Abstract
Background
African American (AA) smokers experience greater tobacco-related disease burden than Whites, despite smoking fewer cigarettes per day (CPD). Understanding factors that influence daily nicotine intake in AA smokers is an important step towards decreasing tobacco-related health disparities. One factor of interest is smoking topography, or the study of puffing behavior. Aims: 1) To create a model using puff characteristics, nicotine dependence, and nicotine metabolism to predict daily nicotine exposure, and 2) to compare puff characteristics and nicotine intake from two cigarettes smoked at different times to ensure the reliability of the puff characteristics included in our model.
Methods
60 AA smokers smoked their preferred brand of cigarette at two time points through a topography device. Plasma nicotine, expired CO, and changes in subjective measures were measured before and after each cigarette. Total nicotine equivalents (TNE) was measured from 24-hour urine collected during ad lib smoking.
Results
In a model predicting daily nicotine exposure, total puff volume, CPD, sex, and menthol status were significant predictors, (R2= .44, p < .001). Total puff volume was significantly greater and inter-puff intervals were significantly shorter after ad lib smoking compared to the first cigarette of the day, but puffing behaviors for both cigarettes were highly correlated (r range= .69–.89, p <.001) within-subjects.
Conclusion
This is the first study, to our knowledge, to show that puff characteristics of individual cigarettes are predictive of daily nicotine intake.
Impact
These findings enhance our understanding of the relationship between smoking behavior and nicotine intake in AA smokers.
Keywords: puff topography, nicotine exposure, African Americans, smoking behavior
INTRODUCTION
Racial differences in smoking behavior are associated with disparities in tobacco-related diseases. Although African Americans (AAs) on average smoke fewer cigarettes per day (CPD),(1–2) they exhibit greater nicotine dependence,(3–5) and have higher risk of developing lung cancer(6) and severe, early onset COPD(7) compared to Whites. The inconsistency across race between cigarette consumption and disease burden highlights the importance of examining other elements of smoking behavior, in addition to CPD, to improve our understanding of smoke/toxicant exposure and associated disease risk. Previous studies from our laboratory reported that AAs smoke cigarettes differently than Whites.(8–9) Specifically, they take in more nicotine per cigarette,8 especially at lower levels of CPD.9 This makes CPD alone a poor indicator of smoke exposure in this population. Given that a person’s intake of tobacco smoke is a product of the number of cigarettes smoked per day and the amount of smoke taken in per cigarette, measuring the latter is a valuable metric in understanding exposure.
The measure of how a person smokes a cigarette has been termed smoking topography. Smoking topography consists of a range of parameters characterizing how one puffs a cigarette. Utilizing computerized smoking topography measurement devices, one can accurately quantify how an individual cigarette is smoked, including the number of puffs, puff duration, puff volume, puff velocity, and inter-puff interval. Total puff volume can be calculated by summing individual puff volumes per cigarette. Together these variables characterize inhalation-based differences between smokers. Although these puffing characteristics can be quite variable between smokers, smoking topography is a useful tool in predicting smoke and nicotine exposure.(10–11) One aim of this research was to determine how smoking topography relates to daily nicotine exposure in AAs, since daily nicotine exposure is a strong predictor of overall tobacco smoke and carcinogen exposure.(8)
While many studies have looked at the relationship between smoking topography characteristics and post-smoking nicotine levels in blood(11–12), the gold standard biomarker of daily nicotine exposure is to measure the sum of nicotine and its metabolites in urine, or total nicotine equivalents (TNE).(13–15) By assessing daily nicotine exposure using TNE from a 24-hour urine collection, Scherer and colleagues showed that machine-derived smoke yields were only weakly associated with overall exposure.(13) Adding measures of smoking topography to machine-derived smoke yields improves the ability to predict post-smoking nicotine blood levels(10) and will likely do the same for predicting daily nicotine exposure using TNE, although to our knowledge this has not been done previously.
Our first objective was to create a model that best predicts daily nicotine exposure, as measured by TNE, to potentially enhance our ability to predict tobacco-related disease risk. There are many relevant factors that may influence one’s daily nicotine exposure. For example, the level of nicotine dependence has been shown to influence TNE in low dependent white smokers, but not in high dependent white smokers or black smokers at either dependence level.(16) In addition to nicotine dependence, differences in nicotine metabolism may influence daily nicotine exposure. Benowitz and colleagues have demonstrated great variability in the rate at which people metabolize nicotine.(17–18) To develop a more complete model of daily nicotine exposure, we included predictors from smoking behavior data (CPD and topography) along with metabolic factors and measures of nicotine dependence.
