Abstract
Objectives:
To investigate differences in management and outcomes for patients admitted to the hospital with TIA according to care on a stroke unit (SU) or alternate ward setting up to 180 days post event.
Methods:
TIA admissions from 40 hospitals participating in the Australian Stroke Clinical Registry during 2010–2013 were assessed. Propensity score matching was used to assess outcomes by treatment group including Cox proportional hazards regression to compare survival differences and other appropriate multivariable regression models for outcomes including health-related quality of life and readmissions.
Results:
Among 3,007 patients with TIA (mean age 73 years, 54% male), 1,110 pairs could be matched. Compared to management elsewhere in hospitals, management in an SU was associated with improved cumulative survival at 180 days post event (hazard ratio 0.57, 95% confidence interval 0.35–0.94; p = 0.029), despite not being statistically significant at 90 days (hazard ratio 0.66, 95% confidence interval 0.33–1.31; p = 0.237). Overall, there were no differences for being discharged on antihypertensive medication or with a care plan, and the 90- to 180-day self-reported outcomes between these groups were similar. In subgroup analyses of 461 matched pairs treated in hospitals in one Australian state (Queensland), patients treated in an SU were more often prescribed aspirin within 48 hours (73% vs 62%, p < 0.001) and discharged on antithrombotic medications (84% vs 71%, p < 0.001) than those not treated in an SU.
Conclusions:
Hospitalized patients with TIA managed in SUs had better survival at 180 days than those treated in alternate wards, potentially through better management, but further research is needed.
Management in a stroke unit (SU) is recommended for patients with acute stroke,1 reducing the odds of death or dependency by more than 20% compared to alternate wards.2 SU care involves management by clinicians with specialist training and expertise in stroke3 who provide greater access to evidence-based care including acute interventions and secondary prevention.4 In alternate wards, patients are managed by a range of health professionals who may not have specific expertise in stroke or TIA.
Data are limited regarding the ideal care pathway for patients with TIA once a decision has been made to admit them to the hospital.5–8 Recent findings from 15 hospitals contributing to a German registry provided evidence that management in an SU was associated with reduced risk of stroke or death at 90 days only in men with TIA.5 In Australia, declining trends in the 90-day risk of stroke were observed in patients with an incident TIA if managed in hospitals with an SU.8
It remains unclear whether patients with TIA have any longer-term benefits associated with management in an SU. To investigate this, we used data obtained prospectively by clinicians or directly from patients or outcome assessors from the Australian Stroke Clinical Registry (AuSCR).9 We hypothesized that, among patients hospitalized for TIA, management in an SU would be associated with (1) fewer deaths within 180 days, and (2) greater utilization of evidence-based processes of care, when compared to management in an alternate ward.
METHODS
Patient population and procedures.
The AuSCR was established in 2009 to routinely monitor processes of care and health outcomes between 90 and 180 days after symptom onset on consecutive, hospitalized cases of stroke or TIA (see protocol9 and www.auscr.com.au). The AuSCR provides a minimum national dataset of process of care indicators that can be used to provide an assessment of differences in the quality of care and outcome of patients with TIA. Briefly, the AuSCR adheres to the Australian guidelines for best practice in clinical quality registries.10 The current analysis incorporates data from all 40 hospitals that contributed data during the period 2010–2013.
Within the AuSCR, TIA is defined using clinical criteria based on an adaption of the more classic epidemiologic approach as a definitive or probable diagnosis at the time of discharge from hospital that is compatible with a TIA using symptoms of neurologic deficits (persisting for <24 hours from onset) and/or neuroimaging evidence (no abnormalities detected). ICD-10 primary discharge codes are also obtained but were not used to classify patients in this study since coding is undertaken by administrative staff and not clinicians within Australia. All clinical staff who collect and enter the AuSCR data are provided with standardized training and a data dictionary. Random audits of medical records are also undertaken by external auditors to verify the quality of the AuSCR data including clinical diagnosis and additional training provided, if needed.9
The processes of care collected in the AuSCR by all hospitals include the following: management on an SU; treatment with IV tissue plasminogen activator if an ischemic stroke; provision of an antihypertensive agent at discharge; and provision of a discharge care plan developed with the patient or family. In hospitals located in one Australian state (Queensland), an additional 4 processes of care have been collected since 2012: mobilized during admission; aspirin administration within 48 hours; swallow assessment and formal speech pathologist review; and discharged on antithrombotic medications. Missing or unknown data were assumed to be negative for processes of care (ranged from 0% to 8%).
The AuSCR protocol incorporates an “opt-out” approach whereby all eligible cases are registered unless the patient or family nominates to have their data excluded via simple, cost-free options (free-call telephone number or postage-paid). This approach ensures that selection bias is minimized.11 To date, <3% of participants have opted out of the AuSCR.
Patients who were discharged from the participating hospitals, and who had not refused follow-up or had not opted out of the registry, were followed up centrally by trained research staff between 90 and 180 days after symptom onset. A modified Dillman protocol was used, whereby 2 attempts by post were made before an attempt by telephone. Although multiple episodes of care are registered in the AuSCR, patients were only followed up after their first episode.9 At follow-up, data on health-related quality of life (HRQoL) are collected using the EuroQoL-5 dimension-3 level (EQ-5D-3L) instrument.12 Index-based values (“utilities”) for the EQ-5D-3L have been reported using health values derived from discrete choice experiment methods in Australia.13 A utility score of 0 corresponds to an HRQoL state equivalent to death, while a score of 1 represents perfect HRQoL. Patient report of subsequent readmission to hospital and stroke is also recorded.
