Abstract
Objective
To evaluate the performance of the Birmingham Vasculitis Activity Score (BVAS) in the assessment of disease activity in giant cell arteritis (GCA).
Methods
Patients with GCA enrolled in a prospective, multicenter, longitudinal study with symptoms of active vasculitis during any visit were included. Spearman’s rank correlation was used to explore the association of the BVAS with other measures of disease activity.
Results
During a mean (SD) follow-up of 2.3 (1.6) years, symptoms of active GCA were present in 236 visits in 136 subjects (100 female, 74%). Median (range) BVAS1 (new/worse symptoms) was 1 (0–10) and median (range) BVAS2 (persistent symptoms) was 0 (0–5). Median (range) physician global assessment (PGA) was 4 (0–9) for disease activity in the past 28 days and 2 (0–9) for activity on the day of the visit. Important ischemic manifestations of active vasculitis not captured by the BVAS included tongue/jaw claudication (27%), upper extremity claudication (15%), lower extremity claudication (5%), carotidynia (7%), ischemic retinopathy (5%). During 25 visits (11%) with active disease, all symptoms of active vasculitis were captured in the “Other” category yet still resulted in a BVAS 1 and BVAS 2 of 0. BVAS1 moderately correlated with PGA for the past 28 days (Spearman’s correlation 0.50) and physician-rated disease activity for the past 28 days (Spearman’s correlation 0.46).
Conclusions
The BVAS has limited utility in GCA. Patients with active GCA can have a BVAS of 0. Many important ischemic symptoms attributable to active vasculitis are not captured in the composite score.
Indexing terms: Giant cell arteritis, disease activity, cohort study, Birmingham vasculitis activity score
INTRODUCTION
Giant cell arteritis (GCA) is a chronic granulomatous vasculitis affecting the aorta and its primary branches. Extra-cranial manifestations of GCA, which occur in about one-third of patients, include large-artery stenosis and aortic disease (1). Glucocorticoids remain the mainstay of treatment for patients with GCA but treatment is associated with morbidity in the majority of patients (2). Additionally, relapses are common (2–10). Several randomized controlled trials have been conducted to evaluate other immunosuppressive therapy in patients with GCA (11–19). A problem common to trials in GCA is the lack of commonly-accepted standardized measures of disease activity (20). Almost all trials have used some measurement of disease activity such as “relapse”, “recurrence”, “flare”, or “remission” but the definitions of these disease states are not uniformly applied across studies, making comparisons challenging (20).
The Birmingham Vasculitis Activity Score (BVAS) is a validated tool for assessment of disease activity in patients with many different forms of vasculitis (21–23). The BVAS includes scored items grouped into 9 organ systems which capture a broad spectrum of clinical manifestations from vasculitis. Only features attributed to active vasculitis are considered. The BVAS is part of the OMERACT core outcome measures for use in clinical trials of anti-neutrophil cytoplasmic antibody (ANCA)- associated vasculitis (24). Previous validation studies of the BVAS included only a small number of patients with large-vessel vasculitis. The aim of this study was to evaluate the BVAS as a measure of disease activity in a prospective observational cohort of patients with GCA. Information gained from such a study could inform future efforts to develop disease activity measures in GCA and clinical trials design.
METHODS
All data for this study was collected from patients enrolled between 2006 and 2013 in the Vasculitis Clinical Research Consortium Longitudinal Study of Giant Cell Arteritis, a multicenter, prospective, observational cohort. All patients in this cohort meet the 1990 ACR classification criteria for GCA (25) which was modified to include patients with large-vessel vasculitis by angiography or biopsy. Inclusion criteria were age above 50 years with ≥2 of the following features: 1) new localized headache, 2) temporal artery abnormality on examination, 3) ESR >40 mm/hour by Westergren method, 4) abnormal temporal artery biopsy, and 5) large vessel vasculitis by angiography or biopsy. All subjects were followed prospectively with standardized clinical assessments including symptoms attributable to vasculitis (over the past 28 days, on the day of evaluation), laboratory findings, BVAS (version 2 which includes 66 items grouped into 9 organ systems) and physician global assessment (PGA, scale of 0–10). Disease activity as assessed also assessed categorically by the treating physician at each visit as “remission” or “active” (low, moderate or high). Disease activity was defined as the presence of new or worsening symptoms attributable to active vasculitis in the 28 days prior to evaluation or on the day of evaluation.
