Abstract
Objective:
We aimed to describe the temporal variation in circulating lipid levels among patients with intracerebral hemorrhage (ICH) and investigate their association with ICH risk.
Methods:
This was a single-center, retrospective, longitudinal, case-control analysis using cases drawn from an ongoing cohort study of primary ICH and controls drawn from a hospital-based clinical data registry. Piecewise linear mixed-effect random coefficient models were used to determine the significance of changes in serum lipid trends on ICH risk.
Results:
Two hundred twelve ICH cases and 301 control individuals were analyzed. Overall trends in serum total cholesterol (TC) and low-density lipoprotein (LDL) levels differed between ICH cases and non-ICH controls (p = 0.00001 and p = 0.0092, respectively). Patients with ICH experience accelerated decline in serum TC and LDL levels during 6 months immediately preceding ICH, compared with levels between 6 and 24 months pre-ICH (TC: −29.25 mg/dL, p = 0.001; LDL: −21.48 mg/dL, p = 0.0038), which was not observed in non-ICH controls. Subgroup analysis confirmed that this phenomenon cannot be attributed to statin or alcohol exposure. Serum triglycerides and high-density lipoprotein trends did not differ between groups.
Conclusions:
Longitudinal lipid levels differ between ICH cases and non-ICH controls, most notably for a decline in serum TC and LDL levels within 6 months preceding primary ICH, independent of statin or alcohol use. These changes in serum TC and LDL trends suggest a biological pathway that precipitates ICH occurrence. Further studies are needed to replicate these results and characterize rate of change in serum lipids as a potential biomarker of impending acute cerebral injury.
Growing evidence supports a paradoxical role of dyslipidemia in cerebrovascular disease.1–6 Antithetical to its role in ischemic stroke, hypercholesterolemia has been associated with decreased primary intracerebral hemorrhage (ICH) risk,7–10 reduced number of cerebral microhemorrhages,11 reduced hemorrhagic conversion after ischemic strokes,12 and improved ICH outcomes.13,14 However, the relationship between serum lipid levels drawn at the time of ICH to individual patients' long-term lipid levels is unclear and likely not representative of long-term exposures.8 Estimates of variation coefficients for serum lipid concentrations range from 5% to 25%,15 reflective of considerable variation due to biological and environmental factors.15–19 Serum lipid levels also undergo considerable variation during acute illness,20–22 hypothesized to be due to catecholamine stress response.21,23 For example, serum cholesterol levels decline precipitously within days after either acute ischemic or hemorrhagic stroke,8,23,24 then subsequently increase above those drawn at the time of stroke by 90 days after the event.8 Given these inter- and intraindividual variabilities, understanding of temporal serum lipid trends in ICH may improve our understanding of the biology of dyslipidemia in ICH and provide better estimates of long-term lipid exposures toward cerebrovascular disease risk.
Our objectives were as follows: (1) to describe temporal trends in serum total cholesterol (TC), low-density lipoprotein (LDL), triglycerides (TGs), and high-density lipoprotein (HDL) over 48 months, before and after ICH; (2) to determine differences in serum lipid trends between patients with ICH and non-ICH controls; and (3) to investigate whether changes in serum lipid trends are associated with increased ICH risk.
METHODS
Study design.
We conducted a single-center, retrospective, longitudinal study utilizing case-control design comparing patients with ICH with patients who were hospitalized for acute noncerebral illnesses as controls (figure 1). Serum lipid values (TC, TGs, LDL, and HDL) for included participants were extracted from review of hospital electronic medical records (EMRs) for all available measurements falling within the interval 2 years before or after the acute event. To make comparisons between the variation in serum lipid trends before and after a random event (hospitalization for acute noncerebral illness vs ICH), individual hospitalization events were time-locked into a balanced design with time periods before and after the acute hospitalization event subdivided into 6-month time intervals. Serum lipid values were segregated into their respective time intervals based on timing of the measurement in relation to the date of acute hospitalization. Means were obtained for repeated serum lipid measurements within the same time interval.