Data linkage.
Personal identifiers of all registrants in the AuSCR were linked to the National Death Index (NDI) using probabilistic matching by the Australian Institute of Health and Welfare. For these analyses, mortality data in the NDI were used. We also report mortality to 90 days because this is common to other studies of TIA.
The socioeconomic status of participants was estimated using the Index of Relative Socio-economic Disadvantage (IRSD) provided by the Australian Bureau of Statistics.14 The IRSD is calculated for State Suburb Codes using national census data on people and households within those areas, including education, occupation, living conditions, and income. Greater IRSD scores indicate lesser relative disadvantage.
Standard protocol approvals, registrations, and patient consents.
Appropriate ethics and/or governance approvals were obtained for all participating hospitals in the AuSCR. Ethical approval was obtained from the Australian Institute of Health and Welfare to conduct data linkage to the NDI.
Statistical analysis.
Kruskal-Wallis tests and χ2 tests were used to assess differences in patient characteristics. Propensity score matching was used to isolate the influence of SU management and improve internal validity. A propensity score was calculated for all patients with TIA based on age, sex, and ability to walk on admission (as a marker of symptom severity/comorbidity at the time of admission to the hospital).15 Each patient who did not receive treatment in an SU was matched to a similar patient with TIA who received treatment in an SU based on this propensity score, and then processes of care and outcomes were compared.
Regression models for the analysis of outcomes using matched pairs were adjusted for age, sex, IRSD, ability to walk on admission, in-hospital TIA, history of stroke, and transfer from another hospital. Outcome data were analyzed by individual patient and not by episode. Cox proportional hazards regression analysis was conducted to assess rates of death within 90 and 180 days. Quantile regression analysis was also conducted to investigate differences in HRQoL utility scores. Random-effects logistic regression analysis was conducted to investigate rehospitalization and occurrence of stroke or recurrent TIA. To adjust for patient clustering by hospital, we used multilevel analyses for logistic regression analyses, but when using Cox and quantile regression, we adjusted for patient clustering directly. As a sensitivity analysis, regression analyses without propensity score matching were also undertaken. Data were analyzed using StataIC 12.1 (2013; StataCorp, College Station, TX).
RESULTS
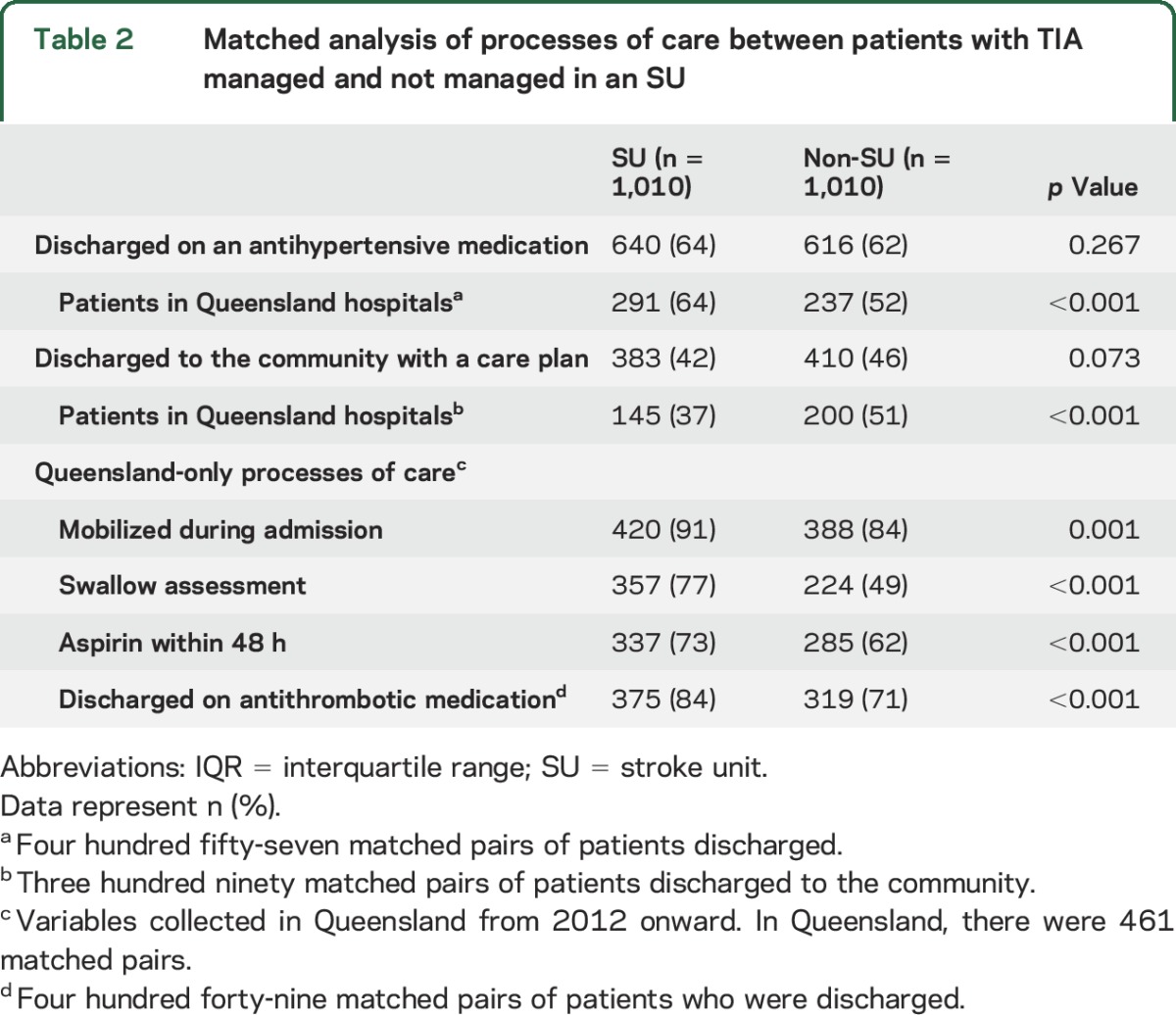
Among the 3,007 registered episodes of TIA (median age 74 years, male 54%), 1,997 were admitted to an SU. Overall, patients with TIA were less often managed in an SU than patients with confirmed stroke (66% vs ischemic stroke 83%, p < 0.001), indicating that they more often received care elsewhere within a hospital. Patients treated in SUs were less disadvantaged, less often born in Australia, less often had an in-hospital event, and were more often discharged home than those not treated in SUs (table 1). In the subset of patients with TIA from Queensland hospitals, those treated in an SU were more often treated with aspirin within 48 hours of admission (73% vs 62%, p < 0.001) and were more often discharged on antithrombotic medication (84% vs 71%, p < 0.001) compared to those treated on an alternate ward (table 2).
