Abstract
Objectives
To determine whether mild, occult sleep-disordered breathing (SDB) moderates the efficacy of cognitive behavioral therapy for insomnia (CBTI) in older adults and to explore whether CBTI reduces the number of patients eligible for PAP therapy.
Methods
Data were analyzed for 134 adults aged ≥ 60 years with insomnia and apnea-hypopnea index (AHI) < 15 who were randomized to a larger study of CBTI versus a sleep education control. Sleep outcomes (sleep onset latency, total wake time, wake after sleep onset, sleep efficiency, Pittsburgh Sleep Quality Index) were compared between CBTI and control at 6-months using repeated measures analysis of variance adjusted for baseline values. AHI ≥ 5 versus <5 was included as an interaction term to evaluate changes in sleep outcomes. The number of participants at baseline and 6 months with mild SDB for whom insomnia was their only other indication for PAP was also compared between CBTI and control.
Results
AHI status (AHI ≥ 5 [75.5% of participants] versus AHI < 5) did not moderate improvements in sleep associated with CBTI (all p values ≥.12). Nine out of 20 participants with mild SDB (45.0%) for whom insomnia was their only other indication for PAP therapy at baseline no longer had another indication for PAP at 6 months, with no significant difference between CBTI and control.
Conclusion
CBTI improves sleep in older veterans with insomnia and untreated mild SDB. Larger trials are needed to assess whether CBTI reduces the number of patients with mild SDB eligible for PAP.
Keywords: sleep apnea syndromes, cognitive behavioral therapy, aged, continuous positive airway pressure
Introduction
Approximately one-third of community-dwelling older adults have symptoms of insomnia,(1) a condition associated with increased healthcare costs and mortality. A wide body of evidence demonstrates that cognitive behavioral therapy for insomnia (CBTI) reduces sleep disturbance among older patients with insomnia (2–4). Efforts are underway to expand CBTI training among mental health providers, and several research studies show that CBTI can be delivered over the telephone and in condensed formats (e.g., Brief Behavioral Treatment for Insomnia (BBTI)) (2, 5, 6). These efforts are providing increased opportunities for patients to access CBTI, which may in turn lead to reductions in sleep disturbance among older adults.
Nearly one-third of older adults with insomnia also have undiagnosed sleep-disordered breathing (SDB), a condition that responds to therapies such as positive airway pressure (PAP) (7, 8). The optimal treatment sequence (concurrent versus sequential SDB treatment and CBTI) for improving sleep disturbance among patients with insomnia and comorbid SDB has only been examined in a limited number of studies, which report inconsistent findings (9–11). One prospective randomized crossover study reported that treating SDB first yielded more improvement in insomnia symptoms than starting with CBTI, suggesting that treating SDB first may be the optimal approach when tackling insomnia complaints among patients with co-occurring insomnia and SDB (10). Another study tested sequential CBTI and SDB therapy in a middle-aged non-veteran population with refractory insomnia and found that fewer than half of participants reached a nonclinical insomnia level after CBTI, whereas 88% achieved this endpoint after adding SDB therapy (9). Yet subgroup analyses from a third study, whose primary findings were that BBTI conducted in an older non-veteran population is associated with remission of insomnia (55% intervention versus 13% control), found no differential effects of BBTI associated with SDB status (11). Several other CBTI trials have excluded older adults with SDB symptoms or SDB diagnoses (12–17).
There are a few scenarios in which a provider might want to begin CBTI prior to initiating SDB treatment. In some instances, insomnia may be the patient’s only indication for treating mild SDB (i.e., the patient may lack excessive daytime sleepiness, impaired cognition, mood disorders, hypertension, ischemic heart disease, or history of stroke, which are other indications for PAP therapy according to the Centers for Medicare and Medicaid Services [CMS] (18)), and therefore, a trial of CBTI may be warranted to see if insomnia symptoms resolve without SDB therapies such as PAP. Second, many patients with psychophysiologic insomnia have difficulty falling asleep at the beginning of the night or falling back asleep after awakening from stimuli,(19) which could be exacerbated by stimuli such as noise from a leaking positive airway pressure [PAP] mask and/or tactile stimulation from PAP headgear, or tethered PAP tubing. Increased length of nighttime awakenings could reduce acceptance of PAP, and since CBTI decreases the length of nighttime awakenings, this could improve adherence to SDB therapies. Finally, there may be delays in accessing SDB therapy due to delays in insurance approvals, crowded appointment schedules at durable medical equipment companies, intermittent interruptions in the supply of PAP units, or difficulty paying out-of-pocket costs associated with SDB therapies (20).
In the context of a larger randomized controlled trial testing CBTI delivered by a supervised sleep coach compared to a sleep education control condition, (21) the primary aim of the current study was to determine whether CBTI results differ based upon SDB status. We hypothesized that, at 6-months follow-up, the presence of untreated mild SDB would not limit the improvements in sleep efficiency, sleep onset latency, wake after sleep onset, total wake time, or Pittsburgh Sleep Quality Index (PSQI) total score among older adults randomized to CBTI versus a sleep education control condition. We also hypothesized that CBTI would decrease the number of patients with mild SDB who meet CMS indications for SDB therapy.