Information on symptoms, laboratory evaluation, PGA, and BVAS during any period of disease activity in the 28 days prior to the visit, or on the date of the visit was reviewed. The distribution of organ involvement as documented in the BVAS was collected. The investigator completing the BVAS separates symptoms that are new or worse from those which have been present within the last 3 months but continue to be present (persistent). There are 2 final BVAS scores; BVAS1 (new/worse) and BVAS2 (persistent). Additional symptoms attributed to vasculitis which were not captured by the BVAS except in the “Other” category were also collected. Manifestations of active vasculitis captured under the “Other” category of BVAS do not add to the total BVAS and are scored as 0. Descriptive statistics were used. Spearman’s rank correlation was used to evaluate the correlation of the BVAS in patients with active disease with other measures of disease activity including PGA, physician rated disease activity (remission, low, moderate or high), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). JMP, Version 11, SAS Institute Inc., Cary, NC was used for all analyses.
The study was approved by Institutional Review Boards at each participating site. All participants provided informed consent.
RESULTS
Data was available for 136 subjects: 100 (74%) female, mean (SD) age at study entry of 71.7 (9.3) years with a mean (SD) follow-up of 2.3 (1.6) years. Symptoms of active GCA were present in 236 visits. Median (range) BVAS1 (new or worse symptoms) was 1 (0–10) and median (range) BVAS2 (persistent symptoms) was 0 (0–5) in the 236 visits with active disease. Median (range) PGA was 4 (0–9) for disease activity in the past 28 days and 2 (0–9) for activity on the day of the visit. Disease activity for the past 28 days was characterized as “remission” for 7 visits, “low” for 75 visits, “moderate” for 72 visits and “high” for 32 visits. Disease activity on the day of evaluation was categorized as “remission” in 48 visits, “low” in 124 visits, “moderate” in 51 visits and “high” in 10 high visits (data missing in 3 cases). On the day of evaluation, median (range) ESR was 21 (1–126) mm/hour with median (range) CRP of 6.65 (0–211.7) mg/L.
In 51 patients (38% patients in this study) with active disease at the time of enrollment, the diagnosis of GCA was made within 3 months of enrollment into the cohort. In these patients, median (range) BVAS1 at first visit was 3 (0–9) with BVAS2 0 (0–5). Disease activity for the past 28 days was rated as “low” in 1 patient, “moderate” in 24 patients and “high” in 24 patients (data missing in 2 patients). Disease activity on day of evaluation (study enrollment) was categorized as “remission” in 15 patients, “low” in 16 patients, “moderate” in 13 patients and “high” in 6 patients. Median (range) PGA for past 28 days was 7 (2–9) and 2 (0–9) for the day of the visit (at study enrollment).
Symptoms of active vasculitis during the 236 visits with active disease and whether they were captured on BVAS are summarized in Table 1. Clinical manifestations of active disease as captured by BVAS for the 236 visits are in Table 2. The BVAS was also separately analyzed for 51 patients with GCA diagnosed within 3 months of enrollment into the cohort (Table 3).
Table 1.