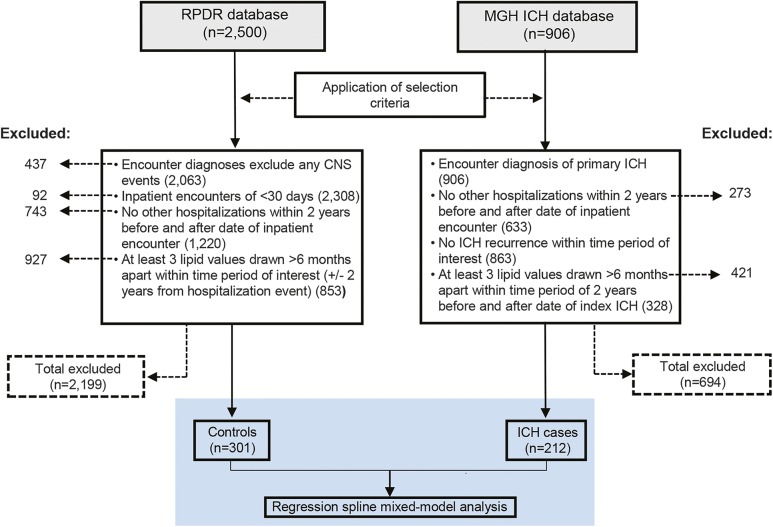
Figure 1. Flowchart describing study cohorts and analysis plan.
ICH = intracerebral hemorrhage; MGH ICH Database = Massachusetts General Hospital–based patients in the Genetics of Cerebral Hemorrhage on Anticoagulation Study; RPDR = MGH Research Patient Data Registry.
Patient selection.
Cases.
Participants (n = 212) were drawn from a previously described, ongoing, longitudinal cohort study of primary ICH.25 Briefly, study participants were patients aged 18 years and older presenting to the Massachusetts General Hospital (MGH) emergency department between June 1993 and June 2014 with imaging-confirmed diagnosis of primary ICH. Exclusion criteria included presence of trauma, brain tumor, hemorrhagic transformation of cerebral infarction, vascular malformation, or any other cause of secondary ICH. Eligibility for participation included survival at 2 years after ICH and at least 3 serum lipid values for each lipid fraction of interest (TC, TGs, LDL, HDL) drawn >6 months apart within 2 years before and after the date of diagnosis. Participants with recurrent ICH or other non-ICH hospitalization events during the time period of interest were excluded to minimize confounding from decreases in serum lipids as part of an acute phase response.8,20–24 Clinical data recorded include information on demographics, medical history, pre-ICH medication use, and health maintenance (cigarette smoking and alcohol use). Statin use for the pre-ICH period was determined at the time of ICH presentation via patient-based questionnaires and scored as “yes/no” variables with constancy assumed for the duration of the study period. The validity and reliability of the questionnaire-based statin use response were internally assessed by comparisons with detailed medication history obtained from hospital EMRs search. A 10% random sampling of the enrolled cohort showed 100% concordance for no statin use.
Controls.
Controls (n = 301) were patients aged 18 years and older admitted to MGH within the same time period, selected via database query from an MGH-affiliated clinical data registry.26 Control selection utilized similar eligibility criteria with an additional criterion of including only patients with inpatient encounters of fewer than 30 days. Patients with any cerebral-related encounter diagnoses and elective admissions were excluded. Clinical data containing covariates of interest identical to cases were concurrently extracted from EMRs and analyzed similarly.
Standard protocol approvals, registrations, and patient consents.
This study was performed with approval of the MGH institutional review board. Data request from the MGH Research Patient Data Registry containing individual-level information was obtained within the confines of the approved institutional review board protocol. Patients with ICH provided informed consent for study participation.
Statistical methods.
Continuous numeric variables were expressed as mean ± SD. Categorical variables were compared using Fisher exact test and continuous variables using Mann–Whitney rank sum or unpaired t test as appropriate. We used a piecewise linear mixed-effects random coefficient model to test the significance of changes in serum lipid trends on ICH risk using acute illness as a critical period on temporal patterns of individual serum lipid fractions at specific time intervals that are fixed in relation to the critical period.27 This allowed modeling of rates of change in mean serum lipid levels in predetermined time intervals of interest to differ within and between cases and controls (e-Methods on the Neurology® Web site at Neurology.org). With the assumption that the time interval immediately before acute illness is likely of most biological relevance, fixed-knots were used to mark transitions in time periods of interest corresponding to the time period 6 to 24 months before acute illness and the time period immediately prior to the acute event (0–6 months before acute illness) (figure e-1). All covariates where p < 0.10 in univariate comparisons or with known potential to influence serum lipid levels were entered into the model. Case-control status and covariates including demographics, clinical, and medication history were included as fixed effects. Inter- and intraindividual variation in serum lipid levels were modeled as random effects. Model validity was examined using the likelihood ratio test. Unstructured covariance was used as the covariance structure model. Comparisons of the difference in rate of change in serum lipid levels (slope) between time intervals of interest in cases and controls were made using the Wald test. Significance threshold was set at p < 0.05 (2-tailed) for univariate analysis and p < 0.0125 (Bonferroni correction for 4 tests) for individual serum lipid fraction mixed-model analyses. All statistical analyses were performed using STATA 10.0 (StataCorp LP, College Station, TX).
