Abstract
Dopamine acts through dopamine type 1 receptors (comprised of D1 and D5 subtypes) and dopamine type 2 receptors (comprised of D2, D3 and D4 subtypes). Intracranial self-stimulation (ICSS) is one experimental procedure that can be used to evaluate abuse-related effects of drugs targeting dopamine receptors. This study evaluated effects of dopamine receptor ligands on ICSS in rats using experimental procedures that have been used previously to examine abused indirect dopamine agonists such as cocaine and amphetamine. Male Sprague-Dawley rats responded under a fixed-ratio 1 schedule for electrical stimulation of the medial forebrain bundle, and frequency of stimulation varied from 56–158 Hz in 0.05 log increments during each experimental session. Drug potency and time course were determined for the D1 ligands A77636, SKF82958, SKF38393, fenoldopam and SCH39166 and the D2/3 ligands sumanirole, apomorphine, quinpirole, PD128907, pramipexole, aripiprazole, eticolopride and PG01037. The high-efficacy D1 agonists A77636 and SKF82958 produced dose-dependent, time-dependent, and abuse-related facilitation of ICSS. Lower efficacy D1 ligands and all D2/3 ligands failed to facilitate ICSS at any dose or pretreatment time. A mixture of SKF82958 and quinpirole produced a mixture of effects produced by each drug alone. Quinpirole also failed to facilitate ICSS after regimens of repeated treatment with either quinpirole or cocaine. These studies provide more evidence for divergent effects of dopamine D1- and D2-family agonists on ICSS procedure in rats and suggest that ICSS may be a useful complement to other approaches for preclinical abuse potential assessment, in part because of the reproducibility of results.
Keywords: Dopamine D1, Dopamine D2, Rat, ICSS, Drug abuse
INTRODUCTION
Indirect dopamine (DA) agonists such as cocaine, amphetamine and cathinone analogs known as “bath salts” are commonly abused drugs (SAMHSA, 2014). In laboratory animals, these indirect DA agonists also produce behavioral effects thought to be related to, and predictive of, their clinical abuse liability. For example, drug self-administration is a type of operant procedure in which experimental subjects engage in a voluntary behavior (e.g. pressing a response lever) to receive delivery of a drug dose, and the primary dependent variable is usually the rate of responding or rate of drug dose delivery. A drug is considered to produce “reinforcing effects” and to function as a “reinforcer” if some dose of drug maintains higher rates of self-administration than vehicle, and many drugs that function as reinforcers in drug self-administration studies also display high abuse liability in humans (Carter & Griffiths, 2009; O'Connor, Chapman, Butler, & Mead, 2011). Intracranial self-stimulation (ICSS) is another operant procedure that has been used for preclinical research on expression and mechanisms of abuse-related drug effects (Carlezon & Chartoff, 2007; Kornetsky, Esposito, McLean, & Jacobson, 1979; Negus & Miller, 2014; Vlachou & Markou, 2011; Wise, 1996). In ICSS procedures, subjects are equipped with microelectrodes that target brain reward regions such as the medial forebrain bundle, and responding produces pulses of electrical brain stimulation delivered via the electrode. Drugs can be administered prior to ICSS test sessions, and many drugs of abuse increase (or “facilitate”) rates of ICSS responding. As a result, drug-induced facilitation of ICSS is often interpreted as an abuse-related effect suggestive of abuse liability. Cocaine, amphetamine, and abused cathinone analogs produce both reinforcing effects in drug self-administration procedures and facilitation of ICSS in ICSS procedures (Gregg & Rawls, 2014; Negus & Miller, 2014; O'Connor, et al., 2011).
Indirect DA agonists act at DA transporters to increase extracellular DA levels, and DA effects are subsequently mediated through activation of five DA receptor subtypes divided into two families, the D1-like family comprised of D1 and D5 subtypes and the D2-like family comprised of D2, D3 and D4 subtypes (Sokoloff & Schwartz, 1995). In contrast to the high abuse liability of many indirect DA agonists, direct agonists at DA receptor subtypes do not appear to have high abuse liability in humans. For example, several D2-family agonists are available clinically for indications that include treatment of Parkinson’s disease (Kehne, Andree, & Heinrich, 2008), but none are scheduled by the Food and Drug Administration or show evidence of abuse in surveys such as those conducted by the Substance Abuse and Mental Health Services Administration. The only clinically available agonist at D1 receptors, the peripherally selective partial agonist fenoldopam (Murphy, Murray, & Shorten, 2001), is also not scheduled, and the centrally acting high-efficacy D1 agonist ABT-491 failed to produce abuse-related subjective effects in human cocaine users (Haney, Collins, Ward, Foltin, & Fischman, 1999). Despite an apparent lack of abuse liability of DA agonists in humans, both D1-family and D2-family agonists are self-administered by laboratory animals, often at rates similar to those maintained by indirect DA agonists like cocaine (Koffarnus et al., 2012; Self & Stein, 1992; Weed & Woolverton, 1995; Wise, Murray, & Bozarth, 1990; Woolverton, Goldberg, & Ginos, 1984). As such, D1- and D2-family agonists represent a class of drugs for which there is some discrepancy between results from preclinical drug self-administration procedures and expression of actual abuse liability in humans.
Effects of DA agonists have also been examined in ICSS procedures, but results have been mixed. For example, D1 agonists have been found to produce both facilitation (Gilliss, Malanga, Pieper, & Carlezon, 2002; Malanga, Riday, Carlezon, & Kosofsky, 2008; Ranaldi & Beninger, 1994) and depression of ICSS (Baldo, Jain, Veraldi, Koob, & Markou, 1999; Hunt, Atrens, & Jackson, 1994), and a similar profile has been observed with D2 agonists (Carr, Kim, & Cabeza de Vaca, 2001; Depoortere, Perrault, & Sanger, 1996; Hatcher & Hagan, 1998; Liebman & Butcher, 1973; Ranaldi & Beninger, 1994; Singh, Desiraju, & Raju, 1996). Procedural variables may influence drug effects on ICSS, and the primary goal of the present study was to systematically compare effects of a range of dopamine receptor ligands on ICSS in rats using procedures that have been used previously to assess and stratify abuse potential of a wide range of indirect DA agonists (Bauer, Banks, Blough, & Negus, 2013; Bonano et al., 2015; Bonano, Glennon, De Felice, Banks, & Negus, 2014; Miller, Altarifi, & Negus, 2015; Miller, Leitl, Banks, Blough, & Negus, 2015; Rosenberg, Carroll, & Negus, 2013). The D1-selective compounds included the high-efficacy agonists SKF82958 (Chausmer & Katz, 2002; Desai, Neumeyer, Bergman, & Paronis, 2007) and A77636 (Chausmer & Katz, 2002), the low-efficacy agonist SKF38393 (Gleason & Witkin, 2004), and fenoldopam (Desai, et al., 2007). The D2/3-selective compounds included the high-efficacy agonists sumanirole (Collins, Jackson, Koek, & France, 2014; Koffarnus et al., 2009), apomorphine (Collins, et al., 2014), quinpirole (Collins, et al., 2014), PD128907 (Gleason & Witkin, 2004) and pramipexole (Koffarnus, et al., 2009) (listed in order from D2 to D3 selective). The low-efficacy D2 agonist aripiprazole (Millan, Iob, Peglion, & Dekeyne, 2007; Thomsen et al., 2008) was also examined. Finally, the D1 antagonist SCH39166 (Ralph & Caine, 2005), D2 antagonist eticlopride (Ralph & Caine, 2005), and D3 antagonist PG01037 (Caine et al., 2012; Higley et al., 2011) were studied for comparison to the agonists.
The D2/3 agonists failed to facilitate ICSS, and as a result, three additional studies were conducted with quinpirole under conditions hypothesized to increase expression of ICSS facilitation. First, previous studies have suggested that combined treatment with D1 and D2 agonists can produce synergistic effects on some endpoints (Longoni, Spina, & Di Chiara, 1987; Schmidt & Pierce, 2006; White, Bednarz, Wachtel, Hjorth, & Brooderson, 1988), so quinpirole was administered in combination with the D1 agonist SKF82958. Second, repeated treatment with some drugs can produce tolerance to ICSS rate-decreasing effects and increased expression of ICSS facilitation by those drugs (Altarifi & Negus, 2011; Freitas, Carroll, & Negus, 2015), so quinpirole was evaluated before, during and after a regimen of repeated quinpirole treatment. Lastly, drug self-administration studies have suggested that self-administration of D2/3 agonists is increased in animals with a cocaine self-administration history (Collins et al., 2012; Collins & France, 2015; Collins & Woods, 2007; Nader & Mach, 1996), so quinpirole was evaluated before and after a regimen of repeated cocaine administration.
