Abstract
Objective
Vaccination rates for influenza, pneumococcus, and zoster in rheumatoid arthritis patients have remained low. Simple electronic or paper reminders have produced only small increases in vaccination rates. We sought to identify a more effective approach to improve vaccination rates.
Methods
We conducted a system-level intervention at an academic rheumatology clinic that included electronic reminders with linked order sets, physician auditing and feedback, patient outreach, and optional printed prescriptions for zoster vaccination at an outside pharmacy.
Results
We targeted 1255 eligible patients with rheumatoid arthritis. There was no change in patients’ self-reported influenza vaccination rates, although the baseline self-reported rate was already high and much higher than that documented in the electronic health record. Pneumococcal vaccination rates increased from 28.7% to 45.8%; in regression analysis, the rate of change in pneumococcal vaccination increased by 9.4% per year above baseline trends (95% CI = 3.9% - 15.5%; p = 0.002). The rate of zoster vaccination increased from 2.5% to 4.5% (p = 0.01) overall and from 3.0% to 6.6% among patients not receiving biologic therapy that preclude zoster vaccination.
Conclusion
Although the intervention improved pneumococcal and zoster vaccination rates, the improvement in pneumococcal vaccination rate was less than expected, and the zoster vaccination rate remained low even for ideal candidates. Likely barriers include lack of familiarity and difficulty using electronic reminders and order sets, uncertainty about the value and safety of recommended vaccines, and uncertainty about patients’ insurance coverage and prior vaccination history. Future interventions should include strategies to address these.
Keywords: Herpes Zoster, Vaccination, Quality Improvement, Electronic Health Records
Introduction
Patients with rheumatoid arthritis (RA) have a risk of infection approximately double that of age and gender-matched controls,(1) which is likely due to both inherent immune dysregulation and chronic immunomodulatory therapy. Because of this increased risk of infection, the Advisory Committee on Immunization Practices (ACIP) recommends that all patients with RA be given influenza vaccination (IVX) and pneumococcal vaccination (PVX) with the 13-strain pneumococcal conjugated vaccine (PCV13) followed by the 23-strain pneumococcal polysaccharide vaccinate (PPSV23).(4, 5) For herpes zoster vaccine (HZVX), ACIP makes no specific recommendation for patients with RA, but states that this live vaccine is safe for patients who are taking less than 20mg/day of prednisone and low-doses of methotrexate (≤0.4 mg/kg/week) or azathioprine (≤3.0 mg/kg/day).(6) If a physician plans to initiate a potent immunosuppressant, ACIP recommends HZVX be given at least 14 days prior to starting the medication.(6)
Vaccination rates appear to be low among patients with RA, despite their high risk of infection and recommendations from national organizations. A recent large study found that only 25% of RA patients were adherent to current vaccine recommendations.(7) Studies have shown vaccination rates among RA patients ranging from 10-34% for IVX, 17-54% for PVX; and 1-21% for HZVX.(7-14) The reasons for these low vaccination rates are not entirely clear.(15) At our institution, vaccination rates for patients with RA measured using electronic health record (EHR) data have been similarly low.
Studies have shown that multifaceted interventions with performance measurement and feedback, EHR reminders, and linked order sets can improve delivery of preventive services.(16-18) However, few studies have examined interventions to improve vaccination rates among patients with rheumatologic disease. Ledwich and colleagues implemented an electronic reminder for PVX and IVX along with linked order sets that allowed physicians to respond easily. Pre-post analyses showed significant increases in vaccination rates from 19 to 41% for PVX and from 47 to 65% for IVX.(15) Desai and colleagues implemented point-of-care paper reminders for PVX for a non-random sample of 35 rheumatologists at five ambulatory rheumatologic clinics. The PVX rate, already high, still increased significantly (from 68% to 80%) in the intervention group but remained stable for physicians who were not selected for the intervention.(19)
These studies show the value of reminders (electronic or paper). However, the improvements were relatively small, and vaccination rates remained suboptimal. We conducted this study to implement and test a system-level intervention that included multiple quality improvement strategies to improve adherence to PVX, annual IVX, and HZVX among patients with RA, including electronic reminders with linked order sets, audit and feedback to rheumatologists about their vaccination rates and how they compare to their peers, and outreach to patients.
