Abstract
Purpose
This work aims to identify differential metabolites and predicting molecular subtypes of breast cancer (BC).
Methods
Plasma samples were collected from 96 BC patients and 79 normal participants. Metabolic profiles were determined by liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry based on multivariate statistical data analysis.
Results
We observed 64 differential metabolites between BC and normal group. Compared to human epidermal growth factor receptor 2 (HER2)-negative patients, HER2-positive group showed elevated aerobic glycolysis, gluconeogenesis, and increased fatty acid biosynthesis with reduced Krebs cycle. Compared with estrogen receptor (ER)-negative group, ER-positive patients showed elevated alanine, aspartate and glutamate metabolism, decreased glycerolipid catabolism, and enhanced purine metabolism. A panel of 8 differential metabolites, including carnitine, lysophosphatidylcholine (20:4), proline, alanine, lysophosphatidylcholine (16:1), glycochenodeoxycholic acid, valine, and 2-octenedioic acid, was identified for the classification of BC subtypes. These markers showed potential diagnostic value with average area under the curve at 0.925 (95% CI 0.867-0.983) for the training set (n=51) and 0.893 (95% CI 0.847-0.939) for the test set (n=45).
Conclusion
Human plasma metabolomics is useful in identifying differential metabolites and predicting breast cancer subtypes.
Keywords: human plasma metabolomics, differential metabolites, molecular subtypes, breast cancer
INTRODUCTION
Breast cancer (BC) is the most common cause of death among women worldwide [1]. Human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER) are the two key molecular biomarkers to segregate the most distinct biologic subgroups of BC [2]. The characteristics of HER2 and ER can be used to roughly divide BC into four major molecular subtypes, including Luminal A (HER2 negative and ER positive), Luminal B (HER2 positive and ER positive), HER2-enriched (HER2 positive and ER negative), and Basal-Like (HER2 negative and ER positive) [3]. Each subtype of BC is accompanied with characteristic molecular features, subsequent metastatic lesions, prognosis and clinical responses to available medical therapies [4].
Determining the molecular subtype of BC is fundamental for personalized treatment. It was demonstrated that the “specific molecular type matched” patients had a higher overall response rate, longer time to treatment failure and longer survival compared to patients whose treatment was not matched to particular molecular abnormality [5]. Repeated biopsies and subsequent histopathology are commonly used to study molecular and genetic information from tumor cells for BC diagnosis and subtype classification. This analysis is invasive and time-consuming [6, 7]. Rapid and sensitive analysis is urgently required in clinic for discrimination of BC subtypes.
Recent studies have shown that genomic alterations in solid cancers can be characterized by bio-fluid metabolome change [8, 9]. Metabolomics is a new, rapidly expanding field dedicated to the global metabolic alterations in biological systems that occur in response to genetic, pathological, and environmental or lifestyle factors. The high-throughput nature of metabolomics makes it applicable to perform diagnostic biomarker screening for diseases or follow drug efficacy [10]. Plasma, a frequently considered pool of metabolites, has been applied to represent systemic metabolic deregulation in cancer patients, and the markers in this biological specimen could present biological mechanisms during cancer progression [9]. Metabolomics has been applied to find urinary biomarkers for BC [11]. Limited data, however, is available to characterize BC molecular subtypes by plasma metabolic profiles.
Gas chromatography coupled with mass spectrometry (GC-MS), liquid chromatography (LC)-MS, and nuclear magnetic resonance (NMR) are the three most commonly used platforms for metabolomic study [12, 13]. LC-MS is the most compatible technique for sensitive detection of biomolecules [14]. GC-MS technique provides a relatively more robust chromatography and greater separation efficiency together with the availability of reference compound libraries [13]. The parallel use of GC-MS and LC-MS could be a good choice to better profile different classes of compounds.
In this study, metabolomics was applied to identify differential metabolites and predicting molecular subtypes of breast cancer. We collected plasma samples from 96 BC patients and 79 normal control (NC) participants. Analysis was performed on ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (UPLC-Q/TOF-MS) and gas chromatography-quadrupole mass spectrometry (GC-Q/MS).
RESULTS
Clinical characteristics of BC patients and NC subjects were summarized in Table 1. Detailed patient information, stages of disease and other parameters were shown in Supplementary Table S1 and Table S2. Typical immunohistochemical pathology of different receptor statuses in accordance with the FDA-approved system was provided in Supplementary Figure S1. Typical total ion chromatograms (TICs) of a BC sample obtained from ESI+, ESI−, and GC-Q/MS were provided in Supplementary Figure S2. As shown in Figure 1, clear discriminations were obtained by ESI+ between BC and NC groups (Figure 1A), HER2-positive and HER2-negative BC groups (Figure 1B), ER-positive and ER-negative BC groups (Figure 1C). Similar discriminations were also observed by ESI− (Supplementary Figure S3) and GC-Q/MS (Supplementary Figure S4). The metabolites with variable importance in the project (VIP) higher than 1 in loading plot were highlighted as biomarker candidates (Supplementary Figure S5). Additionally, Student's t test was used to validate the significance of the difference in intensities between variables.