Symptoms of active giant cell arteritis
| 236 encounters 136 patients with GCA |
51 patients with a new diagnosis of GCA |
|||||
|---|---|---|---|---|---|---|
| Symptoms | Past 28 days |
Today | At diagnosis |
Past 28 days |
Today | Captured on BVAS |
| Weight loss | 17 | 13 | 8 | 8 | 5 | Yes |
| Fever | 7 | 11 | 3 | 3 | 0 | Yes |
| Headache | 98 | 53 | 33 | 32 | 11 | Yes |
| Scalp tenderness | 66 | 30 | 27 | 24 | 6 | Yes (as headache) |
| New temporal artery pain | 43 | 17 | 20 | 18 | 3 | Yes (as headache) |
| Carotidynia | 16 | 7 | 9 | 10 | 5 | No |
| Jaw/tongue claudication | 64 | 29 | 26 | 27 | 9 | No |
| Ischemic retinopathy | 14 | 5 | 10 | 10 | 5 | No |
| Partial vision loss | 14 | 7 | 5 | 8 | 5 | Yes |
| Severe vision loss | 5 | 3 | 5 | 5 | 3 | Yes |
| Diplopia | 9 | 1 | 8 | 8 | 0 | No |
| Polymyalgia rheumatica | 79 | 53 | 16 | 17 | 10 | Yes |
| Arthralgia/Arthritis | 28 | 40 | 11 | 10 | 3 | Yes |
| Upper extremity claudication | 35 | 29 | 9 | 9 | 8 | No |
| Lower extremity claudication | 12 | 9 | 4 | 4 | 3 | No |
| Transient ischemic attack | 1 | 0 | 0 | 0 | 0 | No |
| Lightheadedness | 5 | 5 | 0 | 0 | 0 | No |
| Mesenteric ischemia | 1 | 1 | 1 | 1 | 0 | Yes |
| Other (fatigue, cough, night sweats, anorexia) | 21 | 15 | 4 | 4 | 1 | Yes for fatigue and cough |
Table 2.
Frequency of clinical manifestations in 236 encounters of giant cell arteritis with active disease during observation, as captured by the Birmingham Vasculitis Activity Score.
| BVAS items (by organ system) | New/worse symptoms* | Persistent symptoms* |
|---|---|---|
| General (N=188) | ||
| Malaise | 57 | 17 |
| Myalgia | 51 | 7 |
| Arthralgia/arthritis | 47 | 17 |
| Headache | 108 | 9 |
| Fever (<38.5 degrees Celsius) | 4 | 1 |
| Fever (>38.5 degrees Celsius) | 2 | 2 |
| Weight loss (≥2 kilograms) | 20 | 0 |
| Maximum allowable score on BVAS | 3 | 2 |
| Median (range) BVAS for category | 1 (0–3) | 0 (0–2) |
| Cutaneous (N=2) | ||
| Ulcer | 2 | 0 |
| Maximum allowable score on BVAS | 6 | 3 |
| Median (range) BVAS for category | 0 (0–4) | 0 (0-0) |
| Mucous membranes/eyes (N=35) | ||
| Mouth ulcers | 0 | 1 |
| Blurred vision | 24 | 1 |
| Sudden vision loss | 19 | 0 |
| Maximum allowable score on BVAS | 6 | 3 |
| Median (range) total BVAS for category | 0 (0–6) | 0 (0–2) |
| Ear, Nose, and Throat (N=1) | ||
| Sinus involvement | 0 | 1 |
| Maximum allowable score on BVAS | 6 | 3 |
| Median (range) total BVAS for category | 0 (0-0) | 0 (0–1) |
| Chest (N=9) | ||
| Persistent cough | 8 | 1 |
| Maximum allowable score on BVAS | 6 | 3 |
| Median (range) total BVAS for category | 0 (0–2) | 0 (0–1) |
| Cardiovascular (N=2) | ||
| Congestive heart failure | 0 | 2 |
| Maximum allowable score on BVAS | 6 | 3 |
| Median (range) BVAS for category | 0 (0-0) | 0 (0–2) |
| Abdominal (N=1) | ||
| Severe abdominal pain | 1 | 0 |
| Maximum allowable score | 9 | 4 |
| Median (range BVAS for category) | 0 (0–3) | 0 (0-0) |
| Renal (N=0) | ||
| Maximum allowable score on BVAS | 12 | 6 |
| Median (range) BVAS for category | 0 (0-0) | 0 (0-0) |
| Nervous system (N=4) | ||
| Organic confusion/dementia | 2 | 0 |
| Stroke | 1 | 0 |
| Sensory peripheral neuropathy | 0 | 1 |
| Maximum allowable score on BVAS | 9 | 6 |
| Median (range) BVAS for category | 0 (0–9) | 0 (0–3) |
| Other N=85 | 63 | 22 |
| Total | ||
| Maximum allowable score | 63 | 33 |
| Median (range) total BVAS | 1 (0–10) | 0 (0–5) |
BVAS = Birmingham Vasculitis Activity Score (BVAS); N = total number with any clinical manifestation in that organ system
All median BVAS are scores for 236 encounters with active disease.
