Abstract
Diet affects the risk and progression of prostate cancer (PCa), but the interplay between diet and genetic alterations in this disease is not understood. Here we present genetic evidence in the mouse showing that PCa progression driven by loss of the tumor suppressor Pten is mainly unresponsive to a high fat diet (HFD), but that coordinate loss of the protein tyrosine phosphatase Ptpn1 (PTP1B in human) enables a highly invasive disease. PCa in Pten-/-Ptpn1-/- mice was characterized by increased cell proliferation and Akt activation, interpreted to reflect a heightened sensitivity to IGF-1 stimulation upon HFD feeding. Prostate-specific overexpression of PTP1B was not sufficient to initiate PCa, arguing that it acted as a diet-dependent modifier of prostate cancer development in Pten-/- mice. Our findings offer a preclinical rationale to investigate the anticancer effects of PTP1B inhibitors currently being studied clinically for diabetes treatment as a new modality for management of prostate cancer.
Keywords: PTP1B, Prostate Cancer, PTEN, High Fat Diet, IGF-1
Introduction
Prostate cancer (PCa) is the most frequently diagnosed cancer in North American men, and is the second leading cause of cancer-related deaths (1). Lifetime PCa risk is overwhelmingly associated to environmental factor (2) such as diet (3) and obesity (4). However, the mechanistic links remain elusive. We recently described the protein tyrosine phosphatase 1B (PTP1B; encoded by PTPN1) as an androgen receptor (AR)-regulated phosphatase, which plays a tumor-promoting role in PCa (5), and showed that the PTPN1 gene is co-amplified with the AR in metastatic PCa (6). PTP1B is located at a critical node in signaling pathways that regulate metabolism and cancer and is now a validated therapeutic target for diabetes, obesity and breast cancer (7).
The promise of PTP1B-directed therapeutics prompted us to further characterize the role of PTP1B in PCa initiation and progression using preclinical models. Here we report that prostate-specific overexpression of PTP1B does not lead to prostate transformation, ruling out the possibility that PTP1B alone is capable of inducing PCa. Surprisingly, however, compound Ptpn1-/-; PtenPE-/- (PE: prostate epithelium) mice show a significant increase in PCa invasiveness, but only when these mice are fed a high fat diet (HFD), seemingly through the enhancement of insulin-like growth factor 1 (IGF-1)-mediated Akt activation. Together with the observation that the levels of PTP1B protein are consistently increased following Pten loss, these results suggest that PTP1B acts as a diet-dependent tumor suppressor in the context of PCa that is driven by the absence of Pten. Importantly, our results highlight that cancer progression can be altered by a synergistic cooperation between genetic and environmental factors. Finally, these findings indicate that diet and levels of PTP1B enzymatic activity are important parameters when considering the clinical use of PTP1B-targeted therapeutics.
Materials and Methods
Animal husbandry
Genotypes for Ptpn1, Pten, and PB-Cre4 were determined by polymerase chain reaction (PCR) (Supplementary Table S1). C57BL/6 mice were fed either regular lab chow (Harlan Laboratories, #2920X), or a HFD (Harlan Laboratories, #TD.07011) from the time of weaning. Our animal protocol followed the ethical guidelines of the Canadian Council on Animal Care, and was approved by the McGill University Research and Ethics Animal Committee. Detailed are reported in Supplementary Materials and Methods.
Generation of PTPN1 knock-in mice
We used Gateway-compatible Rosa26 locus targeting vectors to generate a Cre/loxP conditional Rosa26-targeted transgenic mouse that overexpresses human PTP1B (Figure 1A) (8). Detailed procedure is described in Supplementary Materials and Methods.
