Abstract
How synaptic-vesicle release is controlled at the basic release structure, the active zone, is poorly understood. By performing cell-attached current and capacitance recordings predominantly at single active zones in rat calyces, we found that single active zones contained 5-218 (mean, 42) calcium channels and 1–10 (mean, 5) readily releasable vesicles (RRVs) and released 0–5 vesicles during a 2-ms depolarization. Large variation in the number of calcium channels caused wide variation in release strength (measured during a 2-ms depolarization) by regulating the RRV release probability (PRRV) and the RRV number. Consequently, an action potential opened ~1–35 (mean, ~7) channels, resulting in different release probabilities at different active zones. As the number of calcium-channels determined PRRV, it critically influenced whether subsequent release would be facilitated or depressed. Regulating calcium channel density at active zones may thus be a major mechanism to yield synapses with different release properties and plasticity. These findings may explain large differences reported at synapses regarding release strength (release of 0, 1 or multiple vesicles), PRRV, short-term plasticity, calcium transients and the requisite calcium-channel number for triggering release.
The function of the nervous system relies on trillions of synapses that display highly heterogeneous release strength and short-term plasticity. Different release strengths and short-term plasticity profiles of synapses1–5 are used for distinct and essential forms of neural computation in brain circuits6. High-release-strength synapses may initially relay spikes from the presynaptic to the postsynaptic neuron with great reliability but tend to exhibit depressed release with ongoing repetitive firing1–3,5. Such synapses may thereby mediate adaptation of cortical responses to sustained visual, olfactory or auditory stimuli6. By contrast, low-release-strength synapses may behave as silent synapses that can be activated during development, learning and memory formation6; these synapses may also mediate high-pass filtering of spikes owing to their propensity to facilitate during high-frequency firing6. More broadly, release impairment might contribute to the generation of various neurological disorders. All these factors emphasizing the importance of various release profiles have motivated two decades of extensive research of the mechanisms by which synapses elaborate different release strengths1–5,7–23. However, the underlying mechanisms remain obscure, largely owing to the lack of techniques to access a single unit of the release structure, the active zone. Here we addressed this issue by developing a technique to patch predominantly single active zones at the release face of the nerve terminal, where we quantified the number of calcium channels and readily releasable vesicles, and their probability of opening or release.
RESULTS
Cell-attached patch recordings at single active zones
In brainstem slices pretreated with the protease papain (2.5 units ml−1, in bath solution for 5–10 min), we partially exposed the calyx (nerve terminal) release face by applying a pressure (~0.5–1.0 p.s.i.) via a pipette between the calyx and the postsynaptic neuron (Fig. 1a). We used the same pipette to form the cell-attached patch at the release face in the ‘rim’ region within ~0–2 µm under the calyx surface (Fig. 1a). The patch was at the calyx because after whole-cell break-in (with an intracellular solution; Online Methods), a 20-ms depolarization to 0 mV induced a calcium current (ICa) of 1.95 ± 0.12 nA (mean ± s.e.m.) and a capacitance jump (ΔCm ) of 371 ± 35 fF in 8 of 8 patches at 2 mM extracellular calcium, which were similar to those obtained without papain pretreatment and pressure application (ICa, 1.98 ± 0.16 nA; ΔCm, 435 ± 35 fF, n = 5; P > 0.05; Supplementary Fig. 1). Thus, papain pretreatment, which made it easier to expose the release face, and the procedure to expose the release face did not affect ICa and release. Compared to our previously reported method, in which the release face was exposed via removal of the postsynaptic neuron24, the present method detected ICa with far greater consistency, a feature essential for the extensive characterization of release profiles in this study.
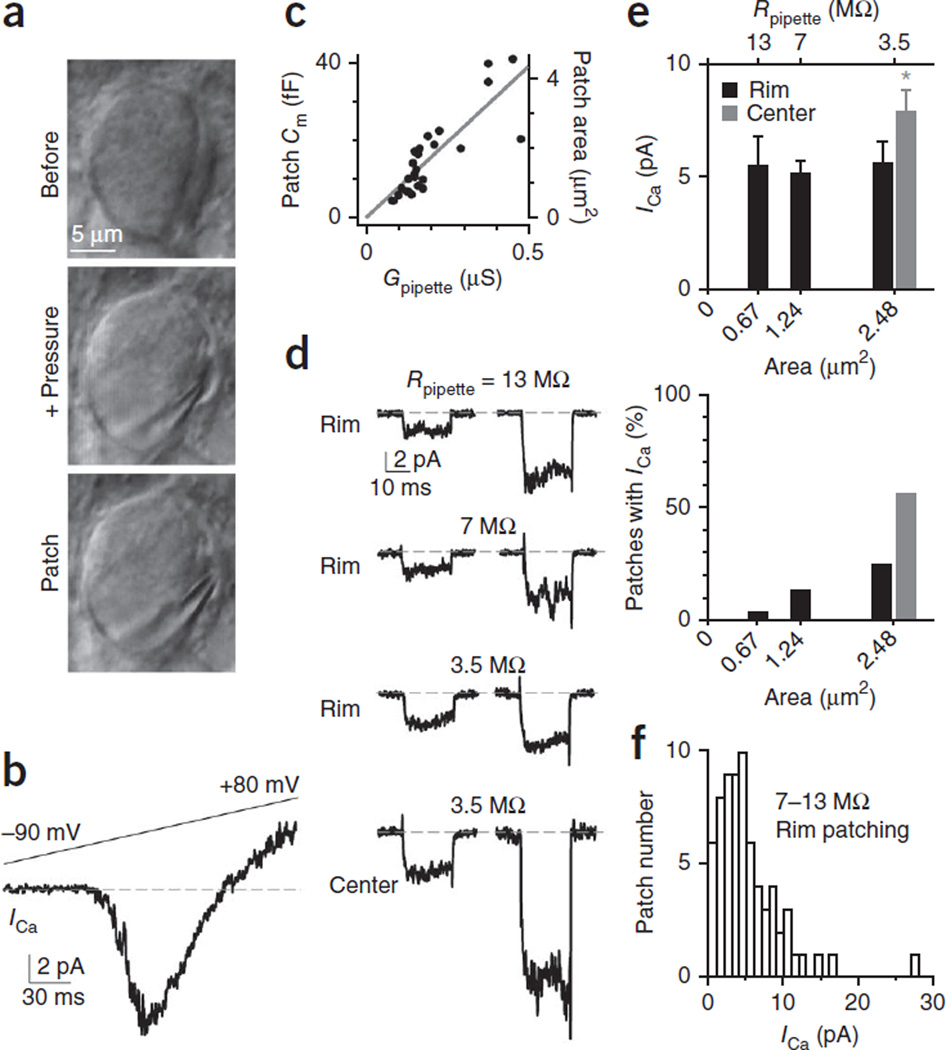
Figure 1.
Cell-attached patch recording of calcium currents at the calyx release face. (a) Differential infrared contrast (DIC) image of a calyx before (top) and after pressure application (middle) via a pipette that partially separated the calyx from the postsynaptic neuron, and after the cell-attached patch at the calyx release face (bottom, same pipette as in the middle). (b) Sampled current response to a ramp voltage from −90 to +80 mV at a cell-attached patch. Dashed line indicates the baseline. (c) Patch Cm and patch area plotted versus the pipette conductance (Gpipette = 1/resistance). The slope of the linear fit was 78 fF µS−1. The specific Cm was 9 fF µ m−2. (d) Sampled ICa induced by a 20-ms depolarization (from −90 to 0 mV) at the rim region (applies if not mentioned) with pipette resistance (Rpipette) of ~13 MΩ, 7 MΩ or 3.5 MΩ and at the central region with ~3.5 MΩ pipettes. All traces were low-pass-filtered at 2 kHz. Each trace was from a different patch. Dashed line indicates the baseline. (e) ICa amplitude (top, mean ± s.e.m.) and percentage (bottom) of patches exhibiting ICa at the calyx rim and central region (>2 µm deeper) are plotted versus the patch mean membrane area or pipette resistance (Rpipette). Top, rim patching: 13 MΩ, n = 12 patches; 7 MΩ, n = 57 patches; 3.5 MΩ, n = 22 patches. Top, central patching: 3.5 MΩ, n = 20. Bottom, rim patching: 13 MΩ, 12/353 patches; 7 MΩ, 57/425 patches; 3.5 MΩ, 22/88 patches. Bottom, central patching: 3.5 MΩn = 20/36 patches. (f) Patch ICa amplitude distribution from 6–8 (n = 57) and 12–14 MΩ (n = 12) pipettes. Patches with 3–4 MΩ (n = 22) pipettes were not included.
