Abstract
Objective
Because depression and anxiety are typically studied in isolation, our purpose was to examine the relative importance of these overlapping emotional factors in predicting incident cardiovascular disease (CVD).
Methods
We examined depression and anxiety screens, and their individual items, as predictors of incident hard CVD events, myocardial infarction, and stroke over eight years in a diverse sample of 2,041 older primary care patients initially free of CVD. At baseline, participants completed self-report depression and anxiety screens. Data regarding CVD events were obtained from an electronic medical record system and the Centers for Medicare and Medicaid Services analytic files.
Results
During follow-up, 683 (33%) experienced a CVD event. Cox proportional hazards models – adjusted for demographic and CVD risk factors – revealed that a positive anxiety screen, but not a positive depression screen, was associated with an increased risk of a hard CVD event in separate models (Years 0–3: Anxiety HR=1.54, p<.001; Years 3+: Anxiety HR=0.99, p=.93; Depression HR=1.10, p=.41), as well as when entered into the same model (Years 0–3: Anxiety HR=1.53, p<.001; Years 3+: Anxiety HR=0.99, p=.99; Depression HR=1.03, p=.82). Analyses examining individual items and secondary outcomes showed that the anxiety-CVD association was largely driven by the feeling anxious item and the myocardial infarction outcome.
Conclusions
Anxiety, especially feeling anxious, is a unique risk factor for CVD events in older adults, independent of conventional risk factors and depression. Anxiety deserves increased attention as a potential factor relevant to CVD risk stratification and a potential target of CVD primary prevention efforts.
Keywords: Anxiety, depression, myocardial infarction, stroke, primary care, prospective
Introduction
Prospective studies of the past 30 years indicate that both depression and anxiety predict the onset of atherosclerotic cardiovascular disease (CVD), including coronary artery disease and cerebrovascular disease. Specifically, meta-analyses have demonstrated that adults with depression have a 57% greater risk and adults with anxiety have a 26% greater risk of developing CVD than those without elevations in these factors (1, 2). Most of this evidence, however, derives from studies in which these emotional factors were examined in isolation – i.e., investigations testing whether depression alone or anxiety alone predicts incident CVD. This approach is problematic due to the substantial overlap that exists between these emotional factors (3). To illustrate, depressive and anxiety disorders are highly comorbid (55–60%) (4), and measures of depressive and anxiety symptoms are moderately to highly correlated (r = 0.45–0.75) (5). As a result of this overlap, studies examining one factor at a time cannot determine whether depression and anxiety are independent CVD risk factors or whether one factor is merely a marker for the other factor that itself is the true CVD risk factor.
While there is a smaller but growing literature examining depression and anxiety as simultaneous predictors of incident CVD, these studies have yielded mixed results, and none has assessed the predictive utility of individual symptoms. For instance, some of these findings indicate that depression predicts incident CVD independent of anxiety (6–8), whereas others suggest that anxiety predicts CVD independent of depression (9–12). Yet another study found that both depression and anxiety predict incident CVD independent of each other (13). Thus, much is left to be learned about the relative importance of these emotional factors and their individual symptoms in predicting the onset of CVD (14). Such knowledge would be of use in (a) identifying patients at greatest CVD risk and in need of early and aggressive primary prevention and (b) informing the development of interventions targeting emotional factors to reduce CVD risk. Accordingly, the aim of the present study was to simultaneously examine depression and anxiety screens, as well as their individual items, as predictors of incident hard CVD events, myocardial infarction (MI), and stroke over eight years in a sample of 2,041 older primary care patients initially free of CVD.
Methods
Participants
Participants were primary care patients who underwent initial screening at the Indiana sites for the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study, a multisite randomized controlled trial of collaborative care for late-life depression (15). From July 1999 to August 2001, all 3,675 patients aged ≥ 60 years attending two large urban primary care clinics in a safety net healthcare system in Indianapolis, IN, were approached for screening during routine visits. About 80% of these patients were economically disadvantaged as indicated by receiving federal or county health insurance assistance (16). Because 172 patients refused the screening and five screens were incomplete, 3,498 (95.2%) patients had complete screens. From this cohort, we excluded (a) 25 (0.7%) patients for whom we could not match their IMPACT trial data with data from other sources (e.g., Medicare/Medicaid claims) and (b) 229 (6.5%) patients who were randomized in the IMPACT trial. We excluded randomized patients because we have previously shown that patients randomized to the IMPACT collaborative care intervention for depression have a significantly lower risk of a hard CVD event than patients randomized to usual depression care (17).