Methods
Overview
Baseline, post-treatment and 6- and 12 months follow-up data for a subset of individuals enrolled in a randomized controlled trial testing a novel delivery model for CBTI (i.e., CBTI provided by a health educator supervised by a sleep psychologist) among older veterans were included for this analysis (21). For the current analyses, we selected the subgroup of participants with an apnea-hypopnea index (AHI) < 15 (mild SDB [AHI ≥ 5 to <15] or no SDB [AHI <5]), to align with the CMS criteria for mild and no SDB (N=134) (18).
Procedures
Participants for the trial were identified through a multi-stage screening process that involved a postal survey, telephone interview, face-to-face interview, home sleep study, and medical chart review. Details of the postal survey and the trial have reported previously (21, 22). Through this screening process, we identified veterans who met the following criteria: aged ≥ 60 years, presence of a chronic insomnia disorder (International Classification of Sleep Disorders-2 (ICSD-2) criteria), no known prior history of sleep apnea (by patient report), Mini-mental State Examination (MMSE) ≥ 24, healthcare visit at our VA between August 2009 and June 2011), no serious physical or mental health issues that would preclude participation in the trial, and AHI < 20 (as described above, only patients with AHI < 15 were included in the current analyses). Eligible patients were randomized to one of three groups: individual CBTI, group CBTI, or a sleep education control condition. CBTI included sleep restriction, stimulus control, and cognitive therapy and was provided by health educators with weekly telephone supervision by a sleep psychologist (18). Controls received general information about sleep. Both the CBTI and control conditions involved 5 sessions over 6 weeks. Assessments were performed at baseline, post-treatment and at 6- and 12 months follow-up. Twelve-month follow-up data were collected to assess maintenance of outcomes. Our predefined primary endpoints were collected at the 6-month follow-up visit.
Instruments
Unattended in-home sleep study
At baseline, participants underwent a single-night, unattended in-home sleep study (WatchPAT, WP100, Itamar Medical). After we visually inspected the recording, the AHI was calculated using the manufacturer’s automated, validated scoring algorithms (23). We grouped patients into two groups: no SDB (AHI 0 to < 5) and mild SDB (AHI ≥ 5 to <15).
Sleep diaries
At each assessment time point, participants kept a sleep diary that included self-reported daily bedtime and rise times, sleep onset latency (SOL), and number of nighttime awakenings. We then computed the average nighttime total sleep time (TST), wake after sleep onset (WASO), total wake time at night (TWT), and sleep efficiency (SE).
Actigraphy
At each assessment time point, participants wore a wrist actigraph (Actiwatch Spectrum, Philips Respironics) on their dominant arm for seven consecutive days and nights. Research staff visually inspected the raw actigraphy data (one minute epoch) to identify artifacts and then scored the recordings. Sleep diaries were used to determine nighttime and daytime periods for scoring and analysis of actigraphy data. We calculated actigraphically-measured sleep efficiency (24).
Other clinical and demographic variables
We collected information on patients’ race/ethnicity, educational level, employment status, and marital status. The VA’s Austin Automation Center administrative database provided each participant’s gender and date of birth, which enabled us to calculate age. At baseline only, participants were asked through a structured interview whether they had a history of hypertension, a history of stroke, or a history of a heart attack. At each assessment time point (including baseline), participants completed the Pittsburgh Sleep Quality Index (PSQI; total possible score 0 – 21; scores > 5 suggest significant sleep disturbance),(25) Insomnia Severity Index (ISI; 0 – 28; scores ≥ 15 suggest clinical insomnia),(26) the Epworth Sleepiness Scale (ESS; 0 – 24; scores ≥ 10 suggest excessive daytime sleepiness),(27) and the Patient Health Questionnaire-9 [PHQ-9; 0–27; scores ≥ 5 are suggestive of depression) (28). At each assessment time point, we also determined whether the participant met ICSD-2 criteria for insomnia, by analyzing the participants’ responses to assessment items (collected through structured interview during the scheduled assessment periods) that were specifically designed by our research team to determine whether the participant met the ICSD-2 General Criteria for Insomnia.
Our predefined primary outcomes were SOL, WASO, TWT, SE (sleep diary and actigraphy), and total PSQI score collected at the 6-month follow-up.
Ethics
All study procedures and materials were approved by VA Greater Los Angeles Healthcare System Institutional Review Board. All participants provided written informed consent.
Statistics
Descriptive statistics (e.g., mean, standard deviations) were calculated to characterize the sample. Demographic characteristics were compared for participants based upon SDB status (AHI < 5 versus AHI ≥ 5) using t-tests for continuous variables and Fisher’s Exact tests for categorical variables.