With 3–14 MΩ pipettes containing 10 mM calcium and a solution that isolated ICa, depolarization from −90 to 0 mV (these conditions apply in all cases unless mentioned otherwise) induced an inward current of 0.6–27.7 pA in 91 of 866 patches (for example, Fig. 1b). The current was mediated by voltage-dependent calcium channels based on multiple criteria15: current was activated at −37 ± 1 mV, peaked at 1 ± 2 mV, reversed direction at 45 ± 2 mV during a voltage ramp (n = 35 patches, Fig. 1b) and was abolished by including 50 µM cadmium in the pipette (n = 119 patches with no detectable currents, P < 0.001, χ2 test; for example, Supplementary Fig. 2). Because only 11% (91 of 866) of the patches exhibited ICa, and we detected no inward currents at the calyx outer face (not apposing the postsynaptic neuron, n = 89 patches, P < 0.01, χ2 test; Supplementary Fig. 3), calcium channels must be clustered at the release face. These results, together with the findings presented below that release only occurs at patches exhibiting ICa, suggest that patches with ICa contain at least one active zone, each with a cluster of calcium channels.
To gauge more precisely the number of active zones encompassed in a rim patch, we characterized ICa using patch pipettes of different sizes with resistances of ~13 MΩ (12–14 MΩ), 7 MΩ (6–8 MΩ) or 3.5 MΩ (3–4 MΩ). To estimate the patched membrane area of these different pipettes, we first measured the patch membrane capacitance of pipettes with different initial resistance (or conductance; Online Methods). We found a linear relation between the patch capacitance and the pipette conductance with a slope of 78 fF µS−1 (Fig. 1c), consistent with previous measurements25. With this slope and the specific membrane capacitance of 9 fF µm−2 (ref. 26), the mean patch membrane areas for 13-MΩ, 7-MΩ and 3.5-MΩ pipettes were ~0.67 µm2, 1.24 µm2 and 2.48 µm2, respectively. Despite a nearly fourfold increase in the patch area (from 0.67 µm2 to 2.48 µm2), mean ICa amplitudes obtained with 13-MΩ, 7-MΩ and 3.5-MΩ pipettes remained essentially unchanged at 5.5 ± 1.3 pA (n = 12), 5.2 ± 0.6 pA (n = 57) and 5.6 ± 0.9 pA (n = 22), respectively (P = 0.89, ANOVA test, Fig. 1d,e); whereas the percentage of patches exhibiting ICa increased from 3.4% (12/353 patches) to 13.4% (57/425 patches) and 25% (22/88 patches, Fig. 1e). These results suggested that each ICa -containing patch encompassed only one active zone. Otherwise, the mean ICa amplitude should have increased with patch area. The ICa amplitude per patch varied widely (Fig. 1f).
Compared to rim patching with ~3.5-MΩ pipettes, patching at >2 µm below the surface (central region) with ~3.5-MΩ pipettes increased the fraction of patches exhibiting ICa from 25% (22/88 patches, rim patching) to 56% (20/36 patches, χ2 test, P < 0.01) and increased the ICa amplitude from 5.6 ± 0.9 pA (n = 22) to 8.0 ± 0.8 pA (n = 20, Fig. 1d,e, P < 0.03). Thus, patches at the central region likely contain multiple active zones, suggesting that active-zone density is higher in central versus rim regions.
To corroborate these interpretations regarding active-zone prevalence, we analyzed previously published three-dimensional reconstructions of calyces obtained with electron microscopy (Fig. 2a)27 or confocal microscopy (for example, Supplementary Fig. 4)28. Computationally identifying the rim region in electron microscopy data as an ~2-µm-wide swath of calyceal release face abutting the outer face (Online Methods and Supplementary Fig. 5), we found an active-zone density of 0.20 active zones µm−2 (Fig. 2a). By contrast, active-zone density in the central region was considerably larger at 0.56 active zones µm−2 (Fig. 2a). We observed similar differences in active-zone density in confocal microscopy reconstruction of calyces (0.17 active zones µm−2 at the rim area, 0.32 active zones µm−2 at the center, n = 5 calyces, P = 0.012, Supplementary Table 1 and Supplementary Fig. 4).
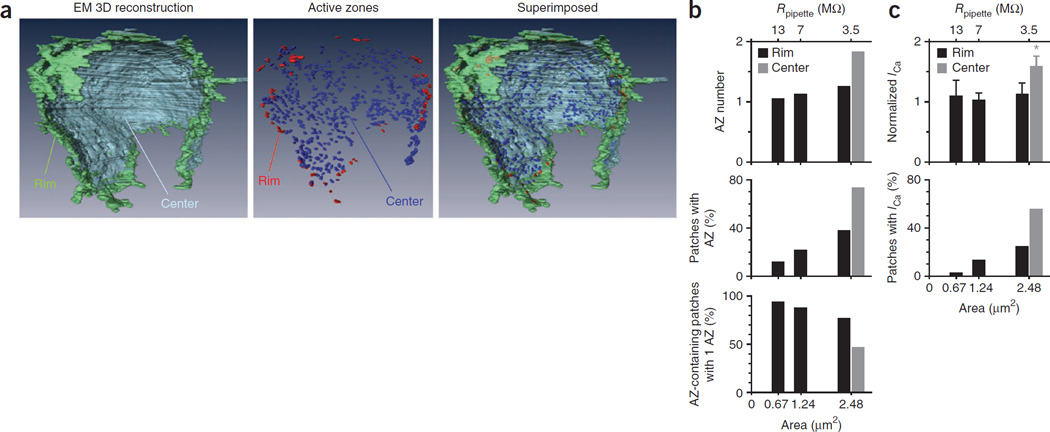
Figure 2.
Active-zone density at the calyx release face matches patch electrophysiology. (a) A calyx three-dimentionally reconstructed with electron microscopy (EM; data from ref. 27) showing rim and center of the calyx release face (left), active zones at the rim and central region (middle; each spot represents an active zone) and the two images superimposed (right). The calyx vertical length is ~18 µm. (b) The mean active zone (AZ) number and percentage of patches containing one or more active zone, and the percentage of patching one active zone in patches containing one or more active zones plotted versus the patch membrane area of 13 MΩ, 7 MΩ and 3.5 MΩ pipettes. Data were obtained from simulation with the rim (0.20 active zone µm−2) or central (0.56 active zone µm−2) active-zone density measured from EM data in a. (c) Normalized ICa amplitude (mean ± s.e.m.) and percentage of patches exhibiting IC at the calyx rim and central region (>2 µm deeper) plotted versus the patch mean membrane area or pipette resistance (Rpipette; 13 MΩ, n = 12; 7 MΩ, n = 57; 3.5 MΩ, n = 22). These plots are the same as those in Figure 1e, except that ICa amplitudes were normalized to the mean ICa obtained with 13-MΩ pipettes for comparison with data in b. We multiplied the normalized value by 1.1 so that the normalized ICa amplitude obtained by the 13-MΩ pipettes was the same as the predicted active-zone number of the corresponding pipette in b.
To determine the probability and the number of active zones being covered by a defined patch membrane size, we performed a numerical simulation using a rim active-zone density of 0.2 active zones µm−2 as derived from the electron microscopy data (Online Methods and Supplementary Fig. 6). With random sampling, our simulation showed that as the patch membrane area increased from 0.67 µm2 (the mean membrane area for 13-MΩ pipettes) to 1.24 µm2 and 2.48 µm2 (for 7-MΩ and 3.5-MΩ pipettes, respectively), the mean active-zone number at active zone-containing patches was rather stable, increasing from 1.1 to only 1.3 active zones per patch, whereas the percentage of patches containing active zones increased by about threefold, and the percentage of patches containing 1 active zone in active zone-containing patches decreased only modestly (Fig. 2b). With the central active-zone density of 0.56 µm−2, simulation with a patch area of 2.48 µm2 (for 3.5-MΩ pipette) predicted a higher percentage of patches containing active zones and higher active-zone number but a lower percentage of getting 1 active-zone in active zone-containing patches when compared to simulation at the active-zone density of 0.20 active zones µm−2 (Fig. 2b). Overall, simulations based on the measured active-zone densities from the reconstructed calyx (Fig. 2a) resulted in outcomes (Fig. 2b) that were closely analogous to those obtained by patch electrophysiology (Fig. 2c, derived from Fig. 1e for side-by-side comparison). We observed a similar match when we used the confocal microscopy data for simulation (0.17 active zones µm−2 at the rim area and 0.32 active zones µm−2 at the center). In fact, the match was better (data not shown) because the active-zone density measured from confocal microscopy data was slightly lower than that measured from electron microscopy data.
The percentage of rim patches showing ICa was lower than the corresponding simulated results (Fig. 2b,c), suggesting that the active-zone density at the patch membrane may be lower than the measured value in the rim region, likely because some presumed rim patch may include the adjacent nonrelease face. This might explain why ICa did not increase, whereas the predicted active-zone number increased as the rim patch area increased (Fig. 2b,c). Alternatively, the increase in predicted active-zone number was too small (only ~18%, from 1.1 to 1.3 active zones) to detect given the large variation of ICa (Fig. 1d,f). Taken together, these results suggest that rim area patching with ~3.5–13-MΩ pipettes contained predominantly single active zones.
