Abstract
The rising incidence of obesity and metabolic diseases such as diabetes mellitus and cardiovascular disease in adolescents and young adults is of grave concern. Recent studies favor role of lifestyle factors over genetics in perpetuation of inflammation, insulin resistance and oxidative stress, which are pathophysiologic processes common to above diseases; furthermore, the importance of dietary factors in addition to calories and physical activity in these processes is being increasingly recognized. Advanced glycation end products (AGEs) belong to a category of dietary oxidants which have been implicated in the pathogenesis of inflammation, oxidative stress, insulin resistance, β-cell failure and endothelial dysfunction. This paper reviews the studies of AGEs with focus on their role in cardiometabolic disease in children.
A MEDLINE search was performed using the keywords childhood obesity, metabolic syndrome and advanced glycation end products. Articles published in English between 1975 and 2015 and their references were reviewed.
While most studies were performed in adults, a few studies also demonstrated a role of AGEs in obesity and associated cardiometabolic comorbidities in the younger population.
Available evidence suggests involvement of AGEs in pathogenesis of adiposity and β-cell failure in children. Potential areas for further research to investigate underlying mechanisms are proposed.
Keywords: obesity, children, insulin resistance, metabolic syndrome, advanced glycation end products, receptor of advanced glycation end products
Introduction
There is a worldwide worsening epidemic of obesity, diabetes mellitus (DM) and cardiovascular disease (CVD) with increasing onset in children and young adults (1). However, knowledge of mechanisms underlying the progression to DM and CVD, particularly in children, is still limited; this represents a barrier for further progress in this field. Strategies to identify the child at risk, mechanisms involved, and how to prevent/treat these conditions in their early stages of development are needed urgently and are vital in effectively preventing or halting the progression of DM and CVD. These multifactorial diseases are known to be associated with low-grade inflammation, insulin resistance (IR) and oxidative stress (OS) across the age spectrum (2).
An increasing number of studies point to a pathogenic role of dietary factors in obesity-associated chronic inflammation particularly with regards to their pro-oxidant properties. Advanced Glycation end Products (AGEs) belong to one such category of oxidants, which may cause β-cell failure, IR and endothelial dysfunction. AGEs are traditionally known to be produced endogenously as a result of hyperglycemia and increased OS. Recently accumulated data additionally indicate that exogenous AGEs ingested with food or smoking represent a major contributor to pool of AGEs in the body. In this article, we will review experimental and human studies looking at the role of AGEs in causing DM and CVD with special emphasis on dietary AGEs and on studies in children. We will also review studies evaluating the role of AGEs and their receptor variants in children with focus on cardiometabolic risk. Finally, we will delineate the challenges of research in the field and present some insights on future directions, particularly in relation to children and adolescents.
I. What are AGEs and what are their pathogenic mechanisms?
Reducing sugars such as glucose and fructose undergo spontaneous reactions with free amino groups on proteins, peptides or amino acids, lipids and nucleic acids to form a heterogeneous group of compounds known as AGEs; this is the classical Maillard reaction. The term AGEs, as currently used, broadly encompasses products of both glycoxidation and lipid peroxidation such as intermediate reactive precursors [1-deoxyglyoxal (1-DG), 3 DG and methylglyoxals (MG)] as well as terminal non-reactive AGEs [carboxymethyllysine (CML), pentosidine]. These reactions increase in the presence of hyperglycemia and OS in vivo. In addition, all these reactions also occur in the environment and accelerate in the presence of high temperatures. For example, cooking food under dry conditions with the application of high heat significantly increases the formation of AGEs.
AGEs, endogenous or exogenous, can produce tissue damage by two main mechanisms. First, AGEs can covalently crosslink proteins and therefore directly alter protein structure and function. Second, through a variety of receptor- and non-receptor mechanisms, AGEs can activate several intracellular pathways that increase generation of reactive oxygen species (ROS) and inflammatory cytokines.
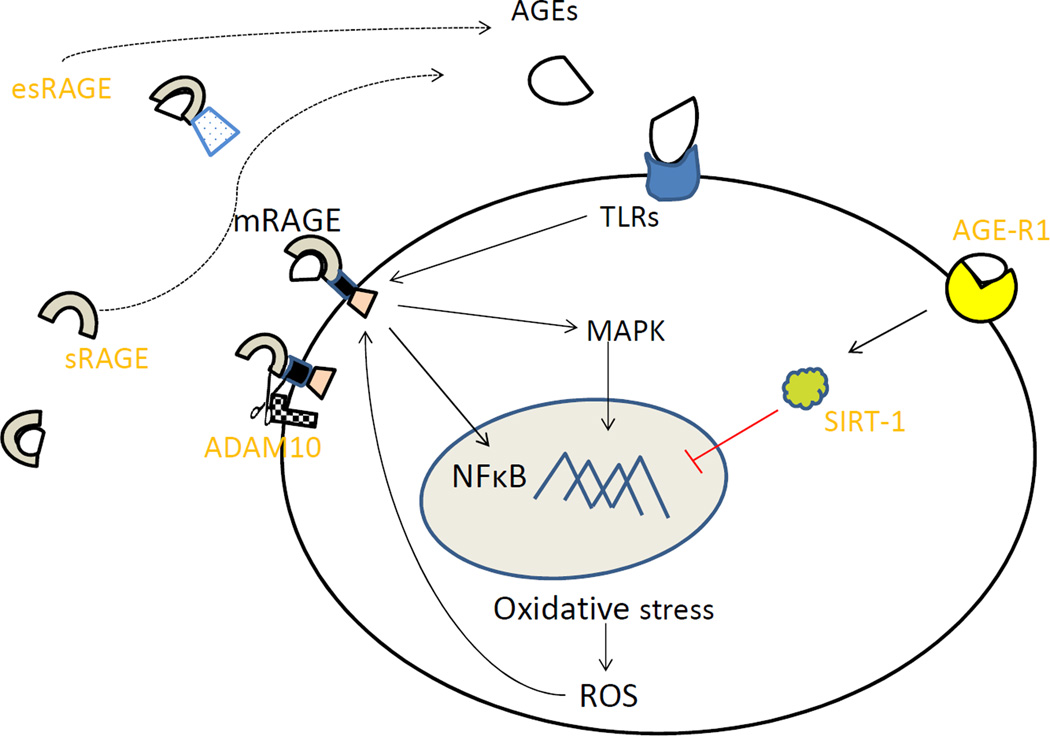