Materials and Methods
Setting
This project was a 12-month, system-level intervention that targeted all patients with RA who were cared for by rheumatologists (and usually a primary care physician) in Northwestern Medical Group (NMG). NMG is an academic, multispecialty group practice staffed by the faculty of the Feinberg School of Medicine, Northwestern University. At the time of this study (October 2013 to October 2014), NMG had eight rheumatologists and four rheumatology fellows. NMG physicians use an EHR (Epic; Epic Systems Corporation; Verona, Wisconsin) for all clinical encounters (in-person and telephone). All prescriptions for disease modifying anti-rheumatic drugs and biologic therapy are initiated and tracked in Epic, including infusions. The Northwestern University Institutional Review Board (IRB) approved the study with a waiver of consent for patients and physicians.
Study Population
Structured query language (SQL) was used to query our EHR data to identify all patients who were eligible for analysis. Patients were eligible if they had a diagnosis of RA (ICD-9 codes 714.0-714.9), at least one visit to the study clinic in each of the previous two years, and were at least 18 years old. A total of 1255 patients were identified as eligible based on EHR data.
Intervention
The intervention included multiple components:
-
1)
Electronic quality measurement - For quality measurement, we used SQL to analyze data in our enterprise data warehouse to determine prior vaccination for influenza (ever and in the prior year), pneumonia, and herpes zoster. We assigned patients to rheumatologists’ panels by determining which physician had provided care most often. We then calculated each rheumatologist's performance based on the proportion of all patients in a panel who had been vaccinated or had a documented medical exception or patient reason (e.g., refusal, financial barriers) for not being vaccinated.
-
2)
Computerized, point-of-care clinical decision support tools - Our EHR has a standard tool called “Best Practice Alerts (BPA)” that allows users to create customizable clinical decision supports, reminders, and linked order sets. We have used these extensively in studies in the NMG General Internal Medicine clinic, and we used the same general approach that we have described previously.(16) Briefly, if a patient has not been vaccinated for one or more of the three vaccinations, the Best Practice Alert tab in the EHR appears in yellow. The clinician can then click on this to see the recommendation and the associated linked order sets; clinicians can then click on one or more order sets to order vaccinations, which are administered by nurses in the clinic. The clinician can also click on options to indicate a medical or patient reason why a vaccination was not given or jump to the immunization section of the EHR to record vaccinations that were previously administered elsewhere. For the IVX BPA, options were available for clinicians to click and record “completed elsewhere” or “prefers to do elsewhere” because so many patients get vaccinated outside of formal medical settings, and recording outside influenza vaccinations in detail did not affect decision-making about annual IVX. We did not make these options available in the BPAs for PVX and HZVX. The IVX alert was active from October 1, 2013 to February 28, 2014. Originally, we planned for PVX vaccine alerts to be for initial vaccination and 5-year revaccination using the 23-valent polysaccharide vaccine (PPSV23). However, around the start of the intervention, the ACIP recommended that immunocompromised patients receive the 13-valent conjugate vaccine (PCV13) followed at least eight weeks later by an initial dose of PPSV23 and a PPSV23 booster five years later.(5) Therefore, we created separate alerts for PCV13 and the initial dose of PPSV23. A training session was held with the rheumatologists on how to use the BPAs and linked order sets, and a team member was available to provide on-site support the week after BPAs were activated.
-
3)
Individual performance feedback to physicians – After assigning each eligible patient to an individual rheumatologist by identifying their provider, we calculated the proportion of each rheumatologist's patients who were up to date on each vaccination (i.e., performance rates). Individualized reports contained graphs showing each doctor's performance and group-level performance (i.e., peer comparison). For the first 6 months, because of our desire to encourage vaccination early during influenza season, reports were presented to each rheumatologist at monthly business meetings. After that, we provided quarterly performance reports.
-
4)
Outreach to patients – In early October, 2013 we mailed a letter to all eligible patients reminding them of the importance of influenza vaccination (see Appendix). The messages for the call and the letter explained to patients that they were immunocompromised, at higher risk for infections and sequelae of infections, and that IVX was effective and safe. A follow-up letter was sent in November or December, 2013 to all eligible patients for whom influenza vaccination had not already been documented. A similar letter was sent to all eligible patients in April, 2014, encouraging them to speak with their rheumatologist about pneumococcal vaccination. Prior to this study, patients in the practice did not routinely receive vaccination reminder letters.