Because our focus is cardiovascular risk and not prognosis, we then excluded 1,203 (34.4%) patients with clinical CVD at baseline. To identify these patients, we merged data from the Regenstrief Medical Record System (18), one of largest and longest operating electronic medical records (earliest data from 1978), with data from the Centers for Medicare and Medicaid Services analytic files (earliest data from 1999). Baseline CVD was defined as the occurrence of any of the following events or procedures in the electronic medical record or Medicare/Medicaid data before the IMPACT screening date: (a) ischemic heart disease diagnosis (ICD-9 codes 410–414, 429.2), (b) laboratory evidence of an acute MI (creatine kinase-myocardial band isoenzyme value >3.0 ng/ml or troponin value >0.3 μg/L), (c) percutaneous coronary intervention (ICD-9 codes 00.66, 36.03, 36.06, 36.07, 36.09; CPT codes 92980–92984, 92995, 92996), (d) coronary artery bypass graft (ICD-9 codes 36.10–36.19; CPT codes 33510–33536), (e) cerebrovascular disease diagnosis (ICD-9 codes 430–434, 436–438), or (f) thrombolytic therapy (CPT code 37195). We used a broad definition of baseline CVD to minimize the likelihood of patients with preexisting clinical CVD being included in our cohort. Our final sample consisted of 2,041 older primary care patients initially free of clinical CVD (see Table 1 for participant characteristics). This represents 58.3% of the patients initially screened, although the vast majority of exclusions were for CVD at baseline. Because our follow-up utilized existing data sources and required no patient contact, there was not any attrition other than the 25 patients mentioned above for whom we could not match data across sources.
Table 1.
Baseline Characteristics of Participants (N = 2,041)
| Demographic Factors | |
| Age (years), mean (SD) | 68.5 (6.9) |
| Female, n (%) | 1,482 (72.6) |
| African American, n (%) | 1,175 (57.6) |
| Cardiovascular Risk Factors | |
| Hypertension, n (%) | 1,458 (71.4) |
| Hypercholesterolemia (total cholesterol ≥ 240 mg/dL), n (%) | 349 (17.1) |
| Diabetes, n (%) | 571 (28.0) |
| Smoker, n (%) | 730 (35.8) |
| Body-Mass Index (kg/m2), mean (SD) | 30.3 (8.0) |
| Depression and Anxiety Screens | |
| Positive PRIME-MD Depression Screen, n (%) | 270 (13.2) |
| Positive PRIME-MD Anxiety Screen, n (%) | 849 (41.6) |
Note. PRIME-MD = Primary Care Evaluation of Mental Disorders.
This follow-up study was approved by the IUPUI Institutional Review Board and the Centers for Medicare and Medicaid Services Privacy Board. The depression and anxiety screens were administered as part of routine care. A waiver of consent was obtained to link electronic medical record and Medicare/Medicaid data.
Measures and Procedures
Depression and Anxiety Screens
During routine primary care visits, patients were administered a modified version of Patient Questionnaire of the Primary Care Evaluation of Mental Disorders (PRIME-MD) (19). The Patient Questionnaire is a self-report symptom measure designed to be an initial screen for common psychiatric disorders in medical populations. Patients responded yes or no to the question: “During the past month, have you been bothered a lot by [symptom]?” Our modified version included both depression items (“little interest or pleasure in doing things” and “feeling down, depressed, or hopeless”) and two of the three anxiety items (“nerves or feeling anxious or on edge” and “worrying about a lot of different things”) of the original measure. Consistent with the PRIME-MD instructions,(19) patients who endorsed either of the two depression or anxiety items were coded as screening positive for depression or anxiety, respectively. When using the complete PRIME-MD procedure, patients who screen positive for a disorder then undergo a structured interview to confirm the diagnosis. This interview was not completed in our study. Even so, the Patient Questionnaire depression screen has moderate sensitivity (69%) and higher specificity (82%) for depressive disorder diagnoses made by mental health professionals, and the anxiety screen has higher sensitivity (94%) and lower specificity (53%) for anxiety disorder diagnoses (19).
Incident Cardiovascular Disease Events
The primary outcome of a hard CVD event was defined as the occurrence of any of the following events in the Regenstrief Medical Record System or Medicare/Medicaid data between the IMPACT screening date and December 31, 2008: (a) fatal MI (ICD-10 codes I21-I22 the first-listed cause of death), (b) acute MI diagnosis (ICD-9 code 410), (c) laboratory evidence of acute MI (creatine kinase-myocardial band isoenzyme value >3.0 ng/ml or troponin value >0.3 μg/L), (d) fatal stroke (ICD-10 codes I60-I64 the first-listed cause of death), or (e) hemorrhagic (ICD-9 codes 430–432) or nonhemorrhagic (ICD-9 codes 433.01, 433.11, 433.21, 433.31, 433.91, 434.01, 434.11, and 434.91) stroke diagnosis. Our secondary outcomes were components of the primary outcome, namely: fatal/nonfatal MI (categories a-c only) and fatal/nonfatal stroke (categories d and e only). Death dates were extracted from the Medicare data, and causes of death were obtained from death certificates provided by the Indiana State Department of Health included in the electronic medical record. Patients were followed for a maximum of 9.5 years (median = 8.3 years).