To be conservative, we examined single active zones with only ~7–13-MΩ pipettes at the rim region in subsequent experiments and data analysis because simulation predicted higher percentages of patching one active zone using 7–13-MΩ pipettes at the rim region (88–94%, Fig. 2b). ICa recorded with ~7–13 MΩ pipettes at the rim region was 5.3 ± 0.5 pA (n = 69) and ranged from 0.6 pA to 27.7 pA. The wide ICa amplitude distribution (Fig. 1f) reflected the large ICa variation from single active zones.
Single-calcium-channel properties at calyx release face
Knowing the ICa amplitude from the patched active zone (Fig. 1f), we sought to count the calcium-channel number (Nchannel) at the patched active zone based on
| (1) |
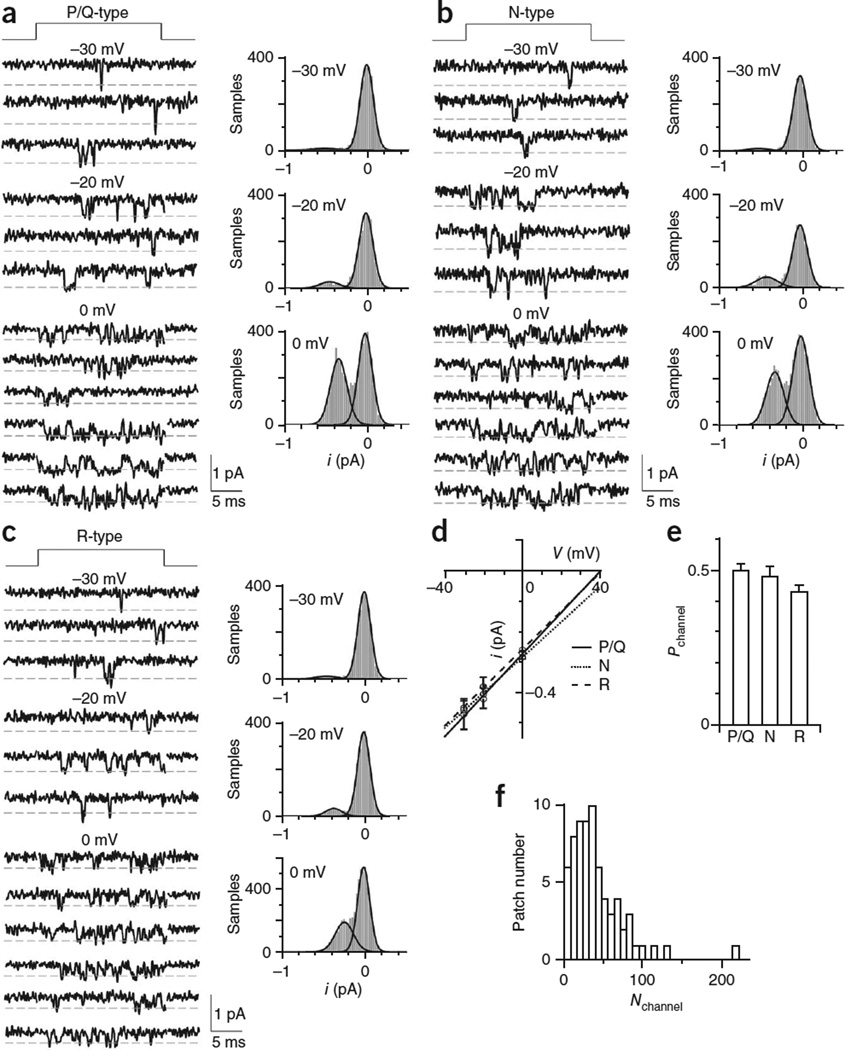
where Nchannel refers to active, but not inactive channels, and imean_ channel (i refers to single-channel current, whereas I refers to current from a population of channels) and Pmean_channel (mean probability of a channel being open) were determined by the following single-channel experiments. In the calyces of postnatal day 8–10 rats we used, P/Q-, N- and R-type calcium channels are all present15. Thus, we used channel type–specific blockers to isolate each type of channel (Fig. 3a–c: see Online Methods for blocker names and concentrations). Depolarization for 20 ms elicited single-channel currents that flickered ‘open’ with durations mostly <3 ms (Fig. 3a–c). For P/Q-, N- and R-type channels, respective single-channel conductances (gchannel) were 6.8 ± 0.2 pS (n = 11 patches), 5.8 ± 0.2 pS (n = 6) and 6.3 ± 0.1 pS (n = 7, Fig. 3d); their elementary-current amplitudes (ichannel) at voltages (V) of 0 mV were 0.27 ± 0.01 pA (n = 11), 0.28 ± 0.01 pA (n = 6) and 0.26 ± 0.01 pA (n = 7, Fig. 3d); and their probabilities of being open (Pchannel) were 0.50 ± 0.02 (51 traces from 11 patches), 0.48 ± 0.03 (33 traces from 6 patches) and 0.43 ± 0.02 (43 traces from 7 patches, Fig. 3e), respectively. As all three types were closely similar in gchannel, ichannel and Pchannel, we obtained the imean_channel (0.27 ± 0.01 pA, n = 3 types) and Pmean_channel (0.47 ± 0.02, n = 3 types) at V = 0 mV without considering their differential contribution. Nchannel, calculated from equation (1) with measured imean_channel, Pmean_channel and ICa, was 42 ± 4 but ranged widely from 5 to 218 (n = 69 patches, Fig. 3f). This distribution represents the active calcium channel distribution at individual active zones. We obtained similar distributions if imean_channel and Pmean_channel for each type were used (Supplementary Fig. 7), suggesting that data in Figure 3f would not be influenced by differential contributions of different types of calcium channels.
Figure 3.
Single calcium-channel conductance and probability of a channel being open (‘open probability’) at the release face. (a–c) Left, sampled single P/Q-type (a), N-type (b) and R-type (c) channel current at indicated voltages (the time of depolarization is shown at the top). Right, all-point histogram of the corresponding traces shown on the left. Curves are Gaussian fits of the data. All traces were low-pass-filtered at 2 kHz. (d) The single-channel i/V curve (mean ± s.e.m.) for P/Q-type (n = 6–11 patches for each data point), N-type (n = 3–6) and R-type (n = 3–7) channels. (e) Pchannel (mean ± s.e.m.) for P/Q-type, N-type and R-type channels 0 mV. (f) Distribution of the patch Nchannel, derived from data in Figure 1f, where ICa was converted to Nchannel using equation (1).
Evoked release at cell-attached patch
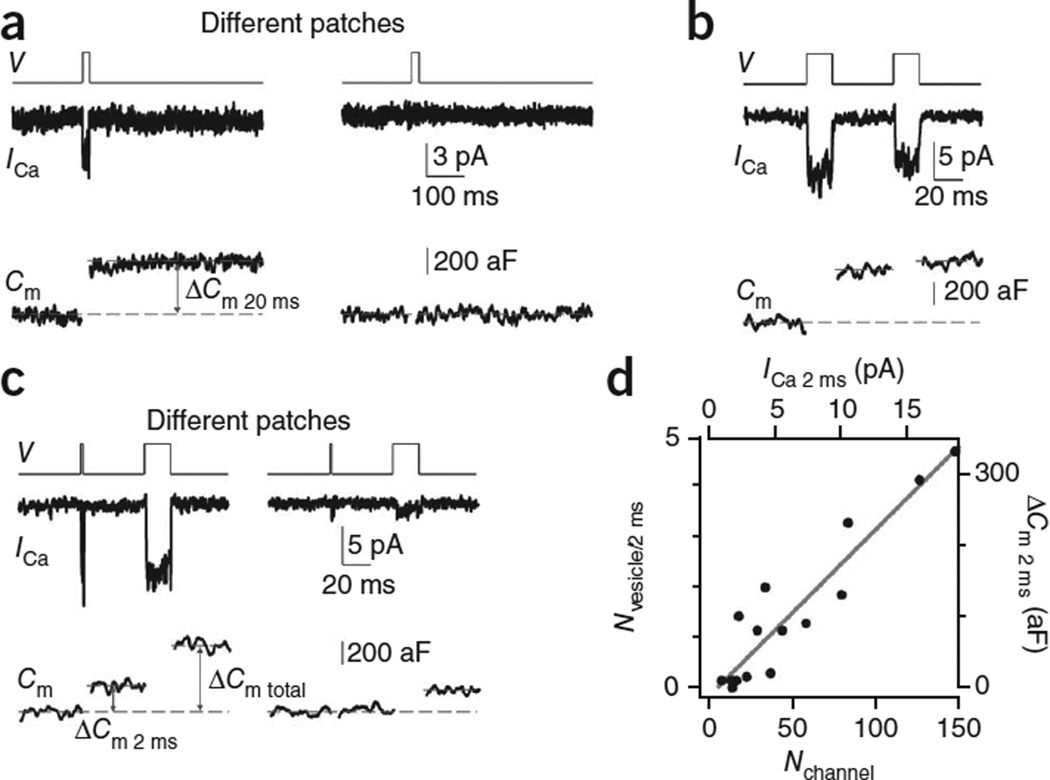
Knowing that Nchannel varied widely at the patched active zone, we studied how this variation affects the release properties at the active zone. We did not include calcium-channel blockers in these experiments. Application of a 20-ms depolarization via the patch pipette induced a capacitance jump (ΔCm 20 ms) of 323 ± 44 aF (n = 10) at patches with detectable ICa (ICa 20 ms ≥ 1 pA), but negligible ΔCm (3 ± 2 aF, n = 226) at patches without detectable ICa 20 ms (< 1 pA, Fig. 4a). For these experiments, the noise in ICa recordings was higher owing to the concurrent capacitance recordings. Typically, we could only detect ICa with 1 pA or larger. However, this should not affect our conclusion, because ICa at most patches was larger than 1 pA when only ICa was measured in low-noise conditions (Fig. 1f). We concluded that release occurred only at patches containing calcium channels. This conclusion supports our suggestion that calcium channels are clustered at the active zone for triggering release.