Processes of Care for Patients Seen Each Week in Clinic
To measure physicians’ response to the intervention, we tracked the weekly “action rate”. The action rate is the proportion of patients who were seen by their rheumatologist who had any of the following after a BPA was displayed: vaccination given, historical vaccination documented, or a documented medical or patient reason (“exception”) for not giving a vaccination. HZVX is not administered in our clinic. Patients receiving a recommendation for HZVX were given a printed prescription stating that their physician felt it was safe for them to receive this vaccine at an outside pharmacy. A link to this prescription was included in the BPA.
Measurement and Analysis of Changes in Influenza Vaccination
The clinical decision support tools in the intervention were expected to increase EHR recording of outside IVX receipt, which could give the spurious impression that our intervention improved IVX use. Therefore, we assumed patient self-reported receipt of IVX was the least biased methodology. In July-September of 2013 (pre-intervention) and 2014 (post-intervention), we surveyed participants about IVX receipt during the prior influenza season. These self-reports were the primary outcome for IVX.
Our full survey methods have been published previously (20) and are only briefly summarized here. We randomly sorted patients for recruitment with a goal of completing 100 interviews each year. Charts were reviewed prior to contacting patients to confirm their RA diagnosis. Patients were called up to six times and invited to participate in a 10-minute structured telephone interview. Patients were offered a $10 gift card for completing the survey. The survey included self-reported IVX any time in the past, during the last year, and the setting in which it was given. Demographic information was obtained at the conclusion of the survey. We analyzed differences in IVX rates for the two seasons using unpaired chi-square tests.
Measurement and Analysis of Changes in Pneumococcal and Herpes Zoster Vaccination
Our primary outcome for PVX and HZVX was vaccination recorded in the EHR. We queried EHR data to determine the proportion of patients who had received PVX or HZVX. After ACIP's new PVX recommendations were published, we also measured the following mutually-exclusive categories of PVX: PPSV23 only, PCV13 only, one or more PPSV23 and PCV13, no PVX with medical exception documented, and no PVX with patient refusal documented. For HZVX the categories were: ever received HZVX, no HZVX with medical exception documented, and no HZVX with patient refusal documented. In addition, we included self-reported PVX and HZVX in the patient survey and used these as secondary outcomes.
We analyzed differences in vaccination rates on October 1, 2013 and October 1, 2014 using paired chi-square tests. In addition, we qualitatively analyzed variation in pre and post-intervention PVX rates for the eight faculty physicians. The Pearson correlation coefficient was calculated to examine the association between physicians’ baseline PVX rates and their degree of improvement. Because simple pre-post analyses do not account for temporal trends, an intervention may appear improve care when care would have improved even without the intervention. We conducted interrupted time series analysis in which two regression models separately investigated clinic-level changes in PVX and HZVX rates for the six-month pre-intervention period and the 12-month intervention period. A linear model was fitted to each series using time as a continuous predictor, intervention as a dichotomous indicator variable, and a term for the interaction between time and intervention. The model adjusted for autoregressive errors. The dependent variable in these models was the proportion of eligible patients who had ever received PVX (any type) and HZVX in each month of the study. The independent variables were time and an interaction term between We examined the 1) monthly change in vaccination rate (i.e. change in slope), and 2) one-time change sustained over the intervention period (i.e. change in intercept). The PVX regression model also tested for a one-time change in the PVX rate after the outreach letter was sent to patients in April 2014. For all analyses, a P value of 0.05 was used to determine statistical significance.
Focus Groups
Following the intervention, we held a focus group of the affected rheumatologists. They were asked a series of structured questions on several elements of the intervention, including the ease of use and impact on visit flow of the best practice alerts, their comfort level with the vaccinations we studied, and their thoughts specific patient and practice issues that may have affected the results.
Results
Among the 1255 eligible patients, 83.5% were female, mean age was 56.8 years (SD 14.5), and 50.4% were White (Table 1). Almost all patients had commercial insurance (57.8%) or Medicare or Medicaid (37.3%). A total of 753 (60.0%) were currently being treated with a biologic agent, and 38.6% had one or more comorbidities (Malignancy, diabetes, hypertension, dementia, coronary artery disease, peripheral vascular disease, congestive heart failure, or chronic pulmonary, renal, or liver disease).