A second objective was to determine if using topography measurements taken at different times affected the validity of our model since few studies have evaluated if smoking topography changes throughout the day. This was important to assess the reliability of a single cigarette topography measure as representative of smoking throughout the day for the model. Although smoking topography variables are highly reliable within subjects when measured at the same time of day, time of day differences may be particularly important when comparing the first cigarette of the day to a subsequent cigarette smoked during ad lib smoking. Grainge et al. assessed temporal differences in puffing characteristics using a portable computerized topography device.(19) Participants smoked most of their cigarettes through the device for a 24-hour period. Cigarettes smoked early in the morning (between 6:00am-10:00am) were smoked less intensively (i.e. lower average puff volumes) than those smoked later in the day. This was most apparent for the first cigarette of the morning, especially when it is smoked within five minutes of waking. Unfortunately, this study did not report the racial breakdown of their participants so it is unclear if these findings are representative of AA smokers.
To address our second objective we evaluated time of day differences in smoking topography in AA smokers, comparing the puff characteristics of a cigarette smoked after waking (Cig 1), to one smoked after four hours of ad lib consumption (Cig 2). In addition, we sought to determine if differences in puffing behavior at each time point were associated with nicotine or CO boost, as well as various characteristics associated with smoking reinforcement such as changes in urge, affect, withdrawal, and smoking satisfaction.
MATERIALS & METHODS
Overview of study design
Sixty African American smokers were recruited through online (Craigslist) and newspaper advertisements to participate in an 11 day smoking study on nicotine metabolism. Prior to enrollment subjects were asked to complete a screening visit in which a brief health evaluation was conducted and participants provided a blood sample and expired CO sample to confirm smoking status. After passing screening, participants completed a baseline orientation visit, a seven day outpatient assessment of daily smoking patterns (not presented here), and three consecutive inpatient sessions evaluating nicotine pharmacokinetics, smoking topography, and daily nicotine exposure. The participants’ preferred brand of cigarettes was provided to them free of charge throughout both the outpatient and inpatient portions of the study. This study was approved by University of California San Francisco Institutional Review Board. All subjects provided written consent prior to participation.
Subject eligibility
Participants were eligible to participate if they were between 18 and 65 years old, medically healthy (by history and physical exam), smoked at least 5 cigarettes per day for the past year (confirmed by either plasma cotinine > 25 ng/ml or expired CO > 10 ppm), and did not use any other nicotine-containing products. Exclusion criteria were a body mass index (BMI) greater than 38 (to gather data most representative of the general population and to avoid possible nicotine toxicity during the nicotine infusion inpatient day, which is administered based on weight), a history of schizophrenia, major depression or psychosis, and current use of illicit substances other than non-daily marijuana use. If subjects reported marijuana use they had to agree to abstain for the duration of the study. Participants were compensated for their time.
Measures
Demographic and smoking history questionnaire
Questionnaires assessed sex, age, race/ethnicity, education, etc. Smoking history questions included age of initiation, number and duration of quit attempts, and machine-determined yield characteristics of usual cigarettes.
Fagerström Test of Cigarette Dependence (FTCD)
(20–21) The FTCD is a six-item validated measure of nicotine dependence. Scores from each item are summed for a total score between 0–10. One item from the FTCD asks about one’s time to first cigarette (TFC). TFC is thought to indicate one’s physical dependence on nicotine, and is predictive of quitting success.(22) This item was asked in an open ended format and then coded for inclusion the total FTCD score.
Minnesota Nicotine Withdrawal Scale (MNWS)
(23) Participants rated eight symptoms of tobacco withdrawal (e.g., depressed mood) in terms of how they are feeling “right now” on scale from 0, “None” to 4, “Severe”. This scale has been shown to have excellent internal consistency and is sensitive to smoking abstinence.(24)
Questionnaire for Smoking Urges- Brief (QSU-B)
(25) This psychometrically valid 10-item measure assesses two factors of smoking urges: pleasurable desire to smoke; anticipation of withdrawal. (26) These items can also be summed to form a total score measure of urge to smoke.
Circumplex Scale of Affective States
(27) This 10-item questionnaire asked participants to rate how they feel “right now” for each affect item on a scale ranging from 1, “Not at all,” to 10, “Very.” Items are divided into positive (i.e. active, peppy, happy) and negative (i.e. bored, sad/depressed) subscales.
Cigarette Satisfaction Item
Participants rated “How satisfying do you find one of these cigarettes” on a scale from 1 “Not at all,” to 5 “Completely satisfying,” after smoking through the topography device.
CReSS Smoking Topography Device (Plowshare Technologies, Baltimore, M.D.)
A cigarette is placed into a plastic mouthpiece that attaches to tubing connected to a pressure transducer where inhalation based changes in pressure are amplified, digitized, and sampled at a rate of 1000 Hz. Measures of number of puffs, puff volume, puff duration, average puff flow, peak puff flow, inter-puff-interval, and smoking duration were collected. Data supporting the validity and reliability of smoking topography assessments have been published.(11, 28–29)
Study Protocol
An outpatient baseline assessment was conducted approximately one week prior to the inpatient sessions. This session included assessments of demographic data and nicotine dependence.
