Abstract
Macro-environmental factors, including a patient’s physical and social environment, play a role in cancer risk and progression. Our previous studies show that living in an enriched environment (EE) providing complex stimuli confers an anticancer phenotype in mice mediated in part by a specific neuroendocrine axis, with brain-derived neurotrophic factor (BDNF) as the key brain mediator. Here we investigated how an EE modulated T-cell immunity and its role in the EE-induced anticancer effects. Our data demonstrated that CD8 T cells were required to mediate the anticancer effects of an EE in an orthotropic model of melanoma. In secondary lymphoid tissue (SLT), an EE induced early changes in the phenotype of T-cell populations, characterized by a decrease in the ratio of CD4 T helper to CD8 cytotoxic T lymphocytes (CTLs). Overexpression of hypothalamic BDNF reproduced EE-induced T-cell phenotypes in SLT whereas knockdown of hypothalamic BDNF inhibited EE-induced immune modulation in SLT. Both propranolol and mifepristone blocked the EE-associated modulation of CTLs in SLT suggesting both the sympathetic nervous system and hypothalamic-pituitary-adrenal axis were involved. Our results demonstrated that enhanced anticancer effect of an EE was mediated at least in part through modulation of T-cell immunity and provided support to the emerging concept of manipulating a single gene in the brain to improve cancer immunotherapy.
INTRODUCTION
Environmental factors profoundly influence carcinogenesis, and epidemiological reports indicate a connection between psychological stress and cancer progression (1-3). Previous research linking stress to cancer growth implicates activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal axis (HPA) (4-6), however the precise molecular mechanisms by which stress regulates cancer progression are not fully understood. Most research has focused on distress, so the impact of eustress, which is associated with health benefits, has not as yet been well investigated. Both the SNS and the HPA axes regulate lymphocyte development and differentiation, and neurological control of immune homeostasis is becoming more widely recognized as a major regulator (7-9). Therefore, the identification of the mechanisms by which different stresses influence T-cell homeostasis in secondary lymphoid tissue (SLT), and the T-cell’s ability to recognize tumors and halt cancer progression, are of significant interest to investigators and clinicians in cancer immunotherapy (10).
To study environmental and psychosocial effects on cancer progression, our lab uses environmental enrichment (EE) as a eustress model. EE is a housing environment for laboratory animals, which, in contrast to the standard environment (SE) cages used in most biomedical research, is socially, physically, and cognitively stimulating. EE has profound impacts on brain structure, function, and progression of neurological diseases (11, 12). We have reported that EE leads to a distinct metabolic phenotype in mice characterized as decreased adiposity, elevated energy expenditure, induction of beige cells in white fat, enhanced insulin sensitivity, improved adipokine profile, and resistance to diet-induced obesity (13). In addition, an EE inhibits tumor progression in an implantation model of melanoma as well as a genetic model of colon cancer (14). Recent reports from others have shown the anticancer effects of an EE in orthotopic and spontaneous tumor models of breast, colon, pancreatic, and melanoma cancers (15, 16). We have identified one mechanism underlying the effects of an EE on metabolism and cancer, the activation of the hypothalamic-sympathoneural-adipocyte (HSA) axis (14). The environmental stimuli provided by an EE upregulate BDNF expression in the hypothalamus and subsequently elevate sympathetic tone preferentially to the white adipose tissue, which has at least two consequences: induction of beige cells and suppression of leptin production and release. The robust drop of leptin in the circulation plays an important role in EE-induced cancer inhibition. However, an EE is associated with other changes such as a modest but significant increase of corticosterone, and enhanced CD8 T-cell cytotoxicity (14). The contribution of an adaptive T-cell immune response, however, is unknown in the context of an EE. Here we investigated how EE modulated T-cell homeostasis in SLT, the role of CD8 cytotoxic T lymphocytes (CTLs) in EE-induced cancer inhibition, and the neuronal substrates of the brain-immune interactions induced by EE.
MATERIALS and METHODS
Environmental Enrichment Protocol
Male 3-week-old C57/BL6 mice were purchased from Charles River (Spencerville, OH) and randomized to live in enrichment or standard laboratory conditions (5 mice per cage). For enrichment housing, mice were housed in groups in cages of 1.5 m × 1.5 m × 1.0 m (10-20 mice per cage) or 73cm × 41cm × 46cm (5 mice per cage) supplemented with running wheels, tunnels, igloos, huts, retreats, wood toys, a maze, and nesting material in addition to standard lab chow and water (17). All mice were housed in temperature- (22-23°C) and humidity-controlled rooms with food and water ad libitum. We carried out all mice experiments in compliance with the regulations of the Ohio State University Institutional Animal Ethics Committees.
Melanoma Implantation and CD8 T-cell depletion
The mouse B16-F10 melanoma cell line was purchased from ATCC (ATCC CRL-6475TM) in 2014 and the culture conditions followed ATCC’s instruction. The B16-F10 cell line was expanded three passages, and the frozen stock was thawed and used for implantation. We housed mice in their respective environments for 5 weeks and then intraperitoneally injected 0.2 mg of anti-CD8 (BioXcell, clone YTS 169.4.2) or IgG (BioXcell) in 200 μl PBS. The day after antibody injection B16-F10 melanoma cells were subcutaneously implanted on the flank (1 × 105 cells/per mouse, n = 10 per group). The antibodies were injected once per week until sacrifice 18 days after melanoma implantation. The tumors were dissected away from neighboring tissues, measured, and weighed.