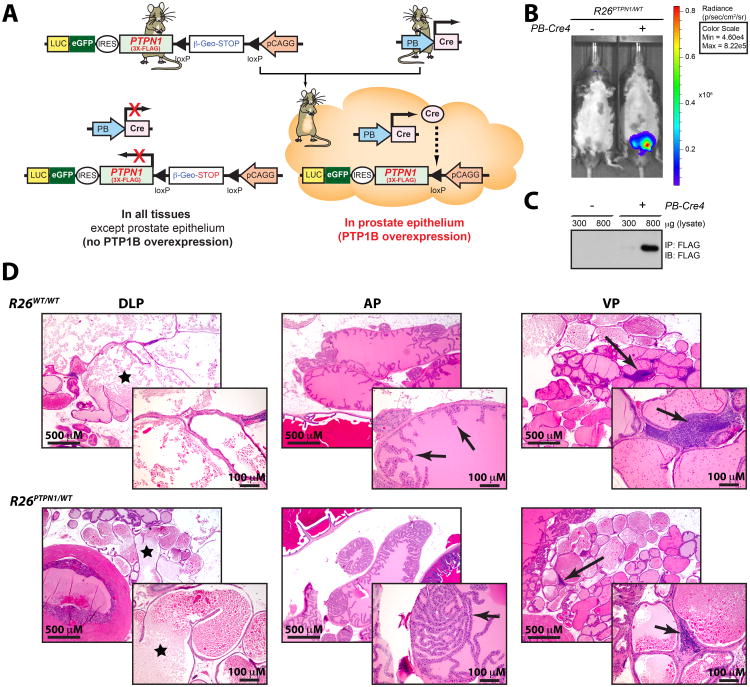
Figure 1. PTP1B overexpression in the prostatic epithelium does not drive cancer.
(A) Rosa26-targeted (R26) pCAGG-promoter-based construct drives strong expression of 3X-FLAG-PTP1B protein (R26PTPN1/WT), together with an eGFP/luciferase reporter in the prostate PE, following breeding with a PB-Cre4 mouse. (B) Validation of the R26PTPN1/WT transgene by live imaging and (C) FLAG immunoprecipitation. (D) Representative photomicrographs of H&E staining to show DLP cystic dilation (left panel; asterisk), normal epithelium in the AP of R26WT/WT mice (middle panel; arrows) along with some regions of focal hyperplasia in R26PTPN1/WT mice (middle panel; arrow) and lymphocytic infiltration in VP (right panel; arrows).
Live imaging
Mice were anesthetized with 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane (isoflurane), injected intraperitoneally with 50 μl luciferin (Caliper), and imaged with the use of the IVIS Spectrum pre-clinical in vivo imaging system (Perkin Elmer), according to the manufacturer's instructions.
Analyses of protein expression
Protein expression was analyzed as described (5). Additional details can be found in Supplementary Materials and Methods.
Histopathological and immunohistochemical analyses
Tissue processing/histopathological examination were done according to conventional procedures, as reported in Supplementary Materials and Methods.
Cell culture and lentiviral infections
Immortalized Ptpn1+/+ and Ptpn1-/- mouse embryonic fibroblasts (MEFs) (9) were infected with a lentiviral shRNA vector against Pten (shPten) or a scramble sequence (kindly provided by Nick R. Leslie). Complementary details can be found in Supplementary Materials and Methods.
Statistics
Statistical analyses were carried out with use of Prism 6.0 GraphPad Software.
Results
To assess whether PTP1B has a PCa-initiating role similar to what we previously observed in breast cancer (10), we generated a Cre/loxP conditional Rosa26-targeted transgenic mouse that overexpresses a FLAG-PTPN1 transgene (R26PTPN1/WT), together with an eGFP/luciferase reporter (Fig. 1A). Prostate-specific PTP1B overexpression was achieved by crossing this model with a PB-Cre4 mouse and transgene activation was validated by live imaging (Fig. 1B) and FLAG immunoprecipitation (Fig. 1C). Histopathological analysis on sections from 1-year old PB-Cre4; R26PTPN1/WT mice that were stained with hematoxylin and eosin (H&E) revealed no signs of cancer lesions (Supplementary Table S2). The PB-Cre4; R26PTPN1/WT mice showed two notable differences from control mice: a moderate incidence of epithelial hyperplasia in the anterior prostate (AP), and a single case of mouse prostatic intraepithelial neoplasia (mPIN) (≤ 5%) in the dorsolateral prostate (DLP; Fig. 1D and Supplementary Table S2). Together, these results suggest that unlike breast cancer, prostate-specific PTP1B overexpression in intact murine prostates is insufficient to initiate PCa.