The receptor for advanced glycation end products (mRAGE) is a well-studied membrane-bound receptor that binds AGEs initiating a cascade of intracellular events leading to inflammation and OS (3). Other receptors of AGEs such as Advanced Glycation End product Receptor-1, 2 and 3 (AGE-R1, AGE-R2 and AGE-R3) and scavenger receptors are considered to be endocytic in nature and also involved in clearance of AGEs. Moreover, AGE-R1 has been shown to participate in pathways that decrease intracellular OS (4). It has been proposed that chronic AGEs’ overload results in unbalanced activation of downstream pro-inflammatory and pro-oxidative pathways. There is also a circulating pool of RAGE, collectively known as soluble RAGE (sRAGE), whose role still remains controversial. Soluble RAGE consists of the isoform derived from membrane bound RAGE by proteolytic action of metalloproteases [such as A Disintegrin and A Metalloprotease-10 (ADAM10) and matrix metalloprotease (MMP-9)] and a minor, alternatively spliced, isoform of RAGE known as endogenously secreted RAGE (esRAGE) (5). In animal studies, administration of sRAGE prevented and stabilized established atherosclerosis (6, 7) and ameliorated retinal neuronal dysfunction in experimental diabetic retinopathy (8). Therefore, sRAGE has been suggested to act as a decoy receptor that binds and eliminates circulating AGEs. A contrary view that has been proposed is that sRAGE may be a marker of tissue RAGE expression and represent disease activity (9). The exact pathophysiological role of these soluble variants remains controversial and is a matter of active investigation. More recently, it has also been shown that AGEs also activate intracellular pathways through Toll Like Receptor-4 (TLR-4) in addition to RAGE (10). Figure 1 shows possible actions of major receptors as a result of interaction with AGEs during normal cellular homeostasis.
Figure 1.
AGEs and their major receptors. AGEs bind to mRAGE and cause activation of inflammatory pathways (MAPK, NF-κB) or are endocytosed and cleared by AGE-R1. AGE-R1 activates sirtuins, a group of deacetylases that suppress NF-κB. ADAM-10 is a metalloprotease that cleaves mRAGE and releases it into circulation as sRAGE. esRAGE is an alternatively spliced form that constitutes approximately 15% of circulating RAGE pool. TLR-4 is another receptor implicated in mediating the action of AGEs. AGE- Advanced glycation end product, mRAGE-membrane bound Receptor of AGE, sRAGE-soluble RAGE, esRAGE-endogenous secretory RAGE, AGE-R1- Advanced Glycation end Product Receptor (OST-48), MAPK-Mitogen-activated protein Kinase, NF-κB -nuclear factor kappa-light-chainenhancer of activated B cells, A Disintegrin and Metalloprotease-10 ADAM-10. TLR-4-Toll like receptor-4.
Dietary AGEs
Food and tobacco are two major environmental sources of AGEs. The dietary content of AGEs depends on the protein, lipid and carbohydrate content of the food as well as on the temperature and conditions of cooking, especially moisture. Animal-derived foods cooked at high temperature, for prolonged time and under dry conditions have the highest content of AGEs (11). Dietary sources of AGEs contain both highly reactive intermediate precursors such as carbonyl derivatives as well as terminal AGEs such as CML. Gastrointestinal absorption of dietary AGEs has been confirmed by the oral administration of double-labeled single protein-AGEs, with or without specific AGE inhibitors, such as aminoguanidine in rats, or the enrichment of low-AGE experimental diets with specific AGEs in mice (4, 12). Chronic studies involving dietary AGEs modification have also confirmed an association between dietary AGEs burden and circulating AGEs levels. An estimated 10% of ingested AGEs are absorbed into the circulation and about 70% percent of those absorbed are retained in the body and contribute to the AGEs pool in the body, where they become indistinguishable from endogenous AGEs both structurally and functionally; kinetic studies in rats have shown that dietary AGEs are bioreactive molecules capable of covalently crosslinking tissue proteins and causing glycoxidative damage similar to glycotoxins produced endogenously (13). AGEs are further metabolized by detoxifying enzymes such as glyoxalases in different tissues as well as excreted by the kidneys. Therefore, AGEs may accumulate with decreased availability of glyoxalases (14) or in conditions of decreased renal clearance.
II. In vitro studies linking AGEs and metabolically active tissues
Adipose cells
Incubation with AGEs prevented differentiation of 3T3-L1 adipocytes, a commonly studied adipose cell line. Also, the cells demonstrated decreased glucose uptake activity and increased ROS in the presence of AGEs. Glucose uptake activity perturbation was reversed by blocking RAGE as well as by N- acetylcysteine, an antioxidant. This suggests that AGEs action on glucose uptake is mediated by RAGE generated intracellular OS. Furthermore, AGEs increased expression of Monocyte Chemoattractant protein-1(MCP-1), an inflammatory marker involved in adipose tissue macrophage infiltration and insulin resistance (15).