Flow cytometry
Spleens and lymph nodes were collected in Eppendorf tubes containing RPMI media. We mechanically dissociated spleens or lymph nodes through a 70 μm strainer to obtain single cell suspensions. Red blood cell (RBC) lysing buffer were used to lyse RBCs. After washing with PBS, cells were counted using hemocytometer. Cells (2 × 106) were pipetted for a flow panel. Conjugated antibodies for CD3-PercpCy5 (Cat. #55116, 145-2C11), CD4-APCH7 (Cat. #560181, GK1.5), CD8-V450 (Cat. #560469, 53-6.7), CD44-FITC (Cat. #553133, IM7), CD122-PE (Cat. #553362, TM-Beta1) were purchased from BD Biosciences. TCRβ-PECy (Cat. #25-5961, H56-597) was purchased from eBioscience. For Treg flow cytometry we used the Mouse Regulatory T-cell Staining Kit from eBioscience (Cat. #88-8111-40) containing CD4-FITC (RM4-5), CD25-APC (PC61.5), and Foxp3-PE (FJK-16s). All flow cytometry was performed using a BD LSRII. The results were analyzed with Flowjo.
rAAV Vector Construction and Packaging
The recombinant adeno-associated virus (rAAV) plasmid contains a vector expression cassette consisting of the CMV enhancer and chicken β-actin (CBA) promoter, WPRE (posttranscriptional regulatory element of woodchuck hepatitis virus) sequence, and bovine growth hormone poly-A region flanked by AAV inverted terminal repeats. Human BDNF cDNA was inserted into the multiple cloning sites between the CBA promoter and WPRE sequence. EGFP (enhanced green fluorescent protein) was cloned into the rAAV plasmid as a control. rAAV serotype 1 vectors were packaged, purified and the vectors were adjusted to 2 × 1013 vg/ml in PBS for injection.
rAAV-mediated BDNF overexpression in hypothalamus
C57BL/6 mice, male, 5 weeks of age, were randomly assigned to receive AAV-BDNF (n = 5–7) or AAV-GFP (n = 5). Mice were anaesthetized with ketamine/xylazine and secured via ear bars and incisor bar on a Kopf stereotaxic frame. A mid-line incision was made through the scalp to reveal the skull and two small holes were drilled into the skull with a dental drill above the injection sites (−1.2 AP, ± 0.5 ML, −6.2 DV, mm from bregma). rAAV vectors were injected bilaterally into the hypothalamus (0.5 μl per site) at a rate of 0.1 μl per minute using a 10 μl Hamilton syringe attached to Micro4 Micro Syringe Pump Controller (World Precision Instruments, Sarasota, FL). At the end of infusion, the syringe was slowly raised from the brain and the scalp was sutured. Animals were placed back into a clean cage and carefully monitored post-surgery until recovery from anesthesia. After three or five weeks, those mice were sacrificed.
AAV-microRNA Experiment
AAV vectors containing microRNA targeting BDNF (miR-Bdnf) and the scramble control (miR-scr) were reported previously(14). We randomly assigned 6-week-old male C57BL/6 mice to receive AAV-miR-Bdnf (n = 15) or AAV-miR-scr (n = 15). We injected 0.7 μl of AAV vectors (1.4 × 1010 per site) bilaterally into the hypothalamus at the stereotaxic coordinates described above. Seven days after surgery, each vector-injected group was split to live in enriched (n = 8 miR-Bdnf, n = 8 miR-scr) or control housing (n = 7 miR-Bdnf, n = 7 miR-scr). Mice were sacrificed after five weeks in the EE.
Propranolol Experiment
We randomly assigned 40 male C57BL/6 mice, 3 weeks of age, to live in an EE or control cages supplied with propranolol (Roxane Laboratories,Inc. Cat# 0054-3727-63) in drinking water (0.5 g/L).
Mifepristone administration
Mifepristone (Abcam Inc. Cat#ab120356) was dissolved in 0.9% NaCl, 0.25% carboxymethylcellulose, 0.2% Tween 20. Male C57BL/6 mice were housed in an EE or control housing and received daily oral gavage of mifepristone (200 mg/kg body weight) or vehicle control for one week.
Statistical analysis
All data were compared using Student t-test and plotted as mean ± SEM in column charts.
RESULTS
EE required CD8 T cells to mediate anticancer phenotype
We have previously reported that CTLs from spleens of EE mice display increased cytotoxicity against B16 melanoma cells in vitro (14). We therefore hypothesized that increased EE-CTL cytotoxicity and tumor infiltration contributed to the slower tumor growth observed in EE mice. To test this hypothesis, we eliminated CTLs prior to melanoma implantation to SE and EE mice using CD8-depleting antibody, with IgG as control. Briefly, 3-week-old C57/B6 (B6) mice were randomly assigned to SE or EE cages for 5-weeks (Fig. 1A), which is the approximate time when EE-associated metabolic changes are observed. EE mice weighed less than SE mice (Supplemental Fig. S1A), and serum leptin concentration was reduced by ~ 70% in EE mice (Supplemental Fig. S1B). Next, EE and SE mice were randomly injected intraperitoneally with either 200 μg of CD8-depleting antibody or IgG control. Twenty-four hours after first dose of antibody, all mice were subcutaneously injected in the hip flank with 1×105 B16 melanoma cells. We confirmed that CTLs were depleted in the blood (Supplemental Fig. S1C). Every seven days, mice were re-injected with their respective antibody. Eighteen days after melanoma implantation all mice were sacrificed.
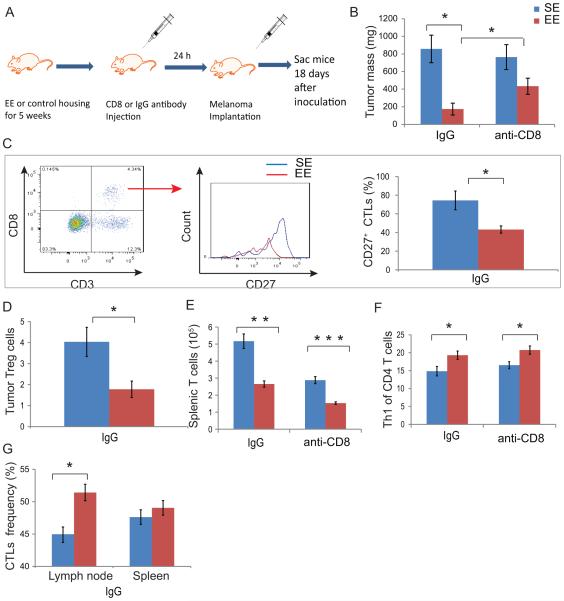
Figure 1. CD8 T cells required to mediate enriched environment’s reduction in tumor growth.
