Abstract
Objective:
To examine the association between consumption of seafood and long-chain n-3 fatty acids with change in 5 cognitive domains over an average of 4.9 years.
Methods:
From an ongoing longitudinal, community-based epidemiologic study of aging and dementia (the Rush Memory and Aging Project), we included 915 participants (age 81.4 ± 7.2 years, 25% men) who had completed at least one follow-up cognitive assessment and dietary data. Diet was assessed by semiquantitative food frequency questionnaire. Scores for global cognitive function and 5 cognitive domains (episodic, semantic, and working memory, perceptual speed, and visuospatial ability) were assessed using 19 cognitive tests. Mixed models adjusted for multiple risk factors of cognitive change were used to assess the associations.
Results:
Consumption of seafood was associated with slower decline in semantic memory (β = 0.024; p = 0.03) and perceptual speed (β = 0.020; p = 0.05) in separate models adjusted for age, sex, education, participation in cognitive activities, physical activity, alcohol consumption, smoking, and total energy intake. In secondary analyses, APOE ε4 carriers demonstrated slower rates of decline in global cognition and in multiple cognitive domains with weekly seafood consumption and with moderate to high long-chain n-3 fatty acid intake from food. These associations were not present in APOE ε4 noncarriers. Higher intake levels of α-linolenic acid were associated with slower global cognitive decline, but also only in APOE ε4 carriers.
Conclusions:
These results suggest protective relations of one meal per week of seafood and long-chain n-3 fatty acids against decline in multiple cognitive domains. The role of APOE ε4 in this association needs further study.
The primary structural component of the brain is the long-chain n-3 fatty acid, docosahexaenoic acid (DHA) (22:6 n-3), of which the direct nutrient source is seafood. DHA is also metabolized in vivo from the n-3 fatty acids eicosapentaenoic acid (EPA) (20:5 n-3) and α-linolenic acid (ALA) (18:3 n-3). The importance of seafood and n-3 fatty acids in the prevention of dementia has been demonstrated through a number of prospective epidemiologic studies,1,2 most of which reported findings for global cognitive functioning. Few examined associations with specific cognitive domains that may provide clues to the underlying biologic mechanisms of effect. Further, we are not aware of a previous study investigating whether APOE, the gene encoding the lipid protein responsible for intercellular trafficking of cholesterol and other lipids involved in brain composition and functioning,3 and more specifically the APOE ε4 genotype, a major risk factor for dementia,4 might modify the relations of seafood/n-3 fatty acid intake to domain-specific cognitive decline. We investigated whether APOE ε4 modifies the association between seafood and n-3 fatty acid intakes and domain-specific cognitive decline in a community-based, prospective study.
METHODS
Study population.
Participants are from the Rush Memory and Aging Project (MAP), an ongoing longitudinal, community-based study of aging and dementia.5 They were recruited from more than 40 retirement and subsidized housing communities across northeastern Illinois, in addition to church groups and social service agencies. Participants were free of known dementia at enrollment into the study and agreed to annual clinical evaluation. From February 2004 to 2013, the MAP study participants were invited to complete food frequency questionnaires. During that period, there were 1,306 active participants, 1,068 of whom completed the dietary questionnaire, and 960 survived and completed at least one follow-up cognitive assessment for the analyses of change in cognition. Of these, 915 had nonmissing data on n-3 fatty acid dietary components.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board of Rush University Medical Center approved this study and all participants signed written informed consent.
Dietary assessment.
Dietary intake was assessed by a semiquantitative food frequency questionnaire (FFQ) that was validated for use in a population-based study of older community residents.6 Participants were asked to report usual frequency of intake of 144 food items over the previous 12 months. Nutrient levels and total energy for each food item were based either on natural portion sizes (e.g., slice of bread) or according to age- and sex-specific portion sizes from national dietary surveys. The FFQ included 4 seafood items: tunafish sandwich, fish sticks/fish cakes/fish sandwich, fresh fish as a main dish, and shrimp/lobster/crab. The use of fish oil supplements was also ascertained. Weekly consumption of seafood was computed by summing the responses to the 4 seafood items. Intakes of n-3 fatty acids were obtained by multiplying the nutrient content of individual food items by the frequency of consumption and summing over all items. For these analyses, we investigated dietary intakes of the long-chain n-3 fatty acids, EPA (20:5 n-3), DHA (22:6 n-3), and ALA (18:3 n-3), a long-chain n-3 fatty acid that can be consumed through plant sources. Nutrient intakes were energy-adjusted by the regression residual method.7
Cognitive testing.
