Abstract
Purpose
Recent controversial publications, citing studies purporting to show that P-gp mediates the transport of propranolol, proposed that passive biological membrane transport is negligible. Based on the BDDCS, the extensively metabolized-highly permeable-highly soluble BDDCS class 1 drug, propranolol, shows a high passive permeability at concentrations unrestricted by solubility that can overwhelm any potential transporter effects. Here we reinvestigate the effects of passive diffusion and carrier-mediated transport on S-propranolol.
Methods
Bidirectional permeability and inhibition of efflux transport studies were carried out in MDCK, MDCK-MDR1 and Caco-2 cell lines at different concentrations. Transcellular permeability studies were conducted at different apical pHs in the rat jejunum Ussing chamber model and PAMPA system.
Results
S-propranolol exhibited efflux ratios lower than 1 in MDCK, MDCK-MDR1 and Caco-2 cells. No significant differences of Papp, B->A in the presence and absence of the efflux inhibitor GG918 were observed. However, an efflux ratio of 3.63 was found at apical pH 6.5 with significant decrease in Papp, A->B and increase in Papp, B->A compared to apical pH 7.4 in Caco-2 cell lines. The pH dependent permeability was confirmed in the Ussing chamber model. S-propranolol flux was unchanged during inhibition by verapamil and rifampin. Furthermore, pH dependent permeability was also observed in the PAMPA system.
Conclusions
S-propranolol does not exhibit active transport as proposed previously. The "false" positive efflux ratio can be explained by the pH partition theory. As expected, passive diffusion, but not active transport, plays the primary role in the permeability of the BDDCS class 1 drug propranolol.
Keywords: propranolol, passive diffusion, carrier-mediated transport, BDDCS, P-gp
INTRODUCTION
Drug transport processes are key determinants of a drug’s pharmacokinetics. Most drugs need to permeate through the intestine to be absorbed, into the liver to be metabolized, into target organs, such as the brain, to be effective, and through the kidney or bile canaliculus to be excreted. The main permeation processes are passive diffusion and carrier-mediated transport. The vital role of passive diffusion has been claimed for a long time based on sound physicochemical principles. In the last 20 years, as an increasing number of carrier proteins were discovered, carrier-mediated transport was increasingly considered. Recently, Kell and his colleagues have published several papers proposing that transport of pharmaceutical drugs is essentially carrier-mediated only, whereas passive transport through biological membrane is negligible (1, 2). Following their papers, there was a passionate debate at the 2013 American Association of Pharmaceutical Scientist (AAPS) annual meeting, as well as further reviews that refuted the proposal of Kell et al., presenting persuasive evidence of passive diffusion (3, 4).
Kell et al. cited a dozen drugs that presumably lacked significant interactions with carriers (2), but then provided references showing that all these drugs were affected by known transporters, arguing that these references denied the role passive diffusion plays in the membrane permeability of all drugs.
From our point of view, it is important to recognize the contribution of both passive diffusion and carrier-mediated transport in the permeation of pharmaceuticals across biological membranes. The Biopharmaceutics Drug Disposition Classification System (BDDCS), which was first introduced in 2005 by Wu and Benet (5), provides a balanced view of carrier-mediated transport and passive diffusion. In this system, drugs are categorized into 4 classes based on drugs’ metabolism and solubility. In each class, the contribution of passive permeation and carrier-mediated transport are disparate, leading to different efflux and absorptive transporter effects. It suggests that for class 1 compounds, which have high permeability rate, extensive metabolism and high solubility, the passive permeability at concentrations unrestricted by solubility overwhelms any potential transporter effects. In this situation, transporter effects will be minimal clinically even for class 1 compounds demonstrated to be substrates of transporters in cellular systems. Some of Kell’s cited drugs (2), such as metoprolol, propranolol and desipramine belong to BDDCS class 1, which implies that even if they are transporter substrates, it is not necessary that transporters will predominate in permeability processes due to their high passive permeability.
Our laboratory has realized that almost all drugs are substrates for transporters since 8 years ago (6). However, this is not to say that transporter effects will always be clinically relevant, especially for BDDCS class 1 drugs. Here, we chose propranolol as an example to further investigate the roles that transporters, as well as passive diffusion, play in drug permeability, and dispute Kell’s views that all the drugs need carrier proteins and that passive diffusion is irrelevant.
Propranolol is a sympatholytic non-selective beta blocker, which is used to treat high blood pressure, abnormal heart rhythms and heart disease. Propranolol has been applied therapeutically as a racemic mixture of 50% R- and 50% S-propranolol-HCl. Animal studies demonstrate that S-propranolol has a beta-blocking activity 100 times more potent than R-propranolol, therefore S-propranolol is believed to be largely responsible for the clinical effects of this racemic drug (7). Also in a study conducted in healthy subjects to compare the hemodynamic effects of propranolol, R-propranolol was found to be ineffective (8). Thus S-propranolol was selected as our investigational drug.
The clinical dosage form of propranolol is propranolol-HCl, which has a high solubility. Regional single-pass perfusion experiments conducted in healthy volunteers showed that propranolol has a high effective permeability of 2.8×10−4 cm/s in the human jejunum (9). The high permeability and high solubility enable propranolol to be almost completely absorbed following oral administration. Subsequently, it undergoes high first-pass metabolism in the liver mediated mainly by CYP2D6, CYP2C19 and CYP1A2, leading to only 25% of the dose reaching the systemic circulation unchanged.
P-glycoprotein (P-gp) and other carrier proteins have been reported to be involved in the transport of propranolol (10–12). Kell et al. (2) referenced these observations as evidence of the vital role carriers play in the transport of propranolol. P-gp is expressed in the intestine, the liver, as well as in the brain, and has an important role in drug absorption, distribution and excretion. In the first of the three papers cited by Kell, Yang et al. (10) demonstrate P-gp efflux in a cultured rabbit conjunctival epithelial cell layer, which was inhibited by an P-gp monoclonal antibody. In the second paper, Wang et al. (11) observed propranolol enantiomer transport in Caco-2 cells with changes in pH and attempts to inhibit transport. Wang et al. (11) attribute the lack of effect of P-gp inhibitors on “the possibility that passive transcellular diffusion dominated the absorptive transport behavior of propranolol”, consistent with the BDDCS class 1 supposition, although Kell et al. (2) cite this paper in support of their hypothesis that passive absorption is nonrelevant. In the third paper cited by Kell et al., D’Emanuele et al. (12) suggested based on Caco-2 studies that dendrimer conjugates may enhance the apical to basolateral permeability of propranolol and “may be useful in overcoming low bioavailability due to P-gp mediated drug elimination…Therapeutic use of these polymers for reducing the effects of intestinal P-gp on drug absorption may improve the oral bioavailability of propranolol and potentially other low solubility drugs that are P-gp substrates.” Of course, propranolol is not a low solubility drug (the reported dose number for propranolol HCl is 0.006 (13)) and although the drug may have a low bioavailability, we are not aware of any data demonstrating that propranolol is not completely absorbed. Whether P-gp controls the transport of propranolol should be clearly characterized. We believe that the ambiguous observations of a propranolol P-gp effect as cited by Kell et al. (2) may mislead drug discovery and development investigators. Moreover, investigation of propranolol permeability mechanisms can provide information for the contribution of passive diffusion and carrier-mediated transport.
