Abstract
The ability to efficiently access cytosolic proteins is desired in both biological research and medicine. However, targeting intracellular proteins is often challenging, because to reach the cytosol, exogenous molecules must first traverse the cell membrane. This review provides a broad overview of how certain molecules are thought to cross this barrier, and what kinds of approaches are being made to enhance the intracellular delivery of those that are impermeable. We first discuss rules that govern the passive permeability of small molecules across the lipid membrane, and mechanisms of membrane transport that have evolved in nature for certain metabolites, peptides, and proteins. Then, we introduce design strategies that have emerged in the development of small molecules and peptides with improved permeability. Finally, intracellular delivery systems that have been engineered for protein payloads are surveyed. Viewpoints from varying disciplines have been brought together to provide a cohesive overview of how the membrane barrier is being overcome.
Keywords: Cell membrane, Permeability, Translocation, Intracellular delivery, Cytosolic delivery, Fluorescent probe, Passive diffusion, Membrane transporter, Endosomal escape
1 Introduction
Molecules that can readily cross cell membranes are frequently needed in biological research and medicine. Permeable molecules that are useful for biological research include indicators of ion concentrations and pH, fluorescent dyes, crosslinking molecules, fluorogenic enzyme substrates, and various protein inhibitors. In medicine, numerous drugs are small molecules acting on intracellular targets, such as statins that inhibit cholesterol production, and reverse transcriptase inhibitors used for the treatment of HIV. Given the high level of interest across multiple areas of study in modulating intracellular targets, a broad overview of cytosolic delivery strategies could contribute to orienting researchers newly entering the field, and bringing together the solutions that have been proposed for various cargo.
This review examines how varying types of molecules—namely, small molecules, peptides, and proteins—are thought to cross the mammalian plasma membrane, how such permeation events are measured experimentally, and what kinds of technologies are being developed to deliver impermeable molecules to the cytoplasm (Fig. 1). Inspired by Stein et al. [1], we first discuss the structure and organization of the cell membrane, and the endocytic and secretory pathways responsible for the uptake and discharge of material. Then, we outline the experimental methods that have been used to determine, qualitatively or quantitatively, whether a molecule has successfully traversed the cell membrane. Next, we survey how various molecules, including ions, small solutes and metabolites, along with bacterial toxins and viruses, are thought to traverse the cell membrane. Lastly, we introduce engineering strategies that have been proposed to improve the permeability of small molecules, peptides, and proteins. Although we do not discuss the delivery of nucleic acids explicitly, certain approaches that have been proposed for introducing peptides and proteins into the cytoplasm have been (or could be) applied to nucleic acids, and vice versa. Overall, significant advances have been made in the prediction and design of permeable small-molecule compounds, and the repertoire of intracellular delivery technologies is steadily expanding for peptide and protein payloads.
Fig. 1.
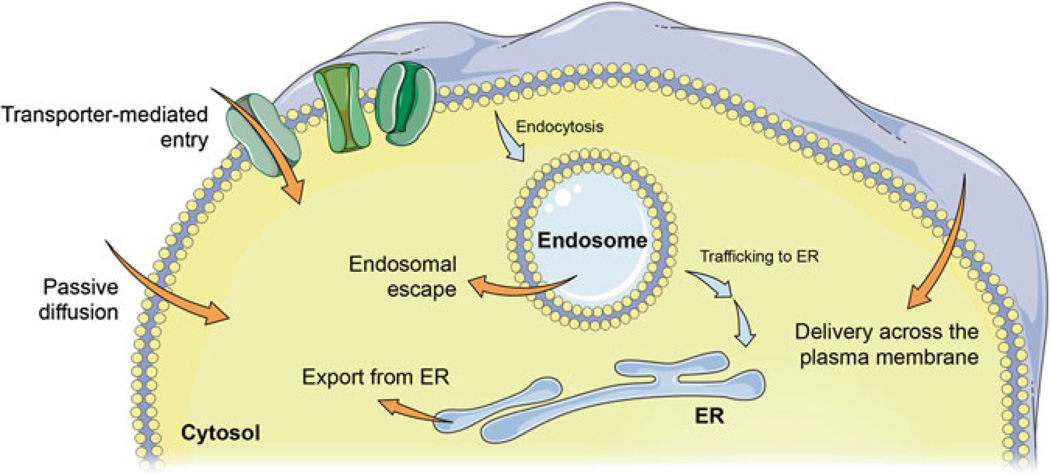
Possible routes of cytosolic entry. Molecules may passively diffuse across the cell membrane, or be shuttled in via natural or artificial delivery mechanisms. Membrane transporters allow the passage of various ions and metabolites. Protein toxins and viruses have evolved complex translocation mechanisms, hijacking the host’s ER transporters in some instances. Engineering approaches to improve a payload’s permeability may involve physically disrupting the membrane, chemically modifying the payload, or attaching the pay-load—covalently or non-covalently—to an intracellular delivery system that can disrupt cell membranes. In any case, the translocation event can occur across the plasma membrane, or across internal cellular membranes following endocytosis (termed “endosomal escape”). Images were adapted from Servier Medical Art
Understandably, much effort has been dedicated towards developing therapeutic entities that can modulate intracellular proteins, and thus we have drawn heavily from the drug development and delivery literature. However, engineering considerations regarding the optimization of in vivo properties were considered beyond the scope of this review and are not discussed. Due to the breadth of subjects we attempted to cover in a small space, we have preferentially referenced pertinent review articles when possible and strongly encourage readers to further explore the primary literature.
2 Cellular Organization
Before discussing membrane transport mechanisms, we will briefly describe the structure of the mammalian plasma membrane and components of the endocytic and secretory pathways. For the purpose of intracellular delivery, it is important to note that the interior of endocytic vesicles and the lumen of the organelles involved in the secretory pathway topologically correspond to the extracellular space.
2.1 The Structure and Organization of the Cell Membrane
The plasma membrane is a complex composite of multiple lipid species and membrane proteins [2]. Three major classes of lipids, including glycerophospholipids, sphingolipids and cholesterol, form a bilayer approximately 5nm in width. Spatially, these lipids are distributed asymmetrically across the bilayer. Additionally, according to the lipid raft hypothesis, the membrane is thought to contain lateral organizations enriched in sphingolipids, cholesterol, and glycosylphosphatidylinositol (GPI)-anchored proteins.
The ratio of protein to lipid in cellular membranes has been approximated to be 1:40 by number [3], suggesting that the membrane may in fact be crowded with proteins [4, 5]. This ratio can vary substantially by cell type, where metabolically active membranes are richer in protein [1, 6]. Membrane proteins can actively influence the organization of the membrane by forming specific and nonspecific interactions with lipids in the immediate boundary [7, 8].
Finally, the plasma membrane is in continuous motion [1], creating a highly dynamic structure. In addition to lateral diffusion, phospholipid flip-flop for some lipids is thought to occur on the order of minutes [9], and faster so for cholesterol [10, 11]. Also, cells constantly internalize and recycle their membranes, as discussed below.
2.2 The Secretory Pathway
In mammalian cells, secretory proteins are translocated across the Endoplasmic Reticulum (ER) membrane into the lumen co-translationally via the translocon complex. Misfolded proteins in the ER are transported back to the cytosol and degraded by the proteasome, a process termed ER-associated degradation (ERAD). Correctly folded proteins are transported across the Golgi network and released into the extracellular space via secretory vesicles. Specialized vesicles also mediate retrograde transport from the golgi to the ER, and from older to newer golgi [2].
2.3 Endocytic Pathways
Multiple endocytic pathways facilitate the internalization of exogenous cargo, creating a complex web of intracellular traffic. The choice of which endocytic pathway is utilized may depend on the cargo [12]. Nonspecific internalization of large volumes of fluid—pinocytosis—occurs in all cells, typically triggered by external stimuli such as growth factors [13]. Clathrin-dependent and independent routes of endocytosis generate primary endocytic vesicles that subsequently fuse with early endosomes, a major sorting station. Traveling down tracks of microtubules towards the perinuclear space, the early endosomes mature into multivesicular bodies (MVB), late endosomes and lysosomes. Endocytosed material that has not been recycled to the plasma membrane or exchanged with the trans golgi network is proteolyzed by hydrolytic enzymes in the lysosome [2].
3 Methods to Measure Membrane Permeation
The permeability of a given molecule can be quantitatively represented by its permeability coefficient (typically in units of cm/s) (Table 1), which is a measure of how fast it can cross a membrane [14]. High-throughput methods to measure the permeability coefficients of small molecules are routinely performed in drug development [15]. Artificial lipid bilayers [16] of various compositions or cell monolayers (typically the colorectal Caco-2 or renal MDCK cell lines) are widely used as model barriers [17]. While the former allows passive permeation only, the latter also allows transporter- mediated permeation. To disentangle these two modes of transport, cell lines that lack certain transporters, such as P-glycoprotein, have been developed [18].
Table 1.