After overnight smoking abstinence (from 10:00 pm the night before), participants arrived at 7:00 am for admission to the inpatient clinical research ward at San Francisco General Hospital where there is a designated smoking room (containing an exhaust and negative pressure system). Abstinence was confirmed with expired CO reading (average < 10 ppm). On the first day nicotine pharmacokinetics were assessed using an infusion of deuterium-labeled nicotine-d2 (3′,3′-dideuteronicotine) and cotinine-d4 (2,4,5,6-tetradeuterocotinine) for 30 minutes as described previously.(9) Blood samples were collected for measurement of nicotine and cotinine levels at 0, 10, 20, 30, 45, 60, 90, 120, 240, 360, and 480 minutes, and then 24, 48, 72, and 96 hours after the infusion to include at least 3 half-lives for cotinine.(9) The analysis of the pharmacokinetic data is being presented elsewhere, but for our model total nicotine clearance and nicotine half-life are presented as measures of the rate of nicotine metabolism. There was also a practice topography session complete with the first cigarette smoked after hospital admission (about 2 hours after the nicotine infusion). Participants were allowed a last cigarette at 11:00 pm, and then their pack was taken from the room by a nurse.
Smoking topography was assessed on the second inpatient day. Participants were awoken at 7:00 am and an indwelling venous catheter was inserted into the forearm. At 8:00 am, participants smoked their preferred brand of cigarette (Cig 1) through the CReSS topography device (Plowshare, Baltimore M.D.). A light breakfast was provided at 8:30 am. Participants completed pre- and post-smoking measures of urge, affect, and withdrawal, and assessed smoking satisfaction after smoking. Plasma nicotine concentrations were measured from samples collected before, and two minutes after smoking each cigarette. Similarly, expired CO breath samples were also taken pre- and post-smoking. These procedures were repeated for a cigarette smoked through the topography device four hours later (Cig 2). During the four hour break participants were allowed to smoke ad libitum without the device.
On the third day participants were allowed to smoke ad libitum. All urine was collected from 8:00am-8:00am to calculate TNE, described below.
Analytical Chemistry
From the first inpatient day, nicotine half-life and nicotine clearance were estimated from plasma nicotine concentrations using a non-compartmental model in Phoenix WinNonlin 6.3 (Pharsight Corporation, Mountain View, CA) as described previously.(18)
Plasma concentrations of pre and post-smoking nicotine levels collected on the second inpatient day were measured by gas chromatography/mass spectrometry (GC-MS) as described previously.(30)
From the third inpatient day urine TNE, (total nicotine equivalents) was calculated. All of the urine excreted on the 2nd-3rd hospital day (8AM to 8AM) was collected and the total volume recorded. After thoroughly mixing the sample, an adequate volume was retained and analyzed urine total (free plus conjugated) concentrations of nicotine (Lower Limit of Quantification, LLOQ, 10 ng/mL), cotinine (LLOQ, 10 ng/mL), trans-3′-hydroxycotinine (LLOQ, 10 ng/mL), and free nicotine N-oxide (LLOQ, 5 ng/mL), and cotinine N-oxide (LLOQ, 5 ng/mL) were measured (by LC-MS/MS).(31) Each concentration was converted to moles and multiplied by the total volume collected over 24 hour to give the amount of each species excreted in 24 hours. These mole amounts were summed to give the TNE excreted in 24 hours.
Data Analysis
For the first aim, a generalized linear regression model was used to estimate daily nicotine exposure using nicotine dependence (i.e. time to first cigarette), the rate of nicotine metabolism, and smoking topography variables. To screen which variables should be included in the final model, Pearson’s correlation coefficients were calculated between the outcome variable, TNE, and all puffing characteristics, measures of nicotine dependence, and measures of the rate of nicotine metabolism.(32) Variables with an association at the p < .1 value were eligible for inclusion. In instances where two variables with high colinearity (r > .5) were eligible, one was selected for inclusion. This was the case with total puff volume, average puff volume, and average puff flow. Total puff volume was selected because it had the highest correlation with TNE of all three variables. Colinearity was also an issue with the measures of nicotine metabolism- nicotine half-life and nicotine clearance. Nicotine half-life was more closely related to TNE than clearance so it was selected. The predictors for the final model were total puff volume, time to first cigarette, cigarettes per day, and nicotine half life. Including total puff volume from either Cig 1 or Cig 2 did not change the results of the analyses, therefore total puff volume from Cig 2 was selected as a more representative parameter of daily smoking behavior. Age, menthol status, and sex were included in the model as covariates.
For the second aim, a generalized linear regression model was used to estimate the effect of time of day on each puffing characteristic using Proc Genmod in SAS v9.4. To account for the non-independence of the data resulting from the repeated measures, generalized estimating equations were used to estimate the standard errors. Tests of the individual regression coefficients were conducted using standard Z-tests. Based on the findings from Grainge et al. presented above, time to first cigarette was included in the model as a covariate.(19) Including CPD, or menthol status had no effect on the outcome.
Lastly, we evaluated the reliability of the puffing characteristics between Cig 1 and Cig 2 using Intraclass Correlation Coefficients (ICCs). All data are presented as means (M) and standard deviations of the mean (SD).