Figure 4.
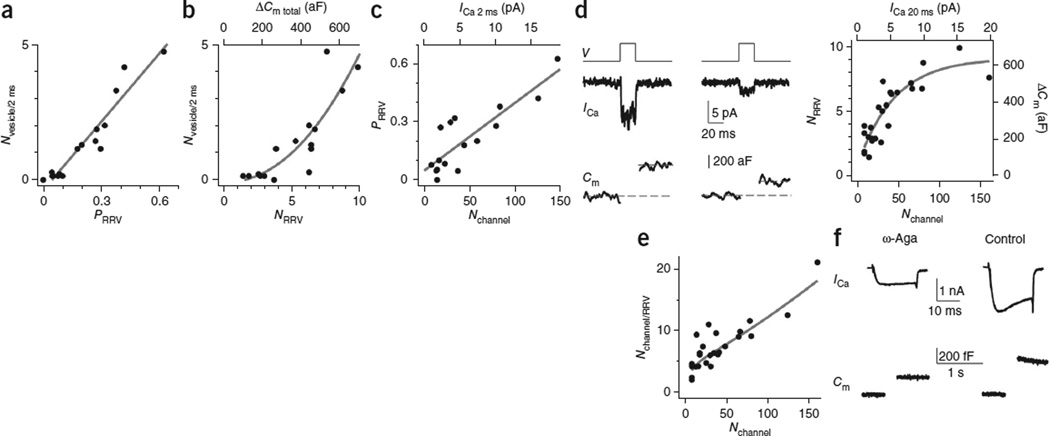
The impact of the calcium-channel number on release strength. (a) ICa (middle) and Cm (lower) induced by a 20 ms depolarization (upper, V) from two patches showing a detectable (left) or undetectable (right) ICa. Dashed lines show how we measured ΔCm. Traces were low-pass–filtered at 1 kHz. The tail current was not as clear as in Figure 1 owing to the higher noise and lower-frequency filtering. (b) Sampled ICa and Cm induced by a pair of 20-ms depolarizations (interval, 50 ms). (c) Sampled ICa and Cm induced by a 2-ms depolarization followed 50 ms later by a 20-ms depolarization from two patches with large (left) and small (right) ICa. (d) Nvesicles/2 ms (ΔCm 2 ms/70 aF) versus Nchannel (calculated from ICa 2 ms using equation (1)). ΔCm 2 ms and ICa 2 ms are also plotted. The line is a linear fit of data.
The 20-ms depolarization depleted the readily releasable vesicles (RRVs), because another 20-ms depolarization applied 50 ms later induced only 17 ± 5% (n = 10) of the first ΔCm (Fig. 4b), similar to observations at the whole-cell configuration16,19,29. As ICa did not decrease as much as the ΔCm during the second depolarizing pulse (Fig. 4b), these results support our previous conclusion that the capacitance jump observed at the cell-attached patch is not a calcium-dependent artifact but reflects exocytosis24,30.
Release strength critically depends on Nchannel
To measure release strength, we applied a 2-ms depolarization to release a fraction of the RRV pool, and a 20-ms depolarization 50 ms later to deplete the remaining RRVs (Fig. 4c). We calculated number of vesicles released by the 2-ms depolarization (Nvesicles/2 ms) as ΔCm 2 ms divided by 70 aF, the vesicle’s mean membrane capacitance at the calyx24,31,32. Nvesicles/2 ms’ which reflected the release strength, ranged from 0 to 5 (Fig. 4d). Nvesicles/2 ms was approximately linearly proportional to the ICa induced by the 2-ms depolarization (ICa 2 ms) or Nchannel (ICa 2 ms/(imean_channel × Pmean_channel)), suggesting that release strength may fundamentally depend on Nchannel (n = 16 patches, Fig. 4d).
In theory, Nchannel in that data shown in Figure 4d should be calculated from ICa 20 ms rather than from ICa 2 ms, because Pmean_channel was measured during a 20-ms depolarization (Fig. 3). However, the amplitude of the ICa during 2-ms depolarization was very similar to that during 20-ms depolarization. Thus, for simplicity of the plot, we used ICa 2 ms instead of the ICa 20 ms.
Nchannel controls RRV release probability and number
How would Nchannel affect the release strength (Fig. 4d)? As Nvesicle/2 ms was the product of the RRV number (NRRV) and PRRV, we addressed this question by first measuring NRRV and PRRV, and then by determining whether these two parameters depend on Nchannel. For the measurement of NRRV, owing to negligible vesicle replenishment, the total ΔCm induced by a 2-ms depolarization and the subsequent 20-ms depolarization applied 50 ms later (ΔCm total = 362 ± 44 aF, n = 16, for example, Fig. 4c) was similar to that induced by a 20-ms depolarization alone (323 ± 44 aF, n = 10; P > 0.1, Fig. 4a,b). Thus, NRRV = ΔCm total/70 aF or NRRV = ΔCm 20 ms/70 aF when the 20-ms depolarization was applied first. Pooling these measurements yielded NRRV = 5.0 ± 0.5 (n = 26) with a range of ~1–10 (see also Fig. 5). For measurements of PRRV, we calculated PRRV as ΔCm 2 ms/ΔCm total from experiments such as those shown in Figure 4c. By analyzing these experiments, we found that Nvesicle/2 ms (or ΔCm 2 ms) was approximately linearly proportional to PRRV (Fig. 5a) and superlinearly proportional to NRRV (Fig. 5b). Thus, Nvesicle/2 ms, an indicator of the release strength, depended on both PRRV and NRRV.
Figure 5.
Impact of calcium-channel number on the release probability and the number of RRVs. (a) Nvesicle/2 ms plotted versus PRRV ( = ΔCm 2 ms/ΔCm total). The line is a linear fit of data from 16 patches like those shown in Figure 4c. (b) Nvesicle/2 ms plotted versus NRRV (ΔCm total/70 aF). ΔCm total (ΔCm induced by 2-ms and 20-ms depolarization) is also shown. The curve is an exponential fit of data (n = 16 patches). (c) PRRV plotted versus Nchannel (n = 16 patches). ICa 2 ms used to derive Nchannel (with equation (1)) is also shown. (d) Left, sampled ICa and Cm induced by a 20-ms depolarization from two patches with different ICa values. Right, NRRV plotted versus Nchannel (n = 26). ΔCm (right) and ICa 20 ms (upper) used to calculate NRRV and Nchannel are also shown. ΔCm included ΔCm total (for example, Fig. 4c, n = 16) and ΔCm 20 ms (for example, left, n = 10) when the 20-ms depolarization was applied alone. The fitted curve (exponential function) shows that NRRV approaches saturation. Dashed lines show how we measured ΔCm. Traces were low-pass–filtered at 1 kHz. (e) Nchannel/RRV plotted versus Nchannel (n = 26, Nchannel/RRV was derived from d, right). The curve was derived from the fitted curve in d, right. (f) Sampled whole-cell ICa and ΔCm induced by a 20-ms depolarization from two calyces, one in the presence of ω-agatoxin-IVA (ω-Aga; 200 nM, bath, left) and the other in control (right). [Ca2+]o = 2 mM.
