Abstract
Objectives
Evaluate the cytotoxicity and genotoxicity of short- and long-term e-cigarette vapor exposure on a panel of normal epithelial and head and neck squamous cell carcinoma (HNSCC) cell lines.
Materials and Methods
HaCaT, UMSCC10B, and HN30 were treated with nicotine-containing and nicotine-free vapor extract from two popular e-cigarette brands for periods ranging from 48 hours to 8 weeks. Cytotoxicity was assessed using Annexin V flow cytometric analysis, trypan blue exclusion, and clonogenic assays. Genotoxicity in the form of DNA strand breaks was quantified using the neutral comet assay and γ-H2AX immunostaining.
Results
E-cigarette-exposed cells showed significantly reduced cell viability and clonogenic survival, along with increased rates of apoptosis and necrosis, regardless of e-cigarette vapor nicotine content. They also exhibited significantly increased comet tail length and accumulation of γ-H2AX foci, demonstrating increased DNA strand breaks.
Conclusion
E-cigarette vapor, both with and without nicotine, is cytotoxic to epithelial cell lines and is a DNA strand break-inducing agent. Further assessment of the potential carcinogenic effects of e-cigarette vapor is urgently needed.
Keywords: oral cancer, head and neck squamous cell carcinoma (HNSCC), electronic cigarettes, smoking, DNA damage, strand breaks, nicotine
Introduction
Electronic cigarettes, or e-cigs, are battery-operated devices that allow users to inhale an aerosolized “e-liquid” in lieu of traditional tobacco smoke. E-liquid typically contains varying compositions of propylene glycol (PG), vegetable glycerin (VG), flavorings, and/or nicotine. Since their introduction to the U.S. in 2007 [1], e-cigs have experienced an exponential surge in popularity, with overall use increasing from 3.3% to 8.5% between 2010 and 2013 [2], and usage among adolescents doubling between 2011 and 2012 alone [3]. E-cigs have gained traction not only among current smokers as a replacement or supplement to traditional cigarettes, but also among non-smokers who may not have otherwise developed smoking habits and nicotine addiction [4]. The rapid rise of e-cigs is often attributed to advertisements portraying e-cigs as a smoking cessation tool or as a completely safe alternative to traditional smoking. However, these claims have been widely found to be controversial and unfounded in scientific evidence [5], [6].
Much remains to be elucidated about the health effects of e-cigs, as existing research is collectively inconclusive. Several studies have substantiated e-cig companies’ claims of negligible e-cig toxicity, with a recent report finding only one e-cig brand marginally cytotoxic to mammalian fibroblasts, and still nearly eight times less potent than conventional cigarettes [7]. E-cigs have also been reported to contain toxicants at trace levels 9-450 times lower than in traditional cigarettes [8]and no toxicological synergies among their compounds [9]. However, numerous other studies suggest otherwise, concluding that e-cigs are hazardous and should be regulated similarly to traditional cigarettes [10], [11], [12]. Heating of e-liquids to high temperatures has been found to release carcinogenic carbonyl compounds, such as formaldehyde, acetaldehyde, and acrolein [13]. The lungs have been shown to be especially vulnerable to e-cig exposure, as e-vapor particles deposit in lungs in a pattern similar to that of regular cigarette smoke particles [11], producing changes in bronchial gene expression [10] and increased oxidative stress and inflammation [12].
Among studies demonstrating the health risks of e-cigs, there is still a paucity of data regarding the cytotoxicity and genotoxicity of e-cig vapor and the role of e-liquid nicotine content in mediating the harmful effects of e-cig exposure. With e-cig usage becoming increasingly prevalent, it is critical to comprehensively evaluate the safety and potency of these devices. In this paper, we sought to investigate the cytotoxic and DNA strand break-inducing effects of nicotine-containing and non-nicotine-free e-cig vapor on a panel of epithelial cell lines. We also assess the contribution of e-cigs to the pathogenesis and progression of head and neck squamous cell carcinoma (HNSCC), a disease for which traditional cigarette smoking is a well-established risk factor yet the potential role of e-cigs has remained entirely unexplored.