Islet cells
Incubation of two insulin secreting cell lines (HIT-T15 and INS-1) with AGEs enhanced cell apoptosis and inhibited insulin secretion in cell culture models (16,17). It was also suggested that AGEs might bind to insulin and decrease its biologic activity. The apoptotic effects of AGEs were shown to be mediated via mitochondrial electron transport chain inhibition as well as the NADPH oxidase-mediated increase in ROS (17). Further, studies in rat islets showed that RAGE blockade could reverse the apoptotic effects of AGEs although the impact of AGEs on glucose stimulated insulin secretion could not be reversed. Interestingly, addition of glucagon like peptide-1 (GLP-1) reversed apoptosis and impaired glucose stimulated insulin secretion in the islets suggesting it has a protective action against AGEs (18). It is not known, however, if this GLP-1 effect is at the receptor-binding site or at a post-receptor level. This suggests that AGEs might act via different receptors to exert their actions on insulin secretion as well as apoptosis.
Hepatic cells
The liver is a major clearing house for AGEs with 85% of intravenously injected AGEs being cleared by sinusoidal cells and Kupffer cells and less than 15% by hepatocytes. AGEs, on the contrary, impair scavenger function of rat hepatic sinusoidal endothelial cells (19). Furthermore, hepatic stellate cells are activated when exposed to triglyceraldehyde-derived AGEs and demonstrate increased expression of RAGE as well as of genes involved in inflammation and fibrogenesis (20). These findings, coupled with elevated levels of triglyceride-derived AGEs in patients with nonalcoholic steatohepatitis (NASH), suggest a role of AGEs in NASH and cirrhosis of the liver (21). In addition, AGEs may cause insulin resistance and upregulate inflammation (as evidenced by increased C-reactive protein levels) in hepatocytes. Both of the above effects are thought to be mediated by activation of Rac-1 kinases followed by I Kappa B kinase and Jun N-terminal Kinase activation and downstream IRS-1 serine phosphorylation in the hepatocyte and adjacent hepatic stellate cells (22).
Muscle cells
Exposure of L6 skeletal muscle cells to human glycated albumin induced insulin resistance (decreased insulin stimulated glucose uptake and decreased glycogen synthase activity) via Protein Kinase C-alpha mediated serine and threonine phosphorylation of Insulin Receptor Substrate -1 (IRS-1) and IRS-2 (23). Endothelial cells: A study with human umbilical vein endothelial cells confirmed that food-derived AGEs induce significant TNF-α activation, as well as cell oxidative and crosslink formation activities and that these actions are mediated by RAGE and non-receptor mechanisms (24).
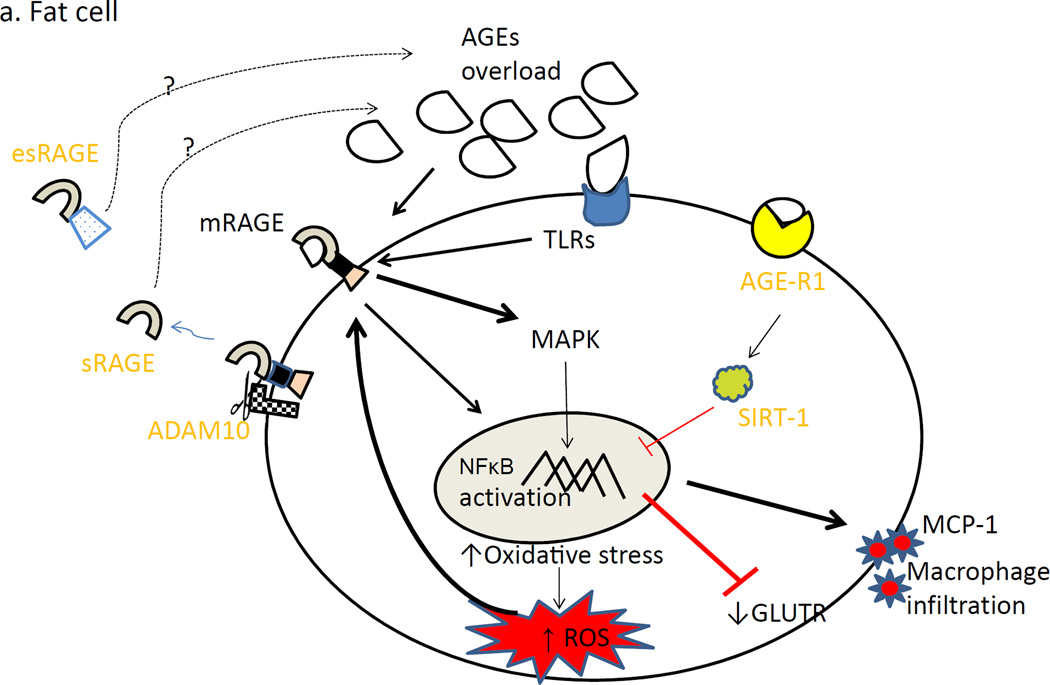
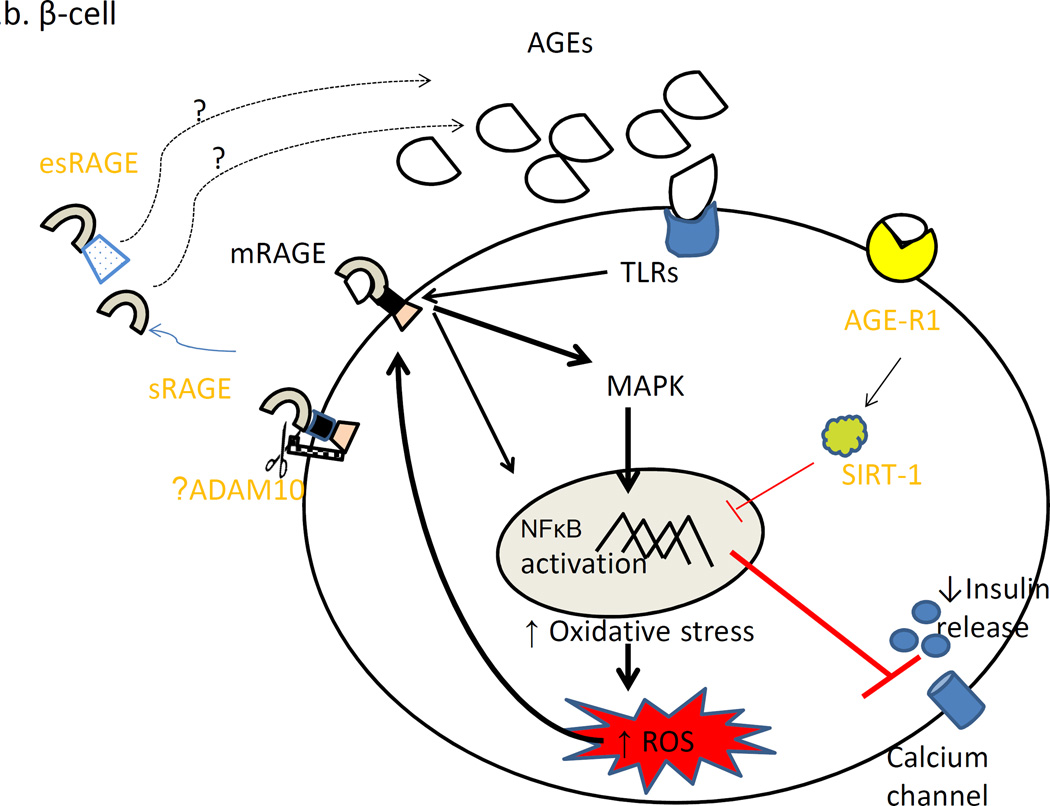
Figure 2 demonstrates hypothesized impact of AGEs ligand excess in fat cell (2a) and β-cell (2a) in the pathogenesis of cardiometabolic risk.
