Abstract
Deep brain stimulation (DBS) is a promising tool for treating drug-resistant epileptic patients. Currently, the most common approach is fixed-frequency stimulation (periodic pacing) by means of stimulating devices that operate under open-loop control. However, a drawback of this DBS strategy is the impossibility of tailoring a personalized treatment, which also limits the optimization of the stimulating apparatus. Here, we propose a novel DBS methodology based on a closed-loop control strategy, developed by exploiting statistical machine learning techniques, in which stimulation parameters are adapted to the current neural activity thus allowing for seizure suppression that is fine-tuned on the individual scale (adaptive stimulation). By means of field potential recording from adult rat hippocampus–entorhinal cortex (EC) slices treated with the convulsant drug 4-aminopyridine we determined the effectiveness of this approach compared to low-frequency periodic pacing, and found that the closed-loop stimulation strategy: (i) has similar efficacy as low-frequency periodic pacing in suppressing ictal-like events but (ii) is more efficient than periodic pacing in that it requires less electrical pulses. We also provide evidence that the closed-loop stimulation strategy can alternatively be employed to tune the frequency of a periodic pacing strategy. Our findings indicate that the adaptive stimulation strategy may represent a novel, promising approach to DBS for individually-tailored epilepsy treatment.
Keywords: Closed-loop strategy, Deep brain stimulation, Epilepsy
Introduction
Epilepsy is a highly-prevalent chronic neurological disorder (Kotsopoulos et al., 2002) and up to 30% of epilepsy patients do not respond to pharmacological treatment (Kwan and Brodie, 2000). Moreover, resective surgery comes at the risk of physical and cognitive impairments. An emerging alternative treatment for drug-resistant patients is deep brain stimulation (DBS), i.e. direct delivery of electrical pulses to the brain, aiming at modulating neuronal excitability and thus halting or even preventing seizures. Recent studies have evaluated the effectiveness of DBS strategies in drug-resistant epileptic patients (Boon et al., 2007; Ellis and Stevens, 2008; Hamani et al., 2009; Marks, 2008). However, conclusive evidence in humans is difficult to demonstrate, due to the large variance in the disease and symptoms among patients; it is therefore difficult to choose a single stimulation protocol that is effective on a collection of individuals.
There are many parameters to select when applying DBS, including the target area for stimulation, as well as the frequency, intensity and pattern of the electrical pulses. These parameters can be specified either through an open-loop paradigm, or as a closed-loop control system. An open-loop strategy uses preset parameters to deliver stimulation, without monitoring electrical cortical activity. Examples of open-loop strategies include fixed-frequency stimulation (periodic pacing), as well as stimulation strategies based on a fixed random process (e.g., Gaussian noise generator) (Durand and Bikson, 2001). In a closed-loop strategy, the stimulation parameters are dynamically changed in response to sensor readings of brain activity. This can be achieved by using software that automatically detects an impending seizure and administering a fixed stimulation protocol designed to terminate the seizure (Cohen-Gadol et al., 2003; Durand and Bikson, 2001; Fountas et al., 2005; Kossoff et al., 2004; Loscher and Schmidt, 2004; Osorio et al., 2005; Theodore and Fisher, 2004). This can also be achieved through more sophisticated feedback control methods (Guez et al., 2008; Pineau et al., 2009).
More substantial evidence for the ability of DBS to successfully reduce epileptic symptoms has been produced using animal models of epilepsy. A number of studies focused on finding the appropriate stimulation site and frequency of stimulation for open-loop (D’Arcangelo et al., 2005; Durand and Bikson, 2001; Ellis and Stevens, 2008; Schiller and Bankirer, 2007) as well as for closed-loop strategies (Bush and Pineau, 2009; Durand and Bikson, 2001; Nakagawa and Durand, 1991; Schiff et al., 1994; Schiller and Bankirer, 2007). By using an in vitro model of limbic ictogenesis, we have previously reported that repetitive low-frequency stimulation, delivered in the subiculum at frequencies similar to those of the CA3-driven interictal discharges, decreases epileptiform synchronization in the entorhinal cortex (EC) (Barbarosie and Avoli, 1997). In particular, we have shown that the 1 Hz frequency exhibits maximal efficacy in reducing EC ictogenesis (D’Arcangelo et al., 2005).
