Figure 1. eIF4B phosphorylation is regulated by PI3K/Akt/mTOR pathway in Abl transformants.
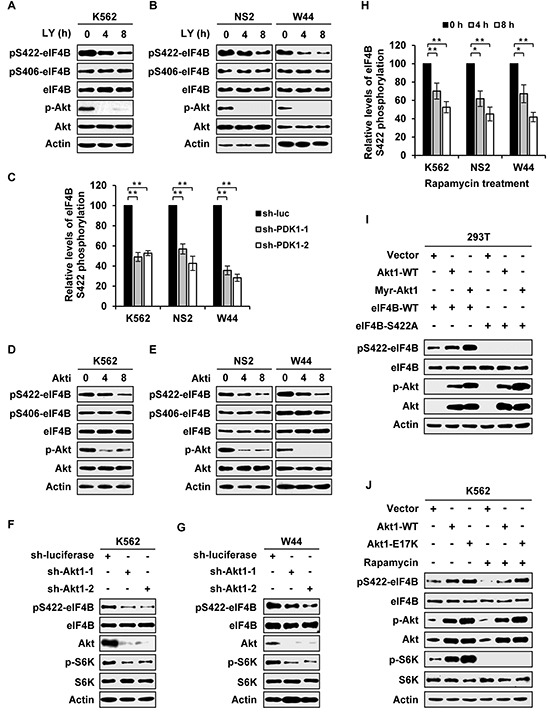
A. Bcr-Abl transformed cells (K562) were treated with LY294002 (10 μM) for indicated time. Whole cell lysates were prepared and examined for eIF4B Ser422 or Ser406 phosphorylation levels by Western blotting. B. v-Abl transformed cells (NS2 and W44) were treated with LY294002 (5 μM) for indicated time. The eIF4B phosphorylation was analyzed as described in A. C. eIF4B Ser422 phosphorylation levels in Supplementary Figure 1B and 1C were quantitated by densitometry and normalized to total protein levels. The eIF4B S422 phosphorylation levels of Abl transformants expressing sh-luciferase were set to 100%. Plotted are results from three independent experiments. Error bars represent SEM, n = 3 (**P < 0.01). D. and E. Bcr-Abl+ cells (D) or v-Abl+ cells (E) were treated with 4 μM (D) or 1.5 μM (E) Akti-1/2 for indicated time. Analysis of eIF4B phosphorylation were performed as described in A. F and G. K562 (F) or W44 (G) cells expressing luciferase-specific shRNA or Akt1-specific shRNAs were analyzed by Western blotting with indicated antibodies. H. eIF4B Ser422 phosphorylation levels in Supplementary Figure 1H and 1I were quantitated by densitometry and normalized to total protein levels. The levels of eIF4B S422 phosphorylation were set to 100% at 0 hour. Plotted are results from three independent experiments. Error bars represent SEM, n = 3 (*P < 0.05, **P < 0.01). I. eIF4B-WT or S422A mutant was co-transfected with empty vector, Akt1-WT, or Myr-Akt1 in 293T cells. Total proteins were extracted and analyzed for eIF4B S422 phosphorylation by Western blotting. J. K562 cells overexpressing Akt1-WT, Akt1-E17K, or control were treated with or without rapamycin (10 μM) for 4h and analyzed as described in Figure 1I with indicated antibodies.