(A) Experimental design of CD8 T cell depletion experiment. (B) Tumor mass among SE-IgG, SE-CD8, EE-IgG, and EE-CD8 groups at time of sacrifice. (C) Tumor infiltrating CTLs were compared for their surface expression of CD27 proteins. (D) Frequency of tumor infiltrating Tregs. (E) T cell numbers in spleens. (F) Th1 phenotype (CXCR3+CCR4−) of helper T cells. In spleens (G) The frequency of CTLs as a proportion of T cells in the lymph node and spleen. Data are represented as the mean ± SEM; n = 9-10 per group. * P <0.05, ** P < 0.01, *** P < 0.001 The experiment was performed once.
Consistent with our previous findings, EE mice receiving IgG (EE-IgG) showed reduced tumor mass by ~ 80% compared to that of SE mice receiving IgG (SE-IgG, Fig. 1B). Although no significant difference was observed between SE mice receiving CD8 (SE-CD8) and IgG (SE-IgG), the CD8 T-cell depletion significantly attenuated EE-induced tumor inhibition (EE-CD8 compared to EE-IgG, Fig. 1B) suggesting an essential role of CD8 T cell in EE-induced tumor inhibition. EE-CD8 tumors were approximately 150% larger than EE-IgG tumors (433±91 mg versus 174±67 mg).
Within the tumor microenvironment, the mononuclear cells per milligram did not significantly differ between SE-IgG and EE-IgG tumors (Supplemental Fig. S1D) nor did the frequency of tumor-infiltrating CD8 CTLs (Supplemental Fig. S1E). We next measured the surface expression of CD27 and PD-1, both of which are targets for clinical immunotherapy (18, 19). CD27 is a member of the tumor necrosis factor receptor superfamily and is expressed on both naïve and long-lived CD8 T cells, but is lost on terminally differentiated effector CTLs (20-22). In the IgG control groups, EE tumors contained a lower frequency of CD27+ effector CTLs when compared to CTLs within SE tumors (Fig. 1C). However, the frequency of CTLs expressing PD-1 or the mean fluorescence intensity of PD-1 expression on CTLs within EE tumors did not change compared to CTLs within SE tumors (Supplemental Fig. S1F). The frequency of Tregs, a helper T cell that inhibits CTLs, was reduced in EE tumors (Fig. 1D).
Total splenocyte (Supplemental Fig. S1G) and total splenic T cell numbers, were reduced in EE mice (Fig. 1E), but the SLT contained T-cell features consistent with greater tumor reactivity. Polarization of CD4 cells toward the helper T cell type 1 (Th1) subset can be defined by surface expression of chemokine receptors CXCR3 and CCR4 (23, 24), and these cells promote protective immunity and induce CTL-mediated tumor eradication (25). CD4 T cells from EE-IgG spleens were polarized towards a Th1 phenotype (Fig. 1F), and this Th1 polarization persisted after CD8 T cell depletion. The frequency of CTLs was increased in the lymph nodes of EE mice (Fig. 1G), and a greater proportion expressed a memory phenotype CD44hiCD122+ (Supplemental Fig. S1H).
EE regulated T-cell phenotypes
We next sought to determine the early effects of EE exposure on T cell homeostasis that may contribute to the anticancer phenotype. We randomly assigned three-week old B6 mice to either SE or EE for one or four weeks. After four weeks exposure to an EE, some characteristics of experiencing the EE were confirmed, including the upregulation of hypothalamic BDNF, the key mediator of EE-induced anticancer and anti-obesity effects (13, 14, 26) (Supplemental Fig. S2A), and reduced body weight (Supplemental Fig. S2B). EE mice showed fewer total splenocytes at four weeks (Supplemental Fig. S2C).
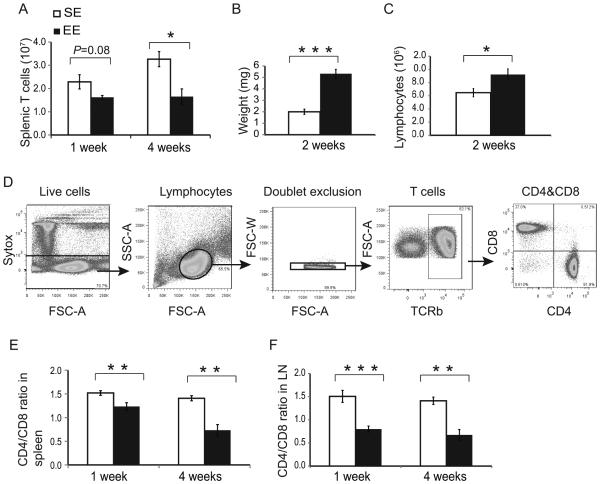
Exposure to an EE for merely one week was sufficient to induce a distinct SLT phenotype in T-cell populations. Consistent with observations from our tumor experiment, total T cells numbers showed a trend of reduction in spleens of EE mice at one week (P = 0.08), and the reduction reached significance by four weeks (Fig. 2A). However, lymph nodes in EE-mice were measurably larger after two-weeks (Fig. 2B) and contained substantially more lymphocytes (Fig. 2C). We used flow cytometry to characterize changes in T-cell populations (Fig. 2D). In EE SLT, the frequency of single positive (SP) CD8 CTLs increased, whereas the frequency of SP CD4 T cells declined, resulting in a depressed CD4:CD8 ratio, and the changes of these populations persisted at four weeks (Fig. 2E). This change of SP CD4:CD8 ratio was also found in lymph nodes at both time points (Fig. 2f). In contrast to the tumor experiment, we did not observe the change in the frequency of memory CTLs in SLT (Supplemental Fig. S2D) suggesting that the presence of the tumor might be responsible for increased memory CTLs in EE mice.
Figure 2. Immune response to short- and long-term environmental enrichment.
