SUMMARY
Striatal medium spiny neurons (MSNs) form inhibitory synapses on neighboring striatal neurons through axon collaterals. The functional relevance of this lateral inhibition and its regulation by dopamine remains elusive. We show that synchronized stimulation of collateral transmission from multiple indirect-pathway MSNs (iMSNs) potently inhibits action potentials in direct-pathway MSNs (dMSNs) in the nucleus accumbens. Dopamine D2 receptors (D2Rs) suppress lateral inhibition from iMSNs to disinhibit dMSNs, which are known to facilitate locomotion. Surprisingly, D2R inhibition of synaptic transmission was larger at axon collaterals from iMSNs than their projections to the ventral pallidum. Targeted deletion of D2Rs from iMSNs impaired cocaine’s ability to suppress lateral inhibition and increase locomotion. These impairments were rescued by chemogenetic activation of Gi-signaling in iMSNs. These findings shed light on the functional significance of lateral inhibition between MSNs and offer a novel synaptic mechanism by which dopamine gates locomotion and cocaine exerts its canonical stimulant response.
INTRODUCTION
MSNs form the two main outputs of the striatum: the direct-pathway formed by long-range axonal projections from dMSNs to the internal segment of the globus pallidus and the substantia nigra pars reticulata, and the indirect-pathway formed by long-range axonal projections from iMSNs, but also dMSNs in the NAc region specially, to the globus pallidum/ventral pallidum (Gerfen and Surmeier, 2011; and see also Kupchik et al., 2015). Previous studies provided functional evidence that these two pathways exert opposing effects on basal ganglia mediated behaviors. Activation of the direct-pathway facilitates locomotion, reward and reinforcement, while activation of the indirect-pathway suppresses these behaviors (Freeze et al., 2013; Kravitz et al., 2010; Kravitz et al., 2012; Lobo et al., 2010). It has been assumed that the long-range connectivity is mainly responsible for this functionally dichotomy. However, in addition to the long-range projections, MSNs extend short-range axonal projections within the striatum to form an extensive collateral plexus. The collateral transmission remains an underappreciated aspect of the striatal circuit and its contribution to regulating the behavioral output of the basal ganglia is unclear.
Initial reports of the anatomical evidence for collateral transmission between MSNs in the dorsal striatum and the NAc appeared decades ago (Chang and Kitai, 1986; Pennartz et al., 1991; Preston et al., 1980; Wilson and Groves, 1980). However, the electrophysiological confirmation of functional inhibitory synapses between MSNs has only recently been shown (Lalchandani et al., 2013; Tunstall et al., 2002). Using paired recordings of unidentified MSNs, the rate of connectivity was found to be variable and low (10 – 25%). This is likely explained by the highly asymmetrical connectivity observed between the two subclasses of MSNs (Taverna et al., 2008; Tepper et al., 2004). Each iMSN forms functional synapses with approximately 1/3 of neighboring dMSNs (iMSN→dMSN) and 1/3 of neighboring iMSNs (iMSN→iMSN); while dMSN collateral connectivity is mainly restricted to other dMSNs and rarely observed with iMSNs (Taverna et al., 2008; Tecuapetla et al., 2009).
Further, it was estimated that each iMSN makes only a few GABA synapses (2-5) with their target neurons (Taverna et al., 2008; Tecuapetla et al., 2009). Thus, the functional relevance of this lateral inhibition to striatal output was considered to be minimal, relative to feed-forward inhibition from local interneurons, and has been largely overlooked. Here, we use optogenetic stimulation to synchronously activate the widespread collateral plexus from iMSNs in order to test its effect on the excitability of neighboring dMSNs and its modulation by dopamine (DA) and cocaine.
Cocaine is an addictive stimulant drug that induces transient increases in locomotion in humans and other mammal species (Julien, 2001). The mechanisms that mediate the locomotor response are not well understood, despite this being one the most well-characterized cocaine behavioral response. It is known that cocaine is high affinity blocker of the monoamine transporters, this includes the DA transporter, and that acute administration of cocaine causes a rapid increase in DA concentration in the NAc, which is reliably observed both in vivo and in vitro within minutes following drug administration(Adrover et al., 2014; Fowler et al., 2001; Zombeck et al., 2009). There is solid evidence that the DA increase in the NAc is required for the cocaine-induced locomotion (Kelly and Iversen, 1976; Longo, 1973; Ziegler et al., 1972) but the synaptic mechanisms downstream of DA elevation that mediate this acute behavioral response in the NAc are still under debate.
DA Gi-coupled D2Rs are mainly expressed in iMSNs and are considered critical for cocaine-induced locomotion (Kita et al., 1999; Ushijima et al., 1995). D2R activation in iMSNs produces well-described changes in intracellular signaling, biochemical pathway and gene expression (Girault and Greengard, 2004; Tritsch and Sabatini, 2012; Walker et al., 2015). The electrophysiological consequences of D2R activation, however, are still controversial (Gaval-Cruz et al., 2014; Hernandez-Lopez et al., 2000; Lemos et al., under review; Nicola et al., 1996) in part because MSNs lack the G-protein inward-rectifying potassium (GIRK) channels that are responsible for generating the D2R-mediated currents in other neurons such as midbrain DA neurons (Beckstead et al., 2004). Adding to the complexity is the diverse expression pattern of D2Rs in the striatum, which has limited the interpretation of experiments relying on pharmacology to characterize the effect of D2R activation in iMSNs because D2Rs are also expressed in cholinergic interneurons (Maurice et al., 2004; Zhang et al., 2009), and in presynaptic terminals from midbrain DA neurons (Adrover et al., 2014; Ford, 2014) and corticostriatal glutamatergic neurons (Bamford et al., 2004a; Bamford et al., 2004b). This study takes advantage of a recently developed mouse model with targeted deletion of D2Rs to iMSNs to uncover a novel synaptic mechanism through which cocaine, and dopamine, regulates intrastriatal connectivity to facilitate locomotion.
RESULTS
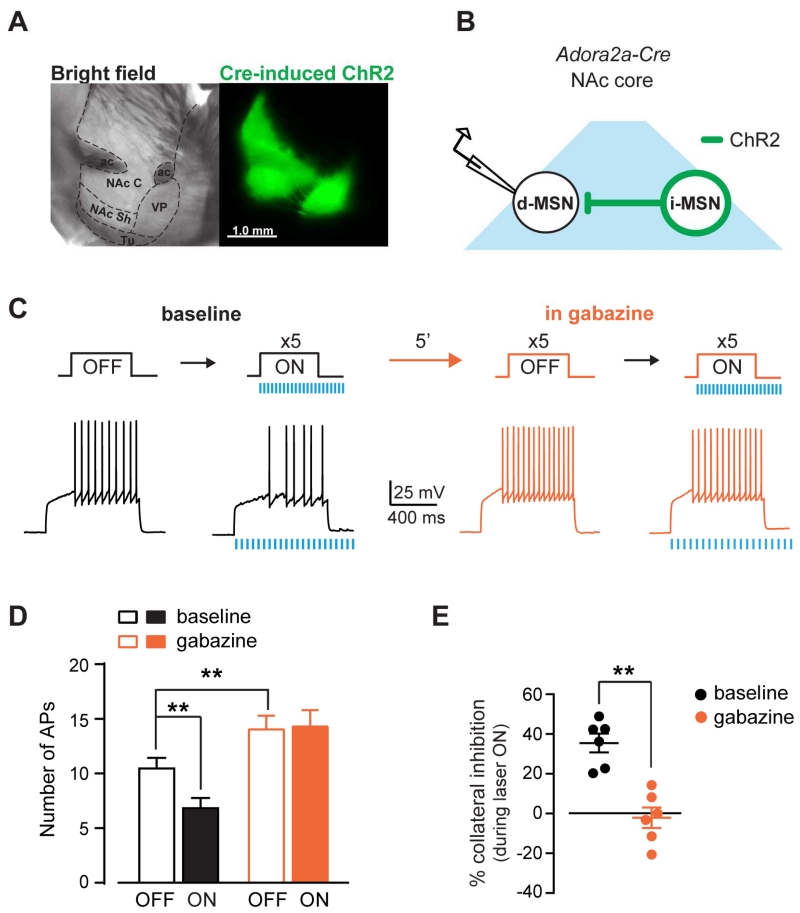
Adora2a-Cre mice were used in this study to target iMSNs. Cre positive neurons in these mice show > 80% co-localization with met-enkephalin, another iMSNs marker and form projection patterns that are consistent with the indirect pathway (Fig. S1; Suppl. Methods) (Lemos et al., under review). Channelrodopsin-2 (ChR2) tagged with a fluorophore was expressed in iMSNs in the NAc using stereotaxic injection of a Cre-dependent viral vector (Fig. 1a). This approach allows for selective stimulation of axonal collaterals from iMSNs within the NAc region in order to evaluate the efficacy of the lateral inhibition in regulating the excitability of neighboring MSNs. Whole-cell current-clamp recordings were performed from MSNs that were negative for ChR2 and are defined here as putative dMSNs (see methods for details). Current steps were delivered to the putative dMSNs to elicit action potentials (AP) in the presence and absence of optogenetic stimulation of collateral transmission (Fig. 1b,c). Before stimulation (laser OFF), each current step (267 ± 18 pA, 800 ms) elicited 10.5 ± 1 APs with a mean latency to fire the first AP of 198 ± 29 ms (n = 6). Optogenetic stimulation (laser ON, 20 pulses of 1-5 ms duration at 16 Hz) applied concurrently with the current step significantly decreased AP firing to 6.8 ± 1 APs (36 ± 5%; 2WRM ANOVA; drug x laser: F1,5 = 21.66, p < 0.01; post-hoc: p < 0.01 ON vs. OFF at baseline) and increased the latency to 239 ± 33 ms (24 ± 8%; 2WRM ANOVA drug x laser: F1,5 = 13.25, p < 0.05; post-hoc: p < 0.05 ON vs. OFF at baseline). Application of the GABAA receptor blocker gabazine (5 μM) increased the number of APs evoked by the current step and decreased the latency during laser OFF, revealing a tonic GABAA mediated-inhibition of AP firing under baseline conditions (APs: from 10.5 ± 1 to 14 ± 1.3; latency: from 198 ± 9 to 157 ± 21 ms after gabazine; post-hoc: APs: p < 0.01; latency: p < 0.05 baseline vs. gabazine during OFF; Fig. 1d). Importantly, gabazine blocked the optical inhibition of AP firing and blocked the increase in latency induced by optogenetic stimulation of iMSNs (post-hoc: APs: p < 0.0001; latency: p < 0.0001 baseline vs. gabazine during ON). The GABAA receptor antagonist abolished the collateral inhibition (from 35.5 ± 4.7% to −2.1 ± 5.2%; paired t-test: t5 = 6.4, p < 0.01; Fig. 1e), demonstrating that GABAergic transmission and GABAA receptor activation are required for the inhibition of dMSN excitability by iMSN stimulation. Altogether, these results show that inhibitory GABAergic collateral transmission by iMSNs can inhibit dMSN excitability.