Other Baseline Factors
Data regarding demographic characteristics (age, sex, and race) and the presence of physician-diagnosed hypertension and diabetes were extracted from the Regenstrief Medical Record System, as has been described elsewhere (16). For each patient, we exacted the most recent total cholesterol value that was within five years of the screening date from the electronic medical record; those with a value ≥ 240 mg/dL were coded as having hypercholesterolemia. Similarly, those with a positive marker for smoking in the medical record during the same 5-year period were coded as smokers. Body mass index (BMI; kg/m2) was computed from height and weight recorded in the medical record. For 12 (0.6%) patients, missing values for height and weight were imputed with sex-specific median values. The PRIME-MD Patient Questionnaire included items assessing general perceived health (“Overall, would you say your health is: excellent, very good, good, fair, or poor.”), tiredness (“feeling tired or having low energy”), and sleep difficulties (“trouble sleeping”).
Data Analysis
To examine the relative importance of depression and anxiety screens in predicting future CVD events, we ran individual and simultaneous-entry Cox proportional hazards models predicting the primary (hard CVD events) and secondary (nonfatal/fatal MI and nonfatal/fatal stroke) outcomes. Patients were censored at date of death or December 31, 2008. Covariates in individual models were demographic factors (age, sex, and race) and CVD risk factors (hypertension, hypercholesterolemia, diabetes, smoking, and BMI). In these models, depression screen and anxiety screen were entered into separate models as the predictor variable. In the simultaneous-entry models, depression screen and anxiety screen were forced into the same model along with the covariates of the individual models to determine which screens were unique predictors of CVD events. The depression screen x anxiety screen interaction was also tested in these models. For screens that were predictors of CVD events, we performed a parallel set of individual and simultaneous-entry Cox models to examine the predictive utility of the screener items. For screener items that predicted CVD events, Kaplan-Meier survival curves were constructed to illustrate the time to first CVD event for patients who endorsed the item versus those who did not.
Because tests of non-proportionality (depression/anxiety variable x log time interactions) were not significant, the proportional hazards assumption was not rejected for models examining depression variables as predictors of hard CVD events (p = .61), nonfatal/fatal MI (p = .61), or nonfatal/fatal stroke (p = .13). This assumption was also not rejected for models examining anxiety variables as predictors of nonfatal/fatal MI (ps ≥.060) or stroke (p = .27). However, this assumption was rejected for models examining anxiety variables as predictors of hard CVD events (anxiety screen: p = .012; feeling anxious: p = .044; worry: p = .028). In these instances, we also calculated separate hazard ratios for Years 0–3 and Years 3+ by including an anxiety variable x time period interaction in the models. These time periods were selected because examination of the hazard ratios for each year of follow-up revealed that there was a consistent decrease in effect sizes across models between Years 3 and 4.
Supplemental analyses were performed to explore key associations. First, to diminish the possibility of reverse causality (e.g., an impending CVD event leading a patient to report depressive or anxiety symptoms), we reran individual Cox models after excluding patients who experienced a MI during the first year of follow-up. Second, to decrease the probability that observed relationships were due to confounding by poor overall health or sleep disturbance, we ran individual Cox models further adjusting for the Patient Questionnaire general perceived health item or the feeling tired and trouble sleeping items. Third, to evaluate whether observed relationships were moderated by sex or race, we tested two- and three-way interactions involving predictor variables, sex, and race. Sensitivity analyses were also performed to explore the effect of including (rather than excluding) depressed patients randomized in the IMPACT trial on the pattern of results. Specifically, we reran the individual Cox models for hard CVD events shown in Table 1, which also included dummy variables adjusting for IMPACT treatment group assignment (randomized to IMPACT group vs. randomized to usual care group; not randomized versus randomized to usual care group). SAS 9.3 software was used to conduct all analyses.
Results
Depression and Anxiety Screens
In our cohort of 2,041 primary care patients, 270 (13.2%) patients screened positive for depression (3.0% little interest item only, 4.6% feeling depressed item only, 5.6% both items), and 849 (41.6%) screened positive for anxiety (10.5% feeling anxious item only, 14.9% worry item only, 16.2% both items). The positive anxiety screen rate (48.6%) reported for the Patient Questionnaire in the PRIME-MD validation study is similar to our rate; however, the positive depression screen rate (32.5%) reported in that study is higher than ours (19). We may have observed a lower rate of positive depression screens than in the validation study due to our older sample (20) and exclusion of patients with clinical CVD (21).