Values are number of encounters during active disease with symptoms captured by that item
Table 3.
Frequency of active disease in 51 patients with newly diagnosed giant cell arteritis, as captured by the Birmingham Vasculitis Activity Score
| BVAS items (by organ system) | New/worse symptoms | Persistent symptoms |
|---|---|---|
| General (N=43) | ||
| Malaise | 16 | 3 |
| Myalgia | 11 | 0 |
| Arthralgia/arthritis | 9 | 1 |
| Headache | 36 | 1 |
| Fever (<38.5 degrees Celsius) | 1 | 1 |
| Fever (>38.5 degrees Celsius) | 1 | 1 |
| Weight loss (≥2 kilograms) | 10 | 0 |
| Median (range) BVAS for category | 1 (0–3) | 0 (0–2) |
| Cutaneous (N=0) | ||
| Median (range) BVAS for category | 0 (0-0) | 0 (0-0) |
| Mucous membranes/eyes (N=18) | ||
| Blurred vision | 16 | 0 |
| Sudden vision loss | 12 | 0 |
| Retinal hemorrhage (N=1) | ||
| Median (range) total BVAS for category | 0 (0–6) | 0 (0-0) |
| Ear, Nose, and Throat (N=0) | ||
| Median (range) total BVAS for category | 0 (0-0) | 0 (0-0) |
| Chest (N=5) | ||
| Persistent cough | 4 | 1 |
| Median (range) total BVAS for category | 0 (0–2) | 0 (0–1) |
| Cardiovascular (N=1) | ||
| Congestive heart failure | 0 | 1 |
| Median (range) BVAS for category | 0 (0-0) | 0 (0–2) |
| Abdominal (N=0) | ||
| Median (range BVAS for category) | 0 (0-0) | 0 (0-0) |
| Renal (N=0) | ||
| Median (range) BVAS for category | 0 (0-0) | 0 (0-0) |
| Nervous system (N=0) | ||
| Median (range) BVAS for category | 0 (0-0) | 0 (0-0) |
| Other N=19 | 19 | 0 |
| Total Median (range) BVAS | 3 (0–9) | 0 (0–5) |
BVAS = Birmingham Vasculitis Activity Score (BVAS); N = total number with any clinical manifestation in that organ system.
All median BVAS are scores for 236 encounters with active disease.
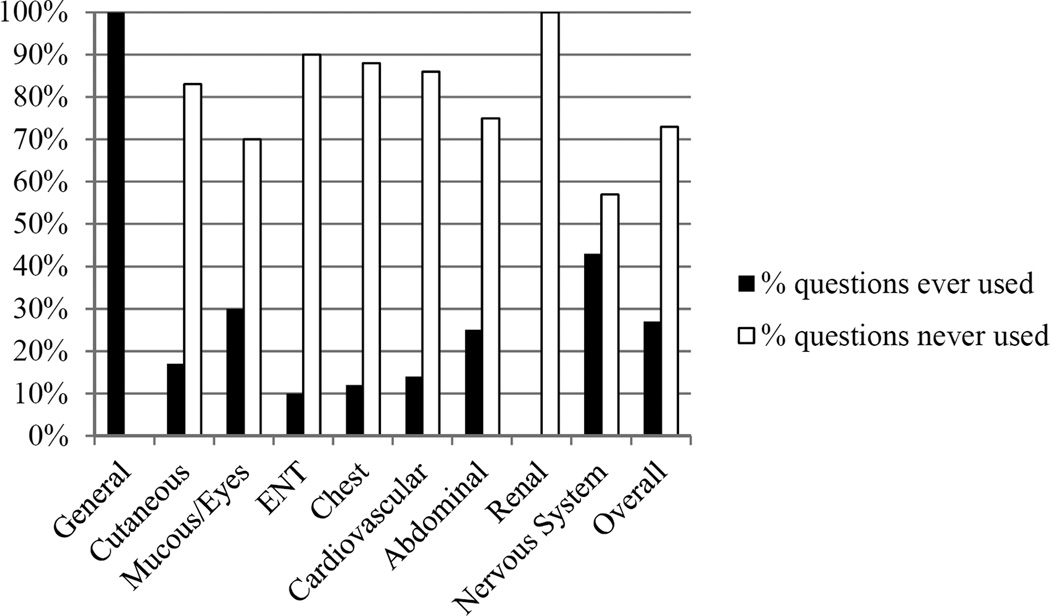
The most utilized categories of BVAS were the “General” category, followed by “Other”, and “Mucus Membranes/Eyes”. Forty-eight (73%) of the 66 items in the 9 organ systems were never used. Furthermore, 57 (86%) of the 66 items in the 9 organ systems were recorded in <3% of visits when active disease was present. The individual questions in the different categories of the BVAS which were applicable to any encounter with symptoms of active vasculitis are in the Figure. All of the components in the “General” section of the BVAS were applicable to patients with GCA while none of the components in the “Renal” section were applicable to patients with active GCA.