Table 1.
Patient Characteristics (N = 1255)
| Female, n (%) | 1048 (83.5) |
| Age, mean (SD) | 56.8 (14.5) |
| Race/Ethnicity, n (%) | |
| Hispanic or Latino | 138 (11.0) |
| White | 633 (50.4) |
| Black | 200 (15.9) |
| Other | 63 (5.0) |
| Unknown/Declined/Missing | 221 (17.6) |
| Insurance, n (%) | |
| Medicare/Medicaid | 468 (37.3) |
| Commercial | 726 (57.8) |
| Uninsured/Self-pay | 61 (4.9) |
| Treated with biologic medication, n (%) | 753 (60.0) |
| Comorbidities, n (%) | |
| 0 | 771 (61.4) |
| 1 | 261 (20.8) |
| 2 or more | 223 (17.8) |
Physician Response to Alerts
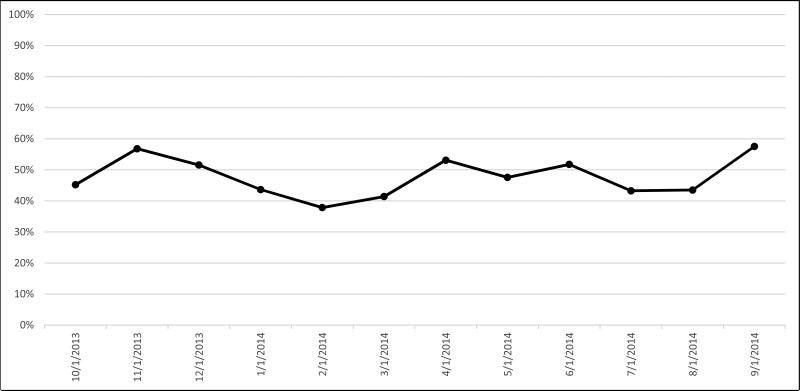
Figure 1 shows the monthly “action rate.” During the first two months that alerts were active, an action was taken in 45% and 57% of patients needing a vaccination, respectively. Over the following 3 months, the action rate steadily decreased to a low of 38%. During the final 6 months of the intervention, the action rate varied from 43-58%, with no clear trend.
Figure 1.
Proportion of Patients Seen in the Rheumatology Clinic Each Study Month Who Had an Action Performed at the Visit (Vaccination, Documentation of a Previous Vaccination or Documentation of a Patient Reason for Not Giving a Vaccination).
Changes in Influenza Vaccination Rate
There was no change in patients’ self-reported influenza vaccination rates (Table 2). For the baseline survey, 79.4% of participants said they received IVX in the previous season, and 90.2% said they had ever received IVX. Among the independent sample of patients interviewed the following year, the results were 78.2% and 86.1%, respectively. Of the 79 patients who reported annual IVX during the second survey, 65 (82.2%) said they were vaccinated between September and December. (This question was not asked in the baseline survey).
Table 2.
Proportion of Patients Vaccinated against Influenza, Pneumococcus, and Herpes Zoster Vaccines Before and After the 12-Month Intervention Period
| Pre-Intervention (%) | Post-Intervention (%) | |
|---|---|---|
| Influenza (N=102, 101)* | ||
| Ever received influenza vaccine | 92 (90.2) | 87 (86.1) |
| Received influenza vaccine in previous season | 81 (79.4) | 79 (78.2) |
| Pneumococcal (N=1255)† | ||
| Ever received any type of vaccine | 360 (28.7) | 575 (45.8) |
| PPSV 23 only | 351 (28.0) | 293 (23.3) |
| PCV 13 only | 5 (0.4) | 151 (12.0) |
| PPSV 23 and PCV 13 | 4 (0.3) | 131 (10.4) |
| No vaccine received, medical reason | 0 | 9 (0.7) |
| No vaccine received, patient reason | 2 (0.2) | 51 (4.1) |
| Herpes Zoster (N=1255)† | ||
| Ever received vaccine | 32 (2.5) | 57 (4.5) |
| Prescription to receive elsewhere, no record of receipt | 0 | 28 (2.2) |
| No vaccine received, medical reason | 0 | 102 (8.1) |
| No vaccine received, patient reason | 0 | 46 (3.7) |
Rates of influenza vaccination are based on self-report from a random sample of 102 patients who were interviewed for the study prior to implementation of the intervention and 101 who were interviewed after implementation of the intervention.