As was expected, depression and anxiety often co-occurred, with 209 (10.2%) patients screening positive for both. This comorbidity rate is similar to that reported in prior studies of primary care patients using brief screeners (22). Despite this comorbidity, correlations between the depression and anxiety variables were small to moderate (phi coefficients ranged from 0.21 to 0.31), indicating that multicollinearity is not an issue with our simultaneous-entry models.
Incident Cardiovascular Disease Events
During the 8-year follow-up period, 683 (33.5%) patients experienced the primary outcome of a hard CVD event, consisting of 487 nonfatal MIs, 172 nonfatal strokes, 10 concurrent nonfatal MIs and strokes, 11 fatal MIs, and 3 fatal strokes as first events. The median time to hard CVD event for those with an event was 3.9 years (IQR: 1.8–6.1 years). A total of 553 (27.1%) patients and 235 (11.5%) patients experienced the secondary outcome of fatal/nonfatal MI and fatal/nonfatal stroke, respectively. CVD event rates were high, which was not surprising given the demographic characteristics (i.e., older age, high percent African American, and lower socioeconomic status) and high CVD risk factor burden in our cohort (see Table 1).
Depression and Anxiety Screens as Predictors of Incident Cardiovascular Disease Events
Event rates for patients with positive versus negative depression screens were 35.2% versus 33.2% for hard CVD events, 28.5% versus 26.9% for MI, and 13.3% versus 11.2% for stroke. For patients with positive versus negative anxiety screens, event rates were 34.9% versus 32.5% for hard CVD events, 28.9% versus 25.8% for MI, and 12.1% versus 11.1% for stroke.
As is shown in Table 2, individual Cox proportional hazards models adjusted for demographic and CVD risk factors revealed that a positive anxiety screen (Years 0–3: p < .001; Years 3+: p = .93), but not a positive depression screen (p = .41), predicted hard CVD events. Specifically, a positive anxiety screen was associated with a 54% greater risk of a CVD event for Years 0–3; however, there was no such association for Years 3+. Of note, we calculated separate hazard ratios for Years 0–3 and Years 3+ because the proportional hazards assumption was rejected for models examining anxiety variables as predictors of hard CVD events (see Data Analysis). The simultaneous-entry Cox models yielded the same pattern of results (see Table 2). A positive anxiety screen (p < .001) was associated with an 53% greater risk of a CVD event for Years 0–3 after adjustment for depression screen, while anxiety screen for Years 3+ (p = .99) and depression screen (p = .82) remained unrelated to CVD events. The depression screen x anxiety screen interaction was not significant for Years 0–3 or Years 3+ (ps = .67 and .82).
Table 2.
Cox Proportional Hazards Models Predicting Incident Cardiovascular Disease (CVD) Events
| Individual Modelsa HR (95% CI) |
Simultaneous-Entry Modelsb HR (95% CI) |
|
|---|---|---|
| Hard CVD Events (683, 33%) | ||
| Depression Screen | 1.10 (0.88–1.36) | 1.03 (0.82–1.29) |
| Little Interest Item | --- | --- |
| Depressed Mood Item | --- | --- |
| Anxiety Screenc | ||
| Years 0–3 | 1.54* (1.21–1.96) | 1.53* (1.20–1.95) |
| Years 3+ | 0.99 (0.81–1.21) | 0.99 (0.80–1.21) |
| Feeling Anxious Itemc | ||
| Years 0–3 | 1.53* (1.19–1.97) | 1.53* (1.19–1.98) |
| Years 3+ | 1.09 (0.87–1.36) | 1.09 (0.86–1.36) |
| Worry Itemc | ||
| Years 0–3 | 1.39* (1.08–1.78) | 1.37* (1.06–1.77) |
| Years 3+ | 0.94 (0.75–1.17) | 0.93 (0.74–1.16) |
| Fatal or Nonfatal MI (553, 27%) | ||
| Depression Screen | 1.07 (0.84–1.37) | 0.99 (0.77–1.28) |
| Little Interest Item | --- | --- |
| Depressed Mood Item | --- | --- |
| Anxiety Screen | 1.22* (1.03–1.45) | 1.22* (1.02–1.46) |
| Feeling Anxious Item | 1.31* (1.09–1.57) | 1.32* (1.09–1.60) |
| Worry Item | 1.07 (0.89–1.29) | 1.06 (0.87–1.28) |
| Fatal or Nonfatal Stroke (235, 12%) | ||
| Depression Screen | 1.20 (0.84–1.71) | 1.13 (0.78–1.64) |
| Little Interest Item | --- | --- |
| Depressed Mood Item | --- | --- |
| Anxiety Screen | 1.20 (0.92–1.56) | 1.17 (0.89–1.54) |
| Feeling Anxious Item | --- | --- |
| Worry Item | --- | --- |
Note. N = 2,041. HR = hazard ratio. CI = confidence interval. MI = myocardial infarction.
