Abstract
We examined 4872 infants born consecutively, 2011-2012, and seen at 3 primary care centers to determine whether area-based socioeconomic measures were associated with noncompletion of common preventive services within the first 15 months. Addresses were geocoded and linked to census tract poverty, adult educational attainment, and household vehicle ownership rates. The quartile of patients in the highest poverty (adjusted odds ratio [aOR] 1.25; 95% confidence interval [CI] 1.01-1.54) and lowest vehicle ownership tracts (aOR 1.32; 95% CI 1.07-1.63) had significantly increased odds of service noncompletion. There were significant spatial clusters of low completion in Cincinnati’s urban core. These findings have implications for preventive service delivery.
Keywords: geographic location, socioeconomic, pediatrics, prevention, primary care, disparities
Where one lives matters for health.1 This impact of place is influenced by underlying socioeconomic factors.2,3 When operationalized as area-based socioeconomic measures, these poverty-related risk factors have been associated with disease at both the patient and population levels.4-8 Poverty rates are among the most useful area-based predictors of health outcomes,9 and census tracts are the most useful geographic grouping.10
Completion of preventive services (eg, immunizations, developmental screening) is increasingly considered an important measure of health care quality. Previous studies have shown associations between individual-level and area-based socioeconomic measures, such as parental income or census tract poverty, and completion of pediatric preventive services.11-16 While studies assessing area-based socioeconomic measures have looked at completion rates across states or cities, to our knowledge, no previous study has evaluated such linkages at the level of a single system of pediatric primary care centers.
Analyses at clinic or health system levels could elucidate variability in preventive service completion that may be relevant to risk-targeted approaches gaining favor in primary care redesign.17,18 Additionally, centers may benefit from information regarding completion of a more broadly defined set of preventive services delivered across early childhood.19 Such an analysis, and identification of the geographic area in which a practice’s children are at highest risk for non-completion of preventive services, could inform the design of place-based interventions. Thus, we sought to identify whether area-level socioeconomic measures would be useful in the prediction of completion, or noncompletion, of preventive services in a single system of pediatric primary care centers.
Methods
Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, free-standing, academic pediatric institution. The CCHMC Division of General and Community Pediatrics oversees 3 primary care centers, 2 urban and 1 suburban. The 3 centers share a leadership structure and use common processes for quality assurance. Attending pediatricians and trainees follow uniform protocols for administering preventive services, resulting in receipt of eligible services at 92% of visits.20 The payer mix at all 3 centers is 85% Medicaid, 5% private insurance, and 10% self-pay.
The study was approved by the CCHMC Institutional Review Board with waived informed consent (#2014-2901).
Study Design
This was a retrospective review of the electronic health record for 5298 infants born consecutively between May 1, 2011 and November 30, 2012 and seen in 1 of 3 primary care centers. Each infant was followed for 15 months. Exclusion of patients with street addresses outside the primary service area of Hamilton and Butler Counties, and those with invalid address data, resulted in a final sample size of 4872 infants. Addresses were geocoded in ArcGIS 10.2 (Redlands, CA) using the address locator toolbox and street data from 2005 maintained by ESRI and TeleAtlas, linking every patient record to a precise geographic location representing their residence. Patients were then connected to the census tract in which the address was located; tract-level socioeconomic variables were appended to the patient record.
Outcomes
The outcome of the study was completion of a set of preventive services, defined a priori, within the first 15 months of life. The measure was defined to be composed of 10 preventive care elements recommended by the American Academy of Pediatrics (Bright Futures),21 including completion of recommended immunizations, lead screening, and developmental screening (Table 1). Infants are scheduled to be seen a minimum of 6 times during the first 15 months, and eligible preventive services can be given at well, ill, or follow-up visits. For this study, services were considered complete if received any time in the first 15 months. In our centers, lead and developmental screenings are routinely completed for all infants at 9 months of age due to our region’s high risk for lead exposure and the recommendation for early intervention.22 Preventive service completion was treated as an all-or-none, dichotomous outcome variable.
Table 1.