To confirm that PTP1B is instead required for PCa progression, as documented with use of cell-based and xenograft assays (5), we generated Ptpn1-/- ; PtenPE-/- compound mice. Homozygous inactivation of Pten in the PE driven by PB-Cre4 is a robust PCa model that yields mPIN by the age of 6 weeks. Following prostate-specific Pten inactivation, histopathological analysis revealed no differences in the number (percentage) of prostate ductules affected by mPIN between Ptpn1+/+ and Ptpn1-/- mice (Supplementary Fig. S1A and B). Likewise, genetic ablation of Ptpn1-/- per se (Ptpn1-/- ; PtenPE+/+ mice) did not lead to prostate gland transformation, nor to any significant alterations beyond mPIN in the AP and ventral prostates (VP) of all 6-week-old mice (Supplementary Fig. S1B). Overall, the impact of Ptpn1-loss in 6-week-old Ptpn1-/- ; PtenPE-/- mice was minor, resulting in a slight increase in the incidence of a desmoplastic reaction, characterized by activation of the stromal tissue surrounding individual prostate ductules in the DLP (Fig. 2A and Supplementary Fig. S2). Microinvasive adenocarcinomas, defined as groups of malignant neoplastic cells crossing the basement membrane, were visible in one Ptpn1-/-; PtenPE-/- mouse at 6 weeks of age (Fig. 2A). Our observations thus suggest that PCa progression driven by Pten-loss is mostly unaltered in the Ptpn1-null background; this conclusion is also supported by the phenotype observed in the DLP (Fig. 2B) and AP (Supplementary Fig. S3A and B) of 12-week-old mice.
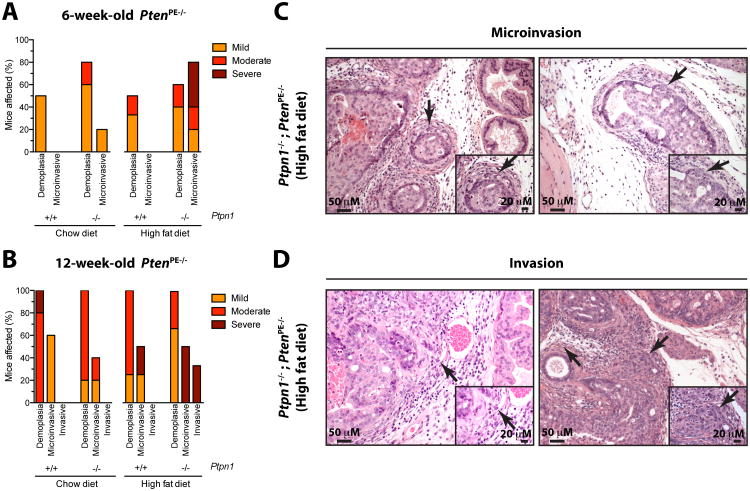
Figure 2. Ptpn1 loss sensitizes PtenPE-/- mice to a HFD, and leads to highly invasive prostate cancer.
(A) Ptpn1 deficiency slightly increases the frequency of desmoplastic reactions when mice are fed chow diet (Ptpn1+/+; PtenPE-/- N=6, Ptpn1-/-; PtenPE-/- N=5); however, when mice are fed a HFD, Ptpn1 deficiency significantly drives the emergence of microinvasive adenocarcinomas in the DLP (Ptpn1+/+; PtenPE-/- N=6, Ptpn1-/-; PtenPE-/- N=5). (B) Only Ptpn1-/-; PtenPE-/- mice fed a HFD present with invasive adenocarcinoma in the DLP at 12 weeks of age (chow diet: N=5, HFD: Ptpn1+/+; PtenPE-/- N=4, Ptpn1-/-; PtenPE-/- N=5). (C and D) Representative H&E-stained sections from (C) microinvasive adenocarcinomas (arrows), and from (D) lymphovascular invasion (tumor cell embolus) (left panel; arrows); an area of invasion, and its extension into widened stroma/connective tissues (right panel; arrows) observed in Ptpn1-/-; PtenPE-/- mice fed on a HFD.
Since PTP1B is central to metabolic homeostasis, we next challenged these mice with a HFD upon weaning. Again, Ptpn1-loss per se did not lead to prostate gland transformation (data not shown) Remarkably, the increased fat intake led to a dramatic increase in the penetrance and score (number of microinvasive foci) of microinvasive adenocarcinomas in Ptpn1-/-; PtenPE-/- mice (Fig. 2A and C); this trend was also confirmed in older mice, whereas 12-week-old Ptpn1-/-; PtenPE-/- mice fed a HFD were the only ones to develop invasive adenocarcinomas defined as a large focus of neoplastic cells invading deeply into a severely desmoplastic stroma or into vessels (Fig. 2B and D). These observations were also mirrored in the AP of 12-week-old animals, in which the development of invasive adenocarcinoma was restricted to the Ptpn1-/-; PtenPE-/- mice (Supplementary Fig. S3). Interestingly, Ptpn1+/+; PtenPE-/- mice were insensitive to HFD with respect to tumor progression in the DLP and AP (Fig. 2A and B and Supplementary Fig. S3A). These findings clearly demonstrate that Ptpn1 deficiency potentiates the aggressiveness of Pten-null tumors, but only for mice that are challenged with a HFD.