Table 1. Clinical characteristics of the patients with breast cancer.
| Samples | HER2 positive (IHC + + + and + + with gene amplification by FISH) |
HER2 negative (IHC−, +, and ++ without gene amplification by FISH) |
Normal control | p | |
|---|---|---|---|---|---|
| Sample No. | 36 | 60 | 79 | ||
| Age(year) | 52.3(31~82) | 53.1(31~78) | 46.4(24~86) | >0.05 | |
| ER positive | n=11 (5 for test set) as Luminal B subtype | n=42 (22 for test set) as Luminal A subtype | \ | ||
| ER negative | n=25 (10 for test set) as HER2-enriched subtype | n=18 (8 for test set) as Basal-like subtype | \ | ||
| TNM classification stage | I | 6 | 25 | \ | |
| IIA | 19 | 20 | \ | ||
| IIIA | 1 | 1 | \ | ||
| IIIB | 10 | 14 | \ | ||
HER2, human epidermal growth factor receptor 2;
ER, estrogen receptor;
IHC, immunohistochemistry;
FISH, fluorescence in situ hybridization.
Figure 1. PLS-DA loading plots and chance permutation test obtained from LC-MS in positive mode.
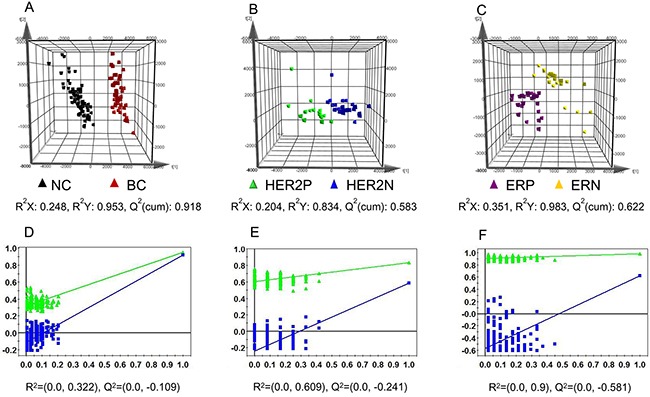
A. Normal control (NC) vs breast cancer (BC) group; B. HER2-positive (HER2P) vs HER2-negative (HER2N) BC group; C. ER-positive (ERP) vs ER-negative (ERN) group. Black triangle corresponds to NC group, red triangle corresponds to BC group, green triangle corresponds to HER2-positive BC patients, blue triangle corresponds to HER2-negative BC subjects, purple triangle corresponds to ER-positive participants, and yellow triangle corresponds to ER-negative patients. Chance permutation at 200 times was used for the discrimination between D. NC vs BC, E. HER2P vs HER2N, and F. ERP vs ERN.
Discrimination of BC and NC groups
A total of 1957 peaks were detected from ESI+ LC-MS, 1329 peaks from ESI− LC-MS, and 123 peaks from GC-MS. The significant ions were then imported into the SIMCA-P 11.5 software package. Figure 1A illustrated score plots of the partial least squares discriminant analysis (PLS-DA) model of BC patients and NC participants. In Figure 1A, BC patients were clearly separated from NC group. The cumulative R2Y and Q2 were 0.953 and 0.918. The chance permutations at 200 times produced R2Y-intercept and Q2-intercept at 0.322 and −0.109 (Figure 1D), indicating that no over-fitting was observed.
Sixty-four significantly altered plasma metabolites in BC patients relative to NC group were identified from the two-component PLS-DA model, in which 32 were further confirmed using reference compounds. The differential metabolites and their pathways were presented in Supplementary Table S3. Their relative normalized quantities were plotted in a heat map in Figure 2A.
Figure 2. The identified differential metabolites between normal control (NC) and breast cancer (BC) groups.
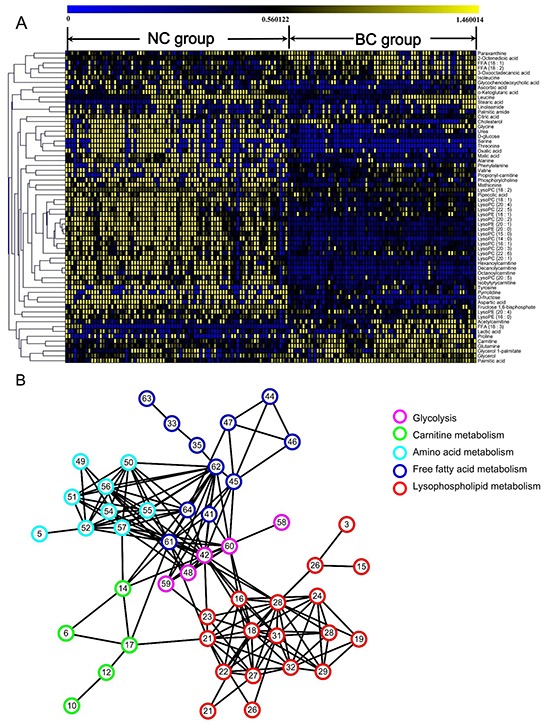
A. Heatmap of 64 differential metabolites between BC and NC participants. The colors from blue to yellow indicate the elevated amount of metabolites. B. Correlation network analysis of differential metabolites. Metabolites with high correlation coefficients were connected by lines.
Correlation network of differential metabolites in BC plasma
A correlation network analysis was established using Cytoscape software in Figure 2B. Highly correlated metabolites were connected with a line. Glycolysis-related metabolites were located in the center of the network with an elevated tendency. A positive correlation was observed between the levels of glycolysis-related metabolites and fatty acids, indicating the high energy consumption from aerobic glycolysis during fatty acid biosynthesis in cancer. Lipids, especially lysophospholipids, exhibited a significantly decreased amount in the network. For most of the amino acids, there was a negatively correlated regulation.