Figure 2.
Chronic AGEs overload leads to increased mRAGE expression leading to oxidative stress. Also, AGE-R1 level is decreased. This is manifested as increased macrophage infiltration and insulin resistance in fat cell (2a) and as decreased insulin secretion in the β-cell (2b).
III. Experimental studies linking dietary AGEs and disease
Studies of various animal models of diabetes, atherosclerosis and kidney disease have demonstrated a negative impact of a high AGE diet and benefits of dietary intervention with a low AGE diet. For example, in both control C57/Bl-6 (25) and spontaneously diabetic db/db mice (26), restriction of AGEs intake decreased serum AGE levels and improved insulin sensitivity. This suggests that dietary AGEs induce and exacerbate IR both under genetic and environmentally acquired conditions that predispose to IR. Furthermore, Lin et al have shown that apolipoprotein-E deficient mice develop atherosclerotic lesions in presence and absence of diabetes when exposed to a high dietary AGEs intake. Interestingly, the lesions decreased significantly by lowering of dietary AGEs (27, 28) suggesting a link of dietary AGEs with atherosclerosis. Glycation of LDL-cholesterol and endothelial dysfunction are some of the proposed mechanisms for AGEs- mediated atherosclerosis.
A low AGEs diet prevented further progression of diabetic nephropathy in both db/db mice and high fat-fed mice models (29) suggesting a pathogenic role of AGEs in renal dysfunction. This was further reinforced by finding that a high AGEs diet administered for a six week period increased proteinuria in 5/6 nephrectomized rats (30).
Interestingly, in a model of autoimmune diabetes, when female NOD mice were maintained on an AGEs restricted diet, the incidence of diabetes dropped from 90% to 30% with reduced insulitis and lower antigenic response (31). It is possible that a low AGEs environment prevented onset of type 1 DM possibly by decreasing T cell stimulus or by inhibiting direct β-cell damage. Furthermore, the impact of dietary restriction of AGEs continued in the next two generations with incidence of diabetes being less than 15% as long as the dams and their offspring were continued on the low AGEs diet. This suggests that avoidance of AGEs during critical developmental periods can prevent their detrimental effects. The preserved transgenerational protective effect of the restricted AGEs intake points to a strong likelihood of reduced transplacental transfer of AGEs with involvement of an epigenetic mechanism. In another study, the F3 offspring of female mice fed an otherwise low AGE diet supplemented with MG, showed an earlier onset of adiposity and IR (32), further reinforcing possibility of epigenetic transmission of the effects of AGEs in offspring. The data from animal studies is summarized in Table 1.
Table 1.
