013). While similar associations were also reported between the slower rate of decline in SS and increase in alcohol use, this evidence was weaker in comparison and not supported in supplemental analyses with non-abstainers. Analogous findings have been reported by Littlefield, Sher, & Wood (2009) in a young adult sample. However, replication of these findings in an early adolescent sample is warranted. According to the neurobehavioral imbalance model, a slower decline in SS is more likely to be associated with experimentation with drugs (Cyders et al., 2009), whereas developmental delays in the maturation of the cognitive control system (associated with impulsivity dimensions of AWT and DD) are more likely to predict chronic and progressive drug use (Ivanov, Schulz, London, & Newcorn, 2008).
Weaknesses in the executive control system, as indexed by WM deficits, have frequently been reported in substance abusing populations (Squeglia, Spadoni, Infante, Myers, & Tapert, 2009). However, in such samples where drug use has already been initiated, it is difficult to establish whether the WM impairments pre-existed or were induced by drug use. In a recent longitudinal study modeling alcohol use trajectories from early to mid-adolescence, Khurana et al. (2013) found that pre-existing weakness in WM predicted individual differences in drinking frequency at baseline as well as the rate of increase in drinking frequency over time. Furthermore, consistent with the neurobehavioral imbalance model, impulsivity dimensions of AWT and DD (indicators of strong reward but weak executive control system) mediated the relation between WM and alcohol use in their sample, whereas SS did not (Khurana et al., 2013). However, this study did not explore the possibility of qualitatively distinct trajectories of alcohol use. It is possible that the average alcohol use trajectory modeled for the entire sample could have included heterogeneous groups that were at low- or high-risk based on the patterns of alcohol use and the underlying vulnerabilities. Furthermore, this study was focused exclusively on alcohol use, developmental trajectories of other addictive drugs commonly used by adolescents, such as cigarettes and marijuana, were not examined. Based on past research documenting similar developmental course and underlying risk profiles for common drugs of onset (Jackson, Sher, & Schulenberg, 2008), it can be expected that an imbalance between the reward and control systems would be associated with escalating use of all three commonly used drugs (alcohol, marijuana, and tobacco) during early adolescence.
Present Study
In this study, we test the implications of the neurobehavioral imbalance model for early drug use progression in human adolescents. We evaluate the level of functioning of the executive control system using individual differences in adolescents’ working memory (WM) ability. Reward motivation is assessed using self-reported SS tendencies, as well as other impulsivity dimensions that uniquely represent both reward sensitivity and loss of executive control. Based on the model, we expect that an imbalance between the two systems, as indexed by low executive control and high reward sensitivity (captured by AWT and DD) will be predictive of early escalation in drug use. On the other hand, occasional experimentation that is not followed by progression in drug use is likely to be associated with a relatively stronger executive control system that is better able to regulate the reward-driven tendencies (captured by SS).
Using a longitudinal sample of 11–13 year olds at baseline, we modeled heterogeneous patterns of common drug use (alcohol, marijuana, and tobacco) from early to middle adolescence. Based on past research (Li et al., 2001; Sher, Jackson, & Steinley, 2011), we expected to observe at least two discrete drug use patterns in our sample – (1) a low-use/low-risk group comprised of abstainers and occasional experimenters, and (2) a high-use/high-risk group characterized by consistent and progressive use of all three drugs. Next, we tested whether baseline differences in WM and associated impulsivity dimensions (SS, AWT, and DD) were able to predict likelihood of membership in these distinct drug use groups. We hypothesized AWT and DD to be predictive of membership in the high-use/high-risk trajectory group as these tendencies represent a lack of executive control as indexed by their negative associations with WM. SS, on the other hand, was hypothesized to be a predictor of membership in the low-use/low-risk group, because occasional experimentation is likely to signify heightened sensitivity to rewards but it does not necessarily represent a lack of executive control. Separate supplemental analyses were conducted as needed to examine differences between the trajectory groups.
Method
Participants
Data were obtained from a five year longitudinal study of an urban, community-based sample of adolescents (N=382), ages 10–12 at baseline (Mean age = 11.4 ± 0.87 years; 52% females). Only the last four annual assessments of this longitudinal study were used in the present analyses, because the low rate (<4%) of any drug use at the first assessment resulted in unstable estimates and poor model convergence. The four time points used in the present analyses will hereafter be referred to as T1 – T4. The mean age of the sample at T1 was 12.4 ± 0.87 years (Range: 11–13 years). Adolescents were recruited mainly through schools in the Philadelphia area, as well as from libraries and community centers. The majority of the sample was from low-middle socio-economic background, assessed using the Hollingshead Two-Factor Index of Social Status (Mean = 47.0±15.8; reverse scored). In terms of racial-ethnic composition, most participants self-identified as non-Hispanic white (56%), followed by non-Hispanic black (26%), Hispanic (9%) and the remaining 9% were categorized in the other category, primarily including Asian and Native American, due to their low representation. Further details regarding recruitment and testing are provided in Romer et al. (2009). There was 6% loss to follow-up over the course of the study. Missing data were handled using full information maximum likelihood (FIML) in Mplus v7. Time-invariant covariates (sex, age, race-ethnicity, and socio-economic background) and predictors (WM, AWT, SS) included in the model were assessed at T1. DD, assessed at T2, was also included in the model.
Measures
Working Memory (WM)
Participant WM was assessed based on performance on the following tasks – (1) Digit span backwards (2) Corsi-block tapping (3) Letter-two-back, and (4) a spatial WM task. All four tasks were found to significantly load on a single latent factor, with loadings ranging from 0.40–0.60. All tasks were scored such that higher scores indicated better WM performance. A brief description of these tasks follows.