Rates of pneumococcal and herpes zoster vaccination are based on electronic health record data for all eligible patients
PPSV 23 – Pneumococcal Polysaccharide Vaccine; PCV 13 - Pneumococcal Polysaccharide Vaccine; HZV – Herpes Zoster Vaccine
Changes in Pneumococcal and Herpes Zoster Vaccination Rates
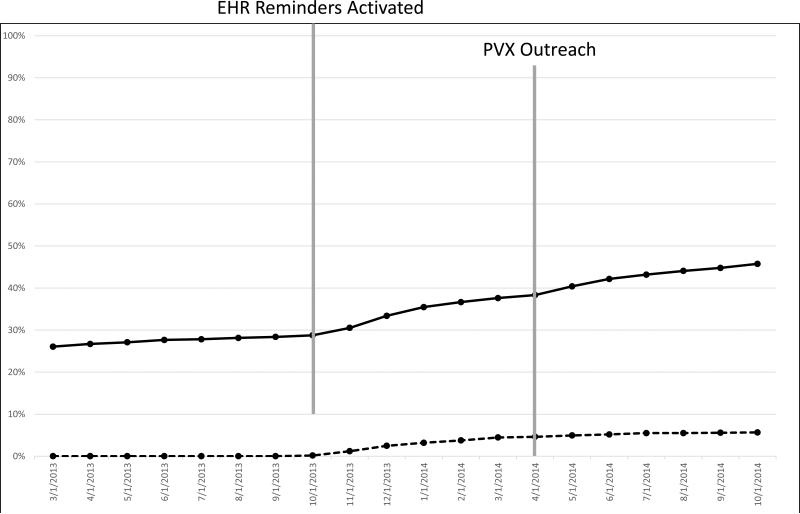
At baseline, 28.7% of patients had PVX documented in the EHR (Table 2). By the end of the one-year intervention, the rate was 45.8% (p < 0.0001). Figure 2 shows monthly PVX rates during the pre-intervention and intervention periods. In regression models, the PVX rate rose by 5.7% per year during the baseline period (95% CI = 2.0% - 9.5%; p=0.005). After the intervention began, the modeled rate of change increased by 9.4% per year (95% CI = 3.9% - 15.5%; p = 0.002) to a cumulative rate of increase of 15.1% per year.
Figure 2.
Pneumococcal Vaccination Rate (solid line) and Documented Exception Rate (dashed line)
Only 0.4% of patients had received PCV13 at baseline (Table 2), and this increased to 12.0% after one year of the intervention. Moreover, the proportion of people who had received both PPSV23 and PCV13 increased from 0.3% to 10.4%. There were 51 (4.1%) patients who had a documented refusal of PVX and 9 (0.7%) had a medical exception recorded, although the specific exception was not documented. In the patient surveys, the self-reported receipt of any PVX was 60.8% in the first sample and 79.2% in the second.
At baseline, 2.5% of patients had HZVX documented in the EHR (Table 2). This increased to 4.5% by the end of the intervention (p = 0.01). Among patients prescribed a biologic medication (N = 753), the HZVX documentation rate increased from 2.4% to 3.5%; among those not prescribed a biologic (N=502), the rate increased from 3.0% to 6.6%. In regression models, the modeled rate of HZVX change increased by 1.7% per year (95% CI = 1.1% - 2.2%; p = < 0.001). In addition to actual vaccinations given, another 2.2% of patients had documentation of a prescription to receive HZVX at a pharmacy, although confirmation of receipt was not recorded in the EHR. There were 8.1% who had a medical exception documented, and 3.7% had a documented patient refusal. In the patient surveys, the self-reported receipt of any HZVX was 7.8% in the first sample and 11.8% in the second.
