Abstract
Purpose of review
Cell-cycle checkpoints are surveillance mechanisms in eukaryotic cells that monitor the condition of the cell, repair cellular damages, and allow the cell to progress through the various phases of the cell cycle when conditions become favorable. We review recent advances in hematopoietic stem cell (HSC) biology, highlighting a mitochondrial metabolic checkpoint that is essential for HSCs to return to the quiescent state.
Recent findings
As quiescent HSCs enter the cell cycle, mitochondrial biogenesis is induced, which is associated with increased mitochondrial protein folding stress and mitochondrial oxidative stress. Mitochondrial unfolded protein response and mitochondrial oxidative stress response are activated to alleviate stresses and allow HSCs to exit the cell cycle and return to quiescence. Other mitochondrial maintenance mechanisms include mitophagy and asymmetric segregation of aged mitochondria.
Summary
Because loss of HSC quiescence results in the depletion of the HSC pool and compromised tissue regeneration, deciphering the molecular mechanisms that regulate the mitochondrial metabolic checkpoint in HSCs will increase our understanding of hematopoiesis and how it becomes dysregulated under pathological conditions and during aging. More broadly, this knowledge is instrumental for understanding the maintenance of cells that convert between quiescence and proliferation to support their physiological functions.
Keywords: aging, checkpoint, hematopoietic stem cell, mitochondria
INTRODUCTION
Hematopoietic stem cells (HSCs) persist throughout the lifespan of an organism to maintain the blood system [1]. Although HSCs possess a robust proliferative capacity that allows them to self-renew and to differentiate, the cell-cycle activity of HSCs changes over the lifetime. During development, nearly all fetal HSCs are actively cycling to meet the physiological demands of the growing organism [2,3]. In contrast, about 90% of adult HSCs exit the cell cycle and reside in the quiescent state under homeostatic conditions [2,4]. HSC quiescence has long been viewed as a result of reduced physiological demands once the organism enters the adulthood. However, it has become increasingly appreciated that the maintenance of HSC quiescence is a highly regulated protective mechanism that prevents cell death and the depletion of the HSC pool [5]. Thus, deciphering the regulation of HSC quiescence will deepen our understanding of hematopoiesis under physiological and pathophysiological conditions.
