Abstract
Identifying mechanisms through which individual differences in reward learning emerge offers an opportunity to understand both a fundamental form of adaptive responding as well as etiological pathways through which aberrant reward learning may contribute to maladaptive behaviors and psychopathology. One candidate mechanism through which individual differences in reward learning may emerge is variability in dopaminergic reinforcement signaling. A common functional polymorphism within the catechol-O-methyl transferase gene (COMT; rs4680, Val158Met) has been linked to reward learning where homozygosity for the Met allele (associated with heightened prefrontal dopamine function and decreased dopamine synthesis in the midbrain) has been associated with relatively increased reward learning. Here, we used a probabilistic reward learning task to asses response bias, a behavioral form of reward learning, across 3 separate samples that were combined for analyses (age: 21.80 ± 3.95; n=392; 268 female; European-American, n=208). We replicate prior reports that COMT rs4680 Met allele homozygosity is associated with increased reward learning in European-American participants (β=0.20, t= 2.75, p< 0.01; ΔR2= 0.04). Moreover, a meta-analysis of 4 studies, including the current one, confirmed the association between COMT rs4680 genotype and reward learning (95% CI −0.11 to −0.03; z=3.2; p<0.01). These results suggest that variability in dopamine signaling associated with COMT rs4680 influences individual differences in reward which may potentially contribute to psychopathology characterized by reward dysfunction.
Keywords: reward, response bias, dopamine, COMT, anhedonia, meta-analysis
Introduction
Blunted reward processing is a cardinal feature of depression that is frequently observed across other forms of psychopathology, including posttraumatic stress disorder (PTSD), schizophrenia, and substance use disorders (Garfield et al., 2014, Gorwood, 2008, Hatzigiakoumis et al., 2011, Pizzagalli, 2014). Consistent with theoretical speculation (Loas, 1996, Meehl, 1975), emerging evidence suggests that diminished hedonic capacity may provide trait-like vulnerability to psychopathology (Corral-Frias et al., 2015, Nikolova et al., 2012), making it important to identify the origin and mechanisms underlying individual differences in reward processing. Guided by evidence that variability in reward processing is heritable (Bogdan & Pizzagalli, 2009, Wichers et al., 2007) and linked to dopaminergic (DA) system function (Schultz, 2015), genetic association studies of reward processing have focused primarily on functional polymorphisms within DA-related proteins (Bogdan et al., 2013, Forbes et al., 2009, Nikolova et al., 2011).
The most studied DA-related polymorphism in psychiatric and behavioral genetics to date is rs4680 (Val158Met) within the catechol-O-methyl transferse (COMT) gene (COMT) (Buckholtz & Meyer-Lindenberg, 2012, Gatt et al., 2015), which codes for a catabolic catecholamine enzyme (Mannisto & Kaakkola, 1999). Along with the DA transporter (DAT), the COMT enzyme is one of the primary synaptic regulators of DA. Unlike DAT, which is primarily expressed in subcortical regions, COMT is widely expressed in the prefrontal cortex (PFC) and is the primary constraint of prefrontal synaptic DA transmission (Tunbridge et al., 2004). Met (A) allele homozygosity at rs4680 is associated with a 40% reduction in COMT activity relative to Val (G) allele homozygosity (Chen et al., 2004).