METHOD
Subjects
A total of 59 adult male Sprague-Dawley rats (Harlan, Frederick, MD) were used. All rats had ad libitum access to food and water and were housed individually on a 12 hr light-dark cycle (6am – 6pm, lights on) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Rats weighed between 300 and 400 g at the time of surgery. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals 8th edition (National Research Council (U.S.), 2011).
Assay of Intracranial Self-Stimulation (ICSS)
Surgery
Rats were anesthetized with 2.5% isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) until unresponsive to toe-pinch prior to implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode, which was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, was stereotaxically implanted into the left medial forebrain bundle at the level of the lateral hypothalamus using previously published coordinates (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull) (Lazenka, Moeller, & Negus, 2015; Paxinos & Watson, 1998). Three screws were placed in the skull, and the anode (0.125 mm diameter, un-insulated) was wrapped around one of the screws to act as a ground. Dental acrylic was used to secure the electrode to the screws and skull. Ketoprofen (5 mg/kg) was administered as a postoperative analgesic immediately and 24 hrs following surgery. Animals were allowed to recover for at least one week before ICSS training.
Apparatus
Operant conditioning chambers consisted of sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 cm × 30.5 cm × 24.1 cm) (Med Associates, St. Albans, VT). Each chamber had a response lever (4.5 cm wide, 2.0 cm deep, 3.0 cm above the floor), three stimulus lights (red, yellow and green) centered 7.6 cm above the lever, a 2-watt house light, and an ICSS stimulator. Bipolar cables routed through a swivel-commutator (Model SL2C, Plastics One) connected the stimulator to the electrode. MED-PC IV computer software controlled all programming parameters and data collection (Med Associates).
Training
The behavioral procedure was identical to that described previously for studies with indirect dopamine agonists (Bauer, et al., 2013; Bonano, et al., 2015; Bonano, et al., 2014; Miller, Leitl, et al., 2015; Rosenberg, et al., 2013). A house light was illuminated during behavioral sessions, and lever-press responding under a fixed-ratio 1 (FR1) schedule produced delivery of a 0.5 s train of square-wave cathodal pulses (0.1 ms per pulse) via the intracranial electrode. During brain stimulation, the stimulus lights over the lever were illuminated, and responding had no scheduled consequences. During initial 60 min training sessions, stimulation intensity was set at 150 µA, and stimulation frequency was set at 158 Hz. Stimulation intensity was then individually manipulated in each rat to identify an intensity that maintained reinforcement rates >30 stimulations/min (range of 100 µA– 295 µA for rats in this study). Once an appropriate intensity was identified, changes in frequency were introduced during sessions consisting of three consecutive 10 min components, each of which contained 10 consecutive 60 s trials. The stimulation frequency was 158 Hz for the first trial of each component, and frequency decreased in 0.05 log unit steps during the subsequent nine trials to a final frequency of 56 Hz. Each trial began with a 10 s time-out period, during which responding had no scheduled consequences, and five non-contingent stimulations at the designated frequency were delivered at 1 s intervals during the last 5 s of the time out. During the remaining 50 s of each trial, responding produced both intracranial stimulation at the designated frequency and illumination of the lever lights under an FR1 schedule as described above. ICSS performance was considered to be stable when frequency-rate curves were not statistically different over three consecutive days of training as indicated by lack of a significant effect of ‘day’ in a two-way analysis of variance (ANOVA) with day and frequency as the main effect variables (see Data Analysis below). All training was completed within six weeks of surgery.
Testing
For dose-effect studies, test sessions consisted of three consecutive ‘baseline’ components followed first by a 10 min time-out period and then by two consecutive ‘test’ components. All drugs were delivered by i.p. injection at the beginning of the time out period. For time-course studies, test sessions consisted of three consecutive baseline components followed first by i.p. administration of the drug and then by pairs of test components beginning 10, 30, 100 and 300 mins after drug administration Test sessions were conducted on Tuesdays and Fridays, and three-component training sessions were conducted on other weekdays. For each drug, dose-effect studies were conducted first, and dose order was varied using a Latin-Square design. Time-course studies were conducted at least 48 hrs after completion of dose-effect testing using a dose identified as behaviorally active during dose-effect studies. Each drug was tested in a group of five to seven rats. Tests with different drugs within a given rat were separated by at least two weeks, and during this inter-drug interval, a vehicle test session was conducted. The drugs and doses tested were as follows: A77636 (0.1–1.0 mg/kg), SKF82958 (0.032–1.0 m mg/kg), SKF38393 (1.0–10 mg/kg), fenoldopam (1.0–10 mg/kg), sumanirole (0.032–1.0 mg/kg), apomorphine (0.01–1.0 mg/kg), quinpirole (0.0032–0.32 mg/kg), PD128907 (0.01–0.1 mg/kg), pramipexole (0.0032–0.1 mg/kg), aripiprazole (0.32–3.2 mg/kg), SCH39166 (0.01–0.1 mg/kg), eticlopride (0.0032–0.1 mg/kg), and PG01037 (10–32 mg/kg). Dose ranges for each drug were based on previous studies in rats with each compound (Carr, et al., 2001; Collins et al., 2007; Desai, et al., 2007; Harrison, Gasparini, & Markou, 2002; Koffarnus, et al., 2009; Ralph & Caine, 2005; Singh, et al., 1996; Thomsen, et al., 2008) and on empirical results.
During these initial studies, quinpirole and the other D2/3 agonists failed to facilitate ICSS. Accordingly, three follow-up studies were conducted in three different sets of drug-naïve rats to examine quinpirole effects under conditions hypothesized to increase expression of quinpirole-induced ICSS facilitation. First, quinpirole was administered in combination with the D1 agonist SKF82958 in a 1:32 quinpirole:SKF82958 mixture (0.001 quinpirole+0.032 SKF82958 to 0.01 quinpirole+0.32 SKF82958; doses in mg/kg; N=5). The dose ratio for the mixture was based on the relative potencies of the two drugs (quinpirole approximately 32-fold more potent than SKF82958), and dose-effect studies with this mixture were conducted using the same procedure described above for dose-effect studies with individual drugs. The other two studies evaluated quinpirole effects before and after regimens of repeated treatment with either quinpirole or cocaine. For repeated dosing studies, test sessions consisted of three consecutive baseline components for three consecutive days, and data across these three days were averaged to yield pre-quinpirole baseline data as described below. Immediately after conclusion of the last baseline component on Day 1, a cumulative quinpirole dose-effect curve was determined during five consecutive test periods. Each 30-min test period consisted of a 10-min time out followed by a pair of 10-min test components. A dose of quinpirole was administered i.p. at the start of each time out, and each dose increased the total cumulative dose by 0.5 log units from 0.0032 to 0.32 mg/kg. On Days 2–7, test sessions consisted of three consecutive baseline components followed first by a 10-min time out and then by a pair of test components, and either 0.032 mg/kg quinpirole (in one group of rats; N=6) or 10.0 mg/kg cocaine (in another group of rats; N=6) was administered at the start of each treatment interval. Finally, on Day 8, the cumulative quinpirole dose-effect curve was redetermined as described above. For repeated quinpirole studies, the study continued, and a higher dose of 0.32 mg/kg quinpirole was administered daily on days 9–14 before a final redetermination of the cumulative quinpirole dose-effect curve on Day 15.
Data Analysis
The first baseline component for each day was considered to be a “warm-up” component, and data were discarded. The primary dependent variable was reinforcement rate in stimulations per min during each frequency trial for all remaining baseline and test components. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (%MCR) for that rat. For dose-effect and time-course studies, MCR was defined as the mean of the maximal rates observed during the second and third baseline components of that test session. For studies of repeated drug administration, MCR was defined as the mean of the maximal rates observed during the second and third baseline components for the three consecutive days preceding repeated drug treatment (six total baseline components). Subsequently, % MCR values for each trial were calculated as [(reinforcement rate during a frequency trial)/(MCR)]×100. For each rat, data from baseline and test components were averaged to yield baseline and test frequency-rate curves. Baseline and test data were then averaged across rats to yield mean baseline and test frequency-rate curves for each manipulation. Results were compared by repeated measures two-way ANOVA with ICSS frequency as one factor and either dose or time as the second factor. A significant ANOVA was followed by the Holm-Sidak post-hoc test, and the criterion for significance was p < .05.