In this review, we summarize the recent advances in the regulation of HSC quiescence and highlight a mitochondrial metabolic checkpoint that is essential for the maintenance of the quiescent state. Beginning with a brief summary of the classic cell-cycle checkpoints, we present evidence for the existence of a mitochondrial metabolic checkpoint in HSCs at the transition between quiescence (G0) and G1 phases of the cell cycle. We further discuss the regulatory mechanisms of the mitochondrial metabolic checkpoint in HSCs. Finally, we speculate on the physiological importance of the mitochondrial metabolic checkpoint by examining how this checkpoint becomes dysregulated in HSCs during the aging process.
CELL-CYCLE CHECKPOINTS
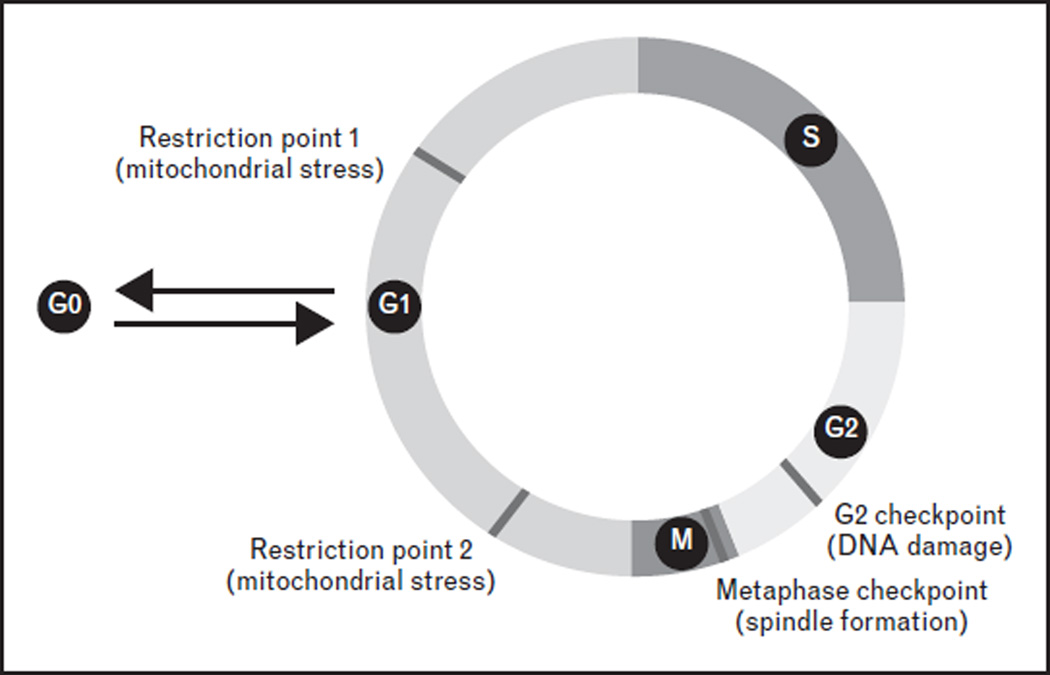
The cell-cycle checkpoints serve at the critical points of the cell cycle when the conditions of the cell are assessed and defects are repaired before the cell progresses to the next phase of the cell cycle [6]. Severe defects that are beyond repair result in cell death. Historically, cell-cycle studies have been focused on understanding how cells maintain their genomic integrity during cell division, because a major event during the cell cycle is the replication of the genome and segregation of the chromosomes into daughter cells. As a result, well established cell-cycle checkpoints include the G2 checkpoint that monitors DNA damage [7] and the metaphase checkpoint that surveils spindle formation [8] (Fig. 1). Molecular control of the DNA damage checkpoint and the spindle checkpoint has been extensively studied, illuminating the actions of sensors, transducers, and effectors that orchestrate the resolution of cellular damage and dictate cell fate decisions.
FIGURE 1.
Cell-cycle checkpoints. The cell-cycle checkpoints serve at critical points of the cell cycle when the conditions of the cell are assessed and defects are repaired before the cell progresses to the next phase of the cell cycle. Classic cell-cycle checkpoints include the G2 checkpoint that monitors DNA damage and the metaphase checkpoint that surveils spindle formation. Another cell-cycle checkpoint is the restriction point at the G1 phase of the cell cycle. Recent advances in hematopoietic stem cell (HSC) quiescence support the existence of a mitochondrial metabolic checkpoint in HSCs at the transition between G0 and G1 phases of the cell cycle (restriction point 1). Studies in human mammary epithelial cells identified a novel exit from mitosis to G0 (restriction point 2), which may also monitor mitochondrial stresses.
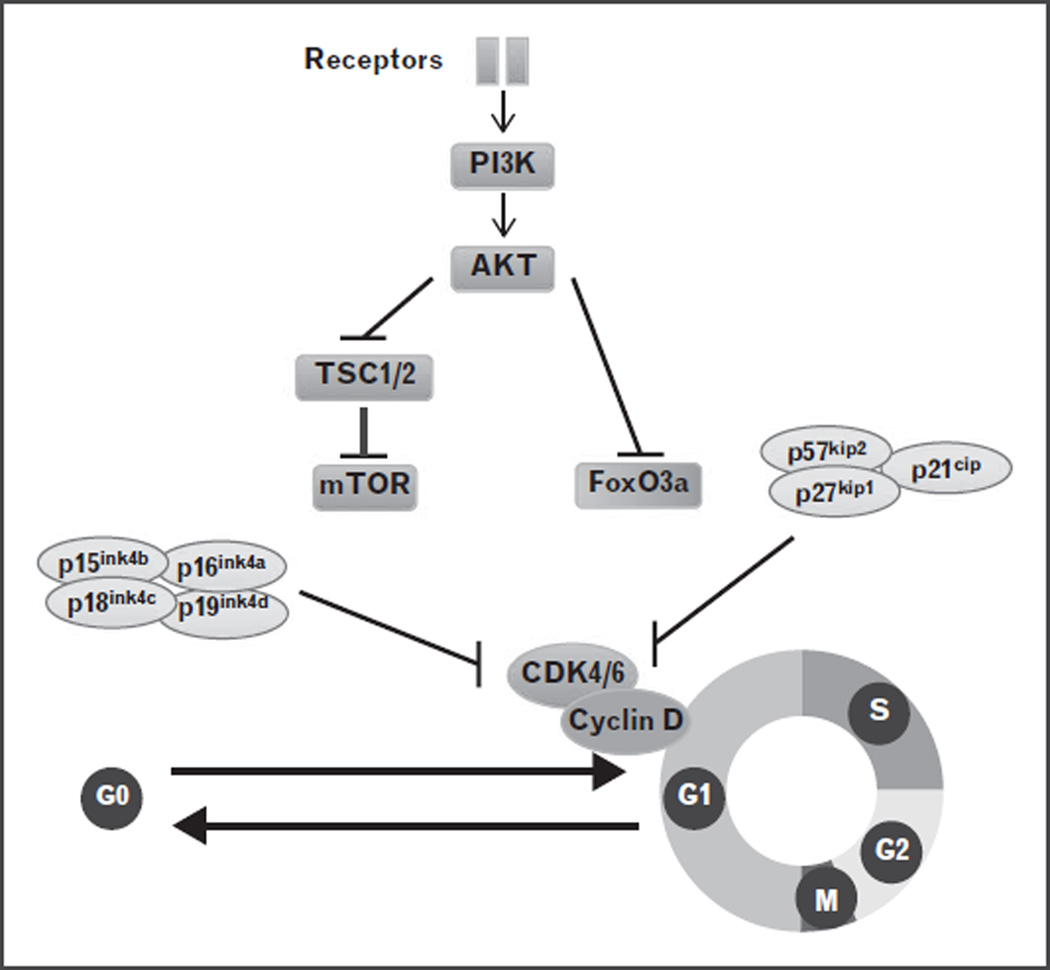
Another cell-cycle checkpoint is the restriction point at the G1 phase of the cell cycle. The entry of a quiescent cell into the G1 phase of the cell cycle requires signals from extracellular growth factors, which elicit a cascade of signaling events, prominently the PI3K/Akt/mTOR pathway, and ultimately result in the regulation of the core cell-cycle machinery, the cyclin D–Cdk4/6 complex [9,10] (Fig. 2). CDK inhibitors, such as Ink4 proteins and p21, counteract the cyclin–cdk activity. Dysregulation of the PI3K/Akt/mTOR pathway or the CDK inhibitors results in loss of HSC quiescence and the depletion of the HSC pool [11–22]. These observations underscore the importance of the regulation of the restriction point for HSC maintenance, but also beg the questions of what cellular conditions are assessed at the restriction point and how the defects are repaired.