Table 1.
Demographic and clinical characteristics of patients with TIA according to management in an SU
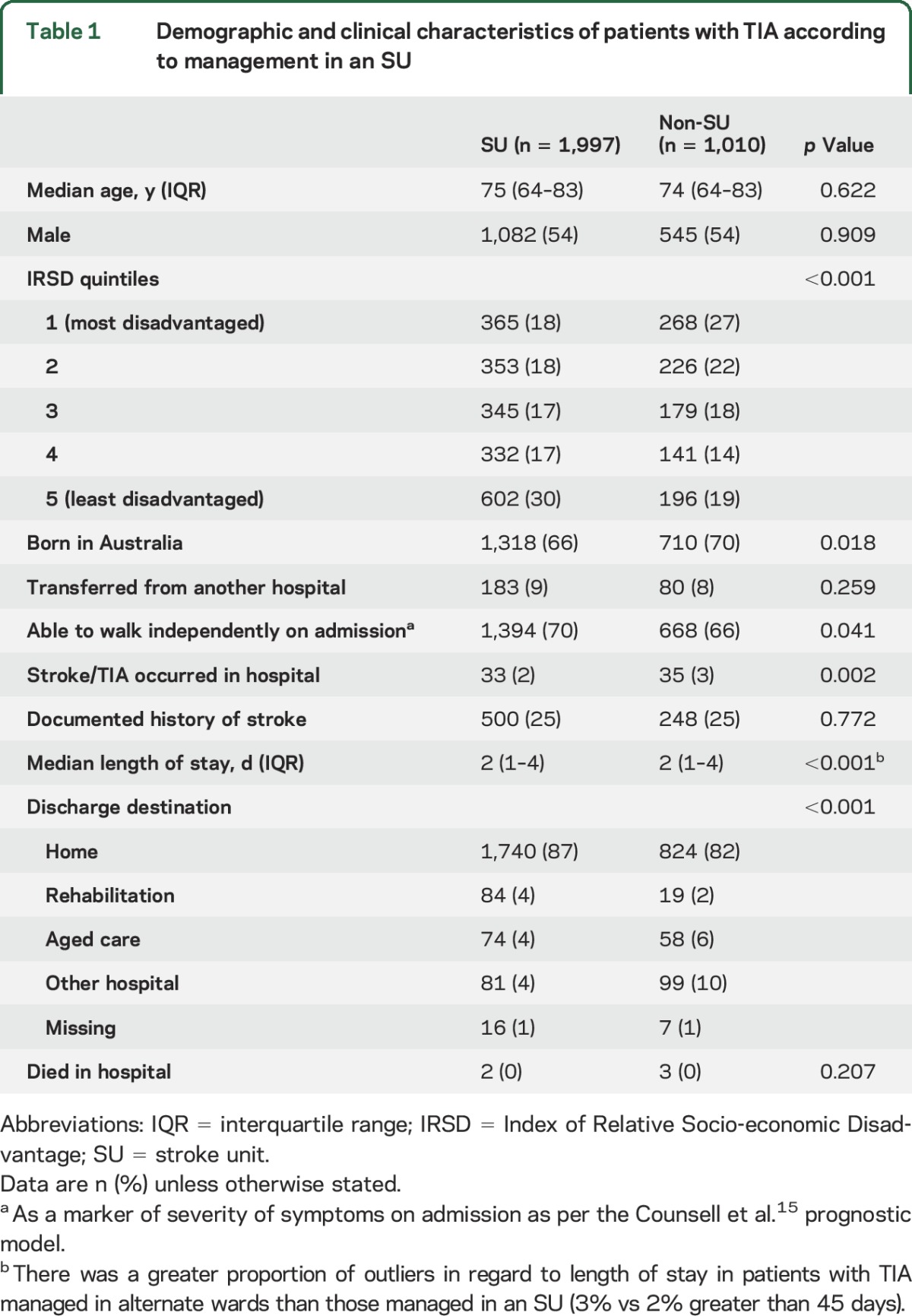
Table 2.