RESULTS
Cohort characteristics.
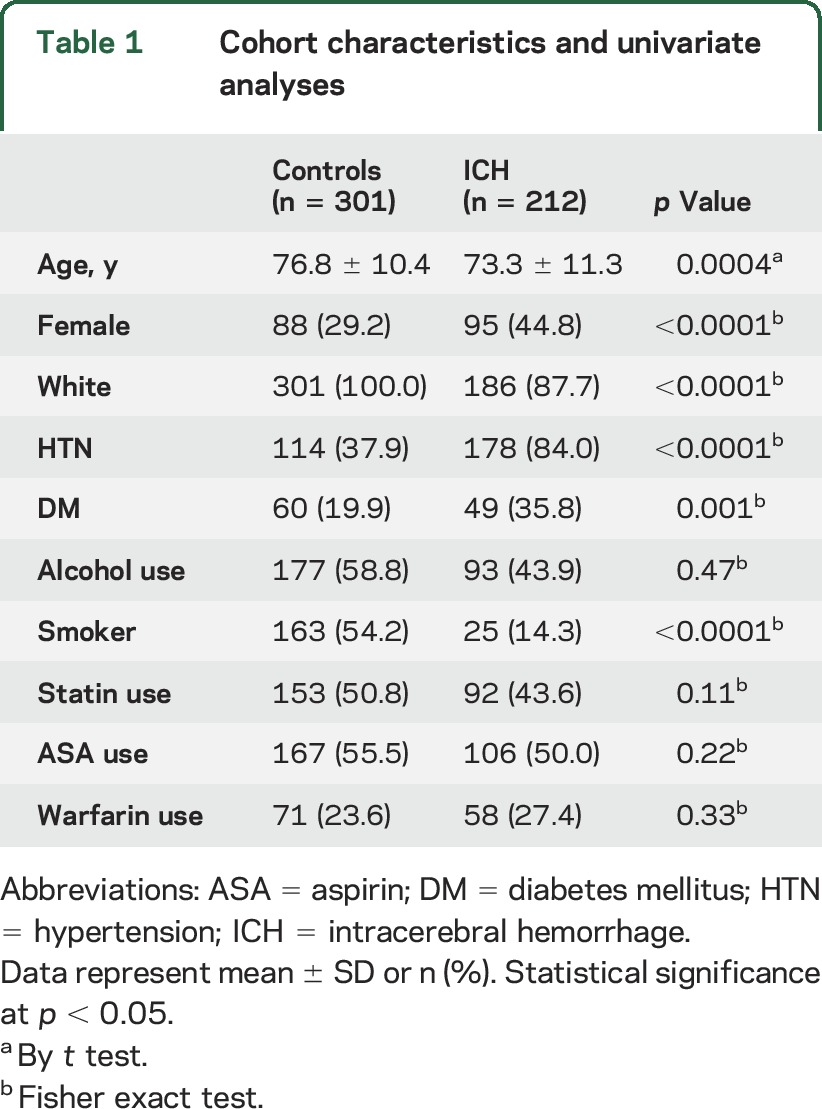
A total of 212 ICH cases and 301 controls with noncerebral acute illnesses were available for analysis (figure 1). Three hundred fifty-one individuals with ICH were removed because of absence of serum lipid data. There were no significant differences in demographics, pre-ICH medical history, or medication use between included and excluded ICH individuals to suggest significant bias from study selection criteria (table e-1). There were significant differences for age, sex, race, smoking status, diabetes, and hypertension between patients with ICH and controls. Risk of coagulopathy represented by rates of aspirin and warfarin use were similar in both groups (table 1). Among cases, ICH location was lobar in 47.6%, with the remainder located in the deep hemispheres and brainstem. Measures of ICH severity were available in 190 patients with ICH. There was a preponderance of mild to moderate ICH cases with admission ICH volume of <30 mL (77.9%) and Glasgow Coma Scale score of >8 (81.6%). Statin exposure pre-ICH did not differ between ICH cases and controls, as well as across ICH subgroups (p = 0.97).
Table 1.
Cohort characteristics and univariate analyses
Temporal trends in serum lipid levels in ICH and acute noncerebral illness.
The number of serum lipid measurements per participant was similar for each time interval in the pre–acute illness period between ICH cases and controls. Conversely, the number of serum lipid measurements was unbalanced in the post–acute illness period, with 25% to 50% fewer measurements per individual in patients with ICH compared with controls (table e-2).
