Abstract
Objective:
To assess the cross-sectional association between multimorbidity and imaging biomarkers of brain pathology in the population-based Mayo Clinic Study of Aging (MCSA).
Methods:
The study consisted of 1,449 MCSA participants who were cognitively normal at the time of MRI. A subset of the participants also had 11C-Pittsburgh compound B (n = 689) and 18fluorodeoxyglucose (n = 688) PET scans available. Information on multimorbidity (defined as ≥2 chronic conditions) in the 5 years prior to the first imaging study was captured from the medical record using ICD-9 codes for chronic conditions and the Rochester Epidemiology Project medical records linkage system. The cross-sectional association of multimorbidity and imaging biomarkers was examined using logistic and linear regression models.
Results:
Among 1,449 cognitively normal participants (mean age 79 years; 50.9% men), 85.4% had multimorbidity (≥2 chronic conditions). Multimorbidity and severe multimorbidity (≥4 chronic conditions) were associated with abnormal Alzheimer disease (AD) signature meta–region of interest (meta-ROI) 18F-FDG hypometabolism (odds ratio [OR] 2.03; 95% confidence interval [CI] 1.10–3.77 and OR 2.22; 95% CI 1.18–4.16, respectively), and with abnormal AD signature MRI cortical thickness (OR 1.53; 95% CI 1.09–2.16 and OR 1.76; 95% CI 1.24–2.51, respectively), but was not associated with amyloid accumulation.
Conclusions:
Multimorbidity was associated with brain pathology through mechanisms independent of amyloid deposition and such neuronal injury and pathology was present before any symptomatic evidence of cognitive impairment. Longitudinal follow-up will provide insights into potential causal associations of multimorbidity with changes in brain pathology.
Multimorbidity has a prevalence of 20%–30% in the general population,1 and increases with age from 55% to 98% among persons older than 60 years.1 Multimorbidity is becoming the norm among adults in primary care settings,2 mandating the need to investigate not only the causes, but also the association with future health outcomes. Multimorbidity is associated with an increased risk for hospitalizations and increased length of stay, worsening quality of life and physical functioning, polypharmacy, mild cognitive impairment,3 and depression, resulting in significant economic costs for the health care system.2 Several of the common chronic conditions that contribute to multimorbidity are established risk factors for mild cognitive impairment (MCI) or dementia (e.g., vascular diseases, cerebrovascular disease, depression, or chronic obstructive pulmonary disease).3–8 We hypothesized, therefore, that multimorbidity may also be associated with abnormal brain imaging findings that are characteristically present in persons with Alzheimer disease (AD) dementia, but this association has not been studied. Thus, the objective of this study was to determine the cross-sectional associations between multimorbidity and in vivo biomarkers of brain pathology (atrophy, increased amyloid accumulation, and reduced metabolism) assessed by MRI, 11C-Pittsburgh compound B (11C-PiB), and 18fluorodeoxyglucose (18F-FDG) PET in Mayo Clinic Study of Aging (MCSA) participants who were cognitively normal at time of imaging.
METHODS
Study population.
The MCSA study design and methodology have been presented in detail previously.9,10 Olmsted County, Minnesota, residents aged 70–89 years on the prevalence (index) date (October 1, 2004) were enumerated using Rochester Epidemiology Project (REP)11 resources. An age- and sex-stratified random sample of eligible Olmsted County residents (without dementia, not terminally ill or in hospice) was invited to participate in person or by telephone.9 To maintain the study sample size, we have ongoing recruitment using the same protocols as in 2004. MRI was offered beginning in 2005 to all MCSA participants and 11C-PiB/18F-FDG PET scans were offered beginning in 2009. The present study includes participants who were recruited between 2004 and 2011, were cognitively normal at time of MRI, and had information on multimorbidity at the time of imaging. Of these, 1,449 had MRI scans, 689 individuals also had 11C-PiB, and 688 participants had 18F-FDG PET scans.
Standard protocol approvals, registrations, and patient consents.
All study protocols were approved by the Institutional Review Boards of the Mayo Clinic and the Olmsted Medical Center, and participants provided written informed consent before participation.
Identification of chronic conditions.
Ascertainment of multimorbidity has been published.3,12 Briefly, the diagnostic indices of the REP medical records linkage system were searched electronically for each participant to identify the ICD-9 codes for 19 chronic conditions13 associated with any health care visit within 5 years before the first imaging (i.e., 5-year capture frame).12 These conditions were proposed by the US Department of Health and Human Services in 2010 to study multimorbidity.13 We excluded dementia diagnoses ascertained through the MCSA study evaluation. A separate 5-year capture frame was created for the MRI scan date and for the 18F-FDG/11C-PiB scan date. A specific chronic condition was assigned to a participant if he or she received 2 ICD-9 codes for the given condition separated by more than 30 days within the 5-year capture frame.3,12 Seventeen (of the 19) chronic conditions were captured in the study sample: hyperlipidemia, diabetes mellitus, hypertension, cardiac arrhythmias, coronary artery disease, stroke, congestive heart failure, cancer, asthma, depression, substance abuse disorders (drugs and alcohol), chronic obstructive pulmonary disease, chronic kidney disease, arthritis, osteoporosis, schizophrenia, and hepatitis. In addition, the 6 most common dyads (any combinations of 2 of the 17 chronic conditions) were identified in persons with multimorbidity.3,12
Identification of MCI/dementia.