A standardized battery including 21 cognitive tests was administered at each annual evaluation, 19 of which were used to measure decline in global cognition and 5 cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability, as previously described.8 For analyses, we computed composite scores of the 5 domains and of all 19 tests to measure global cognition. To compute the composite measures, raw scores on each test were first converted to z scores using the baseline mean and standard deviation for the entire cohort. The z scores were then averaged over all tests. The composite scores minimize floor and ceiling effects of the individual tests as well as other sources of measurement error. The Mini-Mental State Examination9 was also administered for descriptive purposes.
Covariates.
Model covariates were based on information current to the baseline cognitive assessment for these analyses. Age and education were modeled in years. Smoking behavior (never smoked, former smoker, or current smoker) was based on a series of questions. Alcohol consumption (grams per day) was computed from 3 questions on the FFQ about usual frequency of consumption of beer, wine, and liquor. Frequency of participation in cognitively stimulating activities was quantified with a previously established scale8 wherein people rated (5-point scale) how often over the past year they had participated in 7 cognitive activities (reading, library visits, reading newspapers, reading magazines, reading books, writing letters, playing games). Hours of physical activity per week was based on standardized questions10,11 that assessed the sum total of minutes spent on each of 5 activities (walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, swimming or water exercise). Presence of diabetes (self-reported history or antidiabetic medication use), hypertension (self-reported history, measured blood pressure ≥160 mm Hg systolic or ≥95 mm Hg diastolic, or current use of antihypertensive medications), heart disease (self-reported history of myocardial infarction or digitalis use), and stroke (self-reported history and neurologic examination) were modeled as dichotomous variables.
APOE genotype was determined using DNA extracted from peripheral blood lymphocytes as previously described.12 Genotyping was performed by Agencourt Bioscience Corporation (Beverly, MA). Participants with one or more copies of the ε4 allele were considered ε4 carriers.
Statistical analysis.
We used linear mixed models to examine the relations of seafood meals and n-3 fatty acid intake to change in cognitive function. Both linear and nonlinear relations of the n-3 fatty acid nutrient variables were considered by modeling the variables in tertiles and also as linear trend variables in which all participants in a tertile were coded at the median level and modeled as a categorical variable. Seafood consumption was modeled as an indicator variable of one or more (1+) seafood meals per week vs <1 (the referent category) based on findings from previous studies.13–17 Models were adjusted for age (years), sex, education (years), frequency of participation in cognitively stimulating activities, energy intake (only for seafood models), physical activity (hours of activity per week), alcohol consumption (grams per day of beer, wine, and liquor), smoking (never/former/current), time, and time interactions with each model covariate. In a second model, we added covariates for cardiovascular risk factors (hypertension, diabetes, myocardial infarction, and stroke) that may be considered both potential mediators and confounders of the hypothesized relations. We performed tests for statistical interaction by adding to the basic model 2-way multiplicative terms between the dietary variable and APOE ε4 and 3-way multiplicative terms between the dietary variable, APOE-ε4, and time.
Because of significant interactions between intakes of seafood and n-3 fatty acids and APOE ε4 and time for the cognitive outcomes in the basic-adjusted models, we subsequently ran separate models within strata of APOE ε4 carriers and APOE ε4 noncarriers as secondary analyses. All analyses were performed using SAS (SAS Institute, Cary, NC) and p ≤ 0.05 was considered significant.