(A) The total number of T cells in the spleens. (B) Lymph node mass. (C) The total lymphocytes in lymph node. (D) Flow cytometry of T-cell (TCRβ+) populations. (E) CD4:CD8 ratio in spleen (F) CD4:CD8 ratio lymph nodes following 1-week EE or 4-weeks EE exposure. Data are represented as the mean ± SEM; n = 5 per group. * P <0.05, ** P < 0.01, *** P < 0.001
The experiment was performed once at 4-weeks time point and 3 times at 1-week time point.
Hypothalamic overexpression of BDNF mimicked EE-induced T-cell phenotypes
Hypothalamic BDNF is a key mediator linking EE to the leanness and altered adipokine profile (13, 14). We next investigated whether hypothalamic BDNF also mediated the EE-induced T cell population changes. Mice were randomized to receive stereotaxic injection of recombinant adeno-associated virus (rAAV) vector containing BDNF or a green fluorescent protein (GFP) vector as a control to the hypothalamus and maintained in SE housing. GFP fluorescence confirmed that the transgene expression was largely confined to the arcuate nucleus, the ventromedial, and the dorsomedial nuclei of hypothalamus (Supplemental Fig. S3A). The overexpression of BDNF was confirmed by qRT-PCR five weeks after rAAV injection (Supplemental Fig. S3B). Three weeks after rAAV injection, rAAV-BDNF-mice weighed the same as rAAV-GFP-mice (Supplemental Fig. S3C), but had fewer splenocytes (Supplemental Fig. S3D), similar to the changes observed in EE. A feature of long-term EE exposure is the reduced body weight due to the decrease of adiposity (13). In order to investigate whether the immune modulation was dependent on metabolic regulation, we repeated the hypothalamic BDNF overexpression experiment and examined the immune effects 5 weeks post rAAV injection when significant changes in body weight developed between the two groups (Supplemental Fig. S3C).
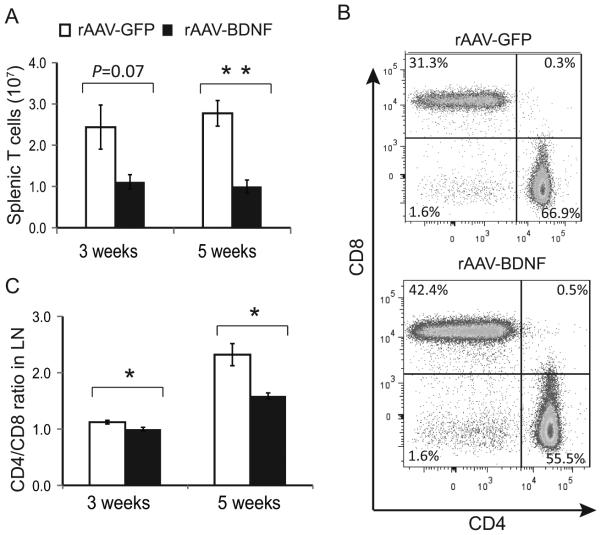
The distribution of T cells within the SLT of BDNF mice showed a similar pattern to EE mice, despite the mice being housed in SE. Total splenic T-cell numbers were reduced by 5 weeks (Fig. 3A). Within the SLT, there was a significant increase in CTL frequency within the T cell population both at 3- and 5-weeks (Fig. 3B and C, Supplemental Fig. S3E, F and G). Similar to EE, there were no changes in chemokine receptors CXCR3, CCR6, and CCR4 (Supplemental Fig. S3H), nor differences in IL-4, IFNγ, or IL-17 by T-helper cells following CD3/CD28 stimulation (Supplemental Fig. S3I). Overall, the results suggested that hypothalamic overexpression of BDNF could mimic the effects of an EE on T-cell populations, and the modulation was independent of changes in body weight.
Figure 3. Overexpressing BDNF in the hypothalamus reproduced EE’s effects on T-cell profile.
(A) TCRβ+ T cells in spleens 3 or 5 weeks post rAAV injection and living in SE housing. (B) Representative flow cytometry of T-cell populations. (C) The T-cell ratio of CD4:CD8 in lymph nodes at 3- and 5-weeks post rAAV injection. The experiment was performed once.
Hypothalamic BDNF depletion inhibited EE-induced CTL phenotypes
To confirm whether hypothalamic BDNF expression is necessary for the EE-induced increase in CTL frequency in SLT, we injected mice with either a rAAV vector expressing a microRNA targeting mouse BDNF (miR-Bdnf) or a microRNA targeting a scrambled sequence (miR-scr) that does not interfere with any known genes as a control. The miR-Bdnf vector was fully characterized previously (14). Mice receiving each rAAV vector were then divided, to live in either SE or EE housing for 5 weeks. We confirmed the knockdown of hypothalamic BDNF by quantitative RT-PCR (Supplemental Fig. S4A). miR-Bdnf blocked the EE-induced weight loss (Supplemental Fig. S4B).
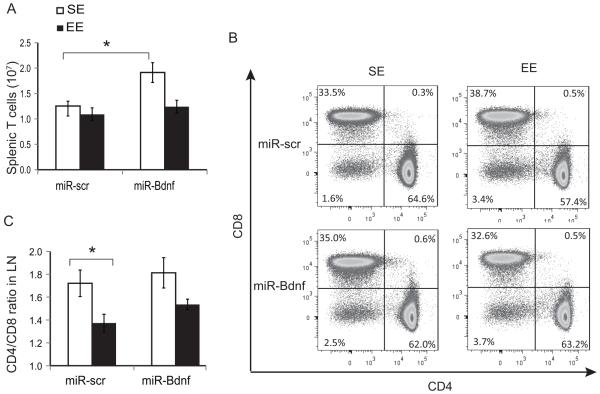
Absolute splenocyte (Supplemental Fig. S4C) and T-cell numbers (Fig. 4A) were increased in miR-Bdnf-SE compared to miR-scr-SE housing. Compared to miR-scr-SE mice, the CD4:CD8 ratio in SLT was reduced in miR-scr-EE mice, whereas miR-Bdnf inhibited this change in T-cell frequency that is closely associated with EE (Fig. 4B, C). Collectively these results showed that knockdown of hypothalamic BDNF prevented the EE-induced changes in CTL frequency within SLT.