Discrimination of HER2-positive and HER2-negative BC
As shown in Figure 1B, we demonstrated significant differences between the HER2-positive patients and HER2-negative BC subjects in the PLS-DA score plot. Permutation results were shown in Figure 1E. Using a combination of the VIP > 1 from PLS-DA with results from Student's t test, 40 metabolites (Table 2) were identified as differential variables. Heatmap of 40 differential metabolites were shown in Supplementary Figure S6. HER2-related altered metabolic pathway network of the significantly regulated metabolites was provided in Figure 3.
Table 2. Differential metabolites identified between HER2 positive breast cancer and HER2 negative breast cancer and their pathway involved.
| No. | tR(min) | m/z | Metabolites | Formula | Fold change a | p value | VIP b | Pathway involved |
|---|---|---|---|---|---|---|---|---|
| ESI+ | ||||||||
| 1 | 0.676 | 147.0604 | Glutamine* | C5H10N2O3 | 1.287 | <0.001 | 1.232 | Alanine, aspartate and glutamate metabolism |
| 2 | 0.733 | 162.1124 | Carnitine* | C7H15NO3 | 1.367 | <0.001 | 2.721 | Fatty acid transportation |
| 3 | 0.743 | 116.0711 | Proline* | C5H9NO2 | 1.217 | 0.007 | 2.771 | Arginine and proline metabolism |
| 4 | 0.959 | 118.0869 | Valine* | C5H11NO2 | 1.187 | 0.002 | 2.749 | Valine, leucine and isoleucine metabolism |
| 5 | 1.015 | 204.1231 | Acetylcarnitine* | C9H17NO4 | 1.666 | 0.003 | 2.337 | Fatty acid transportation |
| 6 | 1.018 | 130.0861 | Pipecolic acid | C6H11NO2 | 0.746 | 0.024 | 1.485 | Protein synthesis, amino acid biosynthesis |
| 7 | 1.242 | 150.0548 | Methionine* | C5H11NO2S | 1.260 | <0.001 | 1.986 | Cysteine and methionine metabolism |
| 8 | 1.468 | 182.0815 | Tyrosine* | C9H11NO3 | 1.227 | 0.030 | 1.036 | Aminoacyl-tRNA biosynthesis |
| 9 | 1.638 | 218.1378 | Propionyl-carnitine | C10H19NO4 | 1.238 | <0.001 | 1.622 | Fatty acid transportation |
| 10 | 1.751 | 166.0871 | Phenylalanine* | C9H11NO2 | 1.375 | <0.001 | 2.221 | Aminoacyl-tRNA biosynthesis |
| 11 | 1.977 | 232.1545 | Isobutyryl-carnitine | C11H21NO4 | 1.334 | 0.011 | 1.454 | Fatty acid transportation |
| 12 | 2.712 | 260.1855 | Hexanoylcarnitine | C13H25NO4 | 1.260 | 0.010 | 1.453 | Fatty acid transportation |
| 13 | 3.448 | 288.2172 | Octanoylcarnitine | C15H29NO4 | 1.254 | 0.019 | 1.839 | Fatty acid transportation |
| 14 | 4.240 | 502.2933 | LysoPE (20:4) | C25H44NO7P | 1.497 | 0.001 | 2.274 | Lysophospholipid catabolism |
| 15 | 4.409 | 316.2485 | Decanoyl-L-carnitine | C17H33NO4 | 1.386 | 0.004 | 1.626 | Fatty acid transportation |
| 16 | 4.636 | 544.3397 | LysoPC(20:4)* | C28H50NO7P | 1.299 | 0.020 | 2.653 | Glycerophospholipid catabolism |
| 17 | 4.749 | 184.0734 | Phosphorylcholine | C5H14NO4P | 0.721 | 0.007 | 1.009 | Glycerophospholipid catabolism |
| 18 | 4.862 | 480.3079 | LysoPE(18:1) | C23H46NO7P | 1.484 | 0.003 | 2.083 | Lysophospholipid catabolism |
| 19 | 5.654 | 522.3550 | LysoPC (18:1) | C26H52NO7P | 1.287 | 0.003 | 2.251 | Glycerophospholipid catabolism |
| ESI− | ||||||||
| 20 | 0.688 | 179.0579 | Paraxanthine* | C7H8N4O2 | 1.129 | 0.004 | 1.141 | Purine metabolism |
| 21 | 0.907 | 145.0139 | α-Ketoglutaric acid | C5H6O5 | 0.713 | 0.038 | 1.101 | Tricarboxylic acid cycle |
| 22 | 1.423 | 133.0139 | Malic acid* | C4H6O5 | 0.461 | 0.004 | 1.571 | Tricarboxylic acid cycle |
| 23 | 1.536 | 130.0871 | Isoleucine* | C6H13NO2 | 1.545 | <0.001 | 1.957 | Valine, leucine and isoleucine metabolism |