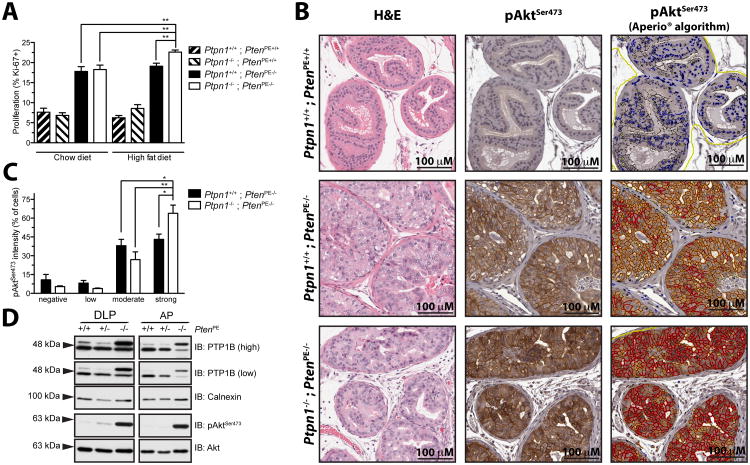
Pten deficiency in the PE is accompanied by an increase in cell proliferation, as indicated by nuclear immunostaining for Ki-67. In line with our histological findings, only Ptpn1-/- ; PtenPE-/- mice fed a HFD demonstrate a significantly higher proportion of Ki-67 positive cells (Fig. 3A). Because hyperactivation of the PI3K/Akt pathway is a major consequence of PTEN-loss, we asked whether the differences in cell proliferation could be due to altered Akt activation. We found that prostatic epithelial cells that retain PTEN expression display a weak and diffuse cytoplasmic staining for pAktSer473, but loss of Pten leads to the recruitment of pAktSer473 to the cell membrane (Fig. 3B, middle panels). Remarkably, the intensity of the pAktSer473 signal is heightened in the DLP of Ptpn1-/-; PtenPE-/- mice that are fed a HFD, as confirmed with the use of a specialized Aperio® algorithm to quantify membrane-bound pAktSer473 (Fig. 3B, right panels). Moreover, while prostatic epithelial cell populations in PtenPE-/- mice have equal distributions of moderate and strong pAktSer473 signal, a significant shift in favor of a dominant cell population with augmented Akt activation is observed in Ptpn1-/-; PtenPE-/- mice that are fed a HFD (Fig. 3C). Collectively, these results suggest an important role for PTP1B in the context of Pten-loss. In this line, increased PTP1B protein levels following loss of Pten are observed in the prostates of 12-week-old mice that are wild-type for Ptpn1 (Fig. 3D), further supporting an intricate crosstalk between both phosphatases.
Figure 3. Prostate cell proliferation and pAkt activation in PtenPE-/- mice is fine tuned by Ptpn1 when mice are fed a HFD.
(A) Ptpn1 deficiency leads to a significant increase in DLP epithelial cell proliferation, as indicated by Ki-67 nuclear staining, but only when PtenPE-/- mice are fed HFD (unpaired t-test; * p<0.05, ** p<0.01; N≥5 +/- SEM for the different PtenPE-/- mice. From left to right: N=4; 4; 6; 5; 4; 4; 6; 5). (B) Akt is further activated upon Ptpn1 loss when mice are fed a HFD, as demonstrated by enhanced staining for pAktSer473. Intensity of the signal is graded and presented in a color-coded overlay (right panel; negative = blue, low = yellow, moderate = orange, strong = red). (C) Quantification of pAktSer473 staining in (B) reveals a shift towards epithelial cells that are highly positive for pAktSer473 in Ptpn1-/-; PtenPE-/- mice (N=5) fed HFD, compared to Ptpn1+/+; PtenPE-/- mice (N=6; unpaired t-test; * p<0.05, ** p<0.01; N≥5 +/- SEM). (D) PTP1B protein level is increased following loss of both Pten alleles in the DLP and AP of 12-week-old mice.