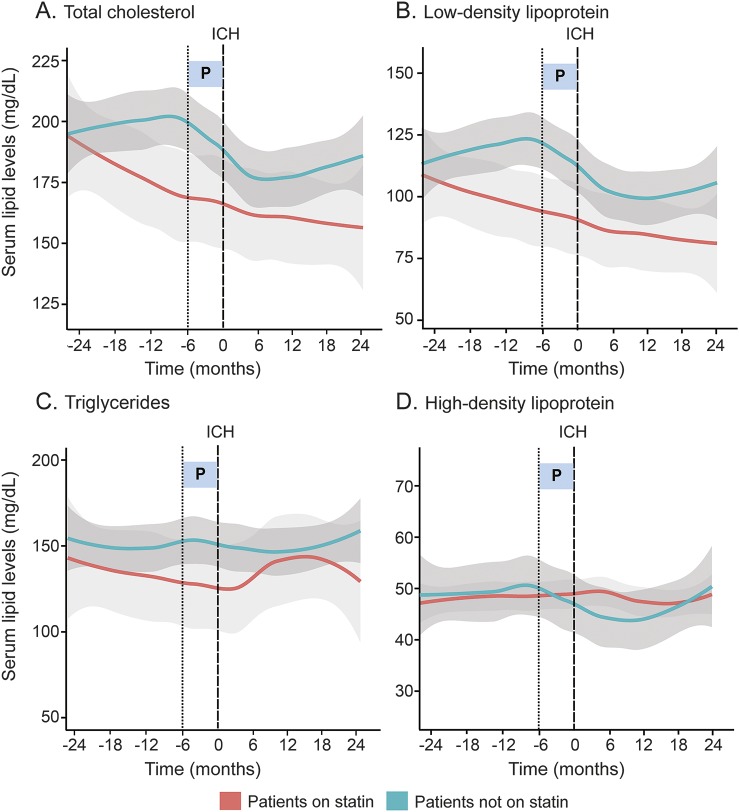
Temporal trends in the mean serum lipid levels between participants hospitalized for ICH and controls hospitalized for acute noncerebral illnesses were assessed visually using Loess-smoothed curves (figure 2) fitted from scatterplots of serum lipid variation over time (figure e-2). Serum TC and LDL trends over the 24-month period before acute illness differ between ICH cases and controls (TC: p = 0.0003; LDL: p = 0.0029). In particular, patients with ICH experienced overall declines in mean serum TC and LDL levels in the 24 months preceding ICH occurrence, with notable acceleration in rates of decline of both lipid fractions beginning 6 months before the ICH event. Following ICH, serum TC and LDL trends became flat with levels remaining depressed over 24 months post-ICH. In contrast, non-ICH controls demonstrated less variability in mean serum TC and LDL levels, which remained largely stable throughout the 4-year period. There were no significant changes in serum TG and HDL trends for all participants regardless of their acute illness diagnoses within the time period examined.
Figure 2. Serum lipid trends in patients with ICH and non-ICH controls.
(A–D) Loess smoothed curves of serum lipid levels (mg/dL) against time (in months) for ICH cases and patients who were hospitalized for acute noncerebral illnesses (controls). Light gray areas indicate SE for controls. Dark gray areas indicate SE for ICH cases. (E) Comparison of difference in rates of change of serum lipid levels (slope) between time interval 6–24 months before acute illness and 0–6 months before acute illness in ICH and non-ICH controls. Test statistic, Wald test; degree of freedom in parentheses. Statistical significance at p < 0.0125 (Bonferroni-corrected). HDL = high-density lipoprotein; ICH = intracerebral hemorrhage; LDL = low-density lipoprotein; P = time interval 0–6 months before acute illness; SE = standard error; TC = total cholesterol; TG = triglyceride.
Change in serum lipid trends in the final 6 months before acute illness and association with ICH risk.