Figure 1.
Percentage of BVAS questionnaire components, arranged by category, which were applicable to any patient with giant cell arteritis and active disease. ENT = ear, nose, and throat; BVAS = Birmingham Vasculitis Activity Score.
In 25 visits (11%) of active disease, all the symptoms of active vasculitis were captured only in the “Other” category resulting in both BVAS1 and BVAS2 of 0. Manifestations of active vasculitis not captured by the BVAS in these 25 visits were tongue/jaw claudication (5 cases, 20%), upper extremity claudication (14 cases, 56%), lower extremity claudication (6 cases, 24%), diplopia (1 case, 4%), and light-headedness (1 case, 4%). Median PGA in these 25 encounters was 3 (0–8) for past 28 days and 3 (0–7) for the day of the evaluation. Disease activity for the past 28 days in these 25 encounters was rated as “remission” in 2visits, “low” in 8 visits, “moderate” in 8 visits, and “high” in 1 visit while disease activity for the day of the evaluation was rated as “remission in 4 visits, “low” in 17 visits, “moderate” in 5 visits, and “high” in 1 visit.
Correlation of the BVAS with other commonly used measures of PGA is in Table 4. Neither the BVAS nor the PGA correlated well with ESR or CRP.
Table 4.
Spearman’s correlations of BVAS with other parameters used to assess disease activity in GCA
| BVAS1 | p-value | BVAS2 | p-value | PGA 28 days |
p-value | PGA today |
p-value | |
|---|---|---|---|---|---|---|---|---|
| PGA past 28 days | 0.50 | <0.001 | −0.20 | 0.003 | - | - | - | - |
| PGA today | 0.01 | 0.62 | −0.08 | 0.26 | 0.29 | <0.001 | - | - |
| ESR | 0.02 | 0.80 | −0.12 | 0.08 | 0.08 | 0.22 | 0.41 | <0.001 |
| CRP | 0.02 | 0.77 | −0.01 | 0.93 | −0.01 | 0.9 | 0.36 | <0.001 |
| Disease activity past 28 days* | 0.46 | <0.001 | −0.18 | 0.001 | 0.90 | <0.001 | - | - |
| Disease activity today | 0.05 | 0.40 | −0.04 | 0.50 | - | - | 0.93 | <0.001 |
BVAS= Birmingham Vasculitis Activity Score; BVAS1= score for new/worse disease activity; BVAS2= score for persistent disease activity; GCA= giant cell arteritis; PGA= physician global assessment; ESR= erythrocyte sedimentation rate; CRP= C-reactive protein.
rated by evaluating physician as remission, low, medium or high.
DISCUSSION
In this large cohort of patients with GCA, the BVAS had limited utility in the assessment of disease activity. Most categories of the BVAS were not applicable in patients with GCA. Additionally, since many components of active vasculitis in GCA were captured in the “Other” category on the BVAS and do not contribute to the total BVAS, some patients had a BVAS of 0 despite active disease. This analysis raises concerns for use of the BVAS in clinical trials of new treatments for GCA.