Corsi-block tapping
This task is a non-verbal variant of the digit span task (Milner, 1971). Participants view a set of identical blocks that are spatially dispersed on the screen, and are individually lit up in a random sequence. Participants are asked to tap each box in the reverse order of the sequence of lit boxes. We used the total correct score, ranging from 0 – 12, as an indicator of WM performance on this task. This task also assesses spatial working memory as the visual sequence must be maintained and reversed in working memory in order to guide the response.
Digit Span Backwards
This task tests the auditory-verbal working memory of participants by having them repeat back in reverse order, sequences of digits to the experimenter. The test was administered in standard format according to the procedures listed in the Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) manual (Wechsler, 2003). The WISC digit span total raw score, ranging from 4 – 14, was used as an indicator of WM performance for this task.
Letter two-back
This task involves monitoring a series of letters for a repeat “two-back.” Letters are presented for 500 milliseconds each, separated by a 1 second interval. Participants must continually update their working memory in order to compare the current letter to the letter shape presented two trials back. This task was adapted for children by Casey et al. (1995). The participants’ total correct score, ranging from 38–122, was used as an indicator of their WM performance on this task.
Spatial Working Memory
This self-directed computerized task requires the participant to search for hidden tokens one at a time within sets of four to eight randomly positioned boxes. Tokens are hidden only once in each box per trial. Working memory skills are tapped as the participant while searching must hold in working memory the locations already checked and as tokens are found, must remember and update information about the locations of those tokens (Owen, Downes, Sahakian, Polkey, & Robbins, 1990). Between-search errors on this task are made if the participant returns to a box where a token had already been found during a previous search sequence. In comparison, a within-search error is made when a participant returns to a box that has already been checked during the same search sequence. Working memory performance was indexed using the between-search errors score, since it places more demands on working memory of participants as they have to remember where the token was found in the last search when beginning a new search for the next token. The between-search error score was reverse scored to indicate better WM performance, and ranged from 0 – 109.
Recent Drug use
Self-reported recent (past 30 days) use of alcohol, marijuana and tobacco was assessed using a binary (yes/no) response variable at each of the four repeated assessments. Even though participants were asked to report their frequency of recent drug use, frequency of use was quite low in this age group producing extremely skewed responses. Hence, we used consistency in the yes/no categorization for the present analyses, which enabled us to observe progression in drug use over time. Responses on recent drug use for the three drugs were summed to create a recent drug use index, ranging from 0 (no use), 1 (used only one drug), 2 (used two of the three drugs), and 3 (used all three drugs) in the past 30 days. This outcome variable was used when modeling cumulative drug use patterns. Descriptive statistics of individual drug use and cumulative drug use patterns across the four time points can be found in Table 1.
Table 1.
Descriptive statistics (mean & sd) of recent (past 30 days) drug use (individual and overall) across the four assessment time points.
| Variable Names | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Alcohol use (Range: 0–1) | 0.08 (0.27) | 0.11 (0.31) | 0.15 (0.36) | 0.27 (0.44) |
| Cigarette use (Range: 0–1) | 0.01 (0.12) | 0.04 (0.19) | 0.07 (0.27) | 0.14 (0.35) |
| Marijuana use (Range: 0–1) | 0.01 (0.09) | 0.03 (0.17) | 0.04 (0.21) | 0.15 (0.36) |
| Overall drug use (Range: 0–3) | 0.10 (0.34) | 0.17 (0.51) | 0.26 (0.63) | 0.47 (0.89) |
Sensation Seeking (SS)
Adolescents’ tendency to seek exciting and novel behaviors was assessed using the brief sensation-seeking scale (Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002). Four items that were representative of each of the four dimensions of SS (i.e., experience seeking, boredom susceptibility, thrill/adventure seeking, and disinhibition) were included in our scale. Respondent’s level of agreement with each of these items was assessed using a Likert-type scale ranging from 1 (strongly disagree) to 4 (strongly agree). Confirmatory factor analysis revealed high item loadings (0.53–0.73), representing a single underlying latent factor, with a rho of 0.77. Participants’ scores on the four items were averaged to create a sensation seeking index that ranged from 1–4.
Acting-Without-Thinking (AWT)
Nine self-report items were used to assess participants’ tendency to act on impulse, without thinking through the consequences (e.g., do you usually do or say things without thinking?) with a binary (yes/no) response option (Eysenck, Easting, & Pearson, 1984; Kuo, Chih, Soong, Yang, & Chen, 2004). The items loaded on a single latent factor, with loadings ranging from 0.37 – 0.71, with a rho of 0.80. Responses on the nine items were averaged to create an AWT score which ranged from 0–1.
Delay Discounting (DD)
Participants’ ability to delay immediate gratification was assessed using a hypothetical monetary choice procedure adapted from Green, Fry, & Myerson (1994). Respondents were asked in the context of payment for a job to identify an amount of money between $10 and $90 that, if received immediately, would be equivalent to receiving $100 six months later. Respondents are initially asked if they would accept a payment of $50 immediately in lieu of being paid $100 in six months. Those who accepted the $50 offer, were then asked if they would accept an amount lower than $50 in $10 decrements. The lowest amount they accepted was taken as their equivalent value. A comparable procedure with successively increasing values was used for those who did not accept $50. Scores on this variable ranged from 10 – 100, which were reverse-scored such that higher scores were indicative of greater delay discounting (i.e. greater inability to delay gratification). Similar procedures have been shown to be valid with this age-group youth (Duckworth & Seligman, 2005) and to be valid indicators of the ability to delay gratification (Reynolds & Schiffbauer, 2005). Research comparing hypothetical with real rewards and delays indicates that this procedure produces comparable estimates of individual differences (Johnson & Bickel, 2002). Delay discounting was assessed starting at Wave 3 of the original study (time point T2 in present analyses).