Participants were evaluated by a nurse or study coordinator and a physician and underwent neuropsychometric testing by a trained psychometrist supervised by a neuropsychologist.9,10 Nine tests included in the neuropsychometric battery assessed performance in memory, language, executive function, and visuospatial skills cognitive domains.9,10,14 The coordinator interview included ascertainment of demographic information, self-reported questions about memory to the participant, and administration of the Clinical Dementia Rating scale15 and the Functional Activities Questionnaire to an informant.16 The physician evaluation included a medical history review, administration of the Short Test of Mental Status,17 and a complete neurologic examination. A diagnosis of MCI,9,16 dementia,18 or normal cognition for each participant was made by consensus decision among the 3 evaluators using published criteria. A cognitively normal diagnosis was assigned to those who performed in the normal range relative to the normative data and did not meet criteria for MCI or dementia.
Participant information collected at the baseline evaluation included age, sex, educational attainment, body mass index (weight in kilograms divided by height in meters squared), and gait speed (meter/second); APOE genotyping was performed at baseline.
Acquisition of MRI measures.
We performed MRI studies at 3T (Signa; GE Healthcare, Waukesha, WI) with an 8-channel phased-array head coil, acquiring both a 3D magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence and a fluid-attenuated inversion recovery sequence.19 From each participant's MPRAGE, we measured hippocampal volume (HV) using FreeSurfer software (version 5.3), which was then adjusted for total intracranial volume.20 Cortical (0.10 mm) and subcortical infarctions (white matter [WM], central gray matter [GM], cerebellum, brainstem) were ascertained by experienced image analysts and confirmed by a radiologist. Technicians who performed the imaging analysis had no knowledge of the clinical characteristics of participants. FreeSurfer (v 5.3) was used to measure cortical thickness. An AD-signature cortical thickness measure21 was computed by averaging the cortical thickness in the following regions of interest (ROIs): entorhinal, inferior temporal, middle temporal, and fusiform cortices. We defined an abnormal AD signature cortical thickness as less than 2.74 mm, corresponding to 90% sensitivity in AD dementia.
18F-FDG PET and 11C-PiB PET acquisition.
PET images were acquired using a PET/CT scanner operating in 3D mode.22 A CT image was obtained for attenuation correction. The 11C-PiB PET scan consisted of four 5-minute dynamic frames acquired 40–60 minutes after injection.23 Participants were injected with 18F-FDG 1 hour after the 11C-PiB scan and imaged after 30–38 minutes, for an 8-minute image acquisition of four 2-minute dynamic frames.23,24 An in-house fully automated image processing pipeline was used for quantitative image analysis for 11C-PiB and 18F-FDG. Each participant's PET images were registered to his or her MRI, which had been labeled with our parcellation atlas.23 A 11C-PiB PET meta-ROI retention ratio (with GM and WM sharpening, partial volume correction) was calculated from the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROIs normalized to the cerebellar GM ROI of the atlas.22 An AD signature 18F-FDG PET ratio (nonsharpened, non–partial volume corrected) was calculated in a similar manner based on glucose metabolic rates from an AD signature meta-ROI and consisted of the average bilateral angular gyri, posterior cingulate, and inferior temporal cortical ROIs from both hemispheres normalized to pons and vermis uptake.25–27
We defined an abnormal 11C-PiB-PET retention ratio (standardized uptake value ratio) as ≥1.40 and an abnormal AD 18F-FDG-PET ratio as ≤1.32, corresponding to 90% sensitivity in AD dementia.28,29
Statistical analysis.
Multimorbidity was defined as having 2 or more chronic conditions in the 5-year capture frame, and categorized as mutually exclusive categories of 0 or 1, 2, 3, and 4 or more conditions. There were only 68 participants with 0 chronic conditions; therefore individuals with 0 and 1 chronic conditions were combined as the reference group.
We examined the cross-sectional association of multimorbidity with the dichotomous imaging measures (i.e., abnormal 11C-PiB PET, abnormal AD signature 18F-FDG PET [hypometabolism], and abnormal AD signature cortical thickness from MRI, and presence of cortical infarctions [cerebrovascular disease]) using multivariable logistic regression models (odds ratios [ORs], 95% confidence intervals [CIs]) adjusted for age, sex, education (basic model), and APOE ε4 allele (full model). Examination of the residuals of regression of multimorbidity on the continuous MRI and PET imaging measures (i.e., AD signature cortical thickness, AD signature 18F-FDG PET ratio, 11C-PiB PET meta-ROI retention ratio, and HV) suggested a linear association. Therefore, we also used multivariable linear regression models to examine the associations (β coefficients, 95% CIs) of multimorbidity with imaging biomarkers in the basic and full models as above. Results from both logistic and linear regression were similar for AD signature cortical thickness, AD signature 18F-FDG PET ratio, and 11C-PiB PET meta-ROI retention ratio; thus, only the estimates from logistic regression analysis are presented in the tables. Potential effect modification by sex, education, and APOE ε4 allele status was also examined.