There are numerous challenges in the clinical assessment of disease activity and an objective measure would be beneficial. Presently, there are no standardized measures of disease activity in GCA. Previous clinical trials have used terms such as “relapses”, “recurrences”, “flares”, or “remission” to define disease activity and often take into consideration markers of inflammation (11–19). GCA is a chronic granulomatous vasculitis with observational cohorts reporting at least one disease relapse in 28–64% patients in (2–5, 7, 9, 10). While markers of inflammation such as sedimentation rate and C-reactive protein are neither sensitive nor specific in the assessment of disease activity (8, 26, 27), they are frequently used to assess disease activity in patients with GCA and may influence treatment decisions. Suspected relapses are often treated with higher doses of glucocorticoids which are associated with significant morbidity in this population of patients (2, 3). The BVAS is a validated tool for assessment of disease activity for systemic vasculitis (21–23). However, previous validation studies evaluating the BVAS for assessment of disease activity in systemic vasculitis have only included a small number of patients with GCA (21, 23). Additionally, in a validation study of BVAS version 3, patients with GCA were excluded due to homogeneity of clinical manifestations and a limited range of abnormalities that would be measured by the BVAS items (23). Few studies have used BVAS in the assessment of disease activity in patients with GCA and again included a small number of patients (28, 29). Our study evaluated BVAS version 2 in a large, multicenter cohort of patients with GCA.
In this study, the total BVAS in patients with GCA during active disease in this study was low with a median score of 1. Even in the subset of patients with newly diagnosed GCA, the BVAS at diagnosis was low despite disease activity being rated by the evaluating physician as moderate to high in most of these patients. Additionally, in the majority of the encounters with active disease, symptoms were new rather than persistent; thus, the BVAS2 was not applicable in most patients with active GCA.
Most categories in the BVAS are not applicable to patients with GCA. The majority of symptoms of active GCA were captured in 2 of the 9 categories on the BVAS (“General” and “Mucus Membranes/Eyes”) and many symptoms fell in the “Other” category on the BVAS which does not contribute to the final score. Nearly 75% of the questions in the BVAS did not apply to any patient with GCA who had active disease, and, >85% of the questions were used in <3% visits when active disease was present.
Furthermore, several common manifestations of GCA are not including among the core elements on the BVAS. For example, ischemic manifestations such as limb claudication, jaw/tongue claudication, carotidynia, ischemic retinopathy and diplopia were not captured except in the “Other” category. A further potential weakness of the BVAS is that the category “Headache” likely lacks specificity for GCA since this term must be used to capture scalp tenderness and temporal artery pain, two symptoms which may not be perceived by patients or physicians as “Headache.” Many symptoms of active GCA were not captured by the BVAS (except in “Other” category). In 11% cases of active disease the BVAS was 0. The median PGA for the patients with a BVAS = 0 was 3 with physician categorized disease activity being rated as “low” or “moderate” in majority of the cases.
There is also not a strong correlation of the BVAS with other commonly used measures to assess disease activity in GCA. (21–23). In one study evaluating magnetic resonance imaging and positron emission tomography, activity scores from imaging findings were compared to clinical measures including BVAS and markers of inflammation (28). Imaging findings showed poor correlation with other clinical measures of disease activity including BVAS and markers of inflammation (28). In the current study BVAS correlated well PGA and physician categorical ratings of disease activity, but only for disease activity in the past 28 days and not for disease activity on the day of evaluation. Treatment changes made before the patient was evaluated may account for this finding. Alternately, symptoms may have spontaneously improved or abated by the time of evaluation. Neither BVAS nor PGA/disease activity in past 28 days correlated with markers of inflammation which again may reflect treatment changes made by the physician based on patient’s symptoms prior to the evaluation visit. In the current study PGA on the day of activity was the only variable of disease activity which correlated with ESR and CRP.