Analytic Approach
The purpose of this study was two-fold. Our first goal was to assess the heterogeneity in the patterns of common drug use in an early adolescent sample. Our second goal, informed by the neurobehavioral imbalance model of drug addiction, was to test the role of weak WM as an underlying risk factor that could serve to distinguish between different drug use trajectory groups and their relationships with the different impulsivity dimensions. To that end, we conducted a conditional Latent Growth Class Analysis (LGCA) to determine the number and nature of latent classes of drug use trajectories in our early adolescent sample and examined the ability of pre-existing weaknesses in WM to distinguish between distinct patterns of early drug use as mediated by SS, AWT, and DD.
LGCA is a group-based modeling approach that helps to identify discrete classes (of individuals) within the population that differ from each other in terms of their starting values and patterns of change on an outcome variable over time. Individuals that are similar to each other in terms of their intercepts and slopes are grouped into one homogeneous latent class. Unlike latent growth curve modeling, which assumes that a single developmental trajectory captures the drug use patterns for the entire sample, LGCA tests for the presence of one or more relatively homogeneous groups within the sample (Bauer & Reyes, 2010). In conditional LGCA models, individual membership in these latent classes can be predicted by model covariates/risk factors (Muthén & Muthén, 2000). This analytic method was chosen because of its utility in identifying population sub-groups based on developmental trajectories of drug use, and examining the underlying vulnerabilities that pre-dispose membership in the high-risk group.
Models with increasing number of latent classes were tested, starting with a single latent class, and the fit statistics were compared to evaluate model fit. Adolescents were not artificially assigned to the latent class to which they had the highest likelihood of belonging. Instead, each individual’s probability of belonging to each class was used as weights in the analyses. All analyses, including descriptive statistics, are based on adolescents’ estimated probability of belonging to the different latent classes. This probabilistic classification of individuals into the different latent classes was also the outcome variable used in the regression analyses. Key socio-demographic variables like age, sex, race-ethnicity, and socio-economic background were included as covariates in the model.
Results
Model Fit
Both linear and quadratic growth curves were estimated for each of the three drugs and the cumulative drug use index. In each case, the linear model provided a better fit to the data. Several model fit criteria specified by Muthén (2004) were used to evaluate the optimal number of classes in our sample, including lower BIC (Bayesian Information Criteria) values; non-significant adjusted LMR LRT (Lo-Mendell Rubin Likelihood Ratio Test) and bootstrap LRT test for deciding on k vs. k + 1 number of classes; high entropy score (with values close to 1), class prevalence (>5%) and class interpretability (based on underlying theory and past research).
A 2-class solution was found to fit the data well for all three drugs individually as well as for the cumulative common drug use index. The latent class distribution and characteristics were found to be similar for the three drugs – alcohol (LC1 = 35%; LC2 = 65%), tobacco (LC1 = 15%; LC2 = 85%), and marijuana (LC1 = 11%; LC2 = 89%). The role of the key predictors (WM and associated impulsivity dimensions) in predicting adolescents’ likelihood of membership in these two latent classes was also comparable for the three drugs. Therefore, instead of focusing on each of these drugs individually, we used the cumulative common drug use index as our final outcome variable.
The following criteria resulted in the selection of a 2-class solution for the cumulative drug use outcome variable – lower BIC value: 14399 (2 class) vs. 14423 (3 class) and 15709 (1 class); non-significant p-values for the 3 vs. 2 class solution using the adjusted LMR LRT test (p = 0.27) and the Bootstrap Likelihood Ratio Test (p = 0.38); and significant p-values (p < 0.001) for 1 vs. 2 class solution using LMR LRT and bootstrap LRT. High classification probabilities (>0.91), high entropy (0.80), and class prevalence (>5% of sample) also suggested that a 2 class solution provided a good fit to the data, with clear distinguishability between the two classes.
Latent Class Characteristics
The first latent class (30.2%, n=115) exhibited a high starting value and a steep slope, with consistent and progressive drug use at each follow-up. In contrast, the second latent class (69.8%, n=267) was characterized by low starting values of drug use with relatively low use at all assessments (see Figure 1). Based on the item response probabilities for the repeated assessments (plotted in Fig 2a & 2b), the first latent class was labeled as ‘Progressors’ given the high probability of use of one or more drugs over the course of the study, while the second latent class was labeled as ‘Low/non-users’ because of the minimal drug use over the course of the study. Although the ‘low/non-users’ latent class included both the ‘experimenters’ and the ‘abstainers’, the variability between the patterns of drug use was not significant enough to necessitate two separate latent classes. More importantly, the experimenters and abstainers did not differ from each other in terms of the underlying risk factors. Sample descriptives based on adolescents’ estimated probabilities of belonging to each of the two latent classes can be found in Table 2.
Figure 1.
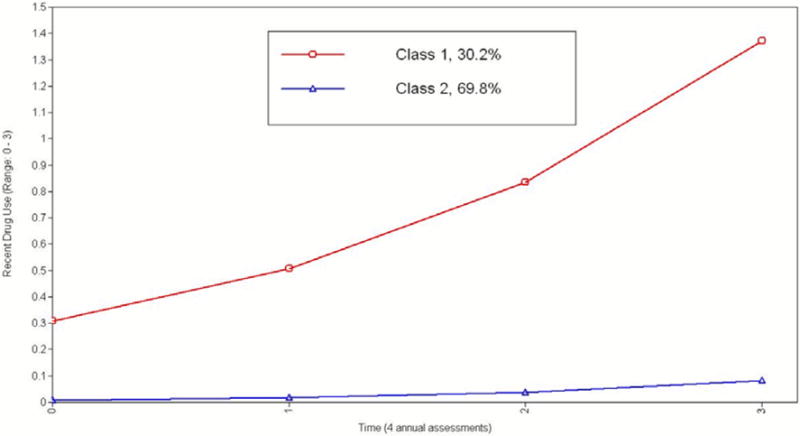
Estimated recent drug use curves from the 2-class latent growth class analysis solution.