A repeated measures analysis of variance model that included an interaction term (SDB status * assigned treatment arm) compared sleep outcomes (between CBTI and controls for patients with versus without mild SDB) at baseline, post-treatment, 6-month follow-up and 12-month follow-up. Using the interaction term in this model, we compared differences in treatment effects for patients with versus without mild SDB.(29) For the six primary (a priori) outcomes, we used a Bonferroni-adjusted alpha level of 0.0083 (0.05/6). For the secondary outcomes (post-treatment and 12-month follow-up outcomes), we used an alpha level = 0.01
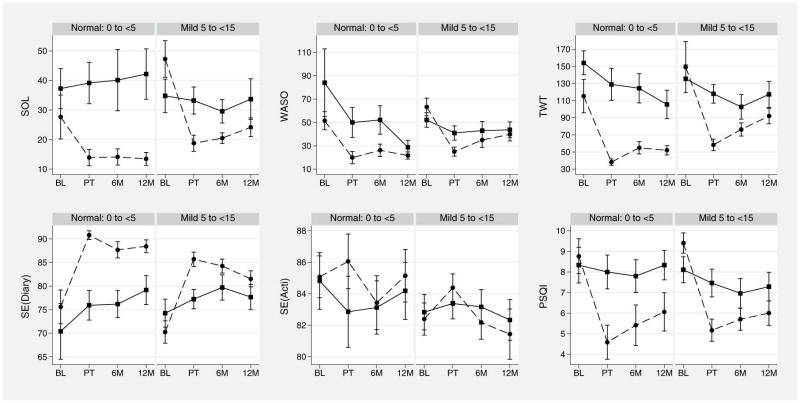
To provide a visual representation of our analyses, we used the means and standard error of the mean generated from the repeated measures ANOVA models to depict the sleep measures as a function of treatment group assignment and SDB status over time (Figure 1).
Figure 1.
Sleep measure means and standard error of the mean, by time (1=baseline, 2=post-treatment, 3=six-month follow-up, 4=twelve-month follow-up), sleep-disordered breathing status (normal, mild), and treatment group assignment (dashed line=cognitive behavioral therapy for insomnia; solid line=sleep education control). Abbreviations: SOL=sleep onset latency; WASO=wake after sleep onset; TWT=total wake time; SE=sleep efficiency; PSQI=Pittsburgh Sleep Quality Index.
We calculated Pearson product-moment correlation coefficients to measure the strength of association between AHI and baseline sleep measures and between AHI and the change in sleep measures from baseline to 6-month follow-up for the CBTI-I and controls.
Finally, to assess for changes in the number of patients with indications for PAP therapy at our pre-defined primary endpoint (6 month follow-up), we identified two groups of patients at baseline: 1) patients with AHI ≥ 5, an ICSD-2 diagnosis of insomnia, and no other indication for PAP therapy at baseline and 2) patients with AHI ≥ 5, an ICSD-2 diagnosis of insomnia, and at least one other indication for PAP therapy at baseline. We operationalized “no other indications for PAP therapy” (based upon CMS criteria) in the following manner: at baseline, the patient denied history of stroke, heart attack, or hypertension and had PHQ-9 < 10, ESS < 10, and MMSE ≥ 24 (MMSE < 24 was an exclusion criterion). To measure the reduction in participants meeting CMS indications for PAP therapy, we calculated the number of patients in group 1 who no longer met ICSD-2 criteria for insomnia at 6 months follow-up. To examine whether CBTI reduces the number of participants meeting CMS indications for PAP therapy, we used Fisher’s Exact test to examine associations between trial arm assignment and presence of insomnia at 6-month follow-up for group 1. Because CBTI could have worsened ESS and PHQ-9 scores (possibly making participants eligible for PAP therapy based upon excessive daytime sleepiness or a mood disorder), we also cross-tabulated the proportion of patients who had ESS ≥ 10 at 6 months follow-up and no other indication for PAP therapy among the patients randomized to CBTI. We repeated this for PHQ-9 ≥ 10.
Results
Baseline
Pretreatment demographic and clinical variables by SDB status are summarized in Table 1. The sample was comprised primarily of non-Hispanic white males. No significant differences in age, gender, educational level, race/ethnicity, or marital status were observed between participants with an AHI < 5 (no SBD) versus 5 ≥ AHI < 15 (mild SDB). Seventy-one percent (N=95) of the analyzed sample had AHI ≥5 (and <15) suggesting mild SDB. Sleep assessments at baseline are summarized in Table 2.
Table 1.