Figure 1. Synchronized activation of GABAergic collateral transmission from iMSNs inhibits dMSN excitability.
A, Sagittal brain sections of Adora2a-Cre mouse in bright field (left) and fluorescence (right) showing ChR2 expression in NAc core (NAc C) and projections to the ventral pallidum (VP). NAc Sh: nucleus accumbens shell, ac: anterior commissure, Tu: olfactory tubercle. B, Schematic of experimental configuration. C, Top, Depolarizing current steps were applied to dMSNs before or during optogenetic stimulation of iMSNs axon collaterals at baseline and in gabazine. Bottom, Representative AP traces recorded in dMSNs under each condition. D, Number of APs during laser OFF and ON at baseline (black) and after gabazine (5 μM, orange) (n = 6). E, Percent inhibition of APs by opto-stimulation of iMSNs collaterals at baseline and gabazine. **p < 0.01. All data expressed as mean ± s.e.m.
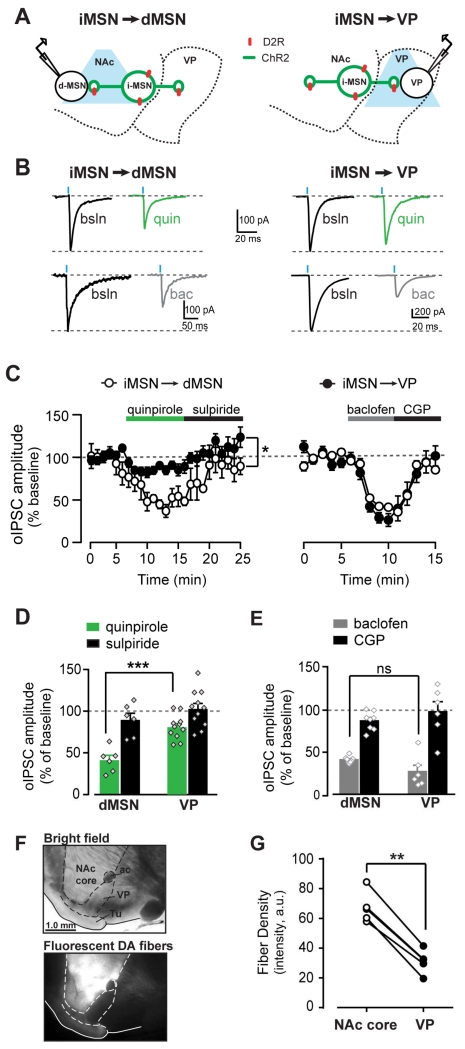
Direct measurement of iMSN→dMSN short-range collateral GABA transmission was performed in voltage-clamp recordings from dMSNs in the NAc core (Fig. 2a). Optogenetic stimulation of iMSNs reliably evoked inhibitory postsynaptic currents (oIPSCs) in neighboring putative dMSNs, which were blocked by 5 μM gabazine (97 ± 0.4% inhibition, n = 37). In agreement with previous reports (Lalchandani et al., 2013; Tecuapetla et al., 2009), the D2-like agonist quinpirole (1 μM) inhibited oIPSC amplitude by 59 ± 6% (n = 6; Fig. 2b-d). Quinpirole showed a trend to increase the paired pulse ratio (baseline: 1.1 ± 0.1, quinpirole: 1.7 ± 0.3; paired t-test, t5 = 2.17, p = 0.08), which was reversed by sulpiride (1.1 ± 0.1), suggesting that quinpirole’s actions are mediated by presynaptic mechanisms that could involve inhibition of GABA release.
Figure 2. Differential quinpirole sensitivity between short-range collateral projections of iMSNs and long-range projections to the VP.
A, Schematic showing ChR2-expressing iMSN (green) and whole-cell recordings from NAc dMSN (left) and VP neuron (right). B, Representative oIPSC traces recorded from dMSNs (left) or VP neurons (right) at baseline (black) or in quinpirole (1 μM, green) or baclofen (1-5 μM, grey). C, Timecourse of oIPSC amplitude recorded from dMSNs in NAc (white, n = 11) or VP neurons (black; n = 7) during quinpirole and sulpiride application (left), or baclofen and CGP application (right). D-E, oIPSC amplitude as percent of baseline when recorded from dMSNs and VP neurons in quinpriole and sulpiride (D), and in baclofen and CGP (E). F, Parasagittal brain section in bright field and fluorescence image showing labeled DA neuron fibers highly concentrated in the NAc core. G, Quantification of fluorescence density in NAc core and VP of individual brain section. *p < 0.05, ***p < 0.001, ns = not significant. All data expressed as mean ± s.e.m.
In parallel experiments under the same conditions we tested the effect of quinpirole on long-range axonal projections to the VP (iMSN→VP synapses). Optogenetic stimulation of iMSN fibers reliably evoked gabazine-sensitive oIPSCs in VP neurons. Quinpirole inhibition was less reliable and smaller in VP neurons compared to dMSNs in the NAc (19 ± 4%, n = 11). Sulpiride reversed the quinpirole inhibition in VP and dMSNs (1 μM; VP: −3 ± 7%; dMSNs: 10 ± 8%). Conversely, the GABAB receptor agonist baclofen (1-5 μM) potently inhibited oIPSC amplitude to a similar extent in dMSNs and VP neurons (62 ± 2% and 73 ± 8% for dMSNs and VP respectively; n = 6-8; 2WRM ANOVA; site x drug: F1,12 = 5.31, p < 0.05; post-hoc: p = NS; Fig. 2c,e). Thus, while modulation by Gi coupled GABAB receptors is similar at both terminals, the magnitude of the D2-like agonist inhibition was significantly greater in short-range collaterals than long-range projections to the VP (2WRM ANOVA; site x drug: F1,15 = 6.33, p < 0.05; post-hoc: p < 0.001, VP vs. dMSNs in quinpirole). These results indicate differential inhibition by a GABAA antagonist and a D2-like receptor on oIPSC amplitude in the VP. While surprising, these results are consistent with the relatively weak DA fiber innervation of the VP compared to the dense innervation of the NAc from midbrain DA neurons (fluorescent fiber density: 67 ± 4.6 and 31 ± 3.5 a.u. for NAc core and VP, respectively; paired t-test: t4 = 13.8, p < 0.001, n = 5; Fig. 2f,g).
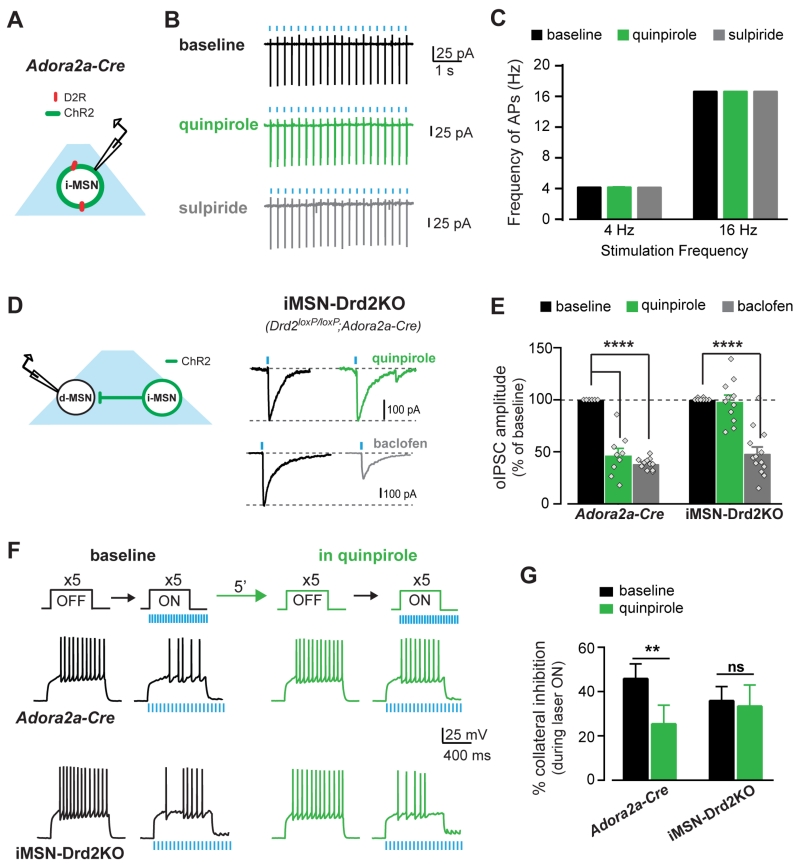
Quinpirole’s effects on the intrinsic excitability of iMSNs could contribute to the inhibition of synaptic transmission and account for its greater efficacy at short-range collaterals vs long-range projections. Using cell-attached recordings and in the presence of synaptic transmission blockers, we tested the effect of quinpirole on the intrinsic excitability of iMSNs by eliciting APs via optogenetic stimulation at different frequencies and under similar conditions as the oIPSC recordings (Fig. 3a). The minimum light pulse duration to elicit 100% fidelity in AP firing was used and delivered at 4 and 16 Hz (4 Hz: 20.1 ± .03 APs; 16 Hz: 20 ± 0 APs, n = 8; Fig. 3a-c). Neither quinpirole (1 μM) nor sulpiride (1 μM) affected AP firing at either frequency (2WRM ANOVA: p’s = NS, n = 5-8), indicating that quinpirole inhibits synaptic collateral GABA transmission downstream of changes in iMSNs excitability.
