Abstract
In this study, EGFR-activating mutation status and DNA copy number abundances of members of ErbB family were measured in 261 lung adenocarcinomas. The associations between DNA copy number abundances of ErbB family, EGFR-activating mutation status, and prognosis were explored. Results showed that DNA copy number abundances of EGFR, ERBB2, ERBB3, and ERBB4 had associations with overall survival in lung adenocarcinoma with EGFR-activating mutations. In the stratification analysis, only ERBB2 showed significant discrepancy in patients carrying wild type EGFR and other members of ErbB family in patients carrying EGFR-activating mutation. This indicated that CNAs of ErbB family had effect modifications of EGFR-activating mutation status. Findings of this study demonstrate potential molecular guidance of patient management of lung adenocarcinoma with or without EGFR-activating mutations.
Keywords: lung adenocarcinoma, DNA copy number abundance, ErbB family, EGFR-activating mutation, prognosis
INTRODUCTION
Lung cancer was the leading cause of cancer death worldwide [1]. It was divided into small-cell lung cancer (SCLC, comprising 20% of lung cancers), and non-small-cell lung cancer (NSCLC, comprising 80% of lung cancers). The global 5-year survival rates of NSCLC remained low, ranging from 10% to 15%[1]. NSCLC is characterized by the accumulation of multiple genetic alterations that results from the inactivation of tumor suppressor genes, activation of oncogenes, and epigenetic changes. Among all subtypes of NSCLC, adenocarcinoma was the most common type found in women and in non-smokers [2, 3]. Currently, surgery remains the gold standard for early stage NSCLC.
The receptor tyrosine kinase (RTK) super-family of cell surface receptors serves as mediators of cell signaling by extra-cellular growth factors [4–6]. ErbB family was one of RTK super-family. The ErbB family including EGFR (also known as ERBB1 or HER1), ERBB2 (also known as HER2), ERBB3 (also known as HER3), and ERBB4 (also known as HER4) had received much attention and their strong association with malignant proliferation had been investigated [6–9]. Activation of ErbB family proteins stimulates many intracellular signaling pathways such as MAPK and (PI3K)–AKT pathways [10, 11]. Other important ErbB signaling effectors are the signal transducer and activator of transcription proteins such as STATs [12], which often associates with EGFR activation [13], SRC tyrosine kinase, the activity of which is increased in response to EGFR and ERBB2 signalling [14], and mammalian target of rapamycin (mTOR), a serine/threonine kinase activates downstream of I3K—AKT and other growth regulators [15].
In this study, EGFR-activating mutation status and DNA copy number abundances of EGFR, ERBB2, ERBB3, and ERBB4 were measured in 261 surgically resected lung adenocarcinomas. In addition, the associations between DNA copy number abundances of above genes, EGFR-activating mutation status, and prognosis were explored. The findings of this study may provide potential biomarkers for drug response and prognosis of lung adenocarcinoma.
EGFR genomic DNA amplification leading to mRNA overexpression was often found in various types of human cancer [16, 17]. Increased mRNA expression levels of EGFR were observed in various cancers such as head and neck, ovary, cervix, bladder, oesophagus, stomach, brain, breast, endometrium, colon and lung, and frequently conferred an adverse prognosis [4, 18].
Extending previous observations of almost two decades ago [19, 20], recent retrospective analyses had reported EGFR overexpression in 62% of NSCLC cases, and its expression was correlated with DNA copy number abundance and poor prognosis [18, 21, 22]. Although DNA copy number abundance of EGFR and ERBB2 had been studied independently [23, 24], the associations between EGFR-activating mutations, whole ErbB family, and clinical prognosis in lung cancer were still needed to be investigated.