Adjusted for age, sex, race, hypertension, hypercholesterolemia, diabetes, smoking, and body-mass index. Age (HR = 1.04, 95% CI: 1.03–1.06, p < .001), being male (HR = 1.28, 95% CI: 1.08–1.51, p = .004), diabetes (HR = 1.55, 95% CI: 1.32–1.82, p < .001), and being a smoker (HR = 1.33, 95% CI: 1.13–1.56, p = .001) at baseline were independent predictors of hard CVD events in the expected directions.
Adjusted for age, sex, race, hypertension, hypercholesterolemia, diabetes, smoking, body-mass index, and depression or anxiety screen.
Because the proportional hazards assumption was rejected, results of piecewise models are presented for the time periods Years 0–3 and Years 3+.
p < .05
We observed similar results for fatal/nonfatal MI (see Table 2). A positive anxiety screen was associated with a 22% greater risk of a MI during the 8-year follow-up period in both individual and simultaneous-entry Cox models (ps = .023 and .028), whereas depression screen was not a predictor in either model (ps = .57 and .95). In contrast, neither depression screen (ps = .32 and .51) nor anxiety screen (ps = .18 and .27) predicted fatal/nonfatal stroke in the individual and simultaneous-entry Cox models (see Table 2). However, three of the four hazard ratios for stroke were comparable in magnitude to those that were significant for other outcomes, suggesting that lower statistical power due to the smaller number of stroke events may be responsible for these null results. The depression screen x anxiety screen interaction was not significant for fatal/nonfatal MI or stroke (ps = .52 and .92).
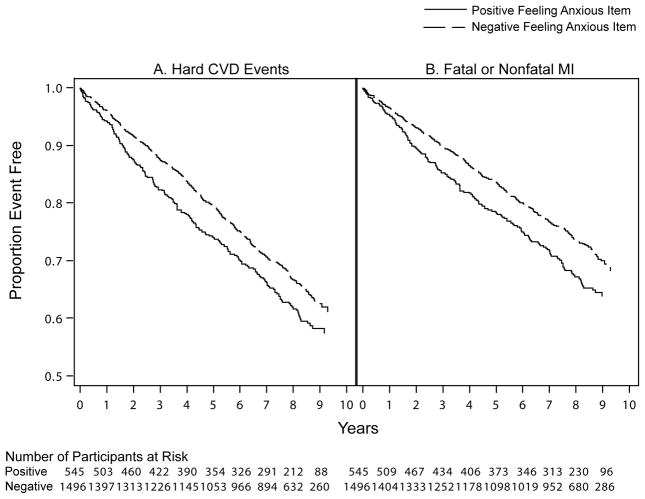
Because anxiety screen was associated with hard CVD events and fatal/nonfatal MI, we examined the utility of the two anxiety items in predicting these outcomes. As can be seen in Table 2, individual Cox models revealed that the feeling anxious item (p < .001) and the worry item (p = .010) were predictors of hard CVD events for Years 0–3. After adjustment for depression screen in the simultaneous-entry Cox models, the feeling anxious item (p = .001) and the worry item (p = .015) remained predictive of hard CVD events. However, when both the feeling anxious item (HR = 1.42, 95% CI: 1.08–1.87, p = .014) and worry item (HR = 1.21, 95% CI: 0.92–1.60, p = .17) were forced into the same simultaneous-entry model, only the feeling anxious item continued to predict. Neither item predicted hard CVD events for Years 3+ across models. For fatal/nonfatal MI, the feeling anxious item (ps = .004 and .005), but not the worry item (ps = .47 and .56), was a predictor in both individual and simultaneous-entry Cox models. Figure 1 displays Kaplan-Meier survival curves illustrating the time to hard CVD events (left panel) and fatal/nonfatal MI (right panel) for patients who endorsed the feeling anxious item (positive) versus those who did not (negative). Given that depression screen did not predict any outcome, analyses examining the depression items were not performed.
Figure 1.
Kaplan-Meier survival curves illustrating the time to (A) hard CVD events and (B) fatal or nonfatal MI over the 8-year follow-up period for primary care patients who endorsed the feeling anxious items (positive) versus those who did not (negative). CVD = cardiovascular disease. MI = myocardial infarction.