A plot of PRRV versus Nchannel from 16 patches similar to those shown in Figure 4c revealed an approximately linear relation between PRRV and Nchannel (calculated from ICa 2 ms, Fig. 5c). Thus, increasing the calcium-channel number at the active zone increased the vesicle’s release probability. A plot of NRRV versus Nchannel from 26 patches such as those shown in Figure 4a–c (see also Fig. 5d) revealed a sub-linear relation between NRRV (calculated from ΔCm total or ΔCm 20 ms alone) and Nchannel (calculated from ICa 20 ms, Fig. 5d). Thus, NRRV was larger as Nchannel increased but approached saturation at larger Nchannel (Fig. 5d). Taken together, Nchannel may regulate release strength (Nvesicle/2 ms, Fig. 4d) by regulating both PRRV and NRRV (Fig. 5c,d).
Mechanisms by which Nchannel regulates PRRV and NRRV
How would Nchannel regulate PRRV? A plot of the calcium-channel number per RRV (Nchannel/RRV = Nchannel/NRRV’ calculated from Fig. 5d) versus Nchannel revealed an approximately linear relation between them (Fig. 5e), suggesting that Nchannel regulates PRRV via influencing the calcium-channel number per RRV and thus the calcium microdomain near the RRV during depolarization.
The correlation between Nchannel and NRRV (Fig. 5d) may not necessarily reflect a causal relation but may suggest that more RRVs need more calcium channels to ensure that RRVs are near calcium channels for release. In support of this possibility, when we applied the high-affinity P/Q-type channel blocker ω-agatoxin IVA (200 nM, bath) to reduce the calcium-channel number33, it reduced the whole-cell ICa 20 ms to 38 ± 5% (n = 5) and the whole-cell ΔCm 20 ms, which reflected the RRV pool size16,34, to 58 ± 5% (n = 5) of control at the extracellular calcium concentration ([Ca2+]o) of 2 mM (Fig. 5f).
The relation between the ΔCm 20 ms and the ICa during ω-agatoxin IVA application (Fig. 5f) did not follow the fourth power relation as demonstrated during brief action potential or 1-ms depolarization15,35. This was because the stimulation was a 20-ms depolarization, which depletes nearly the whole RRV pool16,34,36–38. Even when ICa was reduced by about half, which was similar to the ICa reduction in the presence of ω-agatoxin IVA, the RRV pool can still be depleted by a 20-ms depolarization16. Thus, the reduction of ΔCm 20 ms in the presence of ω-agatoxin IVA (Fig. 5f) reflects a reduction of the RRV pool size. In other words, when ω-agatoxin IVA reduced the calcium-channel number33, some readily releasable vesicles were not near functional calcium channels and thus could not be released by calcium influx during depolarization. We concluded that a larger number of calcium channels is needed to maintain a larger RRV pool.
In the whole-cell configuration, the RRV pool depleted by a 20-ms depolarization is composed of two kinetically different subpools of vesicles, a fast and a slow component34,36,39. This raised the question as to whether a 20-ms depolarization could deplete the entire RRV pool. We included 50 µM 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) in the pipette solution, which was close to the endogenous calcium buffer capacity in the 8–10-day-old calyx40,41. In this condition, the slow component of the RRV pool induced by a 20-ms depolarization was only a minor part (less than ~20%)36. As a result, a 20-ms depolarization depleted nearly all vesicles in the RRV pool, which was mostly composed of the rapid component (see fig. 1 in ref. 36). These results are consistent with earlier findings that the slow release component is minor or even absent upon dialysis of the calyx with 50 µM ethylene glycol tetraacetic acid (EGTA)39 but become more prominent in the presence of 200 µM34 or 500 µM39 EGTA. Even with 200 µM EGTA in the pipette solution, a 20-ms depolarization depleted all the rapid component and a large part of the slow component of the RRVs (see fig. 3 in ref. 34). We concluded that in our recording conditions, a 20-ms depolarization depletes nearly all vesicles in the RRV pool.
Calcium-channel number controls paired-pulse plasticity
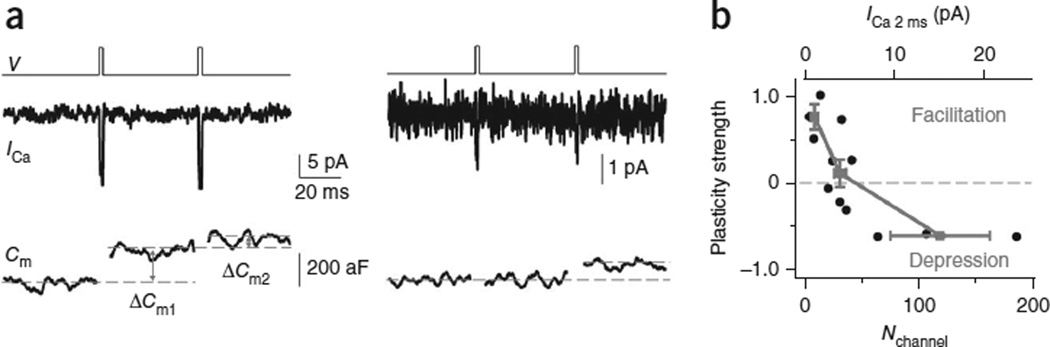
We showed that the release strength was controlled by Nchannel (Fig. 4d) because PRRV, Nchannel/RRV and NRRV depended on Nchannel (Fig. 5c–f). Beyond these baseline release properties, we wondered whether Nchannel would also impact short-term plasticity. Accordingly, we applied two 2-ms depolarizations 50 ms apart (Fig. 6a). We found that depression occurred when Nchannel (or ICa 2 ms) was large (≥64 channels or 8.1 pA; Fig. 6a), facilitation occurred when Nchannel was small (≤13 channels or 1.7 pA, Fig. 6a), and a mixture of depression or facilitation occurred with intermediate ICa 2 ms (Fig. 6b).
Figure 6.
The impact of Nchannel on paired-pulse plasticity and the open channel number during an action potential. (a) ICa and Cm induced by a pair of 2-ms depolarization at 50-ms interval from two patches with large (left) and small (right) ICa (different vertical scales). Dashed lines show how we measured ΔCm. Traces were low-pass-filtered at 1 kHz. (b) Paired-pulse strength plotted versus Nchannel (or ICa 2 ms, top). The paired-pulse strength was calculated as (ΔCm2 - ΔCm1)/(ΔCm2 + ΔCm1), where ΔCm1 and ΔCm2 are the ΔCm induced by the first and the second 2-ms depolarization. We did not use paired-pulse ratio because ΔCm1 was 0 in some patches. Data were also divided into three groups based on ICa 2 ms (≤1.7 pA, 2.6 – 5.2 pA, ≥8.1 pA). Their average values (±s.e.m.) are shown (solid squares). The dashed line indicates zero paired-pulse strength, above which is facilitation and below which is depression.
Our observation that greater depression occurred with larger Nchannel was because larger Nchannel caused larger PRRV (Fig. 5c), resulting in larger depletion of RRVs in the first stimulus (for example, Figs. 4c and 6a). Although we obtained this conclusion with 2-ms depolarization at [Ca2+]o = 10 mM, the same principle should hold for action potential stimulation at [Ca2+]o = 2 mM, except that the curve shown in Figure 6b would be shifted upwards and to the right.
Open calcium channels during an action potential
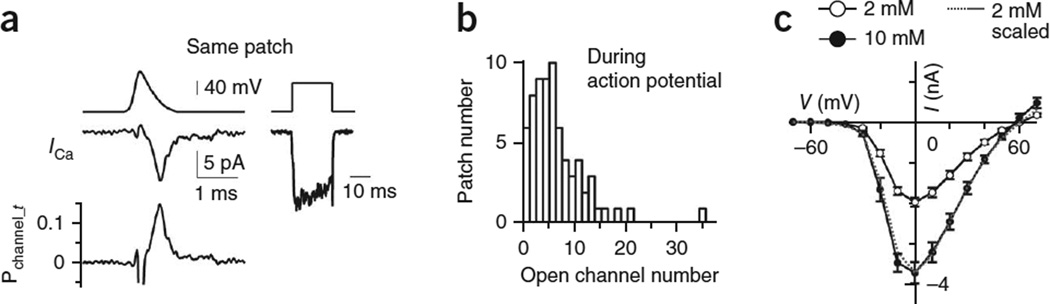
Using step depolarization to activate calcium channels, we observed a profound influence of Nchannel on release properties and short-term plasticity (Figs. 4–6). In physiological conditions, calcium channels are activated by action potentials. To estimate how many calcium channels at an active zone are opened by an action potential, we used an action potential waveform14 to induce ICa (ICa_t, Fig. 7a). For these experiments, we did not record ΔCm because it introduced more noise in ICa recordings and the ΔCm was often too small to be measured reliably. The probability of a channel being open at time t (Pchannel_ t) during the action-potential waveform was calculated based on
| (2) |
where Nopen_channel_t and Vt are the open channel number and voltage at time t during an action potential; Er is the reversal potential (43 mV, Fig. 3d); Pmean_channel/V = 0 mV is the Pmean_channel at V = 0 mV (0.47, Fig. 3e); and ICa V = 0 mV is the ICa induced by a 20-ms depolarization to 0 mV, measured at ~5–10 s before or after the action potential stimulus (for example, Fig. 7a). With this method, we found that the peak Pchannel_t was 0.16 ± 0.02 (n = 5) at V = −45 mV during the action potential-falling phase. With Pchannel_t = 0.16, the data in Figure 3f were converted to the action potential–induced open channel number, which varied from ~0.8 to 35 with a mean of 6.7 per patch (Fig. 7b).