FIGURE 2.
Cell-cycle entry regulated by a network of protein regulators. The entry of a quiescent cell into the G1 phase of the cell cycle requires signals from extracellular growth factors, which elicit a cascade of signaling events, mainly the PI3K/Akt/mTOR pathway, and ultimately result in the regulation of the core cell-cycle machinery, the cyclin D–Cdk4/6 complex. CDK inhibitors, such as Ink4 proteins and p21, counteract the cyclin–cdk activity.
EVIDENCE FOR A MITOCHONDRIAL METABOLIC CHECKPOINT IN HEMATOPOIETIC STEM CELLS
Quiescent HSCs have low numbers of mitochondria [23]. This observation is in keeping with quiescent HSCs’ intrinsic bioenergetic demands and extrinsic environmental cues. HSCs primarily rely on glycolysis for energy production. Compared to mitochondrial oxidative phosphorylation, glycolysis is much less efficient for energy production but is sufficient to support the low energy requirement of quiescent HSCs. HSCs reside in a hypoxic bone marrow niche, which triggers a metabolic switch from oxidative phosphorylation to glycolysis throughHIF1a signaling [23]. This metabolic feature is essential for the maintenance of HSCs, because less reactive oxygen species (ROS), the byproducts of respiration, are produced [24,25▪▪].
A major functional output of mitogenic signals and the subsequent activation of the PI3K/Akt/mTOR pathway is the upregulation of translation, resulting in not only the production of proteins, but increased mitochondrial biogenesis that is necessary for the metabolic switch to the energy efficient oxidative phosphorylation to meet the increasing energy demands [9,26]. Because a major event during the G0 to G1 transition is mitochondrial biogenesis, this raises a possibility that a cellular condition that needs to be assessed at the restriction checkpoint is the health of the mitochondria.
Deletion of TSC1, a negative regulator of mTOR, leads to increased mitochondrial biogenesis, loss of HSC quiescence, and depletion of the HSC pool [11]. Ablation of LKB1, an activator of the energy sensor AMPK and a negative regulator of mTOR, results in increased mitochondrial mass but reduced mitochondrial activity, loss of HSC quiescence, and transient expansion of the HSC pool followed by exhaustion [27–29]. These observations support the existence of a mitochondrial metabolic checkpoint in HSCs at the transition between G0 and G1 phases of the cell cycle, and suggest that the mitochondrial stress response leads to the reduction of mitochondrial mass and the return to quiescence. Failure to repair mitochondrial stresses results in cell death (Fig. 1).