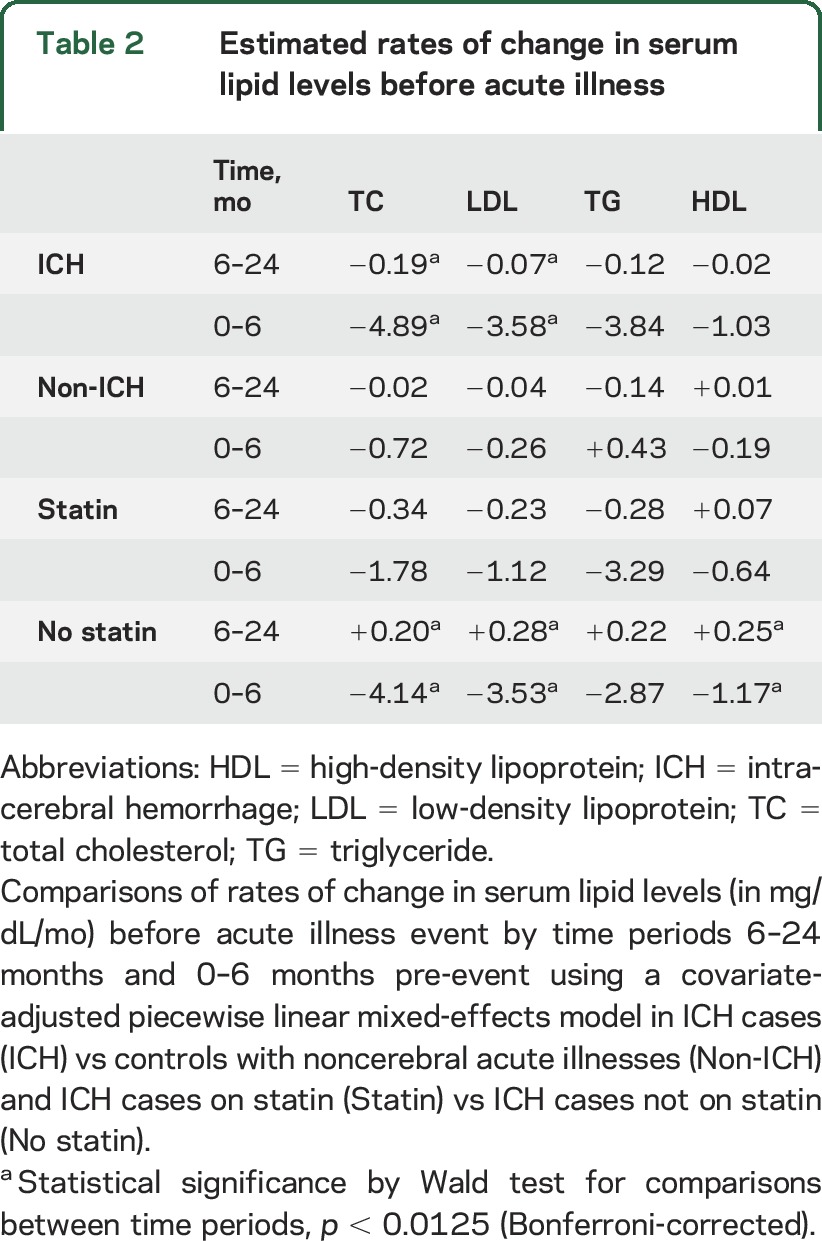
Estimates of rates of change in serum lipid levels in the immediate interval (0–6 months before acute illness) compared with the antecedent interval (6–24 months before acute illness) in ICH cases and controls with acute noncerebral illness are presented in table 2. The rates of decline of mean serum TC and LDL levels in the 6 months immediately preceding ICH increased compared with trends in the antecedent 18-month period (TC: −4.89 mg/dL/mo, p = 0.001; LDL: −3.58 mg/dL/mo, p = 0.0038). In contrast, non-ICH controls experienced negligible change in serum TC and LDL trends within the same time period immediately preceding acute illness (TC: −0.72 mg/dL/mo, p = 0.41; LDL: −0.26 mg/dL/mo, p = 0.85). Serum TG and HDL trends remained unchanged during the 0 to 6 months immediately preceding acute illness for both ICH and non-ICH participants.
Table 2.
Estimated rates of change in serum lipid levels before acute illness
Serum lipid trends pre-ICH are unrelated to environmental exposures.
The observed changes in serum TC and LDL trends in the 6 months immediately preceding ICH remained significant even with the inclusion of variables known to affect serum lipids (tables 2 and e-3). Subgroup analysis of patients with ICH stratified by statin use pre-ICH demonstrated accelerated declines in serum TC and LDL levels in the 6-month interval immediately preceding ICH only in those who were statin-naive (p = 0.0001 and p = 0.0002, respectively) (figure 3, table 2). In contrast, patients with ICH who were on statin medication showed stable serum TC and LDL trends, with gradual decline in serum levels of both lipid fractions over 48 months consistent with known statin effects, and did not experience a more dramatic drop in levels before the ICH. Similar analysis of patients with ICH by cigarette smoking and alcohol use also demonstrated no significant change in serum lipid trends in the 6-month time period immediately before ICH.
Figure 3. Serum lipid trends by statin use in patients with ICH.