Note. Class 1 = “Progressors”; Class 2 = “Low/Non-users”
Figure 2.
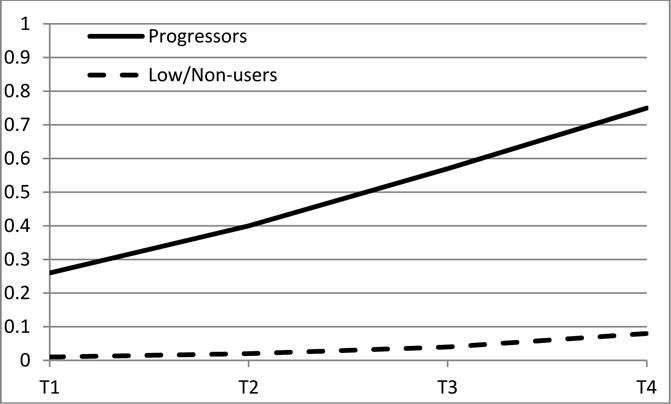
Item response probabilities for recent (past 30 days) drug use:
(a). Class-specific probabilities of at least one drug used in the past 30 days;
(b). Class-specific probabilities of having used more than one drug in past 30 days
Table 2.
Sample statistics (Means/Proportions) weighted by estimated class probabilities
| Non-weighted | Weighted | ||
|---|---|---|---|
|
| |||
| Variable Name |
Full Sample Mean (SD) |
Progressors Means (SD) |
Low/non-users Means (SD) |
| Drug Use (T1; Range: 0–3) | 0.10 (0.34) | 0.29 (0.56) | 0.01 (0.11) |
| Drug Use (T2; Range: 0–3) | 0.17 (0.51) | 0.55 (0.81) | 0.02 (0.12) |
| Drug Use (T3; Range: 0–3) | 0.26 (0.63) | 0.80 (0.91) | 0.03 (0.17) |
| Drug Use (T4; Range: 0–3) | 0.47 (0.89) | 1.38 (1.13) | 0.09 (0.31) |
| Corsi Block Tapping (T1) | 6.14 (3.03) | 5.99 (2.67) | 6.22 (3.18) |
| Digit Span Backwards (T1) | 7.30 (1.78) | 7.21 (1.69) | 7.37 (1.82) |
| Letter-two-back (T1) | 113.33 (9.62) | 113.15 (8.04) | 113.41 (10.25) |
| Spatial WM (T1) | 81.47 (18.50) | 78.51 (18.16) | 83.07 (18.48) |
| AWT (T1; Range: 0–1) | 0.39 (0.28) | 0.56 (0.26) | 0.32 (0.26) |
| DD (T2; Range: 10–100)* | 50.58 (29.0) | 57.84 (28.08) | 47.01 (28.85) |
| SS (T1; Range: 1–4) | 2.20 (0.73) | 2.47 (0.75) | 2.09† (0.69) |
| Age (T1) | 12.4 (0.87) | 12.77 (0.83) | 12.25 (0.84) |
| Sex (%Male) | 48% | 56% | 44% |
| SES (Sample Range: 15–77)* | 47.4 (15.4) | 44.14 (14.01) | 48.77 (15.81) |
Note.
DD and SES were reverse scored such that higher scores are indicative of greater discounting and higher SES respectively.
Within the low/non-users group, the Mean (SD) SS score for abstainers was 2.05 (0.65), while that of experimenters was 2.35 (0.65). The output for SS is not weighted, but is derived from forced assignment of participants into latent classes with highest posterior probabilities.
Predictors of Latent Class Membership
Individual probability of membership in the two latent classes was predicted by baseline scores of WM, AWT and DD. Adolescents who had a high probability of being in the “progressors” group were more likely to have weak WM and had higher scores of AWT and DD at baseline (see Fig 3). The effect of weak WM on higher likelihood of membership in the “progressors” group was significant and fully mediated by the associated impulsivity dimensions of AWT and DD, β (SE) = 0.26 (0.12), p < 0.05. There was no direct effect of WM on latent class membership, β (SE) = −0.23 (0.19), p =0.21. Although SS was correlated with AWT (r = 0.38, p < 0.001), SS did not predict latent class membership, once the effect of AWT and DD was controlled. This was anticipated inasmuch as SS tended to be positively correlated with WM over the waves of the study (r T1 = 0.05, r T2 = 0.17, r T3 = 0.12, r T4 = 0.15), as has been reported in previous analyses with this sample (Romer et al., 2011). Since SS did not have a significant predictive or mediating role, it was not included in the final model.
Figure 3.