This genotype-dependent reduction in COMT activity results in relatively higher DA levels in the PFC (Meyer-Lindenberg et al., 2005, Slifstein et al., 2008). While the direct effect of COMT Val158Met genotype is primarily on cortical DA, there is evidence that such cortical effects may indirectly modulate subcortical DA signaling (Akil et al., 2003, Meyer-Lindenberg et al., 2005, Scornaiencki et al., 2009, Seamans & Yang, 2004); indeed, the Met allele has been associated with decreased midbrain DA synthesis (Akil et al., 2003, Meyer-Lindenberg et al., 2005), which may facilitate the detection of phasic DA shifts critical for reward prediction errors and reinforcement learning (Bilder et al., 2004, Bogdan et al., 2011, Santesso et al., 2008, Schultz, 2002, Schultz, 2007). Consistent with this notion, the Met allele has been linked to heightened behavioral reward learning (Lancaster et al., 2015, Lancaster et al., 2012), elevated positive affect in response to reward (Wichers et al., 2007), and reward-seeking behavior (Lancaster et al., 2012) as well as reduced anhedonic symptoms in relatives of schizophrenia patients (Docherty & Sponheim, 2008). Such individual differences in reward-related behavior may underlie associations between COMT rs4680 genotype and psychopathology (Antypa et al., 2013, Bogdan et al., 2013).
Given emergent evidence linking COMT genotype to reward learning (Frank et al., 2007, Lancaster et al., 2015, Lancaster et al., 2012), and recent concerns of lack replication in behavioral genetics (Duncan & Keller, 2011, Plomin et al., 2016) the present study examined whether COMT genotype (rs4680) is associated with reward learning using data from 3 samples. Based on prior research (Lancaster et al., 2015, Lancaster et al., 2012), we hypothesized that individuals homozygous for the low activity Met allele would have increased reward learning (i.e., greater response bias to more rewarded cues). Lastly, we conducted a meta-analysis of published studies examining associations between COMT rs4680 genotype and behavioral reward learning as measured by a probabilistic reward learning task.
Materials and Methods
Participants
Participants (n=392) were recruited for three independent studies from the general and college community in the greater Boston, Massachusetts (Samples 1–2) and Durham, North Carolina (Sample 3) areas. Following quality control within each sample described below, the final total sample included 303 participants [age: 21.80 ± 3.95; 209 (69%) female; ethnicity: 208 (68.6%) European/European American, 33 (10.9%) African/African-American, 39 (12.9%) Asian/Asian-American, 10 (3.3%) Hispanic, 11 (3.6%) multiracial or other, 2 did not report (0.7%); Supplemental Table 1]. Because the relationship between this polymorphism and response bias has only been characterized in European-American samples (Goetz et al., 2013, Lancaster et al., 2015, Lancaster et al., 2012), and owing to evidence for differential associations in other phenotypes across ancestral origin (Lee & Prescott, 2014), primary analyses were conducted on European-American participants (Table 1) with supplemental analyses conducted in the entire sample (Supplemental Material). All subjects gave written informed consent and studies were approved by the Harvard University and Duke University Institutional Review Boards.
Table 1.
Sample demographics for composite European American sample
|
Sample 1 (n=56) |
Sample 2 (n=119) |
Sample 3 (n=33) |
All Samples (n=208) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Met/Met (n=14) |
Val/Val, Val/Met (n=42) |
Test statistic+ |
Met/Met (n=30) |
Val/Val, Val/Met (n=89) |
COMT Relation |
Met/Met (n=11) |
Val/Val, Val/Met (n=22) |
Test statistic+ |
Met/Met (n=55) |
Val/Val, Val/Met (n=153) |
Test statistic+ |
|
| Age (SD) | 21.8±2.22 | 21.9 ±1.63 | t= 0.18 p= 0.85 |
21.5±3.00 | 23.4 ±4.73 | t= 2.10 p= 0.038* |
19.0±1.09 | 19.6 ±1.21 | t= 1.46 p= 0.15 |
21.0±2.74 | 22.5±4.08 | t= 2.407 p= 0.017* |
| Sex (%female) | 14 (100%) | 42 (100%) | n/a | 13 (43.33%) | 48 (53.93%) | χ2= = 1.00 p = .315 |
5 (45.45%) | 13 (59.09%) | χ2= = 0.55 p = .458 |
32 (58.18%) | 103 (67.32%) | χ2= = 1.48 p= .22 |
= p<.05
Comparison between Met homozygotes and Val carriers
Sample 1
Healthy female participants (n=84) aged 18–25 were recruited from the greater Boston community. Exclusionary criteria included left-handedness, color blindness, past or present neurological, psychiatric, hormonal, or metabolic disturbances, and self-report ethnicity (i.e., only participants with two parents of European ancestry were included). Participants provided written informed consent to a protocol approved by the Committee on the Use of Human Subjects in Research at Harvard University and received either course credit or $10/hour as well as additional compensation earned ($15) during the probabilistic reward learning task (described below). Data were excluded from analyses for the following reasons: genotyping was not conducted (n=19), task non-compliance (i.e., predominantly pressing only one button) or below chance accuracy (n=6), technical difficulties (i.e., equipment did not function properly; n = 3), and failed genotyping (n=1), leaving a final sample of 56 for analyses. Three prior manuscripts have been published using these data evaluating reinforcement learning parameters (Huys et al., 2013) and associations between stress and genetic variation in the hypothalamic-pituitary-adrenal axis (Bogdan et al., 2010, Bogdan et al., 2011).