Materials and Methods
Cell culture
To explore the effects of e-cigs on the oropharynx, in vitro experiments were performed on normal epithelial cells as well as head and neck squamous cell carcinoma (HNSCC) cell lines. We chose to use the widely available cell line HaCat, a spontaneously transformed immortal keratinocyte, to determine the potential effects of e-cig on normal epithelium [14]. We also chose to use the HNSCC cell lines HN30 and UMSCC10B for two reasons. First, these cell lines were originally derived from the oropharynx, and second, we wanted to determine the potential effect of e-cigs on cancerous cell lines, to represent e-cig smokers that already have HNSCC. UMSCC10B was derived from a metastatic lymph node [15]. HN30 was derived from a primary laryngeal tumor [16].
HaCaT, UMSCC10B, and HN30 were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 2% L-glutamine and 2% pen-strep. Media was replaced every three days, and cells were passaged at 90% confluence. All cells were cultured at 37°C and 5% CO2.
E-cigarette, cigarette, and nicotine treatments
E-cigarette vapor was pulled through media using negative pressure, and the resulting extract was filter-sterilized with a 0.2 μm pore-size filter before treating cell cultures. The cigarette-treated media was made similarly using Marlboro Red filter cigarettes, which were determined by the Federal Trade Commission in a 2000 report to contain 1.2 mg of nicotine per cigarette. The e-cig brands V2 and VaporFi, two of the most popular e-cigarettes currently on the market, were chosen for our experiments. Both brands reportedly employ a standard mixture of 70% PG/30%VG liquid formula. For both V2 and VaporFi, we employed 1.2% nicotine e-liquid containing 12 mg of nicotine per mL, as well as the nicotine-free 0% nicotine versions in the same flavor, in order to investigate the properties of e-liquid independently of nicotine content. For VaporFi, the flavor “Classic Tobacco” in Flavor Strength 1 was; for V2, the most similar flavor, “Red American Tobacco,” was used. For nicotine treatment, the calculated amount of nicotine hemisulfate salt solution (Cat # 65-30-5, Sigma-Aldrich, St Louis, MO) for the desired treatment concentration was directly added to the culture media.
Treatment media was replaced every three days with 1% e-cigarette extract. Because of the high toxicity of cigarette smoke extract, cigarette-treated samples of each cell line could only be treated for 24 hours.
Neutral comet assay
HaCaT cells were treated for 8 weeks, and UMSCC10B and HN30 were each treated for a week. At the end of the treatment period, the cells were harvested, lysed, and underwent neutral electrophoresis (Trevigen). Comet tails were counted in multiple fields (>35 cells per sample) and analyzed using CometScore (TriTek Corp).
γ-H2AX immunostaining
Cells were cultured on glass coverslips and treated for one week. Cells were then fixed, permeabilized, and stained with antibody to γ-H2AX. Nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI). Foci were counted in 9 to 13 high-power fields per group using the program FociCounter (SourceForge).
Cell cycle analysis by flow cytometry
After one week of treatment, cells were trypsinized, harvested, and fixed with cold 50% (v/v) ethanol in PBS, and stored at −20 °C for at least 24 hours. The cells were then washed with PBS and resuspended with 80 μg/mL propidium iodide (PI) solution, and the DNA content was measured using flow cytometry.
Trypan blue staining
To evaluate the cytotoxic effects of e-cigarettes, cells treated for 48 hours were trypsinized and the lifted cells resuspended in a 1:1 dilution of 0.4% trypan blue and PBS. The cells were incubated for five minutes at room temperature before visualizing under a light microscope and live and dead cells were counted using a hemacytometer.
Cell survival (clonogenic) assay
In clonal growth assays all populations were plated at 103 cells per 60 mm culture plate and cultured with media supplemented with 0.5% FBS. After 10–12 days of treatment, colonies were fixed with paraformaldehyde for 5 minutes, stained with crystal violet for 30 minutes, and counted. Colonies containing at least 30 cells were considered positive.
Annexin V apoptotic assay
Apoptotic cell death was analyzed using Annexin V-FITC Apoptosis Detection Kit, following the manufacturer’s RAPID Annexin V Binding protocol (EMD Millipore, Cat # PF032). The assay was performed with two-color analysis of FITC-labeled Annexin V binding and PI uptake. Floating cells and adherent cells were counterstained with PI and analyzed by flow cytometry. Positioning of quadrants on Annexin V/PI dot plots was performed and live cells (Annexin V−/PI−, Q3), early/primary apoptotic cells (Annexin V+/PI−, Q4), late/secondary apoptotic cells (Annexin V+/PI+, Q2) and necrotic cells (Annexin V−/PI+, Q1) were distinguished.
Statistical Analysis
All statistical analysis performed and p values given were determined by Student’s t-test.