We have put forward, in previous studies, the use of statistical learning techniques to automatically optimize closed-loop strategies. We showed how the strategies can be learned from field potential recordings in rat brain slices in which epileptiform discharges were induced by superfusion with the convulsant drug 4-aminopyridine (4AP) (Guez et al., 2008; Pineau et al., 2009). These studies suggested that closed-loop DBS could achieve successful suppression of ictal-like activity using fewer pulses than open-loop periodic pacing strategies; however these results were obtained using an in silico model of epilepsy. Here, we have evaluated such statistical learning closed-loop strategies in vitro by using the 4AP model of limbic ictogenesis in adult rat hippocampus–EC slices. Our findings show that the learned controller is able to perform as well as the 1 Hz open-loop paradigm, while reducing the total stimulation delivered in most slices. In contrast, applying periodic pacing at the same effective mean frequency found by the closed-loop controller is not as effective in suppressing ictal-like activity as our adaptive algorithm’s solution.
Methods
Brain slice preparation and maintenance
All efforts were made to minimize the number of animals used and their suffering. All the procedures were carried out in accordance with the Canadian Council on Animal Care and McGill University guidelines. Nineteen male, adult Sprague–Dawley rats (250–300 g) were decapitated under deep isoflurane anesthesia. The brain was quickly removed and placed in cold (0–2 °C) artificial cerebro-spinal fluid (ACSF), continuously bubbled with a gas mixture (CO2 5% and O2 95%) to equilibrate at pH=7.35–7.40, and having the following composition (mM): 124 NaCl, 2 KCl, 2 MgSO4, 2 CaCl2, 1.25 KH2PO4, 26 NaHCO3 and 10 D-glucose. Partially disconnected combined hippocampus–EC slices (450 μm thick) including the most ventral part of the hippocampal formation were cut as previously described (Panuccio et al., 2010) using a VT1000S vibratome (Leica, Germany). In these brain slices fast CA3-driven interictal-like activity disclosed by 4AP application was observed within the hippocampus proper only, i.e. it did not propagate to the EC (cf., Avoli et al., 1996, but see also Avoli et al., 2002). Slices were then transferred to an interface recording chamber, lying between warm (~32 °C) ACSF and humidified gas (CO2 5% and O2 95%), where they were allowed to recover for at least 1 h before beginning continuous bath-application of 4AP. Slices were continuously perfused at ~1 ml/min.
Field potential recording and stimulation paradigms
Field potential recordings were made with ACSF-filled pipettes (tip diameter<10 μm; resistance=5–10 MΩ) pulled from borosilicate capillary tubing (World Precision Instruments Inc., Sarasota, FL, USA) using a P-97 puller (Sutter Instrument, Novato, CA, USA). Extracellular signals were fed to a Cyberamp 380 amplifier (Molecular Devices, Palo Alto, CA) connected to a digital interface device (Digidata 1320A, Molecular Devices). Data were acquired at a sampling rate of 5 kHz (low-pass filtered at 2 kHz), using the software Clampex 8.2 (Molecular Devices), stored on the hard drive and analyzed off-line using pClamp 9.0. Recording electrodes were placed in the deep layers of the medial EC, CA3 stratum pyramidale or radiatum, and the pyramidal layer of the proxymal subiculum (see Fig. 1A). Extracellular current pulses (0.1–2.25 mA, pulse width 100 μs) were delivered in the pyramidal layer of the proximal subiculum through a bipolar concentric Pt–Ir electrode (FHC, Bowdoin, ME, USA) plugged onto a high voltage stimulus isolator unit (A360, WPI Inc., Sarasota, Florida, USA) connected to the pulse generator Pulsemaster A300 (WPI Inc., Sarasota, Florida, USA). The current intensity required to induce a response with failure rate <80% was established before the beginning of any stimulation protocol and was kept constant throughout the experiment. The Cyberamp 380 amplifier was also connected to another digital interface device (USB-6221 M Series, National Instruments, Texas, USA) that acquired data at a sampling rate of 5 kHz using an in-house software. That software was performing signal processing in real time and was querying the adaptive controller software for the stimulation actions. Those stimulation requests were then transmitted digitally from the National Instruments digitizer to the pulse generator.