Ten Recommended Preventive Services Included in Outcome Measure.
| Preventive Service | No. of Doses (or Screens) Required to Be Considered Complete |
|---|---|
| Immunizations | |
| 1. Diphtheria, tetanus, and pertussis | 3 |
| 2. Inactivated polio virus | 3 |
| 3. Haemophilus influenzae type B | 3 |
| 4. Hepatitis B | 2 |
| 5. Pneumococcal conjugate | 3 |
| 6. Rotavirus | 2 |
| 7. Measles, mumps, and rubella | 1 |
| 8. Varicella | 1 |
| Screenings | |
| 9. Lead screening (blood test) | 1 |
| 10. Development screening (using Ages and Stages Questionnaire) | ≥1 |
Predictors
Our primary predictors were census tract rates of poverty, household vehicle ownership, and adult educational attainment. We chose poverty and educational attainment as common markers for socioeconomic status, and household vehicle ownership as a conceived measure of transportation access (and access to care). Variables were obtained from the 2008-2012 American Community Survey.23 For ease of interpretation and given consideration of how such data could be adapted as a tool for clinical usage, each variable was categorized into quartiles to place ~25% of patients in one of four risk groupings. Demographic covariates including race, ethnicity, insurance, and sex were collected from the electronic health record. We also identified the specific primary care center where each patient was seen—defined as center A, B, or C.
Statistical and Spatial Analyses
Bivariate analyses assessed relationships between predictors, and covariates, and our outcome using the chi-square test. Then, multivariable logistic regression with generalized estimating equations was used to assess associations between area-level predictors and preventive service completion, accounting for key covariates and for clustering at the census tract level. Because of the high correlation among the 3 tract-level variables, models were fit for each separately. Covariates included race, ethnicity, insurance, sex, and primary care center.
We then explored spatial patterns in preventive service completion using the Gi* cluster detection statistic. This statistic compares the expected value of a variable at a point across a local area to the expected value of that variable across the entire study area.24 The Gi* value is a standardized z-score. Positive, significant values indicate that a point is part of a cluster of high rates of completion; negative, significant values indicate that a point is part of a cluster of low rates of completion (ie, high rates of noncompletion). To account for multiple testing and spatial dependency, a false detection rate correction was applied, which reduces the critical P-value threshold at which a spatial pattern is considered to be statistically significant.
Results
A total of 4872 patients across 283 census tracts were included (range of 1-176 patients per tract). All recommended preventive service items were completed by 43% of infants (Table 2). Patients were predominantly African American, non-Hispanic, and publicly insured. For patients’ corresponding census tracts, the median poverty rate was 24.9% (range 1% to 84%), the median rate of no household vehicle was 16.7% (range 0% to 71%), and the median rate of less than high school completion was 16.7% (range 0.2% to 41%).
Table 2.
Individual Demographic and Census Tract Socioeconomic Characteristics for Study Population (n = 4872).
| Characteristic | n or median | % or interquartile rangea |
|---|---|---|
| No. of visits in first 15 months | 6.0 | 3.5, 8.5 |
| Preventive services completion | ||
| Complete | 2078 | 42.7 |
| Incomplete | 2794 | 57.4 |
| Race | ||
| Black or African American | 3042 | 62.4 |
| White or Caucasian | 1186 | 24.3 |
| Other | 603 | 12.4 |
| Missing | 41 | 0.8 |
| Ethnicity | ||
| Hispanic | 312 | 6.4 |
| Not Hispanic | 4500 | 92.4 |
| Missing | 60 | 1.2 |
| Insurance | ||
| Public | 4155 | 85.3 |
| Private | 371 | 7.6 |
| Missing | 346 | 7.1 |
| Sex | ||
| Female | 2346 | 48.2 |
| Male | 2525 | 51.8 |
| Primary care center | ||
| Urban, base facility (A) | 2697 | 55.4 |
| Urban, community health center (B) | 883 | 18.1 |
| Suburban, community health center (C) | 1292 | 26.5 |
| Census tract socioeconomic factors | ||
| Percentage of individuals below poverty line | 24.9 | 13.7, 42.0 |
| Percentage of households with no vehicle | 16.7 | 6.9, 30.7 |
| Percentage of adults with below high school education | 16.7 | 10.8, 24.8 |
Percentages may add to more or less than 100 due to rounding.