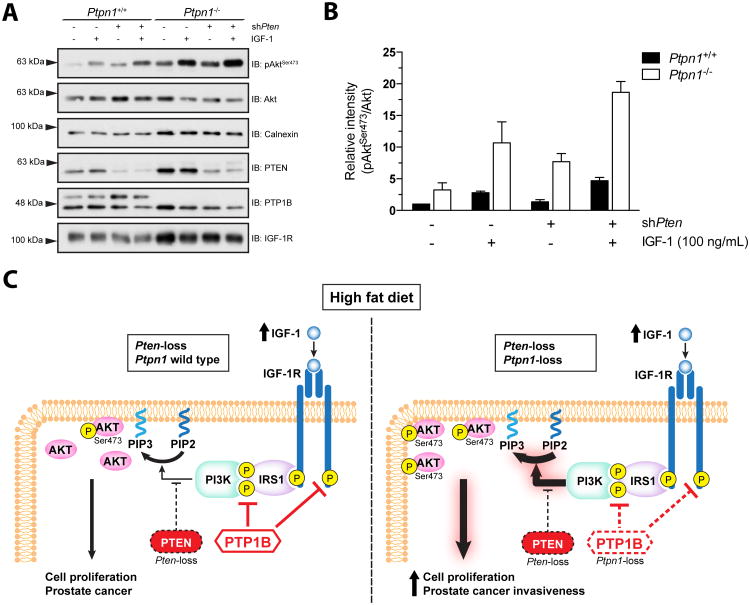
Increased energy consumption leads to higher circulating IGF-1, whereas severe caloric restriction leads to lower IGF-1 levels (11). Since PTP1B dephosphorylates and inactivates the insulin receptor substrate-1 (IRS1) (12) and the β chain of the IGF-1 receptor (IGF-1R) (13), we asked whether greater Akt activation through IGF-1 stimulation could be achieved in a situation where both PTP1B and PTEN expression are compromised. Alone, decreased PTEN expression or Ptpn1-loss only modestly sensitizes cells to IGF-1 treatment. In contrast, Akt activation when both phosphatases were altered was 4-fold greater than the single Pten knockdown (Figure 4A and B). Together, these results provide a mechanistic link between PTP1B and PTEN that may account for the observed increased PCa cell proliferation and invasiveness under HFD (Figure 4C).
Figure 4. Ptpn1 ablation confers increased IGF-1-mediated pAkt activation in PTEN compromised cells.
(A) Sensitization to IGF-1 stimulation in Ptpn1-/- MEFs is further aggravated by diminished PTEN expression as demonstrated by pAktSer473 protein levels (representative experiment). (B) Quantified ratio of pAktSer473 over Akt protein levels in (A) and two other replicates (N=3, mean +/- SEM). (C) Graphical summary.
Discussion
The phosphatidylinositol 3-kinase (PI3K)-Akt signaling axis plays a major role in the development and progression of PCa. Indeed, loss of PTEN function by mutation, deletion or reduced expression, and the consequent activation of PI3K-Akt, is observed in about 40% of PCa cases (14). When taking into account alterations of other modulators of this axis, up to 42% of primary and 100% of metastatic PCa demonstrate an increase in PI3K-Akt activity (15). Notably, hyperactivation of the PI3K-Akt signaling axis desensitizes tumors to dietary modulations: as such, PtenPE-/- tumors are resistant to dietary restriction (16) and to a HFD (Fig. 2A and B and Supplementary Fig. S3A). This feature of Pten-null prostate tumors is reverted when PTP1B-deficient mice are challenged with a HFD, suggesting that nutrient-sensing mechanisms are somehow re-enabled in the absence of PTP1B (17).
Dietary alterations modulate circulating levels of IGF-1 (18) and PTP1B negatively regulates the IGF-1R (13). Strikingly, the hyperactivated PI3K-Akt signaling axis, signature of compromised PTEN expression, is further potentiated by Ptpn1-loss upon IGF-1 stimulation (Figure 4A and B). Together, these results suggest that restoration of nutrient-sensing mechanism in Pten-null prostate tumors following PTP1B-loss occurs through the derepression of the IGF-1/IGF-1R signaling axis.
Interestingly, PTP1B-deficiency did not alter mPIN development in mice that lack a single Pten allele in the PE at 24, 36 and even 48 weeks of age (Supplementary Fig. S4). Additionally, loss of a single Pten allele in the mouse PE is insufficient to drive an increase in the levels of PTP1B protein (Fig. 3D). Finally, 12-week-old PTP1B heterozygous mice when bred on a PtenPE-/- background demonstrate an intermediate phenotype when fed a HFD, suggesting that PTP1B dose might also be an important parameter (Supplementary Fig. S5). These observations suggest that in order to be deleterious, PTP1B-deficiency must be accompanied by both genetic and environmental alterations, in this case a complete loss of Pten plus a HFD. Whether this phenomenon extends to other PCa-relevant genetic alterations such as MYC overexpression remains to be determined.