Sample Characteristics at Baseline
| Variable | Total (N=134) | AHI < 5 (No SDB) (N=39) | 5 ≥ AHI < 15 (Mild SDB) (N=95) | p value |
|---|---|---|---|---|
| Age: mean (SD) | 72.2 (7.7) | 72.0 (6.4) | 71.9 (7.9) | 0.94 |
|
| ||||
| Gender (Male) (%) | 97.0 (130) | 97.4 (38) | 96.8 (92) | > .99 |
|
| ||||
| Race (Non-Hispanic White) (%) | 78.4 (105) | 79.6 (30) | 78.9 (75) | 0.820 |
|
| ||||
| Education (%) | 0.71 | |||
|
| ||||
| Less than High School | 6 (4.5) | 2 (5.1) | 4 (4.2) | |
| High School Graduate | 19 (14.2) | 7 (17.9) | 12 (12.6) | |
| Some College | 60 (44.8) | 14 (35.9) | 46 (48.4) | |
| College Graduate | 25 (18.7) | 8 (20.5) | 17 (17.9) | |
| Post Baccalaureate | 24 (17.9) | 8 (20.5) | 16 (16.8) | |
|
| ||||
| Marital Status (%) | 0.35 | |||
|
| ||||
| Married | 59 (44.0) | 15 (38.5) | 44 (46.3) | |
| Living as Married | 11 (8.2) | 3 (7.7) | 8 (8.4) | |
| Divorced/Separated | 42 (31.3) | 11 (28.2) | 31 (32.6) | |
| Widowed | 9 (6.7) | 3 (7.7) | 6 (6.3) | |
| Single/Never Married | 13 (9.7) | 7 (17.9) | 6 (6.3) | |
|
|
||||
| AHI: mean (SD) | 9.4 (5.3) | 2.4 (1.5) | 10.1 (3.0) | <.01 |
|
| ||||
| PSQI total score: mean (SD) | 9.1 (3.4) | 8.4 (3.6) | 9.2 (3.5) | 0.28 |
|
| ||||
| Epworth Sleepiness Scale score: mean (SD) | 5.1 (3.7) | 4.6 (3.3) | 5.2 (3.8) | 0.43 |
|
| ||||
| PHQ9: mean (SD) | 4.8 (4.3) | 4.5 (4.8) | 4.8 (4.0) | 0.66 |
|
| ||||
| History of hypertension (%) | 87 (64.9) | 26 (66.7) | 61 (64.2) | 0.84 |
|
| ||||
| History of stroke (%) | 8 (6.0) | 1 (2.6) | 7 (7.4) | 0.44 |
|
| ||||
|
History of heart attack (%)
|
19 (14.2) | 6 (15.4) | 13 (13.7) | 0.79 |
|
Weight: mean (SD)
|
185.4 (32.0) | 180.9 (31.9) | 187.3 (32.0) | 0.29 |
| Geriatric Pain Measure: mean (SD) | 14.7 (11.4) | 16.0 (11.8) | 14.2 (11.3) | 0.40 |
AHI=apnea-hypopnea index; PSQI=Pittsburgh Sleep Quality Index; PHQ9=Patient Health Questionnaire-9. Statistical analysis: t-tests for continuous variables and Fisher’s Exact tests for categorical variables
Table 2.
Repeated Measures Analysis of Variance Results: Sleep Measures at Baseline
| Measure | AHI < 5 (No SDB) | 5 ≤ AHI < 15 (Mild SDB) | No SDB vs. Mild SDB | |||||
|---|---|---|---|---|---|---|---|---|
| Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Difference (95% CI) ‡ | P-value Difference | |
| Sleep onset latency (SOL)*, N=99 | 27.7 (11.5, 43.8) | 37.3 (22.7, 51.8) | .47 | 47.3 (34.8, 59.7) | 34.8 (23.2, 46.4) | .14 | −22.1 (−53.3,9.1) | .16 |
| Wake after sleep onset (WASO)*, N=100 | 51.5 (34.8, 68.2) | 84.0 (21.4, 146.6) | .13 | 63.3 (48.1, 78.5) | 52.1 (39.4, 64.9) | .41 | −43.7 (−93.5,6.1) | .085 |
| Total wake time at night (TWT)*, N=98 | 115.3 (72.8, 157.8) | 149.2 (84.8, 213.5) | .33 | 154.1 (126, 182.2) | 135.6 (102.4, 168.8) | .40 | −52.4 (−134.5,29.6) | .21 |
| Sleep Efficiency*, N=98 | 75.6 (67.8, 83.4) | 70.4 (57.6, 83.2) | .41 | 70.3 (65.5, 75.0) | 74.3 (68.1, 80.4) | .32 | 9.2 (−5.6,24.0) | .22 |
| Sleep Efficiency**, N=100 | 85.1 (82.2, 87.9) | 84.8 (80.9, 88.7) | .91 | 82.4 (80.3, 84.5) | 82.8 (80.5, 85.1) | .78 | 0.7 (−4.8,6.2) | .80 |
| Pittsburgh Sleep Quality Index (PSQI), N=107 | 8.8 (7.0, 10.6) | 8.3 (6.5, 10.2) | .72 | 9.4 (8.4, 10.4) | 8.1 (6.8, 9.4) | .11 | −0.9 (−3.7,2.0) | .55 |
From sleep diary data;
From actigraphy data;
This column represents the following: [(CBTI meanmild SDB -Control meanmild SDB) -(CBTI meanNo SDB - Control meanNo SDB)];
CI=confidence interval; CBTI=cognitive behavioral therapy for insomnia; AHI=apnea-hypopnea index; SDB=sleep-disordered breathing. Statistical analysis: Repeated Measures Analysis of Variance.