Permeability coefficients of select molecules
| Species | Molecule | Permeability coefficient (cm/s) |
Membrane type | Reference |
|---|---|---|---|---|
| Ions | Na+ K+ |
5.0 × 10−14 4.7 × 10−14 |
Artificial membrane | Papahadjopoulos et al. [40] |
| Small molecules |
O 2 | 2.3 × 101 | Artificial membrane | Subczynski et al. [36] |
| CO 2 | 3.5 × 10−1 | Artificial membrane | Gutknecht et al. [37] | |
| H 2 O | 3.4 × 10−3 | Artificial membrane | Walter and Gutknecht [38] | |
| EtOH | 2.1 × 10−3 | Erythrocyte membrane |
Stein and Lieb [1] | |
| Steroids | 10−3 to 10−4 | Cell monolayer | Giorgi and Stein [42] | |
| Urea | 4.0 × 10−6 | Artificial membrane | Finkelstein [35] | |
| Glycerol | 5.4 × 10−6 | Artificial membrane | Orbach and Finkelstein [39] | |
| Small molecule drugs |
10−5 to 10−6 | Artificial membrane | Dobson et al. [112] | |
| Peptides | Cyclosporin A | 2.5 × 10−7 | Artificial membrane | Rezai et al. [45] |
| TAT | 2.7 × 10−9 | Artificial membrane | Jones and Howl [81] | |
In contrast to small molecules, the permeability of peptides or proteins across model membranes is rarely reported, reflecting in part the difficulty of translocating such molecules across a lipid membrane. It is technically challenging to accurately quantify the number of functional peptides or proteins that have successfully entered the cytoplasm. Selective isolation of the cytosol (and not endosomal compartments) using cellular fractionation [19, 20] or digitonin-mediated permeabilization of the plasma membrane [21] have been reported. Immunoprecipitation demonstrating the intended disruption of intracellular protein–protein interactions has also been presented as evidence of permeation [22, 23].
Fluorescence microscopy-based methods or biological assays measuring the activity of the payload in the cytoplasm are also employed. In microscopy, diffuse cytosolic staining (indicating endosomal release) is contrasted with punctate signal (indicating endosomal entrapment) to provide a qualitative assessment of permeation. However, it should be noted that payloads in the cytoplasm may also aggregate or associate with subcellular organelles to produce punctate patterns. In some cases, automated image analyses have been reported to identify endosomal release events [24]. In any case, the presence of labeled payload in the cytoplasm does not guarantee that it has retained its function, and the label itself or fixation steps may cause artifacts in cellular distribution [25, 26]. All observations are subject to the detection threshold of the instrument. Flow cytometry may be used as an alternative to microscopy if the fluorescence spectra are distinct in the endosomal compartment and the cytoplasm [27].
Alternatively, cytosolic uptake can be confirmed by measuring a biological effect that is generated only when the payload is in the cytoplasm. For example, peptides have been conjugated to dexamethasone (Dex) derivatives, which bind to transiently expressed gluococorticoid receptor (GR)-fusion proteins in the cytosol to induce a reporter [28] or alter its localization [29]. It should be noted that reporter gene expression inherently amplifies the signal through multiple rounds of transcription and translation [29]. In cases where the biological activity of the pay-load is reported, certain payloads can generate the measured macroscopic effect with fewer numbers. This is particularly true for catalytic proteins. For example, approximately 50 molecules of β-lactamase in a single cell have been reported to generate a detectable signal from catalyzing a fluorogenic substrate, albeit over a long period of time (16 h) [30]. Similarly, in theory, four molecules of Cre recombinase can repeatedly catalyze multiple recombination events to promote recombined gene expression [31]. Single molecules of toxins such as diphtheria and ricin have been estimated to kill a cell [32, 33].
4 Natural Membrane Transport Mechanisms
Small, moderately polar molecules are able to passively diffuse across the cell membrane. To transport larger, more polar compounds such as most sugars, amino acids, peptides, and nucleosides, membrane transporters are utilized. Interestingly, bacteria and viruses have developed sophisticated mechanisms to transport whole organisms, protein toxins, or genetic material into the mammalian cytoplasm.
4.1 Passive Diffusion
Passive diffusion across a cellular membrane is driven by the concentration and electric gradient of the solute and does not require the use of energy. In the simplest terms, passive diffusion is considered a three-step process, where the permeant first partitions into the membrane, diffuses across, and is released into the cytosol (known as the homogeneous solubility-diffusion model) [34, 35].
The most important parameters that govern transmembrane diffusion are polarity and size. For example, small nonpolar gases such as O2 , CO2 , and N2 , and small polar molecules such as ethanol cross lipid membranes rapidly. High permeability coefficients have been reported for such molecules across artificial lipid membranes, such as 2.3 × 101 cm/s for O2 [36] and 3.5 × 10−1 cm/s for CO2 [37]. The small, but highly polar water molecule is still able to diffuse across artificial membranes rapidly with a permeability coefficient of 3.4 × 10−3 cm/s [38].
In comparison, even slightly larger polar metabolites such as urea and glycerol have lower permeability across artificial membranes (approximately 10−6 cm/s) [35, 39]. The plasma membrane is virtually impermeable against larger, uncharged polar molecules and all charged molecules including ions. Indeed, despite their small size, Na+ and K+ have extremely low permeability coefficients (approximately 10−14 cm/s) [40]. Apart from small solutes of moderate polarity, the number of natural molecules known to passively diffuse across the cell membrane is surprisingly limited. Steroid hormones have been assumed to do so [41], although direct experimental evidence is scarce. Permeability coefficients on the order of 10−4 cm/s have been reported for a number of steroids across cell monolayers [42].
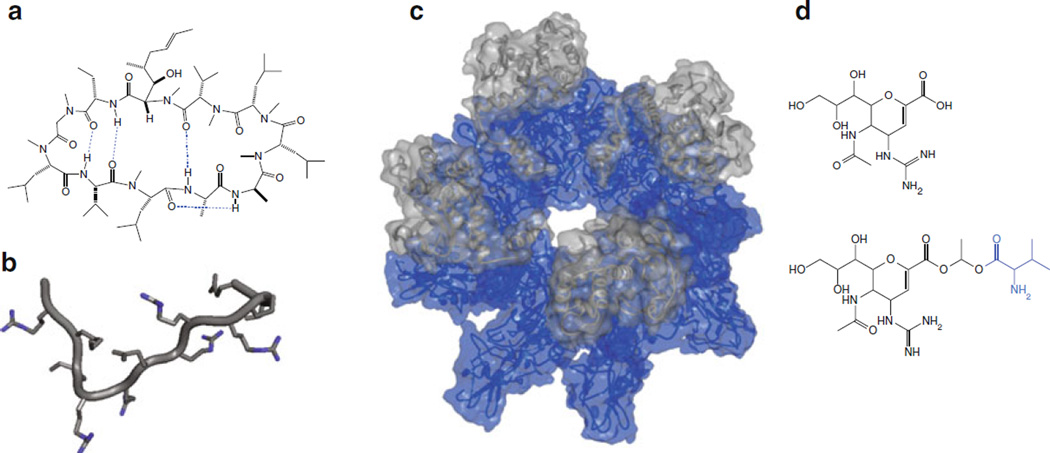
Interestingly, some non-endogenous natural products have been proposed to passively diffuse across the cell membrane despite their relatively higher polarity and size, such as the cyclic peptide Cyclosporin A (CsA) (Fig. 2a). Prescribed as an immunosuppressant, its intracellular mode of action and low EC 50 in cells (7–10 nM) [43] suggests that CsA is capable of passively permeating the cell membrane [43, 44]. Still, the reported permeability coefficient of CsA—2.5 × 10−7 cm/s across artificial membranes [45]—is relatively low compared to those of small molecules that are considered highly permeable (on the order of 10−5 cm/s or higher [46]).
Fig. 2.
(a) Cyclosporin A (CsA) in its closed conformation in nonpolar solvent [186]. The four intramolecular hydrogen bonds (dotted lines in blue) are thought to shield the polarity of the molecule. (b) The TAT peptide segment excerpted from the NMR structure of HIV-1 TAT protein (adapted from PDB 1TIV) [187]. The guanidinium nitrogens (blue) are thought to enhance the interaction between TAT and the cell membrane. (c) A slanted top- down view of the pre-pore formed by anthrax toxin protective antigens (PAs) (blue) in complex with lethal factors (LFs) (gray), which are translocated across the full pore. Shown in the figure are eight molecules of PA bound to four molecules of LF (PA 8(LFN)4) (PDB 3KWV) [188]. (d) The neuraminidase inhibitor Zanamivir (top) and Zanamivir-L-Val (bottom) [189]. The conjugated valine (blue) has been proposed to render Zanamivir into a substrate for amino acid transporters
4.2 Transporter-Mediated Entry
To facilitate the entry or export of molecules that are insufficiently permeable, cells utilize membrane transporters, the expression of which may depend on cell type. Active transporters use energy to translocate substrates against their concentration gradients, whereas passive transporters allow transmembrane diffusion without additional energy. Approximately 10 % of all human genes are transporter related, emphasizing their functional significance [47]. In the following, a selection of transporters is described, ordered according to the size of the substrate. Please refer to the Transporter Classification Database (www.tcdb.org) [48] and the Solute Carrier (SLC) Tables (www.bioparadigms.org) [49] for comprehensive reviews, and detailed information regarding substrate specificity and tissue/cellular distribution.