Figure 3. Quinpirole inhibits collateral GABA transmission to enhance dMSN excitability via D2Rs in iMSNs.
A, Schematic of experimental configuration. B, Traces of cell-attached recordings from iMSNs expressing ChR2 showing high fidelity of AP firing during opto-stimulation at baseline (black) and in quinpirole (1mM, green) followed by sulpiride application (1mM, grey). C, AP firing frequency during trains of light pulses at 4 and 16 Hz under each drug condition (n = 5-8). D, Schematic of experimental configuration and oIPSC traces recorded from dMSNs in iMSN-Drd2KO mice at baseline (black) and during quinpirole (1 μM, green) and baclofen (5 μM, grey) application. E, oIPSC amplitude as percent of baseline during quinpirole and baclofen in control Adora2a-Cre mice (n = 6-11) and iMSN-Drd2KO (n = 8-13). F, Top, Current pulses delivered to dMSNs before and during opto-stimulation of iMSN axon collaterals. Bottom, AP traces recorded from dMSNs in control Adora2a-Cre and iMSN-Drd2KO mice before (OFF) and during (ON) opto-stimulation. G, Percent inhibition of AP firing by activation of iMSN collateral transmission at baseline and in quinpirole for Adora2a-Cre (n = 12) and iMSN-Drd2KO (n = 11). ns = not significant, **p < 0.01, ****p < 0.0001. All data expressed as mean ± s.e.m.
D2-like receptor agonists have poor selectivity between D2Rs and D3Rs, which are both expressed in the NAc. Moreover, due to the complex expression pattern of D2Rs, it is extremely difficult to determine where the D2Rs implicated in this modulation are expressed. To determine whether D2Rs expressed specifically in iMSNs mediate the quinpirole regulation of collateral transmission, we took advantage of a mouse line with a targeted deletion of D2Rs from iMSNs (iMSN-Drd2KO mice Lemos et al., under review). In iMSN-Drd2KO mice, quinpirole did not inhibit oIPSC amplitude (2 ± 6%, n = 11; Fig. 3d,e). This was in contrast to the robust quinpirole-mediated inhibition of oIPSC amplitude in Adora2a-Cre controls (53 ± 7%; n = 9; 2WRM ANOVA; genotype x quinpirole: F1,18 = 30.86, p < 0.0001, post-hoc: Adora2a-Cre: p < 0.0001, iMSN-Drd2KO: p = NS, baseline vs. quinpirole). Conversely, baclofen inhibited oIPSC amplitude by 52 ± 6% in iMSN-Drd2KO mice, which was similar to the baclofen effect Adora2a-Cre mice (n = 13; 2WRM ANOVA; baclofen: F1,22 = 257, p < 0.0001). These experiments demonstrate that the loss of quinpirole inhibition is not due to a general disruption in Gi/o mediated inhibition of GABA release from these terminals and that D2Rs in iMSNs, possibly localized to presynaptic terminals, are required for the suppression of iMSN→dMSN collateral transmission.
We hypothesized that suppression of short range collateral transmission by D2Rs could disinhibit dMSNs and that this effect would be lost in iMSN-Drd2KO mice. These hypotheses were tested using a similar experimental configuration as in Fig. 1. In control Adora2a-Cre mice, optogenetic stimulation of iMSN→dMSN collateral transmission inhibited dMSN firing by 46 ± 7% (OFF: 10.4 ± 0.8 APs; ON: 5.9 ± 0.9 APs, n = 11; 2WRM ANOVA; laser: F1,11 = 30.36, p < 0.001; Fig. 3f,g). In the presence of quinpirole, the optogenetic-mediated inhibition of firing was significantly reduced to 26 ± 8% (paired t-test: t11 = 4.01, p < 0.01; OFF: 9.8 ± 1 APs and ON: 7.4 ± 1.1 APs; n = 12; 2WRM ANOVA; laser x quinpirole: F1,11 = 11.46, p = 0.01; post-hoc: p < 0.05 baseline ON vs. quinpirole ON). Similarly, in iMSN-Drd2KO mice optogenetic stimulation of iMSN→dMSN collateral transmission inhibited dMSN firing (36 ± 6%; OFF: 9.5 ± 1.2 APs; ON: 6 ± 1.1 APs, n = 11; 2WRM ANOVA; ON: F1,10 = 20.52, p = 0.01). In agreement with the lack of a quinpirole effect on oIPSC amplitude, quinpirole also did not affect the optogenetic-mediated inhibition of firing in iMSN-Drd2KO mice (34 ± 10%; paired t-test: p = NS; OFF: 9.5 ± 1.2 APs and ON: 6.3 ± 1.1 APs; n = 11; no effect or interaction; Fig. 3f,g).
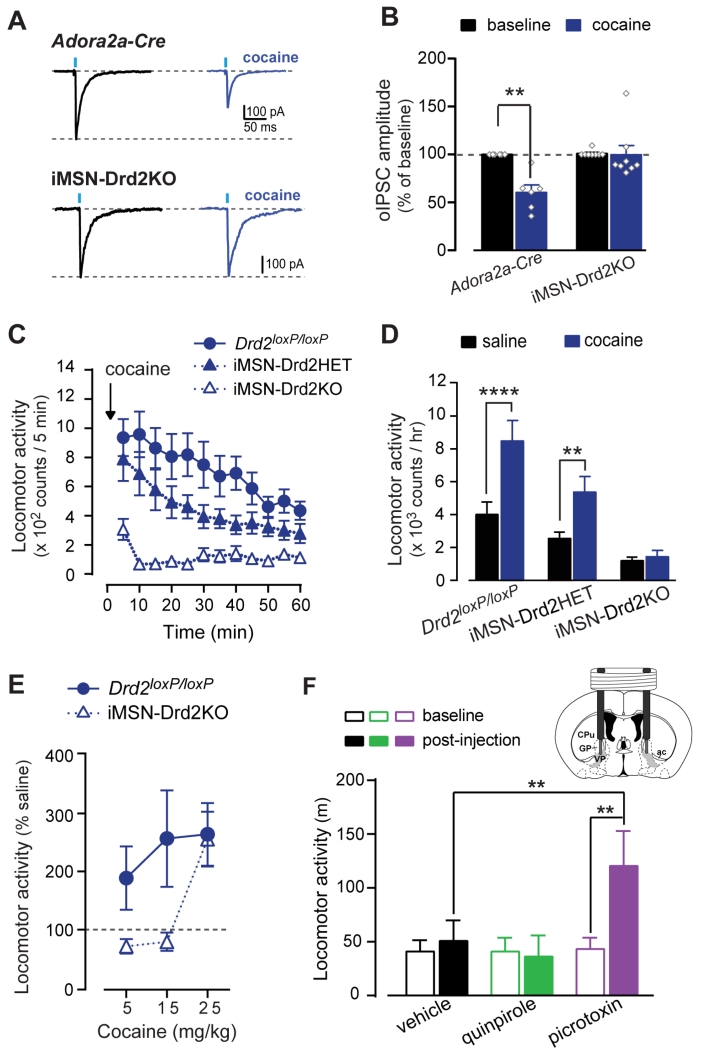
Cocaine increases extracellular DA concentration in the NAc by blocking DA transporters (DAT) (Kiyatkin and Stein, 1995; Rice and Cragg, 2008; Stuber et al., 2005). While this is a very robust, acute effect of cocaine, there are very few reports of other acute cocaine actions in the NAc (Harvey and Lacey, 1996; Nicola et al., 1996). Based on our findings, we hypothesized that cocaine would affect iMSN→dMSN collateral transmission. Indeed, cocaine (10 μM) inhibited oIPSC amplitude recorded in putative dMSNs by 40 ± 8% in control Adora2a-Cre mice (n = 6; 2WRM ANOVA; cocaine x genotype: F1,12 = 10.3, p < 0.01; post-hoc: p < 0.01 baseline vs. cocaine; Fig. 4a,b). Interestingly, cocaine had no effect on oIPSCs in iMSN-Drd2KO mice (0.2 ± 10% inhibition; n = 8; p = NS), indicating that D2Rs in iMSNs mediate the cocaine suppression of iMSN→dMSN collateral transmission.
Figure 4. D2R-mediated inhibition of collateral transmission by cocaine is required for cocaine-induced locomotion.
A, Representative oIPSC traces recorded from dMSNs at baseline and in cocaine in control Adora2a-Cre (top) and iMSN-Drd2KO (bottom). B, Effect of cocaine (10 μM) on oIPSC amplitude (n = 6-8). C, Timecourse of locomotor response to cocaine (15 mg/kg, i.p.) for Drd2loxP/loxP, iMSN-Drd2HET, and iMSN-Drd2KO mice (n = 11, 12, 8, respectively). D, Locomotor activity during the first hour post-saline (black) and post-cocaine (blue) administration. E, Cocaine dose-response in Drd2loxP/loxP (n = 8-14/dose) and iMSN-Drd2KO (n = 7-15/dose). F, Locomotor activity following in vivo bilateral intra-VP microinjection of vehicle (black, n = 5), quinpirole (green, n = 5), or picrotoxin (purple, n = 5). Inset, Schematic of cannula placement for intra-VP microinjections. VP: ventral pallidum (shaded region). ac: anterior commissure; CPu: caudate/putamen; GP: globus pallidus. *p < 0.05, ** p<0.01, **** p< 0.0001. All data expressed as mean ± s.e.m.