Variations in Improvements for Individual Physicians
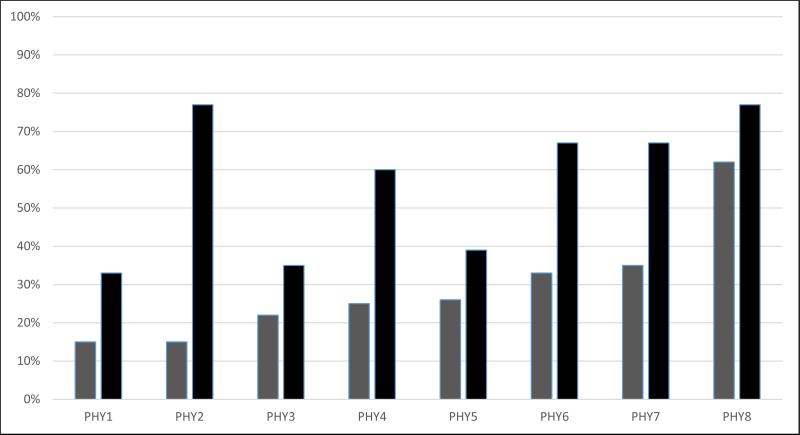
At baseline, the rate of PVX vaccination across physicians ranged from 15% to 62% (Figure 3). One year later, the range was 33% to 77%; improvements in performance ranged from 13% to 62%. Interestingly, there was no significant correlation between baseline PVX rates and the degree of improvement (r = −0.33, p = 0.41).
Figure 3.
Variation in Pneumococcal Vaccination Rate at Baseline (Grey) and Follow-up (Black) for Patients Associated with Individual Attending Rheumatologists (PHY 1-8)
Focus Groups
Six rheumatologists participated in the 1 hour focus group. Several expressed uncertainty about the ACIP recommendations as well as the indications and restrictions on the HZVX. There was also a lack of clarity and agreement on the respective roles of rheumatologists and primary care physicians in administering vaccinations. Representative comments are included in Table 3.
Table 3.
Selected Focus Group Comments from Participating Rheumatologists
| Topic | |
|---|---|
| Provider Education | “I feel I have limited mastery and when my mastery is limited I'm sometimes skeptical about intervening. Because I might not do it right.” |
| Scope of Practice | “From a broader perspective, really prior to this intervention and for many, many years, my view is that my scope of practice doesn't really include preventive things... I'm sort of redefining scope a little, [but] this is not my area. I'm being dragged kicking and screaming into this.” |
| EHR Challenges | “I gave up about a month into it. I couldn't figure it out. Every time I would end up going around and around and around. I gave up. When I gave a vaccination I would just tell the RN to give an injection and document it.” |
| Patient Barriers | “Patients hem and haw- ‘I need to go check with my primary care provider.’ Or they say, ‘I'm going to get it at Walgreens.’ And then when they come back they haven't.” |
| Coordination of Care | “One of my concerns is always coordination of care. What am I going to do with these results? If they are abnormal, want to make sure they'll see their internist. I never know how to make sure that happens.” |
| Performance Reports | “After realizing I'm terrible, I realized I wanted to get better. They provided motivation to improve. And it didn't get as good as I would have liked, but it did improve.” |
Discussion
Our multifaceted intervention that combined audit and feedback of individualized vaccination rates to rheumatologists with peer comparisons, electronic alerts, linked order sets, and patient outreach resulted in a modest improvement in the PVX rate (17.1% absolute increase), a very small improvement in the HZVX rate (2%), and no detectable improvement in the IVX rate over the one-year intervention period. We had hoped that this intensive, multi-level (doctor, system, and patient) intervention would be more effective than previous interventions consisting of paper or electronic alerts.(15) (19)
Based on our own observations and comments from the rheumatologists in the focus group, we believe several factors contributed to the limited success of the intervention. Some rheumatologists may have been uncomfortable giving vaccinations because of limited knowledge of ACIP recommendations, despite our educational intervention at the start of the study. Others expressed doubts about the need for HZVX, its efficacy, and its safety. In addition, two weeks before the start of the intervention, ACIP changed its PVX guideline, which was highly problematic for multiple reasons. First, electronic alerts had to be changed so an initial alert recommended PCV13 and a second alert recommended PPSV23 eight weeks after PCV13. However, many patients did not know whether they had ever received any PVX or the type of PVX they received. Physicians said they did not want to give the wrong vaccine, and the time required to clarify details of past PVX receipt may have discouraged physicians from addressing the issue. Similarly, coverage of HZVX for patients under age 60 is inconsistent across insurers, and some physicians may have been reluctant to recommend HZVX if it might not be covered. It is likely that uncertainty over the safety and efficacy of HZVX was also a barrier in light of the very low HZVX administration rate among patients who were not on a biologic. Finally, some rheumatologists may have felt they did not have adequate time to discuss prevention, and some may have felt that administering vaccinations is the responsibility of primary care providers.