Figure 4. Hypothalamic BDNF knockdown inhibited EE-induced T-cell modulation.
(A) Total splenic T cells following 5 weeks EE. (B) Representative flow cytometry of T-cell populations. (C) The CD4:CD8 ratio in the lymph nodes. Data are represented as the mean ± SEM; n = 5-8 per group. * P < 0.05, ** P < 0.01. The experiment was performed once.
β-adrenergic signaling required for EE-induced SLT immune phenotypes
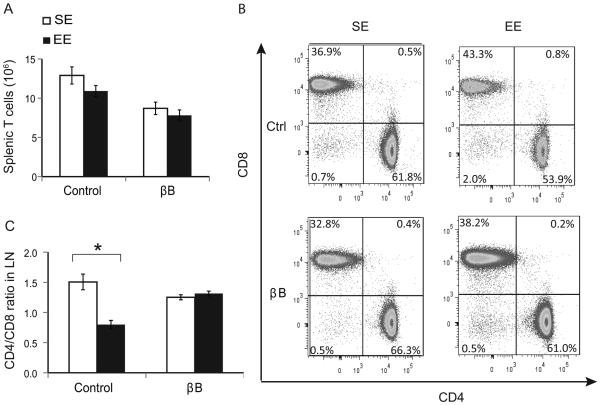
We have previously demonstrated that intact SNS signaling is required for EE’s metabolic and anticancer phenotype (13, 14). We employed the similar approach using the non-specific β-blocker (βB) propranolol to investigate the role of SNS in the EE-induced changes in T-cell homeostasis in SLT. We repeated the experimental design by assigning 3-week-old mice to live in SE or EE cages for one week and half of the SE or EE mice were supplied with propranolol (0.5g/L) in the drinking water, and the other half was supplied with regular water. This dose of propranolol is sufficient to block EE-induced anticancer effects and metabolic regulation (13, 14).
Both propranolol-treated SE and EE mice had similar numbers of T cell (Fig. 5A). However, propranolol completely blocked the EE-induced change in CD4 and CD8 populations in the SLT (Fig. 5B, C).
Figure 5. β-blocker inhibited the EE-induced T-cell phenotypes in SLT.
(A) Absolute number of splenic T cells of mice treated with propranolol and exposed to EE for 1 week. (B) Representative flow cytometry of T-cell populations. (C) The CD4:CD8 ratio in lymph nodes. Data are represented as the mean ± SEM; n = 9-10 per group. * P < 0.05. The experiment was performed 2 times.
HPA axis involved in EE-induced SLT immune phenotypes
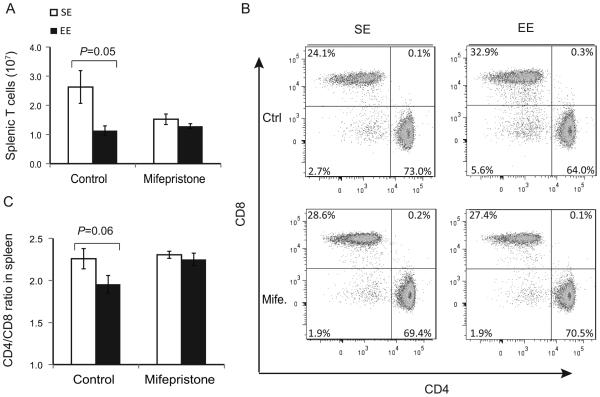
The HPA axis is mildly activated in EE (14). Thus we investigated the role of the HPA axis using mifepristone, an antagonist of cortisol receptors (27). We randomly assigned 3-week old mice to SE or EE housing and administered either mifepristone or solvent as vehicle control by daily oral gavage for one week until sacrifice. Both SE and EE mice receiving mifepristone had similar numbers of T cells (Fig. 6A), but mifepristone completely blocked the EE-induced reduction of the CD4:CD8 T cell ratio in the SLT (Fig. 6B, C).
Figure 6. Mifepristone inhibits the EE effect on CD4:CD8 T cells ratio.
(A) Absolute numbers of splenic T cells of mice treated with mifepristone and exposed to EE for 1 week. (B) Representative flow cytometry of T cell populations. (C) The CD4:CD8 ratio in the spleen. Data are represented as the mean ± SEM; n = 5 per group. The experiment was performed 2 times.
DISCUSSION
In this study, we investigated the contribution of T-cell immunity to the antitumor effect observed in EE mice and defined changes in SLT T-cell homeostasis induced by EE. Our data showed that CTLs were necessary for the anticancer phenotype of an EE in an orthotropic melanoma model and that the tumor microenvironment contained more tumor-responsive T cells in mice living in EE. The EE induced early changes in T-cell homeostasis, including a reduction of the total number of splenic T cells and a shift to CTLs (decreased CD4:CD8 ratio) in SLT. Mechanistically, overexpressing BDNF or knockdown of BDNF in the hypothalamus reproduced or blocked the key features associated with an EE, respectively, including reduced numbers of T cells in SLT and a depressed CD4:CD8 ratio. All primary and secondary immune organs receive substantial sympathetic innervation from sympathetic postganglionic neurons (28, 29). Norepinephrine binds to either α- or β-adrenergic receptors expressed on immune or lymphoid stromal cells, which results in changes in gene expression of various immune cell–derived factors (30, 31). Moreover, the HPA axis is also involved in neuro-immune crosstalk (32). Blockade of the SNS and HPA axes eliminated the two key immunomodulatory features of an EE in SLT: reduced numbers of T cells and a depression of the CD4:CD8 ratio, suggesting both axes contribute to the EE-induced immune phenotypes.