Primary Endpoints (6-month follow-up)
Table 3 (column a) provides the CBTI and sleep education control groups’ outcomes for participants with and without SDB. As compared with participants with mild SDB who received sleep education, participants with mild SDB who received CBTI had significant improvements in sleep onset latency (p=.0078) and PSQI total score (p=0.002), but they did not have significant improvements in wake after sleep onset (p=0.1), total wake time at night (p=0.02), diary-measured sleep efficiency (p=0.01), or actigraphically-measured sleep efficiency (p=.7). The test of the interaction between SDB status and assigned treatment arm resulted in no statistically significant differences in treatment effect associated with CBTI for participants without and with mild SDB.
Table 3.
Repeated Measures Analysis of Variance Results: Sleep Outcomes at 6-month Follow-Up
| Measure | AHI < 5 (No SDB) | 5 ≤ AHI < 15 (Mild SDB) | No SDB vs. Mild SDB | |||||
|---|---|---|---|---|---|---|---|---|
| Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Difference in Treatment Effect (95% CI) ‡ | P-value Difference | |
| Sleep onset latency (SOL)*, N=99 | 14.1 (8.1, 20.1) | 40.1 (17.7, 62.5) | .20 | 20.5 (16.9, 24.1) | 29.6 (21.4, 37.7) | .0078† | 5.2 (−24.4,34.7) | .73 |
| Wake after sleep onset (WASO)*, N=100 | 26.2 (14.8, 37.5) | 52.1 (25.9, 78.3) | .75 | 35.0 (22.2, 47.8) | 43.0 (27.1, 58.9) | .15 | 25.8 (−22.6,74.2) | .29 |
| Total wake time at night (TWT)*, N=98 | 55.0 (39.3, 70.7) | 124.4 (87.5, 161.4) | .22 | 76.4 (60.8, 92.0) | 102.7 (73.4, 132.1) | .016 | 9.3 (−58.1,76.7) | .78 |
| Sleep Efficiency*, N=98 | 87.7 (84.0, 91.4) | 76.2 (70.0, 82.4) | .24 | 84.3 (81.3, 87.2) | 79.7 (74.2, 85.2) | .013 | −2.2 (−14.7,10.3) | .73 |
| Sleep Efficiency**, N=100 | 83.4 (79.8, 87.1) | 83.1 (79.5, 86.8) | 1.0 | 82.2 (80.0, 84.3) | 83.2 (80.9, 85.4) | .65 | 0.6 (−3.8,5.0) | .79 |
| Pittsburgh Sleep Quality Index (PSQI), N=107 | 5.4 (3.3, 7.5) | 7.8 (6.1, 9.5) | .023 | 5.7 (4.6, 6.8) | 7.0 (5.5, 8.4) | .002† | −0.3 (−3.2,2.7) | .86 |
From sleep diary data;
From actigraphy data;
p<.0083;
This column represents the following: [(6 month CBTI meanmild SDB – 6 month Control meanmild SDB) - (Baseline CBTImeanmild SDB - Baseline Control meanmild SDB)]-[(6 month CBTI meanNo SDB – 6 month Control meanNo SDB) – (Baseline CBTImeanNo SDB – Baseline Control meanNo SDB)];
CI=confidence interval; CBTI=cognitive behavioral therapy for insomnia; AHI=apnea-hypopnea index; SDB=sleep-disordered breathing. Statistical analysis: Repeated Measures Analysis of Variance.
CMS indications for PAP therapy
At baseline, 95 participants had mild SDB, 24 of whom had insomnia as their only other indication for PAP therapy. At the 6-month follow-up, insomnia status data were available for 20 participants with mild SDB (14 CBTI, 6 sleep education control), and of these participants, 9 (45%) no longer met CMS indications for PAP therapy (6 CBTI [42.9%]) versus 3 sleep education control [50.0%], Fisher’s exact p > .99). Of the 9 mild SDB participants without insomnia at the 6-month follow-up, none developed an ESS ≥ 10 or PHQ-9 ≥ 10.
Secondary Endpoints
In Tables 4 and 5, we present results from the post-treatment assessments and 12 month assessments, respectively. We found no statistically significant differences in treatment effect associated with CBTI for participants without and with SDB at post-treatment and 12 months, where a p-value < 0.01 was considered statistically significant (alpha=0.01).
Table 4.