Results
E-cigarette vapor extract increases DNA damage via single- and double-strand breaks
One of the most pertinent questions regarding the relative safety of e-cigs is whether or not e-cigs have the potential to cause DNA damage in human cells. To evaluate the ability of e-cigs to induce DNA strand breaks, the normal human epithelial cell line HaCaT and two HNSCC cell lines UMSCC10B and HN30 were treated for one week with two brands of e-cigarette vapor at 1% by volume. Experiments were performed both in normal and cancer cells to assess the effects of e-cigs on healthy cells as well as existing HNSCC. Both the nicotine-free formula and formula containing 12 mg/mL nicotine were tested from each brand to determine if in vitro effects of e-cig vapor are consistent between different brands, and whether the effects are nicotine-dependent. For comparison, cells were also treated with conventional cigarette smoke or with 0.5 mM nicotine, with both nicotine concentrations equivalent to that of the treatment media for nicotine-containing e-cigs.
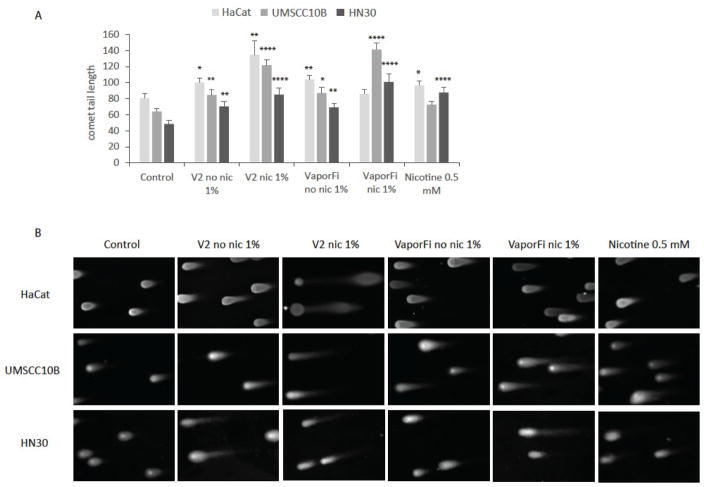
A neutral comet assay was performed on treated cells to evaluate the extent of DNA damage as determined by tail length (Fig 1). Our results show that e-cig vapor results in a statistically significant increase (up to 1.5 fold) in DNA strand breaks as compared to the untreated control. DNA damage is compounded in cells exposed to nicotine-containing e-cig vapor with these cells exhibiting a higher level of damage than cells treated with the equivalent concentration of nicotine alone. At the same time, we also show that DNA strand breaks are sufficiently induced even in the absence of nicotine, with cells exposed to nicotine-free e-cig vapor also exhibiting significant increases in DNA strand breaks relative to the untreated control.
Figure 1. E-cigarette exposure increases DNA damage as measured by neutral comet assay.
A, HaCaT, UMSCC10B, and HN30 were treated with 1% by volume vaporized e-cigarette liquid with brands V2 and VaporFi, and compared to untreated controls. HaCaT cells were treated for 8 weeks, and UMSCC10B and HN30 treated for 1 week each. E-cigarette containing nicotine at 1% by volume treatment was calculated to contain 0.5 mM nicotine, so cells treated at 0.5 mM nicotine directly are shown for comparison. Results are given as mean tail length ± SEM, with at least 35 cells per sample. B, representative images of cells captured following neutral comet assay. *P < 0.05, **P < 0.01, ***P < 0.001, ****P<0.0001
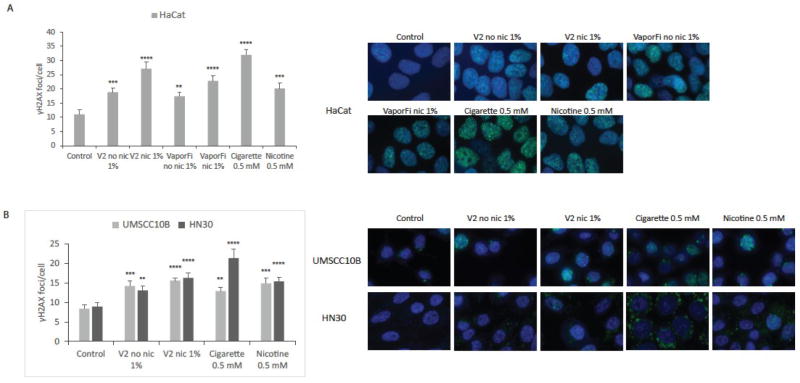
Immunostaining for γ-H2AX was then performed to specifically assess the formation of DNA double-strand breaks (DSBs), which are particularly dangerous to cells as they can lead to irreparable mutations and genomic aberrations. By quantifying the γ-H2AX foci count per cell, we determined that both V2 and VaporFi e-cig brands produced a significant induction of DSBs in HaCaT as compared to the untreated control, with foci number increased by up to 1.5-fold in nicotine-free e-cig-treated cells and up to 3-fold in nicotine-containing e-cig-treated cells (Fig 2A). We observed similar results in the HNSCC lines UMSCC10B and HN30, with cells exposed to V2 e-cig vapor exhibiting a significantly greater number of DSBs – up to 1.5-fold with nicotine-free e-cig and up to 2-fold with nicotine-containing e-cig (Fig 2B). Cells treated with cigarette smoke extract and nicotine at the same concentration of overall nicotine are shown for comparison. Cigarette smoke extract led to the highest number of DSBs in HaCaT and HN30 cell lines, but were not significantly higher than V2 nic 1%.