In addition, we examined the association of the most common chronic conditions (frequency higher than 5%) and the most common dyads of chronic conditions with imaging biomarkers using multivariable logistic regression models. All analyses were considered significant at p < 0.05, and were performed using Stata/IC statistical software version 12.1 (StataCorp LP, College Station, TX) and SAS statistical software version 9.3 (SAS Institute, Cary, NC).
RESULTS
Population characteristics.
Among 1,449 cognitively normal MCSA participants (mean age 79 years; 50.9% men), 1,237 (85.4%) had multimorbidity (≥2 chronic conditions; table 1). Compared to MCSA participants who did not participate in imaging studies, participants who underwent imaging performed better on cognitive tests (global composite scores) from neuropsychometric testing, had a lower frequency of diabetes mellitus or hypertension, and a higher frequency of men, but did not differ in age or frequency of APOE ε4 allele.
Table 1.
Characteristics of cognitively normal participants at baseline MRI
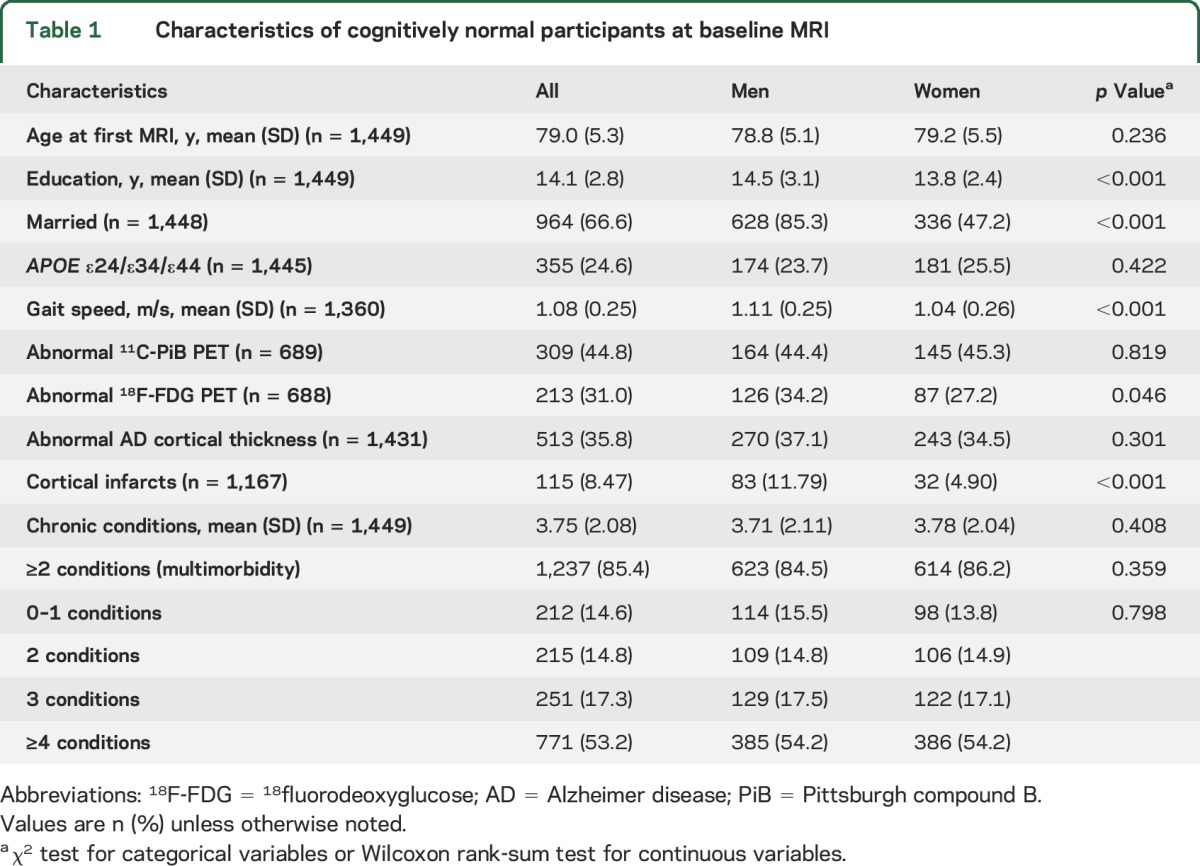
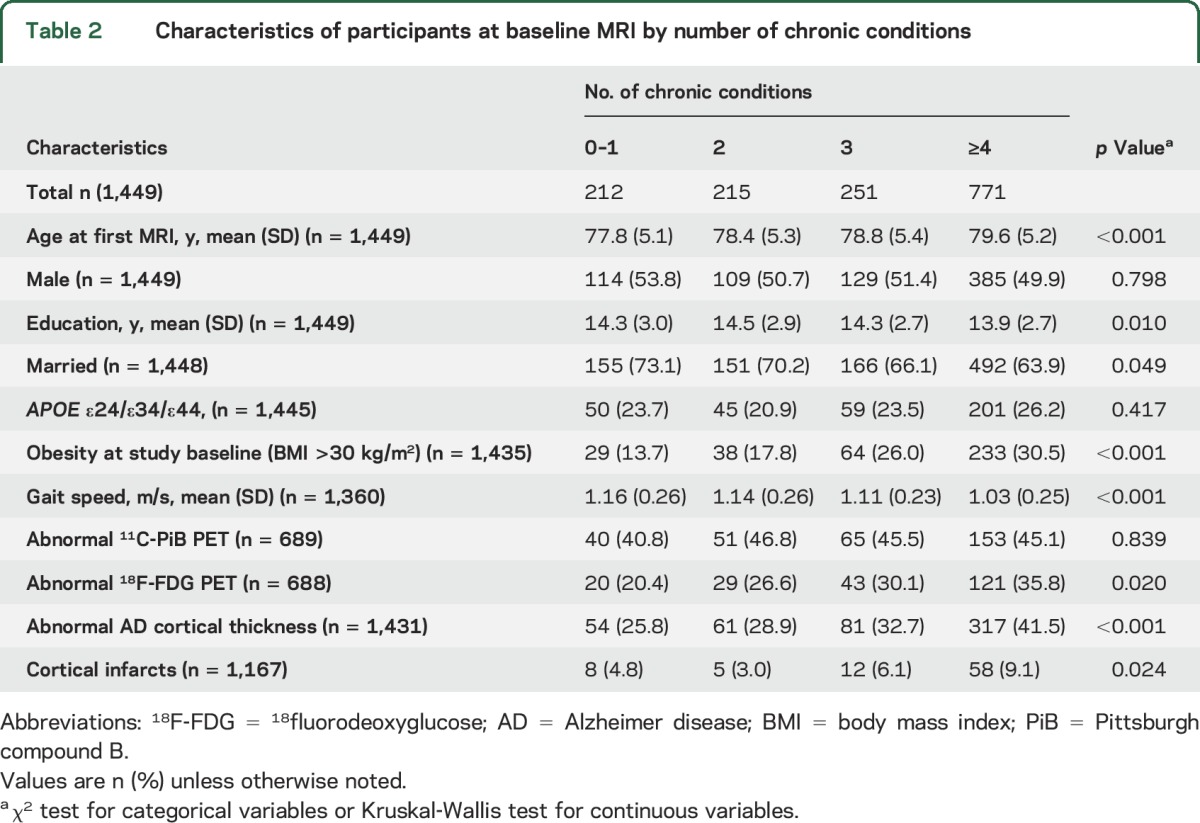