The purpose of this study was to characterize the effect of passive diffusion and P-gp mediated transport on S-propranolol. In this study, we conducted bidirectional transport with and without P-gp inhibition across MDCK, MDCK-MDR1 and Caco-2 cell monolayers to reinvestigate the P-gp effect toward S-propranolol. Also, we performed transport studies at apical side pH 6.5 and 7.4 to assess the pH effect on S-propranolol permeability. Thereafter, the pH dependent permeability observed in cellular systems were validated in Ussing chamber and Parallel Artificial Membrane Permeability Assay (PAMPA) systems. Thus, the dominant permeability mechanism of S-propranolol was characterized.
MATERIALS AND METHODS
Materials
S-Propranolol-HCl, colchicine, phenol red, rifampin, verapamil, digoxin, mannitol and labetolol were obtained from Sigma-Aldrich Inc. (St.Louis, MO). [3H]-S-propranolol, [3H]-digoxin and [14C]-mannitol were obtained from Perkin-Elmer (Boston, MA). GG918 (GF120918) was from Sequoia Research (Oxford, U.K.). Chemicals and solvents of high performance liquid chromatography grade were supplied by Sigma-Aldrich Inc.
Six-well plates were purchased from Corning Life Science (Acton, MA). Falcon polyethylene cell culture inserts (pore size 0.4 µm, growth area 4.2 cm2) were purchased from BD Biosciences (Franklin Lakes, New Jersey). PAMPA system was purchased from BD Biosciences.
The MDCK and MDCK-MDR1 cell lines were a kind gift from Dr. Ira Pastan (National Cancer Institute, Bethesda, MD). The Caco-2 cell line was purchased from American Type Culture Collection (Rockville, MD). Cell culture media and transport buffer were purchased from Cell Culture Facility at the University of California, San Francisco (UCSF, San Francisco, CA).
Cell Culture
Cells were passaged at 90–95% confluence using 0.05% trypsin EDTA (for MDCK and Caco-2) and 0.25% trypsin EDTA (for MDCK-MDR1); 2 mL of cell suspension (approximately 250,000 cells) was plated onto cell culture inserts and 3 mL of grow media was placed in the 6-well plates. Media was replaced every other day. MDCK, MDCK-MDR1 and Caco-2 cell lines were grown for 6, 6 and 21 days, respectively, before the bidirectional transport study.
Bidirectional Transport Studies
Cell monolayers were washed with warm transport buffer (HBSS containing 1% FBS and 25 mM HEPES, pH 7.4) and preincubated for 20 min at 37 °C. The integrity of the cell monolayers was measured using the Millipore Millicell–ERS apparatus (Bedford, MA). Average transepithelial electrical resistance (TEER) values were 1680±95, 420±35 and 500±62 Ωcm2 for MDR1-MDCK, MDCK and Caco-2, respectively. Transport study was initiated by replacing preincubation medium by test solution, with 1.5 mL added to the apical side or 2.5 mL to the basolateral side followed by incubation at 37 °C in an incubator-shaker. At 30, 60, 90 and 120 min, 200 µL samples were removed from the receiver compartment and replaced with fresh medium.
[3H]-digoxin and GG918 were used as a positive control substrate and inhibitor, respectively, for P-gp. S-propranolol transport studies were conducted in MDCK and MDCK-MDR1 cells at concentrations (including a trace of [3H]-S-propranolol) of 25 nM and 50 µM for 2 hours. P-gp inhibition studies were conducted in MDCK-MDR1 and Caco-2 at 50 µM S-propranolol (equivalent to the concentration tested by D’Emanuele et al. (12)) with or without 0.5 µM GG918 in pH 7.4 or pH 6.5 transport buffer.
Animals
Male Sprague-Dawley rats weighing 300–350 g were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in the UCSF Laboratory Animal Resource Center for at least 1 week with a 12 h light/dark cycle and free access to water as well as food prior to sacrifice. Study protocols were approved by the Institutional Animal Care and Use Committee at UCSF.
Transport Study in Ussing Chamber
Rats were anesthetized by intraperitoneal injection of 1 ml/kg mixture that was made of 80 mg/ml ketamine (Butler Schein™ Animal Health, Dublin, OH) and 12 mg/ml xylazine (Lloyd Laboratories, Shenandoah, IA). Procedures for the Ussing chamber studies were the same as those previously reported (14). The proximal jejunum, which was 10 to 40 cm below the duodenum, was used for all studies. A segment free of Peyer’s patches was cut into pieces around 4 cm length and was opened along the mesenteric border to expose the mucosal side. The muscle layer was cut by a blunt blade, then picked up and stripped off carefully by a tweezer. Following this, jejunum was mounted on the modified Ussing chamber (Harvard Apparatus Inc., Hollistion, MA, USA) with 1.03 cm2 of exposed area.
Each chamber compartment was filled with transport buffer (HBSS containing 25 mM HEPES) that had been bubbled with carbogen (5% CO2 – 95% O2) and adjusted pH (pH 6.5 at the mucosal compartment and pH 7.4 at the serosal compartment to simulate assumed physiological conditions). The chambers were placed in a heating block maintained at 37°C. Phenol red was used as a leakage indicator; verapamil and rifampin were used as inhibitors for rat organic cation transporters (Octs) and organic anion transporting polypeptide (Oatps), respectively. Trace [3H]-S-propranolol was added into the 100 µM of S-propranolol to conduct the transport study. Inhibition studies were conducted at 100 µM of S-propranolol with or without 100 µM verapamil or 50 µM rifampin; pH dependent studies were conducted at 100 µM of S-propranolol at mucosal side pH 6.5 or 7.4. Samples (400 µL) were withdrawn at 30, 45, 60, and 90 min with replacement of fresh buffer at the receiver side at each time point.
PAMPA Procedure
In PAMPA system, drugs were dosed at pH 6.5 or pH 7.4 and received at pH 7.4 (pH 6.5→pH 7.4 and pH 7.4→pH 7.4) to simulate the effect of an apical pH change on Papp, A→B; Drugs were dosed at pH 7.4 and received at pH 6.5 or pH 7.4 (pH 7.4→pH 6.5 and pH 7.4→pH 7.4) to simulate the effect of basolateral pH change on Papp, B→A.
The pre-coated PAMPA plate was equilibrated for 60 min at room temperature. Dosing solutions of S-propranolol, mannitol, digoxin or labetalol were prepared in HBSS at both pH 7.4 and pH 6.5. Trace [3H]-S-propranolol, [3H]-digoxin and [14C]-mannitol were added into the dose solutions. Thereafter, 300 µL dosing solutions were applied in the bottom wells; 200 µL blank HBSS at pH 7.4 or pH 6.5 were added to the top wells. After 120 min, 100 µL samples were taken from both the top and bottom wells and prepared for either LC-MS/MS analysis or liquid scintillation counting.
Sample Assay
For [3H]-S-propranolol, [3H]-digoxin and [14C]-mannitol measurements, samples were mixed with 5 mL Econo-Safe scintillation cocktail (RPI Corp., Mount Prospect, IL) in the scintillation vial and analyzed by a liquid scintillation counter (LS 6000TA, Beckman Coulter, Fullerton, CA).
For phenol red, 2% 1 M NaOH was added to keep the solution at a pH higher than 9, a condition where the color of phenol red will not be sensitive to pH changes. The absorption of alkaline phenol red was measured using a Perkin-Elmer Lambda 11 UV-Visible spectrophotometer (Waltham, MA) at 554 nm. For labetalol, measurements were conducted using LC-MS/MS following the procedure previously described (15).