MOLECULAR REGULATION OF THE MITOCHONDRIAL METABOLIC CHECKPOINT IN HEMATOPOIETIC STEM CELLS
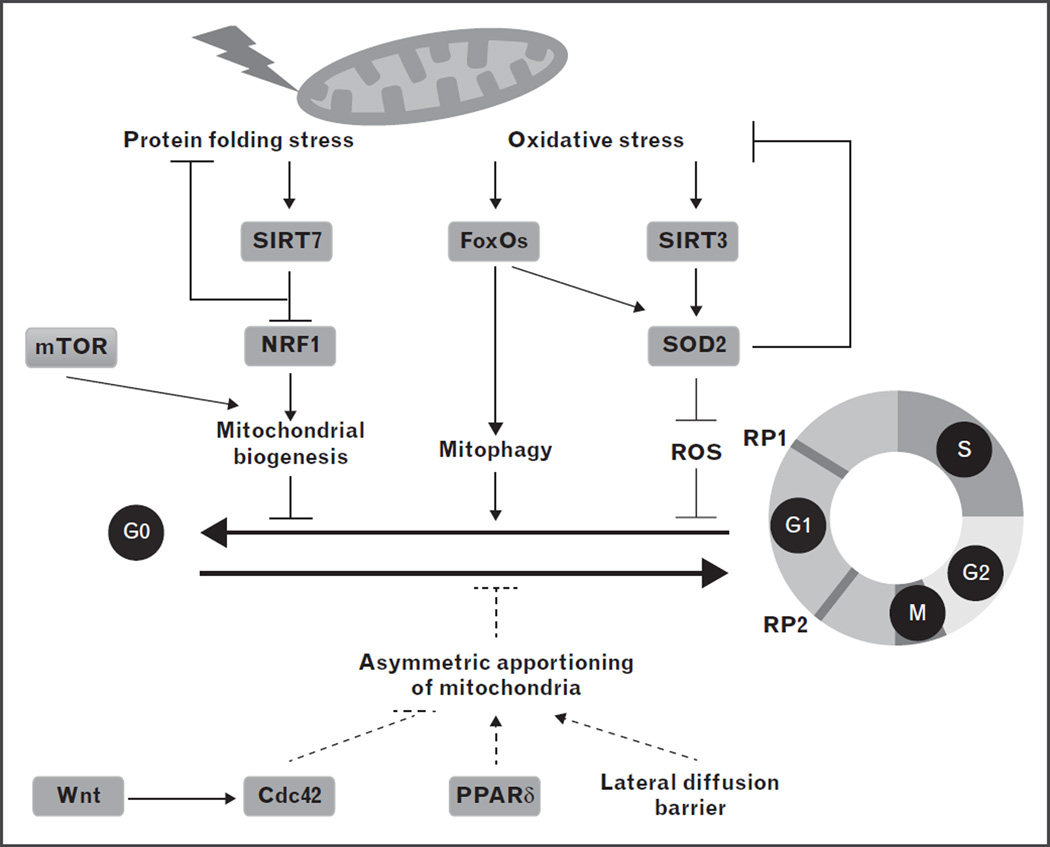
In this section, we highlight recent studies that have uncovered the genes and mechanisms that regulate the mitochondrial metabolic checkpoint in HSCs (Fig. 3). We also propose potential mechanisms based on the studies in other stem cell populations or somatic cells that need to be tested experimentally in HSCs. These discoveries not only solidify the existence of the mitochondrial metabolic checkpoint at the transition between G0 and G1, but also provide valuable information on how mitochondrial stress is sensed and responded to at such a checkpoint.
FIGURE 3.
Molecular regulation of the mitochondrial metabolic checkpoint in hematopoietic stem cells (HSCs). As quiescent HSCs enter the cell cycle, mitochondrial biogenesis is induced, which is associated with increased mitochondrial protein folding stress and mitochondrial oxidative stress. SIRT7 represses the activity of NRF1 to suppress mitochondrial translation and alleviate mitochondrial protein folding stress, and to reduce mitochondrial activity and cell growth. SIRT3 and FoxOs act on SOD2 concertedly to reduce oxidative stress and repress proliferation. In addition to reducing oxidative stress, FoxO3a also directs an autophagy program in HSCs to disassemble damaged mitochondria. Wnt signaling, Cdc42, PPARd, and potentially the lateral diffusion barrier regulate asymmetric division of cellular components, likely including mitochondria, in HSC. Asymmetric apportioning of mitochondria is required to maintain stemness and likely regulates restriction point 2 (RP2). Established links are in solid lines and hypothesized links are in dashed lines. ROS, reactive oxygen species; RP1, restriction point 1.