Ion channels allow the passive diffusion of inorganic ions with high specificity, often in response to stimuli such as changes in transmembrane potential, ligands, light, or mechanical stress [50, 51]. Alternatively, ions can also be actively transported by ion pumps, such as the sodium/potassium pump (the Na+ , K+-ATPase), which transports three Na+ ions extracellularly and two K+ ions intracellularly for every molecule of ATP hydrolyzed [52]. Microbe-synthesized ionophores, such as valinomycin, facilitate the diffusion of ions across the cell membrane by complexing and shuttling ions [53]. Other ionophores such as gramicidin A form channels [54].
Small molecules are also often transported. Water is transported across the membrane by the aquaporin (AQP) family of passive channels. Aquaporins have been reported to transport other gases and solutes as well, such as CO 2 , NO, H 2 O 2 , arsenite, ammonia (in addition to the Rh proteins [55, 56]), urea (in addition to the urea transporters [57]) and glycerol [58]. (This is an abbreviated list excerpted from Bienert et al. [59]).
Sugars, including glucose, galactose, and fructose, are molecules of high polarity and intermediate size, and are imported into the cell by the glucose transporter (GLUT) family of facilitated transporters [60]. Most amino acids are transported across the cell membrane by secondary active transporters that utilize the energy stored in the electrochemical gradient of another solute [61]. Nucleobases and nucleosides also have associated secondary transporters on the plasma membrane [62]. Di- and tri-peptides of various chemical character are transported by the oligopeptide transporter PepT1, which has been reported to transport neutral tripeptide-like β-lactam antibiotics and peptide-like drugs as well [63]. Alternatively, α-Amanitin, a cyclic octapeptide that inhibits eukaryotic RNA polymerase II, has been reported to enter cells via an organic anion transporting polypeptide (OATP) transporter [64].
To note, transporters may mediate the efflux of molecules as well. A variety of structurally unrelated compounds, including small-molecule drugs, are substrates of efflux pumps in the ATP-binding cassette (ABC) transporter family such as the multidrug resistance protein (MRP) family [65], the P-glycoprotein pump (P-gp, also known as multidrug resistance protein 1(MDR1)) [66], and the breast cancer resistance protein (BCRP) [67].
4.3 Other Methods of Cytosolic Entry
A majority of the examples discussed in the following first involve the cargo being internalized into the cell via various endocytic pathways. Reiterating an earlier point, endocytosed cargo are topologically still in an extracellular space separated from the cytoplasm by a lipid membrane. Thus, an additional “endosomal escape” (or “endosomal release”) step is required where the cargo is transported across the membrane to access the cytoplasm. Some peptidic, viral, or bacterial components are thought to accomplish this step, not through passive diffusion or active transport, but by disrupting cellular membranes, allowing the passage of large and charged compounds. The mechanisms of most such processes are not yet fully elucidated and subjects of active research.
4.3.1 Peptides
Cell-penetrating peptides (CPP), also known as peptide transduction domains (PTD), are a diverse class of peptides that have been reported to traverse the cell membrane [68]. Representative members of this family such as the Trans-Activator of Transcription (TAT) peptide (Fig. 2b) and penetratin were initially identified as segments within naturally occurring proteins with proposed membrane permeability [26], such as homeoproteins [69].
The mechanisms of how these peptides cross the cell membrane is still unclear and likely differs amongst peptides. The TAT peptide for example (GRKKRRQRRRPSQ) is rich in arginines, and the delocalized positive charge in their guanidinium moieties has been proposed to allow extensive interactions with negatively charged cell membranes [70, 71]. TAT is thought to bind to the glycosaminoglycans (GAG) on the cell surface such as heparin sulfate [72] or adsorb into the glycerol backbone region of the lipid bilayer [73], eventually being macropinocytosed [74, 75]. Various models of TAT-mediated perturbations of the cell membrane have been proposed, including the formation of transient pores [76–80].
The reported permeability coefficient of TAT across artificial membranes is very low at 2.7 × 10−9 cm/s [81], which may reflect its need for structural features specific to the cell membrane to be able to translocate. Typically, relatively high (µM) concentrations of TAT are required in vitro to observe translocation, and the efficiency of such may depend on the cell line [82, 83]. Also, as mentioned earlier, fixation of cells treated with fluorescently labeled TAT may lead to artifacts in cellular distribution [25], and thus reported results need to be interpreted with caution.
4.3.2 Protein Toxins
A number of plant and bacterial toxins are potent inhibitors of central cellular functions such as protein synthesis. However, before they can have their effect, they must gain access to their cytosolic targets [84]. Typically, a separate domain (typically denoted the B domain, translocation domain, or translocation complex (when an oligomer)) is responsible for binding to cellular receptors and translocating the catalytic domain (the A domain) into the cytoplasm (see [85] for an illustration).
Some toxins form their own pores, such as the diphtheria and anthrax toxins. The translocation domains of anthrax toxin, known as protective antigen (PA), oligomerizes into a prepore complex following proteolytic activation (Fig. 2c). Subsequent internalization and endosomal acidification is thought to trigger its conversion into a full pore, through which catalytic domains escape into the cytosol [86].
A number of other toxins, such as the plant toxin ricin, Pseudomonas exotoxin A, and cholera toxin, take advantage of the ERAD machinery to enter the cell [87–89]. Following binding to gangliosides via its B domains, cholera toxin is internalized and trafficked to the ER where the A domain is reduced and unfolded. This domain is subsequently refolded in the cytoplasm [90, 91].
4.3.3 Viruses
Some viruses enter the cell through the plasma membrane, but more commonly from endocytic compartments after binding to cellular receptors and triggering various endocytic pathways [92]. Viruses can be classified into enveloped viruses, which are encased in a lipid membrane containing glycoproteins, or non- enveloped viruses, which lack a membrane.
In general, enveloped viruses are thought to orchestrate the fusion of host and viral membranes using viral fusion proteins, which expose hydrophobic peptides upon environmental triggers such as receptor binding, low pH, or proteolytic cleavage. For example, influenza A exposes a hydrophobic segment of hemagglutinin (HA) upon endosomal acidification [93]. With this mechanism, there is no need to translocate across the cell membrane.
Non-enveloped viruses, in contrast, have to cross the membrane in order to access the cytoplasm. In general, these viruses are thought to mediate the disruption of cellular membranes by exposing or releasing lytic peptides that are amphipathic or hydrophobic [94–96]. Alternatively, members of the polyomavirus family such as the simian virus (SV40) use a strategy similar to the aforementioned cholera toxin, and hijack the ERAD machinery [97, 98].
5 Approaches to Design and Improve Membrane Permeability
5.1 Small Molecule Cargo
Decades of pharmaceutical research have provided design principles that maximize the chance of obtaining a drug able to efficiently distribute within an organism and permeate through cell membranes. While bioavailability is often the reported parameter of interest, efficient membrane permeation is likely necessary for bio-availability [99]. Therefore, rules that have been devised in medicinal chemistry to achieve favorable bioavailability are a reasonable guide for the design of membrane-permeating small molecules.
5.1.1 Predicting Passive Permeation
Lipinski’s “Rule of 5” has been the most influential framework correlating the physicochemical properties of a given compound with its membrane permeability and bioavailability in the context of small-molecule drug development [100]. It postulates that poor absorption or permeation is more likely when: (1) the calculated lipophilicity (clogP) is over 5; (2) the molecular weight is over 500; (3) there are more than five hydrogen bond donors (well represented by the sum of OH and NH bonds); and (4) there are more than ten hydrogen bond acceptors (represented roughly, by the sum of Ns and Os).
The Rule of 5 has been generally successful at predicting membrane permeability, but not all compounds that comply with the rules are permeable, and permeable compounds that deviate from the rules are not uncommon [46, 101]. Nonetheless, as suggested by Guimarães et al. [46], the Rule of 5 does identify key physicochemical parameters, namely the polarity, size, and lipophilicity of the permeant, that are important for passive diffusion. These interrelated factors can affect the partitioning, diffusion, or both, of the molecule into and across the membrane.
Alternative metrics for these parameters have also been proposed. Regarding polarity, the polar surface area (PSA) of a compound has been used in addition to the number of hydrogen bond donors and acceptors [99, 102]. For molecular size, studies have inversely-correlated the permeability of small solutes with molecular volume [38] or cross- sectional area [103]. A different but related parameter, the number of rotatable bonds, has been suggested as well, where molecules with fewer rotatable bonds and lower PSA were reported to have better permeability across artificial membranes [99]. Additionally, it has also been proposed that conformationally flexible molecules that are able to form intramolecular hydrogen bonds in a low dielectric environment may adaptively reduce their surface polarity for improved permeation [104]. Unsurprisingly, even if the hydrogen bond counts or PSA is low, localized charge or highly polar groups can significantly decrease the permeability of an otherwise permeable parent compound by orders of magnitude [105, 106].