Our previous studies show that metabolic changes are associated with the tumor resistance found after living in an EE (16). In particular, tumor growth is linked with higher β-AR activity in white adipose tissue leading to reduce circulating leptin concentrations (14). Blockade of the leptin drop significantly attenuates the EE-induced tumor inhibition although without full prevention. Our results here demonstrated that T cell immunity played a critical role in the antitumor effects of an EE. Depletion of CTLs also partially but significantly blocked the reduction of tumor mass in EE suggesting that the anti-tumor effects at least partially depend on CTLs. Although CTL expression of PD-1, an immune inhibitory molecule, was unchanged, CTL expression of CD27 was significantly reduced, indicating that the tumor microenvironment in EE mice contains more activated CTLs. In addition, fewer Tregs were found in the tumors of EE mice despite more Tregs in SLT (not shown). No changes of Treg percentage or memory CTL percentage observed in the tumor-bearing mice were found in EE animals in the absence of tumor, suggesting that these differences might be due to T cells responding differently in the context of health and disease. The EE significantly enlarged lymph node mass. The EE led to a higher proportion of CD8 CTLs and lower proportion of CD4 T cells, consistent with our previous finding of enhanced CD8 cytotoxicity in vitro (14).
Overall, the new findings suggest that an EE induces an improved immune microenvironment of tumor, consistent with reports that the increased proportions of CD8+ T cells and decreased Tregs correlate with a better prognosis of cancer (33, 34). Taken together, both metabolic regulation and immune modulation contribute to the robust inhibition of melanoma progression induced by an EE (Fig. 7), supporting the notion that combination therapies targeting multiple cancer-promoting capabilities are likely more effective and durable (35). We have previously reported that EE also enhances natural kill (NK) cells activity (14). A recent report from others has shown that NK cells play a critical role in the anticancer effect of an EE in a mouse model of glioma (36). Investigations on how an EE regulates additional immune cell populations and their roles in cancer inhibition are underway.
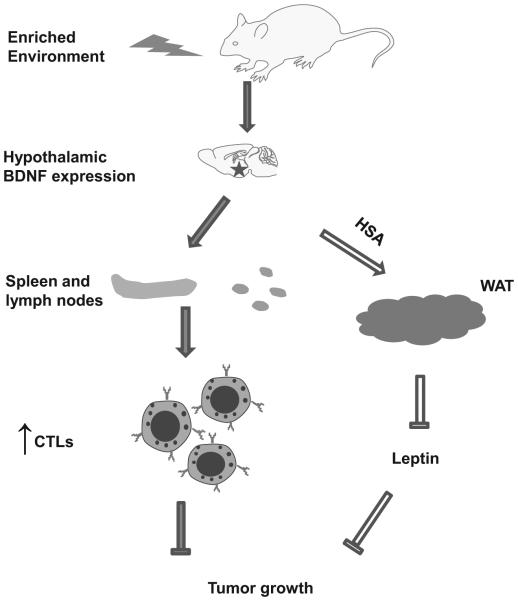
Figure 7. Mechanisms of EE-induced tumor inhibition.
Induction of hypothalamic BDNF expression in response to environmental stimuli leads to the activation of SNS and HPA axes, which enhance T cell immunity to ward off cancer. Our previous finding showed hypothalamic BDNF expression lead to metabolic change and decreased leptin level. All these effects contribute to the inhibition of tumor growth. See discussion for details.
Hypothalamic BDNF expression is necessary for mediating the cancer and metabolic phenotypes seen after living an EE (13, 14). Therefore, we explored whether hypothalamic BDNF could also mediate the EE-induced peripheral CD4:CD8 ratio change. We observed that rAAV-mediated overexpression of BDNF in the hypothalamus lead to a decreased CD4:CD8 ratio in SLT and that depletion of hypothalamic BDNF could eliminate the CD4:CD8 ratio change. These observations indicate that manipulation of a single molecule in the brain could regulate T-cell homeostasis. And this mechanism could potentially be harnessed to strengthen the efficiency of immunotherapies.
Crosstalk between nervous system and immune system has been intensively investigated. However, most research has employed models causing distress, such as social isolation, repeated social defeat, and chronic stress models that often are associated with impairment of immune functions (37-40). In contrast, scarce attention has been paid to eustress that is associated with enhanced adaption to environmental demands, improved general health, and resistance to multiple diseases. An EE is thought to be a good model to study eustress response (41). For example, in contrast to repeated social defeat (42), which stimulates cancer growth through stress-induced dysregulation of inflammatory mediators and the tumor microenvironment (43), EE suppresses cancer progression through enhanced immunity as well as regulation of whole body metabolism and cellular metabolism. Our data showed that some immune phenotypes occurred prior to changes of adiposity or weight in response to EE, suggesting that the immune response and the metabolic response might be parallel. However, the precise relationship between the two phenotypes and whether EE could influence immune cell metabolism require further investigations.
Environmental stress is an important confounding factor of preclinical studies. A recent report demonstrates that thermoneutral ambient temperature (30 °C) increases sensitivity to chemotherapy in a pancreatic cancer mouse model and the underlying mechanism is the activation of β2-adrenergic receptor in tumors (44). In our EE experiments, all mice were housed at 22 °C. It is possible that the extra bedding in EE might provide slightly more additional warmth. However, the EE significantly elevated norepinephrine (NE) in adipose tissue and showed a trend of increased NE in serum (13, 14). Therefore, EE would not antagonize β-adrenergic receptors in tumor cells, on the contrary, there might be a risk of activation. However, the effects of an EE on metabolism (drop of leptin, increase of adiponectin, and enhanced insulin sensitivity) and immune functions (T cells and NK cells) overweigh greatly the potential negative effect of SNS activation directly on tumor cells. Our preliminary data show that an EE under thermoneutral conditions led to the same metabolic effects (leanness, drop of leptin, and induction of beige cells), even to a greater degree, than that at 22 °C (data not shown). This finding suggests that removing cold stress and the associated SNS activation could amplify the HSA axis that elevates the SNS tone preferentially to white fat contributing to the EE-induced tumor resistance. We are investigating other effects associated with an EE, including immune modulations at thermoneutrality.
In summary, an EE beneficially modulated metabolism and immunity, leading to inhibition of tumor progression. Previous research identified hypothalamic BDNF as a key mediator linking social and physical environment to metabolism and cancer progression (13, 14). Our findings showed that hypothalamic BDNF also played an important role in EE-induced immune regulation, suggesting the possibility of manipulating a single molecule in the brain to modulate multiple peripheral systems for therapeutic applications.