RESULTS
Clinical characteristics of patients
Among 261 patients, there were 163 patients (66.3%) with stage I disease, 33 (12.6%) with stage II, and 65 (24.9%) with stage IIIA, respectively. There were 70 patients with EGFR L858R mutation (26.8%), 73 patients with EGFR exon-19-deletion (28.0%), and 118 EGFR wild type patients (45.2%), respectively (Table 1). There were 131 male (50.2%) and 130 female (49.8%). Many male (63.36 %) were current or ex-smoker and only 3 female (2.31 %) were smoker. The percentage of female had EGFR-activating mutation (63.08%) was higher than male (46.56%) (p-value = 0.009, Fisher's exact test). Never smokers had higher EGFR-activating mutations (63.31%) comparing to smokers or ex-smokers (39.53%) (p-value = 0.0005, Fisher's exact test). Patients with EGFR-activating mutation had higher EGFR CNAs than patients without EGFR-activating mutation (p-value = 0.009, student t-test).
Table 1. Clinical characteristics of patients.
| Variable | All (%) | Wild type (%) | L858R (%) | Del-19 (%) |
|---|---|---|---|---|
| Total patients | 261 | 118 | 70 | 73 |
| Gender | ||||
| Male | 131 (50.2) | 70 (59.32) | 24 (34.28) | 37 (50.69) |
| Female | 130 (49.8) | 48 (40.67) | 46 (65.72) | 36 (49.31) |
| Smoking status | ||||
| Non-smoker | 169 (66.3) | 62 (54.38) | 53 (75.71) | 54 (76.05) |
| Ex-smoker | 40 (15.7) | 23 (20.17) | 8 (11.42) | 9 (12.67) |
| Current smoker | 46 (18.0) | 29 (25.43) | 9 (12.85) | 8 (11.26) |
| Smoking years | ||||
| 0 | 169 (66.3) | 62 (54.38) | 53 (75.71) | 54 (76.05) |
| ≤20 | 23 (9.0) | 11 (9.64) | 7 (10.00) | 5 (7.04) |
| 21-40 | 41 (16.1) | 25 (21.92) | 7 (10.00) | 9 (12.67) |
| >40 | 22 (8.6) | 16 (14.03) | 3 (4.28) | 3 (4.22) |
| Dose of cigarette smoking | ||||
| 0 package | 169 (66.3) | 62 (54.38) | 53 (75.71) | 54 (76.05) |
| ≤20 packages | 28 (11.0) | 10 (8.77) | 9 (12.85) | 9 (12.67) |
| 21-40 packages | 26 (10.2) | 18 (13.15) | 5 (7.14) | 3 (4.22) |
| >40 packages | 32(12.6) | 24 (21.05) | 3 (4.28) | 5 (7.04) |
| Smoking-quitted for year | ||||
| Non-smoker | 169 (66.3) | 62 (54.38) | 53 (75.71) | 54 (76.05) |
| Quitted > 15 years | 14 (5.5) | 8 (7.01) | 3 (4.28) | 3 (4.22) |
| Quitted < 15 years | 26 (10.2) | 15 (13.15) | 5 (7.14) | 6 (8.45) |
| Current smoker | 46 (18.0) | 29 (25.43) | 9 (12.85) | 8 (11.26) |
| Histology type | ||||
| Adenocarcinoma | 249 (95.4) | 110 (93.22) | 68 (97.14) | 71 (97.26) |
| BAC | 12 (4.6) | 8 (6.77) | 2 (2.85) | 2 (2.73) |
| Stage | ||||
| I | 163 (62.5) | 67 (56.77) | 47 (67.14) | 49 (67.12) |
| II | 33 (12.6) | 21 (17.79) | 8 (11.42) | 4 (5.47) |
| IIIA | 65 (24.9) | 30 (25.42) | 15 (21.42) | 20 (27.39) |
| Mutation status | ||||
| Wild type | 118 (45.2) | 118 (100) | ||
| L858R | 70 (26.8) | 70(100) | ||
| Exon-19-deletion | 73 (28.0) | 73(100) |
Patients with higher DNA copy number abundance of ErbB family had shorten overall survival
The results of sensitivity analysis showed that 75% percentile of EGFR, ERBB2, ERBB4 and 50% percentile of ERBB3 CNA were the optimal cut-off points for group separation, respectively (Supplementary Tables S1, S2, S3, and S4). Patients with high ErbB family CNAs significantly had shorten overall survival than patients with lower CNAs (Figure 1). Multivariate Cox proportional hazards regression analysis with other clinical covariates adjustments showed that CNAs of ErbB family were all significant prognostic factors (Table 2).