In experiments similar to those performed with quinpirole, cocaine suppressed lateral inhibition and enhanced the excitability of dMSNs. Optogenetic stimulation of iMSNs significantly inhibited dMSN baseline firing from 13.2 ± 2.1 to 7.6 ± 1.6 APs and increased the latency to fire the first AP from 182 ± 38 ms to 307 ± 74 ms (n = 6; paired t-test; t5 = 3.27, p < 0.05; Fig. S2). Cocaine had no effect on AP firing when optogenetic stimulation was off (12.9 ± 1.8 APs), negating a possible anesthetic effect on sodium channels, but attenuated the lateral inhibition during optogenetic stimulation of iMSNs from 45 ± 9% to 32 ± 8% (ON: 9.1 ± 1.7 APs; Fig. S2). Cocaine did not affect optogenetic-induced AP firing in iMSNs (baseline: 20 ± 0.05, cocaine: 20 ± 0.01; n = 17; Fig. S3). Thus, while cocaine does not have a significant effect on baseline dMSN excitability, it acutely diminishes the iMSN→dMSN lateral inhibition through a D2R-dependent mechanism (see below).
As expected based on cocaine’s canonical stimulant effect, in vivo administration of cocaine (15 mg/kg, i.p.) produced a robust increase in locomotion in control Drd2loxP/loxP mice (saline: 4014 ± 758 counts/h; cocaine: 8477 ± 1251 counts/h, n = 11; 2WRM ANOVA; genotype x drug: F2,28 = 4.95, p = 0.01; post-hoc: p < 0.0001 saline vs. cocaine; Fig. 4c,d). However, cocaine failed to stimulate locomotion in iMSN-Drd2KO mice (saline: 1200 ± 216 counts/h; cocaine: 1436 ± 382 counts/h, n = 8, post-hoc: p = NS saline vs. cocaine; p < 0.0001 iMSN-Drd2KO vs Drd2loxP/loxP for cocaine). Heterozygote iMSN-Drd2HET mice showed an intermediate phenotype, indicating that the impairment was dependent on the Drd2 gene dosage (saline: 2545 ± 396 counts/h; cocaine: 5367 ± 951 counts/h; n = 12, post-hoc: p < 0.01 saline vs. cocaine; p < 0.05 iMSN-Drd2HET vs Drd2loxP/loxP for cocaine). There was a rightward shift in the cocaine-induced locomotion dose response in iMSN-Drd2KO mice, indicating that mice lacking D2Rs in iMSNs are less sensitive to the psychomotor activating properties of cocaine (2W ANOVA; genotype: F1,53 = 4.62, p < 0.05; Fig. 4e). Locomotor activity after 5 and 15 mg/kg cocaine was 71 ± 13% and 79 ± 16% of saline in iMSN-Drd2KO mice compared to 189 ± 55% and 257 ± 83% of saline in Drd2loxP/loxP littermates, respectively (n = 7-8). However, 25 mg/kg cocaine increased locomotion in iMSN-Drd2KO mice to a similar extent as seen in Drd2loxP/loxP mice (256 ± 47% vs. 264 ± 54% of saline, respectively; 2W ANOVA; dose: F2,53 = 3.25, p < 0.05, n = 14-15). Thus, while iMSN-Drd2KO mice are less sensitive to the psychomotor activating properties of cocaine, they show enhanced locomotion to a high cocaine dose.
Long-range iMSN→VP projections are known to be important for the behavioral responses to cocaine, and blocking GABA transmission in the VP is sufficient to induce locomotion in rats (Austin and Kalivas, 1989, 1991). Indeed, we found that intra-VP administration of the GABAA receptor antagonist picrotoxin (500 μM) robustly increased locomotion in control Adora2a-Cre mice (baseline: 43 ± 11 m/30 min, post-injection: 120 ± 33 m/h; 2WRM ANOVA: F2,8 = 6.84, p < 0.05; post-hoc: p’s < 0.01, baseline vs. post-injection and picrotoxin vs. vehicle at post-injection, n = 5/group; Fig 4f). Conversely, intra-VP quinpirole (4 mM) did not increase locomotion compared to vehicle (baseline: 41 ± 10 and 41 ± 13 m/30 min, post-injection: 51 ± 19 and 36 ± 20 m/h, for vehicle and quinpirole, respectively; post-hoc: p’s = NS, quinpirole vs vehicle and baseline vs. post-injection for quinpirole or vehicle). In contrast, it is important to note that intra-NAc quinpirole was sufficient to enhance locomotion in mice (Abrahao et al., 2012). Taken together, our results confirm that iMSN→VP projections contribute to locomotion but that the DA modulation of these projections is weak, thus suggesting a more prominent role for D2Rs in regulating short-range collateral transmission and locomotion.
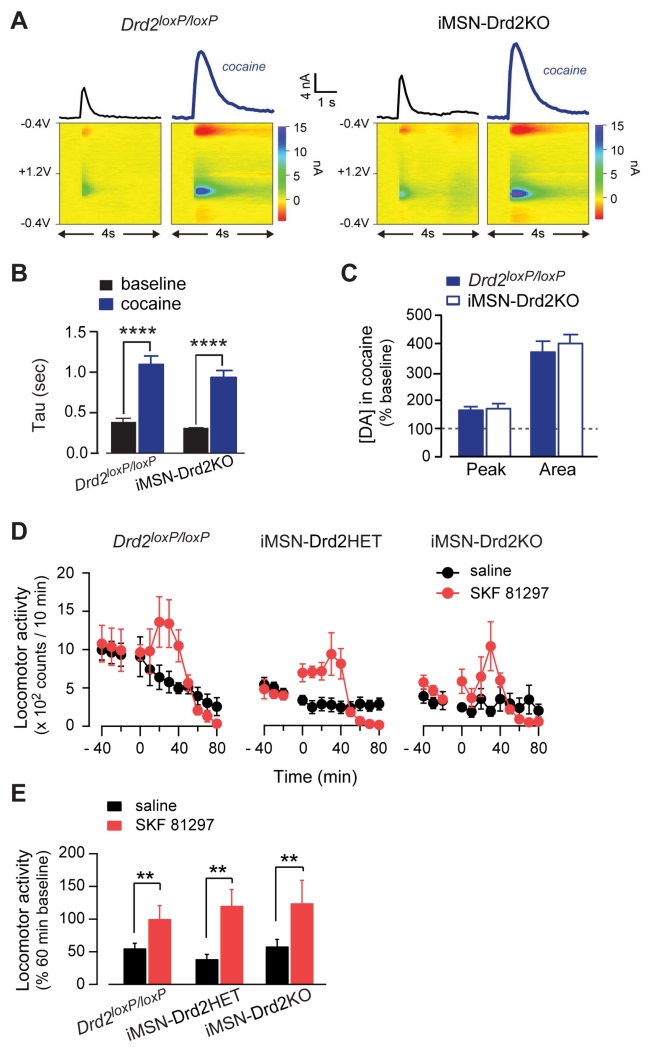
iMSN-Drd2KO mice display reduced basal locomotion (Lemos et al., under review), a motor phenotype that is in agreement with previous reports from global Drd2-KO mice (Baik et al., 1995; Chausmer et al., 2002) and other models of striatal D2R deletion (Anzalone et al., 2012). Since impaired cocaine action on DA transmission could be responsible for the blunted locomotor response seen in iMSN-Drd2KO mice, we examined DA transmission and the effect of cocaine on DA transients. Electrically evoked DA transients were recorded in the NAc core and shell using fast-scanning cyclic voltammetry in brain slices from iMSN-Drd2KO and Drd2loxP/loxP mice. Cocaine prolonged DA transients and increased the tau similarly in both genotypes (952 ± 69 ms and 1114 ± 93 ms for iMSN-Drd2KO and Drd2loxP/loxP, respectively; n = 7-8; 2WRM ANOVA; cocaine: F1,13 = 183.8, p < 0.001; Fig. 5a,b). Cocaine also increased the peak and area of evoked DA transients similarly in both genotypes (peak = 172 ± 16% vs. 166 ± 11%; area = 402 ± 30% vs. 372 ± 37% of baseline for iMSN-Drd2KO and Drd2loxP/loxP, respectively; unpaired t-tests: p’s = NS, n = 7-8; Fig. 5c). These data indicate that cocaine enhances DA levels in the NAc of iMSN-Drd2KO mice and thus rule out altered DA synthesis or release, or impaired cocaine-induced enhancement of extracellular DA levels as potential mechanisms for the blunted locomotor response in iMSN-Drd2KO mice.
Figure 5. No change in cocaine effect on DA signals and the locomotor response to a D1-like agonist in iMSN-Drd2KO mice.
A, DA concentration transients (top) and color plots (bottom) before and after cocaine (bold) for Drd2loxP/loxP (left) and iMSN-Drd2KO mice (right). B-C, Effect of cocaine on the decay time constant Tau (B), and the peak and area (C) of DA signals in Drd2loxP/loxP (n = 8-3) and iMSN-Drd2KO (n = 7-3) mice. D, Timecourse of the locomotor response to the D1-like agonist SKF81297 (7.5 mg/kg, red). E, Locomotor activity during first hour after saline or SKF81297 administration expressed as percent of the activity over 1 hour before injection. **p< 0.01, ****p < 0.0001. All data expressed as mean ± s.e.m.