RESULTS
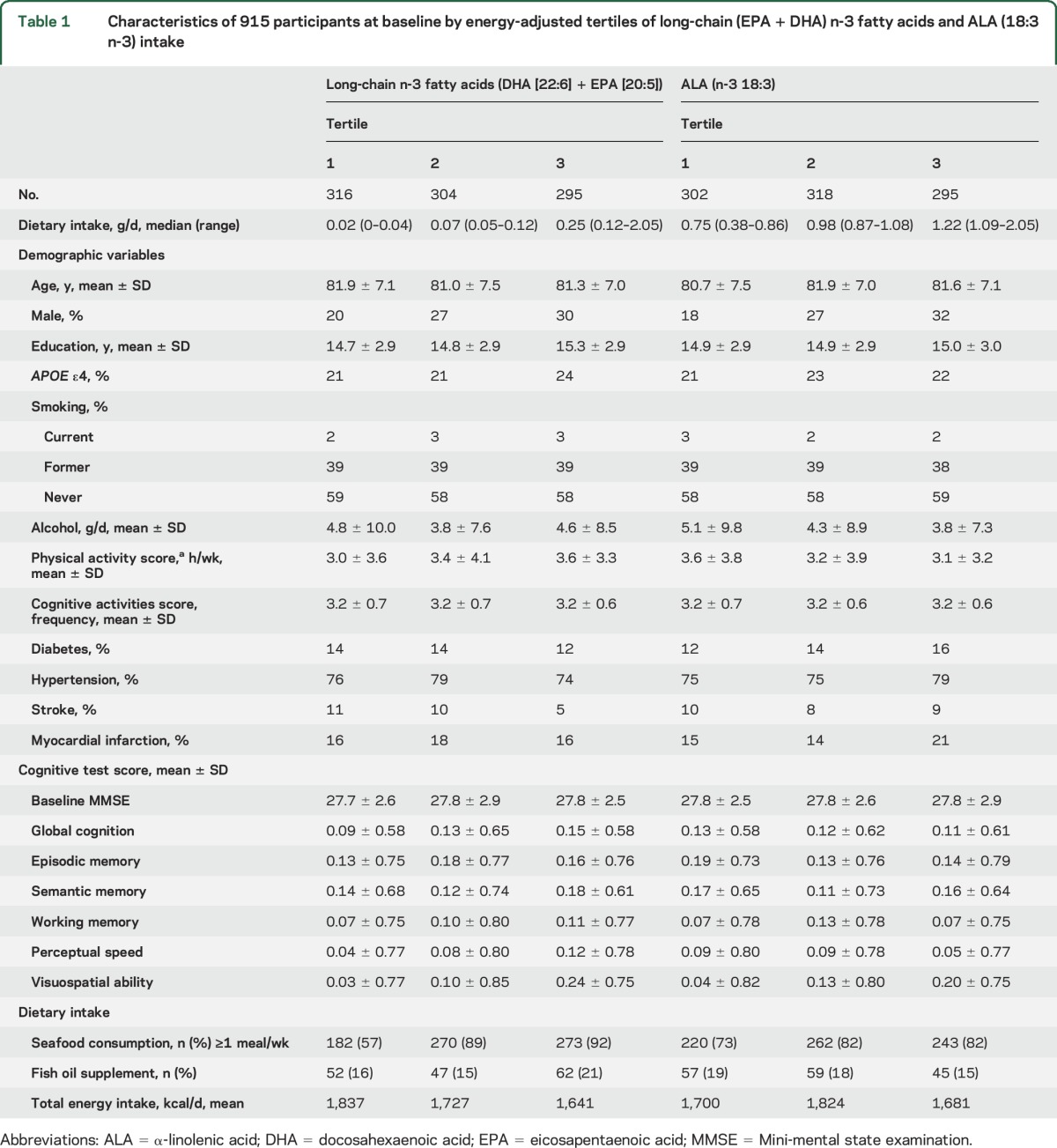
The sample of 915 participants was followed on average 4.9 ± 2.5 years and had a mean of 5.7 cognitive assessments. Mean age of the participants at baseline was 81.4 ± 7.2 years and 25.5% were male. Compared with participants in the lowest tertile of long-chain n-3 fatty acid intake, those in the highest tertile were more likely to be male, APOE ε4 carriers, and physically active, and somewhat less likely to have a history of diabetes, hypertension, or stroke. They also had higher baseline scores on some of the cognitive domains (table 1). Participants in the highest tertile of ALA intake tended to have lower alcohol consumption and to be less physically active than participants in the lowest tertile, and were more likely to have a history of cardiovascular conditions.
Table 1.
Characteristics of 915 participants at baseline by energy-adjusted tertiles of long-chain (EPA + DHA) n-3 fatty acids and ALA (18:3 n-3) intake
Nutrient relations in the total sample.