There were no differences in the frequency of multimorbidity or APOE ε4 allele by sex (table 1). Mean age at imaging and frequency of obesity increased with increasing number of chronic conditions; the inverse was observed for gait speed (table 2). APOE ε4 genotype was not associated with the number of chronic conditions.
Table 2.
Characteristics of participants at baseline MRI by number of chronic conditions
Association of multimorbidity with imaging biomarkers.
The frequency of abnormal AD cortical thickness and abnormal 18F-FDG-PET increased with increasing number of chronic conditions; a similar pattern was not observed for abnormal 11C-PiB-PET (table 2). The frequency of cortical infarcts was highest with severe multimorbidity (≥4 chronic conditions).
In multivariable analyses, multimorbidity (≥2 conditions) was associated with abnormal 18F-FDG-PET (hypometabolism) and abnormal AD signature cortical thickness, but not with abnormal amyloid (table 3); adjustment for APOE ε4 did not change the estimates. Estimates were strongest for severe multimorbidity (≥4 chronic conditions) and patterns of association were similar for analyses using continuous imaging measures.
Table 3.
Association between multimorbidity and abnormal imaging biomarkers in cognitively normal individuals: Mayo Clinic Study of Aging
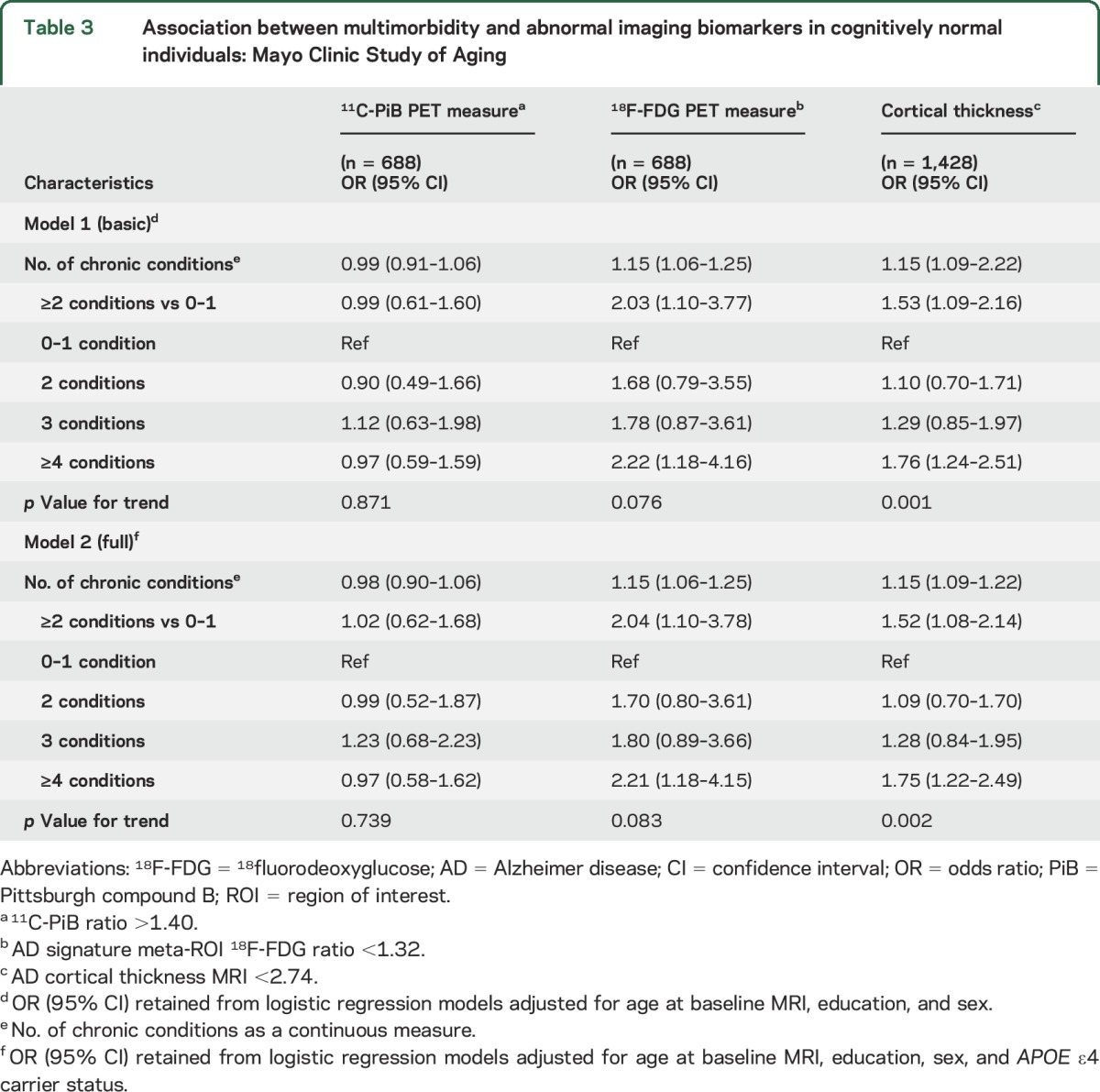