Figure 1. Survival prediction by DNA copy number abundance of ErbB family in 261 patients.
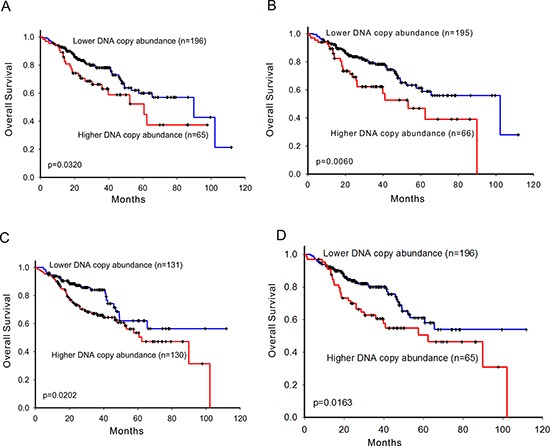
Kaplan-Meier curves for overall survival analysis on A. EGFR, B. ERBB2, C. ERBB3, and D. ERBB4. High- and low-risk groups are divided based on copy number abundance. The optimal cut points were determined by sensitivity analysis which provided the largest discrepancy in overall survival between the low- and high-risk groups on the basis of the log-rank statistic, respectively.
Table 2. Results of multivariate Cox regression.
| Gene | Adjusted HR$ | 95% C.I. | P value | |
|---|---|---|---|---|
| All patients (n = 261) | ||||
| EGFR | 1.89 | 1.16 | 3.10 | 0.011 |
| ERBB2 | 1.68 | 1.03 | 2.74 | 0.038 |
| ERBB3 | 1.65 | 1.02 | 2.68 | 0.042 |
| ERBB4 | 1.62 | 1.01 | 2.61 | 0.047 |
| Wild type (n = 118) | ||||
| EGFR | 1.13 | 0.58 | 2.22 | 0.718 |
| ERBB2 | 1.84 | 0.96 | 3.55 | 0.068 |
| ERBB3 | 0.94 | 0.51 | 1.73 | 0.841 |
| ERBB4 | 1.23 | 0.64 | 2.37 | 0.527 |
| EGFR-activating mutations (n = 143) | ||||
| EGFR | 3.53 | 1.58 | 7.87 | 0.002 |
| ERBB2 | 2.00 | 0.91 | 4.40 | 0.086 |
| ERBB3 | 2.64 | 1.17 | 5.95 | 0.019 |
| ERBB4 | 3.40 | 1.55 | 7.48 | 0.002 |
| L858R (n = 70) | ||||
| EGFR | 2.96 | 1.02 | 8.57 | 0.046 |
| ERBB2 | 2.86 | 0.91 | 9.03 | 0.074 |
| ERBB3 | 3.98 | 1.02 | 15.63 | 0.047 |
| ERBB4 | 7.22 | 2.23 | 23.36 | 0.001 |
| Exon 19 deletion (n = 73) | ||||
| EGFR | 7.25 | 1.74 | 30.27 | 0.007 |
| ERBB2 | 2.47 | 0.64 | 9.60 | 0.192 |
| ERBB3 | 3.39 | 0.88 | 13.03 | 0.076 |
| ERBB4 | 1.88 | 0.53 | 6.71 | 0.332 |
Hazard ratio
+ EGFR-activating mutations include L858R and exon-19-deletion
The association between members of ErbB family DNA copy number abundance and overall survival was evaluated by multivariate Cox hazard regression in all 261 patients, 118 wild type patients, 70 EGFR L858R patients, 73 EGFR exon-19-deletion patients, and all 143 EGFR-activating mutation patients. Potential confounding factors such age, gender, stage, and cell type were adjusted. Hazard ratio, confidence interval, and p-value were shown.