Beyond empirical correlations, molecular dynamics (MD) simulations are increasingly applied to calculate the energetic barrier of transmembrane diffusion, from which permeability coefficients can be derived [15, 107, 108]. Improved computational power and coarse-grained modeling have reduced computing time. However, although these methods are invaluable in estimating permeabilities that are difficult to obtain experimentally, utilizing them on a routine basis is yet hampered by the computational cost and the effort involved in building a suitable representation of the molecule of interest. Estimates for large molecules may be particularly prone to inaccuracy due to insufficient sampling of their conformational space during the simulations.
5.1.2 Predicting Transporter-Mediated Permeation
Designing compounds to be substrates of a specific transporter is currently difficult [109], although indirect approaches have been proposed to identify metabolites that are structurally similar to a given compound [110]. Alternatively, conjugating compounds to known transporter substrates such as amino acids has been reported to improve permeation and oral adsorption by engaging PepT1 [111] (Fig. 2d). In such “prodrug” approaches, the conjugated substrates are designed to be cleaved intracellularly or during circulation to release the free drug [112]. Although designing specific transporter substrates is infeasible at the moment, it should be kept in mind that transporters can affect a compound’s permeation.
5.1.3 Comparing Theory and Empirical Data for Molecular Probes
Empirical permeability data from molecular probes and labeling molecules roughly agree with the theoretical expectations discussed above. Generally, small and uncharged fluorophores, and those whose charge is delocalized over the fluorophore (e.g., TAMRA), are sufficiently membrane-permeable to be used in intra-cellular protein labeling applications [113]. However, fluorescent dyes carrying localized charges (e.g., the sulfonic acid derivatives of Cy3 or Cy5) display low membrane permeability [113]. Esterification of charged groups is one strategy to mask the effects of charge [114].
An example of the size-dependence of membrane translocation is provided by fluorescent dyes modified with long and hydrophobic lipid-like tails. For the voltage-sensitive dyes Di-4-ANEPPS and Di-8-ANEPPS (equipped with two octyl and butyl chains, respectively), a strong decrease in membrane flip-flop was observed across planar black lipid membranes for the long-chain variant [115]. A similar result was obtained for the dyes DiI-C12 and DiI-C18 [116]. The counterintuitive result where increasing the overall hydrophobicity of the molecules strongly reduced the rate of flip-flop is likely due to the concomitant increase in molecular size. A similar result has been reported with anthroyl fatty acids in liposomes, where the rate of flip-flop was observed to be 200-fold faster for a C11-fatty acid compared to a C18-fatty acid [117].
As mentioned earlier, these molecular probes may also be substrates of cellular transporters. For example, acetoxymethyl ester (AM) derivatives of various fluorescent indicators were observed to be actively exported from cells by multidrug transporters [118]. Of note, passive diffusion and active transport may occur concomitantly. Chidley et al. studied the intracellular access of various organic molecules used for protein labeling via the SNAP-tag system in yeast strains that were either wild-type or had three efflux transporters deleted [119]. The study showed a strong decrease in uptake with increasing size and polarity of the labeling molecule, suggesting entry by passive diffusion. Additionally, it showed that labeling efficiency increased in the modified yeast strain, presumably due to reduced active export.
5.2 Peptide Cargo
It is unlikely that peptides will passively diffuse across the cell membrane, but altering their physical properties (such as conformational flexibility and polarity) has been proposed to improve their permeability. Despite interesting findings—a selection of which is discussed in the following—conflicting experimental results have been reported. A straightforward method for converting a non-permeable peptide into an efficiently permeating entity is thus not available so far.
5.2.1 Addressing Conformation and Polarity
Macrocyclic drugs—those with a ring architecture of 12 or more atoms, including cyclic peptides—tend to be larger and more polar than most small-molecule drugs, falling outside the Rule of 5 [120, 121]. Yet some are administered orally [121], suggesting that they may be membrane permeable [43, 99, 122]. In the case of cyclosporin A (Fig. 2a), this is believed to occur by passive diffusion.
Following such examples, cyclizing a given peptide and methylating select amide bond nitrogens have been proposed to improve its membrane permeation and/or bioavailability. Such modifications, when made judiciously [123], are thought to facilitate the formation of intramolecular hydrogen bonds in response to the low dielectric environment of the membrane interior [43, 45, 124]. Passive permeability values ranging from 6.3 × 10−7 cm/s [45] to approximately 7.7 × 10−6 cm/s [124] (estimated from [125]) have been reported for certain hydrophobic cyclic peptides.
Alternatively, cyclization and amidation may alter a compound’s specificity towards membrane transporters. In a study of 54 cyclic alanine hexapeptides containing various degrees of N-methylation, Ovadia et al. reported that none of the tested peptides showed permeation across artificial membranes. However, some peptides were found to be highly permeable across Caco-2 cell monolayers (on the order of 10−5 cm/s), suggesting that transporters may be involved [126].
In some instances, cyclization by covalently linking internal residues has been proposed to increase permeability by changing the peptide’s α-helical content [127]. Such modifications include hydrocarbon “staples” linking the side chains of nonnatural amino acids inserted into the peptide [128], and “hydrogen bond surrogates” replacing a main chain hydrogen bond with a carbon–carbon [129] or disulfide bond [130]. Such modifications have lead to the development of peptide inhibitors against intracellular targets such as the ICN1/CSL complex (involved in the NOTCH signaling pathway) [22], Ras [131] and MDM2/MDMX [23].
However, introduction of a staple alone does not guarantee an improvement in permeability [132–134]. Extensive optimization may still be required for multiple factors such as the position, length, and stereochemistry of the staple [135], as well as the charge and amino acid sequence of the peptide [136].
5.2.2 Designing Cell-Penetrating Peptides
Extensive effort in discovering novel membrane-permeable peptides has generated significant diversity in the physicochemical character of reported CPPs [137]. Methods have been proposed to synthetically design permeable peptides or predict such segments from a given protein sequence [138].
Introducing arginine residues within α-helices has been proposed to improve permeability [139]. In a study of the avian pancreatic polypeptide (aPP), a 36-residue peptide/miniature protein, and CP1, a 28-residue zinc finger, substituting five residues within the α-helix with arginine increased the permeability to that comparable with TAT [139]. The authors estimated that approximately 1–5 % of the internalized peptides were being released into the cytosol.
5.3 Protein Cargo
Proteins cannot passively diffuse across the cell membrane due to their size and polarity. Thus, a delivery system or technique is always required, similar to nucleic acid transfection. However, while nucleic acid transfection reagents are now routinely used in the laboratory, there are no equivalent standards for the delivery of proteins. In the following, we survey strategies that have been proposed to deliver proteins across the cell membrane. Given the physicochemical diversity of proteins and their delicate nature, it is challenging to design a system or method that is readily generalizable to multiple proteins while maintaining the cargo’s respective function and stability. For more comprehensive reviews, please see [140–143], as well as those cited below.
5.3.1 Mechanical Disruption of the Membrane
Varying physical methods of disrupting the cell membrane, such as microinjection and electroporation [144], have been proposed for delivering compounds ranging from small molecules to proteins. Sharei et al. developed a microfluidic device that transiently disrupts the plasma membrane through physical constriction [145]. Silicon “nanowires” that pierce the cell membrane have also been reported [146, 147].
5.3.2 Peptide-Based Strategies
CPPs have been reported to enhance the permeability of various macromolecules, including proteins [148–150]. Early studies showed that the TAT peptide can mediate the translocation of covalently coupled proteins [151, 152]. In later studies, an amphiphilic CPP Pep-1 was reported to noncovalently complex and translocate peptide and protein cargos [153].
Substance P (SP), an 11-residue neuropeptide implicated in cancer progression [154], has been proposed to mediate the cytosolic delivery of synthetic antibody fragments [155] and nucleic acids [156] following covalent conjugation. Its natural GPCR partner, the neurokinin-1 receptor (NK1R), has been suggested to play a role in mediating uptake. The mechanisms by which such peptides mediate translocation remains to be clarified.
5.3.3 Protein-Based Strategies
Various pore- or channel-forming proteins of bacterial origin have been utilized to translocate exogenous proteins. Highly sophisticated secretion systems, which transport proteins directly from the bacterial cytoplasm to the eukaryotic host’s [157], have been reported to deliver proteins to the cytosol of antigen-presenting cells [158]. Doerner et al. reported the functional expression of an engineered bacterial channel (MscL) in mammalian cells, the opening and closing of which could be controlled chemically [159]. Alternatively, the cholesterol-dependent cytolysin (CDC) family of pore-forming toxins, which are capable of forming macropores up to 30 nm in diameter [160], have been proposed as “reversible permeabilization” reagents for delivering exogenous proteins [161, 162].