Data Analysis
Apparent permeability coefficients (Papp) and efflux ratios were calculated for cellular transport studies, Ussing chamber and PAMPA studies. For cellular and Ussing chamber transport studies, Papp was calculated using equation (1):
| (1) |
where dC/dt represents the cumulative concentration in the receiver compartment versus time, Vr the volume of the receiver compartment, A the area of the cell monolayer, and C0 the dosing concentration.
For PAMPA study, Papp was calculated using equation (2)
| (2) |
| (3) |
where VD is donor well volume (0.3 ml), VA is acceptor well volume (0.2 ml), Cequilibrium is the average concentration of both acceptor and donor wells (equation (3)), CA is drug concentration in the acceptor well, CD is drug concentration in the donor well, A is PAMPA filter area (0.3 cm2), and t is incubation time.
Drug efflux ratio was calculated as the ratio of Papp, B->A over Papp, A->B.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Papp and efflux ratios under different tested conditions were compared using Student’s unpaired, two-tailed t-test. P<0.05 was considered to be statistically significant.
RESULTS
P-gp Dependent Transport of Digoxin in Bidirectional Transport Cell Systems
The P-gp substrate, digoxin, was used as a positive control in bidirectional transport study for MDCK-MDR1 and Caco-2 cell lines. As shown in Table I, the efflux ratio of digoxin was 6.75±1.34 in MDCK-MDR1 cell line and 2.57±0.13 in Caco-2 cell line. In the presence of GG918, the efflux ratios in both cell lines diminished to values lower than 2.
Table I.
Digoxin (0.01 µM) Transport in MDCK-MDR1 and Caco-2 Cells in the Presence of GG918 (0.5 µM)
| MDR1-MDCK cells | Caco-2 cells | |||
|---|---|---|---|---|
| Digoxin | +GG918 | Digoxin | +GG918 | |
| Papp A->B (×10−6 cm/s) (±SD) | 0.26±0.02 | 0.41±0.10 | 0.64±0.03 | 1.17±0.07 |
| Papp B->A (×10−6 cm/s) (±SD) | 2.00±0.01 | 0.67±0.06 | 1.64±0.13 | 1.19±0.05 |
| Efflux Ratio | 6.75±1.34 | 1.52±0.16 | 2.57±0.33 | 1. 14±0.13 |
Lack of P-gp Dependent Transport of S-propranolol across MDCK, MDCK-MDR1 and Caco-2 Cell Monolayers
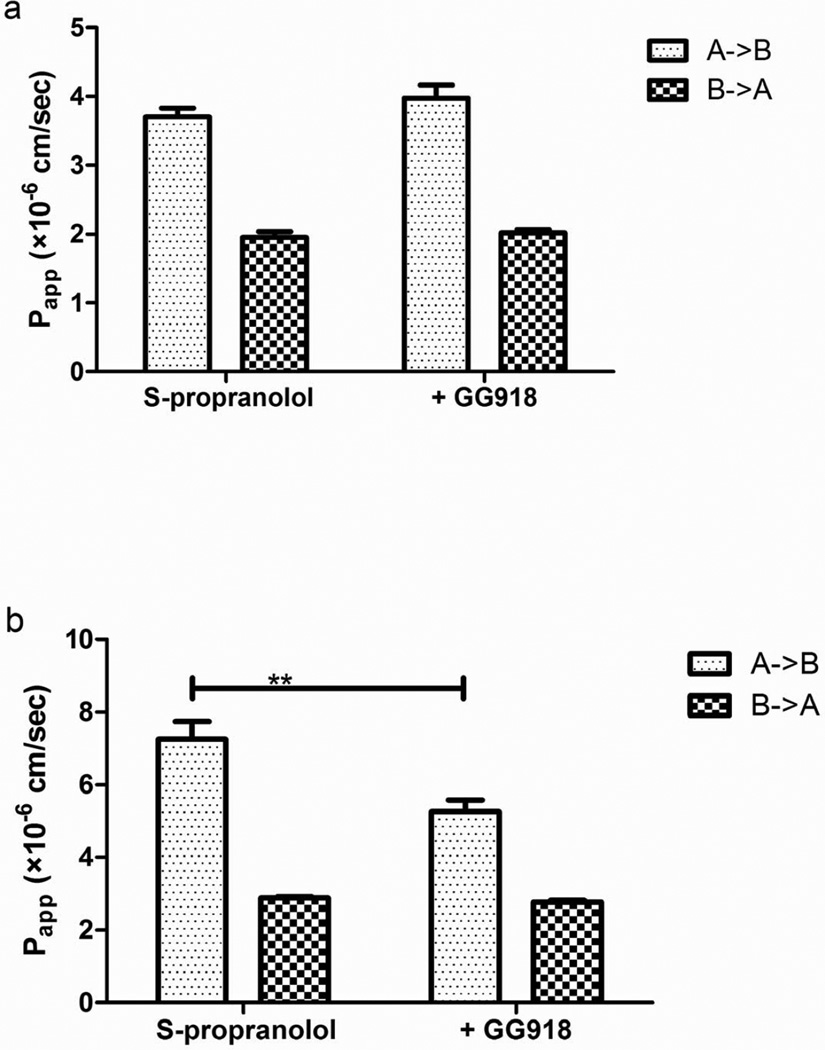
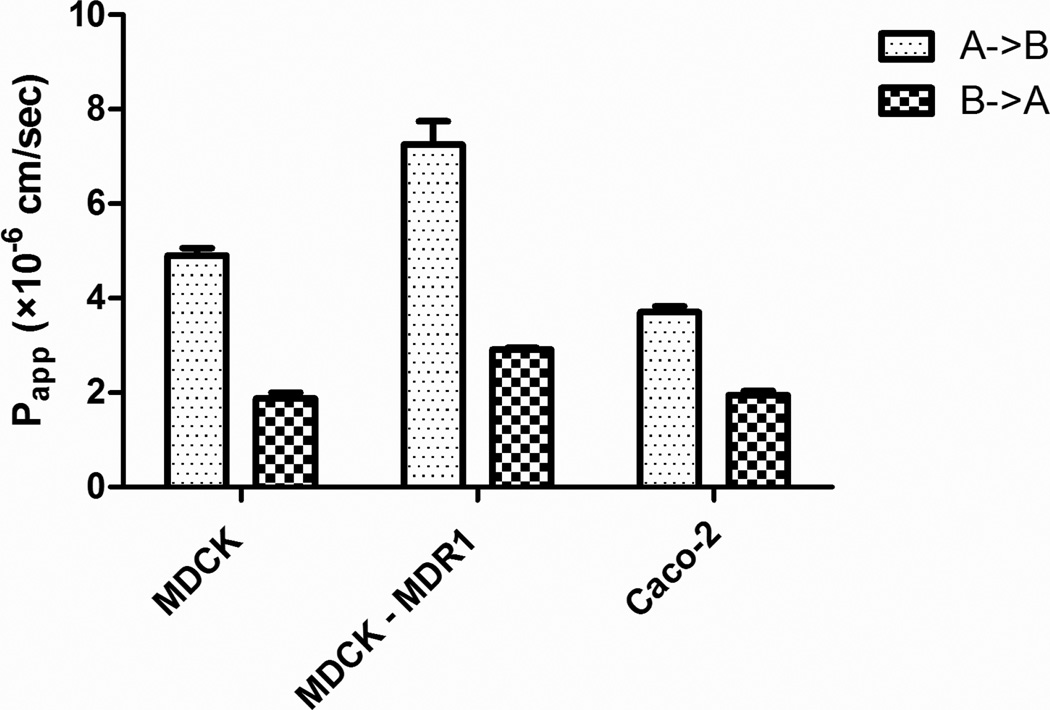
Bidirectional transport and GG918 inhibition studies were conducted across MDCK-MDR1 and Caco-2 cell monolayers at 50 µM S-propranolol. As seen in Figure 1, no significant differences of the basolateral to apical efflux in the absence or presence of GG918 were observed, although an unexplained significant difference in Papp, A→B in the MDCK-MDR1 cell monolayers was observed. Figure 2 shows the bidirectional transport of S-propranolol across MDCK, MDCK-MDR1 and Caco-2 cell monolayers. Fifty micromolar S-propranolol exhibited efflux ratios of 0.37±0.04, 0.42±0.05, 0.52±0.08 in MDCK, MDCK-MDR1 and Caco-2 cell lines, respectively.