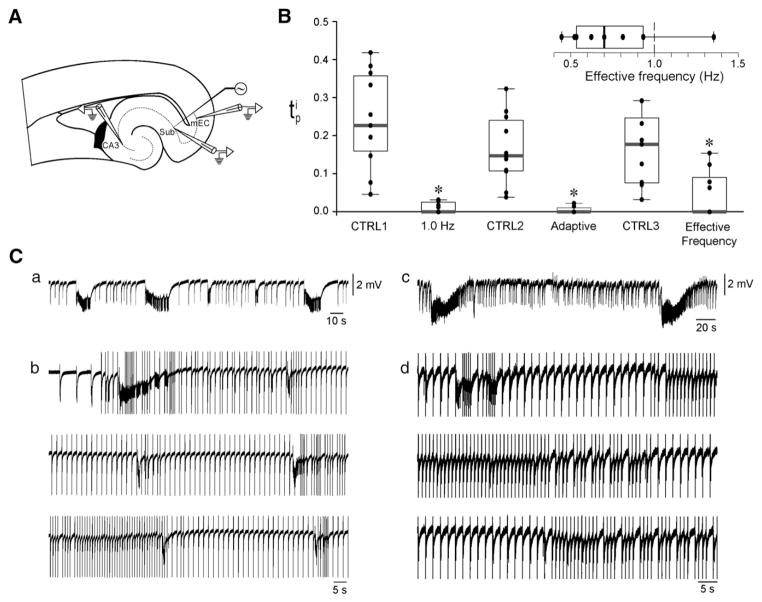
Fig. 1.
Adaptive stimulation performs similarly but is more efficient than low-frequency periodic pacing in controlling ictal activity. A: Brain slice schematic illustrating the position of the recording and stimulating electrodes.2 B: Box plots summarizing on the performance of the three stimulation protocols in terms of suppression of ictal activity as compared to their respective control phases. Each stimulation paradigm significantly decreased the (*p<0.05). However, as further emphasized by the box plot in the inset, the adaptive algorithm requires less stimulation overall. C: Sample recordings from the EC of two different brain slices illustrating the adaptive behavior of the closed-loop controller. In panels a and c are the control phases of the experiments illustrated in panels b and d, respectively. The adaptive stimulation algorithm adjusts the frequency of stimulation in order to dampen or prevent ictal activity. Stimulus artifacts were truncated.
2mEC: medial entorhinal cortex. CA1 and CA3: cornu ammonis 1 and 3, respectively. Sub: subiculum.
Before running the adaptive code, we assessed slice viability in terms of subicular projections to the EC by testing the efficacy of periodic pacing at 1 Hz, which has proved to be the most effective low-frequency periodic pacing protocol in terms of suppression of ictal activity (cf. D’Arcangelo et al., 2005). During the training phase, three different stimulation strategies were applied in 4 slices as follows: (i) periodic pacing at 0.5 Hz, (ii) periodic pacing at 1 Hz, and (iii) periodic pacing at 2 Hz. An early version of the closed-loop controller was also applied to other 4 slices to obtain more relevant training data. Each stimulation paradigm was preceded by a control period and followed by a recovery phase, which also served as the control of the following stimulation protocol. In total, about 12 h of recording (including control and stimulation protocols) was acquired to train our closed-loop controller. During the validation phase, three different stimulation strategies were applied on 11 slices as follows: (i) periodic pacing at 1 Hz, (ii) adaptive stimulation using the closed-loop controller, and (iii) periodic pacing at the average frequency of the adaptive stimulation protocol (we call this the effective frequency stimulation). As above, each stimulation paradigm was preceded by a control period and followed by a recovery phase, which also served as the control of the following stimulation protocol. The effective frequency stimulation, f (Hz), is a periodic pacing strategy. A different effective frequency is computed for each slice after application of the adaptive controller using the following equation:
where ns is the total number of pulses delivered during the duration T, in seconds, of the adaptive stimulation protocol. This strategy effectively includes as many pulses as the adaptive strategy applied to the slice, but distributed in a periodic manner.