Matched analysis of processes of care between patients with TIA managed and not managed in an SU
Few patients with TIA died in the hospital (n = 5). Using the propensity score matched sample, the number of deaths at 90 and 180 days after admission were similarly low for patients with TIA who were and were not admitted to an SU (90 days: no SU care n = 21 [2.2%] and SU care n = 17 [1.8%]; 180 days: no SU care n = 49 [5.2%] and SU care n = 30 [3.2%]). In Cox proportional hazards regression analysis, there were no differences in the hazard of death at 90 days after admission for TIA between those who were and were not admitted to an SU (hazard ratio 0.66, 95% confidence interval 0.33–1.31; p = 0.237) (table 3, figure). However, patients with TIA who were managed in SUs had a reduced hazard of death at 180 days after admission (hazard ratio 0.57, 95% confidence interval 0.35–0.94; p = 0.029) when compared to patients not admitted to an SU.
Table 3.
Survival analysis of patients with TIA based on treatment in stroke units
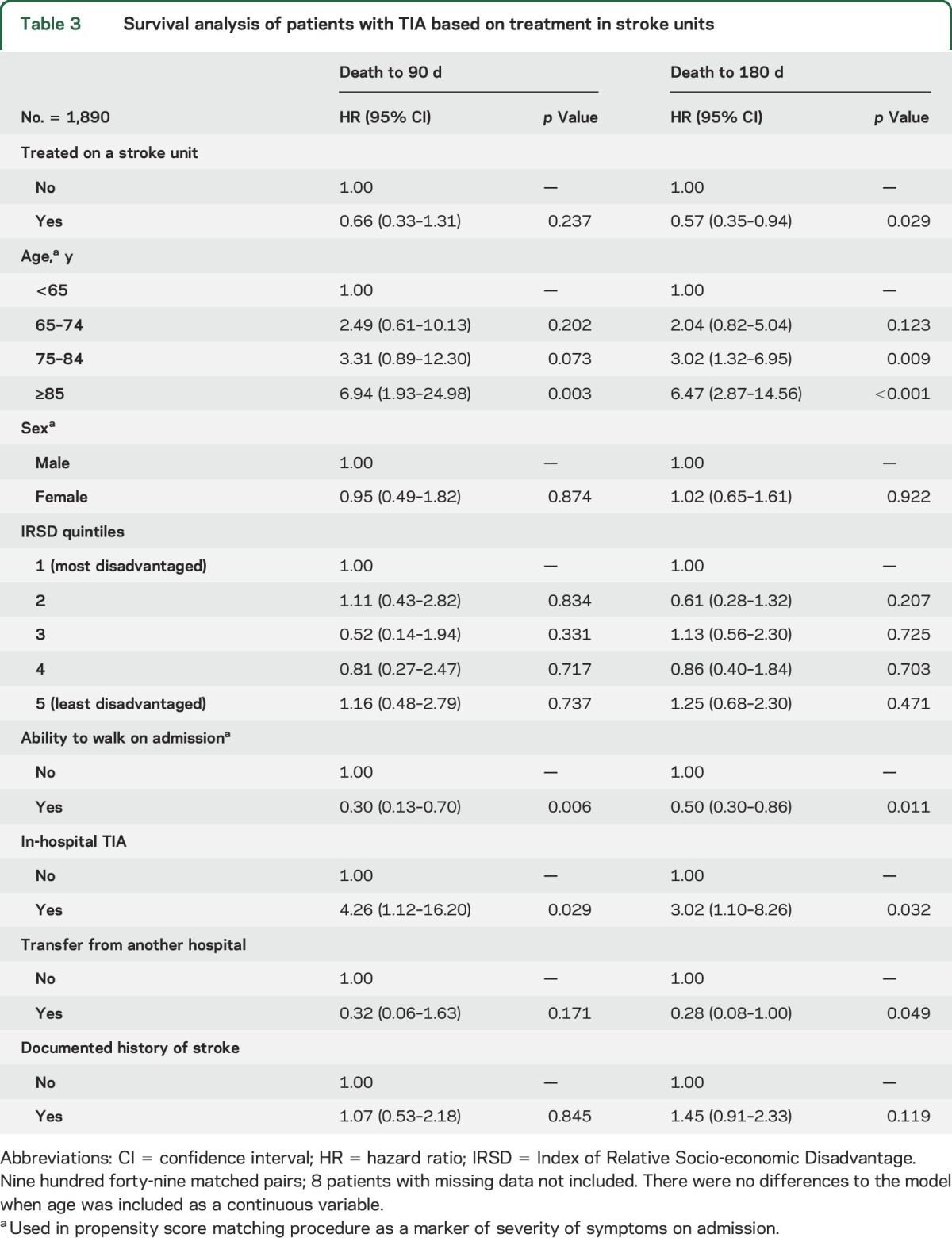
Figure. Cumulative hazard of death to 180 days after TIA according to management in a stroke unit.