To provide an additional summary measure of ICSS performance, the total number of stimulations per component was determined across all 10 frequency trials of each component. Test data were expressed as a percentage of either (a) the average number of total stimulations per component earned during the second and third baseline components for that day (for dose-effect and time-course studies), or (b) the average number of total stimulations per component earned during the second and third components on the three consecutive baseline days preceding repeated drug treatment (for studies with repeated quinpirole and cocaine). Thus, % Baseline Stimulations was calculated as (mean total stimulations during test components/mean total stimulations during baseline components) × 100. These data were then averaged across rats for each experimental manipulation.
Drugs
SCH39166 HBr [(6aS-trans)-11-Chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepin-12-ol hydrobromide], (R)-(−)-apomorphine HCl, eticlopride HCl, pramipexole 2HCl, (+)PD128907 HCl [(4aR,10bR)-3,4a,4,10b-Tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride] and sumanirole maleate were obtained from Tocris (Minneapolis, MN). (−)-Quinpirole HCl, (±)SKF38393 HCl [(±)-1-Phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride, aripiprazole], A77636 HCl [(1R-cis)-1-(Aminomethyl)-3,4-dihydro-3-tricyclo[3.3.1.13,7]dec-1-yl-[1H]-2-benzopyran-5,6-diol hydrochloride], fenoldopam mesylate, β-cyclodextrin and Tween80 were purchased from Sigma-Aldrich (St. Louis, MO). (±)SKF82958 HBr [(±)-6-Chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide] was provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Bethesda, MD). PG01037 HCl [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-trans-but-2-enyl)-4-(pyridine-2-yl)benzamide hydrochloride] was provided by Dr. Amy H. Newman (National Institute on Drug Abuse, Baltimore, MD). (−)-Cocaine HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). PG01037 was dissolved in 10% β-cyclodextrin and 90% sterile water, aripiprazole was suspended in 5% Tween80 and 95% sterile saline, fenoldopam was dissolved in sterile water, and all other drugs were dissolved in sterile saline. All drugs were administered i.p. in a volume of 1 ml/kg except for aripiprazole, which was delivered in a volume of 2 ml/kg.
RESULTS
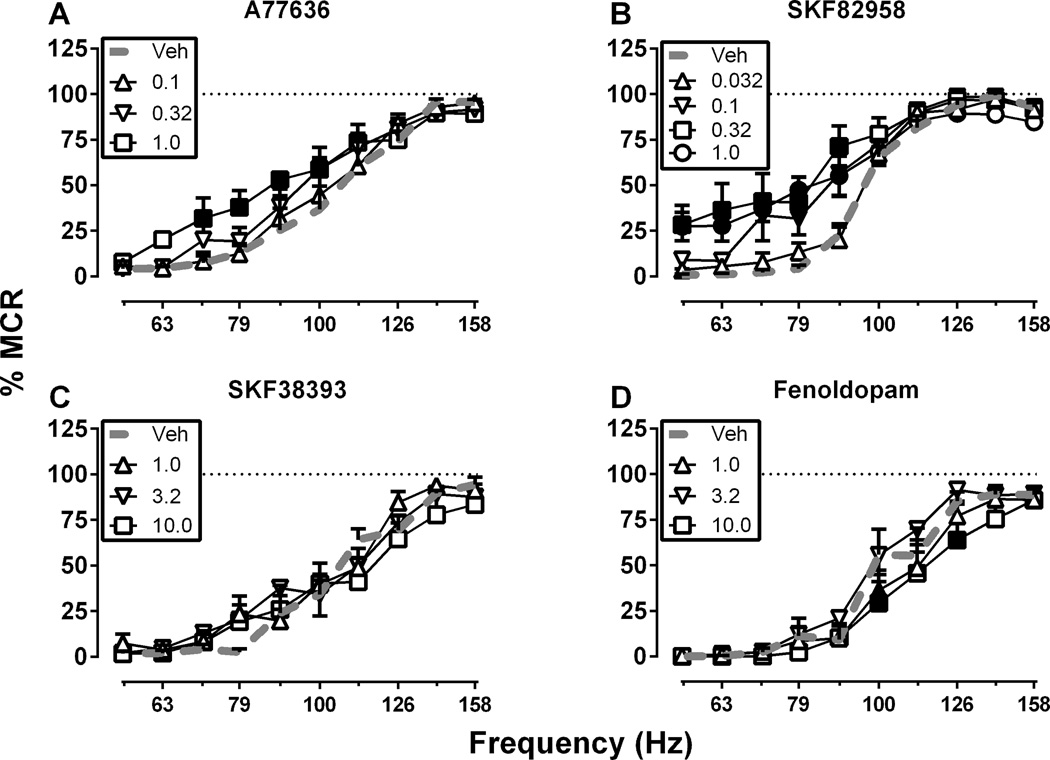
Under baseline conditions, electrical brain stimulation maintained a frequency-dependent increase in reinforcement rates. The mean ± SEM maximum control rate for all rats in the study was 59 ± 0.9 stimulations per trial, and the mean ± SEM number of baseline total stimulations per component was 266 ± 6.5. Figure 1 shows effects of D1 agonists. The high-efficacy D1 agonists A77636 (0.1–1.0 mg/kg; Figure 1A) and SKF82958 (0.032–1.0 mg/kg; Figure 1B) produced dose-dependent ICSS facilitation and leftward shifts in ICSS frequency-rate curves. For A77636, there were significant main effects of frequency, F(9, 45) = 68.59, p < .0001 and dose, F(3, 15) = 5.25, p < .05 and a significant interaction, F(27, 135) = 1.63, p < .05. For SKF-82958, there were also significant main effects of frequency, F(9, 54) = 241.30, p < .0001 and dose, F(4, 24) = 3.99, p < .05 and a significant interaction, F(36, 216) = 3.38, p < .0001. In dose-effect studies, A77636 and SKF82958 were tested up to doses that produced a plateau in ICSS facilitation. A higher dose of A77636 (3.2 mg/kg) was tested in four rats and produced facilitation similar to 1.0 mg/kg. A higher dose of SKF82958 (3.2 mg/kg) was also tested in four rats but produced either complete elimination of responding (three rats) or facilitation similar to 1.0 mg/kg (one rat).
Figure 1. Effects of the D1 agonists (A) A77636, (B) SKF82958, (C) SKF38393 and (D) fenoldopam.
Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from vehicle (Veh) as determined by repeated measures two-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test, p < .05. Data presented are the mean ± SEM of 6–7 rats.