The strengths of this study include the prospective design with standardized serial assessments including the BVAS and detailed questionnaires about symptoms at each visit. This enabled more detailed analysis of symptoms recorded during active disease and comparison to what is captured on BVAS. Details on symptoms were available at different time points including, “in the past 28 days,” and “today” (on the day of evaluation). As a result, symptoms of active vasculitis were captured, including new symptoms which may have resolved by the time of evaluation. The data gathered in a longitudinal manner on each patient in the cohort is more comprehensive and complete than would be available during routine clinical assessment. BVAS version 2 was used since that is the version that was available at the time the cohort was first established. BVAS version 3 has fewer items and a single box for persistent activity but is otherwise similar to the original BVAS (22). Therefore, use of the older version of BVAS should not affect the validity of our findings. Additionally, in a study evaluating different measures of disease activity for ANCA vasculitis, the different measures showed high correlation (30). Additional strengths of this study include the large cohort size helping to ensure uncommon manifestations of GCA were assessed. The conduct of the study at multiple centers in North America adds to the generalizability of the results.
This study has some limitations to consider. The project was not able to assess inter-observer reliability of the BVAS in GCA, although this has been studied in the past (21–23). Symptoms attributed to active GCA by the evaluating clinician were captured as active disease but it is possible that manifestations could be related to other causes in this elderly population. The nature of the cohort is such that the effect of treatment on changes in the BVAS could not be established.
Objective, standardized methods of assessing disease activity in patients with large-vessel vasculitis are greatly needed (20, 31). The BVAS has played an important role in clinical trials in ANCA-associated vasculitis and remains an important contribution in the development of outcome measures in systemic vasculitis. The present study highlights the limitations of this tool in the evaluation of patients with GCA and provides data on aspects of clinical manifestations during active disease in GCA which may be important to include in future measures of disease activity. These findings both highlight why other approaches to disease assessment in GCA are needed and are helpful in informing future efforts to develop and validate measures of disease activity in large-vessel vasculitis.
Acknowledgments
Source of support: Dr. Kermani was supported through a Vasculitis Fellowship by the Vasculitis Clinical Research Consortium (VCRC). The VCRC has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the National Center for Research Resources (U54 RR019497), the Office of Rare Diseases Research, and the National Center for Advancing Translational Science. The VCRC is part of the Rare Diseases Clinical Research Network (RDCRN).
References
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234–245. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 2.Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–708. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 3.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014;93:194–201. doi: 10.1097/MD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Martinez A, Hernandez-Rodriguez J, Espigol-Frigole G, Prieto-Gonzalez S, Butjosa M, Segarra M, et al. Clinical relevance of persistently elevated circulating cytokines (tumor necrosis factor alpha and interleukin-6) in the long-term followup of patients with giant cell arteritis. Arthritis Care & Res. 2010;62:835–841. doi: 10.1002/acr.20043. [DOI] [PubMed] [Google Scholar]
- 5.Hachulla E, Boivin V, Pasturel-Michon U, Fauchais AL, Bouroz-Joly J, Perez-Cousin M, et al. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol. 2001;19:171–176. [PubMed] [Google Scholar]