| 24 | 2.611 | 171.0659 | 2-Octenedioic acid | C8H12O4 | 1.234 | <0.001 | 2.565 | Fatty acid metabolism |
| 25 | 3.573 | 448.3075 | Glycochenodeoxycholic acid* | C26H43NO5 | 0.566 | 0.026 | 1.980 | Bile acid biosynthesis |
| 26 | 6.684 | 277.2174 | FFA (18:3)* | C18H30O2 | 1.422 | 0.007 | 1.746 | Biosynthesis of unsaturated fatty acids |
| 27 | 7.532 | 279.2322 | FFA (18:2)* | C18H32O2 | 1.461 | <0.001 | 1.864 | Biosynthesis of unsaturated fatty acids |
| 28 | 8.833 | 281.2486 | FFA (18:1)* | C18H34O2 | 1.174 | 0.013 | 1.416 | Biosynthesis of unsaturated fatty acids |
| GC-MS | ||||||||
| 29 | 6.299 | Lactic acid* | C3H6O3 | 1.443 | <0.001 | 1.208 | Glycolysis metabolism | |
| 30 | 6.904 | Alanine | C3H7NO2 | 1.023 | <0.001 | 1.190 | Alanine and aspartate metabolism | |
| 31 | 7.117 | Glycine* | C2H5NO2 | 2.252 | <0.001 | 1.961 | Glycine, serine and threonine metabolism | |
| 32 | 8.879 | Urea | CH4N2O | 2.574 | 0.012 | 1.455 | Urea cycle | |
| 33 | 10.612 | Serine* | C3H7NO3 | 2.090 | <0.001 | 1.084 | Glycine, serine and threonine metabolism | |
| 34 | 10.957 | Threonine* | C4H9NO3 | 2.748 | <0.001 | 1.028 | Glycine, serine and threonine metabolism | |
| 35 | 11.871 | Aspartic acid* | C4H7NO4 | 0.559 | <0.001 | 1.162 | Alanine, aspartate and glutamate metabolism | |
| 36 | 16.230 | Citric acid* | C6H8O7 | 0.837 | <0.001 | 1.856 | Tricarboxylic acid cycle | |
| 37 | 17.034 | D-glucose* | C6H12O6 | 0.333 | <0.001 | 1.794 | Glycolysis metabolism | |
| 38 | 18.045 | Palmitic acid* | C16H32O2 | 1.789 | <0.001 | 1.739 | Fatty acid biosynthesis | |
| 39 | 19.862 | Stearic acid* | C18H36O2 | 1.223 | 0.002 | 1.017 | Fatty acid biosynthesis | |
| 40 | 27.843 | Cholesterol* | C27H46O | 0.832 | <0.001 | 2.359 | Hormone biosynthesis and bile acid biosynthesis | |
confirmed with reference standards;
fold change >1 indicates that the average normalized peak area ratio in HER2-positive group is larger than that in HER2-negative group;
variable importance in the projection.
Metabolites in italic were variables with VIP>1.5.
Figure 3. HER2-related altered metabolic pathway network of the significantly regulated metabolites.
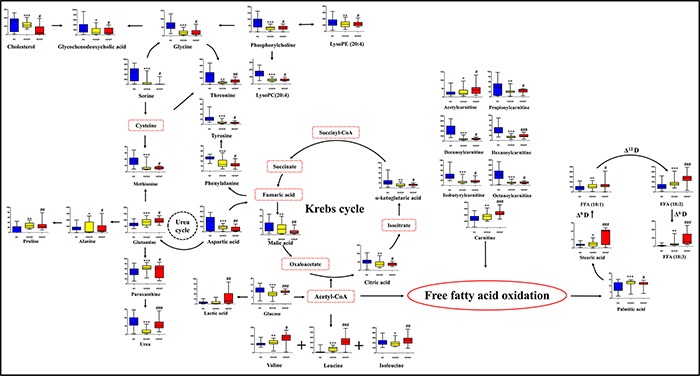
Blue, yellow, and red charts represent the relative intensities of differential metabolites in normal control, HER2-negative, and HER2-positive breast cancer groups. The names with dashed red lines represent the undetected metabolites. The names with full red lines represent the detected metabolic reactions. LysoPC, lysophosphorylcholine; LysoPE, lysophosphoethanolamine; FFA, free fatty acid; Δ6 D, Δ6 desaturase. Acetyl-CoA, acetyl-coenzyme A; Succinyl-CoA, succinyl-coenzyme A. * represents the difference between HER2-negative breast cancer group and normal subjects, *, p < 0.05; **, p < 0.01; ***, p < 0.001, # represents the difference between HER2-positive patients and HER2-negative participants, #, p < 0.05; ##, p < 0.01; ###, p < 0.001.
Discrimination of ER-positive and ER-negative BC
As shown in Figure 1C, significant differences were observed between the ER-positive and ER-negative patients. Table 3 listed the 22 differential metabolites identified with the VIP > 1 from PLS-DA and results from Student's t test. ER-related disturbed metabolic pathways were shown in Figure 4.
Table 3. Differential metabolites identified between ER positive breast cancer and ER negative plasma and their pathway involved.