Fig. 1.
Effects of GG918 on the permeability of S-propranolol (50 µM) across Caco-2(a) and MDCK-MDR1(b) cell monolayers. Data are presented as mean±SD. n=6. **p<0.01
Fig. 2.
Permeability of S-propranolol (50 µM) across MDCK, MDCK-MDR1 and Caco-2 cell monolayers at pH 7.4 on both sides of the barrier. Data are presented as mean±SD. n=6
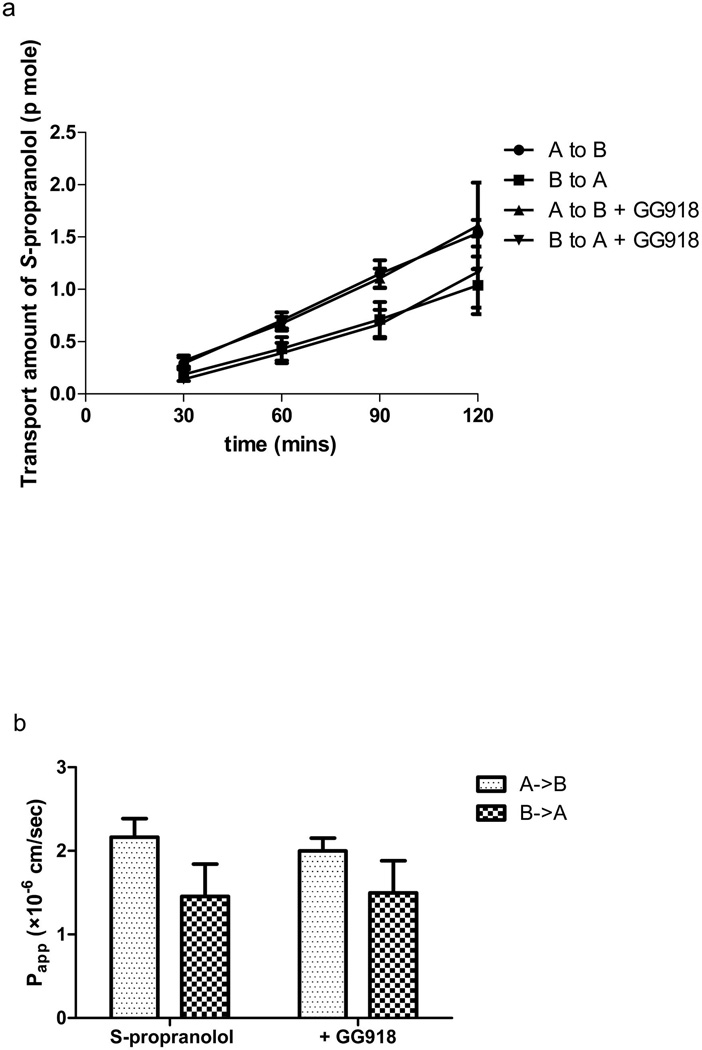
At the 200-fold decreased donor concentration of 25 nM, the efflux ratio remained lower than 1 in MDCK-MDR1 cell lines (Figure 3), and no effect of GG918 was observed at both the basolateral and apical permeabilities (in contrast to the unexplained effect on apical permeability noted in Figure 1b).
Fig. 3.
Effects of GG918 on the permeability rate (a) and Papp (b) of S-propranolol (25 nM) across MDCK-MDR1 cell monolayers at pH 7.4 on both sides of the barrier. Data are presented as mean±SD. n=6.
pH Dependent Permeability of S-propranolol across MDCK-MDR1 and Caco-2 Cell Monolayers
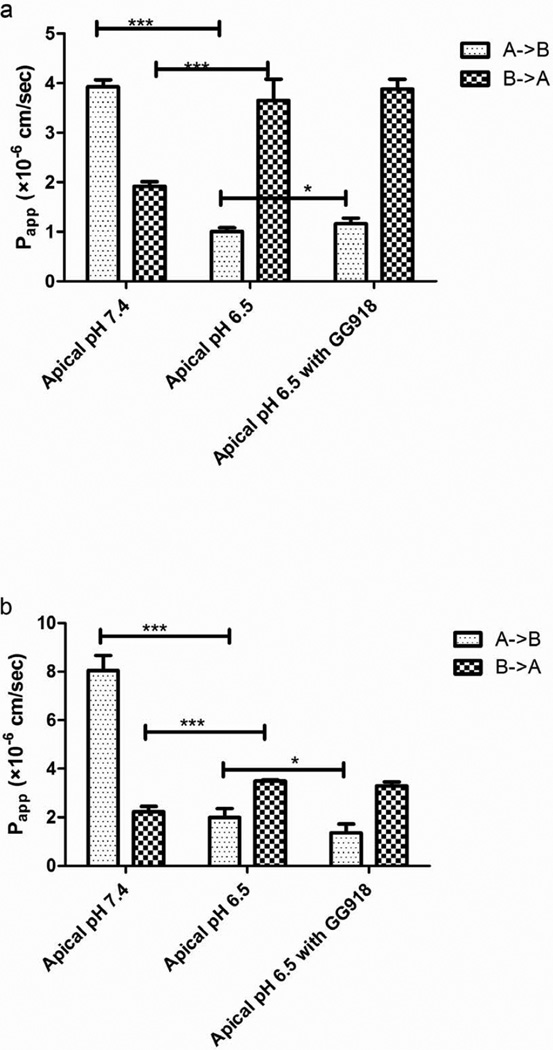
The effects of apical pH on the permeability of S-propranolol were studied in MDCK-MDR1 and Caco-2 cell lines. In Figure 4a, as the apical pH changed from 7.4 to 6.5, the Papp, A->B of S-propranolol was markedly decreased in Caco-2 cell lines, whereas the Papp, B->A was significantly increased, leading to efflux ratios increased from 0.49 to 3.63. The efflux inhibition at apical pH 6.5 showed there were no significant differences of the basolateral to apical efflux in the presence or absence of GG918, although a small but significant increase in Papp, A->B was observed. In MDCK-MDR1 cell lines, similar pH-dependent permeability results were observed, but the change in Papp, A->B decreased significantly in the presence of GG918 (Figure 4b).
Fig. 4.
Effects of pH on the permeability of S-propranolol (50 µM) across Caco-2(a) and MDCK-MDR1(b) cell monolayers. Data are presented as mean±SD. n=6. *p<0.05.***p<0.001
pH Dependent Permeability of S-propranolol in Ussing Chamber
Phenol red was used as an intestine leakage indicator. After 90 min of study, less than 1% phenol red permeated from the mucosal to serosal side.