Sample 2
Participants (n=214; 123 female) aged 18–64 were recruited from Harvard University and the greater Boston community. Exclusionary criteria included current medical illness, attention-deficit hyperactivity disorder (ADHD), head injury, loss of consciousness, seizures, current alcohol/substance abuse or dependence, smoking, use of psychotropic medications during the last 2 weeks, pregnancy, or left handedness. Participants provided written informed consent to a protocol approved by the Committee on the Use of Human Subjects in Research at Harvard University and received course credit or $5 for participation and won additional money (average $6.00; between $5.80-$6.20) while completing the reward task. Collected data were excluded (n=41) from analyses due to task noncompliance (i.e., predominantly pressing only one button, below chance accuracy, or an inadequate reward ratio exposure, n=36), as well as failed genotyping (n=5) leaving a final sample of 173 (105 female) participants (119 European American; 61 female) for the present analyses. Three prior manuscripts have been published using these data evaluating computation reinforcement learning parameters (Huys et al., 2013) associations between stress and genetic variation within the HPA axis (Bogdan et al., 2010) and neural substrates of reward learning (Santesso et al., 2008).
Sample 3
A subset of participants (n=108, 70 females) enrolled in the ongoing Duke Neurogenetics Study (DNS; (Carey et al., 2015, Corral-Frias et al., 2015, Nikolova et al., 2014)) completed the probabilistic reward learning task described below. Participants provided written informed consent to a protocol approved by Duke University and received $5 for their time and won an additional $5 while completing the task. Study exclusion criteria included: medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms; use of psychotropic, glucocorticoid, or hypolipidemic medication; and/or conditions affecting cerebral blood flow and metabolism (e.g., hypertension). As the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology, diagnosis of current DSM-IV Axis I and select Axis II disorders (Antisocial Personality Disorder and Borderline Personality Disorder) were not exclusionary. Collected data were excluded from analyses due to task noncompliance (e.g., pressing one button exclusively, n=6) or because genotyping was not conducted (n=26). The final sample included a total of 74 (48 female) participants (33 European-American, 18 female).