PTP1B is an attractive drug target for the treatment of diabetes, obesity and breast cancer (7,10,19). Because loss of PTEN function is a hallmark of many cancers, if our findings are transposable to the human prostate context, we suggest cautiousness when moving PTP1B-targeted therapeutics from bench to bedside, to avoid the potential risk of inadvertently fueling an undiagnosed PTEN-null cancer of a patient with Western dietary patterns. Unfortunately, we were unable to investigate this issue in our mouse model given the lack of specificity of PTP1B inhibitors available to the research community (they also target TC-PTP or other protein tyrosine phosphatases),(20) as well as the requirement for long-term oral gavage.(10) Nonetheless, due to PTP1B's nutrient sensing capabilities, we suggest that patients involved in these trials be closely monitored for both the levels of PTP1B enzymatic activity and their dietary fat intake.
Supplementary Material
Acknowledgments
The authors thank Serge Hardy, Yevgen Zolotarov, Maxime Bouchard, Jo-Ann Bader, Caroline Thérien, Ailsa Lee Loy, Joseph James Bowden and Bruno Grande (technical assistance and/or helpful discussions), Nick R. Leslie (reagents) and Sonal Jhaveri (critical review of the manuscript). D.P.L. is a recipient of a Canadian Institute of Health Research (CIHR) Frederick Banting and Charles Best doctoral research award and a CIHR/Fonds de recherche du Québec – Santé training grant in cancer research FRN53888 of the McGill Integrated Cancer Research Training Program. M.L.T. is the holder of the Jeanne and Jean-Louis Lévesque Chair in Cancer Research. This work was supported by grants to L.C.T. from the NIH (CA137050) and to M.L.T. from the U.S. Army Department of Defense (#W81XWH-09-1-0259), the CIHR (MOP-62887) and Prostate Cancer Canada (#02013-33).
Financial Support: L.C.T.: NIH R01 (CA137050)
M.L.T.: CIHR operating grant (MOP-62887), U.S. Army DoD award (#W81XWH-09-1-0259) and Prostate Cancer Canada grant (#02013-33).
Footnotes
Conflict of interest: There is no conflict of interest for any authors.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2015;529(7584):43–7. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WCRF/AICR. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington DC: World Cancer Research Fund/American Institute for Cancer Food Research; 2007. p. 517. [Google Scholar]
- 4.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lessard L, Labbé DP, Deblois G, Bégin LR, Hardy S, Mes-Masson AM, et al. PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer. Cancer Res. 2012;72(6):1529–37. doi: 10.1158/0008-5472.CAN-11-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labbé DP, Nowak DG, Deblois G, Lessard L, Giguère V, Trotman LC, et al. Prostate cancer genetic-susceptibility locus on chromosome 20q13 is amplified and coupled to androgen receptor-regulation in metastatic tumors. Mol Cancer Res. 2014;12(2):184–9. doi: 10.1158/1541-7786.MCR-13-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan N, Koveal D, Miller DH, Xue B, Akshinthala SD, Kragelj J, et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat Chem Biol. 2014;10(7):558–66. doi: 10.1038/nchembio.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyabi O, Naessens M, Haigh K, Gembarska A, Goossens S, Maetens M, et al. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37(7):e55. doi: 10.1093/nar/gkp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A, Bal GS, Kennedy BP, Tremblay ML. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J Biol Chem. 2001;276(28):25848–55. doi: 10.1074/jbc.M009734200. [DOI] [PubMed] [Google Scholar]
- 10.Julien SG, Dube N, Read M, Penney J, Paquet M, Han Y, et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39(3):338–46. doi: 10.1038/ng1963. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12(2):84–9. [PubMed] [Google Scholar]
- 12.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem. 2000;275(6):4283–9. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- 13.Buckley DA, Cheng A, Kiely PA, Tremblay ML, O'Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22(7):1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourmand G, Ziaee AA, Abedi AR, Mehrsai A, Alavi HA, Ahmadi A, et al. Role of PTEN gene in progression of prostate cancer. Urol J. 2007;4(2):95–100. [PubMed] [Google Scholar]
- 15.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–31. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517(7534):302–10. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35(11-12):694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 19.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–8. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 20.Labbé DP, Hardy S, Tremblay ML. Protein tyrosine phosphatases in cancer: friends and foes! Prog Mol Biol Transl Sci. 2012;106:253–306. doi: 10.1016/B978-0-12-396456-4.00009-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.