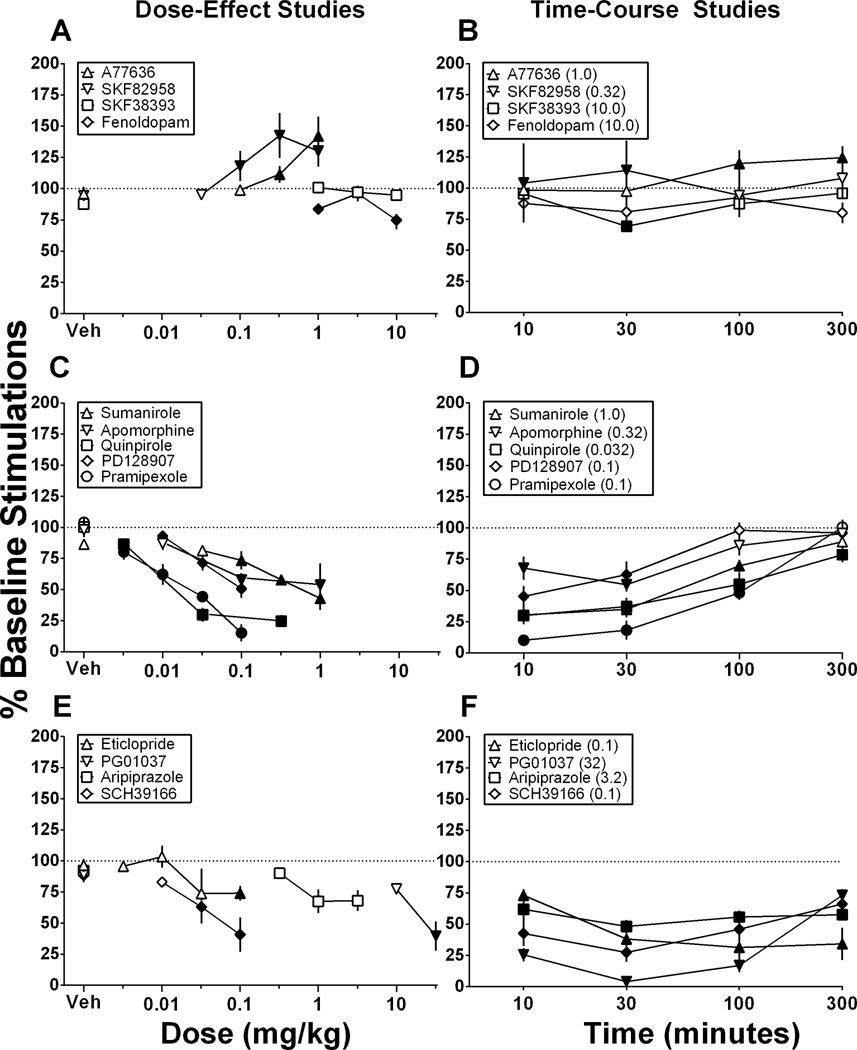
Conversely, the low-efficacy D1 agonist SKF38393 (1.0–10 mg/kg; Figure 1C) did not significantly alter ICSS in this dose-effect study, and the peripherally restricted, low-efficacy D1 agonist fenoldopam (1.0–10 mg/kg; Figure 1D) produced modest but significant depression of ICSS. For SKF38393, there was only a significant main effect of frequency, F(9, 45) = 79.28, p < .0001. For fenoldopam, there were significant main effects of frequency, F(9, 45) = 43.95, p < .0001 and dose, F(3, 15) = 6.29, p < .01 and a significant interaction, F(27, 135) = 1.87, p < .05. Summary data for D1 agonist dose-effect studies are shown in Figure 4A, and the time course of effects produced by selected D1 agonist doses are shown in Figure 4B. In time-course studies, both 1.0 mg/kg A77636 and 0.32 mg/kg SKF82958 produced significant ICSS facilitation, although facilitation was observed only at early time points after SKF82958 (10–30 min) and only at later time points for A77636 (100–300 min), and maximal facilitation in these time-course studies was lower than in dose-effect studies. Conversely, 10 mg/kg SKF38393 produced significant ICSS depression after 30 min, and 10 mg/kg fenoldopam failed to significantly alter ICSS in the time course study.
Figure 4. Summary data for drug effects on ICSS in dose-effect studies (A, C, E) and time-course studies (B, D, F).
Abscissae: Dose in mg/kg (log scale; A, C, E) or time in mins after drug injection (log scale; B, D, F). Ordinates: Percent baseline stimulations per component, a summary measure of ICSS performance across all brain stimulation frequencies. Filled points represent doses that produced a significant change in ICSS relative to vehicle treatment at one or more brain-stimulation frequencies as determined from analysis of full frequency-rate curves. All points show mean ± SEM of 5–7 rats.
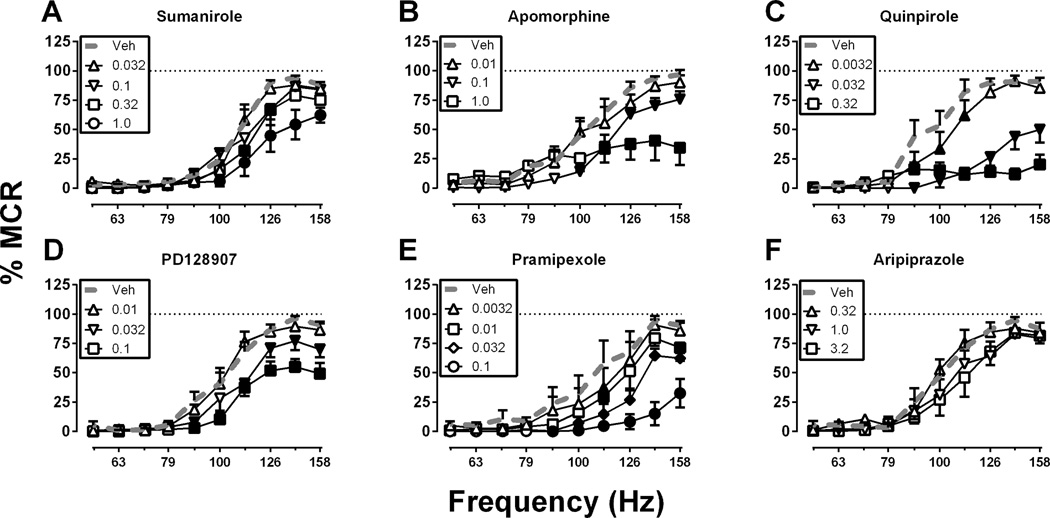
Figure 2 shows effects of agonists with varying degrees of selectivity for D2 vs. D3 receptors. In contrast to high-efficacy D1 agonists, high-efficacy agonists for D2-family receptors produced only dose-dependent depression of ICSS expressed as rightward and downward shifts in ICSS frequency-rate curves. Specifically, only ICSS depression was produced by the following drugs (ranging from most D2 selective to most D3 selective): sumanirole (0.032–1.0 mg/kg; Figure 2A), apomorphine (0.01–1.0 mg/kg; Figure 2B), quinpirole (0.0032–0.32 mg/kg; Figure 2C), PD128907 (0.01–0.1 mg/kg; Figure 2D) and pramipexole (0.0032–0.1 mg/kg; Figure 2E). The low-efficacy D2-family agonist, aripiprazole (0.32–3.2 mg/kg; Figure 2F), did not significantly alter ICSS in the dose-effect study. For sumanirole, there were significant main effects of frequency, F(9, 45) = 73.43 p < .0001 and dose, F(4, 20) = 11.07, p < .0001 and a significant interaction, F(36, 180) = 3.085, p < .0001. For apomorphine, there were significant main effects of frequency, F(9, 45) = 44.58, p < .0001 and dose, F(3, 15) = 3.642, p < .05 and a significant interaction, F(27, 135) = 4.937, p < .0001. For quinpirole, there were significant main effects of frequency, F(9, 54) = 43.90, p < .0001 and dose, F(3, 18) = 46.70, p < .0001 and a significant interaction, F(27, 162) = 11.51, p < .0001. For PD128907, there were significant main effects of frequency, F(9, 45) = 93.44, p < .0001 and dose, F(3, 15) = 17.51, p < .0001 and a significant interaction, F(27, 135) = 4.758, p < .0001. For pramipexole, there were significant main effects of frequency, F(9, 36) = 29.60, p < .0001 and dose, F(4, 16) = 29.01 p < .0001 and a significant interaction, F(36, 144) = 4.691, p < 0.0001. For aripiprazole, there was only a main effect of frequency, F(9, 45) = 48.07, p < .0001. Summary data for these dose-effect studies with D2-family agonists are shown in Figure 4C (aripiprazole in Figure 4E), and the time course of effects produced by selected doses are shown in Figure 4D (aripiprazole in Figure 4F). In general, all the D2-family agonists produced ICSS rate-decreasing effects that peaked after 10–30 min and dissipated after 100–300 min. Aripiprazole (3.2 mg/kg) also significantly depressed ICSS in the time-course study, but this effect endured for the entire 300 min of testing.
Figure 2. Effects of the D2/3 agonists (A) sumanirole (B) apomorphine (C) quinpirole (D) PD128907 (E) pramipexole and (F) aripiprazole.
Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from vehicle (Veh) as determined by repeated measures two-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test, p < .05. Data presented are the mean ± SEM of 5–7 rats.