The mitochondrial unfolded protein response
The mitochondrial unfolded protein response (UPRmt) is activated in response to the perturbation of mitochondrial proteostasis, resulting in the transcriptional upregulation of mitochondrial chaperones or proteases and reestablishement of proteostasis [30,31]. The physiological significance of this pathway was first realized in Caenorhabditis elegans, in which the UPRmt is activated during a developmental stage when a burst of mitochondrial biogenesis takes place and is attenuated when mitochondrial biogenesis subsides [30]. It was therefore hypothesized that in mammals, the UPRmt pathway is important for cells that experience bursts of mitochondrial biogenesis and convert between growth states with markedly different bioenergetic demands, such as stem cells [32▪▪].
A recent study identified a novel regulatory branch of the UPRmt, which is regulated by the interplay of SIRT7, a histone deacetylase, and NRF1, a master regulator of the mitochondria [32▪▪,33]. In response to mitochondrial protein folding stress, SIRT7 represses the activity of NRF1 to suppress mitochondrial translation and alleviate mitochondrial protein folding stress, and to reduce mitochondrial activity and cell growth. Deletion of SIRT7 leads to increased mitochondrial biogenesis and mitochondrial protein folding stress in HSCs, loss of HSC quiescence, and depletion of the HSC pool upon stress [32▪▪].
These findings provide the first evidence that mitochondrial protein folding stress is surveilled at the restriction point and that activation of the UPRmt leads to reduced mitochondrial biogenesis and transition to HSC quiescence. Failure to engage the surveillance of mitochondrial protein folding stress leads to cell death. The role of the canonical UPRmt components, such as the mitochondrial chaperones HSP60 and HSP10, and the mitochondrial protease ClpP, have not been tested in HSCs, but these experiments will be informative in understanding the role of the UPRmt-mediated metabolic checkpoint in regulating HSC quiescence.
The mitochondrial oxidative stress response
Mitochondrial biogenesis leads to increased production of ROS. A tight correlation has been observed between increased ROS levels and HSC proliferation and death in numerous mouse models [11,15,20,34–38]. These observations suggest that mitochondrial biogenesis at the transition from G0 to G1 not only provides HSCs energy supplies to fuel proliferation, but also generates ROS, which can function as signaling molecules to induce proliferation and differentiation [39]. However, accumulation of ROS can lead to oxidative stress and cell death. Thus, one cellular condition that needs to be assessed at the restriction point is the cellular ROS level. The FoxO family of transcription factors, major downstream effectors of the PI3K/Akt pathway, regulate the oxidative stress response by inducing the expression of antioxidants, such as SOD2 [40]. Ablation of FoxOs leads to increased cellular ROS, loss of HSC quiescence, and HSC death [20,37].
SOD2 and likely other cellular antioxidants are modified by acetylation in cells [41–44]. SIRT3, a mitochondrial deacetylase, targets SOD2 for deacetylation at critical lysine residues and enhances the enzymatic activity of SOD2 [43,44]. SIRT3 deficiency results in increased ROS levels, proliferation and death of HSCs under transplantation stress, and the depletion of the HSC pool at an old age [34]. Thus, FoxOs and SIRT3 act on SOD2 concertedly at the transcriptional and posttranslational levels to reduce oxidative stress in HSCs. SIRT3 is highly enriched in HSCs but its expression is much suppressed in the differentiated progeny [34]. Similarly, FoxOs tend to accumulate in the nucleus of HSCs but are found primarily in the cytosol of differentiated cells [45], underscoring the importance of the oxidative stress response in regulating the mitochondrial metabolic checkpoint in HSCs.
Mitophagy
Autophagy is a regulated cellular process that disassembles unnecessary or dysfunctional cellular components [46]. Selective degradation of mitochondria by autophagy, or mitophagy, often occurs to defective mitochondria following damage or stress [47]. Autophagy is induced in HSCs when Lkb1 is ablated and defective mitochondria accumulate [28]. Deletion of Atg7, an essential autophagy gene, in the hematopoietic system results in the accumulation of defective mitochondria and ROS, and a reduction in HSC number [48]. FoxO3a directs an autophagy program in HSCs and in FoxO3a deficient HSCs, autophagy is compromised, contributing in part to loss of HSC quiescence [49]. Together, these studies suggest that HSCs maintain a robust autophagy program, which senses mitochondrial stress, disassembles the damaged mitochondria, and retains HSCs in the quiescent state.