Figure 2. E-cigarette exposure increases DNA double strand breaks as measured by γ-H2Ax immunofluorescence.
A, HaCaT cells were treated for one week at 1% by volume vaporized e-cigarette liquid with brands V2 and VaporFi. E-cigarette containing nicotine at 1% by volume calculated to be equivalent to 0.5 mM nicotine; cultures treated with cigarette smoke at 0.5 mM nicotine and with 0.5 mM nicotine directly are shown for comparison. Graphed results are given as mean foci count per cell ± SEM, with at least 35 cells per sample. Representative images of γ-H2Ax foci formation (green) are shown with nuclei stained with DAPI (blue). B, UMSCC10B and HN30 were treated with the brand V2 at the same concentration and duration. Graphed results are given as mean foci count per cell ± SEM, with at least 35 cells per sample. *P < 0.05, **P < 0.01, ***P < 0.001, ****P<0.0001
Cell lines treated with e-cigarette vapor extract show arrest in G1 and G2, and increased apoptosis and necrosis
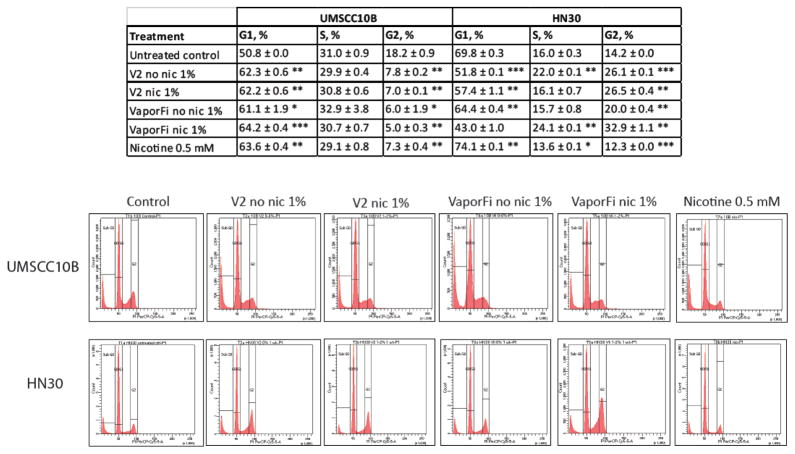
To determine if e-cigarette exposure alters cell cycle profiles, UMSCC10B and HN30 treated with V2 and VaporFi e-cig vapor extract for one week were analyzed for DNA content by flow cytometry (Fig 3). UMSCC10B showed a statistically significant increased accumulation of arrest in G1, and HN30 an increase in G2, both independently of e-cig nicotine content.
Figure 3. HNSCC cell line exposure to e-cigarette vapor results in altered cell cycle profiles.