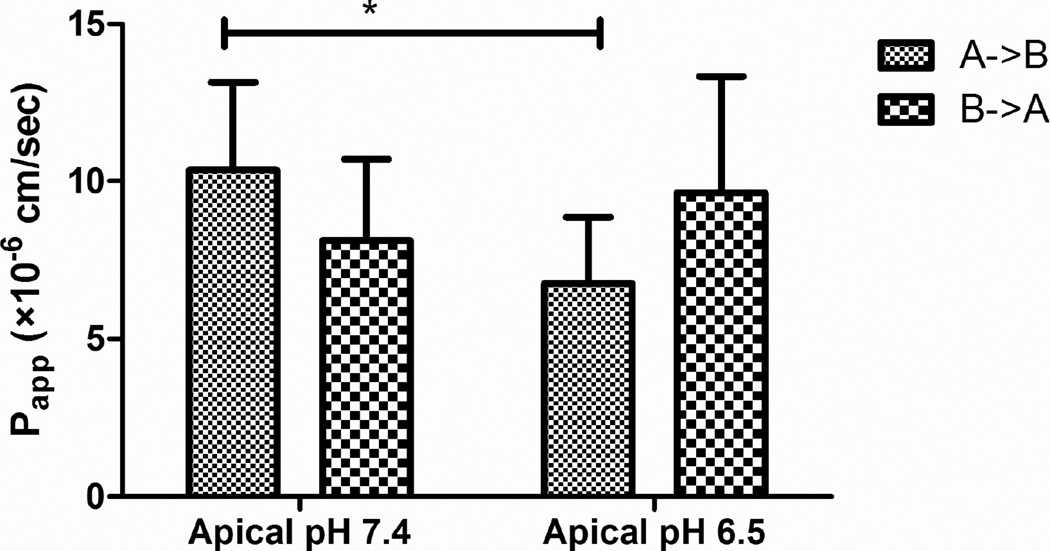
The effects of mucosal pH on transport of S-propranolol across rat intestine are shown in Figure 5. A statistically significant decrease of Papp was observed in the mucosal to serosal direction (absorption). However, the change in mucosal pH didn’t significantly affect serosal to mucosal permeability (secretion) of S-propranolol.
Fig. 5.
Effects of pH on the permeability of S-propranolol (100 µM) across rat intestine. Data are presented as mean±SD. n=6. *p<0.05
Lack of Carrier Dependent Transport of S-propranolol in Ussing Chamber
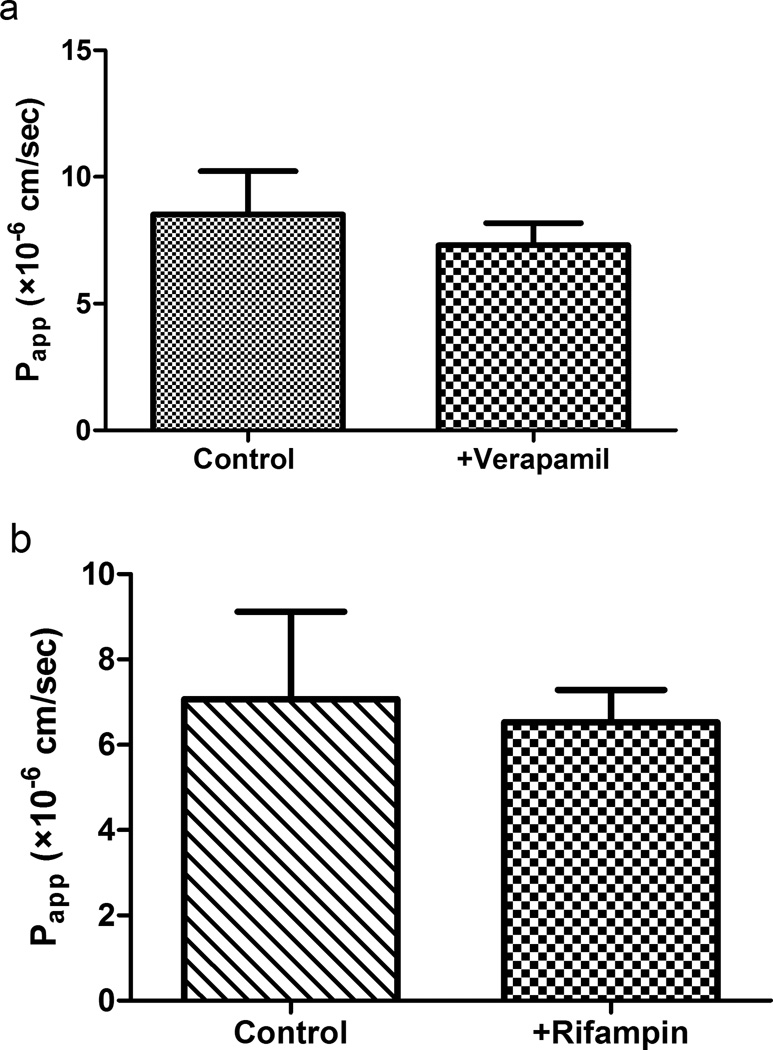
Inhibition studies were conducted in the Ussing chamber to investigate whether intestinal drug transporters are involved in the absorption of S-propranolol. Figure 6a shows that verapamil (OCT/Oct inhibitor) did not alter Papp of S-propranolol in the absorptive direction. Similarly, rifampin (OATP/Oatp inhibitor) shows no inhibitory effect on the absorption of S-propranolol (Figure 6b).
Fig. 6.
(a) Effect of verapamil on S-propranolol absorption in rat intestine. (b) Effect of rifampin on S-propranolol absorption in rat intestine. Data are presented as mean±SD. n=3
pH Dependent Permeability of S-propranolol in PAMPA System
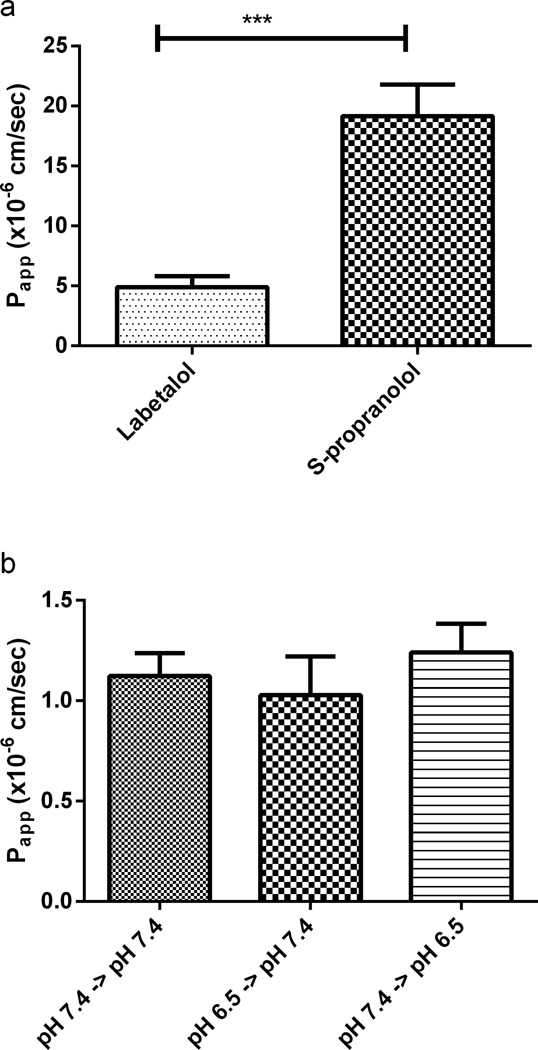
To investigate the effect of apical pH on the passive transcellular passage of S-propranolol, permeability studies were conducted in the PAMPA. Labetalol was used as a high permeability standard.