Studies in various animal models assessing impact of lower dietary AGEs intake
| Animal | Animal model | Age at start | intervention | Duration | Observed effects | Ref |
|---|---|---|---|---|---|---|
| Mouse | db/db C57/BL-6J | adult (4 weeks) | LAGE vs HAGE chow 10-fold | 20 weeks | lower AGE levels, improved insulin sensitivity, glucose tolerance in LAGE diet group better preserved islet architecture |
26 |
| Mouse | C57BL6 | Adult (6 weeks) | LAGE-HF vs HAGE HF diet 2.4-fold | 6 months | HAGE-HF fed mice were heavier, hyperinsulinemic and diabetic compared to LAGE-HF or control diet |
25 |
| Mouse | NOD | Adult/neonatal | LAGE vs HAGE 5- fold | 44 weeks | LAGE diet decreased autoimmune diabetes by 3 fold in F0 females and 5 fold in their first and second generation offspring when kept on LAGE diet |
31 |
| Mouse | NOD,db/db mice | Adult | LAGE vs HAGE 6-fold | 4 or 11 months | LAGE diet mitigated DM nephropathy in both models, increased survival in NOD mice. | 29 |
| Mouse | DM Apo E−/− | 6–8 week | LAGE vs HAGE 4-fold | 2 months | Reduced atherosclerotic lesion with no change in lipids | 27 |
| Mouse | Apo E−/− with initimal injury |
12 week | High AGE vs. Low AGE diet 10-fold followed by arterial injury |
5 weeks | decreased neointimal area, intima/media ratio, number of macrophages, lower AGE levels in blood and endothelial cells and macrophages in the neointimal lesions |
28 |
| Mice | C57/BL6J | Adult/ F3 offspring |
Methylglyoxal added to LAGE diet | 18 months | increased adiposity, AGE, leptin and insulin level, insulin resistance earlier in F3 vs F0 decreased AGER-1 and SIRT-1 levels in adipose tissue, skeletal muscle and liver |
32 |
| Rats | Sprague-Dawley | Adult | Aminoguanidine after AGE albumin | single dose | Aminoguanidine increased urinary exceretion of AGEs and decreased AGE deposits in kidney and liver |
12 |
LAGE-Low AGE, HAGE-high AGE, HF-high fat
NOD-Non obese diabetic F0, F1, F3-successive generations
AGE-advanced glycation end product, AGER-1: advanced glycation end product receptor-1, SIRT-1: survival factor sirtuin 1
IV. Human Studies linking dietary AGEs and disease
Most studies assessing dietary AGEs have involved modification of diet or supplementation with anti-AGE agents and have been performed in adults. Recently, a few studies have examined dietary AGEs in children either directly or indirectly by measuring receptors of advanced glycation end products, especially the soluble form. Table 2 presents a summary of observational and interventional studies of AGEs in children and adults.
Table 2.
Human studies assessing impact of advanced glycation endproducts in healthy subjects as well as various diseases
| Study design | Humans | n | Age | Diet type/Duration | Impact on chronic inflammation/disease | Ref. |
|---|---|---|---|---|---|---|
| Observational | ||||||
| Crossectional | Healthy young and elderly | 325 | 19–90 | Dietary AGEs intake and serum AGEs levels correlate with CRP. and mononuclear TNF a, vCAM-1 and isoprostane level |
38 | |
| Crosssectional | Adults with metabolic syndrome Adults without metabolic syndrome |
130 137 |
49–75 52–85 |
Dietary AGEs intake and CML level higher in those with MS compared to those without MS. |
39 | |
| Longitudinal | Healthy subjects | 49 | Change in AGEs intake correlates with change in AGEs level | 38 | ||
| Crossectional | Obese vs lean children | 18 obese 18 lean |
5–10 4–17 |
CML, FL-AGE levels are lower in obese children despite worse CRP, IL-6 and HOMA-IR |
36 | |
| Crossectional | Obese children | 88 (51M) | 11–15 | CML levels correlate inversely with waist z score, IL-6 as well as isoprostane, TNFa and VCAM-1 |
37 | |
| Interventional | ||||||
| Randomized parallel | Healthy CKD-stage 3 |
30 9 |
18–45, >60 | low AGE vs. standard diet. 4 months low AGE vs. standard diet. 4 months |
CML and RAGE expression ↓, AGER-1, ↑ | 38 |
| Nonrandmized one arm | Healthy | 64 | 18–24 | Heat processed high AGE, 1 month | ↓ CML, improved HOMA IR | 46 |
| Crossover | DM | 11 | 32–52 | low AGE vs. high AGEdiet, 2 weeks | ↓serum AGE levels corresponded with ↓ in dietary AGE | 47 |
| Randomized parallel | DM | 13 | ≈62 | low AGE vs. high AGEdiet, 6 weeks | TNFa, CRP ↑ on HAGE and ↓. on LAGE diet | 47 |
| Randomized parallel | Type 2 DM subjects | 20 | 41–71 | Single High and low AGE | Less endothelial Dysfunction and reactive hyperemia impairment after LAGE diet, also lower markers of endothelial dysfunction and OS |
49 |
| Nonrandmized one arm | Type 2 DM | 13 | 56.9(2.8) | Single high AGE meal | Endothelial dysfunction improved after Benfotiamine treatment prior to a high AGE meal. |
50 |
M=Male, F=female, ↑increased, ↓decreased, ↔ no change, CRP=C-reactive protein, sVCAM-1 soluble vascular cell adjesion molecule-1, TNFa-tumor necrosis factor alpha
PAI-1-plasminogen activator inhibitor-1,soluble sVICAM-1 ,intercellular vascular adhesion molecule-1. IL-6: Interleukin-6
AGE - advanced glycation end products, CML=Carboxymethyl Lysine, FL-Fructose Lysine
HOMA-IR: Homoeostasis Model Assessment of Insulin Resistance
DM=Diabetes Mellitus, CKD-Chronic kidney disease, CVD-Cardiovascular disease, OS-oxidative stress
Studies in children/adolescents
A. Dietary AGEs in children
To date, there have been very few studies assessing AGEs in children/adolescents and they have shown mixed results. A study of mother- infant pairs demonstrated strong correlation of maternal levels of serum AGEs (represented by CML and MG) with those of neonates. Further, introduction of processed infant foods increased dietary AGE consumption, which was also reflected in higher serum AGEs levels in the infants. In addition, levels of serum AGEs correlated inversely with levels of adiponectin, an antiinflammatory adipokine in the infants (33). A recent study by another research group confirmed differences in insulin sensitivity based on AGEs levels in breastfed versus formula fed infants although these differences disappeared at follow up at age 12–14 months (34). Boor et al. recently examined the interaction of AGEs in diet and RAGE gene polymorphisms in infants by comparing formula-fed and breast fed infants although dietary AGE was not measured directly (35). Breast-fed infants carrying major allele of -374A/T RAGE gene polymorphism were noted to be most insulin sensitive while insulin sensitivity improved in formula-fed infants carrying the same major allele. Thus, they concluded that -374A/T RAGE gene polymorphism impacted glucose metabolism and insulin sensitivity in a diet-dependent manner.