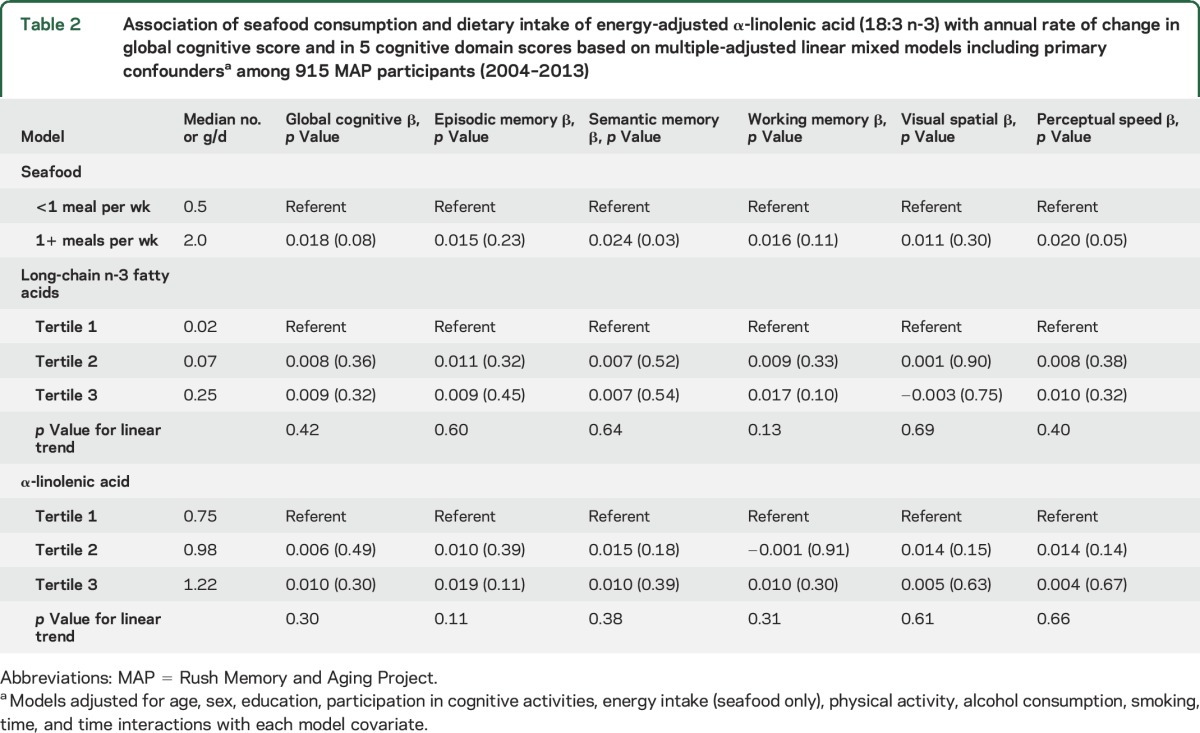
Global cognitive scores in the total sample declined on average by 0.083 standardized units per year (SU/y). When we examined the decline in rates by seafood consumption in models adjusted for age, sex, education, total caloric intake, alcohol consumption, smoking, and participation in cognitive and physical activities, those who consumed one or more seafood meals per week did not demonstrate slower decline in global cognition, but did have significantly slower rates of decline in semantic memory and perceptual speed compared with those who consumed less (table 2). These associations did not change with additional adjustment for cardiovascular risk factors (stroke, hypertension, myocardial infarction, diabetes) that could potentially mediate the relations between seafood and decline (data not shown). Dietary intakes of ALA (table 2) and food sources of the long-chain n-3 fatty acids were not associated with any measure of cognitive decline in the overall sample. However, the group of 161 fish oil supplement consumers had slower rates of decline in the global cognitive measure (β = 0.024, p = 0.02) and in episodic memory (β = 0.027, p = 0.03) compared with the nonconsumers.
Table 2.
Association of seafood consumption and dietary intake of energy-adjusted α-linolenic acid (18:3 n-3) with annual rate of change in global cognitive score and in 5 cognitive domain scores based on multiple-adjusted linear mixed models including primary confoundersa among 915 MAP participants (2004–2013)
Analyses by APOE ε4 status.
We next investigated potential modifications in the associations of the nutrient variables on cognitive decline by APOE ε4 status. Because statistical interactions were observed among all 3 nutrient variables and multiple cognitive measures and time in the basic adjusted models, we conducted secondary analyses stratified by APOE ε4 status (table 3 and table e-1 on the Neurology® Web site at Neurology.org).
Table 3.