Supplementary Material
ACKNOWLEDGEMENT
The study was supported in part by the NIH grant CA166590, CA178227, CA163640 to L.C., and NIH grant CA163205, CA068458 to M.C.
Footnotes
The authors declare no conflict of interest.
References
- 1.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9. Epub 2004/10/07. doi: S1470204504015979 [pii] 10.1016/S1470-2045(04)01597-9. PubMed PMID: 15465465. [DOI] [PubMed] [Google Scholar]
- 2.Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23(1):10–5. doi: 10.1016/j.bbi.2008.06.007. Epub 2008/07/22. doi: 10.1016/j.bbi.2008.06.007 S0889-1591(08)00296-1 [pii]. PubMed PMID: 18638541; PubMed Central PMCID: PMC2630522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–75. doi: 10.1038/ncponc1134. Epub 2008/05/22. doi: 10.1038/ncponc1134 ncponc1134 [pii]. PubMed PMID: 18493231. [DOI] [PubMed] [Google Scholar]
- 4.Obeid EI, Conzen SD. The role of adrenergic signaling in breast cancer biology. Cancer Biomark. 2013;13(3):161–9. doi: 10.3233/CBM-130347. doi: 10.3233/CBM-130347. PubMed PMID: 23912488. [DOI] [PubMed] [Google Scholar]
- 5.De Giorgi V, Grazzini M, Gandini S, Benemei S, Asbury CD, Marchionni N, et al. beta-adrenergic-blocking drugs and melanoma: current state of the art. Expert Rev Anticancer Ther. 2012;12(11):1461–7. doi: 10.1586/era.12.118. doi: 10.1586/era.12.118. PubMed PMID: 23249110. [DOI] [PubMed] [Google Scholar]
- 6.Mussi C, Angelini C, Crippa S, Caprotti R, Fumagalli L, Motta V, et al. Alteration of hypothalamus-pituitary-adrenal glands axis in colorectal cancer patients. Hepatogastroenterology. 2003;50(Suppl 2):ccxxviii–ccxxxi. Epub 2004/07/13. PubMed PMID: 15244187. [PubMed] [Google Scholar]
- 7.Lorton D, Bellinger DL. Molecular mechanisms underlying beta-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci. 2015;16(3):5635–65. doi: 10.3390/ijms16035635. doi: 10.3390/ijms16035635. PubMed PMID: 25768345; PubMed Central PMCID: PMCPMC4394497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. Epub 2000/12/21. PubMed PMID: 11121511. [PubMed] [Google Scholar]
- 9.Schauenstein K, Rinner I, Felsner P, Liebmann P, Haas HS, Wolfler A, et al. The dialogue between the brain and immune system involves not only the HPA-axis. Z Rheumatol. 2000;59(Suppl 2):II/49–53. doi: 10.1007/s003930070018. Epub 2001/01/13. PubMed PMID: 11155804. [DOI] [PubMed] [Google Scholar]
- 10.Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115–28. doi: 10.1007/s00262-014-1617-9. Epub 2014/10/14. doi: 10.1007/s00262-014-1617-9. PubMed PMID: 25307152; PubMed Central PMCID: PMC4325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. Epub 2006/08/23. doi: nrn1970 [pii] 10.1038/nrn1970. PubMed PMID: 16924259. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed AH, Zhu SW, Darmopil S, Hjerling-Leffler J, Ernfors P, Winblad B, et al. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–33. doi: 10.1016/S0079-6123(02)38074-9. Epub 2002/11/16. doi: S0079-6123(02)38074-9 [pii] 10.1016/S0079-6123(02)38074-9. PubMed PMID: 12432766. [DOI] [PubMed] [Google Scholar]
- 13.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–38. doi: 10.1016/j.cmet.2011.06.020. doi: 10.1016/j.cmet.2011.06.020. PubMed PMID: 21907139; PubMed Central PMCID: PMCPMC3172615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142(1):52–64. doi: 10.1016/j.cell.2010.05.029. doi: 10.1016/j.cell.2010.05.029. PubMed PMID: 20603014; PubMed Central PMCID: PMCPMC3784009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachat-Kappes R, Pinel A, Combe K, Lamas B, Farges MC, Rossary A, et al. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One. 2012;7(12):e51525. doi: 10.1371/journal.pone.0051525. doi: 10.1371/journal.pone.0051525. PubMed PMID: 23272114; PubMed Central PMCID: PMCPMC3521763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Gan Y, Fan Y, Wu Y, Lin H, Song Y, et al. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci Rep. 2015;5:7856. doi: 10.1038/srep07856. doi: 10.1038/srep07856. PubMed PMID: 25598223; PubMed Central PMCID: PMCPMC4297951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater AM, Cao L. A Protocol for Housing Mice in an Enriched Environment. J Vis Exp. 2015;(100):e52874. doi: 10.3791/52874. doi: 10.3791/52874. PubMed PMID: 26131694; PubMed Central PMCID: PMCPMC4544994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Ven K, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy. 2015;7(6):655–67. doi: 10.2217/imt.15.32. doi: 10.2217/imt.15.32. PubMed PMID: 26098609. [DOI] [PubMed] [Google Scholar]
- 19.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–7. doi: 10.1158/1078-0432.CCR-06-2746. Epub 2007/04/04. doi: 13/7/2151 [pii] 10.1158/1078-0432.CCR-06-2746. PubMed PMID: 17404099. [DOI] [PubMed] [Google Scholar]
- 20.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16(8):871–9. doi: 10.1038/ni.3224. doi: 10.1038/ni.3224. PubMed PMID: 26147684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35(5):792–805. doi: 10.1016/j.immuni.2011.09.017. doi: 10.1016/j.immuni.2011.09.017. PubMed PMID: 22118527; PubMed Central PMCID: PMCPMC3431922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, et al. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35(11):3131–41. doi: 10.1002/eji.200425770. doi: 10.1002/eji.200425770. PubMed PMID: 16220536. [DOI] [PubMed] [Google Scholar]