Reward Learning Task and Data Processing
The computer task, which was adapted from prior studies (Pizzagalli et al., 2005, Tripp & Alsop, 1999), was presented on a PC using E-prime software (Psychology Software Tools, Inc, Pittsburgh, Pennsylvania). Notably, reward learning as measured by this task is (1) heritable (Bogdan & Pizzagalli, 2009), (2) associated with depression and anhedonia (Luking et al., 2015a, Luking et al., 2015b, Pizzagalli et al., 2005), (3) linked to treatment outcomes (Vrieze et al., 2013)and smoking behaviors in depressed patients (Liverant et al., 2014), (4) associated with depression resistance among anxious individuals (Morris & Rottenberg, 2015), (5) linked to reward-related striatal function (Santesso et al., 2008) and DA release (Vrieze et al., 2013), and (6) blunted by stress (Bogdan & Pizzagalli, 2006, Bogdan et al., 2011) and nicotine withdrawal (Pergadia et al., 2014). Briefly, participants are instructed to press a button on a button box or a keyboard to indicate whether a long or short mouth or nose1 is presented (100 ms) within a schematic face (see Figure 1). Importantly, the small size difference between stimuli and brief exposure time makes it difficult to discern which stimulus is presented. Participants are told that some, but not all correct responses, will result in correct feedback and a monetary reward. One of the stimuli (i.e., either long or short), the “rich” stimulus, is rewarded three times more frequently than the other, “lean” stimulus (stimulus types and buttons were counterbalanced across participants). Under these contingencies, humans and non-human animals develop a response bias for the more frequently rewarded, “rich” stimulus (Der-Avakian et al., 2013, Herrnstein, 1961, Lauwereyns et al., 2002, Pizzagalli et al., 2005, Tripp & Alsop, 1999).
Figure 1. Schematic diagram of the reward learning task.
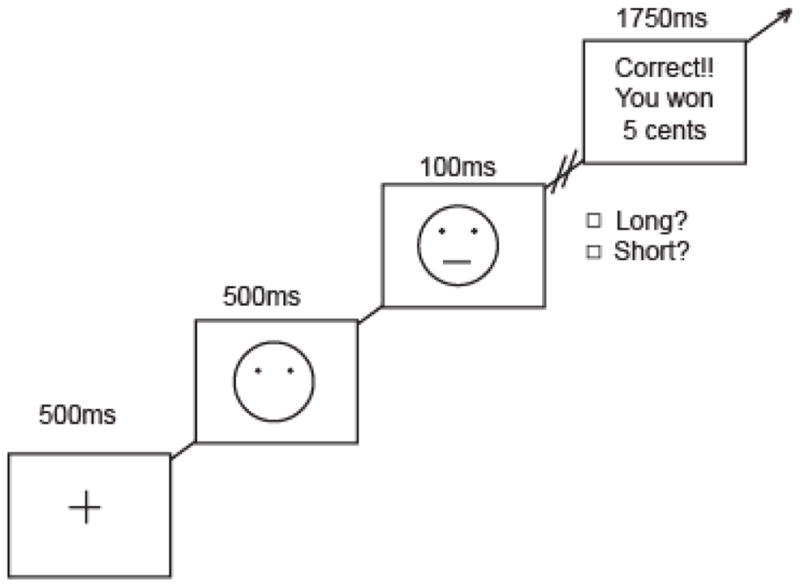
Participants are instructed to press a button on the keyboard to indicate whether a long or short mouth is presented (100 ms) within a schematic face. Following some, but not all correct responses, participants received a monetary reward of 5 cents. One stimulus (rich) was rewarded 3 times more than the other (lean). Figure adapted from (Pizzagalli et al., 2005).
The task consists of three blocks with 40% of trials per block receiving a reward.2 The “rich” and “lean” stimuli were presented with equal frequency, but, unknown to the participants, the reward feedback is asymmetrical in favor of the “rich” stimulus (3 “rich” to 1 “lean” reward ratio).3 Prior to analyses, we implemented a two-step procedure to identify outlier responses (Bogdan & Pizzagalli, 2006, Pizzagalli et al., 2005). First, trials with reaction times (RT) less than 150 ms or longer than 1500 ms were excluded. Second, after removing outliers with step one, we naturally log transformed the remaining trials and calculated the RT mean and standard deviation (SD) for each individual subject; trials that fell outside of the log-transformed mean ± 3 SD were excluded.