There also appear to have been problems with the usability of the EHR tools. Some rheumatologists said they found it difficult to record previous vaccinations, and others said they found the alerts and linked order sets cumbersome to use, although we tried to simplify these tools and provided a demonstration on their use. A practical concern relative to HZVX is the fact that the vaccine is not stocked in the rheumatology clinic. Although we provided clear, written documentation requesting HZVX administration at outside pharmacies, without feedback from these pharmacies we had no way to reliably confirm HZVX administration across the study population.
Despite intensive outreach to patients and point-of-care reminders, the patient-reported IVX rate remained slightly below 80% in the years before and after the intervention (Table 2). This rate is well above the national rate for the 2013-14 season (45.3% for 50-64 year olds and 65.0% for those 65 and older). It may not be possible to increase the IVX rate much above 80% because of patients’ widespread concerns about safety and efficacy of the vaccine and the difficulty of changing these attitudes. Thus, a more practical goal may be to maximize IVX administration early during the influenza season to achieve maximal protection. The vast majority of patients interviewed in the intervention year said they received IVX in the first half of the influenza season (September-December), which was consistent with documented EHR results (data not shown). However, we do not have similar data from the pre-intervention year, so we cannot confirm that the intervention led to IVX administration earlier during influenza season.
There are several limitations to this study. First, it was conducted in a single practice with only 12 rheumatologists. We believe that the barriers to improving vaccination rates described above are likely to be widespread, but additional studies are needed. The findings of increases in PVX rates seen in older studies of simple alerts for PVX may no longer be valid now that there is a more complicated vaccination schedule that requires more time to discern the correct course of action. Second, we were unable to randomize physicians, and we had to use a quasi-experimental study design with interrupted time series analysis to examine trends in PVX and HZVX rates after adjusting for any baseline trends. It remains possible that external factors occurring at the same time as the intervention could have affected our results. Third, the study duration was only one year. We may have seen a larger improvement with a longer duration of the intervention. Conversely, we do not know whether the increased efforts to vaccinate patients would have been sustained if we had a longer observation period. Finally, we analyzed the combined effect of all of our interventions on vaccination rates; we are unable to reliably tease out which specific intervention(s) had the greatest impact.
We believe that future interventions will need to do several things to overcome the obstacles we describe above. First, the health care team and system need to identify the team member responsible for ensuring that patients receive indicated vaccinations. This person could be a rheumatologist, primary care physician, or care manager. Additionally, the use of non-physician members of the health care team for ascertainment of vaccination status may take some of the burden off the physician and improve documentation and compliance. Second, an intensive educational campaign may be necessary to ensure that physicians (rheumatologists and primary care physicians) are more knowledgeable about proper vaccinations for patients with RA and other rheumatologic diseases. Third, EHR systems’ usability must be improved to support the delivery of patient-centered vaccination programs. While it was easy to set up a simple alert for the PCV13, it was not possible to set an alert to fire eight weeks later for the PPSV23, even with the help of our EHR vendor. Similarly, it was not possible to set up an alert for the HZVX that incorporated the exclusions for potent immunosuppressants, as alerts could not integrate the specific dose and timing of some medications. As a consequence, this study's alerts were effectively triggers for the rheumatologist to investigate whether the patient was a candidate for HZVX and/or whether the timing was correct for the PPSV23, thus requiring additional decision making by the physician. Current commercial EHRs are unable to program alerts like these that require advanced logic so that the physician immediately recognizes the proper action to take. Finally, we believe that expanded use of state immunization registries is crucial so that physicians know with certainty what past vaccinations patients have received, allowing them to prescribe quickly and with confidence. The discrepancy between patient reported vaccinations and documentation in the EHR seen in our study has been reported by others.(12) The integration of this state registry data, and, indeed, any improvement of the EHR's ability to easily and accurately document immunization histories could improve physicians’ ability to identify and address incomplete vaccination status. Together, these steps should dramatically increase vaccination rates and help protect patients with RA from debilitating and potentially fatal infections.