Asymmetric apportioning of mitochondria
Stem cells divide asymmetrically to generate two daughter cells with distinct fates. A recent study investigated the organelle distribution during the division of human mammary stem-like cells and found that old mitochondria but not other cellular organelles are asymmetrically apportioned between daughter cells, and daughter cells that received fewer old mitochondria maintained the stem cell fate [50▪▪]. Although this phenomenon has not been tested in HSCs, it raises the possibility that asymmetric apportioning of mitochondria is a potential mechanism for ensuring the mitochondrial integrity of HSCs.
The regulation of asymmetric apportioning of mitochondria is unknown. Much work is needed to elucidate whether and how mitochondria in HSCs are asymmetrically partitioned into daughter cells. A potential clue comes from the studies of the small RhoGTPase Cdc42 and PPARd. Elevated activity of Cdc42 is linked to a loss of polarity in HSCs and their reduced reconstitution capacity [51]. Loss of PPARd or inhibition of mitochondrial fatty acid oxidation induces symmetric division of HSCs and loss of HSC maintenance [52]. An additional lead is a recent finding in neural stem cells, which generate a lateral diffusion barrier in the membrane of the endoplasmic reticulum to promote asymmetric segregation of cellular components [53▪▪].
Another intriguing question is how this mitochondrial surveillance program controls HSC quiescence, because this regulation must not occur at the transition between G0 and G1, but after mitosis. A recent study in human mammary epithelial cells identified a novel exit from the cell cycle into quiescence. Cells decide at the end of mitosis to either start the next cell cycle or to enter a transient G0 state depending on the CDK2 activity (Fig. 1) [54]. It appears that the regulation of quiescence is much more complex than we previously thought and offers ample opportunities for new discoveries.
DEREGULATION OF THE MITOCHONDRIAL METABOLIC CHECKPOINT IN AGED HEMATOPOIETIC STEM CELLS
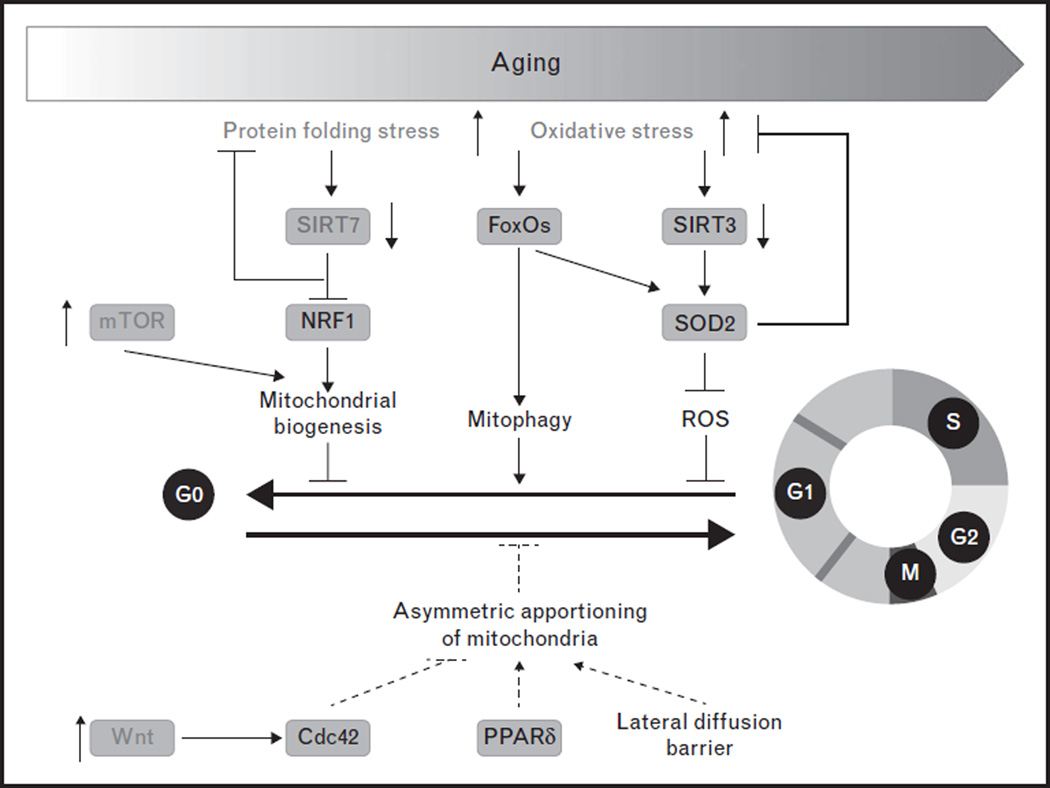
The decline in hematopoietic function during the aging process is attributable to the deterioration of HSCs [14,38,55,56]. Aged HSCs are prone to cell death, have reduced capacity to reconstitute the blood system and skewed differentiation potential from the lymphoid lineage toward the myeloid lineage. Recent advances on HSC aging highlight deregulation of the mitochondrial metabolic checkpoint as an underlying mechanism (Fig. 4). Mitochondrial protein folding stress and ROS levels increase in aged HSCs [32▪▪,36]. The expression of SIRT7 and SIRT3 is reduced with aging in HSCs, whereas mTOR activity is increased in HSCs from old mice compared to those from young mice [11,32▪▪,34,57]. Defects manifested in SIRT7 knockout HSCs resemble essential aspects of aged HSCs whereas SIRT3 knockout mice have aging-dependent defects in HSCs [32▪▪,34]. mTOR activation through conditional deletion of Tsc1 in HSCs mimicks the phenotypes of aged HSCs [11]. These observations suggest that protective programs that safeguard the mitochondrial protein folding stress and oxidative stress are compromised in aged HSCs.
FIGURE 4.