Supplemental and Sensitivity Analyses
Because the primary analyses indicated that the observed associations were largely driven by the link between the feeling anxious item and fatal/nonfatal MI, supplemental analyses examined this relationship only. In the individual Cox model from which we excluded patients who experienced a MI during the first year of follow-up (n = 76), the feeling anxious item remained a predictor of MI (HR = 1.30, 95% CI: 1.07–1.58, p = .009). Similarly, individual Cox models further adjusted for the PRIME-MD general perceived health item or the two items indicative of sleep disturbance revealed that the feeling anxious item continued to predict MI (perceived health-adjusted: HR = 1.25, 95% CI: 1.04–1.51, p = .021; feeling tired- and trouble sleeping-adjusted: HR = 1.25, 95% CI: 1.03–1.51, p = .025). Finally, individual Cox models testing interactions revealed that feeling anxious item x sex (p = .73), x race (p = .93), and x sex x race interactions (p = .42) were all not significant, indicating that sex and race were not moderators. To illustrate, the feeling anxious-MI hazard ratio was 1.33 for African American women, 1.34 for African American men, 1.22 for Caucasian women, and 1.45 for Caucasian men.
In the cohort for the sensitivity analyses that included depressed patients randomized in the IMPACT trial (N = 2,162; 724 hard CVD events), the rate of positive depression (16.7%) and anxiety (43.9%) screens both increased slightly. Individual Cox models revealed that including these patients did not alter the pattern of results. Anxiety screen (Years 0–3: HR = 1.54, 95% CI: 1.22–1.94, p < .001; Years 3+: HR = 1.00, 95% CI: 0.82–1.21, p = .99), the feeling anxious item (Years 0–3: HR = 1.52, 95% CI: 1.20–1.93, p < .001; Years 3+: HR = 1.08, 95% CI: 0.88–1.34, p = .47), and the worry item (Years 0–3: HR = 1.38, 95% CI: 1.09–1.75, p = .008; Years 3+: HR = 0.94, 95% CI: 0.76–1.15, p = .53) – but not depression screen (HR = 1.10, 95% CI: 0.90–1.33, p = .33) – predicted hard CVD events.
Discussion
Our aim was to simultaneously examine depression and anxiety screens and their individual items as predictors of CVD events. We found that a positive screen for anxiety, but not for depression, predicted incident hard CVD events in a large, diverse sample of older primary care patients initially free of CVD. Specifically, patients who screened positive for anxiety had a 53% greater risk of a MI or stroke during Years 0–3 (but not Years 3+) than those who screened negative – after adjustment for demographic factors, conventional risk factors, and depression status. Analyses examining the individual items and secondary outcomes revealed that the association between a positive anxiety screen and CVD events was largely driven by the feeling anxious item and the incident MI outcome. Supplemental results indicated that the feeling anxious-incident MI relationship did not reflect an impending CVD event leading to anxiety, was not due to confounding by poor overall health or sleep disturbance, and did not vary across sex and racial groups. In total, our results suggest that anxiety, in particular feeling anxious, is a risk factor for CVD in older adults, independent of conventional risk factors and depression. Although our findings conflict with studies reporting that depression predicts incident CVD independent of anxiety (6–8), they are in line with studies reporting that anxiety predicts independent of depression (9–12).
Why was anxiety screen, but not depression screen, a predictor of hard CVD events in our study? One possibility is that anxiety may be a stronger trigger of acute coronary syndromes than depression, perhaps because a positive anxiety screen could indicate the presence of anxiety episodes, such as panic attacks. Along with anger outbursts, anxiety episodes have been identified as psychosocial triggers of acute coronary syndromes in vulnerable adults (23). In the Onset Study, episodes of high anxiety were associated with a 60% increase in the risk of MI in the subsequent two hours (24). Conflicting with this possibility, however, is evidence that episodes of depressed mood may also trigger acute coronary syndromes (25). Psychosocial triggers are thought to promote acute coronary syndromes by bringing about pathophysiologic increases in blood pressure, heart rate, vasoconstriction, and coagulation (26). A second possibility is that our depression measure did not capture the most cardiotoxic aspects of this multidimensional construct. Some evidence suggests that the somatic cluster of depressive symptoms (e.g., fatigue, sleep disturbance, and appetite changes) is a stronger predictor of CVD-related outcomes than the cognitive-affective cluster (e.g., depressed mood and trouble concentrating) (27–29). The PRIME-MD, however, assessed only the affective symptoms of depressed mood and anhedonia. Thus, it is unknown whether depression would have also predicted CVD events had a broader assessment been completed. A third possibility is that our depression screen, due to its moderate sensitivity, did not identify patients with subthreshold symptoms as depression cases. This could have weakened depression effects, given that both depressive disorders and subthreshold elevations in depressive symptoms predict incident CVD (2). Conversely, our anxiety screen likely identified patients with subthreshold symptoms as anxiety cases on account of its higher sensitivity, which could have strengthened anxiety effects. A final possibility is that the patients with the most severe depressive symptoms were randomized in the IMPACT trial and, thus, excluded from our primary cohort. This likely restricted the range of depression severity in our cohort, which could have weakened depression effects. Although sensitivity analyses including the randomized patients did not change the pattern of results, half of these patients received the IMPACT intervention. This could have also weakened depression effects, as we have previously shown that this intervention is associated with a lower risk of hard CVD events (17). In addition to being potential explanations for our findings, some of these possibilities could also explain the inconsistent pattern of results across past studies examining depression and anxiety as simultaneous predictors of CVD outcomes.