Patients carrying wild type EGFR with higher DNA copy number abundance of ERBB2 had shorten overall survival
We further investigated the associations between CNAs of ErbB family and overall survival in different EGFR-activating mutation types of patients. In the stratification analysis, 143 patients were grouped into EGFR-activating mutation carrier group (70 in L858R and 73 in exon-19-deletion) and 118 patients were grouped into EGFR wild type carrier group, respectively. In the wild type EGFR group, patients with higher CNA of ERBB2 significantly had shorten overall survival (Figure 2B) while those of EGFR, ERBB3, and ERBB4 did not (Figure 2A, 2B, and 2D). Multivariate Cox proportional hazards regression analysis also showed that ERBB2 was the only marginally significant prognostic factor in the wild type EGFR group (Table 2). This also implied that without driver mutations, such as EGFR-activating mutations, CNA of ERBB2 outperformed CNAs of other ErbB family as a prognostic factor.
Figure 2. Survival prediction by DNA copy number abundance of ErbB family in 118 patients carrying wild type EGFR.

Kaplan-Meier curves for overall survival analysis on A. EGFR, B. ERBB2, C. ERBB3, and D. ERBB4. High- and low-risk groups are divided based on copy number abundance. The optimal cut points were determined by sensitivity analysis which provided the largest discrepancy in overall survival between the low- and high-risk groups on the basis of the log-rank statistic, respectively.
Patients carrying EGFR-activating mutations with higher DNA copy number abundance of EGFR, ERBB3, and ERBB4 had shorten overall survival
In the EGFR-activating mutation carrier group, a different patterns was shown comparing with that in the wild type EGFR group. Patients with higher CNAs of EGFR, ERBB3, and ERBB4 significantly had shorten overall survival (Figure 3A, 3C, and 3D) while that ERBB2 did not (Figure 3B). Furthermore, EGFR, ERBB3, and ERBB4 were significant prognostic factors evaluated by multivariate Cox proportional hazards regression analysis (Table 2). Comparing with results in the wild type EGFR group, this may imply that ERBB2 showed different pattern with other members of ErbB family.
Figure 3. Survival prediction by DNA copy number abundance of ErbB family in 143 patients carrying EGFR-activating mutation.
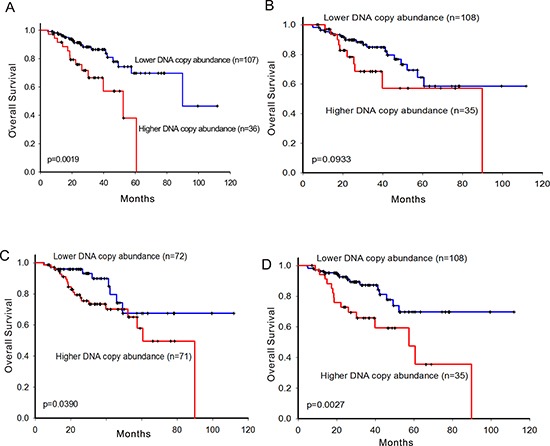
Kaplan-Meier curves for overall survival analysis on A. EGFR, B. ERBB2, C. ERBB3, and D. ERBB4. High- and low-risk groups are divided based on copy number abundance. The optimal cut points were determined by sensitivity analysis which provided the largest discrepancy in overall survival between the low- and high-risk groups on the basis of the log-rank statistic, respectively.
We further divided patientswith EGFR-activating mutations into L858R and exon 19 deletions two sub groups. There were 70 patients carrying L858R mutation and 73 patients carrying exon-19-deletion, respectively. Similar results were also found in the L858R group but not in the exon-19-deletion group (Supplementary Figure S1, Supplementary Figure S2, and Table 2). This may due to the complexity of exon-19-deletion which could be further categorized into many sub-types.
DISCUSSION
The potential confounding factors were adjusted in the Cox proportional hazards regression analysis of testing association between CNAs and overall survival. In this study, the associations between CNAs and prognosis in lung adenocarcinoma were explored. All members of ErbB family showed significant associations between CNAs and overall survival of all patients. Furthermore, patients with higher CNAs of ErbB family showed poor prognosis. In the further stratified analysis of EGFR-activating mutation status, no significant association between CNAs and overall survival was found in the wild type group. It indicated that CNAs of ErbB family had effect modifications of different EGFR-activating mutation status. This may imply that without EGFR-activating mutation, CNAs of ErbB family may not affect the prognosis of patients except ERBB2.