Figure 7.
The open calcium-channel number during an action potential. (a) Left, sampled ICa (middle) and Pchannel_t (lower, calculated from equation (2)) during an action potential waveform stimulus (top). Right, ICa (bottom) induced by a 20-ms depolarization at 5 s after the action potential waveform stimulus (same patch as on the left). (b) Open Nchannel distribution during an action potential, derived from data in Figure 3f (c) The calcium channel I/V curve (mean ± s.e.m.) obtained from whole-cell recordings at 2 mM (n = 5 calyces) and 10 mM (n = 5 calyces) extracellular calcium. The curve at 2 mM was also scaled to that at 10 mM to show similar I/V relation in these two conditions.
Although this action potential waveform measurement was made at [Ca2+]o = 10 mM, we expect similar results at [Ca2+]o = 2 mM because the whole-cell ICa-V curves at 2 mM (n = 5) and 10 mM (n = 5) calcium were amplitude-scale versions of each other (Fig. 7c), thereby excluding appreciable differences in surface-charge screening.
The action potential half-width at [Ca2+]o = 10 mM had been reported to be ~35% wider than at [Ca2+]o = 2 mM42. When we repeated the experiments shown in Figure 7a using a modified action potential waveform with a half-width (0.61 ms) 35% wider than that used for the experiments shown in Figure 7a (0.45 ms), the peak Pchannel_ t was 0.25 ± 0.02 (n = 4, for example, Supplementary Fig. 8). Thus, regardless of the action-potential waveform used, the peak Pchannel_ t was low. Our peak Pchannel_ t (0.16–0.25) was smaller than a prior whole-cell estimate, which was ~69% of the maximum43. However, the maximum probability of channels being open had not been measured in that whole-cell study43. If it was 0.47, as measured here (Fig. 3e), the probability of a channel being open (0.32 = 0.69 × 0.47) was not far from our estimate (0.16–0.25).
DISCUSSION
The present work uncovers the fundamental properties of voltage-dependent calcium channels and transmitter release in single active zones. The work relied on a technique we established to patch predominantly one active zone at the calyx release face. The following arguments strengthen our suggestion that most cell-attached patches do not contain multiple active zones.
If larger NRRV and Nchannel were due to more active zones or a larger fraction of an active zone, NRRV should have been linearly proportional to Nchannel, and PRRV should not have changed with increasing Nchannel. Both predictions contradict our experimental findings (Fig. 5c,d). Furthermore, our patch area (1.24 µm2, ~7 MΩ) was much larger than the area of an individual active zone (~0.05–0.1 µm2)23,27,37, making it unlikely for a patch to contain a fraction of an active zone. Our measured NRRV (5.0 ± 0.5) was similar to the number (~3.3–7) of vesicles anatomically docked within 20 nm from the calyx active-zone membrane27,37. Dividing the whole-cell ICa (3.77 ± 0.25 nA, n = 5, 10 mM extracellular calcium, Fig. 7c) by the reported calyx active-zone number (550)27 yielded a mean ICa per active zone of 6.9 pA, similar to the mean patch ICa we measured (5.3 ± 0.5 pA, Fig. 1f). In conclusion, the technique we developed opens the door to study presynaptic mechanisms at the single active-zone level.
Although only ~2 vesicles contacted the membrane at an active zone within 10 nm from the membrane27, several studies considered vesicles within 20 nm from the membrane as docked vesicles27,37. At the calyx, ~3–7 vesicles per active zone are docked within <20 nm from the plasma membrane27,37, which matched NRRV (~5 vesicles per patch) reported here. Furthermore, based on recordings from the whole calyx (including ~550 active zones), many previous studies showed that there are ~3,000–6,000 vesicles that can be released by a 10–50-ms depolarization, which correspond to ~5–10 RRVs per active zone (≈3,000/550 or 6,000/550)38,39,44,45. This number is much larger than 2 docked vesicles within <10 nm from the active-zone membrane and closer to our estimate of 5 vesicles per patch. These studies suggest that the RRVs released by a 20-ms depolarization may not be identical to the docked vesicles within 10 nm from the plasma membrane, but likely included those docked within 20 nm from the plasma membrane at the calyx.
Calcium-channel number determines release and plasticity
We found that the number of calcium channels at an active zone varied widely from ~5 to 218. This number critically influences release strength (Fig. 4d) by two mechanisms. One mechanism is influence of the RRV release probability (Fig. 5c). As the Nchannel increased, mean Nchannel per RRV increased (Fig. 5e), resulting in an increase in the calcium concentration near the RRV during depolarization and thus an increase of the RRV’s release probability. The other mechanism is ensuring that RRVs are near calcium channels for release (Fig. 5d,f). With these two factors, the number of calcium channels at the active zone may determine whether an active-zone releases 0, 1 or multiple vesicles during a brief 2-ms depolarization (Fig. 4d). By influencing PRRV, Nchannel also critically adjusted the direction and the extent of paired-pulse plasticity at the active zone (Fig. 6). Thus, variation of Nchannel is a major determinant of the heterogeneous release properties and short-term plasticity at single active zones. These results suggest a simple organizing feature of the synapse, whereby controlling Nchannel specifies synapses with diverse release properties. The data shown in Supplementary Figure 9a help to illustrate this point schematically.
Some types of synapses behave like a silent synapse, barely releasing any vesicle, whereas others release multiple vesicles4. Some synapses display short-term facilitation, whereas others, short-term depression9,10. The mechanisms underlying these differences have been intensely studied. At hippocampal and cortical synapses, release strength is proportional to the RRV number1,2, the docked vesicle number and the active-zone area7,8. However, different release strengths are accompanied by similar numbers of docked vesicles at cerebellar synapses3, questioning whether docked vesicles determine release strength. At cortical synapses, different release strengths are correlated with the volume-averaged calcium transient and depend on postsynaptic neurons10. Differences in the calcium transient at cerebellar synapses also have been reported11. However, whether the volume-averaged calcium transient reflects calcium buffers or channel conductance/number remains unclear.
Our finding of Nchannel variation at single active zones may provide an unifying mechanism to explain the heterogeneity of release and short-term plasticity profiles discussed above. Active zones with low Nchannel may behave as a presynaptic silent synapse (Fig. 4d) and show subsequent short-term facilitation (Fig. 6). Active zones with high Nchannel may release one or multiple vesicles (Fig. 4d) and show subsequent short-term depression owing to a large PRRV that causes more depletion of RRVs during repetitive stimulation (Figs. 5c and 6). If Nchannel is low, docked vesicles may not be surrounded by calcium channels and may thus not be releasable (Fig. 5d,f), explaining why release strength is not correlated with docked vesicles at some synapses3. Likewise, a large Nchannel may make all docked vesicles releasable, explaining the correlation between release and docked vesicles7,8.
Heterogeneous PRRV16,18,19 is attributed to the differential distance between calcium channels and RRVs19. Our work suggests that it is due to heterogeneity of active zones having different Nchannel values, which results in different Nchannel per RRV (Fig. 5c,e), and thus different calcium concentrations near the RRV. The seemingly linear relation between PRRV and Nchannel is different from the power relation between release and the calcium concentration5, suggesting that increasing Nchannel raises the calcium concentration near RRVs sub-linearly. The underlying mechanism is unclear. One possibility is that as Nchannel increases, more channels at a longer distance from a releasable vesicle may contribute to the release of it.
Given that Nchannel variation may account for the synapse heterogeneity in releasing 0, 1 or multiple vesicles, calcium transients, short-term plasticity and PRRV, we suggest that the regulation of Nchannel would furnish a simple approach to achieving diverse heterogeneity in synaptic properties. How to regulate Nchannel is not well understood. Recent studies shed light on this issue by showing that Rab3-interacting molecules (RIM) proteins may regulate calcium channels in a population of synapses22,23. Our measurement of Nchannel does not include inactive calcium channels that may be physically present at the active zone. Such inactive calcium channels might be due to regulation by many factors, such as G proteins, soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) proteins, protein kinases and lipids46–48. For example, a recent study showed that reducing the membrane phosphatidylinositol 4,5-bisphosphate (PIP2) may cause reduction of the voltage-dependent calcium current, likely by inactivating calcium channels, within ~100 ms47. Such a dynamic conversion between active and inactive calcium channels might therefore be a potential mechanism to dynamically regulate Nchannel and thus the properties of release and short-term plasticity at individual active zones.