We also wondered whether the motor deficit observed in iMSN-Drd2KO mice could interfere with the ability of DA to activate locomotion in general. We therefore tested the effect of the D1-like receptor agonist SKF81297 on locomotion and found that iMSN-Drd2HET and iMSN-Drd2KO mice show normal locomotor activation compared to littermate controls (121 ± 25%, 125 ± 35% and 101 ± 20% of pre-drug baseline, respectively, n = 4/genotype; 2WRM ANOVA; drug: F1,9 = 17.04, p < 0.01; Fig. 5d,e). Thus, despite depressed basal locomotion, mice with reduced D2Rs in iMSNs respond to dopaminergic drugs with an increase in locomotion.
Substantial evidence implicates the NAc in the behavioral response to cocaine (Di Chiara and Imperato, 1988; Phillips et al., 2003; Taylor and Robbins, 1986; Wise and Bozarth, 1987). To investigate the role of D2Rs specifically in this region, Drd2 was deleted in a spatially- and temporally-restricted fashion in Drd2loxP/loxP mice by expressing Cre bilaterally in the NAc. This resulted in an 80 ± 11% reduction of Drd2 mRNA levels in the NAc 3 weeks after surgery (Lemos et al., under review). Note that this deletion is not specific for iMSNs; however, similar to iMSN-Drd2KO mice, NAc Drd2 knockdown (NAcDrd2KD) mice displayed a blunted locomotor response to acute cocaine compared to control EGFP-expressing mice (NAcDrd2KD: 1603 ± 489, 1759 ± 497 saline vs. cocaine, p = NS; EGFP: 4247 ± 695, 7678 ± 1426 saline vs. cocaine, p < 0.001; n = 10-12; Fig. S4). These results demonstrate that a NAc-specific reduction of D2Rs is sufficient to block the cocaine locomotor response and that D2Rs in the NAc are required for acute cocaine locomotion.
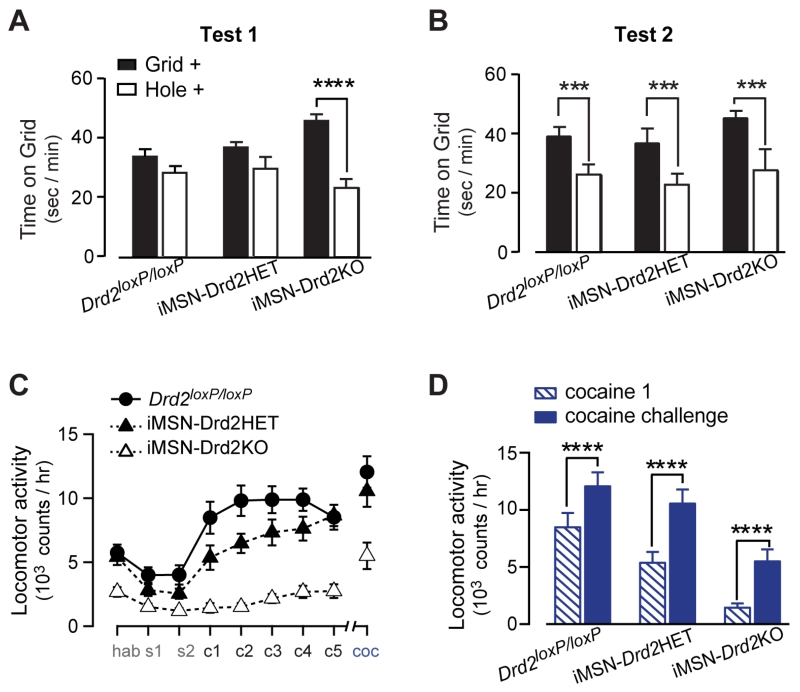
The rewarding properties of cocaine were tested in iMSN-Drd2KO mice using conditioned place preference. Each genotype spent equal time on the two floor types prior to conditioning, indicating no pre-conditioning preference (Fig. S5). iMSN-Drd2KO mice expressed cocaine place preference after 4 conditioning sessions (test1), indicated by significantly more time spent on the grid floor in the Grid+ than the Hole+ conditioning groups (Grid+ 46 ± 2 vs. Hole+ 23 ± 3 sec/min on grid floor; 2W ANOVA; genotype x conditioning: F2,23 = 6.54, p < 0.01; post-hoc: p < 0.0001 Grid+ vs. Hole+; Fig. 6a). Conversely, Drd2loxP/loxP and iMSN-Drd2HET mice did not show a preference at test 1 (Grid+ 34 ± 3 vs. Hole+ 28 ± 2 sec/min on grid floor; Grid+ 37 ± 2 vs. Hole+ 30 ± 4 sec/min on grid floor, respectively; post-hoc: p = NS Grid+ vs. Hole+). Following all 8 conditioning sessions (test 2), all genotypes showed a significant cocaine preference (Grid+ 39 ± 3 vs. Hole+ 26 ± 3 sec/min on grid floor; Grid+ 37 ± 5 vs. Hole+ 23 ± 4 sec/min on grid floor; Grid+ 45 ± 3 vs. Hole+ 28 ± 7 sec/min on grid floor Drd2loxP/loxP and iMSN-Drd2HET and iMSN-Drd2KO, respectively; 2W ANOVA; no genotype effect or interaction; conditioning: F1,23 = 16.48, p < 0.001; Fig 6b). Additionally, all genotypes showed a significant cocaine preference when tested in the presence of cocaine (Fig. S5). These results suggest that, despite having an impaired locomotor response to cocaine, the conditioned rewarding properties of cocaine are intact in mice lacking D2Rs in iMSNs.
Figure 6. Cocaine conditioned reward and locomotor sensitization are intact in iMSN-Drd2KO mice.
A-B, Time spend on Grid floor for animals conditioned in Grid (filled) and Hole (open) floors during test 1 (A) and test 2 (B) of cocaine conditioned place preference (n = 11, 7, 11 mice). C, Locomotor activity during habituation, saline, repeated cocaine administration, and cocaine challenge (n = 11, 12, 8). D, Locomotor activity during the first cocaine administration and during cocaine challenge for each genotype. ***p < 0.001 (b), ****p < 0.0001. All data expressed as mean ± s.e.m.
iMSN-Drd2KO mice had blunted locomotion during conditioning similar to what was shown in Figure 4 (Fig. S5). However, the locomotor response during the cocaine-primed preference test for iMSN-Drd2KO mice was similar to controls. Further, activity was not correlated with percent time on the cocaine-paired floor (p = 0.16, not shown), and none of the genotypes expressed a preference to low dose cocaine (0.5 mg/kg; Fig. S6). These data suggest that blunted locomotor activity was likely not driving place preference.
We further probed the cocaine locomotor response with a cocaine locomotor sensitization protocol (Fig. 6c). All genotypes displayed a sensitized locomotor response to cocaine challenge 2 weeks after repeated cocaine administration (2WRM ANOVA; day x genotype: F16,224 = 4.11, p < 0.0001; post-hoc: p’s < 0.001 cocaine 1 vs. cocaine challenge; Fig. 6d). Mice with a NAc-selective deletion of D2Rs (NAcDrd2KD) did not show cocaine locomotor sensitization (Fig. S4), suggesting that developmental factors or the extended time of the D2R deletion likely contribute to the expression of sensitization. These data indicate that D2Rs in iMSNs are not necessary for the behavioral plasticity and learning induced by repeated cocaine exposures, as measured by cocaine place preference and locomotor sensitization.