RESULTS
Sample Characteristics
Of the 60 African American smokers recruited into the study at baseline, 59 (30 male, 29 female) participated in the inpatient sessions. The participants were on average 34.9 years old (SD= 10.1), smoked an average of 14 cigarettes per day (SD= 6.4) for an average of 16.4 years (SD= 10.2) and had an average plasma cotinine of 206 ng/ml (SD = 103) at screening. They exhibited moderate nicotine dependence (Mean FTCD= 5.1, SD= 2.0). Eighty-three percent smoked menthol cigarettes. Table 1 presents complete baseline demographic, smoking history, metabolism, and daily exposure information.
Table 1.
Demographic, smoking history, dependence, metabolism, and daily nicotine exposure measures.
| Variable | Males (N=30) M (SD) |
Females (N=29) M (SD) |
Overall (N=59) M (SD) |
p-value |
|---|---|---|---|---|
| Age | 35.5 (11.1) | 34.4 (9.2) | 34.9 (10.1) | 0.68 |
| Years of education | 13.1 (1.9) | 12.8 (1.1) | 13.0 (1.5) | 0.51 |
| Cigarettes per day | 14.6 (6.9) | 13.4 (5.9) | 14.0 (6.4) | 0.47 |
| Years smoking | 16.0 (11.2) | 16.8 (9.4) | 16.4 (10.2) | 0.75 |
| FTCD | 4.5 (2.0) | 5.7 (1.8) | 5.1 (2.0) | 0.02* |
| Time to first cigarette (min) | 19.8 (17.3) | 15.6 (16.4) | 18.7 (20.7) | 0.34 |
| % Menthol | 73% | 93% | 83% | |
| Nic. yield of usual† (mg) | 1.2 (.15) | 1.1 (.11) | 1.1 (.13) | 0.10 |
| Tar yield of usual brand† (mg) | 16.0 (1.9) | 15.7 (1.2) | 15.9 (1.6) | 0.43 |
| Baseline plasma cotinine (ng/ml) | 215.7 (100.7) | 196.7 (106.4) | 206.2 (103.1) | 0.48 |
| Nicotine half-life (min) | 133.2 (41.0) | 135.3 (32.9) | 134.2 (36.8) | 0.83 |
| Nicotine clearance (ml/min) | 1441.4 (494.3) | 1194.4 (318.5) | 1317.8 (430.6) | 0.03* |
| Total Nicotine Equivalents (µMol) | 62.5 (30.5) | 45.7 (29.0) | 53.8 (30.7) | 0.04* |
Note. FTCD= Fagerstrom Test for Cigarette Dependence.
Nicotine yield and tar yield are based on U.S. Federal Trade Commission method machine testing. Sex differences compared using an independent samples t-test.
Single asterisks (*) indicates statistical significance at the p <.05 level.
Predicting Daily Nicotine Exposure
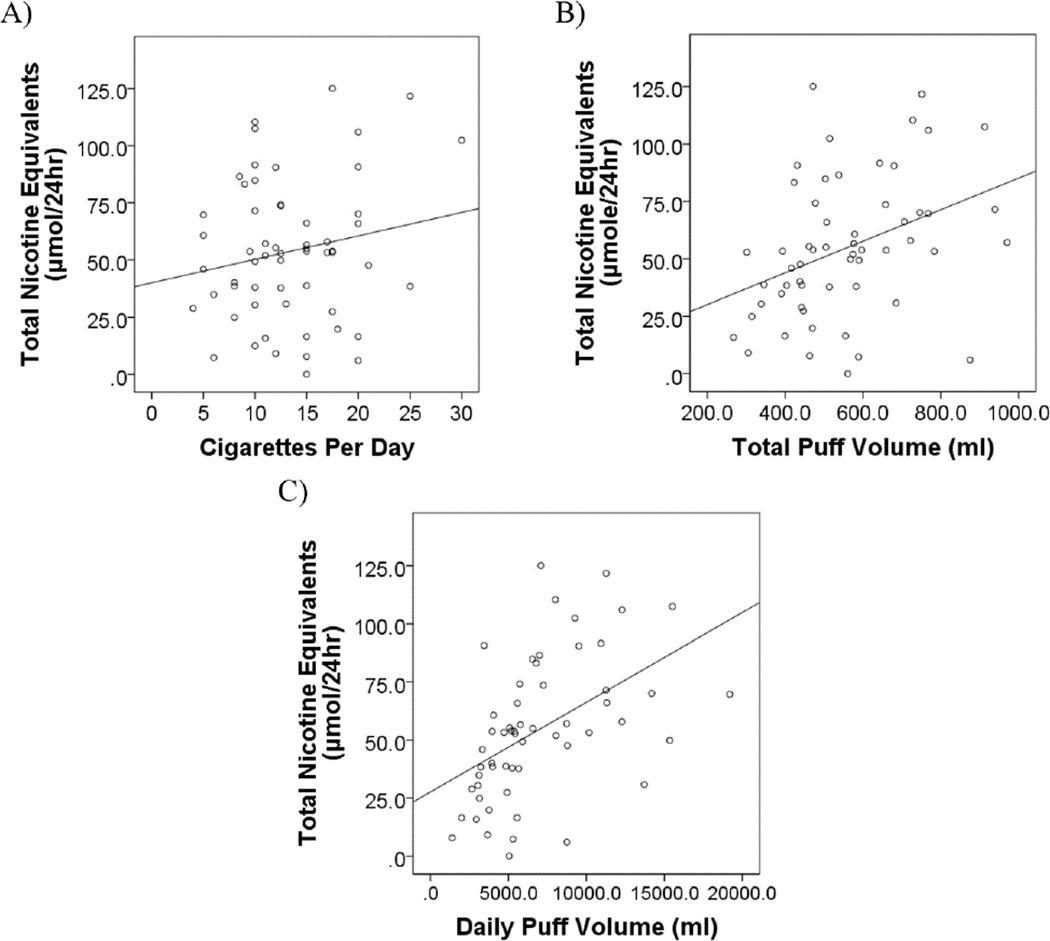