Census tract variables were highly and significantly correlated with one another, with Spearman correlation coefficients of >0.67. When categorized, the number of tracts in each risk quartile ranged from 31 to 132.
In bivariate analyses, African American children had lower completion rates compared with white children (42% vs 49%; P = .009). Non-Hispanic children also had lower completion rates compared to their Hispanic counterparts (42% vs 55%; P < .0001). Primary care center was also associated with completion of services with the rates of completion lowest at the urban, base facility (A) and higher at both the urban, community center (B) and the suburban, community center (C) (39% vs 48% vs 47%; P < .0001). Insurance and sex were not related to our outcome.
In the multivariable generalized estimating equation models, we found that those living in high poverty (quartiles 3 and 4) tracts had significantly higher odds of preventive service noncompletion compared with the lowest poverty quartile (Table 3). Similarly, we found that those living in low vehicle access tracts (quartiles 2, 3, and 4) had significantly higher odds of noncompletion compared with the highest vehicle access quartile. The educational attainment variable was not a significant predictor after adjustment.
Table 3.
Census Tract Variables Associated With Odds of Not Completing Preventive Services Using Logistic Regression With Generalized Estimating Equations to Account for Clustering at the Census Tract Level.
| Census Tract Variable | Odds Ratio | 95% Confidence Interval | Adjusted Odds Ratioa | 95% Confidence Interval |
|---|---|---|---|---|
| Census tract povertyb | ||||
| Quartile 1 (lowest poverty) | Ref | Ref | Ref | Ref |
| Quartile 2 | 1.16 | 0.97-1.39 | 1.13 | 0.94-1.35 |
| Quartile 3 | 1.26 | 1.06-1.49 | 1.22 | 1.01-1.48 |
| Quartile 4 (highest poverty) | 1.27 | 1.06-1.54 | 1.25 | 1.01-1.54 |
| Census tract vehicle accessc | ||||
| Quartile 1 (most households with vehicle) | Ref | Ref | Ref | Ref |
| Quartile 2 | 1.26 | 1.06-1.49 | 1.22 | 1.02-1.46 |
| Quartile 3 | 1.37 | 1.19-1.59 | 1.33 | 1.12-1.59 |
| Quartile 4 (least households with vehicle) | 1.36 | 1.12-1.64 | 1.32 | 1.07-1.63 |
| Census tract educational attainmentd | ||||
| Quartile 1 (highest attainment) | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.99 | 0.82-1.21 | 0.96 | 0.79-1.17 |
| Quartile 3 | 1.20 | 1.01-1.43 | 1.18 | 0.98-1.41 |
| Quartile 4 (lowest attainment) | 1.18 | 0.96-1.44 | 1.12 | 0.90-1.39 |
Adjusted for race, ethnicity, insurance, sex, and primary care center.
Census tract poverty quartiles defined as percentage below poverty line: (1) <13.7%, (2) 13.7% to 24.9%, (3) 24.9% to 42.0%, and (4) >42%.
Census tract vehicle access quartiles defined as percentage of households with no vehicle: (1) <6.9%, (2) 6.9% to 16.7%, (3) 16.7% to 30.7%, (4) >30.7%.
Census tract educational attainment quartiles defined as percentage of adults with less than a high school education: (1) <10.8%, (2) 10.8% to 16.7%, (3) 16.7% to 24.8%, and (4) >24.8%.
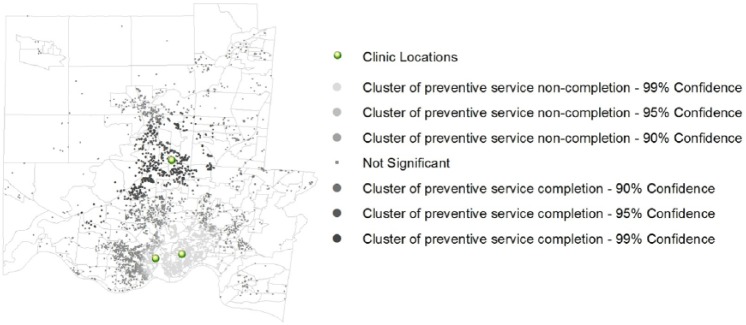
Figure 1 illustrates our spatial analysis. This map displaying the Gi* computation illustrates a cluster of low rates of completion (“hot spot”) in the southern part of the study region (inner-city Cincinnati and adjacent neighborhoods) and a cluster of high rates of completion (“cold spot”) in the center of the study region (suburban Cincinnati). The tested area-based measures seem to drive, at least in part, the geographic patterns in completion across the study region. For example, the tracts included in the cluster of low rates of completion have significantly higher rates of poverty than the tracts located within the cluster of high rates of completion.