Sensitivity analysis was used to explore the optimal CNAs cut-point of prognosis for each gene respectively. The optimal cut-points varied from 50% to 75% in different genes. It was because that CNA was a continuous variable and higher CNA was trend to poor prognosis. Hence, when patients were di-categorized into two groups, it may toward to result in higher cut-off point. Nevertheless, a specific cut-points could be found to discriminate patients into high-risk and low-risk groups significantly having different overall survival time and CNAs of ErbB family were still potentially biomarkers of prognosis in lung cancer.
In 2011, one study, an Asian cohort, showed that EGFR copy number gain per se had no significant associations with relapse-free survival and overall survival [25]. However, this findings may be due from only 34 patients had information on EGFR mutations and large-scale study was still needed. In this study, in term of relapse-free survival, patients with higher EGFR CNA had better relapse-free survival in EGFR-activating mutation group (HR= 1.77, 95% CI=1.00 to 3.12, p-value = 0.05) (Supplementary Table S5). However, other members of ErbB family had no association with relapse-free survival (Supplementary Table S5).
Although the basic structures of genes in the ErbB family are similar, each one has distinct properties, including variation in tyrosine kinase activity[26]. Except ERBB2, all other members of ErbB family showed significant associations between CNAs and prognosis in patients with EGFR-activating mutations. This may due to the biological role of ERBB2. ERBB2 did not directly bind to any known ligand and functioned as a co-receptor binding tightly to other ligand-bound ErbB receptor family members. Such heterodimer may stabilize the ligand binding and may enhance kinase-mediated activation of downstream signaling pathways [6–9].
Among all members of ErbB family, only ERBB2 CNA showed significant discrepancy between high-risk wild type patients and low-risk wild type patients. This may imply that without EGFR-activating mutations, the effect of ERBB2 CNA, as a biomarker of prognosis, outperforms other members of ErbB family. In other studies, ERBB2 had been reported as a significant biomarker of prognosis in other cancer types without EGFR-activating mutation such as breast cancer [27–29]. As a consequence, ERBB2 CNA may be a valuable biomarker of prognosis in lung adenocarcinoma patients without EGFR-activating mutations.
CNAs of ErbB family showed effect modifications between different EGFR-activating mutation status. It indicated that CNAs of ErbB family predicted overall survival in patients with EGFR-activating mutations but not in wild type EGFR. Findings of this study demonstrated that CNAs of ErbB family provided prognosis prediction in patients with EGFR-activating mutations and provided potential molecular guidance of clinical management of lung adenocarcinoma. However, the prediction signature of patients with wild type EGFR is still not clear. It is necessary to collect more CNAs of cancer associated genes for investigation in the future.
MATERIALS AND METHODS
All the lung adenocarcinoma patients underwent surgical resection from January 2001 to March 2009 in Taichung Veterans General Hospital. The lung tumor lesions were completely resected with lymph node dissection. Thepathological diagnoses were based on the 2004 World Health Organization histologic classification system [30]. TNM (tumor, node, and metastases) staging system was used according to the 6th edition of the American Joint Committee for Cancer (AJCC) staging system [31]. Only EGFR exon 19 deletion and L858R point mutation and EGFR wild type patients were included as other EGFR mutations were rare and heterogeneous. Patients with less than 3 months follow-up were excluded. This study was approved by the institutional review boards of Taichung Veterans General Hospital (TCVGH), with written informed consent from all patients.
DNA extraction from frozen tumor tissue for genetic tests
The frozen lung cancer tissues were obtained at surgery, immediately snap frozen in liquid nitrogen and stored until use. Tumor specimens were procured for EGFR gene mutational analysis with previous description [32]. Briefly, DNA was extracted from the tumors using a QIAmp DNA Mini kit (Qiagen, Valencia, CA) following the manufacturer's protocols.