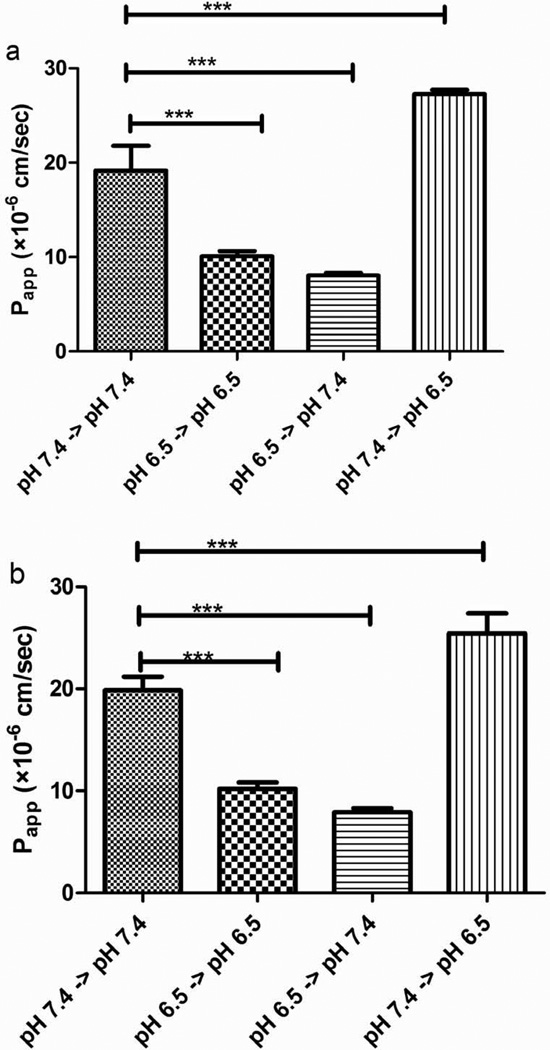
Figure 7a summarizes pH dependent passive permeability of 50 µM S-propranolol in PAMPA system. As apical pH changed from 7.4 to 6.5 while basolateral pH was maintained at 7.4, Papp, A->B decreased from 19.2±2.6 × 10−6 cm/sec to 8.1±0.3 × 10−6 cm/sec (1st and 3rd bars), whereas the simulated Papp, B->A increased from 19.2±2.6 × 10−6 cm/sec to 27.3±0.4 × 10−6 cm/sec when the receiving pH decreased from 7.4 to 6.5 (1st and 4th bars). The resulting simulated ‘efflux ratios’ (Papp, B->A/ Papp, A->B) increased from 1 to 3.39. Moreover, the Papp values were also compared at both ‘apical’ and ‘basolateral’ pH change from 7.4 to 6.5. There is a significant decrease of Papp value at pH 7.4→pH 7.4 compared to pH 6.5→pH 6.5 (1st and 2nd bars). Figure 7b shows that pH dependent transcellular permeability of S-propranolol at 100 µM was almost identical to that observed at 50 µM. The effect of apical pH on the permeability/transport of the poorly permeable compound digoxin was also determined in PAMPA, exhibiting no statistical differences as a function of pH (Figure 8b).
Fig. 7.
Effects of pH on the permeability of S-propranolol in the PAMPA system: (a) 50 µM of S-propranolol; (b) 100 µM of S-propranolol. Data are presented as mean±SD. n=6. ***p<0.001
Fig. 8.
(a) Transcellular permeability of 50 mM labetalol and S propranolol at pH 7.4 in the PAMPA system; (b) Effects of pH on the permeability of 5 µM digoxin in the PAMPA system. Data are presented as mean±SD. n=6.***p<0.001
DISCUSSION
We began these studies to provide further experimental evidence concerning the effect of transporters on the intestinal permeability of propranolol. As noted earlier Kell et al. (2) referenced two studies that suggested that P-gp could affect propranolol intestinal transport. According to our BDDCS system, the class 1 drug propranolol should not exhibit any clinically relevant outcomes related to drug transporters. We note that in one of the references cited, i.e. Wang et al. (11), the authors concluded that P-gp inhibitors lacked an effect on propranolol transport in Caco-2 cells and noted “the possibility that transcellular diffusion dominates the absorptive transport behavior of propranolol. ” However, in the second citation, D’Emanuele at al. (12) found that P-gp inhibition was significant. Since we recognize that experimental results need to be verified by others, and that anomalous significant results can be observed when only one study is carried out in one laboratory (e.g. our unexplained differences noted on the effect of P-gp inhibition on apical to basolateral permeability (Figure 1b)), we began the studies reported here.
As we were preparing this manuscript, Kell and Oliver (16) published a further review, where they now state that “we do not assert that carrier-mediated transport is the only means by which drugs and other xenobiotics gain access to cells…” However, this “straw man” interpretation is not the issue to which the scientific community is reacting, rather it is exactly what Prof. Kell and his colleagues state in their titles and text: “Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule?” (17); “Implications of the dominant role of transporter in drug uptake by cells” (18); “Pharmaceutical drug transport : the issues and the implications that it is essentially carrier-mediated only” (1); “It is clear that the non-carrier mediated permeability of biological membranes to most solutes is, in fact, negligible” (2). Since we have shown that 40% of marketed drugs are BDDCS Class 1 (19), where we believe human data overwhelmingly demonstrates that transporter effects in the intestine and liver are clinically insignificant, we find poor support for Kell and colleagues’ claims for the “dominant role for transporter-mediated uptake of drugs into cells”.
The most recent review (16) reveals a changing approach. It now appears that the authors believe that if they can demonstrate that a drug is a substrate for a transporter in some cellular system, this validates their hypothesis of a “dominant role for transporter-mediated uptake for drugs into cells.” For example, with respect to propranolol Kell and Oliver (16) cite two studies of blood-retinal permeability (20, 21), one LLC-PK1 renal cell line study (22), a K562 cell line (a human immortalized myelogenous leukemia line) study (23) and the same Caco-2 study of Wang et al. (11) where the authors’ conclusion does not support the Kell et al. hypothesis.
To mimic the study conducted by Emanuele et al. (12), the same concentration of 50 µM S-propranolol was dosed in the Caco-2 bidirectional transport model. In our studies, S-propranolol did not exhibit inhibitable P-gp efflux (Figure 1a). It is well known that many proteins are expressed in Caco-2 cells, and thus other potential factors may be involved in the transport of propranolol in Caco-2 cell lines. In contrast, in MDCK and MDCK-MDR1 cell lines, other proteins are expressed at lower levels. In addition, the superior TEER values of MDCK-MDR1 cell line may provide this system an increased sensitivity in identifying P-gp substrates. Therefore, bidirectional transport and transporter inhibition studies of S-propranolol were conducted in MDCK-MDR1 cell lines.
No significant difference was observed in MDCK-MDR1 cell lines in the absence and presence of the P-gp inhibitor GG918 (Figure 1b). Fifty micromolar S-propranolol showed efflux ratios lower than 1 in MDCK, MDCK-MDR1 and Caco-2 cell lines (Figure 2). For class 1 drugs, considering that high passive permeability may overwhelm transporter effects at higher concentrations, permeability studies were further conducted at 25 nM of S-propranolol. However, the presence of GG918 did not enhance the transport of S-propranolol at 25 nM (Figure 3), suggesting to us that S-propranolol is not a P-gp substrate. Moreover, comparing transport permeabilities at 25 nM and 50 µM, showed that both Papp, A->B and Papp, B->A varied little when donor concentrations were 200-fold different, suggesting that carrier-mediated transport did not affect the permeability of S-propranolol in MDCK-MDR1 cell lines.