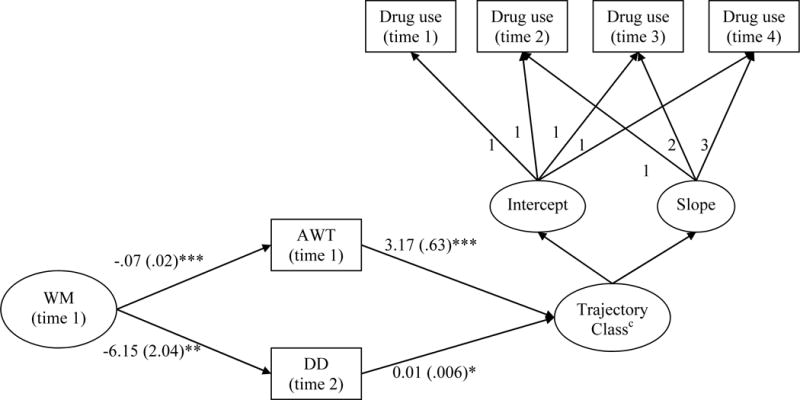
Pre-existing weaknesses in working memory predict latent classes of adolescent drug use trajectories
Note. *p<.05, **p<.01, ***p<.001. WM = Working Memory, AWT = Acting Without Thinking, DD = Delay Discounting.
cThe trajectory class variable represents latent probabilities of belonging to the “progressors” class as compared to the “low/non-users” class. Regression coefficients on significant paths represent the effects of background variables on being in the “progressors” class vs. the “low/non-users” class. Higher scores on AWT (Odds Ratio = 23.8, p < 0.001), and DD (Odds ratio = 1.01, p < 0.05) were associated with greater likelihood of being in the “progressors” than the “low/non-users” class. The mediated effect of WM suggests that high WM at baseline was inversely related to AWT and DD, resulting in a net lower likelihood of belonging to the “progressors” than the “low/non-users” class. There was no left over direct effect of WM on trajectory class membership. The effect of age, sex, and socio-economic background was covaried out.
Given that the experimenters and abstainers were grouped together in the ‘low/non-users’ latent class, in a separate follow-up analysis we further explored the differences between the experimenters (who reported yes to at least one common drug on at least one measurement occasion) and the abstainers (who reported no recent drug use on all occasions), especially in terms of underlying vulnerabilities. The experimenters comprised about 14% of the ‘low/non-users’ class (n=267), and had low and intermittent drug use patterns across all four waves. Findings from a logistic regression analyses conducted with the ‘low/non-users’ group (n=267) revealed that the experimenters had marginally high rates of SS as compared to the abstainers, β (SE) = 0.27 (0.15), p <.08, but were non-distinguishable from the abstainers in terms of their WM, AWT and DD scores. We also compared the experimenters with the progressors, and found that the experimenters did not share the underlying weakness in WM, AWT or DD as was the case with progressors. The net effect of weak WM on the likelihood of being in the “progressors” group as compared to the “experimenters” group, as mediated by AWT, β (SE) = 0.09 (0.03), p <.05, and DD, β (SE) = 0.08 (0.03), p <.01, was significant. Mean SS scores across these two groups were not statistically different.
Discussion
This study was designed to assess the translation of a neurobehavioral imbalance model of addiction derived from research with animals (Belin et al., 2008; Everitt et al., 2008) to the prediction of early drug use patterns in human adolescents. Repeated assessments of drug use in a community sample of early adolescents (ages 11–13 at baseline) were analyzed to distinguish between patterns of early drug use, and to identify the role of WM and mediating forms of impulsivity as predictors of these distinct drug use patterns. We found clear evidence of two latent classes in our early adolescent sample – ‘low/non-users’ and ‘progressors’. ‘Progressors’ had higher baseline drug use scores and were more likely to use multiple drugs persistently and increasingly over the course of the study. The low/non-user group included those who occasionally experimented with drugs (with no consistent pattern of use) as well as those who abstained from any form of drug use over the course of the study. As such, the first main finding of our study is that early use of drugs is not a uniform phenomenon and only some early initiators go on to exhibit progression of drug use during adolescence.
Our findings also supported the neurobehavioral imbalance model, which predicts that forms of impulsivity (AWT and DD) characterized by heightened sensitivity to immediate rewards and weakness in executive control (WM) are important precursors of drug use progression in early adolescence. At the same time, the model predicts that these forms of impulsivity are distinguishable from another personality dimension that has often been identified as a risk factor for drug use, namely SS (Colder et al., 2013). Consistent with what has been observed in research with rats (Belin et al., 2008), we found that SS was marginally predictive of occasional experimentation with drugs in our sample. However, it was not predictive of progression in drug use once the effects for AWT and DD were controlled. These findings suggest that adolescents high in SS may be better able to exert executive control by virtue of their greater WM ability and thus are likely to be protected from early progression of drug use. Impulsivity dimensions characterized by weakness in WM and not merely by heightened reward seeking are predictors of risk for chronic and heavy drug use (Dawes et al., 2000; de Wit & Richards, 2004; Gullo & Dawe, 2008; Tarter, Kirisci, Habeych, Reynolds, & Vanyukov, 2004). Thus, our study provides the first test of the imbalance model for early drug use in humans and shows how an imbalance between the reward and control systems can lead to early progression of drug use.
The findings also extend research done with animals by showing that AWT in addition to DD is an impulsivity dimension that predicts drug use escalation. In the research by Everitt and colleagues, rats that displayed steeper discounting of delayed rewards exhibited greater behavioral risk for addiction while rats with sensation seeking phenotypes did not (Dalley et al., 2011). The present study shows that AWT, which also reflects weakness in executive control, is an additional vulnerability factor for drug use escalation in early adolescence. Recent review of animal models suggests that these two dimensions of impulsivity may play unique roles during the different phases of the addiction process (Winstanley et al., 2010). Specifically, impulsive action (characterized by AWT) appears to be a stronger predictor of drug use progression, whereas impulsive choice (characterized by DD) is more closely related to drug use relapse. Not surprisingly, in our young sample of human adolescents, we find that the effect of AWT on drug use progression tends to be much stronger than DD. Nevertheless, weakness in top-down control as evidenced by low WM and high levels of both types of impulsivity (DD and AWT) was predictive of early progression in drug use.