Figure 1.
Gi* spatial analysis.
Discussion
Preventive services are a critical component of pediatric primary care delivery. We found significant associations between area-based socioeconomic measures and completion patterns of recommended preventive services. Patients living in communities, or census tracts, with higher rates of poverty and lower rates of vehicle access were ~30% less likely to complete key preventive services compared with the lowest poverty and highest vehicle access quartiles, respectively. Such area-based data could be applied at the patient-level to improve completion of preventive services via patient-level risk stratification and tailoring of interventions. Such data could be similarly applied at the primary care center or population level to more effectively target preventive service delivery. Such strategies are especially relevant given renewed focus on preventive service delivery.
Our study adds to previous studies which have shown the impact socioeconomic factors can have on preventive service utilization. Indeed, individual socioeconomic measures like parental employment and access to transportation have been linked to pediatric preventive and ill care utilization as well as immunization rates.25-28 Here, we show that infants from lower socioeconomic tracts are also missing a range of preventive services, including immunizations and key, recommended developmental and lead screens. Past studies have shown that children of lower socioeconomic status are more likely to screen positive on lead and developmental screenings.29,30 Our study makes the unique contribution of illustrating that these children, who are most at risk, are also least likely to complete such screenings. Moreover, variability in completion exists even in a relatively homogenous, high-risk population receiving standardized preventive care processes in a single system.
Our findings suggest that individual primary care centers may be able to use publicly available, area-based socioeconomic measures in novel risk stratification or prediction strategies to improve preventive care delivery for their patient panels. Higher poverty rates, or lower rates of vehicle access, in a patient’s neighborhood could indicate that the patient may benefit from extra screening or intervention (eg, home visits) to ensure receipt of preventive care in the first years of life. Similarly, redesign of well-child care delivery may be indicated for the primary care center as a whole and/or for an entire population or community of interest. For example, perhaps opening smaller clinics in neighborhoods with low rates of completion would improve access to care. Several studies have already begun to explore options for such redesign, suggesting changes in the providers, locations, and formats.17,31-36 Our findings support the need for innovative, place-based strategies to enhance completion of preventive services.
Our study has several limitations. First, center B, which had the highest completion rates of the 3 sites, began implementing outreach efforts to improve attendance at well-child visits toward the end of the study period. Adjustment for primary care center should have accounted for any potential confounding. Second, our results may be subject to the ecological fallacy. If a patient lived in an impoverished census tract, we assigned that value to that patient regardless of the patient’s actual household poverty level. We expect, however, that the homogeneity of census tracts makes it a relatively robust assumption. Third, 7% of the insurance data points were “missing,” which could represent either self-pay status or unmarked information in the electronic health record. Finally, our sample was isolated to patients cared for at 1 of our 3 primary care centers. As such, our findings may not be generalizable to primary care centers in other settings or regions. That said, we believe the methods used in our analyses would be generalizable and potentially useful for a wide range of primary care centers.
Conclusions
Area-based socioeconomic measures may facilitate risk stratification and the targeting of interventions by matching risk level with availability of health-promoting resources. We found clear connections between such measures and preventive service completion within a single system of pediatric primary care centers. Future efforts will focus on exploring geographic variation in preventive services outcomes and determining how such measures could be useful in the redesign of primary care preventive services across a range of clinical settings and communities in ways that are seen as appropriate to families.
Author Biographies
Margaret N. Jones is a medical student at the University of Cincinnati College of Medicine.