Calcium-channel properties at the active zone
We directly measured and provided basic, yet critical parameters for calcium channels that are pivotal in controlling release at single active zones. How many open calcium channels are needed to trigger release has been debated for two decades: 1–125 channels have been proposed at different synapses11,13,14,20,21,49. This large discrepancy might be partly due to the use of indirect techniques such as imaging and modeling. As a result, it has been unclear how many calcium channels are in an active zone and what fraction is activated by an action potential. The present work showed that an active zone contained ~5–218 calcium channels (mean, 42; Fig. 3f). An action potential opened ~16% or 0.8–35 (mean, 6.7) channels at an active zone (Fig. 7). Thus, one or more than ten channels may open depending on how many channels are at an active zone. They may all trigger release during an action potential, although the release probability with one channel should be much lower than that with more than 10 channels (Fig. 5c and Supplementary Fig. 9). This finding may help to reconcile the large differences or discrepancies in the estimate of the number of open calcium channels required to trigger release.
In addition to measuring Nchannel, we measured the single-channel conductance and current amplitude for P/Q-, N- and R-type calcium channels at the active zone. The values were similar for different types of calcium channels, ranging from ~5.8 pS to 6.8 pS for the single-channel conductance and from ~0.26 to 0.28 pA for the single-channel current at 0 mV (Fig. 3). Given that the whole-cell ICa at 0 mV in 2 mM extracellular calcium was ~53% of that in 10 mM extracellular calcium (Fig. 7c), we can derive the single-channel conductance and current at 2 mM extracellular calcium, at which most previous studies of synaptic transmission were performed. The derived single-channel current at 0 mV in 2 mM extracellular calcium was ~0.14–0.15 pA. This value was on the order of those measured from somatic recordings for N- and L-type calcium channels50, suggesting that the single-channel conductance may not be modified substantially by calcium-channel regulators localized at nerve terminals. In addition, calcium-channel currents flickered with a duration mostly less than 3 ms (Fig. 3). These basic properties of calcium channels at the active zone may help to constrain the parameters used in simulation studies of transmitter release in the calcium micro- or nanodomain.
ONLINE METHODS
Slice preparation and solutions
Animal care and use were carried out in accordance with US National Institutes of Health (NIH) guidelines and approved by the National Institute of Neurological Disorders and Stroke Animal Care and Use Committee. Parasagittal brainstem slices (200 µm thick) containing the medial nucleus of the trapezoid body were prepared from 8–10 d old Wistar rats using a vibratome24. For preparing slices, we used a solution containing (in mM): 95 NaCl, 25 NaHCO3, 25 glucose, 50 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.1 CaCl2, and 3 MgCl2, 0.4 ascorbic acid, 3 myo-inositol and 2 sodium pyruvate (95% O2/5% CO2). Slices were incubated for 30 min at 37 °C, during which the protease papain (2.5 unit/ml) was applied for 5–10 min and then held at room temperature (22–24 °C) for recordings with solutions described below.
For cell-attached recordings, the bath solution containing (in mM): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 dextrose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 0.001 tetrodotoxin (TTX; for preventing action potentials), pH 7.4 when bubbled with 95% O2 and 5% CO2. The cell-attached pipette contained (in mM): 10 CaCl2, 10 HEPES and 145 TEA-Cl, pH 7.2, adjusted with TEA-OH (osmolarity was 290–310 milliOsm).
For whole-cell recordings of ICa and ΔCm (Figs. 5f and 7c and Supplementary Fig. 1), the bath solution contained (in mM): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 dextrose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 0.001 TTX and 0.1 3,4-diaminopyridine, pH 7.4 when bubbled with 95% O2 and 5% CO2. The pipette contained (in mM): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES and 0.05 BAPTA, pH 7.2, adjusted with CsOH.
For whole-cell current clamp recordings, the bath solution contained (in mM): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 dextrose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate and 0.001 TTX, pH 7.4 when bubbled with 95% O2 and 5% CO2. The pipette contained (in mM): 125 K-gluconate, 20 KCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES and 0.05 BAPTA, pH 7.2, adjusted with KOH.
Electrophysiology
EPC-10 amplifier (HEKA electronics) was used for whole-cell recordings29, whereas the Axopatch 200B (Molecular Devices) was used for cell-attached ICa recording, which provided a low noise for detection of single channel currents. To achieve low-noise recording, the gain was set at 100 pA/mV. Patch pipettes were pulled from Quartz glass (QF150-75-7.5, Sutter Instrument) with P2000 laser puller (Sutter Instrument) and coated with Sylgard.
For the cell-attached recording, the resting potential was assumed to be −65 mV which was the mean resting potential (-65 ± 2 mV, n = 7) measured at the whole-cell current-clamp mode with the same bath solution. The liquid junction potential was −5 mV, as calculated from the pCLAMP software (Molecular Devices). The resting holding potential was −90 mV at the patch, which took into account of the liquid junction potential (-5 mV) and the resting membrane potential (-65 mV).
For simultaneous ICa and Cm recordings, EPC-8 amplifier (HEKA) and SR830 2-phase lock-in amplifier (Stanford Research Systems) were used. A 20-kHz, 50–200-mV r.m.s. sine wave was superimposed on a command potential. The in-phase and 90°-out-of-phase current outputs of the lock-in amplifier were low-pass filtered at 0.3–1 ms (24 dB). Cell-attached capacitance recordings, phase adjustment, detection and measurement of capacitance up and down steps, and measurements of the patch membrane capacitance were performed as described previously24.
Cell-attached recordings typically lasted for 30–60 s, after which ICa and/or ΔCm ran down with an unknown reason. The fast rundown might explain why ΔCm has never been induced by depolarization applied via the cell-attached patch at any secretory cell. The present work was, to our knowledge, the first to report the ΔCm induced by depolarization via the cell-attached patch. We only analyzed the data collected in the first minute without substantial rundown. The interval between each stimulus was 3–5 s, at which calcium current inactivation was minimal29. The limitation of the recording time, together with the requirement of high signal-to-noise ratio in recording, made our data collection a long and hard process. As a result, one patch could only be used for one type of experiments. For example, data shown in Figures 1, 3, 4, 5, 6 and 7 were from different experiments.
To measure the patch membrane (Fig. 1c), the electrode at the cell-attached configuration was retracted a few micrometers to achieve an inside-out configuration24. A piece of Sylgard was moved toward the pipette, and the tip was pressed a few micrometers into the Sylgard, which resulted in a decrease of the capacitance. When the pipette was retracted from the Sylgard, the capacitance returned to the previous baseline level. The capacitance change during these manipulations was taken as the patch membrane capacitance24.
Statistical analysis
Data are presented as mean ± s.e.m. Unless mentioned, the statistical test was the t-test.
Measurements of single channels and ‘open probability’
For recording single P/Q-type channel currents (Fig. 3a), the pipette contained 5 µM ω-conotoxin-GVIA, 1 µM SNX-482, and 10–50 nM ω-agatoxin-IVA, the latter of which blocked most, but not all P/Q-type channels, which increased the chance of detecting single channels. In addition, the bath contained 1 µM ω-conotoxin-GVIA, 200 nM SNX-482 and 10–50 nM ω-agatoxin-IVA. For recording single N-type channel currents (Fig. 3b), the pipette contained 1 µM ω-agatoxin-IVA and 1 µM SNX-482; the bath contained 200 nM ω-agatoxin-IVA and 200 nM SNX-482. For recording single R-type channel currents (Fig. 3c), the pipette contained 1 µM ω-agatoxin-IVA and 5 µM ω-conotoxin-GVIA; the bath contained 200 nM ω-agatoxin-IVA and 1 µM ω-conotoxin-GVIA.
When blockers of all three types were included in the pipette (1 µM ω-agatoxin-IVA, 5 µM ω-conotoxin-GVIA and 1 µM SNX-482) and in the bath (200 nM ω-agatoxin-IVA, 1 µM ω-conotoxin-GVIA and 200 nM SNX-482), we did not detect single inward channel currents in 91 patches at the release face. This confirmed that single inward channel currents observed in the presence of two types of voltage-dependent calcium channel (VDCC) blockers (Fig. 3) reflected the remaining type of VDCCs. Similar conductance for P/Q, N- and R-type channels observed in Figure 3 may not be surprising because similar ranges have been reported for these channel types (for example, N-type, 13 pS; P/Q-type, 9–19 pS in 110 mM barium)51,52.