In the model evaluating predictors of TNE, a measure of daily nicotine exposure, CPD, total puff volume, sex, and menthol status were all significant (see Table 2). Smoking more cigarettes per day with greater total puff volume, being male, and smoking menthol cigarettes all predicted greater nicotine exposure. Running the model with daily puff volume (a composite measure of CPD x total puff volume) produced similar results, such that daily puff volume, sex, and menthol status were all significant predictors, p’s < .03. Figure 1 depicts the relationship between TNE and CPD, total puff volume, and daily puff volume. Overall both models explained the same proportion of the variance in TNE, R2= .44, F(7,56) = 4.39, p =.001.
Table 2.
Linear regression model predicting urine total nicotine equivalents.
| Parameter | B | Std. Error (B) | β | T | p-value |
|---|---|---|---|---|---|
| Total puff volume | 0.05 | 0.02 | 0.25 | 2.16 | .036* |
| Time to first cigarette | −0.30 | 0.22 | −0.17 | −1.36 | .181 |
| Cigarettes per day | 1.75 | 0.82 | 0.27 | 2.14 | .038* |
| Nicotine half-life | −0.14 | 0.09 | −0.17 | −1.55 | .127 |
| Age | 0.61 | 0.36 | 0.20 | 1.68 | .100 |
| Male Sex | 17.31 | 6.90 | 0.29 | 2.51 | .015* |
| Menthol status | 19.87 | 9.80 | 0.25 | 2.03 | .048* |
Note.
Single asterisks (*) indicates a significant parameter at the p < .05 level.
Figure 1.
The relationship between 24 hr urine Total Nicotine Equivalents and A) Cigarettes per day (r = .44, p = .001; Line of best fit: y=16.3 +0.07x), B) Total puff volume (r = .38, p = .004; Line of best fit: y= 40.0 + 1.03x), and C) Daily puff volume (r = .49, p < .001, Line of best fit: y= 27.6 + .004x). Daily puff volume is the product of cigarettes per day and total puff volume.
Smoking Topography Differences by Time of Day
Time of day was a significant predictor of total puff volume, β = −27.9, z = −2.14, p = .032, and inter-puff interval, β = 2144.6, z = 2.27, p = .023, in the final model. Total puff volume was smaller, and inter-puff intervals were larger for the first cigarette of the day compared to the one smoked after four hours of ad lib consumption. There was also a trend toward time of day predicting average puff flow, β = −1.38, z = −1.83, p = .068, in the final model. Time of day did not significantly predict any of the other puffing characteristics or exposure variables (i.e., nicotine or CO boost). Average values and standard deviations for all topography parameters are presented in Table 3.
Table 3.
Smoking topography and exposure for the first cigarette of the day (Cig 1) and one smoked after 4 hours of ad lib smoking (Cig 2).