Genotyping of EGFR mutation status
The identification of EGFR-activating mutation was genotyped by Matrix Assisted Laser Desorption Ionisation Time-of-Flight Mass Spectrometry (MALDI-TOF MS) or Sanger sequence assays[33]. The MALDI-TOF MS was performed by the MassARRAY system (SEQUENOM, San Diego, CA) followed standard protocol. In the biochemical reaction, polymerase chain reaction (PCR) followed by single nucleotide extension was performed by using primers and corresponding detection probes to amplify the region containing each target mutation. After SpectroClean Resin clean up, samples were loaded onto the matrix of SpectroCHIP® by Nanodispenser (Matrix) and then analyzed by Bruker Autoflex MALDI-TOF MS. Data were collected and analyzed by Typer 4 software (Sequenom, San Diego, CA).
DNA copy number abundance
Due to the heterogeneity of cancer cells, the DNA copy number of cancer cells may not be measured identically in the tumor. It resulted that the copy number may not be an integer comparing with normal cells. Hence, we quantified DNA copy number abundance instead of categorizing CNA value into an integer such as 1, 2, or 3 copies.
The genomic real-time quantitative PCR (qPCR) was performed to measure DNA copy number abundance of each member of ErbB family. The primers and probes of qPCR were designed based on 500 franking nucleotide sequences (250 upstream and 250 downstream nucleotides) of the gene location. Fluorescence emitted by the reporter dye was detected in real time using the ABI prism 7900 sequence detection system (Applied Biosystem, Foster City, CA). Copy-number abundance (CNA) was defined as 2−ΔCt which represents the copy number fold-change between the target gene and the internal control gene GAPDH.
Statistical analysis
Clinical data collected including patient's age, gender, stage, smoking status (nonsmoking defined as patients had never smoked), date of diagnosis, progression, death or last follow-up. Overall survival (OS) was calculated from the date of surgery to the date of death. Patients were classified into the high or low risk groups based on CNAs of ErbB family. The sensitivity analysis was performed to select the optimal cut-off point of the best group separation for each gene, respectively. We screened every 5% from 20% to 75% of CNAs as cut points to evaluate the trend of multivariate Cox proportional hazards regression p-values and found that they gradually descended form 20% to the optimal cut-off point, which p-value was firstly smaller than 0.05, in each gene (Supplementary Tables S1, S2, S3, and S4).
The stratified analysis was performed to analyze the DNA CNAs in 4 groups (L858R, exon 19 deletion, activating mutations, and wild type) of different EGFR mutation status. The Kaplan-Meier method was used to estimate survival curves and the difference between survival curves was evaluated by the log-rank test. Multivariate Cox proportional hazards regression with covariates age, sex, and stage was used to evaluate independent prognostic factors associated with overall survival. All tests were two-tailed and p values less than 0.05 were considered to be significant.
SUPPLEMENTARY FIGURES AND TABLES
Acknowledgments
Integrated Core Facility for Functional Genomics and Pharmacogenomics Lab, and Mathematics in Biology Group of Institute of Statistical Science AS supported genomic, biological, and bioinformatics work.
Footnotes
FUNDING
Supported by grants from Academia Sinica, Institute of Statistical Science AS, AS-100-TP-AB2, NSC 98-3112-B-001-034, NSC 99-2314-B-001-003-MY3, NSC 100-2325-B-001-027, NSC 101-2325-B-002-071, NSC 102-2325-B-002-078, NSC 101-2319-B-002-002, NSC 102-2319-B-002-002, NSC 102-2911-I-002-303, NSC 101-2911-I-002-303, NSC 102-2911-I-002-303, DOH101-TD-B-111-001, 102R7557, NSC 102-2923-B-002-004, NSC 103-2923-B-002-003, MOST 104-2923-B-002-003, MOST 104-2314-B-075A-012, and Taiwan Biosignature Project of Lung Cancer.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers [mdash] a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian J, Govindan R. Lung Cancer in Never Smokers: A Review. Journal of Clinical Oncology. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 4.Krause DS, Van Etten RA. Tyrosine Kinases as Targets for Cancer Therapy. New England Journal of Medicine. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 5.Tao R-H, Maruyama IN. All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. Journal of Cell Science. 2008;121:3207–3217. doi: 10.1242/jcs.033399. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 8.