An increasing number of chronic conditions was associated with presence of cortical infarcts (OR 1.30; 95% CI 1.16 to 1.44; p < 0.001; per unit increase in chronic conditions) and lower hippocampal volume (β = −0.021; 95% CI −0.041 to −0.001; p = 0.04) (results not shown in tables).
We observed interactions between sex and the number of chronic conditions (as a continuous measure) in regard to the association with abnormal 11C-PiB PET (p = 0.037) and between sex and multimorbidity (≥2 chronic conditions) in regard to the abnormal 18F-FDG-PET (p = 0.0496). In sex-stratified analyses, the associations for abnormal 11C-PiB PET were not significant in men or in women; however, the OR of an abnormal 18F-FDG was stronger in women with multimorbidity (OR 6.03; 95% CI 1.40 to 26.03) than in men with multimorbidity (OR 1.38; 95% CI 0.67 to 2.85) compared to those with 0–1 conditions (table e-1 on the Neurology® Web site at Neurology.org). Failure to detect a statistically significant association in men may relate to small sample sizes in some cells, and the wide CI in women raises questions about the precision of the estimates. There were no interactions of multimorbidity with sex in regard to AD signature cortical thickness and hippocampal volume, or with education and APOE ε4 allele status in regard to any of the imaging biomarkers studied.