Repeated Measures Analysis of Variance Results: Sleep Outcomes Post-Treatment
| Measure | AHI < 5 (No SDB) | 5 ≤ AHI < 15 (Mild SDB) | No SDB vs. Mild SDB | |||||
|---|---|---|---|---|---|---|---|---|
| Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Difference in Treatment Effect (95% CI) ‡ | P-value Difference | |
| Sleep onset latency (SOL)*, N=99 | 13.9 (7.9, 19.9) | 39.1 (24.0, 54.2) | .25 | 18.8 (13.2, 24.3) | 33.2 (23.8, 42.6) | .002† | 11.3 (−20.2,42.7) | .48 |
| Wake after sleep onset (WASO)*, N=100 | 19.9 (8.5, 31.2) | 50.0 (22.4, 77.6) | .89 | 25.0 (17.2, 32.8) | 41.0 (28.5, 53.4) | .017 | 29.6 (−11.7,70.8) | .16 |
| Total wake time at night (TWT)*, N=98 | 38.2 (30.1, 46.4) | 129.0 (88.4, 169.6) | .080 | 58.4 (44.8, 72.0) | 118.0 (95.8, 140.1) | <.001† | 21.3 (−54.3,96.8) | .58 |
| Sleep Efficiency*, N=98 | 90.8 (88.8, 92.8) | 75.9 (69.1, 82.7) | .087 | 85.7 (82.6, 88.7) | 77.2 (73.0, 81.4) | <.001† | −2.8 (−15.9,10.4) | .68 |
| Sleep Efficiency**, N=100 | 86.1 (82.4, 89.8) | 82.9 (78.0, 87.7) | .11 | 84.4 (82.6, 86.1) | 83.4 (81.4, 85.4) | .24 | 1.5 (−2.8,5.8) | .49 |
| Pittsburgh Sleep Quality Index (PSQI), N=107 | 4.6 (2.8, 6.3) | 8.0 (6.2, 9.8) | <.001† | 5.2 (4.1, 6.3) | 7.5 (6.1, 8.9) | <.001† | −0.3 (−2.9,2.4) | .85 |
From sleep diary data;
From actigraphy data;
p<.01;
This column represents the following: [(Post-Treatment CBTI meanmild SDB – Post-Treatment Control meanmild SDB) - (Baseline CBTImeanmild SDB - Baseline Control meanmild SDB)]-[(Post-Treatment CBTI meanNo SDB – Post-Treatment Control meanNo SDB) – (Baseline CBTImeanNo SDB – Baseline Control meanNo SDB)];
CI=confidence interval; CBTI=cognitive behavioral therapy for insomnia; AHI=apnea-hypopnea index; SDB=sleep-disordered breathing. Statistical analysis: Repeated Measures Analysis of Variance.
Table 5.
Repeated Measures Analysis of Variance Results: Sleep Outcomes at 12-month Follow-Up
| Measure | AHI < 5 (No SDB) | 5 ≤ AHI < 15 (Mild SDB) | No SDB vs. Mild SDB | |||||
|---|---|---|---|---|---|---|---|---|
| Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Sleep Education Control Mean (95% CI) | CBT-I Mean (95% CI) | P-value CBT-I vs. Control | Difference in Treatment Effect (95% CI) ‡ | P-value Difference | |
| Sleep onset latency (SOL)*, N=99 | 13.5 (8.7, 18.3) | 42.2 (23.8, 60.6) | .14 | 24.2 (17.7, 30.6) | 33.7 (19.6, 47.8) | .008† | 2.9 (−27.5,33.3) | .85 |
| Wake after sleep onset (WASO)*, N=100 | 21.7 (15, 28.4) | 28.7 (15.8, 41.7) | .24 | 39.8 (28.4, 51.1) | 43.7 (29.8, 57.6) | .28 | 40.5 (−10.4,91.4) | .12 |
| Total wake time at night (TWT)*, N=98 | 52.0 (40.3,63.8) | 105.5 (69.7,141.3) | .54 | 92.1 (73.7,110.4) | 117.3 (86.1,148.5) | .033 | 24.2 (−50.7,99.1) | .52 |
| Sleep Efficiency*, N=98 | 88.4 (85.5, 91.4) | 79.2 (72.5, 85.8) | .48 | 81.5 (78.1, 85) | 77.7 (72.2, 83.2) | .037 | −3.7 (−17.5,10.0) | .59 |
| Sleep Efficiency**, N=100 | 85.1 (81.6, 88.7) | 84.2 (80.3, 88.1) | .78 | 81.4 (78.2, 84.7) | 82.3 (79.7, 85.0) | .77 | 1.2 (−4.6,7.0) | .69 |
| Pittsburgh Sleep Quality Index (PSQI), N=107 | 6.1 (4.1, 8.0) | 8.3 (6.8, 9.9) | .030 | 6.0 (4.8, 7.2) | 7.3 (5.9, 8.7) | .002† | −0.1 (−3.1,2.8) | .93 |
From sleep diary data;
From actigraphy data;
p<.01;
This column represents the following: [(12 month CBTI meanmild SDB – 12 month Control meanmild SDB) - (Baseline CBTImeanmild SDB - Baseline Control meanmild SDB)]- [(12 month CBTI meanNo SDB – 12 month Control meanNo SDB) – (Baseline CBTImeanNo SDB – Baseline Control meanNo SDB)];
CI=confidence interval; CBTI=cognitive behavioral therapy for insomnia; AHI=apnea-hypopnea index; SDB=sleep-disordered breathing. Statistical analysis: Repeated Measures Analysis of Variance.