Association of seafood and of energy-adjusted long-chain (EPA + DHA) n-3 fatty acids and ALA (18:3 n-3) with annual rate of change in global cognitive score and in 5 cognitive domain scores among 178 MAP participants who were APOE ε4–positive based on multiple-adjusted linear mixed modelsa (2004–2013)
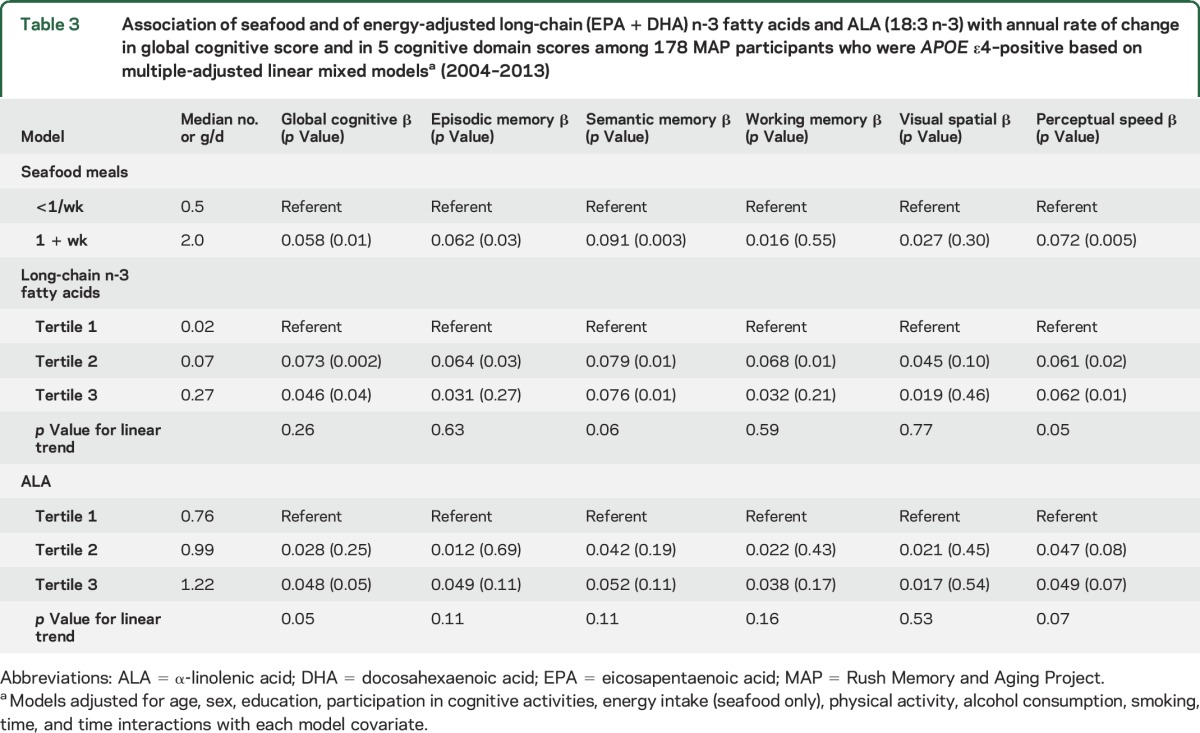
Among the 178 APOE ε4 carriers, the mean rate of decline in global score was −0.126 SU/y. Weekly seafood consumption in this group was associated with slower declines in global cognitive score (figure), episodic memory, semantic memory, and perceptual speed compared with less consumption (table 3). APOE ε4 carriers also demonstrated linear protective relations with increased intakes of long-chain n-3 fatty acids reflected as slower declines in semantic memory and perceptual speed. However, nonlinear associations for the long-chain n-3 fatty acids were observed for global cognition, episodic memory, and working memory, such that participants in the second tertile of intakes had significantly slower declines, but there were no benefits with higher intakes. The plant-based 18:3 n-3 fatty acid, ALA, had marginally linear protective relations with global cognitive score (p = 0.05) and perceptual speed (p = 0.07) in the group of APOE ε4 carriers.
Figure. Association of seafood consumption with rate of change in global cognitive score among APOE ε4–positive and APOE ε4–negative participants.
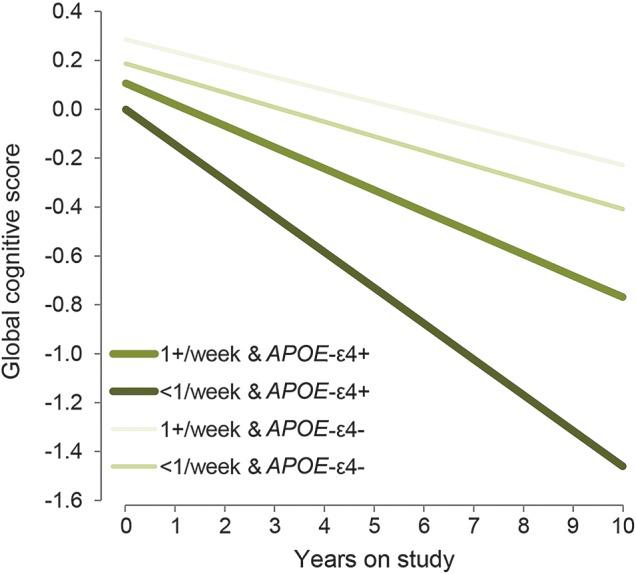
Association of <1 seafood meal per week and 1 or more seafood meals per week with annual rate of change in global cognitive score among 178 Rush Memory and Aging Project (MAP) participants who were APOE ε4–positive and among 632 MAP participants who were APOE ε4–negative based on multiple-adjusted linear mixed models, 2004–2013.