Digoxin was used as a positive control in the above bidirectional transport studies conducted in MDCK-MDR1 and Caco-2 cell lines. In Table I, the significant efflux of digoxin was inhibited by GG918, indicating that the bidirectional transport in MDCK-MDR1 and Caco-2 cell lines in our laboratory is an reliable model to investigate P-gp transport in vitro.
Propranolol is absorbed mainly in jejunum, where the physiological pH is 6.5. Further studies were carried out in Caco-2 and MDCK-MDR1 cell lines to investigate the effect of apical pH on the transport of S-propranolol. Results showed that the apical pH plays an important role in the transcellular transport of S-propranolol (Figure 4). At apical pH 6.5, the bidirectional transport yielded an efflux ratio higher than 2. This high efflux ratio may mislead one to conclude that S-propranolol is a P-gp substrate, but the lack of any marked changes in the presence of GG918 belie such a conclusion.
To investigate if this pH dependent transport is caused by carriers, further transport studies across rat jejunum were conducted in the Ussing chamber model. Phenol red was used as an intestine leakage indicator. The propranolol dose concentration of 100 µM, which is the highest concentration that can dissolve in less than 0.1% DMSO, was selected to simulate the potential high concentration in the human gastrointestinal tract. Transport studies conducted in Ussing chamber showed that pH dependent permeability occurred in the absorption direction, but not in secretion direction (Figure 5). The changes due to pH dependent permeability in Ussing chamber were not as pronounced as in MDCK-MDR1 or Caco-2 cell monolayers. This can be explained by the multilayer characteristics of the intestine. Even though the muscle layer was removed, multilayers can still prevent the fast pH equilibration between the cell surface and transport buffer. In contrast, the cultured monolayer cells readily equilibrate with buffer solution.
As reported by the International Transporter Consortium, many transporters are expressed in the intestinal epithelia (24), some of which might be involved in the pH dependent transport of S-propranolol. In an analysis of the main transporters expressed in intestine, P-gp and BCRP were ruled out due to the insignificant effect on efflux in the presence of GG918. The multidrug resistance-associated protein family (MRPs) are observed to transport drugs conjugated to glutathione, sulfate or glucuronide and a variety of endogenous compounds (25); the proton-coupled oligopeptide transporter 1 (PEPT1) mediates the cellular uptake of di/tripeptides and peptide-like drugs (26); the monocarboxylic acid transporter (MCT) is responsible for the absorption of short chain fatty and monocarboxylates (27); the apical sodium/bile acid co-transporter (ASBT) mediates bile acid absorption across the apical membranes (28). These transporters were also excluded because propranolol is not within their substrate specificity. Hence, the effects of inhibition of Octs or Oatps on absorption of S-propranolol in rat intestine were evaluated, exhibiting no changes in S-propranolol flux in the presence of these inhibitors (Figure 6). The lack of significant transport inhibition effects suggests that intestinal transporters would not affect the absorption of S-propranolol or contribute to the observed pH dependent permeability changes. Our inhibition study in the Ussing chamber correlates well with the carrier inhibition studies of S-propranolol conducted in Caco-2 cell monolayer by Wang et al. (11), which has been referenced by Kell and collegues as evidence for tranporter effects on propranolol. In the Wang et al. study, carriers such as P-gp, Octs, Mrps as well as Na+/K+ ATPase showed no inhibitory effect on S-propranolol permeability. Thus, we believe that the driving force for the observed pH dependent permeability of propranolol is due to passive transcellular pathways.
To test this hypothesis, permeability studies at different pH were conducted in PAMPA, which is a parallel artificial membrane devoid of carrier-mediated and paracellular processes. In our PAMPA studies, 25 nM, 50 µM and 100 µM S-propranolol were dosed, whereas only results at 50 µM and 100 µM were evaluated due to the high mass retention that occurred at 25 nM (58±4%) compared to 50 µM (12%±1) and 100 µM (8%±1%). That is, high adsorption may occur at low concentrations, especially for hydrophobic compounds, yielding unreliable results. The transport of the high permeability standard labetalol was also determined in the PAMPA system. At both apical and basolateral sides pH 7.4, 50 µM S-propranolol exhibited a higher permeability than 50 µM labetalol (Fig. 8a), which is in accordance with the BDDCS class 1 category of S-propranolol. At 50 µM and 100 µM S-propranolol, the directional transport study in PAMPA exhibited similar permeablity trends to that observed in the cell systems as apical pH decreased from 7.4 to 6.5. In contrast, the neutral compound digoxin did not show different permeability results at different apical pHs. This observation confirmed our hypothesis that pH dependent transport of S-propranolol is mainly caused by passive diffusion. The good correlation between the PAMPA system and cell bidirectional transport systems makes PAMPA a suitable model for ionized compounds to assess the contribution of passive transcellular passage to the whole permeability process.
Our observation that P-gp does not affect the transport of S-propranolol correlates well with the study conducted by Stephens et al. (29) using mdr1a (−/−) mice intestine that found propranolol permeability is not influenced when comparing animals where P-gp is expressed or knocked out.
Based on the pH partition hypothesis, for weak base compounds such as propranolol, a greater fraction of nonionized molecules will exist at the higher pH value. When S-propranolol is dosed at apical side and received at basolateral side, an apical pH change from 7.4 to 6.5 (with basolateral pH maintained at 7.4) provides a decreased driving force for membrane permeability; therefore, Papp A->B decreases (Figure 7, 1st and 3rd bars). In contrast, when the dosing pH is maintained at pH 7.4 and the receiving pH is changed from 7.4 to 6.5, permeability increases (Figure 7, 1st and 4th bars). In Caco-2 bidirectional transport study, the observed “efflux ratio” of 3.6 at the apical pH 6.5 is actually caused by passive diffusion. This leads to the caution that pH condition must be taken into consideration since Papp values are significantly different at variable pHs. Therefore, in terms of substrate identification for efflux transporters, isocratic pH should be applied in both sides of the membrane to avoid producing false positive efflux ratios. Neuhoff et al. has investigated pH-dependent transport of weakly basic drugs in Caco-2 cell lines. They found that the efflux ratio of P-gp substrates talinolol and quinidine significantly increased when the apical pH decreased from 7.4 to 6.5 and basolateral pH was maintained at 7.4, which probably is caused by the pH-partitioning effect and the compounds transited across the cells through transcellular passive diffusion (30). However, they also observed an aqueous boundary layer effect and did report evidence of an uptake transporter effect on the positively charged form of propranolol under completely unphysiologic conditions by stirring at 450rpm (30). This is consistent with our belief that transporter effects may be demonstrated for almost all compounds, but that under physiologic conditions passive diffusion is the predominant membrane transit mechanism for BDDCS class 1 compounds such as propranolol.
Our present results imply that the pH dependent transport of S-propranolol is caused overwhelmingly by passive diffusion, rather than carrier-mediated transport. Carriers, known or unknown, are potentially involved in the permeability of S-propranolol. However, being substrates of carrier transporters doesn’t necessarily mean that carriers have significant effects on permeability due to the high passive diffusion. For example, studies have shown that both human and rat OCTs/Octs mediate the transport of propranolol (21, 22). However, here we conducted an Oct inhibition study with verapamil in intestine, showing that verapamil did not affect the absorption of S-propranolol in Ussing chamber. This observation further strengthens the importance of passive diffusion.