A related question is: Why did the feeling anxious item, but not worry item, predict CVD events in our study? We offer three potential explanations, although future research is needed to examine these possibilities. One, the feeling anxious item (“nerves or feeling anxious or on edge”), versus the worry item (“worrying about a lot of different things”), may have been a stronger marker of the presence of the anxiety episodes discussed above, which in turn could trigger acute coronary syndromes. Two, similar to depression, recent findings suggest that the somatic symptoms of anxiety (e.g., palpitation, sweating, and trembling) may be more predictive of CVD events than the cognitive-affective symptoms (e.g., worry and anxious mood) (11). It is plausible that the feeling anxious item primarily captures the somatic symptoms of anxiety, whereas the worry item largely reflects the cognitive-affective symptoms. Three, the feeling anxious item could be marker of pre-existing autonomic dysregulation or poor overall health. However, we reduced the likelihood of such reverse causality by excluding patients who experienced a MI during the first year and by adjusting for general perceived health and sleep disturbance in the supplemental analyses.
Our study has limitations that should be noted. First, we used brief screeners to detect depression and anxiety cases, rather than a structured clinical interview. This certainly led to some misclassification – i.e., patients with subthreshold symptom elevations being classified as cases, particularly when it comes to anxiety. The anxiety screen of PRIME-MD Patient Questionnaire has been found to have higher sensitivity (94%) and lower specificity (53%) (19). This higher sensitivity and lower specificity, however, may not be problematic here and, in fact, may have increased the predictive utility of the anxiety screen, given that subthreshold elevations in anxiety symptoms have been found to predict incident CVD (14). Nonetheless, future studies utilizing clinical interview-based measures are needed to simultaneously examine depressive and anxiety disorders as predictors of incident CVD events. Second, due to the lower number of events, our analyses predicting incident stroke appear to be underpowered. We failed to detect effects that are potentially clinically meaningful and that were similar in magnitude to effects that were significant for the other CVD outcomes. Thus, conclusions involving the stroke outcome should be drawn with caution. Third, our last date of follow-up was in 2008. However, it is doubtful that this influenced our results. Although the CVD death rate has been on the decline (30), this trend is thought to be attributable to improvements in traditional risk factor management and secondary prevention, not the detection and treatment of psychological factors like depression and anxiety. Finally, because our sample consisted of older primary care patients, most of whom were economically disadvantaged, future studies are needed to determine whether our results generalize to younger adults and those of higher socioeconomic status.
In addition to the limitations, it is worth noting our study’s unique methodological strengths. One, our sample of primary care patients with high CVD risk factor burden enhances the clinical relevance of our findings, as CVD risk stratification and primary prevention often occur in this patient population and clinical setting (31). Two, our sample includes high percentages of traditionally underrepresented groups – namely, African Americans and the medically uninsured/underinsured. Three, we identified a high number of incident CVD events using multiple data sources, reducing the likelihood of outcome misclassification and allowing us to predict incident MI and stroke together and separately. Four, our comprehensive analytic approach (i.e., individual, simultaneous-entry, and item-level analyses) provide refined views of the depression-CVD and anxiety-CVD relationships.
In summary, we found that anxiety, especially feeling anxious, is a unique risk factor for hard CVD events in older adults with high CVD risk factor burden, independent of conventional risk factors and depression. Our findings, combined with those of similar studies, indicate that anxiety deserves increased attention as a potential factor relevant to CVD risk stratification and a potential target of CVD primary prevention efforts. For instance, primary care patients with anxiety may be a subpopulation at heightened risk for CVD events for whom earlier and more aggressive primary prevention is warranted. In addition, there is a current need for treatment studies to evaluate whether efficacious anxiety interventions, delivered before the onset of clinical CVD, can decrease CVD risk markers or prevent incident CVD events.
Acknowledgments
Source of Funding: This research was supported by the National Institutes of Health through the National Institute on Aging (C.M.C., AG031222, AG024078).
Some data management tasks were performed by Joseph G. Kesterson, MA, Regenstrief Institute, Inc., Indianapolis, IN.