| No. | tR(min) | m/z | Metabolites | Formula | Fold change a | p value | VIPb | Pathway involved |
|---|---|---|---|---|---|---|---|---|
| ESI+ | ||||||||
| 1 | 0.676 | 147.0604 | Glutamine* | C5H10N2O3 | 1.159 | 0.019 | 1.650 | Alanine, aspartate and glutamate metabolism |
| 2 | 0.733 | 162.1124 | Carnitine* | C7H15NO3 | 1.187 | 0.006 | 2.373 | Fatty acid transportation |
| 3 | 0.959 | 118.0869 | Valine* | C5H11NO2 | 0.682 | <0.001 | 2.813 | Valine, leucine and isoleucine metabolism |
| 4 | 1.242 | 150.0548 | Methionine* | C5H11NO2S | 1.284 | 0.025 | 1.942 | Cysteine and methionine metabolism |
| 5 | 4.296 | 468.3086 | LysoPC(14:0) | C22H46NO7P | 0.751 | 0.001 | 2.257 | Glycerophospholipid catabolism |
| 6 | 4.466 | 494.3242 | LysoPC(16:1)* | C24H48NO7P | 1.268 | 0.002 | 2.748 | Glycerophospholipid catabolism |
| 7 | 4.692 | 454.2957 | LysoPE (16:0) | C21H44NO7P | 1.227 | 0.027 | 1.111 | Lysophospholipid catabolism |
| 8 | 4.805 | 542.3224 | LysoPC (20:5) | C28H48NO7P | 0.649 | 0.037 | 2.358 | Glycerophospholipid catabolism |
| 9 | 4.805 | 482.3233 | LysoPC(15:0) | C23H48NO7P | 0.870 | 0.003 | 2.152 | Glycerophospholipid catabolism |
| 10 | 4.862 | 570.3547 | LysoPC(22:5) | C30H52NO7P | 0.799 | <0.001 | 1.076 | Glycerophospholipid catabolism |
| 11 | 5.145 | 546.3559 | LysoPC(20:3) | C28H52NO7P | 0.794 | 0.006 | 1.701 | Glycerophospholipid catabolism |
| ESI− | ||||||||
| 12 | 0.688 | 179.0579 | Paraxanthine* | C7H8N4O2 | 1.169 | 0.003 | 1.445 | Purine metabolism |
| 13 | 0.914 | 175.0248 | Ascorbic acid* | C6H8O6 | 0.750 | 0.014 | 1.330 | Ascorbic acid |
| 14 | 1.423 | 133.0139 | Malic acid* | C4H6O5 | 0.464 | <0.001 | 1.373 | Tricarboxylic acid cycle |
| 15 | 1.536 | 130.0871 | Isoleucine* | C6H13NO2 | 0.875 | 0.002 | 1.209 | Valine, leucine and isoleucine metabolism |
| 16 | 2.611 | 171.0659 | 2-Octenedioic acid | C8H12O4 | 1.104 | <0.001 | 2.511 | Fatty acid metabolism |
| 17 | 3.573 | 448.3075 | Glycochenodeoxycholic acid* | C26H43NO5 | 1.265 | 0.002 | 3.035 | Bile acid biosynthesis |
| GC-MS | ||||||||
| 18 | 6.904 | Alanine* | C3H7NO2 | 2.056 | <0.001 | 2.891 | Alanine and aspartate metabolism | |
| 19 | 9.304 | Leucine* | C6H13NO2 | 0.452 | <0.001 | 1.085 | Valine, leucine and isoleucine metabolism | |
| 20 | 9.403 | Glycerol* | C3H8O3 | 1.069 | 0.004 | 1.476 | Glycerophospholipid metabolism | |
| 21 | 17.034 | D-glucose* | C6H12O6 | 1.218 | 0.003 | 2.193 | Glycolysis metabolism | |
| 22 | 18.045 | Palmitic acid* | C16H32O2 | 0.556 | <0.001 | 1.900 | Fatty acid biosynthesis | |
confirmed with reference standards;
fold change >1 indicates that the average normalized peak area ratio in ER-positive group is larger than that in ER-negative group;
variable importance in the projection.
Metabolites in italic were variables with VIP>1.5.
Figure 4. Disturbed metabolic pathways in ER-positive compared with ER-negative BC.
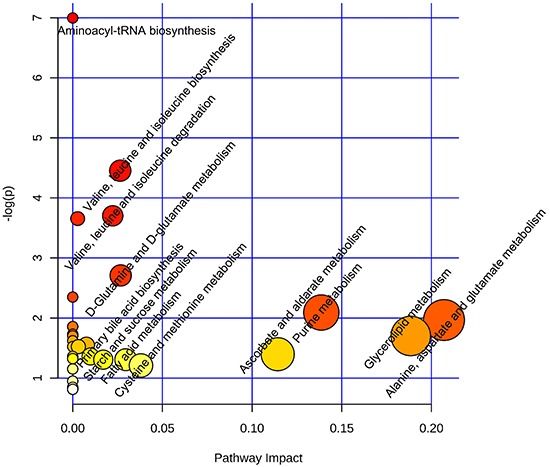
Metaboanalyst (http://www.metaboanalyst.ca) generated topology map described the impact of baseline metabolites identified between ER-positive vs ER-negative groups with high VIP values (VIP>1) on metabolic pathways.
Diagnostic potential of differential metabolites for subtype classifications
The HER2 and ER statuses are the key to the classification of BC subtypes. The metabolites with VIPs > 2.5 responsible for the discrimination of HER2 in Table 2 and ER statuses in Table 3 were selected for potential diagnosis. A combinational panel of 8 metabolites was assigned as candidate markers shown in Figure 5A, including carnitine, lysophosphatidylcholines (lysoPC) (20:4), proline, alanine, lysoPC (16:1), glycochenodeoxycholic acid (GDCA), valine, and 2-octenedioic acid (2-OA). The performances of these 8 metabolites in the diagnosis of four clinical BC subtypes were conducted by ROC analysis. As shown in Figure 5B, the panel of 8 metabolites provided diagnostic abilities with average area under the curve at 0.925 (95% CI 0.867-0.983) for the training set (n=51) and 0.893 (95% CI 0.847-0.939) for the test set (n=45). Based on the highest prediction sensitivity and specificity of the ROC on the training set, we calculated the optimal cut-off values at 0.376 for Luminal A, 0.132 for Luminal B, 0.288 for HER2-enriched, and 0.342 for Basal-like subtypes (Figure 6). Using the optimal cut-off values, prediction accuracies in Figure 6A showed 88.3% for Luminal A subjects in the training set and 84.4% in the test set. Predictive accuracies in Figure 6B showed 92.2% for Luminal B patients in the training set and 88.9% for the test set. As shown in Figure 6C, predictive accuracies at 89.3% for HER2-enriched group in training set and 82.2% in the test set was obtained. In Figure 6D, we observed predictive accuracies at 84.3% for basal-like participants in the training set and 86.7% in the test set.