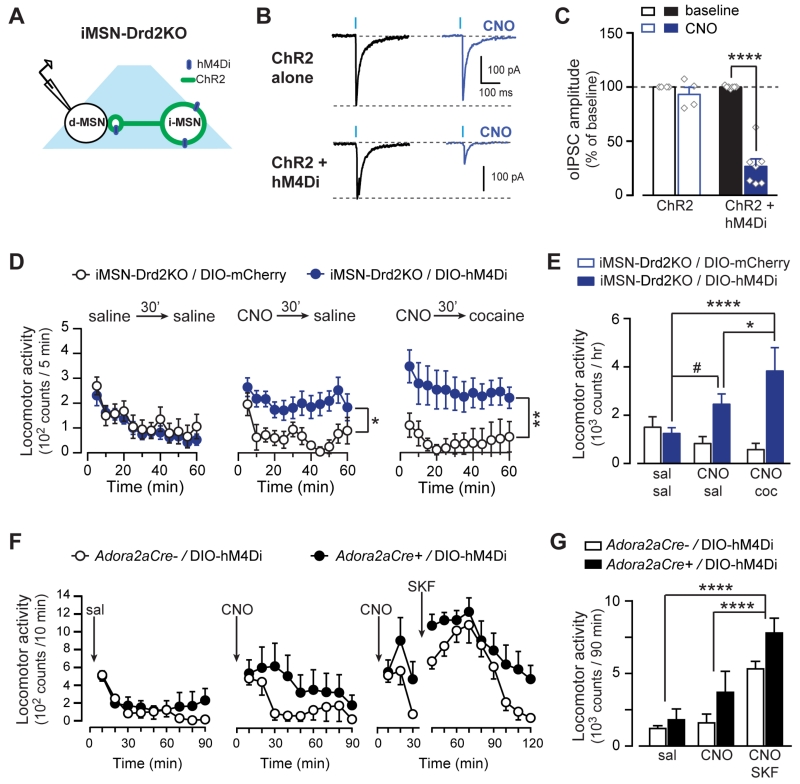
Thus far the data indicate that D2Rs in the NAc are necessary for the acute cocaine locomotor response, but not for locomotor sensitization or place preference following repeated cocaine administration; however, it is still unclear whether activation of D2Rs in iMSNs is sufficient to restore cocaine-induced locomotion in iMSN-Drd2KO mice. To this end, the recombinant Gi coupled DREADD hM4Di was targeted to iMSNs in the NAc core of iMSN-Drd2KO mice (Fig. 7a). Bath application of the synthetic agonist clozapine-N-oxide (CNO, 1 μM) inhibited oIPSCs by 73 ± 7% in neighboring putative dMSNs from iMSN-Drd2KO mice expressing hM4Di+ChR2, but had no effect in mice expressing ChR2 alone (7 ± 7%; 2WRM ANOVA; hM4Di x CNO: F1,9 = 40.04, p < 0.001, n = 4-7; Fig. 7b,c). Similar to the experiment presented in Figure 3, CNO had no effect on optogenetic-induced (16 Hz) AP firing of iMSNs expressing hM4Di (baseline: 19.3 ± 1.5, CNO: 20.3 ± 0.7; n = 6; Fig. S3). Thus, activation of Gi signaling in iMSNs rescued the inhibition of iMSN→dMSN collateral transmission in iMSN-Drd2KO mice without affecting the iMSN excitability. Locomotor activity was then assessed in iMSN-Drd2KO mice expressing hM4Di or control mCherry in NAc iMSNs. Locomotor activity was indistinguishable between these two groups after pretreatment with vehicle (saline, 30 min) followed by saline administration (1244 ± 240 vs. 1503 ± 432 counts/h for hM4Di and mCherry controls respectively; 2WRM ANOVA; time: F11,253 = 7.97, p < 0.0001; n = 9-16; Fig. 7d). Pretreatment with CNO (1 mg/kg, 30 min), increased locomotion in iMSN-Drd2KO-hM4Di mice compared to control iMSN-Drd2KO-mCherry mice (2451 ± 431 vs. 829 ± 288 counts/h; 2WRM ANOVA; vector: F1,23 = 6.90, p < 0.05; time: F11,253 = 2.63, p < 0.05). Importantly, pretreatment with CNO followed by cocaine (15 mg/kg, i.p.) further enhanced locomotion compared to saline/saline and CNO/saline treatment in iMSN-Drd2KO-hM4Di but not iMSN-Drd2KO-mCherry mice (3825 ± 974 vs. 580 ± 260 counts/h; 2WRM ANOVA; day x vector: F2,46 = 7.64, p < 0.01; post-hoc: p < 0.0001 CNO/cocaine vs. saline/saline; p < 0.05 CNO/cocaine vs. CNO/saline for iMSN-Drd2KO-hM4Di; Fig. 7e). Thus, activation of Gi-signaling in NAc iMSNs is sufficient to induce a locomotor response and, importantly, it rescued the cocaine locomotor response in iMSN-Drd2KO-hM4Di mice. We speculate that activation of Gi signaling in iMSNs gates cocaine-induced locomotion by suppressing the collateral inhibition onto neighboring D1R-containing dMSNs, which then permits cocaine-induce activation of D1Rs on dMSNs. To test this hypothesis, hM4Di was targeted to iMSNs in the NAc core of Adora2a-Cre+ or control Adora2a-Cre- mice and locomotion was examined following administration of CNO alone or CNO + SKF81297. Following saline, locomotion was similar between Adora2a-Cre+ or Adora2a-Cre- mice (1812 ± 750 vs. 1212 ± 191 counts/90 min, respectively; 2WRM ANOVA; time: F8,80 = 7.858, p < 0.0001, n = 6/genotype; Fig. 7f). Administration of CNO (3 mg/kg) induced a modest increase in locomotion in Adora2a-Cre+ mice relative to Adora2a-Cre- mice (3707 ± 1451 vs. 1599 ± 605 counts/90 min, respectively; 2WRM ANOVA; time: F8,80 = 2.95, p < 0.01). This modest effect of CNO in Adora2a-Cre mice compared to iMSN-Drd2KO mice is possibly due to the endogenous activation of D2Rs occluding the hM4Di effect. Interestingly, CNO + SKF81297 further increased locomotion in Adora2a-Cre+ relative to Adora2a-Cre- mice (7794 ± 1022 vs. 5317 ± 521 counts/90 min, respectively; 2WRM ANOVA; drug: F2,20 = 40.68, p < 0.0001; post-hoc: p < 0.0001 vs. saline and CNO). Thus, CNO and a D1-like receptor agonist showed an additive effect, similar to CNO and cocaine in iMSN-Drd2KO mice.
Figure 7. Activation of Gi/o signaling in iMSNs rescues the acute cocaine locomotor response.
A, Schematic of experimental configuration. B, Representative oIPSC traces and the effect of CNO in iMSN-Drd2KO mice expressing ChR2 alone or ChR2 + hM4Di in iMSNs. C, Effect of CNO (1 μM) on oIPSC amplitude as percent of baseline in iMSN-Drd2KO mice expressing ChR2 (open, n = 7) or ChR2 + hM4Di (filled, n = 5). D, Time course of the locomotor activity following saline (left), CNO (1 mg/kg, middle) or CNO followed by cocaine (15 mg/kg, right). E, Locomotion during 1 hour after sal/sal, CNO/sal, and CNO/cocaine administration for iMSN-Drd2KO mice expressing hM4Di (blue) or mCherry (white) in iMSNs. F, Time course of the locomotor activity following saline (left), CNO (1 mg/kg, middle) or CNO followed by SKF81297 (2.5 mg/kg, right). E, Locomotion during 1 hour after saline, CNO, and CNO+ SKF81297 administration for control mice expressing hM4Di (black) or not (white). *p < 0.05, **p < 0.01. ****p < 0.0001, #p = 0.058. All data expressed as mean ± s.e.m.
DISCUSSION
This study offers important insight into the functional relevance of lateral inhibition between MSNs by showing it can regulate MSN excitability and contribute to the behavioral response to cocaine. Synchronous activation of axon collaterals from iMSNs reliably produced a large inhibitory postsynaptic response in the vast majority (> 90%) of neighboring putative dMSNs and was sufficient to inhibit dMSN AP firing. These results suggest a significant degree of conversion of synaptic inputs from multiple iMSNs onto each dMSN, which could not have been predicted from previous studies that demonstrated only a small number of synaptic contacts between each MSN pair (Taverna et al., 2008; Tecuapetla et al., 2009). Further, our findings suggest that synchronized activation of multiple iMSNs can exert powerful inhibition of dMSN excitability in vivo and restrain direct-pathway output.
The study demonstrates that activation of D2Rs expressed in iMSNs potently inhibits synaptic transmission from these neurons onto neighboring MSNs. By regulating the lateral inhibition, D2R activation disinhibits dMSN firing and could facilitate direct-pathway output. These findings are very relevant because they show a reliable electrophysiological signal in response to activation of striatal D2Rs expressed in iMSNs, which lack inward-rectifying potassium channels responsible for mediating currents following D2R activation in other neurons, such as midbrain DA neurons (Beckstead et al., 2004). Interestingly, while a D2-like receptor agonist prominently suppressed short-range iMSN→dMSN collateral transmission, it had a minimal effect on long-range GABA transmission to the VP. This differential efficacy of the D2R agonist is supported by the anatomical evidence showing a weaker DA fiber innervation of the VP compared to the NAc. Furthermore, intra-VP administration of a D2-like receptor agonist did not affect locomotion, while blocking GABA transmission to the VP strongly potentiated locomotion, in agreement with previous reports (Austin and Kalivas, 1989, 1991). Thus, while these long-range projections to the VP can regulate striatal output and locomotion, the regulation of these projections by D2Rs is minor compared to its modulation of axon collaterals in the NAc.
The exact mechanism for the differential effect of D2-like agonist remains unclear but we speculate that differences in the efficiency of D2R coupling to signaling transduction pathway and/or expression levels of D2Rs between the two axonal projections could be responsible. Another possibility is that one subpopulation of iMSNs forms only local collateral synapses within the NAc and a different subpopulation forms mainly long-range projections. While possible, the anatomical studies that currently exist do not support this idea and in fact indicate that MSNs in the dorsal striatum, while differing in their axon projection patterns, all form short-range collateral connections before proceeding to form long-range projections to the globus pallidus/entopenduncular nucleus/substantia nigra (Kawaguchi et al., 1990; Preston et al., 1980; Wilson and Groves, 1980).
Cocaine suppressed iMSN→dMSN collateral transmission through activation of D2Rs in iMSNs and facilitated dMSN firing only when iMSN collateral transmission was stimulated. It had no effect on baseline dMSN excitability in this preparation, which possibly explains the lack of previous reports of this cocaine effect in the NAc. In brain slice preparations, the contribution of iMSN→dMSN collateral transmission to the overall spontaneous GABAergic transmission is expected to be negligible due to the hyperpolarized resting membrane potential and the absence of AP firing in iMSNs. In contrast, gabazine had a potent effect on dMSN excitability under baseline conditions, revealing a tonic level of GABAA-mediated inhibition of dMSNs in the slice preparation. We speculate that GABA release from striatal interneurons (likely the spontaneously active interneurons) largely contributes to the tonic GABAergic-mediated inhibition and is insensitive to DA or cocaine. In vivo, however, when iMSN activity is higher (Freeze et al., 2013; Gremel and Costa, 2013; Tecuapetla et al., 2014), in part due to active glutamatergic afferents, GABAergic collateral transmission from iMSNs is expected to be engaged and play a role in inhibiting dMSN activity. Thus, under conditions in which collaterals are active, cocaine-induced DA elevation and subsequent activation of D2Rs causes a suppression of lateral inhibition and disinhibition of dMSNs.