Correlations between baseline AHI and baseline sleep measures and between AHI and the change in sleep measures from baseline to 6-month follow-up are presented in Tables S1–S3, Supplemental Digital Content 1. Baseline AHI was not significantly correlated with any of the baseline sleep measures or with the change in sleep measures for either the CBT-I or control groups. As expected, diary-measured sleep efficiency and total wake time at night, which are both commonly-reported in insomnia trials, were highly correlated at baseline (r=−.96), and changes in sleep efficiency (from sleep diary) and changes in total wake time from baseline to 6-month follow-up were highly correlated for the CBT-I group (r=−.95) and control group (r=−.98).
Discussion
In this study, we examined whether the presence of mild, occult SDB limits the efficacy of CBTI. We found that participants with mild SDB reported substantial reductions in sleep onset latency and improvements in sleep quality associated with CBTI and that the effect of CBTI is similar for older veterans with and without untreated mild SDB. We also found that for 24 (15.4%) mild SDB participants, insomnia was their only CMS indication for PAP therapy at baseline, and of the mild SDB participants followed up at 6 months, 45% no longer met CMS indications for PAP therapy.
Few other studies have analyzed whether CBTI is efficacious in patients with mild, occult SDB and none have specifically studied older veterans, a group that may have increased opportunity to receive behavioral treatments for their sleep disturbance due to efforts to expand access to CBTI (10, 30). Our results suggest that patients with insomnia and mild, occult SDB are likely to see improvements in their sleep when they are treated with CBTI, with reductions in total wake time at night, improved sleep efficiency, and better sleep quality. Although our study was not designed to test acceptance or adherence to PAP therapy, the improvements in sleep quality have the potential to reduce awakenings associated with stimuli from SDB therapies such as PAP-related noise. Our findings are similar to those reported by Buysse et al, who found that non-veterans with SDB did not have different BBTI results than those without SDB (11). However, our study differs in that our participants underwent CBTI, not BBTI, and our intervention was provided by health educators who were supervised by a sleep psychologist during the delivery of the intervention.
Our findings may also have implications for treating older veterans with mild, occult SDB that occurs in the context of insomnia. Currently, CMS pays for PAP therapy if patients with mild sleep apnea have another indication for therapy such as insomnia, excessive daytime sleepiness, impaired cognition, mood disorder, hypertension, ischemic heart disease, or history of stroke (18). A large percentage of the mild SDB patients in our study for whom insomnia was their only CMS indication for PAP therapy no longer had another indication for PAP therapy at 6-months follow-up. We did not find that CBTI was significantly associated with a larger reduction in the number of mild SDB patients meeting CMS criteria PAP therapy, but this result may have been due to insufficient power to detect a difference. Given the high rates of non-adherence associated with PAP therapy and the long-term costs associated with PAP equipment replacement and maintenance, an attractive alternative for mild SDB patients and their payers may be to first address the patient’s insomnia when there are no other indications for a PAP prescription.
Limitations of our study include our population, which was primarily comprised of non-Hispanic males. In addition, we used unattended, in-home sleep study to measure participants’ AHI, which may have resulted in incorrectly categorizing patients as having mild SDB (or as normal). However, unattended, in-home sleep studies are increasingly used in routine clinical practice to make determinations of patients’ SDB status and severity. We were unable to assess differences in dichotomous treatment remission and dichotomous treatment response outcomes due to limited sample size. Another limitation is that the number of participants for whom insomnia was their only other indication for PAP therapy was small, due to high rates of comorbid conditions in older adults (particularly older veterans) such as hypertension, which is another indication for PAP therapy in patients with mild SDB.
The current study provides support for employing CBTI in older adults with mild, occult SDB to improve sleep outcomes and further suggests that a subset of older adults with mild, occult SDB might no longer meet CMS indications for PAP therapy after successful CBTI. The effect of CBTI on AHI in patients with mild, occult SDB should be studied. Additional studies with larger sample sizes are needed to identify the success rate of CBTI for improving sleep disturbance among patients with mild, occult SDB and no other indications for PAP therapy.
Supplementary Material
Acknowledgments
Sources of Funding: Funded by Veterans Administration Health Services Research and Development (Alessi IIR 08-295), Veterans Administration Greater Los Angeles Geriatric Research, Education and Clinical Center, American Federation for Aging Research (to CHF), American Sleep Medicine Foundation Physician Scientist Training Award (to CHF), and National Institute on Aging of the National Institutes of Health under Award Number K23AG045937 (to CHF)/ The Beeson Career Development in Aging Research Award Program (supported by NIA, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies; to CHF). JMD was supported by UCLA Claude Pepper Older Americans Independence Center (5P30AG028748) and National Center for Advancing Translational Sciences UCLA CTSI (UL1TR000124). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, U.S. Department of Veterans Affairs or the United States Government.