In further analyses we investigated an intermediary role of cardiovascular conditions on the observed associations among the APOE ε4 carriers. The addition of covariates for stroke, myocardial infarction, diabetes, and hypertension to basic-adjusted models reduced the protective association of seafood consumption on global cognitive decline (β = 0.047, p = 0.06), and that with episodic memory was reduced and no longer remained (β = 0.048, p = 0.12). By contrast, the protective associations with semantic memory (β = 0.086, p = 0.01) and perceptual speed (β = 0.066, p = 0.02) remained. In parallel analyses of the long-chain n-3 fatty acids, the associations with cognitive decline and the second tertile of intakes changed little and remained significant. The exception was working memory, for which there was a 32% diminution in the estimated effect that was no longer significant. (β = 0.046, p = 0.11). The marginal associations for ALA intake were further weakened after the adjustments for cardiovascular conditions (global cognition β = 0.089, p = 0.10; perceptual speed β = 0.107, p = 0.08).
To investigate the extent to which the findings could be explained by dietary changes or to invalid reporting among individuals with ongoing disease processes, we reanalyzed the data after eliminating those in the lowest 10% of baseline cognitive scores. There were no appreciable changes in the estimated effects of the second tertile of long-chain n-3 fatty acid intake for any cognitive measure except for a marginal association for episodic memory (β = 0.054, p = 0.07). For seafood consumption, only the association for semantic memory remained (β = 0.049, p = 0.03) and the previously observed associations for ALA no longer lasted (all p > 0.15).
In APOE ε4 noncarriers, the average decline in global cognitive score was −0.078 SU/y. There was no evidence of association for any of the nutrient exposures and cognitive measures. (table e-1) Further, there was no evidence of statistical interaction between fish oil supplement use and APOE ε4 on cognitive decline.
DISCUSSION
The present study is one of a few that investigated associations between seafood and long-chain n-3 fatty acid intake with change in cognitive domains. Our findings support the hypothesis that consumption of seafood and long-chain n-3 fatty acids reduces age-related decline in multiple cognitive domains. The study findings suggest that the relation may be more pronounced in individuals who have the APOE ε4 genotype.
APOE ε4 is associated with increased brain neuroinflammation and deposition of Aβ in senile plaques.18 DHA is a major polyunsaturated fatty acid in the brain that is obtained through diet and has potent anti-inflammatory and antioxidative properties that have been demonstrated in some brain animal models.19 One such study of APOE ε4 and APOE ε3 targeted replacement mice found that the phenotypic deleterious effects of APOE ε4 on cognition and brain neuropathology were prevented by a DHA-rich diet.20
Our associations of higher dietary n-3 fatty acids with slower cognitive decline among APOE ε4 carriers are in accordance with a prior observational study showing associations in APOE ε4 carriers between higher plasma EPA and DHA and slower decline in visual working memory.21 Yet several other observational studies reported associations of higher fish consumption or erythrocyte levels of EPA/DHA with dementia in noncarriers of APOE ε4.22–24 However, the interaction terms in these studies were not significant at p ≤ 0.05, inconsistent per outcome measure, or based on very small numbers, so the evidence restricting the association to APOE ε4 noncarriers is not strong. The robustness of the current associations is reinforced by our findings in a subsample of deceased participants with autopsy data, in which we recently showed associations between higher seafood consumption with lower levels of Alzheimer disease neuropathology also in APOE ε4 carriers only.25 A very recent study observed associations between fish oil supplement use and less cognitive decline and less brain atrophy in the entire study cohort that, after stratification, remained significant in the APOE ε4 noncarriers only.26 Results from randomized placebo-controlled intervention trials are also mixed, observing positive effects of long-chain n-3 fatty acids among APOE ε4 carriers27,28 or in APOE ε4 noncarriers.29 One possible explanation for the previous findings of association among APOE ε4 noncarriers is the larger number of participants being APOE ε4 noncarriers. Our contrasting result with the majority of previous observational and intervention studies may also be due to the relatively older study sample (mean age at baseline was 81.4 years), or could also be a chance finding. The interaction between APOE ε4 status and long-chain n-3 fatty acid intake is not fully understood. It has been suggested that APOE ε4 carriers may have compromised brain reserves or poor brain protection and repair mechanisms, making them more vulnerable to detrimental factors, but also to beneficial factors, such as long-chain n-3 fatty acid intake.30 It has also been proposed that EPA and DHA may help to compensate brain glucose hypometabolism.31 Alternately, it has also been hypothesized that APOE ε4 carriers may modulate n-3 fatty acid metabolism.32
A number of studies have reported protective associations of seafood intake on slower global cognitive decline.17,33–35 Most observed protective benefits at the level of one seafood meal per week, which is consistent with our findings. In addition, our finding of slower decline (in APOE ε4 carriers) among persons in the second tertile of long-chain n-3 fatty acid intake (range 0.35–0.84 g/wk) also approximates the DHA/EPA content of one 3-oz serving of seafood per week (e.g., shrimp, 0.23 g; pollock, 0.43 g; sockeye salmon, 1.04 g; bluefin tuna, 1.28 g). To illustrate the relevance of our findings, we compared the mixed model effect estimates for age over time on global cognition β = −0.004 to that for weekly seafood consumption (β = 0.058). Based on the comparison of these effect estimates, the rate of cognitive decline among weekly seafood consumers was the equivalent of being 14.5 years younger in age.