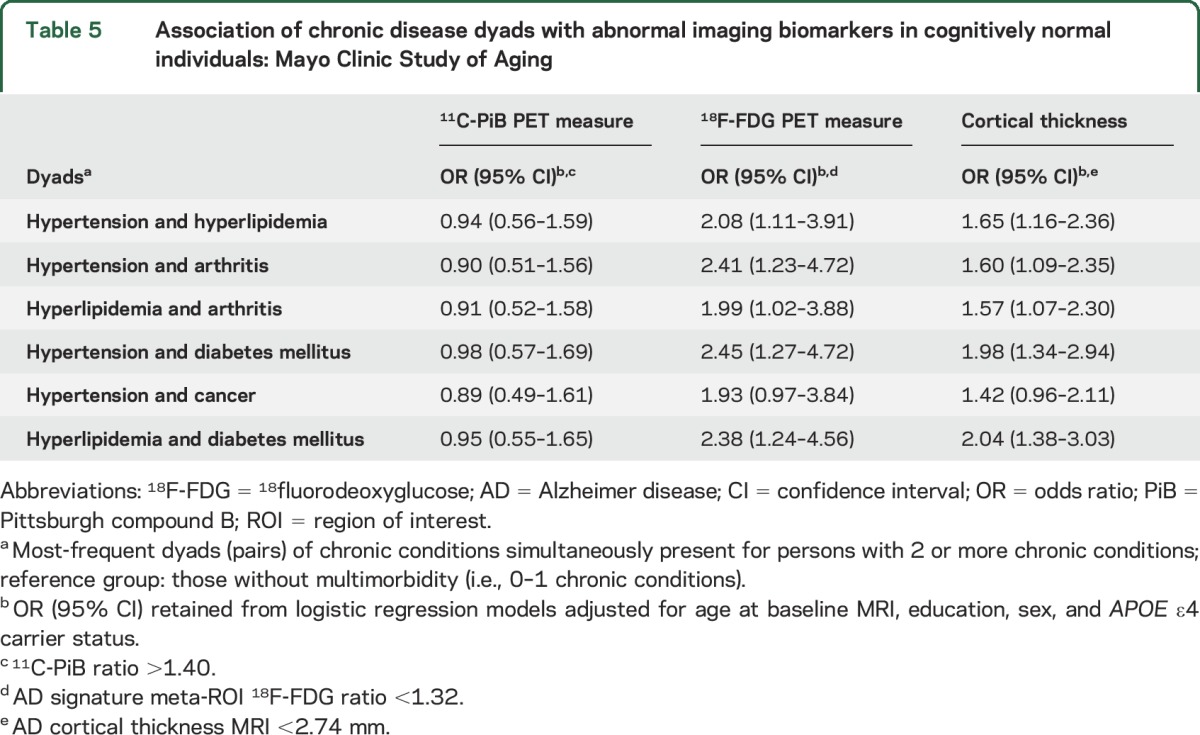
Several of the most frequent single chronic conditions were associated with abnormal AD signature cortical thickness or abnormal 18F-FDG-PET in multivariable models (e.g., hypertension, arthritis, diabetes mellitus, cancer, arrhythmia, coronary artery disease) (table 4). However, residual confounding could be present since these models do not take into account coexisting chronic conditions as covariates in the model. Most of the 6 common pairs (dyads) were associated with abnormal AD signature cortical thickness or abnormal 18F-FDG-PET but not with abnormal amyloid in multivariable analyses (table 5). The associations with abnormal 18F-FDG-PET were stronger in dyads that included diabetes mellitus.
Table 4.
Association between single chronic conditions and abnormal imaging biomarkers in cognitively normal individuals: Mayo Clinic Study of Aging
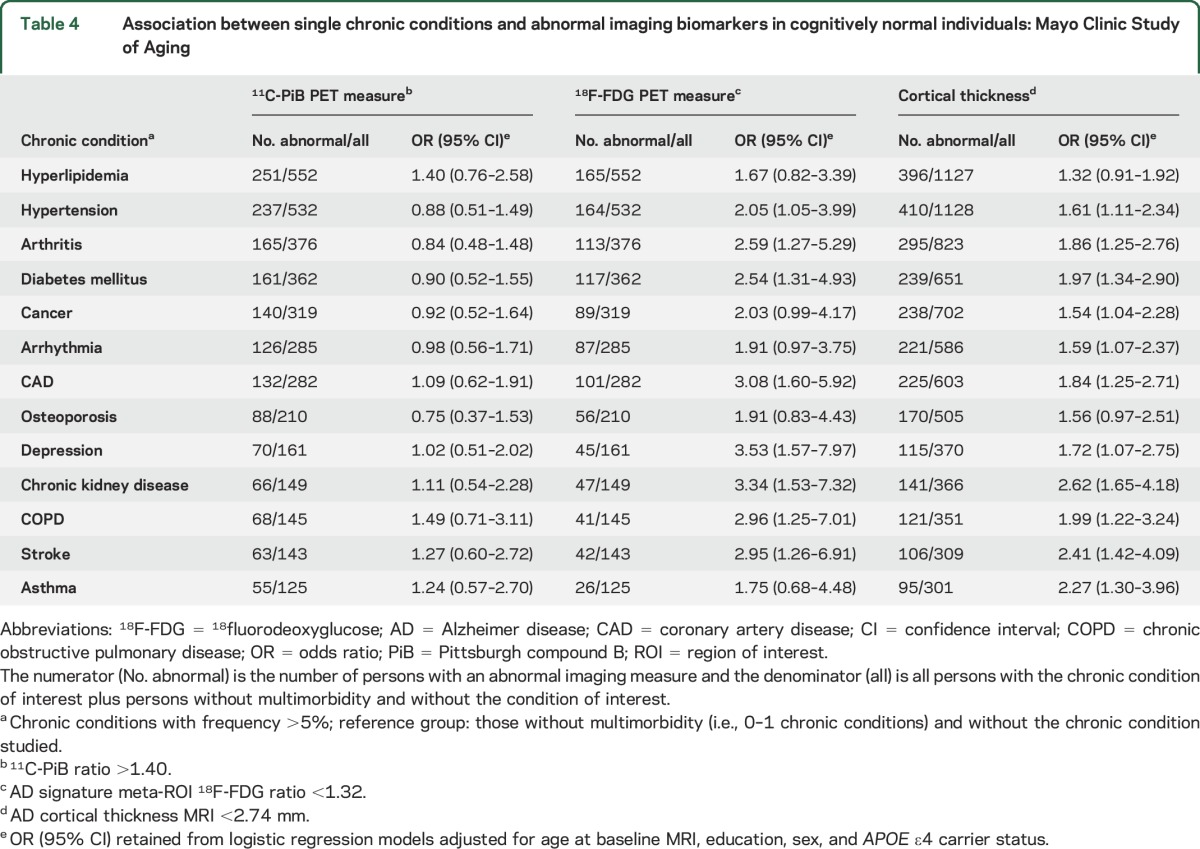
Table 5.