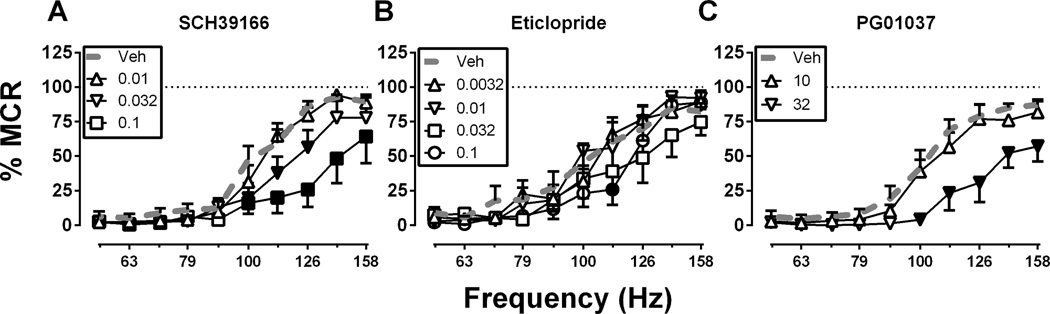
Figure 3 shows effects of D1, D2 and D3 receptor antagonists. Only ICSS depression was produced by the D1 antagonist SCH39166 (0.01–0.1 mg/kg; Figure 3A), the D2 antagonist eticlopride (0.0032–0.1 mg/kg; Figure 3B), and the D3 antagonist PG01037 (10–32 mg/kg; Figure 3C). For SCH39166, there were significant main effects of frequency, F(9, 45) = 53.48, p < .0001 and dose, F(3, 15) = 7.23, p < .0001 and a significant interaction, F(27, 135) = 8.55, p < .0001. For eticlopride, there were significant main effects of frequency, F(9, 54) = 41.81, p < .0001 and dose, F(3, 18) = 3.381, p < .05. For PG01037, there were significant main effects of frequency, F(9, 36) = 49.11, p < .0001 and dose, F(4, 16) = 65.03, p < 0.0001 and a significant interaction, F(36, 144) = 14.52, p < .0001. A dose of 0.32 mg/kg SCH39166 and eticlopride were tested in a subset of animals (N=4) and these doses completely eliminated responding for ICSS within 10 minutes (data not shown). Summary data for antagonist dose-effect studies are shown in Figure 4E, and the time course of effects produced by selected antagonist doses are shown in Figure 4F. All three antagonists produced long-lasting ICSS depression throughout the 300 min testing period.
Figure 3. Effects of (A) the D1 antagonist SCH39166, (B) the D2 receptor antagonist eticlopride, and (C) the D3 antagonist PG01037.
Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from vehicle (Veh) as determined by repeated measures two-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test, p < .05. Data presented are the mean ± SEM of 5–6 rats.
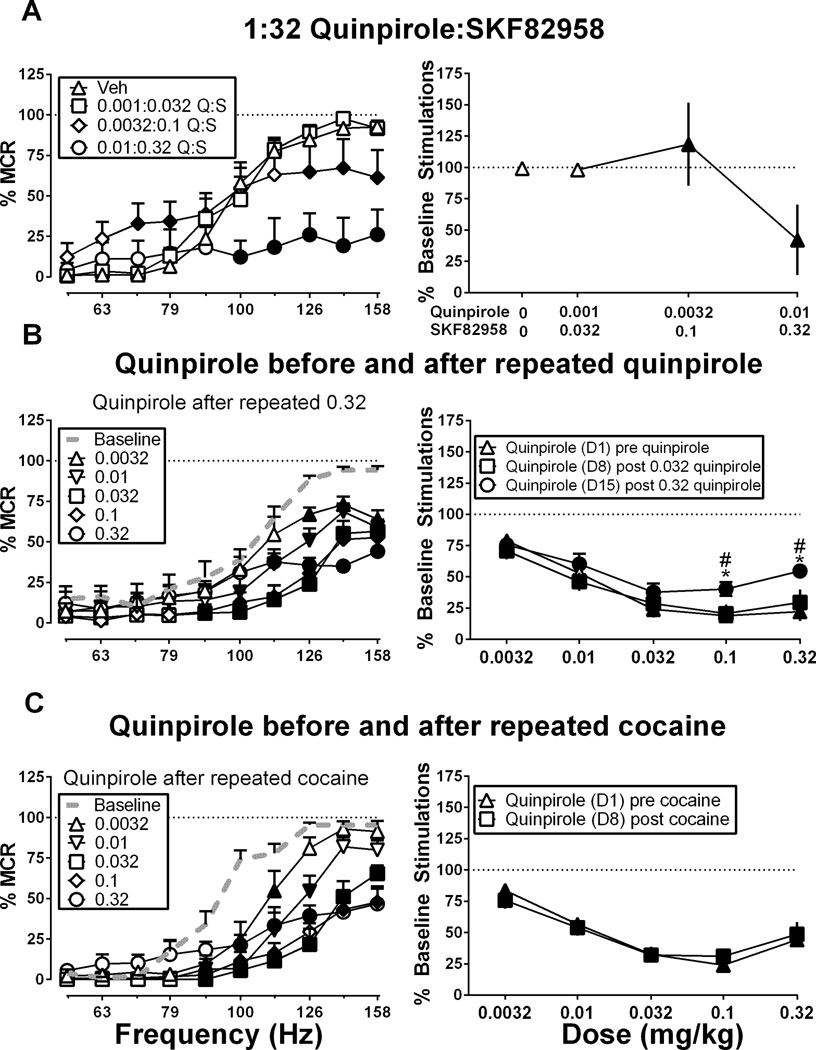
Figure 5 shows effects of quinpirole under three sets of conditions hypothesized to increase expression of quinpirole-induced ICSS facilitation. This hypothesis was not supported for any of the three conditions. First, Figure 5A–B shows the effect of administration of quinpirole in combination with SKF82958 in a 1:32 quinpirole:SKF82958 mixture, and there was a significant main effect of frequency, F(9, 36) = 71.76, p < .0001 and a significant interaction between frequency and dose, F(27, 108) = 8.53, p < .0001]. A low dose of 0.001 mg/kg quinpirole + 0.032 mg/kg SKF82958 did not produce any effect on ICSS. A higher dose of 0.0032 mg/kg quinpirole + 0.1 mg/kg SKF82958 produced both facilitation of low ICSS rates maintained by low brain stimulation frequencies (similar to the effect of 0.1 mg/kg SKF82958 alone) and a decrease in high ICSS rates maintained by high brain stimulation frequencies (similar to the effect of 0.0032 mg/kg quinpirole alone). At the highest dose of 0.01 mg/kg quinpirole + 0.32 mg/kg SKF82958, only depression was seen similar to a dose of 0.01 mg/kg quinpirole administered alone.
Figure 5. Effects of quinpirole on ICSS when quinpirole was administered (A) in combination with SKF82958 in a 1:32 quinpirole:SKF82958 mixture, (B) before, during and after a regimen of repeated quinpirole treatment, or (C) before and after a regimen of repeated cocaine treatment.
Left panels show full frequency-rate curves from selected conditions, and right panels show summary data for all treatment conditions. Abscissae: frequency of electrical brain stimulation in Hz (log scale; left panels) and dose in mg/kg (log scale; right panels). Ordinates: Percent maximum control reinforcement rate (% MCR) (left panels) and percent baseline stimulations per component (right panels). In left panels, filled symbols show significant differences from vehicle (Veh, A) or baseline (B,C) as determined by repeated measures two-way analysis of variance (ANOVA) followed by the Holm-Sidak post-hoc test, p < .05. In right panels, filled symbols represent doses that produced a significant change in ICSS relative to vehicle (A) or baseline (B, C) at one or more brain-stimulation frequencies as determined from analysis of full frequency-rate curves. In addition, in Panel B, * represents a significant difference from Day 1 and # represents a significant difference from Day 8 for that dose as determined by two-way ANOVA followed by the Holm-Sidak post-hoc test, p < .05. Data presented are the mean ± SEM of 5–6 rats.