Acknowledgements
We would like to thank Alpa Patel and NMG's Epic support team for their programming assistance. We would also like to thank the NMG rheumatologists for their willing participation in this project.
Funding
Pfizer award #: 8392087
NIH Grant: P60AR064464
References
- 1.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli MA, Moreland LW, Brick JE. Herpes zoster in patients with rheumatoid arthritis treated with weekly, low-dose methotrexate. Am J Med. 1991;90:295–8. [PubMed] [Google Scholar]
- 3.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–8. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 4.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 7.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73:62–8. doi: 10.1136/annrheumdis-2013-204223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumentals WA, Arreglado A, Napalkov P, Toovey S. Rheumatoid arthritis and the incidence of influenza and influenza-related complications: a retrospective cohort study. BMC Musculoskelet Disord. 2012;13:158. doi: 10.1186/1471-2474-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haroon M, Adeeb F, Eltahir A, Harney S. The uptake of influenza and pneumococcal vaccination among immunocompromised patients attending rheumatology outpatient clinics. Joint Bone Spine. 2011;78:374–7. doi: 10.1016/j.jbspin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JR, Arora T, Narongroeknawin P, Taylor A, Bingham CO, 3rd, Cush J, et al. The delivery of evidence-based preventive care for older Americans with arthritis. Arthritis Res Ther. 2010;12:R144. doi: 10.1186/ar3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowden E, Mitchell WS. An audit of influenza and pneumococcal vaccination in rheumatology outpatients. BMC Musculoskelet Disord. 2007;8:58. doi: 10.1186/1471-2474-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai SP, Turchin A, Szent-Gyorgyi LE, Weinblatt M, Coblyn J, Solomon DH, et al. Routinely measuring and reporting pneumococcal vaccination among immunosuppressed rheumatology outpatients: the first step in improving quality. Rheumatology (Oxford) 2011;50:366–72. doi: 10.1093/rheumatology/keq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13:R174. doi: 10.1186/ar3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hechter RC, Tartof SY, Jacobsen SJ, Smith N, Tseng HF. Trends and disparity in zoster vaccine uptake in a managed care population. Vaccine. 2013;31:4564–8. doi: 10.1016/j.vaccine.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 15.Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61:1505–10. doi: 10.1002/art.24873. [DOI] [PubMed] [Google Scholar]
- 16.Persell SD, Kaiser D, Dolan NC, Andrews B, Levi S, Khandekar J, et al. Changes in performance after implementation of a multifaceted electronic-health-record-based quality improvement system. Med Care. 2011;49:117–25. doi: 10.1097/MLR.0b013e318202913d. [DOI] [PubMed] [Google Scholar]
- 17.Baker DW, Persell SD, Kho AN, Thompson JA, Kaiser D. The marginal value of pre-visit paper reminders when added to a multifaceted electronic health record based quality improvement system. J Am Med Inform Assoc. 2011;18:805–11. doi: 10.1136/amiajnl-2011-000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean-Jacques M, Persell SD, Thompson JA, Hasnain-Wynia R, Baker DW. Changes in disparities following the implementation of a health information technology-supported quality improvement initiative. J Gen Intern Med. 2012;27:71–7. doi: 10.1007/s11606-011-1842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai SP, Lu B, Szent-Gyorgyi LE, Bogdanova AA, Turchin A, Weinblatt M, et al. Increasing pneumococcal vaccination for immunosuppressed patients: a cluster quality improvement trial. Arthritis Rheum. 2013;65:39–47. doi: 10.1002/art.37716. [DOI] [PubMed] [Google Scholar]
- 20.Sandler D, Ruderman E, Brown T, Lee J, Ozanich A, Liss D, et al. Understanding Vaccination Rates and Attitudes among Patients with Rheumatoid Arthritis. Am J Manag Care. 2015 in press. [PubMed] [Google Scholar]