In conclusion, the studies reported here confirm that the permeability of S-propranolol is primarily due to passive diffusion, and that P-gp, octs and other carriers are unimportant. Passive diffusion, instead of being viewed as negligible, is of vital importance for propranolol.
Supplementary Material
Acknowledgments
We thank the Chinese Scholarship Council for providing financial support for Yi Zheng to study and carry out these studies in Dr. Benet’s laboratory at the University of California, San Francisco. The studies in Dr. Benet’s lab were funded in part by NIH grants RR031474 and GM061390.
ABBREVIATIONS
- P-gp (MDR1)
P-glycoprotein
- BCRP
breast cancer resistance protein
- BDDCS
Biopharmaceutics Drug Disposition Classification System
- PAMPA
Parallel Artificial Membrane Permeability Assay
- MDCK
Madin-Darby canine kidney
- TEER
transepithelial electrical resistance
- Papp
apparent permeability coefficient
- Papp, A->B
apical-to-basolateral Papp
- Papp, B->A
basolateral-to-apical Papp
- OCT
organic cation transporter
- OATP
organic anion transporting polypeptide
- HBSS
Hank’s balanced salt solution
- FBS
fetal bovine serum
- PEPT
proton-coupled oligopeptide transporter
- ASBT
apical sodium/bile acid co-transporter
- MCT
monocarboxylic acid transporter
- MRP
multidrug resistance like protein
- GG918(GF120918)
N-{4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)-ethyl]-phenyl}-9,10-dihydro-5-methoxy-9-oxo-4-acridinecarboxamide
REFERENCES
- 1.Kell DB, Dobson PD, Oliver SG. Pharmaceutical drug transport: the issues and the implications that it is essentially carrier-mediated only. DRUG DISCOV TODAY. 2011;16(15–16):704–714. doi: 10.1016/j.drudis.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kell DB, Dobson PD, Bilsland E, Oliver SG. The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: what we (need to) know and how we can do so. DRUG DISCOV TODAY. 2013;18(5–6):218–239. doi: 10.1016/j.drudis.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, et al. Coexistence of passive and carrier-mediated processes in drug transport. NAT REV DRUG DISCOV. 2010;9(8):597–614. doi: 10.1038/nrd3187. [DOI] [PubMed] [Google Scholar]
- 4.Di L, Artursson P, Avdeef A, Ecker GF, Faller B, Fischer H, et al. Evidence-based approach to assess passive diffusion and carrier-mediated drug transport. DRUG DISCOV TODAY. 2012;17(15–16):905–912. doi: 10.1016/j.drudis.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 6.Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. ADV DRUG DELIVER REV. 2008;60(6):717–733. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett AM, Cullum VA. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. Br J Pharmacol. 1968;34(1):43–55. doi: 10.1111/j.1476-5381.1968.tb07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoschitzky K, Lindner W, Rath M, Leitner C, Uray G, Zernig G, et al. Stereoselective hemodynamic effects of (R)-and (S)-propranolol in man. N-S ARCH PHARMACOL. 1989;339(4):474–478. doi: 10.1007/BF00736064. [DOI] [PubMed] [Google Scholar]
- 9.Lennernas H, Nylander S, Ungell AL. Jejunal permeability: a comparison between the ussing chamber technique and the single-pass perfusion in humans. Pharm Res. 1997;14(5):667–671. doi: 10.1023/a:1012121632357. [DOI] [PubMed] [Google Scholar]
- 10.Yang JJ, Kim KJ, Lee VH. Role of P-glycoprotein in restricting propranolol transport in cultured rabbit conjunctival epithelial cell layers. Pharm Res. 2000;17(5):533–538. doi: 10.1023/a:1007508714259. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Cao J, Wang X, Zeng S. Stereoselective transport and uptake of propranolol across human intestinal Caco-2 cell monolayers. Chirality. 2010;22(3):361–368. doi: 10.1002/chir.20753. [DOI] [PubMed] [Google Scholar]
- 12.D'Emanuele A, Jevprasesphant R, Penny J, Attwood D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J Controlled Release. 2004;95(3):447–453. doi: 10.1016/j.jconrel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura T, Kato Y, Amano N, Ono M, Kubo Y, Kimura Y, et al. Species difference in intestinal absorption mechanism of etoposide and digoxin between cynomolgus monkey and rat. Pharm Res. 2008;25(11):2467–2476. doi: 10.1007/s11095-008-9658-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Okochi H, Benet LZ, Zhai SD. Sotalol permeability in cultured-cell, rat intestine, and PAMPA system. Pharm Res. 2012;29(7):1768–1774. doi: 10.1007/s11095-012-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kell DB, Oliver SG. How drugs get into cells: tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion. Front Pharmacol. 2014;5:231. doi: 10.3389/fphar.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? NAT REV DRUG DISCOV. 2008;7(3):205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 18.Dobson PD, Lanthaler K, Oliver SG, Kell DB. Implications of the dominant role of transporters in drug uptake by cells. CURR TOP MED CHEM. 2009;9(2):163–181. doi: 10.2174/156802609787521616. [DOI] [PubMed] [Google Scholar]
- 19.Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J PHARM SCI. 2013;102(1):34–42. doi: 10.1002/jps.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo Y, Kusagawa Y, Tachikawa M, Akanuma S, Hosoya K. Involvement of a novel organic cation transporter in verapamil transport across the inner blood-retinal barrier. Pharm Res. 2013;30(3):847–856. doi: 10.1007/s11095-012-0926-y. [DOI] [PubMed] [Google Scholar]
- 21.Kubo Y, Shimizu Y, Kusagawa Y, Akanuma S, Hosoya K. Propranolol transport across the inner blood-retinal barrier: potential involvement of a novel organic cation transporter. J PHARM SCI. 2013;102(9):3332–3342. doi: 10.1002/jps.23535. [DOI] [PubMed] [Google Scholar]
- 22.Dudley AJ, Bleasby K, Brown CD. The organic cation transporter OCT2 mediates the uptake of beta-adrenoceptor antagonists across the apical membrane of renal LLC-PK(1) cell monolayers. Br J Pharmacol. 2000;131(1):71–79. doi: 10.1038/sj.bjp.0703518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng XL, Yu QQ, Wang Y, Zeng S. Stereoselective accumulation of propranolol enantiomers in K562 and K562/ADR cells. Chirality. 2013;25(6):361–364. doi: 10.1002/chir.22178. [DOI] [PubMed] [Google Scholar]
- 24.International Transporter C. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. Membrane transporters in drug development. NAT REV DRUG DISCOV. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15(20):1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 26.Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34(2–3):323–336. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Juttner M, Kullak-Ublick GA, Eloranta JJ. Regulation of the gene encoding the intestinal bile acid transporter ASBT by the caudal-type homeobox proteins CDX1 and CDX2. AM J PHYSIOL-GASTR L. 2012;302(1):G123–G133. doi: 10.1152/ajpgi.00102.2011. [DOI] [PubMed] [Google Scholar]
- 29.Stephens RH, Tanianis-Hughes J, Higgs NB, Humphrey M, Warhurst G. Region-dependent modulation of intestinal permeability by drug efflux transporters: in vitro studies in mdr1a (−/−) mouse intestine. J PHARMACOL EXP THER. 2002;303(3):1095–1101. doi: 10.1124/jpet.102.041236. [DOI] [PubMed] [Google Scholar]
- 30.Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res. 2003;20(8):1141–1148. doi: 10.1023/a:1025032511040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.