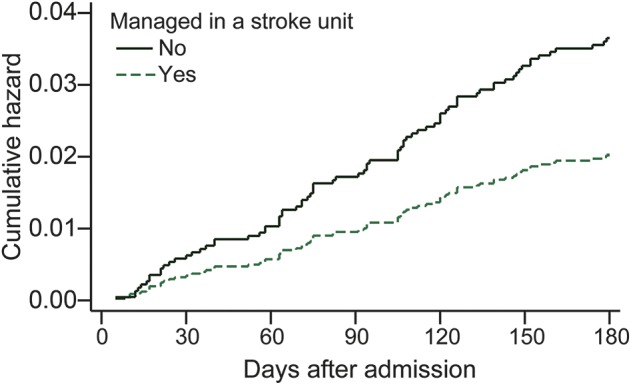
Adjusted for age, sex, place of birth, socioeconomic status, ability to walk on admission, in-hospital TIA, history of stroke, and transfer from another hospital using a matched 1:1 cohort based on propensity score methods. Cumulative hazard is the number of events that would be expected for each individual in a group at a given time if the event were a repeated process. No. = 1,890 (945 matched pairs).
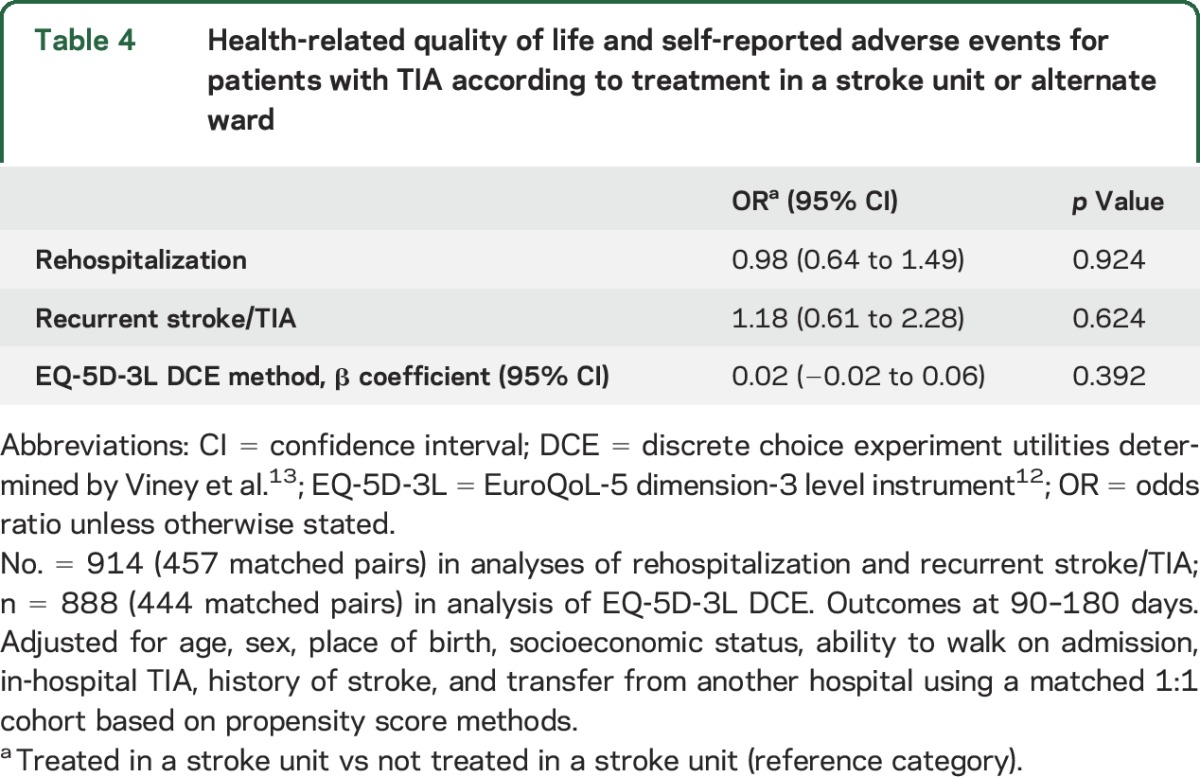
A greater proportion of patients with TIA treated in an SU completed a follow-up interview than those not treated in an SU (SU 59% vs 48%, p < 0.001). The median time to follow-up was 101 days (interquartile range 96–107). There was no statistical difference in the proportion reporting readmission to the hospital based on care setting (SU 22% vs non-SU 24%, p = 0.432). Similarly, there were no detectable differences based on care setting in reports of new strokes since admission (8% in both groups, p = 0.806). The median EQ-5D utility score was 0.79 for patients who were treated in an SU and 0.81 for those who were not treated in an SU. There were no detectable differences in these outcomes between groups after adjustment (table 4).
Table 4.
Health-related quality of life and self-reported adverse events for patients with TIA according to treatment in a stroke unit or alternate ward
The results from the propensity score matching methods were robust. Results were similar when the full sample of 3,007 TIA patients were included in standard logistic regression multilevel models (e.g., difference in HRQoL β coefficient 0.03, 95% confidence interval 0.00–0.05; p = 0.051), except that those managed in SUs were more often discharged on antihypertensive medications (70% vs 61%, p < 0.001). The hazard of death was similar after exclusion of TIAs that occurred while in the hospital for another condition and when adjusting for symptom onset to arrival time (see tables e-1 and e-2 on the Neurology® Web site at Neurology.org).
DISCUSSION
This is the largest reported study of outcomes after TIA according to the setting of management after admission to the hospital. Compared to alternate wards, treatment in an SU was associated with a 45% reduced cumulative hazard of death at 180 days, but no difference was observed at 90 days. Our results were comparable to studies of the benefits of rapid-access TIA clinics,16–18 since our 90-day survival rates fell within their 95% confidence limits.