- 23.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108(9):1331–9. doi: 10.1172/JCI13543. doi: 10.1172/JCI13543. PubMed PMID: 11696578; PubMed Central PMCID: PMCPMC209443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Lukacs NW, Lawless VA, Kunkel SL, Kaplan MH. Cutting edge: differential expression of chemokines in Th1 and Th2 cells is dependent on Stat6 but not Stat4. J Immunol. 2000;165(1):10–4. doi: 10.4049/jimmunol.165.1.10. Epub 2000/06/22. doi: ji_v165n1p10 [pii]. PubMed PMID: 10861028. [DOI] [PubMed] [Google Scholar]
- 25.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi: 10.1038/nature03337. doi: 10.1038/nature03337. PubMed PMID: 15744305. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Sanroman D, Sanchis-Segura C, Toledo R, Hernandez ME, Manzo J, Miquel M. The effects of enriched environment on BDNF expression in the mouse cerebellum depending on the length of exposure. Behav Brain Res. 2013;243:118–28. doi: 10.1016/j.bbr.2012.12.047. Epub 2013/01/09. doi: 10.1016/j.bbr.2012.12.047 S0166-4328(12)00837-6 [pii]. PubMed PMID: 23295397. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois S, Pfahl M, Baulieu EE. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. EMBO J. 1984;3(4):751–5. doi: 10.1002/j.1460-2075.1984.tb01879.x. PubMed PMID: 6327286; PubMed Central PMCID: PMCPMC557421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21(6):736–45. doi: 10.1016/j.bbi.2007.03.008. doi: 10.1016/j.bbi.2007.03.008. PubMed PMID: 17467231; PubMed Central PMCID: PMCPMC1986730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7(9):2255–61. doi: 10.1016/0306-4522(82)90135-x. Epub 1982/01/01. doi: 0306-4522(82)90135-X [pii]. PubMed PMID: 7145093. [DOI] [PubMed] [Google Scholar]
- 30.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79(6):1093–104. doi: 10.1189/jlb.1105625. Epub 2006/03/15. doi: jlb.1105625 [pii] 10.1189/jlb.1105625. PubMed PMID: 16531560. [DOI] [PubMed] [Google Scholar]
- 31.Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(3):1177–200. doi: 10.1002/cphy.c130051. doi: 10.1002/cphy.c130051. PubMed PMID: 24944034; PubMed Central PMCID: PMCPMC4374437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melmed S. Series introduction. The immuno-neuroendocrine interface. J Clin Invest. 2001;108(11):1563–6. doi: 10.1172/JCI14604. Epub 2001/12/06. doi: 10.1172/JCI14604. PubMed PMID: 11733548; PubMed Central PMCID: PMC201000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–71. doi: 10.1158/0008-5472.CAN-11-3687. doi: 10.1158/0008-5472.CAN-11-3687. PubMed PMID: 22549946; PubMed Central PMCID: PMCPMC3342842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. doi: 10.1371/journal.pone.0080063. PubMed PMID: 24244610; PubMed Central PMCID: PMCPMC3828213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. doi: 10.1016/j.cell.2011.02.013. PubMed PMID: 21376230. [DOI] [PubMed] [Google Scholar]
- 36.Garofalo S, D'Alessandro G, Chece G, Brau F, Maggi L, Rosa A, et al. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623. doi: 10.1038/ncomms7623. doi: 10.1038/ncomms7623. PubMed PMID: 25818172; PubMed Central PMCID: PMCPMC4389244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W, Yamaura T, Murakami K, Murata J, Matsumoto K, Watanabe H, et al. Social isolation stress enhanced liver metastasis of murine colon 26-L5 carcinoma cells by suppressing immune responses in mice. Life Sci. 2000;66(19):1827–38. doi: 10.1016/s0024-3205(00)00506-3. Epub 2000/05/16. doi: S0024320500005063 [pii]. PubMed PMID: 10809180. [DOI] [PubMed] [Google Scholar]
- 38.Cruces J, Venero C, Pereda-Peeez I, De la Fuente M. The effect of psychological stress and social isolation on neuroimmunoendocrine communication. Curr Pharm Des. 2014;20(29):4608–28. doi: 10.2174/1381612820666140130205822. Epub 2014/03/05. doi: CPD-EPUB-58922 [pii]. PubMed PMID: 24588822. [DOI] [PubMed] [Google Scholar]
- 39.Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–42. doi: 10.1016/j.neuroscience.2015.01.001. Epub 2015/01/18. doi: 10.1016/j.neuroscience.2015.01.001 S0306-4522(15)00013-5 [pii]. PubMed PMID: 25596319; PubMed Central PMCID: PMC4536813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. Epub 2004/07/15. doi: 10.1037/0033-2909.130.4.601 2004-15935-004 [pii]. PubMed PMID: 15250815; PubMed Central PMCID: PMC1361287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;35:331–45. doi: 10.1146/annurev-neuro-062111-150546. doi: 10.1146/annurev-neuro-062111-150546. PubMed PMID: 22462541. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Liu BJ, Ji S, Wu JF, Xu CQ, Du YJ, et al. Social defeat stress promotes tumor growth and angiogenesis by upregulating vascular endothelial growth factor/extracellular signal-regulated kinase/matrix metalloproteinase signaling in a mouse model of lung carcinoma. Mol Med Rep. 2015;12(1):1405–12. doi: 10.3892/mmr.2015.3559. doi: 10.3892/mmr.2015.3559. PubMed PMID: 25824133. [DOI] [PubMed] [Google Scholar]
- 43.Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(Suppl):S41–7. doi: 10.1016/j.bbi.2012.06.015. doi: 10.1016/j.bbi.2012.06.015. PubMed PMID: 22790082. [DOI] [PubMed] [Google Scholar]
- 44.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nat Commun. 2015;6:6426. doi: 10.1038/ncomms7426. doi: 10.1038/ncomms7426. PubMed PMID: 25756236; PubMed Central PMCID: PMCPMC4471870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.