UMSCC10B and HN30 were treated for one week at 1% by volume vaporized e-cigarette liquid with brands V2 and VaporFi, and analyzed by flow cytometry for DNA content. Results are given as mean percentage ± SEM. Peaks generated by flow cytometry analysis are shown for each of the permutations. *P < 0.05, **P < 0.01, ***P < 0.001
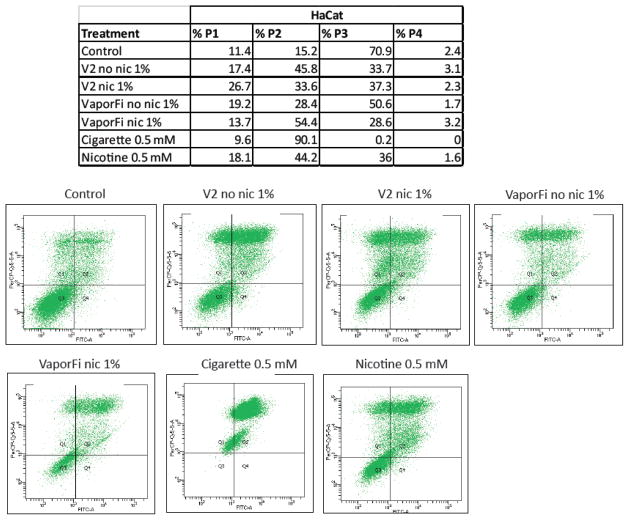
Next, to evaluate the extent and mechanisms of cell death resulting from e-cig vapor exposure, HaCaT cells were treated for one week with nicotine-containing and nicotine-free V2 and VaporFi e-cig vapor, and analyzed by flow cytometry using a conjugated Annexin V antibody and PI staining (Fig 4). This enabled differentiation among live, necrotic, early apoptotic, and late apoptotic cells. HaCaT cells were also treated with cigarette smoke extract and nicotine for comparison. HaCaT samples exposed to nicotine-free e-cig vapor extract showed a 53–68% increase in necrotic cells and a 120–200% increase in late/secondary apoptotic cells as compared to the untreated control, suggesting that e-cig vapor induces cell death both by necrosis and by apoptosis. HaCaT treated with nicotine-containing e-cig vapor extract exhibited a 20–134% increase in necrotic cells and a 121–258% increase in late/secondary apoptotic cells as compared to the control.
Figure 4. E-cigarette treated cells exhibit increased apoptosis and necrosis.
HaCaT cells were treated for one week at 1% by volume vaporized e-cigarette liquid with brands V2 and VaporFi, and analyzed by flow cytometry after staining with Annexin V-FITC and counterstaining with PI. Results for cultures treated with cigarette smoke at 0.5 mM nicotine and with 0.5 mM nicotine directly are shown for comparison. Positioning of quadrants on Annexin V/PI dot plots was performed and live cells (Annexin V−/I−, P3), early/primary apoptotic cells (Annexin V+/PI−, P4), late/secondary apoptotic cells (Annexin V+/PI+, P2) and necrotic cells (Annexin V−/PI+, P1) were distinguished.
E-cigarette exposure results in increased cell death
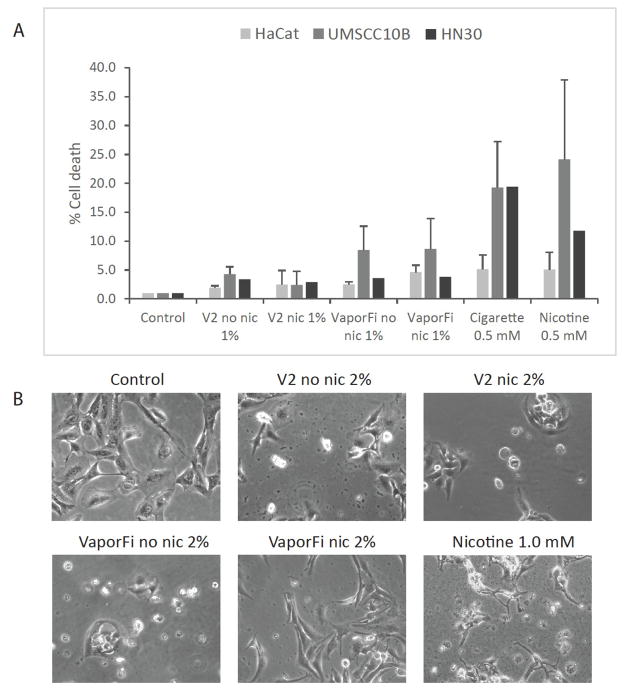
For all cell lines, e-cig vapor exposure, independent of nicotine content, resulted in increased cell death as measured by trypan blue exclusion assay. HaCaT, UMSCC10B, and HN30 were treated for 48 hours with 1% by volume V2 and VaporFi with and without nicotine prior to staining and counting (Fig 5A). Our results show that e-cig exposure induces a 5-fold increase in cell death without nicotine, and a 10-fold increase with nicotine as compared to the untreated control. Representative images of HaCaT treated at 2% by volume e-cig vapor for 2 weeks were taken at 200X bright field, showing significant cell death and changes in cell morphology as compared to the control (Fig 5B).