| Variable | Male M (SD) |
Cig 1 Female M (SD) |
Overall M (SD) |
Male M (SD) |
Cig 2 Female M (SD) |
Overall M (SD) |
Overall z-statistic (p-value) |
|---|---|---|---|---|---|---|---|
| Number of puffs |
11.4 (2.8) |
13.4 (4.6) |
12.4†(3.9) | 12.2 (3.2) |
13.7 (4.7) |
12.9 (4.0) |
−1.7 (.09) |
| Total puff volume (ml) |
559.5 (200.9) |
512.5 (148.1) |
536.4 (177.0) |
582.2 (171.8) |
526.7 (155.3) |
554.5 (164.7) |
−2.1 (.03)* |
| Avg. puff volume (ml) |
50.9 (17.7) |
40.7 (13.7) |
45.9† (16.6) |
52.2 (17.6) |
40.9 12.0) |
46.6† (16.0) |
−0.7 (.48) |
| Avg. puff duration (sec) |
1.7 (0.5) |
1.4 (0.4) |
1.5† (0.1) |
1.7 (0.5) |
1.3 (0.4) |
1.5† (0.5) |
0.2 (.84) |
| Avg. puff flow (ml/sec) |
31.4 (10.5) |
31.1 (5.8) |
31.3 (8.4) |
31.9 (11.0) |
32.2 (5.9) |
32.1 (8.8) |
−1.8 (.07) |
| Avg. IPI (sec) |
21.2 (7.3) |
21.6 (11.3) |
21.4 (9.4) |
18.7 (8.4) |
20.0 (8.4) |
19.4 (8.4) |
2.3 (.02)* |
| Nicotine boost (ng/ml) |
16.9 (11.0) |
14.4 (8.3) |
15.7 (9.8) |
15.1 (8.8) |
12.9 (6.8) |
14.1 (7.9) |
1.1 (.24) |
| CO boost (ppm) |
8.0 (4.0) |
8.2 (5.3) |
8.1 (4.7) |
9.8 (4.7) |
8.5 (6.3) |
9.2 (5.5) |
−1.6 (.09) |
Note. Avg.= Average. IPI= Interpuff Interval. CO= expired Carbon Monoxide.
Single cross (†) denotes significant sex differences at the p < .05 level.
Single asterisks (*) denotes significant differences between Cig 1 and Cig 2 at the p < .05 level.
Subjective Reactions to Smoking by Time of Day
There was a significant effect of time of day on craving reduction after smoking, such that craving decreased more after the first cigarette of the day compared to the one smoked after the four hour ad lib period, as noted in Table 4. However, there was a trend toward greater smoking satisfaction after the afternoon cigarette compared to the first cigarette of the day. There was no main effect on affect ratings or nicotine withdrawal symptoms.
Table 4.
Subjective reactions to smoking after Cig 1 and Cig 2.
| Variable |
Cig 1 Mean (SD) |
Cig 2 Mean (SD) |
z-statistic (p-value) |
|---|---|---|---|
| Smoking Satisfaction | 2.9 (1.4) | 3.2 (1.2) | −1.9 (0.06) |
| Change in Craving | −1.8 (1.6) | −1.1 (1.5) | −3.2 (0.002)** |
| Change in Positive Affect | −0.7 (8.7) | −0.7 (6.7) | 0.01 (0.99) |
| Change in Negative Affect | −1.2 (5.9) | −0.1 (3.8) | −1.1 (0.25) |
| Change in Withdrawal | −2.6 (4.7) | −1.5 (3.7) | −1.6 (0.11) |
Note. Change scores were calculated by taking the post-smoking and subtracting the pre-smoking rating. Negative vales indicate a reduction in that item after smoking.
Double asterisks (**) denotes significance at the p < .01 level.
Reliability of Smoking Topography by Time of Day
The high ICCs, presented in Table 5, indicate excellent (ICC > .75) test-retest reliability for puffing variables, and fair-to-good range reliability (.4< ICC <.75) for CO and nicotine boost.
Table 5.
Test-retest reliability for puff variables, nicotine, and smoking exposure.
| Variable | ICC | 95% CI |
|---|---|---|
| Total Puff Volume | .84 | [.75, .90] |
| Avg. Puff Volume | .89 | [.82, .93] |
| Avg. Puff Velocity | .79 | [.67, .87] |
| Avg. Puff Duration | .89 | [.82, .93] |
| Avg. IPI | .76 | [.59, .83] |
| CO Boost | .51 | [.29, .68] |
| Nicotine Boost | .55 | [.34, .70] |
Note. ICC= Intraclass Correlation Coefficient. CI= Confidence Interval. IPI= Interpuff Interval. CO= expired Carbon Monoxide.
DISCUSSION
We present a novel model indicating that smoking more cigarettes per day, having greater total puff volume during ad lib smoking, smoking menthol cigarettes, and being male were all significant predictors of greater daily nicotine exposure, as measured by TNE, in African American smokers. Consistent with research showing that actual smoking behavior correlated better with post smoking nicotine blood level than machine indicated yield(33), we found that one’s total puff volume per cigarette is an important predicator of daily nicotine exposure. Furthermore, the finding that males have greater nicotine exposure is supported by previous research indicating that males take in larger puff volumes.(34)
The finding that menthol smokers yielded greater TNE is supported by some previous research suggesting menthol smokers take in greater puff volumes,(35) but contradicts research from the Wang et al. from Altria Client Services who found no significant differences in daily nicotine exposures in African American menthol and non menthol smokers.(36) It should be noted that 83% of the current sample were menthol smokers. In addition, previous research from our lab, in a sample of non-Hispanic White and AA smokers, indicated that menthol smokers had less urine TNE than non-menthol smokers.(37) However, there is significant disparity in the literature on the effect of mentholated cigarettes on puff topography and nicotine exposure (for a review see Laurence at al., 2011).(38) This is both due to differences in methodology (i.e., comparing established menthol and non-menthol smokers or evaluating switching from menthol to non-menthol brands within subjects) and sample characteristics. For example, Strasser et al. found no differences in puff topography or cigarettes smoked per day when menthol smokers were switched from a laboratory menthol cigarette to a laboratory non-menthol cigarette.(39) More research is needed on possible moderating effect of menthol status on puff topography and subsequent nicotine exposure.