Figure 5. Combinational panel of 8 biomarkers and their diagnostic outcomes.
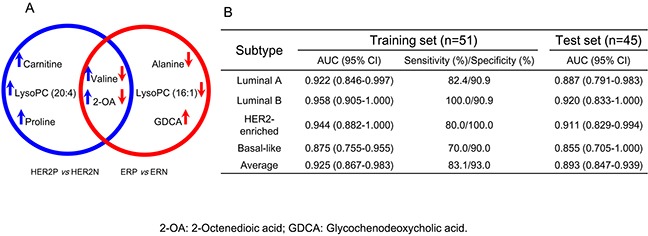
A. Venn diagram of the differential metabolites panels generated from the discrimination of different HER2 and ER statuses in breast cancer. The upward arrow represents an increased level of metabolite with the overexpression of HER2 (blue arrow) and ER (red arrow). LysoPC: lysophosphatidylcholine; 2-OA: 2-octenedioic acid; GDCA: glycochenodeoxycholic acid. B. Areas under the curve provided by the 8 biomarkers for the discrimination of BC subtypes in the training set and test set.
Figure 6. Prediction accuracies for BC subtypes based on the eight biomarkers.
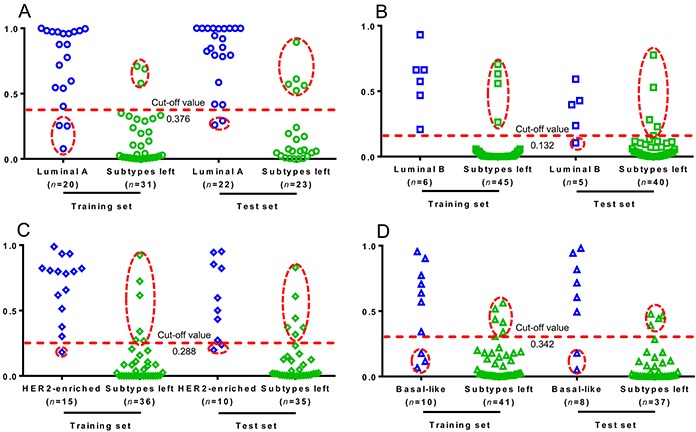
The prediction plots based on the optimal cut-off value for A. Luminal A, B. Luminal B, C. HER2-enriched, and D. Basal-like BC subtypes. Plot in hollow dashed circle represents samples with false prediction.
DISCUSSION
This study describes for the first time the plasma metabolic profiling change specifically associated with BC subtypes. Metabolic phenotypes revealed significant pattern differences between BC and NC groups, HER2-positive and HER2-negative BC groups, ER-positive and ER-negative BC groups. In these datasets, there were few misclassifications by unbiased analysis. Progesterone receptor (PR) status should be considered in classification of breast cancer. Expressional consistency of ER and PR was observed in all samples.
The parallel use of LC-MS and GC-MS provided comprehensive distinct metabolites. We observed 64 most significantly regulated plasma metabolites between BC patients and NC group. They were classified as amino acids, free fatty acids (FFA), lysoPCs, lysophosphatidylethanolamines (lysoPEs), carnitines, and organic acids.
Comparison between HER2-positive and HER2-negative BC patients generated 40 differential metabolites. The principal metabolic changes in HER2-positive BC compared with HER2-negative BC included elevated aerobic glycolysis, enhanced gluconeogenesis, and increased fatty acid biosynthesis with reduced Krebs cycle and Δ9 desaturase. The elevated level of lactic acid and decreased D-glucose in plasma of HER2-positive BC characterized the strong aerobic glycolysis (Warburg effect) in cancer cells [15]. Gluconeogenesis in HER2-positive BC was upregulated for energy supply, resulting in enriched consumption of amino acids in gluconeogenesis [16]. A significant enrichment in unsaturated fatty acids (UFAs) was found in HER2-positive BC, implying the increased UFAs probably resulted from the de nevo biosynthesis of fatty acids and enhanced Δ-dehydrogenase during the cell proliferation and metastasis of HER2-positive BC [17].
The ER statuses in BC were considered. The present data suggested that the major altered pathways in ER-positive BC patients included elevated alanine, aspartate and glutamate metabolism, decreased glycerolipid catabolism, and enhanced purine metabolism, when compared with ER-negative group (Figure 4). Similar to HER2-positive BC group, lysoPCs were at low levels in ER-positive patients, corresponding to the strong negative correlation between cPLA2α mRNA expression and ER expression levels [18]. Elevated level of glutamine in ER-positive patients compared to ER-negative participants clearly point to the perturbation of glutamate-to-glutamine ratio. This result is in agreement with previous observations [19].