Deregulation of the mitochondrial metabolic checkpoint in aged hematopoietic stem cells (HSCs). Mitochondrial protein folding stress and mitochondria oxidative stress are increased in aged HSCs because of deregulation of the mitochondrial metabolic checkpoint in HSCs, such as repression of SIRT3 and SIRT7, and activation of mTOR and noncanonical Wnt. ROS, reactive oxygen species.
The observations that aged mice and genetically engineered mouse models that manifest mitochondrial stresses in HSCs display myeloid-biased differentiation potential raise a possibility that HSCs with lymphoid potential are particularly sensitive to mitochondrial stresses. A recent study of mitofusin 2, a protein involved in mitochondrial fusion, has provided direct evidence. Gain-of-function and loss-of-function studies revealed that mitofusin 2 is specifically required for the maintenance of HSCs with extensive lymphoid potential but less so for myeloid primed HSCs [58▪▪]. How mitochondria stresses specifically affect lymphoid primed HSCs is unclear. One potential explanation is that lymphoid-biased HSCs divide more frequently than myeloid-biased HSCs [59].
Studies of Wnt signaling in HSCs revealed an unexpected shift from canonical to noncanonical Wnt signaling during physiological aging, which is causally linked to apolarity and functional deterioration of HSCs via activation of Cdc42 [60]. It remains to be tested whether asymmetrical apportioning of mitochondria is compromised in aged HSCs. Together, these findings suggest that deregulation of the mitochondrial metabolic checkpoint is a contributing factor to HSC aging and these deregulated pathways may be exploited to reverse HSC aging. Indeed, overexpression of either SIRT3 or SIRT7 rejuvenated aged HSCs [32▪▪,34]. Inhibition of Cdc42 activity or repression of Wnt5a improved the functional capacity of aged HSCs [51,60]. Pharmacological inhibition of mTOR restores the functionality of aged HSCs [11].
CONCLUSION
Recent advances in the molecular regulation of the mitochondrial metabolic checkpoint represent the tip of the iceberg of deciphering this complex process. Future studies will unravel new regulators and mechanisms that control the mitochondrial stress responses and elucidate how mitochondrial stresses result in HSC quiescence and how severe mitochondrial stresses lead to the death of HSCs. Studies in HSCs are instrumental to the understanding of stem cell biology and tissue regeneration. Much work is needed to determine whether the molecular regulation of the mitochondrial metabolic checkpoint in HSCs is conserved in stem cells in other tissues. The importance of the mitochondrial metabolic checkpoint for stem cell maintenance and tissue homeostasis motivates the search for small molecules targeting this pathway for therapeutic purposes.
KEY POINTS.
A mitochondrial metabolic checkpoint regulates HSC quiescence.
Mitochondrial unfolded protein response and mitochondrial oxidative stress response regulate the mitochondrial metabolic checkpoint in HSCs.
Mitophagy and likely asymmetric mitochondrial apportioning safeguard mitochondrial health and stemness of HSCs.
The mitochondrial metabolic checkpoint is deregulated in aged HSCs
Acknowledgments
The authors thank H. Luo and J. Shin for comments.