Figure 5. E-cigarette exposure induces cell death.
A, HaCaT, UMSCC10B, and HN30 cells were treated at 1% by volume vaporized e-cigarette liquid with brands V2 and VaporFi for 48 hours before trypan blue staining. Cultures treated with cigarette smoke at 0.5 mM nicotine and with 0.5 mM nicotine directly are shown for comparison. Cell death was normalized to the untreated control cell cultures. Results are shown as mean percentage of cell death per sample ± SEM. B, representative images of the cell cultures treated at 2% by volume vaporized e-cigarette or 1.0 mM nicotine for 2 weeks taken at 20X to show cell death and changes in cell morphology.
E-cigarette exposure decreases clonogenic survival
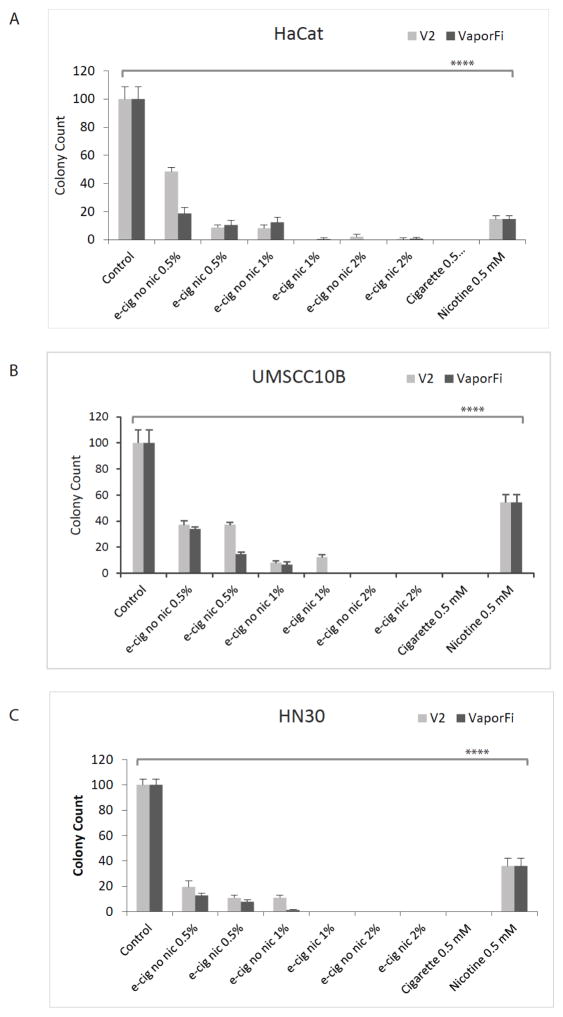
To evaluate the survival of normal and HNSCC cells with varying doses of e-cig vapor treatment, a clonogenic assay was performed using cells treated with 0.5%, 1.0%, and 2.0% by volume V2 and VaporFi e-cigarette vapor (Fig 6A and B, respectively). Cells treated with cigarette smoke extract and nicotine at 0.5 mM are shown for comparison. HaCaT cells were treated for 10 days, and UMSCC10B and HN30 for 12 days prior to colony enumeration. In UMSCC10B and HN30, 2% by volume vaporized e-cig treatment resulted in 100% cell death. Our results also show stepwise decrease in colony count and decreased survival with increasing e-cig doses in both brands independently of nicotine, with a greater than 2-fold decrease in survival in all cell lines after exposure to only 0.5% by volume nicotine-free e-cig vapor.
Figure 6. E-cigarette exposure decreases clonogenic survival in both normal and HNSCC cell lines.