Second, Figure 5C–D shows the effects of cumulative quinpirole administered before a regimen of repeated quinpirole treatment (Day 1, D1), after 6 days of treatment with an intermediate dose of 0.032 mg/kg/day quinpirole (Day 8, D8), and after 6 additional days of treatment with a high dose of 0.32 mg/kg/day quinpirole (Day 15, D15). Results during the intervening days are shown in Supplemental Figure 1. Figure 5C shows full frequency-rate curves only for results determined on Day 15, but Figure 5D shows summary data for all three determinations. On Day 1 there were significant main effects of frequency, F(9, 45) = 56.38, p < .0001 and dose, F(5, 25) = 22.00, p < .0001 and a significant interaction, F(45, 225) = 6.47, p < .0001 as cumulative quinpirole produced dose-dependent depression of ICSS as described above. During days 2–7, 0.032 mg/kg quinpirole produced a consistent depression of ICSS that did not change in magnitude across days (Supplemental Figure 1). On Day 8 there were significant main effects of frequency, F(9, 45) = 36.27, p < .0001 and dose, F(5, 25) = 14.60, p < .0001 and a significant interaction, F(45, 225) = 8.02, p < .0001, and after 6 days of repeated treatment with 0.032 mg/kg/day quinpirole, the cumulative quinpirole dose-effect curve was also unchanged. During days 9–14, 0.32 mg/kg quinpirole produced a depression of ICSS that again did not change significantly in magnitude across days (Supplemental Figure 1). On day 15 there were significant main effects of frequency, F(9, 45) = 24.94, p < .0001 and dose, F(5, 25) = 27.55, p < .0001 and a significant interaction, F(45, 225) = 4.75, p < .0001], and cumulative quinpirole again produced only dose-dependent depression of ICSS. However analysis of summary data in Figure 5D indicated significant main effects of dose, F(4, 20) = 21.90, p < .0001 and day, F(2, 10) = 6.99, p < .05 and a significant interaction F(8, 40) = 2.59, p < 0.05. The highest doses of 0.1 and 0.32 mg/kg quinpirole produced less ICSS depression on day 15 than on days 1 or 8. Thus, despite modest tolerance to its rate-decreasing effects after 2 weeks of repeated treatment, quinpirole failed to facilitate ICSS at any frequency of brain stimulation at any time before, during or after repeated quinpirole treatment. Following determination of the last cumulative quinpirole dose-effect curve, rats were tested with 10 mg/kg cocaine as a positive control, and cocaine facilitated ICSS and significantly increased the % Baseline Number of Stimulations per component to 140.9% ± 18.5 (mean ± SEM, data not shown).
Finally, Figure 5E–F shows the effects of cumulative quinpirole administered before and after a regimen of repeated cocaine treatment (10 mg/kg/day). Effects on intervening days are shown in Supplemental Figure 2. Figure 5E shows full frequency-rate curves only for results determined after cocaine, but Figure 5F shows summary data for both determinations. On Day 1, before repeated cocaine, cumulative quinpirole produced dose-dependent depression of ICSS as described above. The dose of 10 mg/kg/day cocaine produced significant facilitation of ICSS each day, and the effects did not differ across days (Supplemental Figure 2). On Day 8, after 6 days of repeated treatment with 10 mg/kg/day cocaine, the cumulative quinpirole dose-effect curve was unchanged. Quinpirole failed to facilitate ICSS at any frequency of brain stimulation at any time before or after repeated cocaine treatment. For quinpirole effects before and after repeated cocaine treatment, there were significant main effects of frequency, F(9, 45) = 109.3, p < .0001 and dose, F(5, 25) = 66.95, p < .0001 and a significant interaction, F(45, 225) = 13.03, p < .0001 on Day 1 and significant main effects of frequency, F(9, 45) = 80.70, p < .0001 and dose, F(5, 25) = 47.55, p < .0001 and a significant interaction, F(45, 225) = 10.16, p < .0001 on Day 8 during determination of each cumulative quinpirole dose-effect curve. Analysis of summary data in Figure 5F indicated a significant main effect of quinpirole dose F(4, 20) = 39.59, p < .0001], but there was no effect of treatment day, and the interaction was not significant.
DISCUSSION
This study compared effects of a range of DA receptor agonists on ICSS in rats using a frequency-rate ICSS procedure and a strategy for data analysis that we have used previously to evaluate effects of indirect DA agonists. There were three main findings. First, D1-selective ligands produced efficacy-dependent changes in ICSS, such that high-efficacy agonists facilitated ICSS, whereas lower efficacy agonists and the D1 antagonist depressed ICSS. Second, D2/D3-selective ligands only depressed ICSS, and this effect was observed for high-efficacy agonists regardless of D2/D3 receptor selectivity, for the partial agonist aripiprazole, and for the antagonists. Lastly, the D2/3 agonist quinpirole still failed to facilitate ICSS when it was administered in combination with a D1 agonist or after regimens of repeated quinpirole or cocaine treatment. Taken together, these results provide evidence for dissociable effects of D1 vs. D2/3 receptor stimulation on ICSS in rats.
Effects of D1 dopamine receptor ligands
The present results agree with previous evidence for a positive relationship between in vitro measures of D1 agonist efficacy and in vivo effectiveness to facilitate ICSS in rats and mice. For example, the high-efficacy D1 agonists SKF82958 (Gilliss, et al., 2002) and A77636 (Carr, et al., 2001) produced significant facilitation of ICSS in mice and rats, respectively, responding under frequency-rate procedures similar to the one used in this study. Conversely, the lower efficacy agonist SKF38393 and the antagonist SCH23390 did not significantly facilitate ICSS but rather produced significant ICSS depression in rats (Nakajima & O'Regan, 1991). The present results also support other characteristics of ICSS facilitation reported previously for high-efficacy D1 agonists. In particular, both SKF82958 and A77636 in the present study increased low rates of responding maintained by low brain-stimulation frequencies at doses that did not decrease high rates of ICSS maintained by high frequencies of brain stimulation. This profile is similar to the effects of abused indirect DA agonists like cocaine and amphetamine, although as in previous studies, the maximal facilitation produced by high-efficacy D1 agonists was less than that produced by cocaine and other abused indirect agonists (for example, compare Figure 1 and Supplemental Figure 2). The magnitude of facilitation produced by the high-efficacy D1 agonists was also less consistent than cocaine (for example, compare Figure 4A with 4B and Supplemental Figure 2). The present study expanded on these previous results in three ways. First, this is the first study to compare effects of D1 ligands with a broad range of efficacies in a single study using a common ICSS procedure. The results suggest that previous evidence for efficacy-dependent ICSS facilitation by D1 ligands cannot be attributed solely to methodological differences across studies. Second, this study identified different time courses for ICSS facilitation by SKF82958 and A77636, with the former having a more rapid onset and shorter duration of action. To our knowledge, this is the first direct comparison of the time course of effects produced by these two agonists in rats. Lastly, this is the first report of effects produced by fenoldopam and SCH39166 on ICSS. Results with fenoldopam in particular are relevant insofar as this is the only D1 agonist available clinically.
The efficacy-dependent effects of D1 ligands on ICSS are also consistent with evidence for efficacy-dependent self-administration of D1 ligands by nonhuman primates (Grech, Spealman, & Bergman, 1996; Weed, Paul, Dwoskin, Moore, & Woolverton, 1997; Weed & Woolverton, 1995). For example, studies conducted in rhesus monkeys found that high-efficacy D1 agonists including SKF82958 maintained high rates of drug self-administration, whereas lower efficacy agonists including SKF38393 did not (Weed, et al., 1997; Weed & Woolverton, 1995). Moreover, stimulant-naïve monkeys acquired self-administration of a high-efficacy D1 agonist (Weed & Woolverton, 1995), a result that provides additional evidence for effectiveness of high-efficacy D1 agonists to function as reinforcers and that may also distinguish D1 agonists from D2-agonists (see below). Studies in rats have yielded less consistent evidence for the reinforcing effects of D1 agonists. For example, one study (Self & Stein, 1992) reported self-administration of both SKF82958 and of SKF77434, a D1 agonist that has lower efficacy at D1 receptors than SKF38393 and that did not maintain self-administration by nonhuman primates (Grech, et al., 1996; Weed & Woolverton, 1995). Conversely, neither SKF82958 nor SKF77434 maintained self-administration in another study in rats (Caine, Negus, Mello, & Bergman, 1999). Determinants of the discrepancies between these two studies in rats and with studies in nonhuman primates remain to be determined. Overall, then, results of the present study cannot be readily compared to an inconsistent literature on D1 agonist self-administration in rats. However, the present results from this ICSS procedure do parallel efficacy-dependent self-administration of D1 ligands by nonhuman primates and additional evidence for efficacy-dependent rewarding effects of D1 ligands under a place conditioning procedure in rats (Abrahams, Rutherford, Mallet, & Beninger, 1998).