Special thanks to Sergio Martinez, Terry Vandenberg, and Simone Vukelich.
Abbreviations
- AHI
Apnea-hypopnea index
- BBTI
Brief Behavioral Treatment for Insomnia
- CBTI
Cognitive behavioral therapy for insomnia
- CI
Confidence interval
- CMS
Centers for Medicare and Medicaid Services
- ESS
Epworth Sleepiness Scale
- ICSD-2
International Classification of Sleep Disorders-2
- ISI
Insomnia Severity Index
- MMSE
Mini-mental State Examination
- N=
Number equals
- PAP
Positive airway pressure
- PHQ-9
Patient Health Questionnaire-9
- PSQI
Pittsburgh Sleep Quality Index
- SD
Standard deviation
- SDB
Sleep-disordered breathing
- SE
Sleep efficiency
- SOL
Sleep onset latency
- TST
Total sleep time
- TWT
Total wake time
- VA
Department of Veteran Affairs
- WASO
Wake after sleep onset
Footnotes
All work was completed at VA Greater Los Angeles Healthcare System
Conflicts of Interest: No conflict of interest declared for any of the manuscript’s authors. This study did not involve any off-label or investigational use.
References
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, Begley A, Houck PR, Mazumdar S, Reynolds CF, III, Monk TH. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 4.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Manber R, Carney C, Edinger J, Epstein D, Friedman L, Haynes PL, Karlin BE, Pigeon W, Siebern AT, Trockel M. Dissemination of CBTI to the non-sleep specialist: protocol development and training issues. J Clin Sleep Med. 2012;8(2):209–18. doi: 10.5664/jcsm.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36(3):353–62. doi: 10.5665/sleep.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooneratne NS, Gehrman PR, Nkwuo JE, Bellamy SL, Schutte-Rodin S, Dinges DF, Pack AI. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166(16):1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31(11):1527–33. doi: 10.1093/sleep/31.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, Begley A, Houck PR, Mazumdar S, Reynolds CF, III, Monk TH. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 13.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, Nielsen GH, Nordhus IH. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 14.Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aguillard RN. Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J Consult Clin Psychol. 2001;69(2):227–39. doi: 10.1037//0022-006x.69.2.227. [DOI] [PubMed] [Google Scholar]
- 15.McCurry SM, Logsdon RG, Vitiello MV, Teri L. Successful behavioral treatment for reported sleep problems in elderly caregivers of dementia patients: a controlled study. J Gerontol B Psychol Sci Soc Sci. 1998;53(2):122–9. doi: 10.1093/geronb/53b.2.p122. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. J Consult Clin Psychol. 1988;56(5):748–53. doi: 10.1037//0022-006x.56.5.748. [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61(1):137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Local Coverage Determination (LCD) for Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea(L11528) Centers for Medicare and Medicaid Services; 2012. p. L11528. [Google Scholar]
- 19.Haynes SN, Fitzgerald SG, Shute G, O’Meary M. Responses of psychophysiologic and subjective insomniacs to auditory stimuli during sleep: a replication and extension. J Abnorm Psychol. 1985;94(3):338–45. doi: 10.1037//0021-843x.94.3.338. [DOI] [PubMed] [Google Scholar]
- 20.Tzischinsky O, Shahrabani S, Peled R. Factors affecting the decision to be treated with continuous positive airway pressure for obstructive sleep apnea syndrome. Isr Med Assoc J. 2011;13(7):413–9. [PubMed] [Google Scholar]
- 21.Alessi CA, Martin J, Fiorentino L, Fung C, Dzierzewski J, Rodriguez J, Josephson K, Jouldjian S, Mitchell M. Cognitive behavioral therapy for insomnia in older veterans: Final results of a randomized trial. Sleep. 2014;37(Abstract Supplement):A172. [Google Scholar]
- 22.Fung CH, Martin JL, Dzierzewski JM, Jouldjian S, Josephson K, Park M, Alessi C. Prevalence and symptoms of occult sleep disordered breathing among older veterans with insomnia. J Clin Sleep Med. 2013;9(11):1173–8. doi: 10.5664/jcsm.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 24.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CFI, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rausch JR, Maxwell SE, Kelley K. Analytic methods for questions pertaining to a randomized pretest, posttest, follow-up design. J Clin Child Adolesc Psychol. 2003;32(3):467–86. doi: 10.1207/S15374424JCCP3203_15. [DOI] [PubMed] [Google Scholar]
- 30.Espie CA, Kyle SD, Williams C, Ong JC, Douglas NJ, Hames P, Brown JS. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–81. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.