A, HaCaT cells were treated for 10 days at 0.5, 1.0, and 2.0% by volume vaporized e-cigarette liquid. Colony counts were normalized to the untreated control cell cultures. Graphed results are given as mean colony count ± SEM. B and C, the same treatments were replicated for 12 days using UMSCC10B and HN30 respectively. ****P<0.0001
Discussion
Although e-cigs have skyrocketed in popularity and have been widely marketed as a safe alternative to traditional cigarettes, their safety and long-term effects have remained shrouded in controversy. In this paper, we have demonstrated cytotoxicity of short-term e-cig vapor exposure on a panel of normal epithelial and HNSCC cell lines. Through Annexin V staining, trypan blue exclusion, and colony forming assays, we have shown that the cytotoxic effects of e-cig vapor are mediated through nicotine as well as non-nicotine components of the e-liquid. The specific substances in e-cig liquids are still under investigation, as many formulations are proprietary information. However, our findings are consistent with previous assessments of e-cig effects on pulmonary tissue and cell lines, which implicated flavoring compounds as primary toxicants within e-cigs [17–19]. Additionally, numerous studies have reported the presence of other hazardous substances in e-cig vapor and heated e-liquid emissions, including the carcinogenic carbonyl compounds formaldehyde and acetaldehyde [8, 13, 20], and oxidants and reactive oxygen species, heavy metals, and volatile organic compounds such as toluene [8, 21, 22].
Because formaldehyde, acetaldehyde, and free radical species are well-known DNA damaging agents, especially in the context of conventional cigarette-associated diseases [25, 26], we decided to assess the induction of DNA strand breaks in normal and HNSCC cell lines following both short- and long-term e-cig exposure. Our study is the first to conclusively link e-cigarettes to DNA breakage. Neutral comet assay and immunofluorescence staining for the DNA double-strand break (DSB) marker γ-H2AX [27] revealed significantly increased tail length and γ-H2AX foci numbers, respectively, in cells exposed to e-cig vapor, regardless of nicotine concentrations. Although the mechanism by which vaporized e-cig components induce strand breaks is still unclear, it is likely that ROS are implicated in the process. Lerner et al demonstrated that the vaporization of e-cig liquids produced ROS which resulted in an inflammatory response in both human epithelial cells and mouse models [21]. More importantly, the presence of ROS is linked to single and double strand breaks, as well as oxidative DNA damage, as they modify nitrogenous bases by oxidation. The most common mutation is guanine oxidized to 8-oxo-7,8-dihydroguanine, which creates 8-oxo-deoxy guano sine (8-oxo-dG) [23]. The 8-oxo-dG is capable of base pairing with deoxyadenosine, instead of pairing correctly with deoxycytotidine, resulting in the replicated DNA containing a point mutation [24].
The accumulation of DSBs in e-cig-treated cells is particularly suggestive of the carcinogenic potential of e-cigs. DSBs are predominantly repaired through the non-homologous end joining pathway, a notoriously error-prone process associated with the acquisition of genomic instability. Furthermore, the increased rates of G1 and G2 arrest we observed among cells exposed to e-cig suggest that higher-fidelity DSB repair mechanisms such as homologous recombination, which is primarily active in the S phase [28], are even less utilized in e-cig-treated cells. While the maximum exposure period for our cell lines was two months, increased induction of DNA breakage was visible even after short-term (one-week) e-cig treatment. Our findings therefore pose especially alarming implications for the genotoxic and carcinogenic effects of chronic e-cigarette use. Repeated introduction of DNA strand breaks due to long-term e-cig exposure, accompanied by successive rounds of dysfunctional DNA repair, would generate accumulated mutations and other genomic alterations in an inevitable progression towards cancer.
Moreover, given the immense variability of e-cig designs and usage habits, these potential health risks of e-cigarettes may even be understated. Regulation, quality control standards, and safety assessments for e-cig products have become further complicated by the rapid evolution of new e-cig devices and e-liquid formulations. These newer generations of e-cigs, in contrast to the “first-generation” V2 and VaporFi brands used in our study, often boast larger batteries with adjustable voltages and a wider range of flavors, thus appealing more broadly to e-cig users [29]. Next-generation models have been shown to deliver greater doses of nicotine to users [30], as well as higher levels of hazardous carbonyls and other toxins with increasing voltages [13, 20]. Meanwhile, an increasing number of users report customizing their e-liquids or introducing multi-wick heating coils and multi-chamber atomizers to their e-cig devices, typically to achieve stronger, more concentrated vapors [31]. Varied e-cig usage patterns, such as different puff durations or average “vaping” session lengths, have also been associated with differential absorption of nicotine and e-cig vapor in users [32]. These recent trends in e-cig use and design combinatorially diversify the studies that must still be undertaken to accurately capture all health risks experienced by e-cig users. While focused safety assessments still remain elusive for most of the e-cig user demographic, our study nevertheless suggests that e-cigarettes should be viewed as far from risk-free or harmless in the interim.