Adaptive stimulation design
The stimulation patterns for the adaptive stimulation strategy are computed in real-time based on a function relating the observed neural activity and optimal stimulation parameters. This function is learned a priori using data collected from the training slices. This function defines the parameters of the adaptive stimulation strategy over the full range of observed neural conditions. The function is then used on the validation slices, to match the observed neural signal with the optimal stimulation parameters. A full description of the mathematical and computational methods underlying the adaptive controller can be found in Guez et al. (2008) and Pineau et al. (2009). Here, we briefly summarize the mathematical method.
Field potential recordings were processed using fast Fourier transforms over different window lengths to extract spectral features forming the state vector s on which the adaptive controller based its stimulation decision at each time step. A cost c was associated with performing a stimulation action a in a state s, whose cost was mainly influenced by the presence of epileptiform activity in s and also by the frequency of stimulation described by a.
The controller could then choose, by the mean of an action a at a state s, between not stimulating and stimulating at either 0.5 Hz, 1 Hz, or 2 Hz for the duration of the next reference window. The goal of the controller was to reduce the long-term accumulation of those costs. To achieve that, a sophisticated regression tool, called extremely randomized trees (Geurts et al., 2006), was used to learn the long-term cumulative cost Q(s,a) of applying an action a in any state s using the field potential recordings in the training dataset. In the validation phase, an optimal stimulation action at, could then be selected based on the current neuronal network state st, using the best learned controller function Q(st,at)=maxa Q(st,a).
Data and statistical analysis
We arbitrarily defined ictal-like (hereafter termed ictal) discharges those epileptiform events resembling EEG ictal activity and lasting longer than 3 s. We used the following parameters as performance indicators of the adaptive controller:
, which denotes the proportion of ictal time during protocol p for the slice i, where for each slice the following protocols are executed in the following sequence: control (CTRL1) → 1 Hz stimulation→recovery (CTRL2)→adaptive stimulation→recovery (CTRL3)→effective frequency stimulation.
is the mean frequency that a stimulation protocol p resorts to for the i-th slice, and measures the amount of stimulation used by the protocol. By definition, the adaptive stimulation protocol and the effective frequency protocol share the same value of for any particular slice.
Data throughout the text are expressed as mean±SEM and n indicates the number of slices unless otherwise specified. Significance was set at p<0.05.
Results
Fig. 1A illustrates the brain slice preparation used in this study and the positions of the recording and the stimulating electrodes. In Fig. 1B, the estimate is computed for each slice in the validation data set under the different protocols; the quartiles are reported as box-plots for each protocol. Ictal activity generated by each slice did not significantly change throughout the three control phases, suggesting that electrical stimulation did not induce any detectable modification in the functionality of EC neuronal networks (Duration: CTRL=40.4±10.1 s, CTRL2=35±5.6 s, CTRL3=33±6.7 s; interval: CTRL1=170.4±38.1 s, CTRL2=193.3±33 s, CTRL3=180.1±31.5 s; n=11, 11 and 9, respectively). During 1 Hz stimulation, 5 out of 11 slices generated a total of 11 ictal discharges, which emerged mostly during the early stimulation phase. Ictal events lasted 11±3 s and occurred at an interval of 209±46 s. During adaptive stimulation, 3 out of 11 slices generated 13 ictal discharges lasting 12±6 s and occurring every 306±131 s. During periodic pacing at the effective frequency, 4 out of 9 slices generated 19 ictal discharges that were 19±5 s long and occurred at an interval of 167±18 s. Statistical comparison of values indicated that all stimulation protocols significantly decreased ictal activity as compared to the control (CTRL1=0.24± 0.04, 1 Hz: 0.02±0.01, n=11, p<0.001; CTRL2=0.16±0.03, adaptive stimulation: 0.01±0.01, n=11, p<0.001; CTRL3=0.16±0.03, effective frequency: 0.05±0.02, n=9, p<0.01, Wilcoxon–Mann–Whitney two-sample rank-sum test).