Courtney M. Brown, MD, MSc, is an assistant professor in the Department of Pediatrics, Division of General & Community Pediatrics at the University of Cincinnati College of Medicine. She is also an attending pediatrician at Cincinnati Children’s Hospital Medical Center.
Michael J. Widener, PhD, is an assistant professor of Geography & Planning at the University of Toronto.
Heidi J. Sucharew, PhD, is an assistant professor in the Department of Pediatrics, Division of Biostatistics & Epidemiology at the University of Cincinnati College of Medicine and Cincinnati Children’s Hospital Medical Center.
Andrew F. Beck, MD, MPH, is an assistant professor in the Department of Pediatrics, Divisions of General & Community Pediatrics and Hospital Medicine at the University of Cincinnati College of Medicine. He is also an attending pediatrician at Cincinnati Children’s Hospital Medical Center.
Footnotes
Authors’ Note: Michael J. Widener is presently at the Department of Geography & Planning, University of Toronto, Ontario, Canada.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this work was provided through the Arnold P. Gold Foundation (M. Jones, Student Summer Research Fellowship) and the National Institutes of Health (A. Beck, Grant #1K23AI112916). Funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1. Richardson DB, Volkow ND, Kwan MP, Kaplan RM, Goodchild MF, Croyle RT. Medicine. Spatial turn in health research. Science. 2013;339:1390-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cook JT, Frank DA. Food security, poverty, and human development in the United States. Ann N Y Acad Sci. 2008;1136:193-209. [DOI] [PubMed] [Google Scholar]
- 3. Malat J, Oh HJ, Hamilton MA. Poverty experience, race, and child health. Public Health Rep. 2005;120:442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. Geographic variability in childhood asthma prevalence in Chicago. J Allergy Clin Immunol. 2008;121:639-645.e1. [DOI] [PubMed] [Google Scholar]
- 6. Shankardass K, Jerrett M, Dell SD, Foty R, Stieb D. Spatial analysis of exposure to traffic-related air pollution at birth and childhood atopic asthma in Toronto, Ontario. Health Place. 2015;34:287-295. [DOI] [PubMed] [Google Scholar]
- 7. Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: a multilevel analysis of Massachusetts births, 1989-1991. Am J Epidemiol. 2006;164:823-834. [DOI] [PubMed] [Google Scholar]
- 8. Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children’s health. Pediatrics. 2008;121:337-344. [DOI] [PubMed] [Google Scholar]
- 9. Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95:312-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geocoding Project (US). J Epidemiol Community Health. 2003;57:186-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chi DL, Momany ET, Jones MP, et al. An explanatory model of factors related to well baby visits by age three years for Medicaid-enrolled infants: a retrospective cohort study. BMC Pediatr. 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feemster KA, Spain CV, Eberhart M, Pati S, Watson B. Identifying infants at increased risk for late initiation of immunizations: maternal and provider characteristics. Public Health Rep. 2009;124:42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kattan JA, Kudish KS, Cadwell BL, Soto K, Hadler JL. Effect of vaccination coordinators on socioeconomic disparities in immunization among the 2006 Connecticut birth cohort. Am J Public Health. 2014;104:e74-e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Berckelaer AC, Mitra N, Pati S. Predictors of well child care adherence over time in a cohort of urban Medicaid-eligible infants. BMC Pediatr. 2011;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitehead SJ, Cui KX, De AK, Ayers T, Effler PV. Identifying risk factors for underimmunization by using geocoding matched to census tracts: a statewide assessment of children in Hawaii. Pediatrics. 2007;120:e535-e542. [DOI] [PubMed] [Google Scholar]
- 16. Williams IT, Milton JD, Farrell JB, Graham NM. Interaction of socioeconomic status and provider practices as predictors of immunization coverage in Virginia children. Pediatrics. 1995;96(3 pt 1):439-446. [PubMed] [Google Scholar]
- 17. Coker TR, Moreno C, Shekelle PG, Schuster MA, Chung PJ. Well-child care clinical practice redesign for serving low-income children. Pediatrics. 2014;134:e229-e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coker TR, Windon A, Moreno C, Schuster MA, Chung PJ. Well-child care clinical practice redesign for young children: a systematic review of strategies and tools. Pediatrics. 2013;131(suppl 1):S5-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown CM, Samaan ZM, Morehous JF, Perkins AA, Kahn RS, Mansour ME. Development of a bundle measure for preventive service delivery to infants in primary care. J Eval Clin Pract. 2015;21:642-648. [DOI] [PubMed] [Google Scholar]
- 20. Samaan ZM, Brown CM, Morehous JF, Perkins AA, Kahn RS, Mansour ME. Implementation of a preventive services bundle in academic pediatric primary care centers. Pediatrics. 2016. [DOI] [PubMed] [Google Scholar]
- 21. Bright Futures. 2014. http://brightfutures.aap.org/index.html. Accessed August 20, 2014.