In addition to pore- or channel-forming proteins, the membrane- translocating domains of bacterial toxins have been proposed as a modular tool that can be fused to, and enhance the intracellular delivery of, other proteins [163, 164]. In instances where the receptor-binding domain of the toxin is physically distinct from the translocation domain, the former has been replaced with alternative targeting moieties to generate immunotoxins. Immunotoxins retain the cytotoxicity of the parent toxin but are directed at specific cell types [165, 166].
Additionally, “supercharged” GFP, a variant engineered to have high net positive charge (+36) [167], and certain human proteins with naturally high positive charge [168, 169] have been reported to translocate across the cell membrane. Curiously, 3E10, an autoantibody proposed to bind to dsDNA [170], has been proposed to penetrate into the nucleus and impair DNA repair [171], or translocate an exogenous phosphatase across the cell membrane [172].
5.3.4 Virus-Based Strategies
Packaging proteins in virus-like particles [173] or attaching them to an engineered bacteriophage T4 head [174] has been reported to enhance cytosolic delivery. In addition, although not yet utilized as a delivery system, it has been reported that virus-bound antibodies co-internalize into the cytoplasm along with the virus [175].
5.3.5 Lipid-and Polymer-Based Strategies
With lipid-based materials, the protein cargo is either encapsulated in liposomes [176] or complexed with lipids. Regarding the latter strategy, lipid formulations that have been successful in the transfection of DNA have been attempted for the protein delivery. For example, a formulation based on a mixture of cationic and neutral lipids was reported to translocate negatively charged proteins [177].
Similarly, polymer-based formulations that have been successfully used for nucleic acid transfections have also been examined for their ability to “transfect” proteins. The “proton sponge effect” is an influential hypothesis still undergoing debate [178], which states that materials such as polyethylenimine (PEI) that are rich in protonatable amines, will cause a significant buffering of protons and subsequent osmotic swelling in endosomes. The endosome is then proposed to stall its maturation and eventually rupture [179]. Poly-β-amino esters (PBAEs), successfully developed for the transfection of nucleic acids [180], are thought to take advantage of this proton sponge effect. Su et al. reported that biodegradable PBAE nanoparticles enhance the endosomal escape of various cargos, including proteins, when co-administered [181].
Alternatively, Yan et al. reported a technology to encapsulate single proteins in a polymeric shell (termed “nanocapsule”) after attaching the monomeric building blocks of the polymer directly to the protein [182, 183]. Such nanocapsules, designed to be degraded in response to environmental stimuli such as protease activity or changes in pH or redox potential, were reported to deliver proteins including transcription factors [184].
5.3.6 Inorganic Material-Based Strategies
A variety of inorganic materials have also been proposed to translocate protein cargo, including silica, carbon nanotubes, quantum dots, and gold nanoparticles (see [142, 185]).
6 Conclusions
The plasma membrane of a mammalian cell is an intricate composite of multiple lipid and protein species continually undergoing endocytosis and exocytosis. Whereas small molecules with moderate polarity are able to diffuse through the cell membrane passively, most metabolites and short peptides require specialized membrane transporters for translocation. Proteins are generally unable to cross the cell membrane, with protein toxins being exceptions where sophisticated (and yet to be fully elucidated) mechanisms have been evolved for translocation.
In this review, we have surveyed proposed strategies on how to obtain membrane-permeable molecules that utilize passive diffusion, membrane transporters, or engineered delivery systems (Fig. 1). For passive diffusion, the Rule of 5 and its derivatives provide a rough guide for design. Minimizing the size of the desired permeant and its effective polarity is recommended. The latter can be achieved by minimizing the number of polar groups and localized charges in the molecule, or by cyclization, amidation and esterification strategies that shield polar groups in the interior of the molecule or mask a charge. It is currently unfeasible to explicitly design molecules as transporter substrates, although conjugation strategies have been proposed to known transporter substrates. When interpreting experimental results showing the entry or export of molecules, the possible contribution from membrane transporters should be kept in mind. Finally, various methods and delivery systems have been proposed to transport proteins across the cell membrane, from mechanical disruption to utilizing delivery systems that are either covalently attached or noncovalently complexed to the protein of interest.
In addition to providing an overview of engineering approaches, we have strived to provide a framework for evaluating the effectiveness of such strategies. Here, it cannot be understated that the experimental assay employed to demonstrate cytosolic delivery, depending on its sensitivity and mode of detection, could greatly impact the perceived efficacy of the delivery method. Particularly with protein delivery, an easy-to- implement, standardized assay that can accurately quantify delivery performance would be invaluable in objectively comparing different platforms. Also, while frequently overlooked, cytotoxicity caused by the delivery vehicle, if any, should be explicitly addressed. Finally, the mechanism(s) of action allowing delivery should be thoroughly investigated to avoid experimental artifacts.
In summary, we have summarized strategies employed by nature or devised by man to transport small molecules, peptides, and proteins across cell membranes. We hope this review will provide scientists interested in designing cell- permeable probes or effector molecules with a starting point to approach the task. Second, we hope it will aid in bringing together the concepts and solutions generated for diverse payloads. Although overcoming the membrane barrier remains a challenging and incompletely solved problem, significant progress continues to be made towards enabling potentially powerful applications in biological research and medicine.
Acknowledgments
The authors thank Bradley Pentelute, Alessandro Angelini, Sandrine Sagan, Alexander H. de Vries, and Christopher Chidley for helpful discussions and critical reading of the manuscript.
References
- 1.Stein WD, Lieb WR. Transport and diffusion across cell membranes. 1st. Orlando, FL: Academic; 1986. [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. 5th. New York: Garland Science; 2007. [Google Scholar]
- 3.Di L, Artursson P, Avdeef A, et al. Evidence-based approach to assess passive diffusion and carrier-mediated drug transport. Drug Discov Today. 2012;17:905–912. doi: 10.1016/j.drudis.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 6.Koichi K, Michiya F, Makoto N. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim Biophys Acta. 1974;369:222–233. [PubMed] [Google Scholar]
- 7.Marsh D, Horváth LI. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim Biophys Acta. 1998;1376:267–296. doi: 10.1016/s0304-4157(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 9.Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993;294:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leventis R, Silvius JR. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys J. 2001;81:2257–2267. doi: 10.1016/S0006-3495(01)75873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steck TL, Ye J, Lange Y. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys J. 2002;83:2118–2125. doi: 10.1016/S0006-3495(02)73972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 13.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 14.Alberts B, Johnson A, Lewis J, et al. [Accessed 27 Feb 2014];Molecular biology of the cell. 2002 http://www.ncbi.nlm.nih.gov/books/NBK21054/
- 15.Orsi M, Essex JW. Passive permeation across lipid bilayers: a literature review. Molecular simulations and biomembranes: from biophysics to function. 2010:76–90. [Google Scholar]
- 16.Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem. 1998;41:1007–1010. doi: 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
- 17.Sugano K, Kansy M, Artursson P, et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov. 2010;9:597–614. doi: 10.1038/nrd3187. [DOI] [PubMed] [Google Scholar]
- 18.Di L, Whitney-Pickett C, Umland JP, et al. Development of a new permeability assay using low-efflux MDCKII cells. J Pharm Sci. 2011;100:4974–4985. doi: 10.1002/jps.22674. [DOI] [PubMed] [Google Scholar]
- 19.Shamu CE, Story CM, Rapoport TA, Ploegh HL. The pathway of Us11-dependent degradation of Mhc class I heavy chains involves a ubiquitin-conjugated intermediate. J Cell Biol. 1999;147:45–58. doi: 10.1083/jcb.147.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartz R, Fan H, Zhang J, et al. Effective siRNA delivery and target mRNA degradation using an amphipathic peptide to facilitate pH-dependent endosomal escape. Biochem J. 2011;435:475–487. doi: 10.1042/BJ20101021. [DOI] [PubMed] [Google Scholar]