(A–D) Loess smoothed curves of serum lipid levels (mg/dL) against time (in months) for comparison between ICH cases that were either on statin medication or statin-naive. (A) Total cholesterol; (B) low-density lipoprotein; (C) triglycerides; (D) high-density lipoprotein. Dark gray areas indicate SE for statin-naive ICH cases. Light gray areas indicate SE for ICH cases on statin. ICH = intracerebral hemorrhage; SE = standard error.
DISCUSSION
We identified a novel behavior in serum lipid trends in primary ICH in which serum TC and LDL levels decline precipitously within a 6-month time interval immediately preceding the occurrence of primary ICH. This observation was distinct for ICH as similar changes in serum lipid trends were not demonstrated in the same time period before noncerebral acute illnesses, which served as controls. Furthermore, the change in serum TC and LDL trends preceding primary ICH is independent of measured environmental exposures known to influence serum lipid variability and raises the question of whether a systemic process may contribute toward the occurrence of ICH.
Rapid declines in serum lipid levels have been observed in the context of acute illnesses20–24 and are thought to be associated with active inflammation.28,29 Previous studies of prestroke serum lipid association with ICH have been inconclusive,30–32 being dependent on serum lipid concentrations measured at a single time point. Given our observations of a temporal association, we speculate that the decline in serum lipids may represent an increase in systemic inflammatory response preceding the occurrence of ICH.
There are several strengths to our study. First, we constructed a unique dataset of longitudinal lipid data to describe temporal lipid patterns before ICH. The relatively large sample size of the cohort and the inclusion of serial serum lipid measurements both before and after ICH in the present cohort allow for more accurate representation of longitudinal lipid trends in ICH in comparison with previous studies that lack data on prestroke serum lipid measurements.8 Second, our results derived from biannual serum lipid measurements over 4 years are focused on understanding the broader temporal variation in lipid trends in ICH, which potentially improves biological relevance in lipids–disease risk associations, in contrast with short-term lipid variance, which may be more unstable.8,30–32 Third, the use of a piecewise mixed-effects model in our analysis allowed for investigation of individual, distinct, biologically relevant time periods,27 as separate slopes can be fitted to observations representing periods before and after the time period of interest. Also, the broad inclusion criteria for inpatient diagnoses (table e-4) in control participants attempted to best reflect the mixed biology of acute illnesses that are known to influence serum lipid variability.20–24 Acute illnesses including myocardial infarction21 and ischemic strokes,23,24 among others,20,22 have all been associated with rapid and large declines in serum lipids immediately following the event. Hence, the selection of severely ill individuals including those with cardiac arrest or those who required cardiac procedures, mainly due to acute myocardial infarction in our cohort, has the additional benefit of building an inherent bias toward the null hypothesis of no difference in serum lipid trends between ICH cases and controls, and thus allows for robust comparisons of ICH-specific effects on changes in serum lipid trends.
Our analysis has important limitations. As our case cohort included only patients with ICH, we cannot speculate that these effects are unique to ICH. Declining serum lipid trends have been observed following acute cerebrovascular diseases including TIAs and ischemic strokes,33 similar to prior observations after ICH.8 It remains a possibility that this phenomenon of declining serum lipids heralding ICH may instead reflect more general processes influencing the occurrence of acute cerebral illnesses, which may be addressed by further studies. However, the inclusion of control individuals with increased likelihood of ischemic cerebral insults such as patients with cardiac arrest or who underwent cardiac procedures for acute myocardial infarction failed to demonstrate the phenomenon of declining serum lipids preceding the acute event in the control group, which would be expected of non–ICH-specific phenomena. Second, causal inference is limited by the retrospective design. A similar study in a prospective cohort will be ideal but extremely challenging and unfeasible in practice given low ICH event rates (17.0 per 100,000 person/years).34 Third, in our case-control comparison of lipid trends, serum lipid measurements during the post–acute illness period were unbalanced between case-control cohorts, which increases the risk of asymptotic bias in estimates of serum lipid trends during that same period. This may account for the discrepancy in our observation of depressed serum TC and LDL levels up to 2 years postdischarge in ICH cases compared with a prior report of serum TC elevations by 90 days post-ICH.8 However, we attempted to minimize such biases using complete case analysis for multivariable comparisons in order to preserve comparability at the expense of reduction in statistical power. Furthermore, serum lipid measurements during the pre–acute illness period related to our primary finding were balanced across both case-control cohorts and among individual time intervals. Fourth, our case-control cohorts were similarly unbalanced for the majority of clinical characteristics because of prioritization in matching by statin-use status. However, we adjusted for these covariates as fixed effects in our final multivariate model to account for the differences. Fifth, selection biases are present both from subject-specific indications for serial lipid measurements and inclusion of predominantly small- to moderate-sized ICH cases, which were necessitated by the study question and design. Hence, our findings of decline in serum TC and LDL preceding ICH occurrence cannot be generalized toward all patients with primary ICH although the likelihood of significant differences in dyslipidemia in ICH pathophysiology by ICH size or severity would seem less biologically plausible. Future validation and confirmation of these results in larger, prospective cohorts will be needed to surmount these sampling biases and current limitations in relevance toward predominantly mild to moderately severe ICH.