We identified a panel of 8 potential small-molecule biomarkers for the diagnosis of BC subtypes. Carnitine, as an essential for the entry of fatty acid into the mitochondria for β-oxidation [20], was observed at a high level (FC=1.367, P<0.001) in HER2-positive group, which might lead to the activated metabolism of fats. LysoPC (20:4), metabolic products of PC by hydrolysis of phospholipase A2 [18], were at a high level (FC=1.299, P=0.020) in HER2-positive patients. The results corresponded to an increased expression of cytosolic phospholipase A2-α in HER2 over-expression BC cell lines [21]. The elevated amount of proline (FC=1.217, P=0.007) might indicate a suppressed proline oxidase in HER2-positive group [22]. Alanine was the most significantly decreased metabolite (FC=0.544, P<0.001) in ER-positive participants compared with ER-negative group [23]. The reduced lysoPC (16:1) (FC=0.786, P=0.002) in ER-positive patients showed relation with the activity inhibition of phospholipase A2 in MCF-7 BC cells [18]. The increased GDCA (FC=1.265, P=0.002) in ER-positive group was highly related to the enhanced proliferation of cancer cells, corresponding to its higher morbidity [24]. Valine and 2-OA were the co-markers in the discrimination of BC with different HER2 and ER expression levels. They are significantly increased in HER2-postive compared to HER2-negative but decreased remarkably in ER-positive compared to ER-negative groups. The abnormalities of valine suggested the disorder of energy supply in HER2-postive (FC=1.187, P=0.002) and ER-positive (FC=0.682, P<0.001) patients. The marked regulation of 2-octenedioic acid was an indicator for the abnormal fatty acid metabolism in HER2-positive (FC=1.234, P<0.001) and ER-positive (FC=0.833, P<0.001) subjects [25].
The clinical predictive potential of the identified 8 biomarkers was highlighted in this work for BC subtypes. Average predictive accuracies at 88.5% (95% CI 83.3%-93.7%) were obtained for the training set and 85.6% (95% CI 80.9%-90.1%) for the test set. We also used a panel of 29 metabolites with VIPs>1.5 (metabolites in italic in Tables 2 and 3) instead of 8 metabolites with VIPs>2.5 for prediction of breast cancer subtypes. The average predictive accuracies increased to 97.1% (95% CI 93.0%-100.0%) for training sets and 95.6% (95% CI 92.7%-98.5%) for test sets. In consideration of the clinical use of 29 metabolites is difficultly popularized due to the limited standards, 8 metabolites with VIPs>2.5 were more applicable as the diagnostic biomarkers.
In conclusion, this study is a first clinical metabolic research for BC subtype classification. We demonstrate a clear move toward discovering the metabolomic drivers for the various BC subtypes. We suggest that plasma metabolomic test is faster, less costly, and noninvasive, and could be used as a pre-screen to other forms of more invasive or uncomfortable screening. These metabolomic data can also help to identify new therapeutic pathways from which novel agents might be developed. In future, we will undertake study of a larger prospective cohort to further validate the accuracy of this test. An evaluation of the mechanisms of BC subtypes by general and targeted metabolomics as well as other systematic biological approaches could be used.
MATERIALS AND METHODS
Clinical sample collection
We collected plasma samples from 175 participants and all the subjects signed the informed consents before sample collection. In total, 96 BC patients, aged 31 to 82 years old, were enrolled in this work. This study was conducted with the guide of the Helsinki Declaration and the International Conference on Harmonization-Good Clinical Practices (ICH-GCP). This study was approved by the Institutional Review Boards of the First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital with approval number 2011-SRFA-058. The patients selecting protocol was set as follows: all the participants should sign the informed consent; patients diagnosed with BC should be confirmed by histology; patients should receive no surgical operation before this research; participants have sufficient heart, lung, liver, kidney, and hematopoietic functions with Eastern cooperative oncology group (ECOG) performance status ≤ 2, and weight loss < 10% in recent 6 months. Cancer stage was classified according to the 2002 Tumor Nodes Metastasis (TNM) staging system. Particularly, BC patients diagnosed with HER2 (−), HER2 (+), or HER2 (+ +) without gene amplification were defined as HER2-negative BC. Patients diagnosed with HER2 (+ + +) or HER2 (+ +) with gene amplification were classified into the HER2-positive BC group. NC samples were collected from a total of 79 healthy volunteers between the ages of 24 and 86 according to the same sample collection protocol. All the samples were randomly classified into training set and test set. Detailed baseline characteristics of patients enrolled in this study were provided in Table 1.
Fasting blood samples collected in the morning from all the subjects were stored in K2 EDTA vacutainer tubes and cooled down in freezer (4°C) at once. They were then centrifuged at 3000 × g for 10 min at 4°C within 2 h. Supernatants (plasma) were transferred into new vials, and immediately stored frozen (−80°C) until sample preparation. The sample pretreatment methods for LC-MS and GC-MS were detailed in the methods provided in Methods S1.
As part of the quality control (QC) and system conditioning process, a pooled QC sample was prepared by mixing equal volumes (10 μL) of the collected 175 samples.
Statistical analysis
The acquired MS data from GC-Q/MS and UPLC-Q/TOF-MS in both positive and negative ion modes were imported into the SIMCA-P software (version 11.5, Umetrics) for multivariate analysis. GraphPad Prism 5 package was applied to plot the relative amount of each metabolite. Heatmaps and hierarchical cluster analysis (HCA) were conducted using the MeV software package (version 4.6.0), and the correlation network was established using the Cytoscape software package. ROC analysis and binary logistic regression were applied using SPSS version 19.