It may be expected that the three stimulation policies perform differently in terms of suppression of ictal activity. Statistical analysis using repeated measure Friedman test (n=9, k=3) returned a value of p=0.13, thus suggesting that the three paradigms performed similarly possibly due to the limited data set used in this study. Moreover, it is important to stress that the median value of for each simulation protocol was 0 (i.e., complete suppression). The fundamental difference between the 1 Hz protocol and the other protocols stems from the rate of stimulation as measured by the effective frequency (Fig. 1B, inset). A Wilcoxon signed rank test determined that the distribution of for the adaptive controller is unlikely to have at least 1 Hz as median (n=11, p<0.03), i.e., the rate of stimulation employed by the adaptive controller is mostly slower than 1 Hz. Therefore, the closed-loop strategy proposed here is more efficient than low-frequency periodic pacing in that it requires less stimulation. We also provide preliminary evidence that the closed-loop stimulation strategy can alternatively be employed to tune the frequency of a periodic pacing strategy.
Fig. 1C showcases different scenarios that occurred when running the adaptive controller. Sample traces are recordings from the medial EC, and panels a and c are the control phases of the experiments illustrated in panels b and d, respectively (stimulus artifacts were truncated). The adaptive code dynamically changes the stimulation frequency in order to entrain network activity and dampen (Fig. 1Ca,b) or even prevent (Fig. 1Cc,d) the generation of ictal events.
Discussion
In this work we have leveraged statistical machine learning techniques to learn a closed-loop stimulation strategy from in vitro electrophysiology data. The result is an adaptive stimulation algorithm that can control epileptiform activity. Our key findings can be summarized as follows. (i) The adaptive stimulation strategy has similar efficacy as low-frequency periodic pacing in suppressing ictal events in an in vitro animal model of ictogenesis. (ii) The adaptive stimulation strategy is more efficient than periodic pacing in that it requires less stimulation overall.
We show here that applying the mean stimulation frequency of the adaptive protocol for a given slice, referred to as effective frequency stimulation, decreases ictal activity with performance similar to the adaptive strategy. However, it is not possible to predict a priori the effective periodic pacing frequency in any given experiment; there is no analytic formula to calculate this. Rather, the effective periodic pacing frequency for a particular subject (or brain slice) can be discovered by applying the adaptive stimulation strategy on and calculating the rate of stimulation. In the future, employing the adaptive controller to probe the network activity and automatically tune the frequency of a periodic pacing protocol may represent an attractive alternative to running the adaptive strategy at all times. In contrast to our in vitro model, where periodic pacing at 1 Hz consistently achieves significant reduction of ictal activity, optimization of DBS parameters in epileptic animals and, more importantly, in human epileptic patients, is an open question. It is unlikely that a single non-adaptive stimulation strategy will work well across subjects. Therefore, automatically tuning, or learning, the DBS parameters for a particular subject becomes highly desirable. Learning the DBS parameters in an efficient way online (as opposed to training based on a fixed set of data) is a challenging problem in many respects. One difficulty is that the controller needs to acquire information about the subject by probing (i.e. applying pulses at varying times to observe the effects), while simultaneously maintaining a tolerably low level of ictal activity. In practice, achieving these two goals simultaneously may be challenging. Statistical machine learning techniques can provide significant guidance to tackle this problem by designing controllers that probe and adapt parameters in a principled way. These controllers should have the potential to learn at different timescales to continuously adapt to a given patient, as well as incorporate additional relevant factors, such as the time of the day, or the level or type of physical activity of the subject (e.g., exercise vs. sleep). In another work, we have provided a useful approach for inferring a sufficient state representation from data to characterize the neural system, including predicting network activity and the response to electrical stimulation (Bush et al., 2012).
Acknowledgments
The authors would like to thank their colleague Keith Bush for his valuable comments and suggestions.
Grants
The authors gratefully acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), grant RGPIN 311949; the Canadian Institutes of Health Research (CIHR), grants MOP 8109 and MOP 97907; and the National Institutes of Health (NIH), grant R21 DA019800. Gabriella Panuccio was a fellow of Epilepsy Canada and of the Savoy Foundation.