The main variable of interest was response bias, an empirically-based measure of reward learning, which measures the propensity to select a stimulus based on prior reinforcement history. Higher response bias values are reflective of a tendency to select the “rich” stimulus as being displayed. Response bias was calculated according to the following formula:
This formula illustrates that increased response bias results from: 1) a high quantity of correct identifications of the rich stimulus and misses for the lean stimulus (i.e. incorrectly identifying the lean stimulus as the rich stimulus) resulting in a large numerator, and 2) a low number of misses for the rich stimulus and correct identifications of the lean stimulus, resulting in a smaller denominator. The addition of 0.5 to each cell in this formula allows for the inclusion of data in which there were no incorrect responses.
To test the specificity of putative findings, control analyses were performed on discriminability, which provides a measure of the ability to discriminate between the two stimuli and is a measure of overall task performance or difficulty. Discriminability was calculated according to the following formula:
Both measures, response bias and discriminability, were derived from the behavioral model of signal detection (Macmillan, 2005).
Procedure
Sample 1
Participants completed two separate sessions. In the first session, the Structured Clinical Interview for the DSM-IV (SCID; (First et al., 1997) was administered to ensure no past or current Axis I disorder was present (participants with past minor alcohol abuse, i.e., one symptom meeting threshold more than 2 years ago, were included, n = 2). Eligible participants then completed a battery of questionnaires and provided a saliva sample for DNA analysis. During the second session participants performed the probabilistic reward task under a stress (threat-of-shock) and no-stress condition. In the stress condition, which was excluded from the present analyses, two electrodes were attached to the back of participants’ right hand and participants were instructed that they would receive one to three electrical shocks during the stress condition and that the intensity of shocks would increase over time. For a complete description of this procedure please see (Bogdan et al., 2011). The order of the stress and no stress condition was counterbalanced across participants. Only data from the no-stress condition was used for analyses.
Sample 2
Participants completed two separate sessions. In the first, the SCID (First et al., 1997) was administered to ensure that participants had no past or present Axis I disorders. Participants then completed several self-report measures assessing mood and stress, and provided a saliva sample for DNA analysis. In the second session, participants completed the probabilistic reward task. For a complete description of this procedure please see (Santesso et al., 2008).
Sample 3
Participants were recruited to complete the probabilistic reward learning task from a large ongoing study, the Duke Neurogenesis Study (DNS), which assesses a wide range of behavioral, experiential, and biological phenotypes among young-adult college students (Carey et al., 2015, Corral-Frias et al., 2015, Nikolova et al., 2014, Yacubian et al., 2007). Diagnosis of current DSM-IV Axis I and select Axis II disorders (Antisocial Personality Disorder and Borderline Personality Disorder) was assessed with the electronic Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and Structured Clinical Interview for the DSM-IV Axis II (SCID; (First et al., 1997). These disorders were not exclusionary, as the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology. After completing the initial portion of the study, some participants completed the probabilistic reward learning task on an additional day.
Genotyping
Samples 1 and 2
DNA obtained from saliva samples (OG-100; OG-25; Oragene; DNA Genotek) was purified, extracted, and hydrated; it was stored at −80°C when not in use. Primers were designed using Spectro DESIGNER software (Sequenom). Following a PCR, an iPLEX mass EXTEND reaction was performed. After baseline correction and peak identification, Sequenom SPECTROTYPER software was used to analyze resulting spectra. Concordance for duplicate DNA in the current sample was 100%. COMT rs4680 did not deviate from Hardy–Weinberg equilibrium (HWE; all ethnicities: χ2=0.372, p=.54; European American sample only: χ2=1.20, p=.27; sample 1: χ2=.055, p=.82; sample 2: χ2=1.388, p=.24).
Sample 3
DNA from participants within the DNS cohort was isolated from saliva derived from Oragene DNA self-collection kits (DNA Genotek) customized for 23andMe (www.23andme.com). DNA extraction and genotyping were performed by the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. The Illumina HumanOmniExpress BeadChips and a custom array containing an additional ~300,000 SNPs were used to provide genome-wide data. COMT rs4680 did not deviate from HWE (all ethnicities: χ2=.118, p= 0.73; European-American sample only: χ2=2.44, p= 0.12).