Metabolites identification
GC-Q/MS metabolites were identified by comparing the mass fragmentations with NIST 05 Standard mass spectral databases in NIST MS search 2.0 (NIST, Gaithersburg, MD) software with a similarity of more than 70% and finally verified by available reference standards. Differential metabolites obtained from positive and negative ion modes of UPLC-Q/TOF-MS analyses were identified with available reference standards in our lab and the web-based resources such as the Human Metabolome Database (http://www.hmdb.ca/) and METLIN (http://metlin.scripps.edu/index.php) data source.
Metabolomics pathway analysis
Database sources, including the KEGG (http://www.genome.jp/kegg/), MetaboAnalyst (http://www.metaboanalyst.ca/MetaboAnalyst/), Human Metabolome Database, and METLIN, were used for the identification of affected metabolic pathways.
SUPPLEMENTARY FIGURES AND TABLES
Acknowledgments
The authors thank Professor Chun-Su Yuan and Chong-Zhi Wang from the University of Chicago for their revisions on the manuscript. Special thanks go to Professor Feng-Guo Xu from China Pharmaceutical University for his help in the data analysis by SIMCA software (version 13.0.2, Umetrics). This work was financially supported in part by the National Natural Science Foundation of China (No. 81222052, 81421005, 81201705), and Jiangsu Province Science Fund for Distinguished Young Scholars (BK20130025).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no non-financial competing interests.
Authors' contributions
L.-W.Q, P.L, W.Z, and M.-D.L. designed the research. Q.L and Y.C coordinated the experiments. Y.F performed the experiments and analyzed the data. Z.C and J.L coordinated the experiments and data analysis. X.Z and T.-S.X provided the blood samples. Y.F, and L.W.Q wrote the manuscript. R. N. A and M.-D.L. edited the manuscript. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Tibshirani R, Parker J, Hastie T, Marron J, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proceedings of the National Academy of Sciences. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clinical Cancer Research. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou A-M, Iskander NG, Hong DS, Wheler JJ, Falchook GS, Fu S, Piha-Paul S, Naing A, Janku F, Luthra R. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clinical Cancer Research. 2012;18:6373–6383. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murtaza M, Dawson S-J, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin S-F, Kingsbury Z, Wong AS. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Yun JS, Jeong P, Kim YI, Kim WJ, Park S. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. 2014;5:1636–1645. doi: 10.18632/oncotarget.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 10.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nature reviews Drug discovery. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 11.Slupsky CM, Steed H, Wells TH, Dabbs K, Schepansky A, Capstick V, Faught W, Sawyer MB. Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clinical Cancer Research. 2010;16:5835–5841. doi: 10.1158/1078-0432.CCR-10-1434. [DOI] [PubMed] [Google Scholar]
- 12.Bales JR, Higham DP, Howe I, Nicholson JK, Sadler PJ. Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clinical chemistry. 1984;30:426–432. [PubMed] [Google Scholar]
- 13.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Wang X. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Molecular & Cellular Proteomics. 2011;10:M110. doi: 10.1074/mcp.M110.004945. 004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Tan Y, Yin P, Ye G, Gao P, Lu X, Wang H, Xu G. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer research. 2013;73:4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 15.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 16.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O'Connor STF, Li S, Chin AR. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nature cell biology. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Currie E, Schulze A, Zechner R, Walther TC, Farese RV. Cellular fatty acid metabolism and cancer. Cell Metabolism. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caiazza F, Harvey BJ, Thomas W. Cytosolic phospholipase A2 activation correlates with HER2 overexpression and mediates estrogen-dependent breast cancer cell growth. Molecular Endocrinology. 2010;24:953–968. doi: 10.1210/me.2009-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budczies J, Pfitzner BM, Györffy B, Winzer KJ, Radke C, Dietel M, Fiehn O, Denkert C. Glutamate enrichment as new diagnostic opportunity in breast cancer. International Journal of Cancer. 2015;136:1619–1628. doi: 10.1002/ijc.29152. [DOI] [PubMed] [Google Scholar]
- 20.Mazar J, Zhao W, Khalil AM, Lee B, Shelley J, Govindarajan SS, Yamamoto F, Ratnam M, Aftab MN, Collins S. The functional characterization of long noncoding RNA SPRY4-IT1 in human melanoma cells. Oncotarget. 2014;5:8959. doi: 10.18632/oncotarget.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caiazza F, McCarthy NS, Young L, Hill AD, Harvey BJ, Thomas W. Cytosolic phospholipase A2-α expression in breast cancer is associated with EGFR expression and correlates with an adverse prognosis in luminal tumours. British journal of cancer. 2011;104:338–344. doi: 10.1038/sj.bjc.6606025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Togashi Y, Arao T, Kato H, Matsumoto K, Terashima M, Hayashi H, de Velasco MA, Fujita Y, Kimura H, Yasuda T. Frequent amplification of ORAOV1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ROS production. Oncotarget. 2014;5:2962. doi: 10.18632/oncotarget.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budczies J, Brockmöller SF, Müller BM, Barupal DK, Richter-Ehrenstein C, Kleine-Tebbe A, Griffin JL, Orešič M, Dietel M, Denkert C. Comparative metabolomics of estrogen receptor positive and estrogen receptor negative breast cancer: alterations in glutamine and beta-alanine metabolism. Journal of proteomics. 2013;94:279–288. doi: 10.1016/j.jprot.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. Journal of Clinical Oncology. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 25.Trushina E, Dutta T, Persson X-MT, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. Plos One. 2013;8:e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