Support for the imbalance model also highlights the reality that not all adolescents are subject to heightened risk taking as many have suggested (Casey & Jones, 2010; Steinberg, 2004). Although sensitivity to reward increases during adolescence as indexed by increased attraction to novel and exciting experience (Romer & Hennessy, 2007), youth with relatively weak executive control systems are far more susceptible to adverse consequences of risk taking. In our study, adolescents who occasionally experimented with drugs (but were not on a progressive drug use trajectory) did not exhibit weaknesses in WM at baseline. In fact, from a purely analytic perspective, the occasional experimenters were indistinguishable from the abstainers in terms of patterns of drug use and the underlying vulnerabilities. The heightened novelty-seeking that characterizes adolescence (and “occasional experimenters” in our study) is an evolutionarily adaptive tendency that encourages separation from parents and exploration of new identities (Spear, 2010). In contrast, the more adverse forms of risk-taking observed during adolescence, such as early progression in drug use, are linked with deficits in impulse control and other risk factors that are evident even prior to adolescence (Moffitt et al., 2011).
The finding that early weakness in WM and associated forms of impulsivity were predictive of progression in use of all three drugs adds to the conclusion that weakness in executive control can predispose to risk for SUD. According to the imbalance model, increasing use of addictive drugs will eventually undermine executive control over the use of those drugs, a phenomenon observed in both animals (Dalley et al., 2011) and humans (George & Koob, 2010). Given that all three drugs were involved in the progressive trajectory, the risk for loss of control over drug use will likely be magnified in adolescents using more than one drug at the same time. Although we did not assess SUD at this young age, our findings suggest that youth on the high risk trajectory, given their chronic patterns of drug use, will be at greater risk for SUD as they reach late adolescence. Likewise, in a longer follow-up it may be possible to examine the adverse impact of chronic drug use on impulsivity (Goldstein & Volkow, 2002) and the underlying executive control system (Squeglia, Schweinsburg, Pulido, & Tapert, 2011).
A longer follow-up may also reveal that some of the ‘occasional experimenters’ in our sample exhibit a developmentally limited rise of drug use during late adolescence, resembling the ‘adolescent-limited’ group in Moffitt’s theory (Moffitt, 1993). The ‘early progressors’, on the other hand, are more likely to have a developmental trajectory of drug use that is akin to the “life-course persistent” group. Although SS may become a significant predictor of drug use frequency during later years (Cyders et al., 2009), the imbalance model predicts that this rise would be limited to the adolescent period and would decline along with the decline in SS by early adulthood (Littlefield et al., 2009).
Although studies modeling heterogeneity in early drug use behaviors are fewer in number, the latent classes obtained in our analyses are consistent with past findings, with a few exceptions of studies that involved larger sample sizes (Colder et al., 2002; Tucker, Ellickson, Orlando, Martino, & Klein, 2005). Our sample is also unique in that it is a community-based urban sample and thus relatively higher risk as compared to school-based samples used in previous studies. Therefore, the typical variations in normative patterns of drug use may be limited in our sample. Nevertheless, our 2-class solution is in line with the developmental patterns of early drug use (Maggs & Schulenberg, 2005) and resembles past findings with early adolescent samples (Chassin, Flora, & King, 2004; Li et al., 2001; White, Pandina, & Chen, 2002). Furthermore, our classes were of meaningful sizes (>5%) and had practical implications, a criterion that is often overlooked resulting in over-extraction of classes (Bauer & Reyes, 2010). During later years, we do expect the number of drug use trajectory classes in our sample to increase. Specifically, the experimenter group is expected to diverge into two groups - the consistently low users/abstainers and the developmentally limited high users.
Implications for Intervention
By empirically testing the neurobehavioral imbalance model of the etiology of SUD, the present study identified the individual vulnerabilities that precede and predict dysfunctional forms of early drug use and serve to distinguish it from the more normative experimentation with drugs. The core deficits in top-down control over impulsive tendencies that we examined can be detected at an early age and are found to act as “developmental snares” (Moffitt et al., 2011) predisposing the adolescent to other risks besides SUD (Krueger et al., 2002). Therefore, targeting these weaknesses at an early stage may prove to be more advantageous than simply delaying the age at onset of drug use. Since many of the risk factors that predispose youth to risk for SUD were shared by the “early progressors” and not “occasional experimenters” (George & Koob, 2010), our results tend to suggest that the robust association observed between early drug use initiation and SUD (Grant & Dawson, 1997; Hawkins et al., 1997) is more likely attributable to early escalation in drug use than mere experimentation. Interventions that can reduce adolescents’ vulnerability to progressive drugs use during younger years may also help reduce their risk of developing SUD later in life.
Our findings pertaining to the role of WM highlight the potential of cognitive training interventions as a means for enhancing executive control (Diamond & Lee, 2011). WM training-based interventions (Jaeggi, Buschkuehl, Jonides, & Shah, 2011; Klingberg, 2010) could be considered as a potential means to reduce problematic drug use during adolescence and later SUD risk. To improve chances of far-transfer effects on to behavioral control, WM training could be delivered in combination with problem-solving and social-skill building interventions that provide a context to translate cognitive skills into behavioral change (Botvin & Griffin, 2004; Greenberg, Kusche, Cook, & Quamma, 1995). Interventions that can improve self-control can be especially helpful for preventing adolescents from embarking on potentially harmful forms of drug use by targeting an important underlying vulnerability (Moffitt et al., 2011). Early detection can also help in delivering interventions at an early stage before the addictive and damaging drug use processes become more established.