- 21.Bittner MA, Holz RW. Effects of tetanus toxin on catecholamine release from intact and digitonin-permeabilized chromaffin cells. J Neurochem. 1988;51:451–456. doi: 10.1111/j.1471-4159.1988.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Moellering RE, Cornejo M, Davis TN, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang YS, Graves B, Guerlavais V, et al. Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc Natl Acad Sci. 2013;110:E3445–E3454. doi: 10.1073/pnas.1303002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonner DK, Leung C, Chen-Liang J, et al. Intracellular trafficking of polyamido-amine-poly(ethylene glycol) block copolymers in DNA delivery. Bioconjug Chem. 2011;22:1519–1525. doi: 10.1021/bc200059v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard JP, Melikov K, Vives E, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 26.Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Cebrian I, Visentin G, Blanchard N, et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Yu P, Liu B, Kodadek T. A high-throughput assay for assessing the cell permeability of combinatorial libraries. Nat Biotechnol. 2005;23:746–751. doi: 10.1038/nbt1099. [DOI] [PubMed] [Google Scholar]
- 29.Holub JM, LaRochelle JR, Appelbaum JS, Schepartz A. Improved assays for determining the cytosolic access of peptides, proteins, and their mimetics. Biochemistry (Mosc) 2013;52:9036–9046. doi: 10.1021/bi401069g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlokarnik G, Negulescu PA, Knapp TE, et al. Quantitation of transcription and clonal selection of single living cells with β-lactamase as reporter. Science. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]
- 31.Bordonaro M. Modular Cre/lox system and genetic therapeutics for colorectal cancer. J Biomed Biotechnol. 2009 doi: 10.1155/2009/358230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment a introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 33.Eiklid K, Olsnes S, Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res. 1980;126:321–326. doi: 10.1016/0014-4827(80)90270-0. [DOI] [PubMed] [Google Scholar]
- 34.Diamond JM, Katz Y. Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. J Membr Biol. 1974;17:121–154. doi: 10.1007/BF01870176. [DOI] [PubMed] [Google Scholar]
- 35.Finkelstein A. Water and nonelectrolyte permeability of lipid bilayer membranes. J Gen Physiol. 1976;68:127–135. doi: 10.1085/jgp.68.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subczynski WK, Hyde JS, Kusumi A. Oxygen permeability of phosphatidylcholine- cholesterol membranes. Proc Natl Acad Sci. 1989;86:4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutknecht J, Bisson MA, Tosteson FC. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977;69:779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter A, Gutknecht J. Permeability of small nonelectrolytes through lipid bilayer membranes. J Membr Biol. 1986;90:207–217. doi: 10.1007/BF01870127. [DOI] [PubMed] [Google Scholar]
- 39.Orbach E, Finkelstein A. The nonelectrolyte permeability of planar lipid bilayer membranes. J Gen Physiol. 1980;75:427–436. doi: 10.1085/jgp.75.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papahadjopoulos D, Nir S, Oki S. Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta. 1972;266:561–583. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- 41.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 42.Giorgi EP, Stein WD. The transport of steroids into animal cells in culture. Endocrinology. 1981;108:688–697. doi: 10.1210/endo-108-2-688. [DOI] [PubMed] [Google Scholar]
- 43.Bockus AT, McEwen CM, Lokey RS. Form and function in cyclic peptide natural products: a pharmacokinetic perspective. Curr Top Med Chem. 2013;13:821–836. doi: 10.2174/1568026611313070005. [DOI] [PubMed] [Google Scholar]
- 44.Augustijns PF, Bradshaw TP, Gan LSL, et al. Evidence for a polarized efflux system in Caco-2 cells capable of modulating cyclosporine A transport. Biochem Biophys Res Commun. 1993;197:360–365. doi: 10.1006/bbrc.1993.2487. [DOI] [PubMed] [Google Scholar]
- 45.Rezai T, Bock JE, Zhou MV, et al. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: successful in silico prediction of the relative permeabilities of cyclic peptides. J Am Chem Soc. 2006;128:14073–14080. doi: 10.1021/ja063076p. [DOI] [PubMed] [Google Scholar]
- 46.Guimarães CRW, Mathiowetz AM, Shalaeva M, et al. Use of 3D properties to characterize beyond rule-of-5 property space for passive permeation. J Chem Inf Model. 2012;52:882–890. doi: 10.1021/ci300010y. [DOI] [PubMed] [Google Scholar]
- 47.Hediger MA, Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med. 2013;34:95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saier MH, Reddy VS, Tamang DG, Vastermark A. The transporter classification database. Nucleic Acids Res. 2013;42:D251–D258. doi: 10.1093/nar/gkt1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hediger MA. The ABCs of membrane transporters in health and disease (SLC series) Mol Aspects Med. 2013;34(2–3):95–752. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kew JNC, Davies CH. Ion channels: from structure to function. Oxford: Oxford University Press; 2010. [Google Scholar]
- 51.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 52.Toyoshima C, Kanai R, Cornelius F. First crystal structures of Na+, K+-ATPase: new light on the oldest ion pump. Structure. 2011;19:1732–1738. doi: 10.1016/j.str.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Duax WL, Griffin JF, Langs DA, et al. Molecular structure and mechanisms of action of cyclic and linear ion transport antibiotics. Pept Sci. 1996;40:141–155. doi: 10.1002/(SICI)1097-0282(1996)40:1%3C141::AID-BIP6%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 54.Wallace BA. Recent advances in the high resolution structures of bacterial channels: gramicidin A. J Struct Biol. 1998;121:123–141. doi: 10.1006/jsbi.1997.3948. [DOI] [PubMed] [Google Scholar]
- 55.Zheng L, Kostrewa D, Bernèche S, et al. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrade SLA, Einsle O. The Amt/Mep/Rh family of ammonium transport proteins. Mol Membr Biol. 2007;24:357–365. doi: 10.1080/09687680701388423. [DOI] [PubMed] [Google Scholar]
- 57.Shayakul C, Clémençon B, Hediger MA. The urea transporter family (SLC14): physiological, pathological and structural aspects. Mol Aspects Med. 2013;34:313–322. doi: 10.1016/j.mam.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Ishibashi K, Hara S, Kondo S. Aquaporin water channels in mammals. Clin Exp Nephrol. 2009;13:107–117. doi: 10.1007/s10157-008-0118-6. [DOI] [PubMed] [Google Scholar]
- 59.Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schweikhard ES, Ziegler CM. Amino acid secondary transporters: toward a common transport mechanism. Curr Top Membr. 2012;70:1–28. doi: 10.1016/B978-0-12-394316-3.00001-6. [DOI] [PubMed] [Google Scholar]
- 62.Young JD, Yao SYM, Baldwin JM, et al. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med. 2013;34:529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Smith DE, Clémençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–336. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z-S, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–3245. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:27–34. doi: 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83:1084–1103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langel U. Handbook of cell-penetrating peptides. 2nd. Boca Raton: CRC Press; 2010. [Google Scholar]
- 69.Sagan S, Burlina F, Alves ID, et al. Homeoproteins and homeoprotein-derived peptides: going in and out. Curr Pharm Des. 2013;19:2851–2862. doi: 10.2174/1381612811319160002. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt N, Mishra A, Lai GH, Wong GCL. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 71.Futaki S, Hirose H, Nakase I. Arginine-rich peptides: methods of translocation through biological membranes. Curr Pharm Des. 2013;19:2863–2868. doi: 10.2174/1381612811319160003. [DOI] [PubMed] [Google Scholar]
- 72.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 73.Su Y, Waring AJ, Ruchala P, Hong M. Membrane-bound dynamic structure of an arginine-rich cell-penetrating peptide, the protein transduction domain of HIV TAT, from solid-state NMR. Biochemistry (Mosc) 2010;49:6009–6020. doi: 10.1021/bi100642n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 75.Nakase I, Tadokoro A, Kawabata N, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry (Mosc) 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 76.Yesylevskyy S, Marrink S-J, Mark AE. Alternative mechanisms for the interaction of the cell-penetrating peptides penetratin and the TAT peptide with lipid bilayers. Biophys J. 2009;97:40–49. doi: 10.1016/j.bpj.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herce HD, Garcia AE, Litt J, et al. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys J. 2009;97:1917–1925. doi: 10.1016/j.bpj.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra A, Lai GH, Schmidt NW, et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc Natl Acad Sci. 2011;108:16883–16888. doi: 10.1073/pnas.1108795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawamoto S, Miyakawa T, Takasu M, et al. Cell-penetrating peptide induces various deformations of lipid bilayer membrane: inverted micelle, double bilayer, and trans-membrane. Int J Quantum Chem. 2012;112:178–183. [Google Scholar]
- 80.Huang K, García AE. Free energy of translocating an arginine-rich cell-penetrating peptide across a lipid bilayer suggests pore formation. Biophys J. 2013;104:412–420. doi: 10.1016/j.bpj.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones S, Howl J. Enantiomer-specific bioactivities of peptidomimetic analogues of mastoparan and mitoparan: characterization of inverso mastoparan as a highly efficient cell penetrating peptide. Bioconjug Chem. 2012;23:47–56. doi: 10.1021/bc2002924. [DOI] [PubMed] [Google Scholar]