Abbreviations
- DBS
Deep brain stimulation
- EC
Entorhinal cortex
References
- Avoli M, Barbarosie M, Lucke A, Nagao T, Lopantsev V, Kohling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal–entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt T, Dewaele I, Achten R, Wadman W, Dewaele F, Caemaert J, Van Roost D. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Bush K, Pineau J. Manifold embeddings for model-based reinforcement learning under partial observability. In: Bengio Y, Schuurmans D, Lafferty J, Williams CKI, Culotta A, editors. Advances in Neural Information Processing Systems. 2009. pp. 189–197. [Google Scholar]
- Bush K, Panuccio G, Avoli M, Pineau J. Evidence-based modeling of network discharge dynamics during periodic pacing to control epileptiform activity. J Neurosci Methods. 2012;204:318–325. doi: 10.1016/j.jneumeth.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Stoffman MR, Spencer DD. Emerging surgical and radiotherapeutic techniques for treating epilepsy. Curr Opin Neurol. 2003;16:213–219. doi: 10.1097/01.wco.0000063773.81810.fe. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Panuccio G, Tancredi V, Avoli M. Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol Dis. 2005;19:119–128. doi: 10.1016/j.nbd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Durand MD, Bikson M. Suppression and control of epileptiform activity by electrical stimulation: a review. Proc IEEE. 2001;89:1065–1082. [Google Scholar]
- Ellis TL, Stevens A. Deep brain stimulation for medically refractory epilepsy. Neurosurg Focus. 2008;25:E11. doi: 10.3171/FOC/2008/25/9/E11. [DOI] [PubMed] [Google Scholar]
- Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, Jenkins PD. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83:153–158. doi: 10.1159/000088656. [DOI] [PubMed] [Google Scholar]
- Geurts P, Ernst D, Wehenkel L. Extremely randomized trees. Mach Learn. 2006;63:3–42. [Google Scholar]
- Guez A, Vincent RD, Avoli M, Pineau J. Adaptive treatment of epilepsy via batch-mode reinforcement learning. Proceedings of the Twentieth Innovative Applications of Artificial Intelligence Conference; 2008. pp. 1671–1678. [Google Scholar]
- Hamani C, Andrade D, Hodaie M, Wennberg R, Lozano A. Deep brain stimulation for the treatment of epilepsy. Int J Neural Syst. 2009;19:213–226. doi: 10.1142/S0129065709001975. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, Spencer DD, Bergey GK. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos IA, van Merode T, Kessels FG, de Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43:1402–1409. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schmidt D. New horizons in the development of antiepileptic drugs: the search for new targets. Epilepsy Res. 2004;60:77–159. doi: 10.1016/j.eplepsyres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Marks WJ. Deep brain stimulation in epilepsy. In: Tarsy DV, JL, Starr P, Okun M, editors. Deep Brain Stimulation in Neurological and Psychiatric Disorders. Humana Press; New York: 2008. pp. 561–569. [Google Scholar]
- Nakagawa M, Durand D. Suppression of spontaneous epileptiform activity with applied currents. Brain Res. 1991;567:241–247. doi: 10.1016/0006-8993(91)90801-2. [DOI] [PubMed] [Google Scholar]
- Osorio I, Frei MG, Sunderam S, Giftakis J, Bhavaraju NC, Schaffner SF, Wilkinson SB. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258–268. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- Panuccio G, D’Antuono M, de Guzman P, De Lannoy L, Biagini G, Avoli M. In vitro ictogenesis and parahippocampal networks in a rodent model of temporal lobe epilepsy. Neurobiol Dis. 2010;39:372–380. doi: 10.1016/j.nbd.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau J, Guez A, Vincent R, Panuccio G, Avoli M. Treating epilepsy via adaptive neurostimulation: a reinforcement learning approach. Int J Neural Syst. 2009;19:227–240. doi: 10.1142/S0129065709001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Jerger K, Duong DH, Chang T, Spano ML, Ditto WL. Controlling chaos in the brain. Nature. 1994;370:615–620. doi: 10.1038/370615a0. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Bankirer Y. Cellular mechanisms underlying antiepileptic effects of low- and high-frequency electrical stimulation in acute epilepsy in neocortical brain slices in vitro. J Neurophysiol. 2007;97:1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]