Financial support and sponsorship
This work was supported by the NIH R01 AG040990 (D.C.), RO1 DK101885 (D.C.), T32 AG000266 (M.M.), Siebel Stem Cell Institute (D.C. and M.M), Ellison Medical Foundation (D.C.), Glenn Foundation (D.C.), and PackerWentz Endowment (D.C.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Bowie MB, McKnight KD, Kent DG, et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nygren JM, Bryder D, Jacobsen SE. Prolonged cell cycle transit is a defining and developmentally conserved hemopoietic stem cell property. J Immunol. 2006;177:201–208. doi: 10.4049/jimmunol.177.1.201. [DOI] [PubMed] [Google Scholar]
- 4.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term selfrenewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 6.Alberts B, et al. Molecular biology of the cell. 5th. New York: Garland Science; 2007. [Google Scholar]
- 7.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Burke DJ, Stukenberg PT. Linking kinetochore-microtubule binding to the spindle checkpoint. Dev Cell. 2008;14:474–479. doi: 10.1016/j.devcel.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Liu Y, Liu R, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng T, Rodrigues N, Dombkowski D, et al. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 14.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 15.Juntilla MM, Patil VD, Calamito M, et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalaitzidis D, Sykes SM, Wang Z, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kentsis A, Look AT. Distinct and dynamic requirements for mTOR signaling in hematopoiesis and leukemogenesis. Cell Stem Cell. 2012;11:281–282. doi: 10.1016/j.stem.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kharas MG, Okabe R, Ganis JJ, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee JA, Ikenoue T, Nakada D, et al. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415–428. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Shen H, Franklin DS, et al. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 23.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broxmeyer HE, O’Leary HA, Huang X, Mantel C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22:273–278. doi: 10.1097/MOH.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161:1553–1565. doi: 10.1016/j.cell.2015.04.054. This study highlights the important role of hypoxia in maintenance of HSCs in vivo, and implicates ROS and the induction of the mitochondrial transition pore in the HSC cellular response to oxygen shock.
- 26.Morita M, Gravel SP, Chénard V, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Gan B, Hu J, Jiang S, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurumurthy S, Xie SZ, Alagesan B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes CM, Ron D. The mitochondrial UPR—protecting organelle protein homeostasis. J Cell Sci. 2010;123(Pt 22):3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q, Wang J, Levichkin IV, et al. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohrin M, Shin J, Liu Y, et al. Stem cell aging: a mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi: 10.1126/science.aaa2361. This study identifies a novel branch of the mitochondrial UPR and provides the first evidence that mitochondrial protein folding stress is a trigger of a metabolic checkpoint that regulates HSC quiescence and that deregulation of mitochondrial UPR is a contributing factor for HSC aging.
- 33.Shin J, He M, Liu Y, et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep. 2013;5:654–665. doi: 10.1016/j.celrep.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown K, Xie S, Qiu X, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 36.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Zhang J, Lin Y, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SC, Sprung R, Chen Y, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Qiu X, Brown K, Hirschey MD, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naka K, Hoshii T, Muraguchi T, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 46.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 47.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warr MR, Binnewies M, Flach J, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katajisto P, Döhla J, Chaffer CL, et al. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–343. doi: 10.1126/science.1260384. This study shows that mammary stem-like cells symmetrically segregate mitochondria into daughter cells based upon the age of the mitochondria; the cells with less old mitochondria maintain stemness.
- 51.Florian MC, Dörr K, Niebel A, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito K, Carracedo A, Weiss D, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moore DL, Pilz GA, Araúzo-Bravo MJ, et al. A mechanism for the segregation of age in mammalian neural stem cells. Science. 2015;349:1334–1338. doi: 10.1126/science.aac9868. This study discovers that neural stem cells asymmetrically segreate cellular components upon cellular division via a lateral diffusion barrier in the endoplasmic reticulum membrane, and that this process is degraded with age.
- 54.Spencer SL, Cappell SD, Tsai FC, et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155:369–383. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 56.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers SM, Shaw CA, Gatza C, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luchsinger LL, de Almeida MJ, Corrigan DJ, et al. Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature. 2016;529:528–531. doi: 10.1038/nature16500. This study demonstrates that mitochondrial perturbation affects lymphoid primed HSCs, but not myeloid primed HSCs.
- 59.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Florian MC, Nattamai KJ, Dörr K, et al. A canonical to noncanonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503:392–396. doi: 10.1038/nature12631. [DOI] [PMC free article] [PubMed] [Google Scholar]