We attempted to address confounding of serum lipid levels by including variables known to influence serum lipid variability. Contrary to known effects of statin on lowering serum TC and LDL levels, subgroup analysis of ICH cases by statin-use status demonstrated that serum lipid declines preceding ICH were unrelated to statin exposure. Only individuals who were statin-naive experienced a more pronounced decline in trends of both TC and LDL. Because of limitations in information collected through the study protocol, we could not account for the additional effects of statin dose or nonstatin lipid-lowering agents. However, as the latter are typically less potent than statin in their lipid-lowering effects, they are unlikely to behave as major confounders in the absence of statin effects on serum lipid declines. Because of the broad range of environmental exposures that can influence serum lipid variations, we were unable to exhaustively address additional potential confounders. We did not have nutritional data for our cohorts, but severe acute malnutrition would not be expected based on our selection criteria and we would also expect serum TGs to be affected by dietary changes.15 Fluctuations in menstrual cycles, pregnancy, menopause, and seasonal variations, which can influence serum lipid levels, are unlikely to have a predominant role in serum lipid trends observed given the elderly age of our cohort, while the long study duration of 48 months would be expected to limit the effect of seasonal variations in serum lipid levels.
Our results have implications for ongoing efforts in dissecting the role of dyslipidemia in cerebrovascular disease risk. Our finding of significant declines in serum lipid levels within 6 months preceding primary ICH suggests an association between accelerations in serum lipid decline and ICH risk, and that temporal lipid trends may augur a generalized process that precipitates ICH. Our observations also suggest that absolute levels of serum lipid measurements may not be biologically relevant for stroke risk associations.35 This may be of relevance in informing designs of future prospective clinical trials for lipid biomarkers or lipid-lowering agents in stroke. Given our study limitations, these findings should be considered hypothesis-generating at present, and future studies are needed to replicate these results in prospective cohorts, probe for potential mediation effects between TC and LDL, and further characterize changes in serum lipid trends as a potential biomarker of impending acute cerebral injury.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Daniel Woo for his helpful discussions regarding serum lipid confounders.
GLOSSARY
- EMR
electronic medical record
- HDL
high-density lipoprotein
- ICH
intracerebral hemorrhage
- LDL
low-density lipoprotein
- MGH
Massachusetts General Hospital
- TC
total cholesterol
- TG
triglyceride
Footnotes
Supplemental data at Neurology.org
Editorial, page 2028
AUTHOR CONTRIBUTIONS
Dr. C.-L. Phuah participated in study design, data acquisition, statistical analyses, drafting and revision of the manuscript. M.R. Raffeld and A.M. Ayres were responsible for data collection. Drs. A. Viswanathan, S.M. Greenberg, and Dr. J. Rosand participated in final editing of the manuscript. Dr. C.D. Anderson participated in study design and revision of the manuscript.
STUDY FUNDING
This work was supported by funding from the National Institute for Neurologic Disorders and Stroke (R01 NS059727 and K23 NS086873). All funding entities had no involvement in study design, data collection, analysis, interpretation, writing of the report, and in the decision to submit the paper for publication.