- 82.Tréhin R, Krauss U, Beck-Sickinger AG, et al. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat(47–57) through well-differentiated epithelial models. Pharm Res. 2004;21:1248–1256. doi: 10.1023/b:pham.0000033013.45204.c3. [DOI] [PubMed] [Google Scholar]
- 83.Foerg C, Merkle HP. On the biomedical promise of cell penetrating peptides: limits versus prospects. J Pharm Sci. 2008;97:144–162. doi: 10.1002/jps.21117. [DOI] [PubMed] [Google Scholar]
- 84.Sandvig K, van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- 85.Falnes PØ, Sandvig K. Penetration of protein toxins into cells. Curr Opin Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 86.Collier RJ. Membrane translocation by anthrax toxin. Mol Aspects Med. 2009;30:413–422. doi: 10.1016/j.mam.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Virgilio M, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-inactivating proteins: from plant defense to tumor attack. Toxins. 2010;2:2699–2737. doi: 10.3390/toxins2112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spooner RA, Lord JM. How ricin and shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. In: Mantis N, editor. Ricin shiga toxins. Berlin: Springer; 2012. pp. 19–40. [DOI] [PubMed] [Google Scholar]
- 89.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013;140:317–326. doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 90.Wernick NLB, Chinnapen DJ-F, Cho JA, Lencer WI. Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins. 2010;2:310–325. doi: 10.3390/toxins2030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho JA, Chinnapen DJ-F, Aamar E, et al. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front Cell Infect Microbiol. 2012 doi: 10.3389/fcimb.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 93.Sriwilaijaroen N, Suzuki Y. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:226–249. doi: 10.2183/pjab.88.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai B. Penetration of nonenveloped viruses into the cytoplasm. Annu Rev Cell Dev Biol. 2007;23:23–43. doi: 10.1146/annurev.cellbio.23.090506.123454. [DOI] [PubMed] [Google Scholar]
- 95.Johnson J, Banerjee M. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr Protein Pept Sci. 2008;9:16–27. doi: 10.2174/138920308783565732. [DOI] [PubMed] [Google Scholar]
- 96.Moyer CL, Nemerow GR. Viral weapons of membrane destruction: variable modes of membrane penetration by non-enveloped viruses. Curr Opin Virol. 2011;1:44–99. doi: 10.1016/j.coviro.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inoue T, Tsai B. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb Perspect Biol. 2013;5:a013250. doi: 10.1101/cshperspect.a013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suomalainen M, Greber UF. Uncoating of non-enveloped viruses. Curr Opin Virol. 2013;3:27–33. doi: 10.1016/j.coviro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Veber DF, Johnson SR, Cheng H-Y, et al. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 100.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 101.Faller B, Ottaviani G, Ertl P, et al. Evolution of the physicochemical properties of marketed drugs: can history foretell the future? Drug Discov Today. 2011;16:976–984. doi: 10.1016/j.drudis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 103.Xiang T-X, Anderson BD. Influence of chain ordering on the selectivity of dipalmi-toylphosphatidylcholine bilayer membranes for permeant size and shape. Biophys J. 1998;75:2658–2671. doi: 10.1016/S0006-3495(98)77711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuhn B, Mohr P, Stahl M. Intramolecular hydrogen bonding in medicinal chemistry. J Med Chem. 2010;53:2601–2611. doi: 10.1021/jm100087s. [DOI] [PubMed] [Google Scholar]
- 105.Mayer PT, Xiang T-X, Anderson BD. Independence of substituent contributions to the transport of small-molecule permeants in lipid bilayer. AAPS Pharm Sci. 2000;2:40–52. doi: 10.1208/ps020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulander J, Haymet ADJ. Permeation across hydrated DPPC lipid bilayers: simulation of the titrable amphiphilic drug valproic acid. Biophys J. 2003;85:3475–3484. doi: 10.1016/S0006-3495(03)74768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiang T-X, Anderson BD. Liposomal drug transport: a molecular perspective from molecular dynamics simulations in lipid bilayers. Adv Drug Deliv Rev. 2006;58:1357–1378. doi: 10.1016/j.addr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Bennett WFD, MacCallum JL, Hinner MJ, et al. Molecular view of cholesterol flip-flop and chemical potential in different membrane environments. J Am Chem Soc. 2009;131:12714–12720. doi: 10.1021/ja903529f. [DOI] [PubMed] [Google Scholar]
- 109.Maeda K, Sugiyama Y. Transporter biology in drug approval: regulatory aspects. Mol Aspects Med. 2013;34:711–718. doi: 10.1016/j.mam.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Dobson PD, Patel Y, Kell DB. “Metabolite-likeness” as a criterion in the design and selection of pharmaceutical drug libraries. Drug Discov Today. 2009;14:31–40. doi: 10.1016/j.drudis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 111.Dahan A, Khamis M, Agbaria R, Karaman R. Targeted prodrugs in oral drug delivery: the modern molecular biopharmaceutical approach. Expert Opin Drug Deliv. 2012;9:1001–1013. doi: 10.1517/17425247.2012.697055. [DOI] [PubMed] [Google Scholar]
- 112.Majumdar S, Duvvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv Drug Deliv Rev. 2004;56:1437–1452. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Keppler A, Arrivoli C, Sironi L, Ellenberg J. Fluorophores for live cell imaging of AGT fusion proteins across the visible spectrum. Biotechniques. 2006;41:167–170. doi: 10.2144/000112216. 172, 174–175. [DOI] [PubMed] [Google Scholar]
- 114.Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- 115.Ries RS, Choi H, Blunck R, et al. Black lipid membranes: visualizing the structure, dynamics, and substrate dependence of membranes. J Phys Chem B. 2004;108:16040–16049. [Google Scholar]
- 116.Melikyan GB, Deriy BN, Ok DC, Cohen FS. Voltage-dependent translocation of R18 and DiI across lipid bilayers leads to fluorescence changes. Biophys J. 1996;71:2680–2691. doi: 10.1016/S0006-3495(96)79459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleinfeld AM, Chu P, Storch J. Flip-flop is slow and rate limiting for the movement of long chain anthroyloxy fatty acids across lipid vesicles. Biochemistry (Mosc) 1997;36:5702–5711. doi: 10.1021/bi962007s. [DOI] [PubMed] [Google Scholar]
- 118.Homolya L, Holló Z, Germann UA, et al. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 119.Chidley C, Haruki H, Pedersen MG, et al. A yeast-based screen reveals that sulfasalazine inhibits tetrahydrobiopterin biosynthesis. Nat Chem Biol. 2011;7:375–383. doi: 10.1038/nchembio.557. [DOI] [PubMed] [Google Scholar]
- 120.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat Rev Drug Discov. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 121.Giordanetto F, Revell JD, Knerr L, et al. Stapled vasoactive intestinal peptide (VIP) derivatives improve VPAC2 agonism and glucose-dependent insulin secretion. ACS Med Chem Lett. 2013;4:1163–1168. doi: 10.1021/ml400257h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bock JE, Gavenonis J, Kritzer JA. Getting in shape: controlling peptide bioactivity and bioavailability using conformational constraints. ACS Chem Biol. 2013;8:488–499. doi: 10.1021/cb300515u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwon Y-U, Kodadek T. Quantitative comparison of the relative cell permeability of cyclic and linear peptides. Chem Biol. 2007;14:671–677. doi: 10.1016/j.chembiol.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 124.White TR, Renzelman CM, Rand AC, et al. On-resin N-methylation of cyclic peptides for discovery of orally bioavailable scaffolds. Nat Chem Biol. 2011;7:810–817. doi: 10.1038/nchembio.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malakoutikhah M, Prades R, Teixidó M, Giralt E. N-methyl phenylalanine-rich peptides as highly versatile blood-brain barrier shuttles. J Med Chem. 2010;53:2354–2363. doi: 10.1021/jm901654x. [DOI] [PubMed] [Google Scholar]
- 126.Ovadia O, Greenberg S, Chatterjee J, et al. The effect of multiple N-methylation on intestinal permeability of cyclic hexapeptides. Mol Pharm. 2011;8:479–487. doi: 10.1021/mp1003306. [DOI] [PubMed] [Google Scholar]
- 127.Azzarito V, Long K, Murphy NS, Wilson AJ. Inhibition of α-helix-mediated protein- protein interactions using designed molecules. Nat Chem. 2013;5:161–173. doi: 10.1038/nchem.1568. [DOI] [PubMed] [Google Scholar]
- 128.Kim Y-W, Grossmann TN, Verdine GL. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat Protoc. 2011;6:761–771. doi: 10.1038/nprot.2011.324. [DOI] [PubMed] [Google Scholar]
- 129.Patgiri A, Menzenski MZ, Mahon AB, Arora PS. Solid-phase synthesis of short α-helices stabilized by the hydrogen bond surrogate approach. Nat Protoc. 2010;5:1857–1865. doi: 10.1038/nprot.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller SE, Kallenbach NR, Arora PS. Reversible alpha-helix formation controlled by a hydrogen bond surrogate. Tetrahedron. 2012;68:4434–4437. doi: 10.1016/j.tet.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the Ras-Sos interaction. Nat Chem Biol. 2011;7:585–587. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okamoto T, Zobel K, Fedorova A, et al. Stabilizing the pro-apoptotic BimBH3 Helix (BimSAHB) does not necessarily enhance affinity or biological activity. ACS Chem Biol. 2013;8:297–302. doi: 10.1021/cb3005403. [DOI] [PubMed] [Google Scholar]
- 133.Bird GH, Gavathiotis E, LaBelle JL, et al. Distinct BimBH3 (BimSAHB) stapled peptides for structural and cellular studies. ACS Chem Biol. 2014;9:831–837. doi: 10.1021/cb4003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okamoto T, Segal D, Zobel K, et al. Further insights into the effects of pre-organizing the BimBH3 helix. ACS Chem Biol. 2014;9:838–839. doi: 10.1021/cb400638p. [DOI] [PubMed] [Google Scholar]