Our findings of associations with multiple cognitive domains are supported by previous prospective studies that found associations of higher concentrations of n-3 fatty acids measured in diet or plasma with slower declines in perceptual speed,33 semantic memory,35 verbal memory,36 and verbal fluency.37,38 Together, the findings that n-3 fatty acids are associated with multiple cognitive domains involving multiple brain regions and cognitive systems suggest that they may play a fundamental role in brain neuroprotection.
There are many strengths of the study that lend confidence in the overall findings. The prospective design and annual cognitive assessments using a comprehensive battery of tests over 2–10 years allowed for more precise measurement of individual cognitive trajectories to test dietary associations. This precision was also provided by the use of multiple tests to measure specific cognitive domains. The relatively long follow-up period (mean of 4.9 years) after the dietary assessment reduced the potential for bias due to dietary changes caused by underlying diseases or cognitive changes. Further, the results for n-3 fatty acid intake remained after exclusion of the lowest (10%) baseline cognitive scores, i.e., those with poorer cognition, which could have impaired recall on the FFQ. Whereas dietary intake was assessed with a FFQ that was validated in a community sample of older Chicago residents,6,39 there was likely error in the measurement of n-3 fatty acid intake due to nonspecific information obtained on the type of fresh fish consumed as a main dish. This type of measurement error would tend to bias the results toward the null and thus likely had minimal influence on the study findings. As with all observational studies, despite adjustment for the most relevant potential confounders, residual confounding is still possible. Another limitation of the study is the relatively small sample size to detect modest associations within subgroups. Therefore, it is possible that a protective relation of n-3 fatty acids on cognitive decline also exists among APOE ε4 noncarriers, but the power of the study was insufficient to detect an association. The average rate of cognitive decline in this group was substantially less (−0.078) than the rate in the APOE ε4 carriers (−0.126).
Loss in cognitive abilities and dementia are among the most feared and debilitating conditions of aging and currently there are no effective treatments. The role of seafood consumption may be differential depending on APOE ε4 status. Randomized trials would be required to further clarify this possible distinction. Dietary behaviors, such as the consumption of one seafood meal per week, are relatively simple measures for individuals to take with few negative consequences and potentially many established health benefits, if not cognitive.40
Supplementary Material
ACKNOWLEDGMENT
The authors thank the men and women from across northeastern Illinois participating in the Rush Memory and Aging Project.
GLOSSARY
- ALA
α-linolenic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
- MAP
Rush Memory and Aging Project
- SU
standardized units
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
O.v.d.R. commissioned the statistical analyses and drafted the manuscript. Y.W. performed the statistical analyses. M.C.M. supervised the data analysis and interpretation. All authors critically reviewed the manuscript and approved the final draft.