The above studies suggest a possible relationship of dietary AGEs and insulin sensitivity in infancy. Also, they raise important questions about the possible post-natal programming effects of AGEs when considered in conjunction with the animal studies discussed previously that span multiple generations (31, 32) although a causal effect cannot be yet established.
B. Observational studies of serum AGEs in children
A study comparing obese and lean children showed lower serum AGEs levels in children with obesity than in lean children despite their higher levels of C-reactive protein (CRP) and interleukin-6 (IL-6) and comparable renal function. The authors suggested renal hyper-filtration of AGEs as a compensatory mechanism that might explain the results (36). A similar negative correlation of CML with adiposity and inflammatory markers has been reported in a cross-sectional study of middle school children with obesity (37). These findings are contrary to those in adults (38, 39, 52) and need further investigation for underlying mechanisms. An interesting suggested explanation is trapping of AGEs in adipose tissue macrophages but this needs further confirmation.
Another study of population-based and twin cohorts of children with T1DM showed elevated serum CML levels to be a strong predictor of type 1 diabetes. Genetic model fitting suggested CML levels to be an environmentally acquired risk factor for diabetes (40). These results combined with studies of animal models suggest an important role of AGEs in autoimmune diabetes that needs to be further investigated. Further, children and adolescents with T2DM (41) lose their β-cells at a faster pace compared to adults (42,) for reasons that are not yet clear. An important consequence to consider is the adverse impact of AGEs on glucose metabolism in obese children predisposed to develop T2DM; dietary AGEs can provide a further hit to the already stressed β-cell burdened to produce extra insulin in order to compensate for the obesity-associated insulin resistance. Therefore, it would be important to study the effect of a low AGE dietary intervention in this population as a potential approach to mitigate the epidemic of T2DM that threatens future generations.
C. AGE receptor variants in children with obesity
A common approach in previous studies has been to measure levels of circulating soluble RAGE instead of actual serum AGEs levels and trying to correlate them with severity of different diseases. An important problem here is that the exact role these circulating forms of RAGE play in the AGE-RAGE axis remains elusive. A study of prepubertal children with obesity demonstrated a significant negative correlation of sRAGE and esRAGE levels with birth weight; children born small and large for gestational age had lower sRAGE and esRAGE levels compared to their appropriate for gestational age counterparts (43). Whether these observations are related to genetic factors or point to an epigenetic phenomenon is yet to be determined. In addition, sRAGE and esRAGE had a negative correlation with IR independent of birth weight. Other studies in prepubertal obese children showed significant negative correlation of sRAGE and esRAGE levels with carotid intima -media thickness (44) as well as presence of hepatic steatosis, independent of BMI (45). In the previously mentioned study of middle school children with obesity (37), authors reported a significant negative correlation of sRAGE with adiposity and of both sRAGE and esRAGE with acute insulin response, which is consistent with observations in adults with diabetes.
Interventional studies
Healthy adults habitually consuming “high normal” dietary AGEs were randomized to either continuing their high intake of AGEs or to a low AGEs diet. Those on the low AGEs diet were noted to have significant reductions in circulating AGEs levels after four months of intervention with parallel decrease in markers of OS and inflammation. Of note, there were no changes in energy or nutrient consumption during the study (38). In another trial of 62 healthy adults, utilizing a randomized crossover design with two diets, one with high amount of Maillard reaction products (MRP) and another milder diet incorporating steam-based cooking, BirlouezAragon et al. (46) demonstrated a decrease in insulin sensitivity but increase in triglycerides and cholesterol after one month on the high MRP diet. While CML levels correlated strongly with cholesterol and triglycerides, they did not correlate with insulin sensitivity suggesting possible involvement of other MRP’s.
Studies in patients with DM have also shown significant decrease in CRP, Vascular Cell Adhesion Molecule-1(VCAM-1), and AGEs levels as early as six weeks (47) as well as a decrease in IR after four months of intervention with a low AGEs diet (48). In addition, in patients with type 2 DM, a low AGEs meal was noted to be associated with less impairment of macrovascular and microvascular endothelial function than a similar meal enriched in AGEs by cooking with heat (49). Interestingly, pretreatment with benfotiamine (a liposoluble vitamin B1) prevented the increase in serum levels of AGEs and markers of OS as well as endothelial dysfunction associated with a high AGEs diet in diabetic patients (50).
Interventional studies in children
A recent study in pre-pubertal obese children with hepatic steatosis demonstrated an increase in esRAGE levels in response to daily supplementation with 600 mg of Vitamin E (51). Serum or dietary AGEs levels were not measured in this study. Future studies will be needed to elucidate mechanism underlying improved esRAGE level in response to vitamin E and its significance in the pathogenesis of hepatic steatosis. As mentioned previously, although not interventional in design, studies by Mericq and Boor et al. (33, 35) have suggested effects of RAGE polymorphisms and AGEs in diet on glucose metabolism in infants.
The above studies suggest that circulating AGEs and RAGE variants are perhaps affected by both genetic and environmental factors and may be markers of cardiometabolic disease risk. One interesting possibility that has not been studied so far is that AGEs derived from the diet may contribute to the changes in levels of circulating RAGE variants since AGEs stimulate RAGE directly. These studies further support the hypothesis that AGE-RAGE axis alterations occur in early childhood although many issues remain undefined.