Our results may partly be explained by our low event rates. The proportion of patients in our study who reported having had a recurrent stroke at 90 to 180 days follow-up (8%) was less than that reported in a systematic review of 18 studies (average approximately 11% at 90 days, range 0.6%–20.6%)19 but appeared to be greater than that reported from studies on the benefits of rapid-access TIA clinics,16–18 including the Australian M3T model.20 It is possible that a different case mix of patients may have contributed to this observed difference in outcome since patients seen in rapid-access TIA clinics tended to be younger (by up to 10 years) than in our hospitalized sample. Furthermore, the large proportion of patients with TIA in our sample who were unable to walk on admission suggests selection bias toward admission of those with more severe or persistent symptoms, and potentially greater comorbidity or frailty, both of which may be associated with greater incidence of subsequent events.
A small proportion of patients with TIA were discharged to an aged care facility, and this was less often observed if they were managed in an SU.4 This is consistent with the findings of another study in which patients with stroke receiving care in an SU were more often discharged directly home compared to those who received care on a general ward. It was anticipated that few patients with TIA would be discharged to aged care, but this is difficult for us to reconcile because the AuSCR does not collect preadmission residence and so we were unable to identify patients who were living in an aged care facility before their TIA. We observed that patients who were treated in an SU were less often disadvantaged than those who were not. However, we found no association between socioeconomic status and outcomes. Nevertheless, this may point to a disparity in health care delivery, which warrants further investigation.
Processes of care that are characteristic of SUs that potentially improve outcomes include delivery of more appropriate early secondary prevention, assessment for etiology, early management, and discharge care planning.4 In this study, important processes of care, such as assessment of swallow function and hyperacute aspirin therapy, differed between those treated in an SU and those who were not. However, commencement of early secondary prevention before discharge was mixed. There was no difference in antihypertensive medication prescription at discharge between groups overall, but a difference was observed in the Queensland subgroup. When compared to patients with TIA managed in an alternate ward, patients with TIA who were managed in an SU were more often treated with antithrombotic medications, which is a potential explanatory factor for the observed difference in survival. However, the use of antithrombotic medication was only captured in hospitals in Queensland (58% of total AuSCR hospitals and 45% of all TIA episodes in AuSCR) and these patients may not be representative of all patients from hospitals using AuSCR. In addition, it remains unclear what happens in primary care or the outpatient setting post discharge, since only hospital discharge medications are captured in this study. It is possible that communication with primary care doctors or follow-up care is better for patients experiencing TIA who are treated in an SU than those who are not, potentially resulting in a longer-term survival advantage.
Of note, there are different models of care for patients with TIA in Australia.21 Because of the milder symptoms associated with TIA, hospitals may have policies to admit these cases to short-stay units, protocol-based observation units, or outpatient clinics that provide a similar quality at a reduced cost to hospitals than using an admit-all approach.22,23 Patients with TIA who are admitted are likely to have different characteristics than patients with TIA seen in outpatient clinics. Several authors have developed algorithms to identify people with TIA who are at greatest risk of early stroke to help fast track the patients who require rapid investigation or admission to hospital.24,25 While some groups have demonstrated safe management of TIA without admission to the hospital, this is still a specialized alternative.20 Improving availability of specialized stroke care in Australia, and elsewhere, is likely to improve the management of patients hospitalized with TIA. Electronic clinical support tools may assist health professionals in providing appropriate outpatient care to patients with TIA in locations where access to specialists is limited.26
The strengths of this study include the large sample size from all 40 hospitals participating in the AuSCR during the study period (21% of 195 hospitals known to admit people with acute stroke and TIA in Australia)27 located in urban and rural locations, and the confirmation of death status in all patients using linked data from the NDI. In addition, appropriate statistical techniques for nonrandomized data, including propensity score matching, were used to maximize comparability between patients who were and were not managed in an SU. Therefore, the between-group imbalances in variables used to match are unlikely to account for these results. The results from this model were consistent with those found using standard analytic methods, thereby demonstrating the robustness of our findings.
There are some limitations to our study. In particular, there were limited covariates available in the AuSCR minimum dataset to use in the multivariable analyses, thereby raising the potential for residual confounding. For example, established prognostic factors such as hypertension, diabetes, TIA duration, and clinical symptoms were unavailable. One complexity that arises with this, or other standard multivariable statistical approaches, is that we cannot accurately account for unmeasured confounding factors. For example, the more gradual accumulation of deaths in the non-SU group over time may have been attributable to greater preadmission comorbidity that we were unable to take into account. However, we have adjusted for a prognostic measure of severity of disease validated for stroke when investigating outcomes.15 In future work, we will be able to expand our registry variables through person-level linkage to other health datasets to enable our models to account for important prestroke comorbidity.28
Another limitation is that those who were followed up were more often treated in an SU than those who were not. This response bias may explain why we did not observe differences in self-reported readmissions, recurrent stroke, and HRQoL. In addition, because death, recurrent stroke, and readmissions were relatively few within the follow-up time frame, we were likely underpowered to detect a difference in these outcomes at 90-day follow-up. Unfortunately, not all patients were able to be followed up for HRQoL data because of resource constraints. In addition, it was also not possible to adjust for the different policies for management used by hospitals to admit patients with TIA because information on this is not collected in the AuSCR. Lastly, duration of care in an SU was not recorded and therefore we were unable to investigate a dose response.