Limitations
Our findings should however be interpreted in light of the study limitations. First, due to lack of information, we could not account for the influence of other variables besides WM, such as family history of substance abuse (Dawson & Grant, 1998) and deviant peer influences (Dishion & Owen, 2002), that can impact adolescent drug use trajectories. Second, although our measure of recent (past 30 days) drug use was less subject to recall decay, it is limited in the amount of information it can provide us about the drug use patterns during the 12 month time period between assessments. Finally, we did not examine reverse effects of substance use on WM (Brown & Tapert, 2004) and impulsivity (Bickel & Yi, 2008), since that was not the focus of this study and these effects were unlikely to have already developed given the still low levels of drug use in our early adolescent sample. By examining the effects of both WM and impulsivity prior to the escalation of drug use, we can be relatively confident that the patterns we observed were at least partly attributable to early vulnerabilities in WM and associated forms of impulsivity.
Conclusion
In sum, by testing an emerging neurobehavioral model (De Wit & Richards, 2004; Everitt & Robbins, 2005), our findings reveal that individual differences in the balance between the reward motivation system and the executive control system influence early drug use patterns in human adolescents, much as they appear to influence the progression of drug use in rats. Thus, our findings highlight fundamental similarities in the functioning of mammalian brain systems that control the onset and progression of drug use. Our study also highlights the critical role played by WM as an indicator of the executive control system in predicting early forms of drug use, through its divergent associations with SS and impulsivity dimensions of AWT and DD. Specifically, we found evidence that impulsivity dimensions associated with weak executive control predated drug use onset and were associated with progressive patterns of drug use over time. Future research would benefit from systematically examining the role of distinct impulsivity dimensions and the underlying neural systems as predisposing risk factors in the development of drug use behaviors.
Acknowledgments
The project was supported by grants R01DA018913 and R01DA033996 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45(1):143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion and Personality. Amsterdam, North Holland: Elsevier Science; 1985. pp. 137–146. [Google Scholar]
- Bauer DJ, Reyes HLM. Modeling variability in individual development: Differences of degree or kind? Child Development Perspectives. 2010;4(2):114–122. doi: 10.1111/j.1750-8606.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R. Temporal discounting as a measure of executive function: Insights from the competing neuro-behavioral decision system hypothesis of addiction. Advances in health economics and health services research. 2008;20:289–309. [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: Personality and cognitive correlates. Experimental and Clinical Psychopharmacology. 2009;17(1):51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvin GJ, Griffin KW. Life skills training: Empirical findings and future directions. The Journal of Primary Prevention. 2004;25(2):211–232. doi: 10.1023/B:JOPP.0000042391.58573.5b. [DOI] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: Basic to clinical studies. Annals of the New York Academy of Sciences. 2004;1021(1):234–244. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329(5991):532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113(4):483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: Multiple trajectories and their psychosocial correlates. Health Psychology. 2000;19(3):223–231. doi: 10.1037/0278-6133.19.3.223. [DOI] [PubMed] [Google Scholar]
- Colder CR, Campbell RT, Ruel E, Richardson JL, Flay BR. A finite mixture model of growth trajectories of adolescent alcohol use: Predictors and consequences. Journal of Consulting and Clinical Psychology. 2002;70(4):976–985. doi: 10.1037/0022-006X.70.4.976. [DOI] [PubMed] [Google Scholar]
- Colder CR, Hawk LW, Lengua LJ, Wiezcorek W, Eiden RD, Read JP. Trajectories of reinforcement sensitivity during adolescence and risk for substance use. Journal of Research on Adolescence. 2013;23(2):345–356. doi: 10.1111/jora.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(20):5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104(2):193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes MA, Antelman SM, Vanyukov MM, Giancola P, Tarter RE, Susman EJ, Clark DB. Developmental sources of variation in liability to adolescent substance use disorders. Drug and Alcohol Dependence. 2000;61(1):3–14. doi: 10.1016/S0376-8716(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF. Family history of alcoholism and gender: Their combined effects on DSM-IV alcohol dependence and major depression. Journal of Studies on Alcohol and Drugs. 1998;59(1):97–106. doi: 10.15288/jsa.1998.59.97. [DOI] [PubMed] [Google Scholar]
- De Wit H, Richards JB. Dual determinants of drug use in humans: Reward and impulsivity. Nebraska Symposium on Motivation. 2004;50:19–55. [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American Journal of Psychiatry. 2000;157(5):745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15(2):217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Owen LD. A longitudinal analysis of friendships and substance use: Bidirectional influence from adolescence to adulthood. Developmental Psychology. 2002;38(4):480–491. doi: 10.1037/0012-1649.38.4.480. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychological Science. 2005;16(12):939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Strycker LA. Alcohol use from ages 9 to 16: A cohort-sequential latent growth model. Drug and Alcohol Dependence. 2006;81(1):71–81. doi: 10.1016/j.drugalcdep.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: Impulsive versus sensation-seeking personality traits. Biological Psychiatry. 2010;68(8):770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Easting G, Pearson PR. Age norms for impulsiveness, venturesomeness and empathy in children. Personality and Individual Differences. 1984;5(3):315–321. doi: 10.1016/0191-8869(84)90070-9. [DOI] [Google Scholar]
- Eysenck SBG, Eysenck HJ. Impulsiveness and venturesomeness in children. Personality and Individual Differences. 1980;1(1):73–78. doi: 10.1016/0191-8869(80)90006-9. [DOI] [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. Early adolescent through young adult alcohol and marijuana use trajectories: Early predictors, young adult outcomes, and predictive utility. Development and Psychopathology. 2004;16(01):193–213. doi: 10.1017/S0954579404044475. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews. 2010;35(2):232–247. doi: 10.1016/j.neubiorev.2010.05.002. doi:10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10(3):203–205. doi: 10.1111/1467-9280.00135. [DOI] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. doi:16/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5(1):33–36. doi: 10.1111/j.1467-9280.1994.tb00610.x. [DOI] [Google Scholar]
- Greenberg MT, Kusche CA, Cook ET, Quamma JP. Promoting emotional competence in school-aged children: The effects of the PATHS curriculum. Development and Psychopathology. 1995;7(01):117–136. doi: 10.1017/S0954579400006374. [DOI] [Google Scholar]