- 135.Verdine GL, Hilinski GJ. Stapled pep-tides for intracellular drug targets. In: Dane Wittrup K, Verdine GL, editors. Methods enzymol. New York: Academic; 2012. pp. 3–33. [DOI] [PubMed] [Google Scholar]
- 136.Bird GH, Christian Crannell W, Walensky LD. Chemical synthesis of hydrocarbon-stapled peptides for protein interaction research and therapeutic targeting. Curr Protoc Chem Biol. 2011;3(3):99–117. doi: 10.1002/9780470559277.ch110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides: design synthesis and applications. ACS Nano. 2014 doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 139.Appelbaum JS, LaRochelle JR, Smith BA, et al. Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm. Chem Biol. 2012;19:819–830. doi: 10.1016/j.chembiol.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marschall ALJ, Frenzel A, Schirrmann T, et al. Targeting antibodies to the cytoplasm. mAbs. 2011;3:3–16. doi: 10.4161/mabs.3.1.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gu Z, Biswas A, Zhao M, Tang Y. Tailoring nanocarriers for intracellular protein delivery. Chem Soc Rev. 2011;40:3638–3655. doi: 10.1039/c0cs00227e. [DOI] [PubMed] [Google Scholar]
- 142.Du J, Jin J, Yan M, Lu Y. Synthetic nanocarriers for intracellular protein delivery. Curr Drug Metab. 2012;13:82–92. doi: 10.2174/138920012798356862. [DOI] [PubMed] [Google Scholar]
- 143.Salmaso S, Caliceti P. Self assembling nanocomposites for protein delivery: supra-molecular interactions of soluble polymers with protein drugs. Int J Pharm. 2013;440:111–123. doi: 10.1016/j.ijpharm.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y, Yu L-C. Microinjection as a tool of mechanical delivery. Curr Opin Biotechnol. 2008;19:506–510. doi: 10.1016/j.copbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 145.Sharei A, Zoldan J, Adamo A, et al. A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci. 2013;110:2082–2087. doi: 10.1073/pnas.1218705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shalek AK, Robinson JT, Karp ES, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yosef N, Shalek AK, Gaublomme JT, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lo SL, Wang S. Peptide-based nano-carriers for intracellular delivery of biologically active proteins. Organelle-specific pharmaceutical nanotechnology. 2010:323–336. [Google Scholar]
- 149.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 150.Nakase I, Tanaka G, Futaki S. Cell-penetrating peptides (CPPs) as a vector for the delivery of siRNAs into cells. Mol Biosyst. 2013;9:855–861. doi: 10.1039/c2mb25467k. [DOI] [PubMed] [Google Scholar]
- 151.Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nagahara H, Vocero-Akbani AM, Snyder EL, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 153.Morris MC, Depollier J, Mery J, et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 154.Harford-Wright E, Lewis KM, Vink R, Ghabriel MN. Evaluating the role of substance P in the growth of brain tumors. Neuroscience. 2014;261:85–94. doi: 10.1016/j.neuroscience.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 155.Rizk SS, Luchniak A, Uysal S, et al. An engineered substance P variant for receptor-mediated delivery of synthetic antibodies into tumor cells. Proc Natl Acad Sci. 2009;106:11011–11015. doi: 10.1073/pnas.0904907106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rizk SS, Misiura A, Paduch M, Kossiakoff AA. Substance P derivatives as versatile tools for specific delivery of various types of biomolecular cargo. Bioconjug Chem. 2011;23:42–46. doi: 10.1021/bc200496e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chatterjee S, Chaudhury S, McShan AC, et al. Structure and biophysics of type III secretion in bacteria. Biochemistry (Mosc) 2013;52:2508–2517. doi: 10.1021/bi400160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carleton HA, Lara-Tejero M, Liu X, Galán JE. Engineering the type III secretion system in non-replicating bacterial minicells for antigen delivery. Nat Commun. 2013;4:1590. doi: 10.1038/ncomms2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doerner JF, Febvay S, Clapham DE. Controlled delivery of bioactive molecules into live cells using the bacterial mechanosensitive channel MscL. Nat Commun. 2012;3:990. doi: 10.1038/ncomms1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunstone MA, Tweten RK. Packing a punch: the mechanism of pore formation by cholesterol dependent cytolysins and membrane attack complex/perforin-like proteins. Curr Opin Struct Biol. 2012;22:342–349. doi: 10.1016/j.sbi.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Provoda CJ, Stier EM, Lee K-D. Tumor cell killing enabled by listeriolysin O-liposome-mediated delivery of the protein toxin gelonin. J Biol Chem. 2003;278:35102–35108. doi: 10.1074/jbc.M305411200. [DOI] [PubMed] [Google Scholar]
- 162.Pirie CM, Liu DV, Wittrup KD. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol Cancer Ther. 2013;12:1774–1782. doi: 10.1158/1535-7163.MCT-12-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sandvig K, van Deurs B. Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol. 2002;18:1–24. doi: 10.1146/annurev.cellbio.18.011502.142107. [DOI] [PubMed] [Google Scholar]
- 164.Johannes L, Römer W. Shiga toxins— from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 165.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 166.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J Am Chem Soc. 2007;129:10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cronican JJ, Thompson DB, Beier KT, et al. Potent delivery of functional proteins into mammalian cells in vitro and in vivo using a supercharged protein. ACS Chem Biol. 2010;5:747–752. doi: 10.1021/cb1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cronican JJ, Beier KT, Davis TN, et al. A class of human proteins that deliver functional proteins into mammalian cells in vitro and in vivo. Chem Biol. 2011;18:833–838. doi: 10.1016/j.chembiol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weisbart RH, Noritake DT, Wong AL, et al. A conserved anti-DNA antibody idiotype associated with nephritis in murine and human systemic lupus erythematosus. J Immunol. 1990;144:2653–2658. [PubMed] [Google Scholar]
- 171.Hansen JE, Chan G, Liu Y, et al. Targeting cancer with a lupus autoantibody. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004385. 157ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lawlor MW, Armstrong D, Viola MG, et al. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum Mol Genet. 2013;22:1525–1538. doi: 10.1093/hmg/ddt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaczmarczyk SJ, Sitaraman K, Young HA, et al. Protein delivery using engineered virus-like particles. Proc Natl Acad Sci. 2011;108:16998–17003. doi: 10.1073/pnas.1101874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tao P, Mahalingam M, Marasa BS, et al. In vitro and in vivo delivery of genes and proteins using the bacteriophage T4 DNA packaging machine. Proc Natl Acad Sci. 2013;110:5846–5851. doi: 10.1073/pnas.1300867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mallery DL, McEwan WA, Bidgood SR, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discov Today Technol. 2008;5:e95–e103. doi: 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 177.Zelphati O, Wang Y, Kitada S, et al. Intracellular delivery of proteins with a new lipid-mediated delivery system. J Biol Chem. 2001;276:35103–35110. doi: 10.1074/jbc.M104920200. [DOI] [PubMed] [Google Scholar]
- 178.Benjaminsen RV, Mattebjerg MA, Henriksen JR, et al. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Behr J-P. The proton sponge: a trick to enter cells the viruses did not exploit. Chim Int J Chem. 1997;51:34–36. [Google Scholar]
- 180.Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 181.Su X, Yang N, Wittrup KD, Irvine DJ. Synergistic antitumor activity from two-stage delivery of targeted toxins and endosome-disrupting nanoparticles. Biomacromolecules. 2013;14:1093–1102. doi: 10.1021/bm3019906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gu Z, Yan M, Hu B, et al. Protein nanocapsule weaved with enzymatically degradable polymeric network. Nano Lett. 2009;9:4533–4538. doi: 10.1021/nl902935b. [DOI] [PubMed] [Google Scholar]
- 183.Yan M, Du J, Gu Z, et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53. doi: 10.1038/nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- 184.Biswas A, Joo K-I, Liu J, et al. Endoprotease-mediated intracellular protein delivery using nanocapsules. ACS Nano. 2011;5:1385–1394. doi: 10.1021/nn1031005. [DOI] [PubMed] [Google Scholar]
- 185.Malmsten M. Inorganic nanomaterials as delivery systems for proteins, peptides, DNA, and siRNA. Curr Opin Colloid Interface Sci. 2013;18:468–480. [Google Scholar]
- 186.Loosli H-R, Kessler H, Oschkinat H, et al. Peptide conformations. Part 31. The conformation of cyclosporin a in the crystal and in solution. Helv Chim Acta. 1985;68:682–704. [Google Scholar]
- 187.Bayer P, Kraft M, Ejchart A, et al. Structural studies of HIV-1 tat protein. J Mol Biol. 1995;247:529–535. doi: 10.1006/jmbi.1995.0158. [DOI] [PubMed] [Google Scholar]
- 188.Feld GK, Thoren KL, Kintzer AF, et al. Structural basis for the unfolding of anthrax lethal factor by protective antigen oligomers. Nat Struct Mol Biol. 2010;17:1383–1390. doi: 10.1038/nsmb.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Varghese Gupta S, Gupta D, Sun J, et al. Enhancing the intestinal membrane permeability of zanamivir: a carrier mediated prodrug approach. Mol Pharm. 2011;8:2358–2367. doi: 10.1021/mp200291x. [DOI] [PMC free article] [PubMed] [Google Scholar]