Other predictors of interest were not associated with daily nicotine exposure in our sample, including measures of nicotine dependence (FTCD or TFC). This finding is consistent with previous research that found no relationship between nicotine dependence measure and TNE in AA smokers, despite evidence of a positive relationship in low-dependent whites.(16) Lastly, we found no relationship between TNE and measures of the rate of nicotine metabolism (specifically nicotine half-life or clearance). This is somewhat surprising as we would expect slower metabolizers to take in less nicotine and have less urine TNE than fast metabolizers. However this effect has been reported primarily in whites and Asians, and may not be occurring in AAs.(40–41)
Although AAs tend to smoke fewer CPD than non-Hispanic Whites(2), they experience greater nicotine dependence and greater difficulty quitting. (3–5) This indicates that AAs smoke cigarettes differently from their White counterparts. Research has shown AAs take in 30% more nicotine per cigarette than whites.(9) Therefore, predictors of smoke exposure and disease risk are likely to differ by race.(8) In this study we found that total puff volume per cigarette was a significant predictor of nicotine exposure in AAs, and may provide a more precise measure of smoking behavior, and greater insight for disease risk when combined with cigarettes per day.
Furthermore, we present novel data indicating that AA smokers smoke the first cigarette of the day less intensely than cigarettes smoked during ad libitum smoking. Specifically AA smokers take in lower total puff volumes per cigarette and wait longer between each puff, which is similar to research assessing time of day differences in puff topography in a group of smokers from the United Kingdom.(19) Although the first cigarette of the day was smoked less intensely, it produced a greater reduction in craving compared to the mid-day cigarette. Taken together, these characteristics suggest that despite abstinence-induced craving and withdrawal; smokers regulate their nicotine intake in the morning by decreasing the overall amount of smoke inhaled. This is somewhat unexpected since elevated craving and withdrawal symptoms were reported prior to the first cigarette of the morning. However, smokers may be experiencing an increase in nicotine sensitivity after overnight abstinence, getting a greater pharmacologic effect from a lower dose of nicotine, and subsequently smoke less intensely.
It should be noted that although all participants were allowed a final cigarette at 11:00 pm the night prior to the topography day, we did not collect the exact time of participant’s final cigarette. Therefore although everyone had nine hours of abstinences, these results may lack ecological validity since some participants may have had a longer abstinence period, or may have woken up earlier than expected and had to wait for their first cigarette at 8am.
While it is informative to know that African Americans smoke the first cigarette of the day by taking in less smoke volume and waiting longer between puffs, overall smoking topography variables from Cig 1 and Cig 2 indicated excellent (ICC > .75) reliability within individuals. This finding is consistent with previous research evaluating within subject reliability of puff characteristics over the course of four days.(28) Thus, although the first cigarette of the day was smoked less intensely and provided greater craving relief, its topography characteristics were highly correlated with those of the mid-day cigarette indicating that either cigarette can be used to represent an individual’s overall puffing pattern.
Strengths of our study include characterization of nicotine pharmacokinetic parameters and the measurement of TNE in a 24 hour urine collection, the gold standard biomarker of daily nicotine exposure. A potential limitation of our research design is that to assess TNE participants were confined to an inpatient research ward, which may have altered their normal smoking behavior and subsequently reduce the ecological validity of the findings. It should also be noted that the extent to which puff topography and lung inhalation correlate is unknown. People inhale varying amounts of what they take into their mouth (i.e. puff), and this most likely explains some of the variation in the relationship between total puff volume and TNE. Lastly, this research was conducted with an all AA sample. Since smoking behavior differs in AA from other smokers, similar studies need to be done in other racial/ethnic groups, as well as in AA smokers from other geographical areas.
Understanding nicotine exposure, a surrogate for tobacco smoke exposure, in AA smokers is an important area of research since this population bears a disproportionate amount of the disease risk associated with smoking. We found that in addition to knowing the sex of a participant, gathering information on one’s level of cigarette consumption (cigarettes per day), smoking topography (total puff volume), and menthol status contributed significant information in understanding nicotine exposure. Together these predictors account for 44% of the variance in daily nicotine exposure. Since daily nicotine exposure is an important predictor of disease risk, this data represents an important step toward understanding the relationship between smoking behavior and the tobacco related health disparities experienced by AA smokers.
Acknowledgments
We would like to thank Sandra Tinetti for research coordination, Olivia Yturralde, Trisha Mao and Lita Ramos for analytical chemistry, and Faith Allen for data management. We appreciate the assistance of the nurses on the Clinical Research Center-Clinical and Translational Science Institute research ward at San Francisco General Hospital.