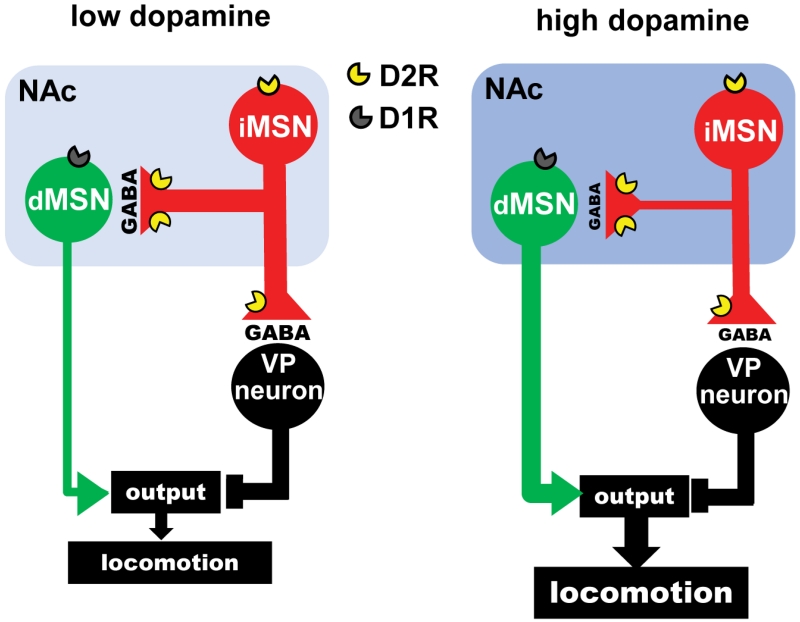
Cocaine, by elevating DA levels in the striatum, also activates Gs/olf-coupled D1Rs expressed in dMSNs, which are known to contribute to cocaine psychomotor activation and reinforcement (Freeze et al., 2013; Gore and Zweifel, 2013; Kita et al., 1999; Kravitz et al., 2010; Kravitz et al., 2012; Pierce et al., 1996; Ushijima et al., 1995). We speculate that cocaine induces a synchronized activation of D1Rs and D2Rs which contributes to increased output of the direct-pathway. This idea is supported by the results of Figure 7f that shows an additive effect of D1R activation and Gi signaling in iMSNs. Further, in mice lacking D2Rs in iMSNs, the impaired locomotor response to cocaine was recovered by Gi-DREADD activation, which by itself had a smaller effect. Based on these findings we propose that suppression of the lateral inhibition between iMSNs and dMSNs contributes to gating the stimulatory behavioral response to acute cocaine administration (Fig. 8).
Figure 8.
Model: Lateral inhibition can control the firing of dMSNs. Dopamine D2Rs in iMSNs suppress the lateral inhibition to gate basal ganglia output and locomotion. Left, Under conditions of low DA levels in the NAc (light blue), a strong GABAergic collateral transmission from indirect-pathway MSNs (iMSNs) restrains the excitability of direct-pathway MSNs (dMSNs) and reduces locomotion. Right, When DA levels are high (dark blue), such as when cocaine is present, activation of D2Rs localized in iMSNs suppresses GABA release from iMSNs to disinhibit dMSNs. DA/cocaine facilitates direct pathway output via two independent mechanisms: 1) indirectly, via D2Rs in iMSNs that caused disinhibition, and 2) directly, via activation of D1Rs in dMSNs.
We also showed that D2Rs in iMSNs in the NAc are required for the cocaine locomotor response. This conclusion is also supported by another study (Anzalone et al., 2012); however, this study did not provide a mechanism to account for lack of cocaine locomotor response. Our findings significantly add to the field by providing a novel understanding of how cocaine and DA regulate intra-striatal connectivity to suppress lateral inhibition and regulate direct-pathway output. Furthermore, by inducing D2R deletion in adulthood, our study shows that the requirement of D2Rs for cocaine-induced locomotion is not due to D2R regulation of striatal circuitry during development.
Activation of Gi signaling in iMSNs specifically within the NAc rescued the cocaine locomotor response in mice lacking D2Rs in iMSNs, further demonstrating that accumbal D2R activation is critical for this stimulant response to cocaine. Moreover, iMSN-Drd2KO mice exhibit reduced locomotion (Lemos et al., under review), similar to global D2R knockout mice (Baik et al., 1995; Chausmer et al., 2002), and activation of Gi signaling in iMSNs was sufficient to restore locomotion close to levels of control Drd2loxP/loxP mice, revealing its importance in mediating locomotion. Importantly, pairing activation of Gi signaling in iMSNs with cocaine or D1-like agonist further increased locomotion, suggesting that D1R activation by cocaine also contributes to cocaine-induced locomotion. We interpret these results as evidence that cocaine, by enhancing DA activation of D2Rs in iMSNs, suppresses lateral inhibition to enable or gate dMSN activation, which is driven by DA activation of D1Rs. These findings also provide a mechanism for the previous observation of a synergistic effect following co-administration of D1R and D2R agonists in the striatum (Gerfen et al., 1995).
iMSN-Drd2KO mice showed cocaine locomotor sensitization, and cocaine place preference. It is likely that the potentiation of cortical glutamatergic inputs onto dMSNs that take place following repeated cocaine administration can act to overcome the lateral inhibition from iMSNs and facilitate cocaine seeking and cocaine locomotor sensitization.
Taken together, D2Rs are required for the acute cocaine locomotor response in large part because of the D2R-mediated suppression of lateral inhibition between MSNs within the NAc. On the other hand, the suppression of long-range projections in the VP, a mechanism already known to affect locomotion via GABA and opioid receptors (Kupchik et al., 2014; Stefanik et al., 2013; Wang et al., 2004), is only weakly modulated by D2Rs. Our findings offer a novel mechanism by which cocaine mediates the canonical behavioral response, which involves relieving lateral inhibition via D2R activation from iMSNs to disinhibit dMSNs in the NAc and promote locomotion.
EXPERIMENTAL PROCEDURES
Animals
Experiments were performed in accordance with guidelines from the National Institute on Alcohol Abuse and Alcoholism’s Animal Care and Use Committee. Heterozygote Adora2a-Cre mice (B6.FVB(Cg)-Tg(Adora2a-Cre)KG139Gsat/Mmucd, GENSAT, 036158-UCD) (Gerfen et al., 2013) were used to target transgenes to iMSNs. iMSN-Drd2KO and iMSN-Drd2HET mice are cell-specific D2R knockout and heterozygote mice, respectively, and were generated by crossing Drd2loxP/loxP mice (B6.129S4(FVB)-Drd2tm1.1Mrub/J, JAX020631) with Adora2a-Cre mice (details in Suppl. Methods). To quantify DA neuron projections in NAc and VP, DATiRES-Cre mice (B6.SJL-Slc6a3tm1.1(cre)Bkmn/J, JAX006660) (Backman et al., 2006) were crossed with Ai80D mice (B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J; JAX025109). Mice were randomized and counterbalanced for sex and treatment and group housed (unless otherwise stated) under a 12h:12h light cycle (6:30 ON/18:30 OFF) with food and water available ad libitum.
Stereotaxic surgeries
Adult mice (6-8 weeks) were placed in the stereotax and kept under isoflurane anesthesia while viral vectors were infused (250-400 nl/side; 100 nl/min) in the NAc core (from bregma: AP, +1.3 - +1.1; ML, ±1.0; DV, −4.7 - −4.5 mm). Detailed information on all vectors used can be found in Suppl Methods. For intra-VP microinjections, bilateral cannula (Plastics One) were implanted above the VP (AP, −0.1; ML, ± 1.5; DV, −3.6 mm) and dummy stylets placed when cannula not used. Experiments were performed at least 3 weeks after surgery. Viral expression was confirmed by fluorescence and/or immunohistochemistry. Subjects with no viral expression were excluded from data analysis.
Electrophysiology
Sagittal slices (240 μm) were prepared from 10 ± 1 week old mice in cold cutting solution (in mM: 225 sucrose, 119 NaCl, 2.5 KCl, 0.1 CaCl2, 4.9 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, 1.25 glucose, and 3 kynurenic acid; (Dobi et al., 2011). During recording, slices were maintained at 31 – 33 °C and perfused with oxygenated aCSF (in mM: 124 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, and 20 glucose). Whole cell recordings were performed from MSNs in the NAc core with glass electrodes (2.5-3.5 MΩ) filled with K+-based internal solution for current-clamp (in mM: 120 KMeSO4, 20 KCl, 10 HEPES, 0.2 K-EGTA, 2 MgCl2, 4 Na-ATP, and 0.4 Na-GTP; pH = 7.25, ~290 mOsm) and with CsCl internal solution for voltage-clamp experiments (in mM: 120 CsCl, 10 HEPES, 0.2 EGTA, 4 Na-ATP, 0.4 Na-GTP, and 10 phosphocreatine, pH = 7.24, ~290 mOsm). Recordings were restricted to fluorescent negative MSNs (putative dMSNs) that lacked ChR2 current (estimated error ~ 16%). Cells with Ri > 500 MΩ, Rm > −75 mV, or spontaneously active were excluded. When required, current steps (200-400 pA, 800 ms) were delivered to elicit 10 – 15 APs in the presence or absence of optogenetic stimulation. The following synaptic blockers were used to isolate GABAergic responses: 5 μM NBQX, 10 μM CPP, and 2 μM CGP. Cells were held at −55 mV and optogenetic stimulation (0.2 ms – 5 ms duration, 2 pulses, 200 ms ISI) was delivered every 20 s through a fiber optic (200 μm/0.22 NA, ThorLabs) coupled to 473 nm laser (25 mW, CrystaLaser). Light pulse duration was adjusted to evoke an oIPSC of 400-1200 pA (mean = 958 ± 75 pA). Data were acquired using Multiclamp 700B (Molecular Devices), filtered at 1 kHz and digitized at 5 kHz. All data were analyzed using pClamp (Clampfit, v. 10.3) and IgorPro (Wavemetrics). Quinpirole (1 μM), cocaine (10 μM), gabazine (5 μM), baclofen (1-5 μM), or CNO (1 μM) were bath applied for 5-10 minutes. Gabazine was added to the other synaptic blockers for the cell-attached recordings. APs were evoked by trains (20 light pulses, 0.2 – 1 ms duration, at 4 Hz or 16 Hz) and minimal pulse duration was used to evoke APs with 100% fidelity. For all experiments, sample sizes are the cells and collected from at least 3 mice.