Conclusions
In summary, we believe there are enough data suggesting a role for dietary AGEs in inducing low-grade chronic inflammation, oxidative stress, insulin resistance and vascular dysfunction in adults although similar studies in younger populations are lacking. In view of the rising incidence of obesity and the associated comorbidities of DM and CVD in children and potential role of AGEs in adiposity and β-cell damage, further studies to help define whether dietary AGEs play a similar role in children and adolescents are definitely warranted. Several research gaps remain to be addressed. Given the challenges of recruitment in children and sample size needed, a collaborative multi-center effort by investigators interested in studying this field can provide a useful strategy towards achieving meaningful results with following common goals: 1. To characterize the response of serum AGEs levels to dietary AGEs intake in healthy and obese children; 2. To determine if serum AGEs are associated with measures of inflammation or IR in children with obesity independent of energy intake as they do in adults; 3. To investigate if general dietary counseling practices adequately modify dietary AGEs content and intake in this population as previously shown in adults; 4. To study the impact of changes in other antioxidant nutrients on AGEs levels; and finally 5. To clearly define the relationship between AGEs levels in diet and serum and RAGE variants including sRAGE, esRAGE and RAGE at cellular level as well as with RAGE gene polymorphisms in children, both healthy and those with obesity.
Acknowledgments
Sources of funding: AG was supported by KL2TR000057 as part of Institutional CTSA award to VCU by National Insititutes of Health. The sponsor did not have any role in any part of this manuscript’s preparation.
Non-standard abbreviations
- IR
insulin resistance
- OS
oxidative stress
- CVD
cardiovascular disease
- ROS-, AGEs
Advanced glycation end products
- MRPs
Maillard reaction products
- MG
Methylglyoxal
- CML
Carboxymethyllysine
- RAGE
Receptor of advanced glycation end products
- sRAGE
soluble RAGE
- esRAGE
Endogeneous secreted RAGE
- AGER-1
Advanced glycation end product receptor-1
- IL-6
Interlekin-6
- CRP C
reactive protein
- TNF-α
Tumor Necrosis Factor- alpha
- IRS-1
Insulin Receptor Substrate-1
- MCP-1
Monocyte Chemoattractant Protein-1
- TLR-4
Toll like receptor-4
- GLP-1
Glucagon Like Peptide-1
- VCAM-1
Vascular Cell Adhesion Molecule-1
- NASH
Non-alcoholic Steatohepatitis
- MAPK
Mitogen-activated protein Kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
Footnotes
Statement of authorship: AG researched the literature, drafted and edited the manuscript. JU researched the literature, assisted with editing the manuscript. Both authors read and approved the final manuscript.
Conflict of Interest Statement: AG and JU have no financial conflicts to disclose.
References
- 1.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A Potential Decline in Life Expectancy in the United States in the 21st Century. New Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Berenson GS, Wattigney WA, Tracy RE, Newman WP, 3rd, Srinivasan SR, Webber LS, Dalferes ER, Jr, Strong JP. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (the Bogalusa Heart Study) Am J Cardiol. 1992;70:851–858. doi: 10.1016/0002-9149(92)90726-f. [DOI] [PubMed] [Google Scholar]
- 3.Yan SF, Ramsamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H. AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol. 2008;294:C145–C152. doi: 10.1152/ajpcell.00350.2007. [DOI] [PubMed] [Google Scholar]
- 5.Kalea AZ, Schmidt AM, Hudson BI. RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (Lond) 2009;116:621–637. doi: 10.1042/CS20080494. [DOI] [PubMed] [Google Scholar]
- 6.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 7.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 8.Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi S, Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front Biosci. 2010;E2:1184–1195. doi: 10.2741/e178. [DOI] [PubMed] [Google Scholar]
- 10.Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8:e66611. doi: 10.1371/journal.pone.0066611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Goldberg T, Cai W, Peppa M, Dardaine V, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 12.He C, Sabol J, Mitsuhashi T, Vlassara H. Dietary glycotoxins: inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes. 1999;48:1308–1315. doi: 10.2337/diabetes.48.6.1308. [DOI] [PubMed] [Google Scholar]
- 13.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JC, Li XH, Peng YD, Wang JB, Tang JF, Wang YF. Association of two glyoxalase I gene polymorphisms with nephropathy and retinopathy in Type 2 diabetes. J Endocrinol Invest. 2011;34:e343–e348. doi: 10.3275/7856. [DOI] [PubMed] [Google Scholar]
- 15.Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76:236–244. doi: 10.1016/j.diabres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Luciano Viviani G, Puddu A, Sacchi G, Garuti A, Storace D, Durante A, Monacelli F, Odetti P. Glycated fetal calf serum affects the viability of an insulin-secreting cell line in vitro. Metabolism. 2008;57:163–169. doi: 10.1016/j.metabol.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Lin N, Zhang H, Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012;38:250–257. doi: 10.1016/j.diabet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Puddu A, Storace D, Durante A, Odetti P, Viviani GL. Glucagon-like peptide-1 counteracts the detrimental effects of Advanced Glycation End-Products in the pancreatic beta cell line HIT-T 15. Biochem Biophys Res Commun. 2010;398:462–466. doi: 10.1016/j.bbrc.2010.06.100. [DOI] [PubMed] [Google Scholar]
- 19.Hansen B, Svistounov D, Olsen R, Nagai R, Horiuchi S, Smedsrod B. Advanced glycation end products impair the scavenger function of rat hepatic sinusoidal endothelial cells. Diabetologia. 2002;45:1379–1388. doi: 10.1007/s00125-002-0912-8. [DOI] [PubMed] [Google Scholar]