SU management was associated with reduced mortality at 180 days after admission for TIA. Given the nonrandomized study design, this finding requires confirmation. In addition, whether or not the benefit of SU care persists beyond 180 days after admission for TIA should be investigated. Furthermore, it will be important to determine the effect of SU care on other outcomes in these patients, such as the adherence to secondary prevention therapies and the frequency of future stroke and acute myocardial infarction. Linkage of the AuSCR data to hospital administrative databases will enable better assessments of subsequent cardiovascular events in these patients.28 These findings provide evidence that supports the treatment of TIA in an SU, where admission to the hospital is warranted, as this may improve longer-term survival outcomes for patients.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Joyce Lim, Sabrina Small, Francis Kung, Karen Moss, Steven Street, and Renee Stojanovic from the AuSCR Office who contributed to AuSCR operations during this study period. Staff from The George Institute, the Florey, and the National Stroke Foundation are acknowledged for their contributions to patient follow-up. The authors thank the hospital staff for their diligence regarding data collection for AuSCR. Current hospital site investigators who provided data between 2010 and 2013 are also acknowledged (see coinvestigator list on the Neurology Web site at Neurology.org). The authors also acknowledge Leonid Churilov for his advice on propensity score matching analyses.
GLOSSARY
- AuSCR
Australian Stroke Clinical Registry
- EQ-5D-3L
EuroQoL-5 dimension-3 level
- HRQoL
health-related quality of life
- ICD-10
International Classification of Diseases, Tenth Revision
- IRSD
Index of Relative Socio-economic Disadvantage
- NDI
National Death Index
- SU
stroke unit
Footnotes
Supplemental data at Neurology.org
Editorial, page 2030
Contributor Information
Collaborators: Australian Stroke Clinical Registry Consortium, James Hughes, Martin Jude, Fiona Ryan, Melissa Gill, Geoffrey Herkes, Andrew Wong, Noel Saines, Richard Geraghty, Pradeep Bambery, Christopher Staples, Amanda Siller, Richard White, Arman Sabet, Eva Salud, Martin Dunlop, Nisal Gange, Paula Easton, Graham Mahaffey, Graham Hall, Carolyn De Wytt, Sean Butler, Paul Laird, Karen Hines, David Douglas, M Admin, Suzana Milosevic, Joel Iedema, Stephen Read, Francis Hishon, David Blacker, Tim Bates, Helen Castley, Mark Mackay, Christopher Bladin, Ernie Butler, Peter O’Brien, Douglas Crompton, Sharan Ermel, Michael Pollack, Frances Simmonds, Julie Bernhardt, Erin Lalor, John McNeil, Mark Simcocks, Andrew Lee, Richard Lindley, Greg Cadigan, Peter Hand, Andrew Evans, Andrew Wesseldine, and Susan Hillier
AUTHOR CONTRIBUTIONS
D.A.C.: conceptualization and design of the study, drafting of the manuscript, supervision of analyses, interpretation of the data. J.K.: drafting of the manuscript, literature review, analysis of data, interpretation of the data. N.A.L.: conceptualization and design of the study, revisions, and interpretation of the data. C.R.L.: conceptualization and design of the study, revisions, and interpretation of the data. H.M.D.: conceptualization and design of the study, revisions, and interpretation of the data. K.H.: conceptualization and design of the study, revisions, and interpretation of the data. S.F.: conceptualization and design of the study, revisions, and interpretation of the data. N.E.A.: revisions to the manuscript, contribution to data analysis methods, interpretation of the data. M.F.K.: revisions to the manuscript, contribution to data analysis methods, interpretation of the data. R.G.: conceptualization and design of the study, revisions, and interpretation of the data. A.G.T.: conceptualization and design of the study, revisions, and interpretation of the data. B.G.: oversight of AuSCR operations including supervision of data management and data collection process, revisions to the manuscript. S.M.: conceptualization and design of the study, revisions, and interpretation of the data. C.S.A.: conceptualization and design of the study, revisions, and interpretation of the data. G.A.D.: conceptualization and design of the study, drafting of the manuscript, interpretation of the data.
STUDY FUNDING
D.A.C. was supported by a fellowship from the National Health and Medical Research Council (NHMRC; 1063761 cofunded by the National Heart Foundation). A.G.T. was supported by a fellowship from the NHMRC (1042600). N.E.A. was supported by an NHMRC Early Career Fellowship (1072053). C.S.A. holds an NHMRC Senior Principal Research fellowship (1081356). AuSCR was supported by grants from the NHMRC (1034415), Allergan, Ipsen, Boehringer Ingelheim, Monash University, Queensland Health, and the National Stroke Foundation.