Acronyms
- CVD
cardiovascular disease
- MI
myocardial infarction
- IMPACT
Improving Mood-Promoting Access to Collaborative Treatment
- ICD-9
International Classification of Diseases, 9th Revision
- ICD-10
International Classification of Diseases, 10th Revision
- CPT
current procedural terminology
- PRIME-MD
Primary Care Evaluation of Mental Disorders
- BMI
body mass index
- HR
hazard ratio
- CI
confidence interval
Footnotes
Conflicts of Interest: None of the authors has any conflicts of interest to declare.
References
- 1.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. International Journal of Geriatric Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 3.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 5.Clarke LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 6.Davidson KW, Mostofsky E, Whang W. Don’t worry, be happy: positive affect and reduced 10-year incident coronary heart disease: the Canadian Nova Scotia Health Survey. Eur Heart J. 2010;31:1065–70. doi: 10.1093/eurheartj/ehp603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mykletun A, Bjerkeset O, Dewey M, Prince M, Overland S, Stewart R. Anxiety, depression, and cause-specific mortality: the HUNT study. Psychosom Med. 2007;69:323–31. doi: 10.1097/PSY.0b013e31803cb862. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker KS, Krantz DS, Rutledge T, Johnson BD, Wawrzyniak AJ, Bittner V, Eastwood JA, Eteiba W, Cornell CE, Pepine CJ, Vido DA, Handberg E, Merz CN. Combining psychosocial data to improve prediction of cardiovascular disease risk factors and events: The National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2012;74:263–70. doi: 10.1097/PSY.0b013e31824a58ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Caminero A, Blumentals WA, Russo LJ, Brown RR, Castilla-Puentes R. Does panic disorder increase the risk of coronary heart disease? A cohort study of a national managed care database. Psychosom Med. 2005;67:688–91. doi: 10.1097/01.psy.0000174169.14227.1f. [DOI] [PubMed] [Google Scholar]
- 10.Kubzansky LD, Cole SR, Kawachi I, Vokonas P, Sparrow D. Shared and unique contributions of anger, anxiety, and depression to coronary heart disease: A prospective study in the normative aging study. Ann Behav Med. 2006;31:21–9. doi: 10.1207/s15324796abm3101_5. [DOI] [PubMed] [Google Scholar]
- 11.Nabi H, Hall M, Koskenvuo M, Singh-Manoux A, Oksanen T, Suominen S, Kivimaki M, Vahtera J. Psychological and somatic symptoms of anxiety and risk of coronary heart disease: the health and social support prospective cohort study. Biol Psychiatry. 2010;67:378–85. doi: 10.1016/j.biopsych.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64:1153–60. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 13.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28:125–30. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston RC, Rewak M, Kubzansky LD. An anxious heart: anxiety and the onset of cardiovascular diseases. Prog Cardiovasc Dis. 2013;55:524–37. doi: 10.1016/j.pcad.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C Treatment IIIM-PAtC. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 16.Sha MC, Callahan CM, Counsell SR, Westmoreland GR, Stump TE, Kroenke K. Physical symptoms as a predictor of health care use and mortality among older adults. Am J Med. 2005;118:301–6. doi: 10.1016/j.amjmed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76:29. doi: 10.1097/PSY.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald CJ, Tierney WM, Overhage JM, Martin DK, Wilson GA. The Regenstrief Medical Record System: 20 years of experience in hospitals, clinics, and neighborhood health centers. MD Computing. 1992;9:206–17. [PubMed] [Google Scholar]
- 19.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 20.Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–89. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, Fauerbach JA, Bush DE, Ziegelstein RC. Prevalence of depression in survivors of acute myocardial infarction. Journal of General Internal Medicine. 2006;21:30–8. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613–21. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 23.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–72. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 24.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–5. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 25.Steptoe A, Strike PC, Perkins-Porras L, McEwan JR, Whitehead DL, Steptoe A, Strike PC, Perkins-Porras L, McEwan JR, Whitehead DL. Acute depressed mood as a trigger of acute coronary syndromes. Biological psychiatry. 2006;60:837–42. doi: 10.1016/j.biopsych.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med. 1999;61:476–87. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med. 2012;74:33–8. doi: 10.1097/PSY.0b013e3182405ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins MA, Callahan CM, Stump TE, Stewart JC. Depressive symptom clusters as predictors of incident coronary artery disease: a 15-year prospective study. Psychosom Med. 2014;76:38–43. doi: 10.1097/PSY.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–33. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 31.van Lieshout J, Wensing M, Campbell SM, Grol R. Primary care strength linked to prevention programs for cardiovascular disease. Am J Manag Care. 2009;15:255–62. [PubMed] [Google Scholar]