- 20.Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during trans-differentiation to myofibroblasts. Hepatology. 2001;34:943–952. doi: 10.1053/jhep.2001.28788. [DOI] [PubMed] [Google Scholar]
- 21.Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, Ochi H, Nonaka M, Nabeshima Y, Inoue M, Ishitobi T, Chayama K, Tazuma S. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112–1119. doi: 10.1111/j.1440-1746.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyogo H, Yamagishi H. Advanced Glycation End Products (AGEs) and their Involvement in Liver Disease. Curr Pharm Des. 2008;14:969–972. doi: 10.2174/138161208784139701. [DOI] [PubMed] [Google Scholar]
- 23.Miele C, Riboulet A, Maitan MA, Oriente F, Romano C, Formisano P, Giudicelli J, Beguinot F, Van Obberghen E. Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C alpha-mediated mechanism. J Biol Chem. 2003;278:47376–47387. doi: 10.1074/jbc.M301088200. [DOI] [PubMed] [Google Scholar]
- 24.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8(7):337–346. [PMC free article] [PubMed] [Google Scholar]
- 25.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–2089. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 27.Lin RY, Choudhury RP, Cai WJ, Lu M, Fallon JT, Fisher EA, et al. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168:213–220. doi: 10.1016/s0021-9150(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 28.Lin RY, Reis ED, Dore AT, Lu M, Ghodsi N, Fallon JT, Fisher EA, Vlassara H. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis. 2002;163:303–311. doi: 10.1016/s0021-9150(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 29.Zheng F, He CJ, Cai WJ, Hattori M, Steffes M, Vlassara H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–237. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- 30.Sebekova K, Fais V, Hofmann T, Schinzel R, Heidland A. Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am J Kidney Dis. 2003;41:S48–S51. doi: 10.1053/ajkd.2003.50084. [DOI] [PubMed] [Google Scholar]
- 31.Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H. Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes. 2003;52:1441–1448. doi: 10.2337/diabetes.52.6.1441. [DOI] [PubMed] [Google Scholar]
- 32.Cai WJ, Ramdas M, Zhu Li, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin-1. Proc Natl Acad Sci USA. 2012;109:15888–15893. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mericq V, Piccardo C, Cai W, Chen X, Zhu L, Striker GE, Vlassara H, Uribarri J. Maternally transmitted and food-derived glycotoxins: a factor preconditioning the young to diabetes? Diabetes Care. 2010;33:2232–2237. doi: 10.2337/dc10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klenovics KS, Boor P, Somoza V, Celec P, Fogliano V, Sebekova K. Advanced glycation end products in infant formulas do not contribute to insulin resistance associated with their consumption. PLoS One. 2013;8:e53056. doi: 10.1371/journal.pone.0053056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boor P, Celec P, Klenovicsova K, Vlkova B, Szemes T, Minarik G, Turna J, Sebekova K. Association of biochemical parameters and RAGE gene polymorphisms in healthy infants and their mothers. Clin Chim Acta. 2010;411:1034–1040. doi: 10.1016/j.cca.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 36.Sebekova K, Somoza V, Jarcuskova M, Heidland A, Podracka L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int J Pediatr Obes. 2009;4:112–118. doi: 10.1080/17477160802248039. [DOI] [PubMed] [Google Scholar]
- 37.Accacha S, Rosenfeld W, Jacobson A, Michel L, Schnurr FJ, Shelov S, Ten S, Boucher-Berry C, Carey DE, Speiser PW, Lowell B, Conroy R, Klein M, Fennoy I, Rapaport R, Rosenbaum M. Plasma advanced glycation end products (AGEs), receptors for AGEs and their correlation with inflammatory markers in middle school-age children. Horm Res Paediatr. 2013;80:318–327. doi: 10.1159/000354831. [DOI] [PubMed] [Google Scholar]
- 38.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, Yee K, Tansman L, Chen X, Mani V, Fayad ZA, Vlassara H. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab. 2015;100:1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyan H, Riese H, Hawa MI, Beretta G, Davidson HW, Hutton JC, Burger H, Schlosser M, Snieder H, Boehm BO, Leslie RD. Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes: a twin and population study. Diabetes. 2012;61:1192–1198. doi: 10.2337/db11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 43.de Giorgis T, D'Adamo E, Giannini C, Chiavaroli V, Scarinci A, Verrotti A, Chiarelli F, Mohn A. Could receptors for advanced glycation end products be considered cardiovascular risk markers in obese children? Antioxid Redox Signal. 2012;17:187–191. doi: 10.1089/ars.2012.4525. [DOI] [PubMed] [Google Scholar]
- 44.Chiavaroli V, D'Adamo E, Giannini C, de Giorgis T, De Marco S, Chiarelli F, Mohn A. Serum levels of receptors for advanced glycation end products in normal-weight and obese children born small and large for gestational age. Diabetes Care. 2012;35:1361–1363. doi: 10.2337/dc11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Adamo E, Giannini C, Chiavaroli V, de Giorgis T, Verrotti A, Chiarelli F, Mohn A. What is the significance of soluble and endogenous secretory receptor for advanced glycation end products in liver steatosis in obese prepubertal children? Antioxid Redox Signal. 2011;14:1167–1172. doi: 10.1089/ars.2010.3719. [DOI] [PubMed] [Google Scholar]
- 46.Birlouez-Aragon I, Saavedra G, Tessier FJ, Galinier A, Ait-Ameur L, Lacoste F, Niamba CN, Alt N, Somoza V, Lecerf JM. A diet based on high heat treated foods promotes risk factors for DM and CVD. Am J Clin Nutr. 2010;91:1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 47.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 50.Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 51.D'Adamo E, Marcovecchio ML, Giannini C, de Giorgis T, Chiavaroli V, Chiarelli F, Mohn A. Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic Res. 2013;47:146–153. doi: 10.3109/10715762.2012.755262. [DOI] [PubMed] [Google Scholar]