Association of chronic disease dyads with abnormal imaging biomarkers in cognitively normal individuals: Mayo Clinic Study of Aging
DISCUSSION
In this sample of cognitively normal elderly individuals, we investigated the cross-sectional associations of multimorbidity with known AD-related neuroimaging measures including brain cortical thickness, amyloid accumulation, and hypometabolism. Multimorbidity (≥2 chronic conditions) was associated with hypometabolism and decreased cortical thickness in AD signature regions, and this association was likely driven by the strong associations observed for severe multimorbidity (i.e., ≥4 chronic conditions). Failure to observe associations of multimorbidity with increased amyloid suggests that the observed associations with other biomarkers of brain pathology may involve mechanisms independent of amyloid deposition. This pathology may be present before symptomatic evidence of cognitive impairment.
Although the exact mechanism for the association of multimorbidity with neuroimaging measures of neurodegeneration cannot be delineated in the current study, we hypothesize that known mechanisms governing the association of chronic diseases, specifically, vascular conditions such as diabetes30,31 or vascular disease,29,30,32 with antemortem30,31 imaging or postmortem32 biomarkers could be involved in these pathologic processes. In the present study, the most common dyads (i.e., hypertension and hyperlipidemia, hypertension and arthritis, hyperlipidemia and arthritis, hypertension and diabetes mellitus, cancer and hypertension, hyperlipidemia and diabetes mellitus) that were associated with hypometabolism and decreased cortical thickness in AD signature regions all included vascular conditions. This is consistent with dyads previously found to be associated with risk of incident MCI in the MCSA cohort.3
A second hypothesis is that the aggregate effect of multiple co-occurring chronic conditions on brain pathology may differ from the simple summation of their individual effects—as suggested for example by investigators32 for the co-presence of multiple vascular risk factors and their aggregate effect on brain pathology. As such, the insight provided by the association of multiple co-occurring conditions (i.e., multimorbidity) with antemortem brain pathology is informative and relevant for early detection and intervention for those at increased risk of brain pathology.
The underlying mechanism for the interaction of sex with FDG metabolism is unclear. However, it suggests that multimorbidity may be differentially associated with brain pathology in men vs women as observed for other conditions. For instance, cardiac disease was associated with nonamnestic MCI in women but not in men,33 and atrial fibrillation with cognitive decline in women but not in men.34 The interaction may also be due in part to different combinations of medical conditions reported in men vs women,35 or to sex differences in cerebral glucose metabolism. The borderline interaction and wide CI in estimates for women suggest a low precision, and these cross-sectional findings should be regarded with caution. In particular, higher estimates of brain hypometabolism reported in men30 and a stronger association of multimorbidity with MCI in men than women3 would suggest a stronger association of multimorbidity with brain hypometabolism in men.
Our findings that multimorbidity is not associated with biomarkers of amyloid deposition are in agreement with previous studies suggesting that cardiovascular disease may not directly affect amyloid accumulation.32,36 However, vascular disease could have effects on WM, and likely is associated with cortical atrophy.32 In a clinicopathologic imaging study,37 subcortical vascular pathology and atherosclerosis contributed to lower cortical GM volume after AD pathology was considered, further suggesting the importance of vascular pathology to neurodegenerative processes. Diabetes mellitus and hypertension have also been associated with brain pathology such as ischemic lesions or atrophy in imaging studies.31 These conditions could cause small vessel disease and other cerebrovascular damage or promote neurodegenerative processes through interactions at the cellular level with neurons or synapses. By contrast, while the exact etiology of brain amyloidosis is unclear, it is multifactorial and includes genetics (APOE ε4), inflammation, oxidative damage/stress, and small or large vessel disease. The relative contribution of vascular disease to development of amyloidosis is unclear, but may explain the lack of an association of multimorbidity, which primarily consisted of vascular conditions, with amyloid accumulation. We did not observe any confounding or effect modification by APOE ε4 that could explain the lack of association of multimorbidity with amyloid accumulation.
Limitations of the study warrant consideration. Because of its cross-sectional design, we cannot assess causality. However, longitudinal follow-up of the cohort will enable us to identify the effects of multimorbidity on changes in imaging biomarkers. The exact mechanism of the association of multimorbidity with neuroimaging measures of neurodegeneration cannot be delineated in the current study and findings may not necessarily be generalized in older populations with different distribution and prevalence of chronic conditions. Multimorbidity was assessed later in life and those with earlier onset of comorbidities who had more severe disease may have died or declined to participate; this would result in an underestimate of the associations toward the null, i.e., we could expect even stronger estimated associations had these participants been included. In addition, participants with imaging studies might not be completely representative of the total MCSA population; nonparticipants in imaging studies had a higher frequency of diabetes and hypertension, but did not differ in age or APOE ε4 allele. These differences in the frequency of chronic conditions may bias the association between multimorbidity and imaging biomarkers toward the null. We cannot account for the effects related to efficiency of management of multimorbidity, or adequacy of control of conditions such as diabetes or blood pressure on the observed associations.