DISCLOSURE
D. Cadilhac: restricted educational grants from Allergan Australia (2009, 2010), Ipsen (2010), and Boehringer (2013) awarded to the Management Committee of the Australian Stroke Clinical Registry (AuSCR) to improve aspects of the data tool or as contributions to supporting an annual national workshop. Data custodian for the AuSCR. J. Kim reports no disclosures relevant to the manuscript. N. Lannin: restricted educational grants from Allergan Australia (2009, 2010) and Ipsen (2010) awarded to the Management Committee of the AuSCR to improve aspects of the data tool. C. Levi: as per N.L. above regarding industry support for AuSCR. member, Clinical Council, National Stroke Foundation. H. Dewey: as per N.L. above regarding industry support for AuSCR. K. Hill reports no disclosures relevant to the manuscript. S. Faux: as per N.L. above regarding industry support for AuSCR. N. Andrew, M. Kilkenny, and R. Grimley report no disclosures relevant to the manuscript. A. Thrift: board member, National Stroke Foundation. B. Grabsch reports no disclosures relevant to the manuscript. S. Middleton: member, Clinical Council, National Stroke Foundation. C. Anderson: as per N.L. above regarding industry support for AuSCR. G. Donnan: as per D.A.C. above regarding industry support for AuSCR. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 2.Stroke Unit Trialists Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;9:CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorne P, Pollock A. What are the components of effective stroke unit care? Age Ageing 2002;31:365–371. [DOI] [PubMed] [Google Scholar]
- 4.Cadilhac DA, Pearce DC, Levi CR, Donnan GA; Greater Metropolitan Clinical Taskforce, New South Wales Stroke Services Coordinating Committee. Improvements in the quality of care and health outcomes with new stroke care units following implementation of a clinician-led, health system redesign programme in New South Wales, Australia. Qual Saf Health Care 2008;17:329–333. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khaled M, Matthis C, Eggers J. The prognostic impact of the stroke unit care versus conventional care in treatment of patients with transient ischemic attack: a prospective population-based German study. J Vasc Interv Neurol 2013;5:22–26. [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Khaled M, Matthis C, Seidel G. The prognostic impact of the stroke unit concept after transient ischemic attack. Clin Neurol Neurosurg 2013;115:725–728. [DOI] [PubMed] [Google Scholar]
- 7.Walter A, Seidel G, Thie A, Raspe H. Semi-intensive stroke unit versus conventional care in acute ischemic stroke or TIA: a prospective study in Germany. J Neurol Sci 2009;287:131–137. [DOI] [PubMed] [Google Scholar]
- 8.Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke 2014;45:3214–3218. [DOI] [PubMed] [Google Scholar]
- 9.Cadilhac DA, Lannin NA, Anderson CS, et al. Protocol and pilot data for establishing the Australian Stroke Clinical Registry. Int J Stroke 2010;5:217–226. [DOI] [PubMed] [Google Scholar]
- 10.Evans SM, Scott IA, Johnson NP, Cameron PA, McNeil JJ. Development of clinical-quality registries in Australia: the way forward. Med J Aust 2011;194:360–363. [DOI] [PubMed] [Google Scholar]
- 11.Tu JV, Willison DJ, Silver FL, et al. Impracticability of informed consent in the Registry of the Canadian Stroke Network. N Engl J Med 2004;350:1414–1421. [DOI] [PubMed] [Google Scholar]
- 12.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke? Stroke 1997;28:1876–1882. [DOI] [PubMed] [Google Scholar]
- 13.Viney R, Norman R, Brazier J, et al. An Australian discrete choice experiment to value EQ-5D health states. Health Econ 2014;23:729–742. [DOI] [PubMed] [Google Scholar]
- 14.Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA), Australia, 2011. Canberra: Australian Bureau of Statistics; 2011. [Google Scholar]
- 15.Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke 2002;33:1041–1047. [DOI] [PubMed] [Google Scholar]
- 16.Dutta D, Bowen E, Foy C. Four year follow up of transient ischemic attacks, strokes, and mimics: a retrospective transient ischemic attack clinic cohort study. Stroke 2015;46:1227–1232. [DOI] [PubMed] [Google Scholar]
- 17.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS Study): a prospective population-based sequential comparison. Lancet 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 19.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007;6:1063–1072. [DOI] [PubMed] [Google Scholar]
- 20.Sanders LM, Srikanth VK, Jolley DJ, et al. Monash transient ischemic attack triaging treatment: safety of a transient ischemic attack mechanism-based outpatient model of care. Stroke 2012;43:2936–2941. [DOI] [PubMed] [Google Scholar]
- 21.Price CJ, Blacker DJ, Grimley RS, et al. National survey of management of transient ischaemic attack in Australia: take immediate action. Med J Aust 2009;191:17–20. [DOI] [PubMed] [Google Scholar]
- 22.Nahab F, Leach G, Kingston C, et al. Impact of an emergency department observation unit transient ischemic attack protocol on length of stay and cost. J Stroke Cerebrovasc Dis 2012;21:673–678. [DOI] [PubMed] [Google Scholar]
- 23.Sanders LM, Cadilhac DA, Srikanth VK, Chong CP, Phan TG. Is nonadmission-based care for TIA patients cost-effective? A microcosting study. Neurol Clin Pract 2014;5:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu AYX, Coutts SB. Stroke: risk assessment to prevent recurrence after mild stroke or TIA. Nat Rev Neurol 2015;11:131–133. [DOI] [PubMed] [Google Scholar]
- 25.Sanders LM, Srikanth VK, Blacker DJ, Jolley DJ, Cooper KA, Phan TG. Performance of the ABCD2 score for stroke risk post TIA: meta-analysis and probability modeling. Neurology 2012;79:971–980. [DOI] [PubMed] [Google Scholar]
- 26.Ranta A, Cariga P. Who should manage transient ischemic attacks? A comparison between stroke experts, generalists, and electronic decision support. N Z Med J 2013;126:25–31. [PubMed] [Google Scholar]
- 27.National Stroke Foundation. National Stroke Audit: Organisational Survey Report 2013. Melbourne: National Stroke Foundation; 2013. [Google Scholar]
- 28.Cadilhac DA, Sundararajan V, Andrew N, et al. Using linked data to more comprehensively measure the quality of care for stroke: understanding the issues [online]. Australas Epidemiol 2013;20:15–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