DISCLOSURE
C. Phuah, M. Raffeld, and A. Ayres report no disclosures relevant to the manuscript. A. Viswanathan is supported by NIH-NINDS K23 AG028726. S. Greenberg is supported by NIH-NINDS U10 NS077360, R01 AG026484, R01 NS070834. A. Biffi reports no disclosures relevant to the manuscript. J. Rosand is supported by NIH-NINDS U01 NS069208, R01 NS073344, and R01 NS059727. C. Anderson is supported by NIH-NINDS K23 NS 086873. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gordon T. Mortality experience among the Japanese in the United States, Hawaii and Japan. Public Health Rep 1957;72:543–553. [PMC free article] [PubMed] [Google Scholar]
- 2.Kuller L, Reisler DM. An explanation for variations in distribution of stroke and arteriosclerotic heart disease among populations and racial groups. Am J Epidemiol 1971;93:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Yano K, Reed DM, MacLean CJ. Serum cholesterol and hemorrhagic stroke in the Honolulu Heart Program. Stroke 1989;20:1460–1465. [DOI] [PubMed] [Google Scholar]
- 4.Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the Multiple Risk Factor Intervention Trial. N Engl J Med 1989;320:904–910. [DOI] [PubMed] [Google Scholar]
- 5.Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ 1994;309:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iribarren C, Jacobs DR, Sadler M, Claxton AJ, Sidney S. Low total serum cholesterol and intracerebral hemorrhagic stroke: is the association confined to elderly men? The Kaiser Permanente Medical Care Program. Stroke 1996;27:1993–1998. [DOI] [PubMed] [Google Scholar]
- 7.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757–767. [DOI] [PubMed] [Google Scholar]
- 8.Segal AZ, Chiu RI, Eggleston-Sexton PM, Beiser A, Greenberg SM. Low cholesterol as a risk factor for primary intracerebral hemorrhage: a case-control study. Neuroepidemiology 1999;18:185–193. [DOI] [PubMed] [Google Scholar]
- 9.Woo D, Kissela BM, Khoury JC, et al. Hypercholesterolemia, HMG-CoA reductase inhibitors, and risk of intracerebral hemorrhage: a case-control study. Stroke 2004;35:1360–1364. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 11.Wieberdink RG, Poels MM, Vernooij MW, et al. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol 2011;31:2982–2989. [DOI] [PubMed] [Google Scholar]
- 12.Bang OY, Saver JL, Liebeskind DS, et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology 2007;68:737–742. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Luna D, Rubeira M, Ribo M, et al. Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke 2011;42:2447–2452. [DOI] [PubMed] [Google Scholar]
- 14.Mustanoja S, Strbian D, Putaala J, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke 2013;44:2330–2332. [DOI] [PubMed] [Google Scholar]
- 15.Durrington PN. Biological variation in serum lipid concentrations. Scand J Clin Lab Invest Suppl 1990;198:86–91. [PubMed] [Google Scholar]
- 16.Schaefer EJ, Lamon-Fava S, Cohn SD, et al. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res 1994;35:779–792. [PubMed] [Google Scholar]
- 17.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993;98:83–90. [DOI] [PubMed] [Google Scholar]
- 18.Ockene IS, Chiriboga DE, Stanek EJ, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med 2004;164:863–870. [DOI] [PubMed] [Google Scholar]
- 19.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. Br Med J 1989;298:784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Man EB, Bettcher PG, Cameron CM, Peters JP. Plasma amino acids, nitrogen and serum lipids of surgical patients. J Clin Invest 1946;25:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore JM, Goldberg RJ, Matsumoto AS, Castelli WP, McNamara AB, Dalen JE. Validity of serum total cholesterol level obtained within 24 hours of acute myocardial infarction. Am J Cardiol 1984;54:722–725. [DOI] [PubMed] [Google Scholar]
- 22.Keele KD, Stern PR. Serum lipid changes in relation to pain. J R Coll Physicians Lond 1973;7:319–329. [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez I, Hachinski V, Wolfe B. Serum lipids after stroke. Neurology 1987;37:507–511. [DOI] [PubMed] [Google Scholar]
- 24.Hollanders FD, Burton P, Shafar J. Serum lipid changes following completed stroke syndrome. Postgrad Med J 1975;51:386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genes for Cerebral Hemorrhage on Anticoagulation (GOCHA) Collaborative Group. Exploiting common genetic variation to make anticoagulation safer. Stroke 2009;40(suppl 3):S64–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a research patient data repository. AMIA Annu Symp Proc 2006;2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 27.Naumova EN, Must A, Laird NM. Tutorial in biostatistics: evaluating the impact of “critical periods” in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol 2001;30:1332–1341. [DOI] [PubMed] [Google Scholar]
- 28.McGillicuddy FC, de la Llera Moy M, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009;119:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteve E, Ricart W, Fernandez-Real JM. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 2005;24:16–31. [DOI] [PubMed] [Google Scholar]
- 30.Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007;38:2718–2725. [DOI] [PubMed] [Google Scholar]
- 31.Noda H, Iso H, Irie F, et al. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation 2009;119:2136–2145. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Neurology 2008;24:2364–2370. [DOI] [PubMed] [Google Scholar]
- 33.Woo J, Lam CWK, Kay R, Wong HY, Teoh R, Nicholls MG. Acute and long-term changes in serum lipids after stroke. Stroke 1990;21:1407–1411. [DOI] [PubMed] [Google Scholar]
- 34.Rincon F, Mayer SA. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocrit Care 2013;19:95–102. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Koh SJ, Yoo SY, et al. Characteristics of subjects with very low serum low-density lipoprotein cholesterol and the risk for intracerebral hemorrhage. Korean J Intern Med 2012;27:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.