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends in Cell Biology. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13:663–673. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- 10.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 11.Schlessinger J. Common and Distinct Elements in Cellular Signaling via EGF and FGF Receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 13.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proceedings of the National Academy of Sciences. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bjornsti M-A, Houghton PJ. The tor pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 16.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre P-L, Burkhard C, Schüler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, et al. Genetic Pathways to Glioblastoma: A Population-Based Study. Cancer Research. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 17.Sunpaweravong P, Sunpaweravong S, Puttawibul P, Mitarnun W, Zeng C, Barón A, Franklin W, Said S, Varella-Garcia M. Epidermal growth factor receptor and cyclin D1 are independently amplified and overexpressed in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:111–119. doi: 10.1007/s00432-004-0610-7. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson RI, Gee JMW, Harper ME. EGFR and cancer prognosis. European Journal of Cancer. 2001;37(Supplement 4):9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 19.Veale D, Ashcroft T, Marsh C, Gibson GJ, Harris AL. Epidermal growth factor receptors in non-small cell lung cancer. Br J Cancer. 1987;55:513–516. doi: 10.1038/bjc.1987.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haeder M. Epidermal growth factor receptor expression in human lung cancer cell lines. Cancer Res. 1988;48:1132–1136. [PubMed] [Google Scholar]
- 21.Ohsaki Y. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep. 2000;7:603–607. doi: 10.3892/or.7.3.603. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch FR. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 23.Al-Saad S, Al-Shibli K, Donnem T, Andersen S, Bremnes RM, Busund L-T. Clinical Significance of Epidermal Growth Factor Receptors in Non-small Cell Lung Cancer and a Prognostic Role for HER2 Gene Copy Number in Female Patients. Journal of Thoracic Oncology. 2010;5:1536–1543. doi: 10.1097/JTO.1530b1013e3181ea1510a. [DOI] [PubMed] [Google Scholar]
- 24.Liang Z, Zhang J, Zeng X, Gao J, Wu S, Liu T. Relationship between EGFR expression, copy number and mutation in lung adenocarcinomas. BMC Cancer. 2010;10:376. doi: 10.1186/1471-2407-10-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh Y, Jang B, Jeon YK, Kim TM, Lee S-H, Kim D-W, Chung DH, Kim YT, Kim YW, Heo DS. EGFR Gene Copy Number Gain is Related to High Tumor SUV and Frequent Relapse after Adjuvant Chemotherapy in Resected Lung Adenocarcinoma. Japanese Journal of Clinical Oncology. 2011;41:548–554. doi: 10.1093/jjco/hyq248. [DOI] [PubMed] [Google Scholar]
- 26.Inamura K, Ninomiya H, Ishikawa Y, Matsubara O. Is the Epidermal Growth Factor Receptor Status in Lung Cancers Reflected in Clinicopathologic Features? Archives of Pathology & Laboratory Medicine. 2010;134:66–72. doi: 10.5858/2008-0586-RAR1.1. [DOI] [PubMed] [Google Scholar]
- 27.Mitri Z, Constantine T, O'Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemotherapy Research and Practice. 2012;2012:7. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burstein HJ. The Distinctive Nature of HER2-Positive Breast Cancers. New England Journal of Medicine. 2005;353:1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 29.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. International Journal of Gynecology & Obstetrics. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Seminars in Roentgenology. 40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 31.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. [Google Scholar]
- 32.Shih JY, Gow CH, Yu CJ, Yang CH, Chang YL, Tsai MF, Hsu YC, Chen KY, Su WP, Yang PC. Epidermal growth factor receptor mutations in needle biopsy/aspiration samples predict response to gefitinib therapy and survival of patients with advanced nonsmall cell lung cancer. Int J Cancer. 2006;118:963–969. doi: 10.1002/ijc.21458. [DOI] [PubMed] [Google Scholar]
- 33.Su K-Y, Chen H-Y, Li K-C, Kuo M-L, Yang JC-H, Chan W-K, Ho B-C, Chang G-C, Shih J-Y, Yu S-L, Yang P-C. Pretreatment Epidermal Growth Factor Receptor (EGFR) T790M Mutation Predicts Shorter EGFR Tyrosine Kinase Inhibitor Response Duration in Patients With Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