- 22. Raymond J, Wheeler W, Brown MJ, Centers for Disease Control and Prevention. Lead screening and prevalence of blood lead levels in children aged 1-2 years—Child Blood Lead Surveillance System, United States, 2002-2010 and National Health and Nutrition Examination Survey, United States, 1999-2010. MMWR Surveill Summ. 2014;63 (suppl 2):36-42. [PubMed] [Google Scholar]
- 23. American FactFinder. 2013. http://factfinder2.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t. Accessed July 12, 2013.
- 24. Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geogr Anal. 1992;24:189-206. [Google Scholar]
- 25. Bardenheier BH, Yusuf HR, Rosenthal J, et al. Factors associated with underimmunization at 3 months of age in four medically underserved areas. Public Health Rep. 2004;119:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hambidge SJ, Phibbs SL, Davidson AJ, et al. Individually significant risk factors do not provide an accurate clinical prediction rule for infant underimmunization in one disadvantaged urban area. Ambul Pediatr. 2006;6:165-172. [DOI] [PubMed] [Google Scholar]
- 27. Holl JL, Oh EH, Yoo J, Amsden LB, Sohn MW. Effects of welfare and maternal work on recommended preventive care utilization among low-income children. Am J Public Health. 2012;102:2274-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jhanjee I, Saxeena D, Arora J, Gjerdingen DK. Parents’ health and demographic characteristics predict noncompliance with well-child visits. J Am Board Fam Pract. 2004;17:324-331. [DOI] [PubMed] [Google Scholar]
- 29. Jones RL, Homa DM, Meyer PA, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988-2004. Pediatrics. 2009;123:e376-e385. [DOI] [PubMed] [Google Scholar]
- 30. Potijk MR, Kerstjens JM, Bos AF, Reijneveld SA, de Winter AF. Developmental delay in moderately preterm-born children with low socioeconomic status: risks multiply. J Pediatr. 2013;163:1289-1295. [DOI] [PubMed] [Google Scholar]
- 31. Bundy DG, Randolph GD, Murray M, Anderson J, Margolis PA. Open access in primary care: results of a North Carolina pilot project. Pediatrics. 2005;116:82-87. [DOI] [PubMed] [Google Scholar]
- 32. Hambidge SJ, Phibbs SL, Chandramouli V, Fairclough D, Steiner JF. A stepped intervention increases well-child care and immunization rates in a disadvantaged population. Pediatrics. 2009;124:455-464. [DOI] [PubMed] [Google Scholar]
- 33. Heath G, Greenfield S, Redwood S. The meaning of ‘place’ in families’ lived experiences of paediatric outpatient care in different settings: a descriptive phenomenological study. Health Place. 2015;31:46-53. [DOI] [PubMed] [Google Scholar]
- 34. Margolis P, Halfon N. Innovation networks: a strategy to transform primary health care. JAMA. 2009;302:1461-1462. [DOI] [PubMed] [Google Scholar]
- 35. Margolis PA, Stevens R, Bordley WC, et al. From concept to application: the impact of a community-wide intervention to improve the delivery of preventive services to children. Pediatrics. 2001;108:E42. [DOI] [PubMed] [Google Scholar]
- 36. Mooney K, Moreno C, Chung PJ, Elijah J, Coker TR. Well-child care clinical practice redesign at a community health center: provider and staff perspectives. J Prim Care Community Health. 2014;5:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]