STUDY FUNDING
Supported by grants (R01AG031553 and R01AG17917) from the National Institute on Aging. A Scientific Travel Grant provided by the Judith Zwartz Foundation financed travel expenses of Dr. O. van de Rest to Rush University Medical Center in Chicago.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Huang TL. Omega-3 fatty acids, cognitive decline, and Alzheimer's disease: a critical review and evaluation of the literature. J Alzheimers Dis 2010;21:673–690. [DOI] [PubMed] [Google Scholar]
- 2.van de Rest O, van Hooijdonk LW, Doets E, Schiepers OJ, Eilander A, de Groot LC. B vitamins and n-3 fatty acids for brain development and function: review of human studies. Ann Nutr Metab 2012;60:272–292. [DOI] [PubMed] [Google Scholar]
- 3.Solfrizzi V, D'Introno A, Colacicco AM, et al. Circulating biomarkers of cognitive decline and dementia. Clin Chim Acta 2006;364:91–112. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 2003;158:1213–1217. [DOI] [PubMed] [Google Scholar]
- 7.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005;11:400–407. [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 10.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72. [PubMed] [Google Scholar]
- 11.1985 Health Interview Survey. 1985 Health Interview Survey. Hyattsville, MD: National Center for Health Statistics; 1985. [Google Scholar]
- 12.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord 2009;23:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ 2002;325:932–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 1997;145:33–41. [DOI] [PubMed] [Google Scholar]
- 15.Larrieu S, Letenneur L, Helmer C, Dartigues JF, Barberger-Gateau P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging 2004;8:150–154. [PubMed] [Google Scholar]
- 16.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–946. [DOI] [PubMed] [Google Scholar]
- 17.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62:1849–1853. [DOI] [PubMed] [Google Scholar]
- 18.Egensperger R, Kosel S, von Eitzen U, Graeber MB. Microglial activation in Alzheimer disease: association with APOE genotype. Brain Pathol 1998;8:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs 2008;9:735–743. [PubMed] [Google Scholar]
- 20.Kariv-Inbal Z, Yacobson S, Berkecz R, et al. The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. J Alzheimers Dis 2012;28:667–683. [DOI] [PubMed] [Google Scholar]
- 21.Samieri C, Feart C, Proust-Lima C, et al. Omega-3 fatty acids and cognitive decline: modulation by ApoEepsilon4 allele and depression. Neurobiol Aging 2011;32:2317 e13–2317 e22. [DOI] [PubMed] [Google Scholar]
- 22.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 2007;69:1921–1930. [DOI] [PubMed] [Google Scholar]
- 23.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–1414. [DOI] [PubMed] [Google Scholar]
- 24.Whalley LJ, Deary IJ, Starr JM, et al. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr 2008;87:449–454. [DOI] [PubMed] [Google Scholar]
- 25.Morris MC, Brockman J, Schneider JA, et al. Association of seafood consumption, brain mercury level and APOE-ε4 status with brain neuropathology in older adults. JAMA 2016;315:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daiello LA, Gongvatana A, Dunsiger S, Cohen RA, Ott BR; Alzheimer's Disease Neuroimaging Initiative. Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement 2015;11:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stonehouse W, Conlon CA, Podd J, et al. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr 2013;97:1134–1143. [DOI] [PubMed] [Google Scholar]
- 28.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008;71:430–438. [DOI] [PubMed] [Google Scholar]
- 29.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304:1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schipper HM. Apolipoprotein E: implications for AD neurobiology, epidemiology and risk assessment. Neurobiol Aging 2011;32:778–790. [DOI] [PubMed] [Google Scholar]
- 31.Cunnane SC, Plourde M, Pifferi F, Begin M, Feart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer's disease. Prog Lipid Res 2009;48:239–256. [DOI] [PubMed] [Google Scholar]
- 32.Hennebelle M, Plourde M, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P, Cunnane SC. Ageing and apoE change DHA homeostasis: relevance to age-related cognitive decline. Proc Nutr Soc 2014;73:80–86. [DOI] [PubMed] [Google Scholar]
- 33.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–280. [DOI] [PubMed] [Google Scholar]
- 34.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr 2007;85:1142–1147. [DOI] [PubMed] [Google Scholar]
- 35.Eskelinen MH, Ngandu T, Helkala EL, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry 2008;23:741–747. [DOI] [PubMed] [Google Scholar]
- 36.Kim DH, Grodstein F, Rosner B, et al. Seafood types and age-related cognitive decline in the Women's Health Study. J Gerontol A Biol Sci Med Sci 2013;68:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 2007;85:1103–1111. [DOI] [PubMed] [Google Scholar]
- 38.Beydoun MA, Kaufman JS, Sloane PD, Heiss G, Ibrahim J. n-3 Fatty acids, hypertension and risk of cognitive decline among older adults in the Atherosclerosis Risk in Communities (ARIC) study. Public Health Nutr 2008;11:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr 2004;134:927–934. [DOI] [PubMed] [Google Scholar]
- 40.Harris WS, Mozaffarian D, Lefevre M, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr 2009;139:804S–819S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