- Gullo MJ, Dawe S. Impulsivity and adolescent substance use: Rashly dismissed as “all-bad”? Neuroscience & Biobehavioral Reviews. 2008;32(8):1507–1518. doi: 10.1016/j.neubiorev.2008.06.003. doi:16/j.neubiorev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. The Journal of Neuroscience. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol and Drugs. 1997;58(3):280. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WYW, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biological Psychiatry. 2010;68(3):287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences. 2002;32(3):401–414. doi: 10.1016/S0191-8869(01)00032-0. [DOI] [Google Scholar]
- Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. The American Journal of Drug and Alcohol Abuse. 2008;34(3):239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE. Conjoint developmental trajectories of young adult substance use. Alcoholism: Clinical and Experimental Research. 2008;32(5):723–737. doi: 10.1111/j.1530-0277.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/PL00005483. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction. 2013;108(3):506–515. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in Cognitive Sciences. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. doi: 10.1037/0021-843X.111.3.411. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Chih YC, Soong WT, Yang HJ, Chen WJ. Assessing personality features and their relations with behavioral problems in adolescents: tridimensional personality questionnaire and junior eysenck personality questionnaire. Comprehensive Psychiatry. 2004;45(1):20–28. doi: 10.1016/j.comppsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Li F, Duncan TE, Hops H. Examining developmental trajectories in adolescent alcohol use using piecewise growth mixture modeling analysis. Journal of Studies on Alcohol and Drugs. 2001;62(2):199–210. doi: 10.15288/jsa.2001.62.199. [DOI] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK. Is “maturing out” of problematic alcohol involvement related to personality change? Journal of abnormal psychology. 2009;118(2):360–374. doi: 10.1037/a0015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK. Impulsivity: the behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- Maggs JL, Schulenberg JE. Initiation and course of alcohol consumption among adolescents and young adults. In: Galanter M, Lowman C, Boyd GM, Faden VB, Witt E, Lagressa D, editors. Recent Developments in Alcoholism. Vol. 17. New York: Kluwer Academic Publishers-Plenum Publishers; 2005. pp. 29–47. Retrieved from http://www.springerlink.com/content/l8n514715822044j/ [DOI] [PubMed] [Google Scholar]
- Magid V, MacLean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive behaviors. 2007;32(10):2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism, Clinical and Experimental Research. 2001;25(8):1156–1165. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. doi: 10.1037/0033-295X.100.4.674. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The SAGE Handbook of Quantitative Methodology for the Social Sciences. Thousand Oaks, CA: SAGE; 2004. pp. 345–368. [Google Scholar]
- Muthén Bengt, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research. 2000;24(6):882–891. doi: 10.1111/j.1530-0277.2000.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, Moffitt TE. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychological Science. 2008;19(10):1037–1044. doi: 10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–1034. doi: 10.1016/0028-3932(90)90137-D. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behaviour. Trends in Pharmacological Sciences. 2008;29(4):192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcoholism: Clinical and Experimental Research. 1999;23(1):101–107. doi: 10.1111/j.1530-0277.1999.tb04029.x. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Harden KP. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Development and Psychopathology. 2013;25(s1):223–239. doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Reynolds C, Venables PH, Mednick SA. Stimulation seeking and intelligence: a prospective longitudinal study. Journal of Personality and Social Psychology. 2002;82(4):663–674. [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Delay of gratification and delay discounting: A unifying feedback model of delay-related impulsive behavior. Psychological Record. 2005;55(3):439. [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, De Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences. 2012;16(1):81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11(2):250–257. doi: 10.1016/S0959-4388(00)00204-X. [DOI] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology. 2010;52(3):263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47(13):2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Yang W, Hurt H. Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Developmental Science. 2011;14(5):1119–1133. doi: 10.1111/j.1467-7687.2011.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prevention Science. 2010;11(3):319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Hennessy M. A Biosocial-Affect Model of Adolescent Sensation Seeking: The Role of Affect Evaluation and Peer-Group Influence in Adolescent Drug Use. Prevention Science. 2007;8(2):89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Gray JR. Individual differences in delay discounting. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Jackson KM, Steinley D. Alcohol use trajectories and the ubiquitous cat’s cradle: Cause for concern? Journal of Abnormal Psychology. 2011;120(2):322–335. doi: 10.1037/a0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14(2):155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Spear L. The Behavioral Neuroscience of Adolescence. New York, NY: W. W. Norton & Company; 2010. [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35(10):1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, Merikangas KR. Use and abuse of alcohol and illicit drugs in US adolescents: Results of the National Comorbidity Survey-Adolescent Supplement. Archives of General Psychiatry. 2012;69(4):390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and alcohol dependence. 2004;73(2):121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: A maturational perspective. Development and Psychopathology. 1999;11(04):657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Orlando M, Martino SC, Klein DJ. Substance use trajectories from early adolescence to emerging adulthood: a comparison of smoking, binge drinking, and marijuana use. Journal of Drug Issues. 2005;35(2):307–332. doi: 10.1177/002204260503500205. [DOI] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children® — Fourth Edition (WISC®-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- White HR, Pandina RJ, Chen PH. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug and Alcohol Dependence. 2002;65(2):167–178. doi: 10.1016/S0376-8716(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism: Clinical and Experimental Research. 2010;34(8):1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. The Journal of Neuroscience. 2008;28(53):14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol–disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Development Perspectives. 2011;5(4):248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