Financial Support: The research was supported from US Public Health Service grants DA02277 (N.L. Benowitz, D.A. Dempsey, K.Delucchi), DA 020830 (N.L. Benowitz) and DA 12393 (N.L. Benowitz) from the National Institute on Drug Abuse. K.C. Ross was supported by the National Cancer Institute grant CA-113710. G. St.Helen was supported by the University of California’s Tobacco-Related Disease Research Program Postdoctoral fellowship #22FT-0067. Clinical studies were performed at the General Clinical Research Center at San Francisco General Hospital Medical Center with support of grant UL1 RO24131 from the National Institutes of Health/National Center for Research Resources (N.L. Benowitz).
Footnotes
Conflicts of Interest: Dr. Benowitz has consulted for pharmaceutical companies Pfizer and GlaxoSmithKline that market smoking cessation products and has been an expert witness in litigation against tobacco companies. The other authors have no conflicts.
REFERENCES
- 1.US Department of Health and Human Services. National Center for Chronic Disease Prevention and Health Promotion, editor. Washington, DC: Centers for Disease Prevention and Control; 1998. Tobacco Use Among U.S. Racial/Ethnic Minority Groups: A Report of the Surgeon General. [Google Scholar]
- 2.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 3.Collins CC, Moolchan ET. Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addict Behav. 2006;31:1460–1464. doi: 10.1016/j.addbeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Luo Z, Alvarado GF, Hatsukami DK, Johnson EO, Bierut LJ, Breslau N. Race differences in nicotine dependence in the Collaborative Genetic study of Nicotine Dependence (COGEND) Nicotine Tob Res. 2008;10:1223–1230. doi: 10.1080/14622200802163266. [DOI] [PubMed] [Google Scholar]
- 5.Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102:1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 6.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 7.Foreman MG, Zhang L, Murphy J, Hansel NN, Make B, Hokanson JE, et al. Early-Onset Chronic Obstructive Pulmonary Disease Is Associated with Female Sex, Maternal Factors, and African American Race in the COPDGene Study. Am J Respir and Crit Care Med. 2011;184(4):414–420. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Stable EJ, Herrera B, Jacob IP, Benowitz NL. NIcotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 10.Herning RI, Jones RT, Benowitz NL, Mines AH. How a Cigarette Is Smoked Determines Blood Nicotine Levels. Clin Pharmacol Ther. 1983;33:84–90. doi: 10.1038/clpt.1983.12. [DOI] [PubMed] [Google Scholar]
- 11.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Pickens RW, Svikis DS, Hughes JR. Smoking topography and nicotine blood levels. Addict Behav. 1988;13:91–95. doi: 10.1016/0306-4603(88)90031-7. [DOI] [PubMed] [Google Scholar]
- 13.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharm. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, et al. Smokers with the CHRNA Lung Cancer-Associated Variants Are Exposed to Higher Levels of Nicotine Equivalents and a Carcinogenic Tobacco-Specific Nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., 3rd Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19:1160–1166. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Helen G, Dempsey D, Wilson M, Jacob P, 3rd, Benowitz NL. Racial differences in the relationship between tobacco dependence and nicotine and carcinogen exposure. Addiction. 2013;108:607–617. doi: 10.1111/j.1360-0443.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 19.Grainge MJ, Shahab L, Hammond D, O’Connor RJ, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009;101:191–195. doi: 10.1016/j.drugalcdep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence - a Revision of the Fagerstrom Tolerance Questionnaire. Brit J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 21.Fagerström K. Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 22.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 24.Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21:216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 26.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 27.Toll BA, Schepis TS, O’Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relapse: a preliminary study. Drug Alcohol Depend. 2007;89:302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 31.Jacob P, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the Nicotine Metabolites Cotinine and Trans-3′-Hydroxycotinine in Biologic fluids of Smokers and Non-Smokers using Liquid Chromatography - Tandem Mass Spectrometry: Biomarkers for Tobacco Smoke Exposure and for Phenotyping Cytochrome P450 2A6 Activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrell FE. Regression Modeling Strategies: Sprinter International Publishing. 2015 [Google Scholar]
- 33.Djordjevic MV, Hoffmann D, Hoffmann I. Nicotine regulates smoking patterns. Prev Med. 1997;26:435–440. doi: 10.1006/pmed.1997.0184. [DOI] [PubMed] [Google Scholar]
- 34.Eissenberg T, Adams C, Riggins EC, 3rd, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1:317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- 35.Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24:115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Roethig HJ, Appleton S, Werley M, Muhammad-Kah R, Mendes P. The effect of menthol containing cigarettes on adult smokers’ exposure to nicotine and carbon monoxide. Regul Toxicol Pharm. 2010;57:24–30. doi: 10.1016/j.yrtph.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., 3rd Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19:3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence D, Cadman B, Hoffman A. Sensory properties of menthol and smoking topography. Tob Induc Dis. 2011;9:S3. doi: 10.1186/1617-9625-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenet Genomics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Benowitz NL, Pérez-Stable EJ, Herrera B, Jacob P. Slower Metabolism and Reduced Intake of Nicotine From Cigarette Smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]