Data Analysis
Because participants in each sample completed the same task with minor variations and response bias across these 3 samples did not differ, we combined samples. Kolmogorov-Smirnov test statistics indicated that the data did not significantly deviate from a normal distribution (D=.04; p=.20). As such, linear regressions (SPSS v.21) were used to test the association between COMT genotype and total response bias in the combined sample as well as in each sample individually. We used total response bias as our index of reward learning because this reflects the overall bias developed across the task. In addition, this metric has been previously associated with rs4680 genotype (Lancaster et al., 2012) and is robust (i.e. does not produce lower estimates) even in cases where individuals learn contingencies quickly. Given prior evidence that Met homozygotes have higher response bias relative to Val carriers, participants were separated into Val-allele carriers (Val/Val and Val/Met) and Met homozygotes (Met/Met) (Goetz et al., 2013, Lancaster et al., 2015, Lancaster et al., 2012). Additional results reporting an additive model are reported in Supplemental Materials. Covariates included sex, study, and ethnicity (when applicable). Additionally, due to differences in age across samples and evidence that COMT enzyme activity differs according to age (Tunbridge et al., 2007), we also included age as a covariate. Finally, since some of the participants in our sample met criteria for one or more Axis I disorders (4.6%) according to a diagnostic interview (Supplemental Tables S2 and S3), psychiatric diagnosis was also added as a covariate. Because the association between Val158Met genotype and response bias has only been reported in European-American samples and due to population stratification concerns (Thomas & Witte, 2002), primary analyses were conducted in European-American participants only (n=208). Supplemental analyses were conducted in the full population (see Supplemental Materials).
Meta-analysis
Literature Search and Analyses
We performed PubMed and Google scholar searches to identify COMT genotype and reward learning studies, using the probabilistic reward learning task of interest (Pizzagalli et al., 2005), published before December 2015. Search words included “COMT genotype”, “COMT Val158Met”, “rs4680”, “reward”, “reward learning”, “response bias”, and “probabilistic reward task”. This search yielded a total of 3 studies that were published between 2012 and 2015 ((Goetz et al., 2013, Lancaster et al., 2015, Lancaster et al., 2012); Table 2). A weighted average for total response bias for Val carriers was calculated utilizing the means for Val/Val and Val/Met participants for a study that implemented an additive model (Goetz et al., 2013).
Table 2.
Descriptive characteristics of studies included in meta-analysis
| Study | Year | Age | Sex (%female) | Ancestry | Country of Origin | Allele frequency | Total N | ||
|---|---|---|---|---|---|---|---|---|---|
| Met/Met | Val/Met | Val/Val | |||||||
| Lancaster et al | 2012 | 22.7 ±4.2 | 61.42% | European | England | 19 | 25 | 26 | 70 |
| Goetz et al | 2013 | 21.3 ±2.7 | 59.32% | European-American | United States | 14 | 28 | 17 | 59 |
| Lancaster et al | 2015 | 22.2 ±4.6 | 43.56% | European | England | 24 | 54 | 20 | 98 |
| Current Mauscript | 21.8 ± 3.9 | 64.9% | European-American | United States | 55 | 97 | 56 | 208 | |
Analyses were performed using Revman 5.3 software (Cochrane IMS, Oxford, UK). The pooled effect was reported as a weighted mean difference (MD) with the corresponding 95% CI. Heterogeneity was assessed using I2 and χ2 tests, and a p value < 0.10 was considered to be significant. Since heterogeneity was not present in this meta-analysis, the pooled effect size was calculated through a fixed-effects model. Forest plots were constructed with p < 0.05 considered to be significant.