- 6.Liozon E, Roblot P, Paire D, Loustaud V, Liozon F, Vidal E, et al. Anticardiolipin antibody levels predict flares and relapses in patients with giant-cell (temporal) arteritis. A longitudinal study of 58 biopsy-proven cases. Rheumatology (Oxford) 2000;39:1089–1094. doi: 10.1093/rheumatology/39.10.1089. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Lado L, Calvino-Diaz C, Pineiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore) 2011;90:186–193. doi: 10.1097/MD.0b013e31821c4fad. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000;43:1041–1048. doi: 10.1002/1529-0131(200005)43:5<1041::AID-ANR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Nesher G, Nesher R, Mates M, Sonnenblick M, Breuer GS. Giant cell arteritis: intensity of the initial systemic inflammatory response and the course of the disease. Clin Exp Rheumatol. 2008;26:S30–S34. [PubMed] [Google Scholar]
- 10.Kermani TA, Warrington KJ, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et al. Disease Relapses among Patients with Giant Cell Arteritis: A Prospective, Longitudinal Cohort Study. J Rheumatol. 2015;42:1213–1217. doi: 10.3899/jrheum.141347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med. 1975;82:613–618. doi: 10.7326/0003-4819-82-5-613. [DOI] [PubMed] [Google Scholar]
- 12.Schaufelberger C, Andersson R, Nordborg E. No additive effect of cyclosporin A compared with glucocorticoid treatment alone in giant cell arteritis: results of an open, controlled, randomized study. Br J Rheumatol. 1998;37:464–465. doi: 10.1093/rheumatology/37.4.464. [DOI] [PubMed] [Google Scholar]
- 13.De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45:136–138. doi: 10.1136/ard.45.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A, Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–114. doi: 10.7326/0003-4819-134-2-200101160-00010. [DOI] [PubMed] [Google Scholar]
- 15.Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA) Clin Exp Rheumatol. 2001;19:495–501. [PubMed] [Google Scholar]
- 16.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–1318. doi: 10.1002/art.10262. [DOI] [PubMed] [Google Scholar]
- 17.Schaufelberger C, Mollby H, Uddhammar A, Bratt J, Nordborg E. No additional steroid-sparing effect of cyclosporine A in giant cell arteritis. Scand J Rheumatol. 2006;35:327–329. doi: 10.1080/03009740500474537. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Taboada VM, Rodriguez-Valverde V, Carreno L, Lopez-Longo J, Figueroa M, Belzunegui J, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis. 2008;67:625–630. doi: 10.1136/ard.2007.082115. [DOI] [PubMed] [Google Scholar]
- 20.Direskeneli H, Aydin SZ, Kermani TA, Matteson EL, Boers M, Herlyn K, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol. 2011;38:1471–1479. doi: 10.3899/jrheum.110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–678. [PubMed] [Google Scholar]
- 22.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 23.Suppiah R, Mukhtyar C, Flossmann O, Alberici F, Baslund B, Batra R, et al. A cross-sectional study of the Birmingham Vasculitis Activity Score version 3 in systemic vasculitis. Rheumatology (Oxford) 2011;50:899–905. doi: 10.1093/rheumatology/keq400. [DOI] [PubMed] [Google Scholar]
- 24.Merkel PA, Aydin SZ, Boers M, Direskeneli H, Herlyn K, Seo P, et al. The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol. 2011;38:1480–1486. doi: 10.3899/jrheum.110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 26.Kyle V, Cawston TE, Hazleman BL. Erythrocyte sedimentation rate and C reactive protein in the assessment of polymyalgia rheumatica/giant cell arteritis on presentation and during follow up. Ann Rheum Dis. 1989;48:667–671. doi: 10.1136/ard.48.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pountain GD, Calvin J, Hazleman BL. Alpha 1-antichymotrypsin, C-reactive protein and erythrocyte sedimentation rate in polymyalgia rheumatica and giant cell arteritis. Br J Rheumatol. 1994;33:550–554. doi: 10.1093/rheumatology/33.6.550. [DOI] [PubMed] [Google Scholar]
- 28.Both M, Ahmadi-Simab K, Reuter M, Dourvos O, Fritzer E, Ullrich S, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis. 2008;67:1030–1033. doi: 10.1136/ard.2007.082123. [DOI] [PubMed] [Google Scholar]
- 29.Henes JC, Mueller M, Pfannenberg C, Kanz L, Koetter I. Cyclophosphamide for large vessel vasculitis: assessment of response by PET/CT. Clin Exp Rheumatol. 2011 Jan-Feb;29(1 Suppl 64):S43–S48. [PubMed] [Google Scholar]
- 30.Merkel PA, Cuthbertson DD, Hellmich B, Hoffman GS, Jayne DR, Kallenberg CG, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis. 2009;68:103–106. doi: 10.1136/ard.2008.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aydin SZ, Direskeneli H, Sreih A, Alibaz-Oner F, Gul A, Kamali S, et al. Update on Outcome Measure Development for Large Vessel Vasculitis: Report from OMERACT 12. J Rheuamtol. 2015 Jun 15; doi: 10.3899/jrheum.141144. [E-pub]. [DOI] [PMC free article] [PubMed] [Google Scholar]