Locomotor activity
Acute and Sensitized Cocaine Locomotion: Mice were habituated to chambers, handling and injection (saline) (Days 1 – 3) then received 15 mg/kg cocaine for 5 consecutive days (Days 4 – 8), with acute cocaine locomotion measured on Day 4. Locomotor sensitization to a cocaine challenge (15 mg/kg) was assessed two weeks after the last cocaine injection. Cocaine dose-response: Mice were habituated as above, then received escalating doses of cocaine (5, 15, 25 mg/kg) over 3 consecutive days or a single injection of 25 mg/kg cocaine. Intra-VP agonist locomotor response: Baseline locomotion was recorded for 30 min before drug administration (200 nl/side; 100 nl/min) then locomotion was recorded for 60 additional min. Mice moved freely in the chamber during injection (details in Suppl Methods). All mice received vehicle (aCSF), 4 nM quinpirole, or 500 μM picrotoxin in a randomized order with 24 h between each test. SKF81297 Activity: Mice were habituated as above. On Day 4, after baseline (30 min) mice received saline or 7.5 mg/kg SKF81297. DREADD rescue: Mice were habituated for 4 days. iMSN-Drd2KO mice received 1 mg/kg CNO in the home cage 30 min before saline or 15 mg/kg cocaine on Days 5 and 6, respectively. Adora2a-Cre mice received saline (Day 5), 3 mg/kg CNO (Day 6), and 3 mg/kg CNO 30 min before 2.5 mg/kg SKF81297 (Day 7).
Conditioned place preference
An unbiased conditioning procedure and a 2-chamber apparatus with distinct tactile floor cues (Grid or Hole) was used. Mice were habituated (5 min, saline, paper floor), received a pre-conditioning test (30 min, saline), 8 conditioning trials (15 min; 1/day, alternating 0.5 mg/kg or 15 mg/kg cocaine and saline) and 3 preference tests (30 min; after 4 and 8 conditioning trials and 2 days after test 2). For conditioning, cocaine and saline was paired with one side and floor type. For preference tests, mice received saline (pre-test, tests 1-2) or cocaine (0.5 or 15 mg/kg, test 3) and had access to both sides and floor types. Place preference is expressed as the time on the grid floor for each cocaine conditioning group: Grid+ or Hole+. A significant difference between these subgroups in the time spent on the grid floor indicates place conditioning (Cunningham et al., 2003).
Fast scanning cyclic voltammetry
Brain slices and solutions were prepared as in the electrophysiology experiments and recordings performed as previously described (Adrover et al., 2014). Carbon fiber (7 μm diam., Goodfellow) electrodes were fabricated with glass capillary (602000, A-M Systems) using a Sutter P-97 puller. Fiber tips were hand cut to 100-150 μm past the capillary tip. The carbon-fiber electrode was held at −0.4 V and a voltage ramp to and from 1.2 V (400V/s) was delivered every 100 ms (10 Hz). Before recording, electrodes were conditioned with this ramp (60 Hz for 15 min then 10 Hz for 15 min) and calibrated daily with 1 μM DA freshly prepared from light protected frozen stock (10 mM). DA transients were evoked by electrical stimulation delivered through a glass microelectrode filled with aCSF placed ~100 μm from carbon fiber (1 pulse of 300 μA, 0.2 ms duration) every 2 min. Cocaine (10μM) was bath applied for 10-20 min.
Statistics
Analyses were performed in Prism (GraphPad). Two-way (2W), 2-way repeated measures (2WRM) or 1-way (1W) ANOVAs, and paired or unpaired 2-tailed t-tests corrected for unequal variance (Welch’s correction) were used when appropriate. Significant interactions were followed up with pairwise t-tests and corrected for multiple comparisons. Experimental design determined the statistical test used and all data met assumptions for the test. Results were considered significant at an alpha of 0.05. All data are presented as mean ± SEM.
Supplementary Material
HIGHLIGHTS.
Synchronized activation of GABA transmission from multiple iMSNs inhibits APs in dMSNs
Cocaine suppresses lateral inhibition via D2Rs in iMSNs to disinhibit dMSNs
D2R agonist show higher efficacy at axon collaterals than at projections to VP
D2Rs in iMSNs are required for the stimulant effect of cocaine on locomotion
Acknowledgements
This study was funded by the Intramural Programs of NIAAA and NINDS (ZIA-AA000421) to VAA, and by ANPCYT-Mincyt of Argentina (MR), Universidad de Buenos Aires (MR) and Tourette Syndrome Association (MR). We are grateful to Dr. Fumihito Ono for access to the confocal microscope and to the members of the Alvarez lab for helpful comments and discussion. We also thank Dr. B. Roth (UNC) and K. Deisseroth (Stanford) who generously provided the hM4Di and ChR2 constructs, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
Author Contributions: Conceptualization: VAA, M.R., L.K.D. and A.R.L. Methodology: M.R., V.A.A., L.K.D., A.V.K. and J.C.L. Investigation, Validation and Analysis: L.K.D., A.V.K., J.C.L. and A.M. Writing: L.K.D., A.R.L., J.C.L and V.A.A Funding: V.A.A., M.R. Resources: V.A.A. Supervision: V.A.A.
References
- Abrahao KP, Quadros IM, Andrade AL, Souza-Formigoni ML. Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology. 2012;62:882–889. doi: 10.1016/j.neuropharm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Adrover MF, Shin JH, Alvarez VA. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci. 2014;34:3183–3192. doi: 10.1523/JNEUROSCI.4958-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Blockade of enkephalinergic and GABAergic mediated locomotion in the nucleus accumbens by muscimol in the ventral pallidum. Jpn J Pharmacol. 1989;50:487–490. doi: 10.1254/jjp.50.487. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Dopaminergic involvement in locomotion elicited from the ventral pallidum/substantia innominata. Brain Res. 1991;542:123–131. doi: 10.1016/0006-8993(91)91005-l. [DOI] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004a;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004b;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kitai ST. Intracellular recordings from rat nucleus accumbens neurons in vitro. Brain Res. 1986;366:392–396. doi: 10.1016/0006-8993(86)91326-0. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology. 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Gatley SJ, Logan J. [(11)]Cocaine: PET studies of cocaine pharmacokinetics, dopamine transporter availability and dopamine transporter occupancy. Nucl Med Biol. 2001;28:561–572. doi: 10.1016/s0969-8051(01)00211-6. [DOI] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Goertz RB, Puttick DJ, Bowles DE, Meyer RC, Hall RA, Ko D, Paladini CA, Weinshenker D. Chronic loss of noradrenergic tone produces beta-arrestin2-mediated cocaine hypersensitivity and alters cellular D2 responses in the nucleus accumbens. Addiction biology. 2014 doi: 10.1111/adb.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Archives of neurology. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Gore BB, Zweifel LS. Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. J Neurosci. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol. 1996;492(Pt 1):143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien RM. A Primer of Drug Action: a concise nontechnical guide to the actions, uses, and side effects of psychoactive drugs. 9th edn Worth Publishers; New York: 2001. [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64:599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015 doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, Kalivas PW. Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J Neurosci. 2014;34:1057–1066. doi: 10.1523/JNEUROSCI.4336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalchandani RR, van der Goes MS, Partridge JG, Vicini S. Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J Neurosci. 2013;33:14075–14086. doi: 10.1523/JNEUROSCI.0692-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, Alvarez VA. Striatal D2 receptors constrain local inhibitory transmission to set in vivo firing and spontaneous basal movement. Neuron. under review. [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science (New York, NY. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VG. Central effects of 6-hydroxydopamine. Behav Biol. 1973;9:397–420. doi: 10.1016/s0091-6773(73)80061-6. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Boeijinga PH, Kitai ST, Lopes da Silva FH. Contribution of NMDA receptors to postsynaptic potentials and paired-pulse facilitation in identified neurons of the rat nucleus accumbens in vitro. Experimental brain research Experimentelle Hirnforschung. 1991;86:190–198. doi: 10.1007/BF00231053. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. The Journal of pharmacology and experimental therapeutics. 1996;278:384–392. [PubMed] [Google Scholar]
- Preston RJ, Bishop GA, Kitai ST. Medium spiny neuron projection from the rat striatum: an intracellular horseradish peroxidase study. Brain Res. 1980;183:253–263. doi: 10.1016/0006-8993(80)90462-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain research reviews. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Koos T, Tepper JM, Kabbani N, Yeckel MF. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci. 2009;29:8977–8990. doi: 10.1523/JNEUROSCI.6145-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Matias S, Dugue GP, Mainen ZF, Costa RM. Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nat Commun. 2014;5:4315. doi: 10.1038/ncomms5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends in neurosciences. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. Journal of neurophysiology. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Carino MA, Horita A. Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacol Biochem Behav. 1995;52:737–741. doi: 10.1016/0091-3057(95)00167-u. [DOI] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Heller EA, Nestler EJ. Regulation of chromatin states by drugs of abuse. Current opinion in neurobiology. 2015;30:112–121. doi: 10.1016/j.conb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ng K, Hayes D, Gao X, Forster G, Blaha C, Yeomans J. Decreased amphetamine-induced locomotion and improved latent inhibition in mice mutant for the M5 muscarinic receptor gene found in the human 15q schizophrenia region. Neuropsychopharmacology. 2004;29:2126–2139. doi: 10.1038/sj.npp.1300502. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. The Journal of comparative neurology. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H, Del Basso P, Longo VG. Influence of 6-hydroxydopamine and of -methyl-p-tyrosine on the effects of some centrally acting agents. Physiology & behavior. 1972;8:391–396. doi: 10.1016/0031-9384(72)90317-4. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology. 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.