If the all-points frequency histogram taken from recorded current traces showed a clear single peak, the amplitude of which was similar to that judged by eye from recorded traces, the current was identified as the single-channel current, and the peak current (Fig. 3a–c) was ichannel. Otherwise, data were not used. The single-channel conductance ( gchannel) was obtained by linear regression fit of the single channel i/V curve (Fig. 3d). During single-channel recording, Pchannel (Fig. 3e) was calculated as the mean current during 20-ms depolarization to 0 mV (integral of the current from 2 ms to 19.5 ms divided by 17.5 ms) divided by ichannel, the single channel current amplitude at V = 0 mV Owing to the limited time of patching, only 1–2 traces (one trace corresponded to currents in response to a 20-ms depolarization) were obtained in some patches. Thus, the mean Pchannel was calculated from all traces in all patches, which was similar to the mean value from patches where more than four traces were collected.
Measurement of the active zone density
We measured the active zone density at the rim region, ~0–2 µm below the calyx surface where we patched (Fig. 1a), from two published data sets of 3D reconstructed calyces27,28. One data set was based on calyces expressing mGFP to visualize the membrane and anti-Bassoon and anti-Piccolo three-dimensional immunolocalization to visualize the active zones with confocal microscopy28. The other was derived from a serial sectioning electron microscopy reconstruction and manual tracing of the membrane and active zones. The latter data set was converted into a binary image stack to permit application of the same algorithms to both data sets.
The rim area was identified using a weakly supervised perceptual segmentation algorithm described below. Binary calyx image stacks generated by the algorithm were imported into the visualization software Amira 4.1.1 (Mercury Computer Systems). Active zones positioned in the central volume (including those with a larger fraction in the central region) were classified as central active zones, and those in the rim volume belonged to rim active zones. The active zone numbers were calculated using the ConnectedComponents function of Amira. The volume and surface were determined using the SurfaceGen function of Amira with no filtering. The surface of each compartment was subtracted by the connecting surface generated by cutting the original calyx into two compartments. The calyx surface oriented toward the postsynaptic cell was approximated by dividing the surface of center and rim by 2. Active-zone densities were calculated as the active zone number divided by the rim or the central surface area.
The weakly supervised perceptual segmentation algorithm was composed of three steps. First, using an image sequence of calyx sections, a sliding window was moved along both x-y and z directions in the image sequence to cluster and classify the whole image into two subgroups: the rim and the central group. The sliding window measures a set of properties for each labeled region on each calyx section. The measured properties were stored in a structured array, which denoted and described different measurements for each region (for example, perimeter, area and orientation). Then we applied a clustering method to classify the calyx sections into two subgroups corresponding to rim and central regions.
Second, we considered the labeled calyx region in each section as the composite of rim regions R and central regions C in linear blending of intensity values based on ith pixel-level information. The sum of rim αR and central parts (1 - α)C was equal to the entire labeled calyx region (E),
| (3) |
where α is the fraction of the rim and (1 - α) is the remaining part of the central region. This equation simplifies computational complexity and avoids oversegmentation. The α is crucial to define the size of the rim regions and is derived from the size of the calyx section and its shape (Supplementary Fig. 5a).
To extract the correct position of two ending points for defining and segmenting the rim, we developed a skeleton-based ending point search algorithm that can identify ending points. A medial axis–based skeleton is a curve locally centered with respect to the shape boundary and the capture of the shape topology (Supplementary Fig. 5b). The smoothed medial axis along the shape of a calyx section was extracted by using anisotropic diffusion and morphological image processing (Supplementary Fig. 5c). This way, individual pieces of calyx regions can be connected into one continuous calyx region, which is useful for searching only two ending points.
Third, we applied the weakly supervised global visual quality control for segmenting rim and center. The weakly supervised control requires less human efforts and provides less bias than traditional supervised methods. In equation (3), α is controlled by measuring the size of neighborhood regions of each section. The α is then used to control the size of radius of a labeling circle (Supplementary Fig. 5d). The rim part is defined as the inside of the circle. However, owing to the variations and complexity of each calyx section, often more than two ending points were found per section (Supplementary Fig. 5c). To solve this problem, we manually selected the two correct ending points as labeled information and then propagated the label information along the z-direction through the whole image stack. The selected ending points were used as seed information to perceptually segment the rim and central parts.
Estimate of the probability of patching at the active zone
Simulation was performed using a custom-made Program (Igor Pro) with a square of 1,000 µm2 (31.6 µm × 31.6 µm) to represent the calyx release face (Supplementary Fig. 6), whose surface area was reported to be ~1,000–1,400 µm2 (refs. 27,28). We used a dot to represent an active zone (Supplementary Fig. 6) because our patch-membrane area (0.67 µm2, 1.24 µm2 or 2.48 µm2) was much larger than the active zone size (0.05–0.1 µm2) and the calcium channels might cluster in even a smaller area in the active zone53. The active zone position was randomly generated inside the 1,000 µm2 area. The mean active zone density was set as the measured mean active zone density at the calyx rim area or the center (Supplementary Table 1).
The patch membrane was set as round with an area of 0.67 µm2, 1.24 µm2 or 2.48 µm2, the measured mean patch membrane area for the 12–14, 6–8 or 3–4 MΩ pipettes. The position (center) of the round patch membrane was generated randomly within the 1,000 µm2 square for 100,000 times. For each time, if an active zone dot was within the round patch membrane, that active zone was counted as within the patch membrane. The results obtained from these 100,000 repeats were then summarized (Fig. 2b).
Supplementary Material
Acknowledgments
We thank K. Sätzler and J. Lübke for providing the electron microscopy data for analysis, and J. Diamond, J. Xu and M. Baydyuk for comments on the manuscript. This work was supported by the US National Institute of Neurological Disorders and Stroke Intramural Research Program.
Footnotes
Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS
J.S. performed most experiments, designed experiments and participated in writing the paper. L.H. designed experiments and developed the method of patching single active zones. L.X. recorded whole-cell calcium currents in the presence of the P/Q-type channel blocker. F.L. participated in designing experiments. H.Z. and T.K. performed computational analysis of the active-zone density. D.T.Y. ensured low-noise single-channel recordings and contributed to experimental design. W.S. and T.S. assisted the project. L.-G.W. designed experiments, supervised the project and wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 2.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 3.Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J. Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 5.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 6.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 7.Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurorsci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 9.Reyes A, et al. Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 10.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- 11.Brenowitz SD, Regehr WG. Reliability and heterogeneity of calcium signaling at single presynaptic boutons of cerebellar granule cells. J. Neurosci. 2007;27:7888–7898. doi: 10.1523/JNEUROSCI.1064-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 14.Borst JGG, Sakmann B. Calcium infux and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 15.Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurorsci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu LG, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- 17.Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 18.Moulder KL, Mennerick S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons. J. Neurosci. 2005;25:3842–3850. doi: 10.1523/JNEUROSCI.5231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadel K, Neher E, Sakaba T. The Coupling between Synaptic Vesicles and Ca(2+) Channels Determines Fast Neurotransmitter Release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J. Neurosci. 2006;26:13240–13249. doi: 10.1523/JNEUROSCI.1418-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat. Neurosci. 2010;13:19–21. doi: 10.1038/nn.2461. [DOI] [PubMed] [Google Scholar]
- 22.Kaeser PS, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 25.Sakmann B, Neher E. Geometric parameters of pipettes and membrane patches. In: Sakmann B, Neher E, editors. Single-channel Recording. Plenum Press; 1995. pp. 637–650. [Google Scholar]
- 26.Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys. J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sätzler K, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J. Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dondzillo A, et al. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. J. Comp. Neurol. 2010;518:1008–1029. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 30.He L, et al. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009;459:93–97. doi: 10.1038/nature07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- 32.Wu XS, et al. The origin of quantal size variation: vesicular glutamate concentration plays a significant role. J. Neurosci. 2007;27:3046–3056. doi: 10.1523/JNEUROSCI.4415-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of held synapse. J. Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J. Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- 36.Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J. Neurosci. 2009;29:11038–11042. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taschenberger H, Leao RM, Rowland KC, Spirou GA, Von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 38.Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynatpic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 39.Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 40.Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J. Physiol. (Lond.) 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helmchen F, Borst JGG, Sakmann B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys. J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 43.Borst JGG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J. Physiol. (Lond.) 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neher E, Sakaba T. Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of held. J. Neurosci. 2001;21:444–461. doi: 10.1523/JNEUROSCI.21-02-00444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wölfel M, Schneggenburger R. Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J. Neurosci. 2003;23:7059–7068. doi: 10.1523/JNEUROSCI.23-18-07059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanley EF, Mirotznik RR. Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature. 1997;385:340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- 47.Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67:224–238. doi: 10.1016/j.neuron.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. J. Neurosci. 2011;31:11268–11281. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber AM, et al. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat. Neurosci. 2010;13:1348–1350. doi: 10.1038/nn.2657. [DOI] [PubMed] [Google Scholar]
- 51.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 52.Usowicz MM, Sugimori M, Cherksey B, Llinás RR. P-type calcium channels in the somata and dendrites of adult cerebellar purkinje cells. Neuron. 1992;9:1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- 53.Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of held governed by nonuniform channel-vesicle topography. J. Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.