Taken together, these preclinical studies suggest that high-efficacy D1 agonists might have abuse liability in humans and that activation of D1-family receptors may contribute to abuse-related effects of indirect DA agonists. High-efficacy D1 agonists are not currently available clinically, nor have they appeared as “designer” drugs in illicit drug markets and drugs such as SKF82958 that produce reliable abuse-related effects preclinically have not been tested in humans. However, another selective high-efficacy D1 agonist, ABT-431, was tested in experienced cocaine smokers, and it did not produce subjective effects suggestive of abuse liability (e.g. ratings of “High” or “Good Drug Effect”) (Haney, et al., 1999). The peripherally restricted low-efficacy D1 agonist fenoldopam is the only D1 agonist available clinically, and the failure of fenoldopam to facilitate ICSS is consistent with the status of fenoldopam as a non-scheduled drug by the Food and Drug Administration.
Effects of D2/3 dopamine receptor ligands
The present study also evaluated effects of D2-family agonists with differing selectivity for the D2 and D3 receptors based on in vivo data comparing the effects of these agonists on D2-induced hypothermia and D3-induced yawning in rats (Collins, et al., 2007), with sumanirole being the most selective for D2 and pramipexole the most selective for D3. All of these compounds produced only dose-dependent rate-decreasing effects regardless of dose, pretreatment time, selectivity for D2 vs. D3 receptors, or efficacy at D2 family receptors. These results agree with previous studies in finding that D2-family agonists fail to facilitate low ICSS rates maintained by low brain-stimulation frequencies at doses below those that depress high ICSS rates maintained by high brain-stimulation frequencies (Depoortere, et al., 1996; Hall & Stellar, 1996; Hatcher & Hagan, 1998; Knapp & Kornetsky, 1996; Strecker, Roberts, & Koob, 1982). This profile of effects distinguishes D2-family agonists from both high-efficacy D1 agonists like SKF82958 and from indirect DA agonists like cocaine and amphetamine, and it further suggests that stimulation of D2-family receptors is not sufficient to mediate abuse-related ICSS facilitation by abused indirect DA agonists. However, the present results with relatively high D2/3 agonist doses differ from some previous findings. Specifically, in the present study, high doses that depressed high ICSS rates maintained by high brain-stimulation frequencies either depressed or did not alter low ICSS rates maintained by low brain-stimulation frequencies. In contrast, previous studies have occasionally reported more complex effects, in which depression of high ICSS rates was accompanied by facilitation of low ICSS rates (Fouriezos & Francis, 1992; Malanga, et al., 2008; Nakajima & O'Regan, 1991; Ranaldi & Beninger, 1994) As one example of this phenomenon, Malanga et al. (2008) evaluated effects of quinpirole in an ICSS frequency-rate procedure in mice. Quinpirole doses of 0.1–0.3 mg/kg failed to facilitate low ICSS rates but depressed high ICSS rates. Higher quinpirole doses of 1–10 mg/kg produced both facilitation of low ICSS rates and depression of high ICSS rates, although facilitation of low ICSS rates had a slower onset than depression of high rates. Overall, then, the present study did not observe ICSS facilitation by D2/3 agonists under any condition, and previous studies have reported ICSS facilitation by D2/3 agonists only at high doses similar to or greater than those that also depress high ICSS rates.
We have reported previously that these profiles of drug effects on ICSS (either no ICSS facilitation or facilitation of low ICSS rates only in the presence of simultaneous depression of high ICSS rates) are usually predictive of weak reinforcing effects in drug self-administration procedures and low abuse liability in humans (Bauer et al., 2013; Negus and Miller, 2014). However, D2/3 agonists appear to represent an exception to this pattern. Clinically available D2-family agonists such as apomorphine and pramipexole are not scheduled by the Food and Drug Administration and have yielded no apparent evidence for diversion or abuse in the general population (although abuse is reported in ~3–4% of Parkinson’s patients) (Cilia et al., 2014; O'Sullivan, Evans, & Lees, 2009). In contrast, D2-family agonists have repeatedly been shown to maintain drug self-administration in both rats and non-human primates (Caine, et al., 1999; Grech, et al., 1996; Woolverton, et al., 1984). Thus, D2-family agonists have generally produced more robust signals for abuse potential in drug self-administration studies than in ICSS studies or in human patterns of drug use.
Given the discrepancy in abuse-related effects of D2-family agonists in preclinical assays of ICSS and drug self-administration, three additional studies were conducted under conditions hypothesized to increase expression of ICSS facilitation by the representative D2-family agonist quinpirole. First, quinpirole was combined with the D1 agonist SKF82958, because D1 and D2 agonists have been reported to produce synergistic effects on some points including motor stereotypies (Longoni, et al., 1987; White, et al., 1988) and reinstatement of extinguished cocaine self-administration (Schmidt & Pierce, 2006). However, administration of both drugs produced only a net effect that integrated effects of each drug alone (i.e. facilitation of low ICSS rates similar to effects of SKF82958 alone and depression of high ICSS rates similar to effects of quinpirole alone). Moreover, with a high dose of the mixture (0.01 mg/kg quinpirole + 0.32 mg/kg SKF82958), the rate-decreasing effects of quinpirole predominated and prevented expression of ICSS facilitation by SKF82958. Thus, there was no evidence to suggest that co-administration of quinpirole with SKF82958 enhanced ICSS facilitation by the D1 agonist or unmasked ICSS facilitation by quinpirole. Second, quinpirole was administered repeatedly because previous studies have found that repeated administration of agonists at some other receptor types (e.g. at mu opioid or nicotinic acetylcholine receptors) can produce tolerance to rate-decreasing drug effects and unmask ICSS facilitation (Altarifi & Negus, 2011; Freitas, et al., 2015). In the present study, repeated treatment with the highest quinpirole dose did produce modest tolerance to quinpirole’s rate-decreasing effects; however, even under these conditions, quinpirole failed to facilitate ICSS. Lastly, quinpirole effects were determined before and after a regimen of repeated cocaine administration because previous studies have found that self-administration of D2 agonists is enhanced in animals with a history of cocaine self-administration (Collins, et al., 2012; Collins & France, 2015; Collins & Woods, 2007; Nader & Mach, 1996). In the present study, cocaine facilitated ICSS, and in agreement with previous studies (e.g. Bauer, Banks, & Negus, 2014), this facilitation was sustained during repeated cocaine treatment; however, this treatment regimen did not alter quinpirole effects. It has been suggested that self-administration of D2-family agonists may result not from primary reinforcing effects of the D2-family agonist but rather from stimulation of responding maintained by conditioned stimuli (e.g. cue lights) previously paired with self-administered cocaine doses. Notably, D2-family agonists like quinpirole are less effective or ineffective to stimulate responding maintained by conditioned stimuli previously paired with other positive reinforcers including ketamine and food (Collins & Woods, 2007, 2009). The present results suggest that quinpirole also fails to increase responding maintained by stimuli paired with rewarding brain stimulation.
Summary
The present results support previous evidence to suggest that D1-family agonists produce relatively weak but significant efficacy-dependent abuse-related effects whereas D2-family agonists do not. Importantly, D2 agonists failed to produce an abuse-related facilitation of ICSS across a broad range of experimental conditions, suggesting that this frequency-rate ICSS procedure is less vulnerable than conventional drug self-administration procedures to methodological confounds that may overestimate abuse potential of D2-family agonists. In addition to providing evidence for modest abuse potential for high-efficacy D1 agonists, the present results also suggest that D1-family receptors may play a more important role than D2-family receptors in mediating abuse-related ICSS facilitation by indirect agonists such as cocaine or amphetamine. These results provide further confirmation that ICSS is a useful complement to drug self-administration studies, and provide additional evidence that effects of DA agonists in this procedure are reproducible.