The study has several important strengths. The population-based design reduced the potential for selection bias that could occur from recruiting volunteers or studying clinical cohorts only. The state-of-the-art multimodal imaging studies used to ascertain brain pathology provide reliable measures of brain pathology and thereby yields valid estimates of associations. In addition, multimorbidity was assessed using the REP medical records linkage system and was not dependent on self-report and eliminated recall bias.
Given that many of the predictors of chronic conditions are modifiable, preventive strategies targeting the decrease or postponement of multimorbidity may be beneficial for neurodegeneration and neuronal injury that are independent of Alzheimer pathology.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dana Swenson-Dravis, operations manager of the Mayo Clinic Study of Aging; the staff of the Abigail Van Buren Alzheimer's Disease Research Center for recruitment and evaluation of study participants; and study participants for their participation.
GLOSSARY
- 18F-FDG
18fluorodeoxyglucose
- AD
Alzheimer disease
- CI
confidence interval
- GM
gray matter
- HV
hippocampal volume
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- MPRAGE
magnetization-prepared rapid-acquisition gradient echo
- ICD-9
International Classification of Diseases–9
- OR
odds ratio
- PiB
Pittsburgh compound B
- REP
Rochester Epidemiology Project
- ROI
region of interest
- WM
white matter
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Roberts had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Vassilaki and Roberts. Acquisition of data: Drs. Vassilaki, Roberts, Knopman, Geda, Machulda, Mielke, Jack, Lowe, and Petersen. Analysis and interpretation of data: Drs. Vassilaki, Aakre, Kremers, and Roberts. Drafting of the manuscript: Dr. Vassilaki. Critical revision of the manuscript for important intellectual content: Drs. Roberts, Aakre, Alhurani, Knopman, Mielke, Geda, Machulda, Kremers, Jack, Lowe, and Petersen. Statistical analysis: Drs. Vassilaki, Aakre, Kremers, and Roberts. Obtained funding: Drs. Roberts, Mielke, Knopman, Petersen, Jack, and Lowe. Administrative, technical, or material: Drs. Vassilaki, Roberts, and Petersen. Study supervision: Dr. Roberts.
STUDY FUNDING
The study was supported by NIH grants U01 AG006786, K01 AG028573, P50 AG016574, R01 AG011378, and R01 AG041851, and the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program; and was made possible by the Rochester Epidemiology Project (R01 AG034676). This publication was also made possible by support from the Clinical and Translational Science Award Grant Number UL1 TR000135, supporting the Mayo Clinic Center for Clinical and Translational Science (CCaTS), from the National Center for Advancing Translational Sciences (NCATS), a component of NIH.
DISCLOSURE
M. Vassilaki and J. Aakre report no disclosures relevant to the manuscript. M. Mielke receives research grants from NIH/NIA. Y. Geda, W. Kremers, and R. Alhurani report no disclosures relevant to the manuscript. M. Machulda receives research support from the NIH. D. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals, and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH. R. Petersen serves on data monitoring committees for Pfizer, Inc. and Janssen Alzheimer Immunotherapy; is a consultant for Roche, Inc., Merck, Inc., Genentech, Inc., Biogen, Inc., and Eli Lilly and Co.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH. V. Lowe serves on scientific advisory boards for Bayer Schering Pharma and Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI). C. Jack has provided consulting services for Eli Lily and owns stock in Johnson & Johnson. He receives research funding from the NIH (R01-AG011378, RO1 AG041851, U01-AG06786, U01-AG024904, R01 AG37551, R01AG043392) and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. R. Roberts receives research support from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430–439. [DOI] [PubMed] [Google Scholar]
- 2.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 2014;9:e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015;63:1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology 2013;80:2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013;29:753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B, Mielke MM, Parsaik AK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol 2014;71:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H. Future directions in Alzheimer's disease from risk factors to prevention. Biochem Pharmacol 2014;88:661–670. [DOI] [PubMed] [Google Scholar]
- 8.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014;88:640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc 2014;89:1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivnik RJ, Malec JF, Smith GE, et al. Mayo Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol 1992;6:1–104. [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 17.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status: correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol 2015;72:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology 2014;82:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 2015;138(pt 12):3747–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 2009;50:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology 2009;73:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol 2014;13:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 2014;55:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014;82:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bangen KJ, Nation DA, Delano-Wood L, et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer's disease. Alzheimers Dement 2015;11:394–403.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts RO, Geda YE, Knopman DS, et al. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA Neurol 2013;70:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke 1997;28:316–321. [DOI] [PubMed] [Google Scholar]
- 35.Schafer I, Hansen H, Schon G, et al. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care: first results from the multicare cohort study. BMC Health Serv Res 2012;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchant NL, Reed BR, Sanossian N, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol 2013;70:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