Conclusion
In conclusion, our study strongly suggests that electronic cigarettes are not as safe as their marketing makes them appear to the public. Our in vitro experiments employing 2 brands of e-cigs show that at biologically relevant doses, vaporized e-cig liquids induce increased DNA strand breaks and cell death, and decreased clonogenic survival in both normal epithelial and HNSCC cell lines independently of nicotine content. Further research is needed to definitively determine the long-term effects of e-cig usage, as well as whether the DNA damage shown in our study as a result of e-cig exposure will lead to mutations that ultimately result in cancer.
Highlights.
Normal and HNSCC cell lines were cultured in e-cigarette vapor pulled through media
Cell cultures exposed to e-cigarette vapor show increased DNA double strand breaks
E-cigarette vapor induces increased cell death as compared to control
E-cigarette vapor decreases clonogenic survival as compared to control
Acknowledgments
This work was supported by funding from the National Institutes of Health, grant number DE023242 to W.M.O.; by funding from the Department of Veterans Affairs (VA) BLR&D Career Development Award (CDA)-2, award number 1IK2BX001313 to L.C.A.; and by a grant from the Brandon C. Gromada Head and Neck Cancer Foundation to W.M.O. This work was also performed with the support of the Flow Cytometry Core at the UC San Diego Center for AIDS Research (P30 AI036214). The HaCaT cell line was a generous gift from Dr. Victor Nizet at the Center for Immunity, Infection, and Inflammation of the UC San Diego School of Medicine. UMSCC-10B was a gift from Dr. Tom Carey at the University of Michigan, and HN-30 was provided by Dr. JS Gutkind (NIDCR).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regan AK, et al. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 2.King BA, et al. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–27. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notes from the field: electronic cigarette use among middle and high school students - United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62(35):729–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr. 2014;168(7):610–7. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grana RA, Ling PM. “Smoking revolution”: a content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46(4):395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao T, et al. A content analysis of electronic cigarette manufacturer websites in China. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romagna G, et al. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–61. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 8.Goniewicz ML, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meo SA, Al Asiri SA. Effects of electronic cigarette smoking on human health. Eur Rev Med Pharmacol Sci. 2014;18(21):3315–9. [PubMed] [Google Scholar]
- 11.Bertholon JF, et al. Comparison of the aerosol produced by electronic cigarettes with conventional cigarettes and the shisha. Rev Mal Respir. 2013;30(9):752–7. doi: 10.1016/j.rmr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Kang GS, et al. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect. 2011;119(2):176–81. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosmider L, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–26. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson VG. Growth and differentiation of HaCaT keratinocytes. Methods Mol Biol. 2014;1195:33–41. doi: 10.1007/7651_2013_42. [DOI] [PubMed] [Google Scholar]
- 15.Wu W, et al. Identification and validation of metastasis-associated proteins in head and neck cancer cell lines by two-dimensional electrophoresis and mass spectrometry. Clin Exp Metastasis. 2002;19(4):319–26. doi: 10.1023/a:1015515119300. [DOI] [PubMed] [Google Scholar]
- 16.Yeudall WA, et al. Functional characterization of p53 molecules expressed in human squamous cell carcinomas of the head and neck. Mol Carcinog. 1997;18(2):89–96. doi: 10.1002/(sici)1098-2744(199702)18:2<89::aid-mc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Cervellati F, et al. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro. 2014;28(5):999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behar RZ, et al. Identification of Toxicants in Cinnamon-Flavored Electronic Cigarette Refill Fluids. Toxicol In Vitro. 2013 doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Willershausen I, et al. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head Face Med. 2014;10:39. doi: 10.1186/1746-160X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen RP, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–4. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 21.Lerner CA, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams M, et al. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke MS, et al. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb j. 2003;17(10):1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 24.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19(3):169–85. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 25.Yoshie Y, Ohshima H. Synergistic induction of DNA strand breakage by cigarette tar and nitric oxide. Carcinogenesis. 1997;18(7):1359–63. doi: 10.1093/carcin/18.7.1359. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama T, et al. Cigarette smoke induces DNA single-strand breaks in human cells. Nature. 1985;314(6010):462–4. doi: 10.1038/314462a0. [DOI] [PubMed] [Google Scholar]
- 27.Celeste A, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5(7):675–9. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 28.Mao Z, et al. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7(18):2902–6. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yingst JM, et al. Factors Associated With Electronic Cigarette Users’ Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farsalinos KE, et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisko JG, et al. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farsalinos KE, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers) Sci Rep. 2015;5:11269. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]