Supplementary Material
Abbreviations
- ANOVA
analysis of variance
- DA
Dopamine
- D1
dopamine D1
- D2
dopamine D2
- D3
dopamine D3
- FR
fixed-ratio
- Hr
hour
- ICSS
Intracranial self-stimulation
- I.p.
intraperitoneal
- Min
minutes
- S
seconds
- Veh
vehicle
References
- Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. European Journal of Pharmacology. 1998;343:111–118. doi: 10.1016/s0014-2999(97)01531-8. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behavioural Pharmacology. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Jain K, Veraldi L, Koob GF, Markou A. A dopamine D1 agonist elevates self-stimulation thresholds: comparison to other dopamine-selective drugs. Pharmacology, Biochemistry, and Behavior. 1999;62:659–672. doi: 10.1016/s0091-3057(98)00206-8. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British Journal of Pharmacology. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology. 2014;231:2461–2470. doi: 10.1007/s00213-013-3405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Negus SS. Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. British Journal of Pharmacology. 2015;172:2433–2444. doi: 10.1111/bph.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic "bath salts" cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. The Journal of Pharmacology and Experimental Therapeutics. 1999;291:353–360. [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Xu M. Cocaine self-administration in dopamine D(3) receptor knockout mice. Experimental and Clinical Psychopharmacology. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology. 2001;154:420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105(Suppl 1):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer AL, Katz JL. Comparison of interactions of D1-like agonists, SKF 81297, SKF 82958 and A-77636, with cocaine: locomotor activity and drug discrimination studies in rodents. Psychopharmacology. 2002;159:145–153. doi: 10.1007/s002130100896. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Canesi M, Zecchinelli AL, De Gaspari D, Natuzzi F, Pezzoli G. Dopamine dysregulation syndrome in Parkinson's disease: from clinical and neuropsychological characterisation to management and long-term outcome. Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85:311–318. doi: 10.1136/jnnp-2012-303988. [DOI] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology. 2012;219:123–135. doi: 10.1007/s00213-011-2382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, France CP. Determinants of conditioned reinforcing effectiveness: Dopamine D-like receptor agonist-stimulated responding for cocaine-associated stimuli. European Journal of Pharmacology. 2015 doi: 10.1016/j.ejphar.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Jackson JA, Koek W, France CP. Effects of dopamine D(2)-like receptor agonists in mice trained to discriminate cocaine from saline: influence of feeding condition. European Journal of Pharmacology. 2014;729:123–131. doi: 10.1016/j.ejphar.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. The Journal of Pharmacology and Experimental Therapeutics. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behavioural Pharmacology. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacology. 1996;124:231–240. doi: 10.1007/BF02246662. [DOI] [PubMed] [Google Scholar]
- Desai RI, Neumeyer JL, Bergman J, Paronis CA. Pharmacological characterization of the effects of dopamine D(1) agonists on eye blinking in rats. Behavioural Pharmacology. 2007;18:745–754. doi: 10.1097/FBP.0b013e3282f14ee6. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, Francis S. Apomorphine and electrical self-stimulation of rat brain. Behavioural Brain Research. 1992;52:73–80. doi: 10.1016/s0166-4328(05)80326-2. [DOI] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Negus SS. Comparison of Effects Produced by Nicotine and the alpha4beta2-Selective Agonist 5-I-A-85380 on Intracranial Self-Stimulation in Rats. Experimental and Clinical Psychopharmacology. 2015 doi: 10.1037/pha0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology. 2002;163:238–248. doi: 10.1007/s00213-002-1153-8. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Witkin JM. Effects of dopamine D1 receptor full agonists in rats trained to discriminate SKF 38393. Behavioural Pharmacology. 2004;15:85–89. doi: 10.1097/00008877-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Grech DM, Spealman RD, Bergman J. Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology. 1996;125:97–104. doi: 10.1007/BF02249407. [DOI] [PubMed] [Google Scholar]
- Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sciences. 2014;97:27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Stellar JR. Measurement issues in curve-shift analysis of apomorphine effects on rewarding brain stimulation. Pharmacology, Biochemistry, and Behavior. 1996;53:417–423. doi: 10.1016/0091-3057(95)02007-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hatcher JP, Hagan JJ. The effects of dopamine D3/D2 receptor agonists on intracranial self stimulation in the rat. Psychopharmacology. 1998;140:405–410. doi: 10.1007/s002130050782. [DOI] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. Journal of Psychopharmacology. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM, Jackson DM. Reward summation and the effects of dopamine D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacology, Biochemistry, and Behavior. 1994;48:853–862. doi: 10.1016/0091-3057(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Andree TH, Heinrich JN. D2 receptor partial agonists: treatment of CNS disorders of dopamine function. Current Topics in Medicinal Chemistry. 2008;8:1068–1088. doi: 10.2174/156802608785161394. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Kornetsky C. Low-dose apomorphine attenuates morphine-induced enhancement of brain stimulation reward. Pharmacology, Biochemistry, and Behavior. 1996;55:87–91. doi: 10.1016/0091-3057(96)00073-1. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Collins GT, Rice KC, Chen J, Woods JH, Winger G. Self-administration of agonists selective for dopamine D2, D3, and D4 receptors by rhesus monkeys. Behavioural Pharmacology. 2012;23:331–338. doi: 10.1097/FBP.0b013e3283564dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Greedy B, Husbands SM, Grundt P, Newman AH, Woods JH. The discriminative stimulus effects of dopamine D2- and D3-preferring agonists in rats. Psychopharmacology. 2009;203:317–327. doi: 10.1007/s00213-008-1323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Archives of General Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, Moeller FG, Negus SS. Effects of caffeine and its metabolite paraxanthine on intracranial self-stimulation in male rats. Experimental and Clinical Psychopharmacology. 2015;23:71–80. doi: 10.1037/pha0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM, Butcher LL. Effects on self-stimulation behavior of drugs influencing dopaminergic neurotransmission mechanisms. Naunyn-Schmiedeberg's Archives of Pharmacology. 1973;277:305–318. doi: 10.1007/BF00505669. [DOI] [PubMed] [Google Scholar]
- Longoni R, Spina L, Di Chiara G. Permissive role of D-1 receptor stimulation for the expression of D-2 mediated behavioral responses: a quantitative phenomenological study in rats. Life Sciences. 1987;41:2135–2145. doi: 10.1016/0024-3205(87)90532-7. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biological Psychiatry. 2008;63:214–221. doi: 10.1016/j.biopsych.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Iob L, Peglion JL, Dekeyne A. Discriminative stimulus properties of S32504, a novel D3/D2 receptor agonist and antiparkinson agent, in rats: attenuation by the antipsychotics, aripiprazole, bifeprunox, N-desmethylclozapine, and by selective antagonists at dopamine D2 but not D3 receptors. Psychopharmacology. 2007;191:767–782. doi: 10.1007/s00213-006-0567-0. [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Experimental and Clinical Psychopharmacology. 2015;23:405–414. doi: 10.1037/pha0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS. Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain. 2015;156:175–184. doi: 10.1016/j.pain.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MB, Murray C, Shorten GD. Fenoldopam: a selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. The New England Journal of Medicine. 2001;345:1548–1557. doi: 10.1056/NEJMra010253. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Nakajima S, O'Regan NB. The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacology, Biochemistry, and Behavior. 1991;39:465–468. doi: 10.1016/0091-3057(91)90209-k. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Guide for the care and use of laboratory animals. 8th. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological Reviews. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neuroscience and Biobehavioral Reviews. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SS, Evans AH, Lees AJ. Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs. 2009;23:157–170. doi: 10.2165/00023210-200923020-00005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. San Diego: Academic Press; 1998. [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Research. 1994;651:283–292. doi: 10.1016/0006-8993(94)90708-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. The Journal of Pain. 2013;14:246–259. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. (NSDUH Series H-48) 2014 Retrieved from http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf.
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Research. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Singh J, Desiraju T, Raju TR. Dose-response functions of apomorphine, SKF 38393, LY 171555, haloperidol and clonidine on the self-stimulation evoked from lateral hypothalamus and ventral tegmentum. Indian Journal of Physiology and Pharmacology. 1996;40:15–22. [PubMed] [Google Scholar]
- Sokoloff P, Schwartz JC. Novel dopamine receptors half a decade later. Trends in Pharmacological Sciences. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Roberts DC, Koob GF. Apomorphine-induced facilitation of intracranial self-stimulation following dopamine denervation of the nucleus accumbens. Pharmacology, Biochemistry, and Behavior. 1982;17:1015–1018. doi: 10.1016/0091-3057(82)90487-7. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DP, Wortwein G, Sager TN, Holm R, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal models of drug addiction. New York: Humana Press; 2011. pp. 3–56. [Google Scholar]
- Weed MR, Paul IA, Dwoskin LP, Moore SE, Woolverton WL. The relationship between reinforcing effects and in vitro effects of D1 agonists in monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1997;283:29–38. [PubMed] [Google Scholar]
- Weed MR, Woolverton WL. The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1995;275:1367–1374. [PubMed] [Google Scholar]
- White FJ, Bednarz LM, Wachtel SR, Hjorth S, Brooderson RJ. Is stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors? Pharmacology, Biochemistry, and Behavior. 1988;30:189–193. doi: 10.1016/0091-3057(88)90442-x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annual Review of Neuroscience. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1984;230:678–683. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.