Results
Response bias
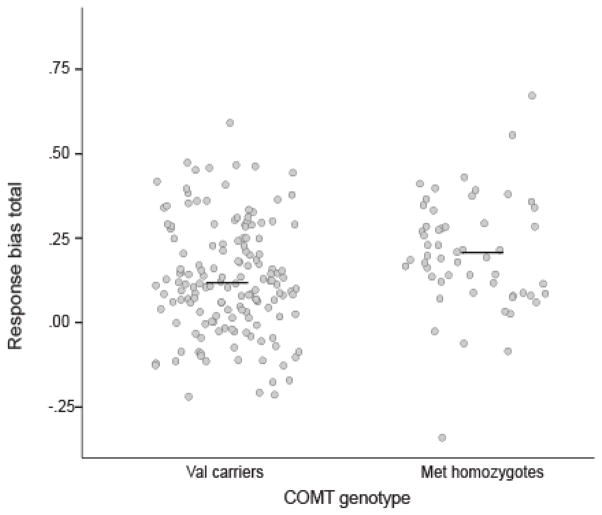
COMT rs4680 (Val158Met) genotype was significantly associated with total response bias (β=0.20, t= 2.75, p<0.01; ΔR2= 0.04; Figure 2) in the combined European-American samples (n=208). Consistent with prior literature (Lancaster et al., 2015, Lancaster et al., 2012), Met allele homozygotes demonstrated relatively higher response bias (M = 0.19 SD = 0.15; n = 55) compared to Val allele carriers (M = 0.12 SD = 0.16; n = 153). The directionality of this relationship was also consistent when participants of all ethnicities were included in the analysis (n=303); however, the effect of genotype was no longer significant (β= 0.09, t=1.56, p=0.12; Supplemental Figure 2). Although an additive genetic model showed consistent directional effects, these effects did not reach significance (see Supplemental Materials and Supplemental Figure 1).
Figure 2. COMT genotype associated with differences in total response bias in the probabilistic reward task.
A. In the European-American sample Met/Met participants (n = 55) demonstrated significantly greater total response bias than Val carriers (n = 153) (β=.20, t=2.75, p<.01; ΔR2= .04). Data points are jittered to allow for distribution visualization.
The main effect of COMT rs4680 genotype on response bias was significant in the European-American population within the largest sample (sample 2: β= 0.19, t= 1.99, p= 0.04; n=119) but was not significant in sample 3 (β= 0.29, t=1.50; p=0.14; n=33) or 1 (β= 0.15, t= 1.0; p= 0.32; n=56). Consistent with analyses combining data across samples, meta-analysis of all 3 independent samples from this study showed that Met-allele homozygotes had heightened response bias compared to Val carriers (MD: −0.07; 95% CI −0.12 to −0.02; p < 0.01; n=208).
To ensure that our findings were specific to response bias and not due to differences in the ability to discriminate between the two different stimuli (i.e., discriminability), we conducted regression analyses using COMT rs4680 genotype as a predictor of discriminability. Highlighting the specificity of the response bias findings in this European American sample, COMT rs4680 genotype was not significantly associated with discriminability (Discriminability total: β= −0.07, t= −1.60, p= 0.10).
Meta-analysis
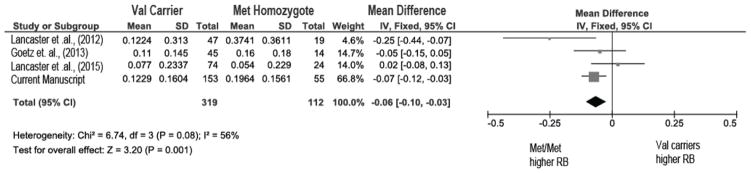
A pooled analysis of four studies (Goetz et al., 2013, Lancaster et al., 2015, Lancaster et al., 2012), present study, n=431) of European/European-American participants revealed that response bias was significantly increased among Met-allele homozygotes (n = 112) compared to Val-allele carriers (n = 319; MD: −0.07; 95% CI −0.10 to −0.03; p < 0.01; Figure 3). The test for heterogeneity was not significant (I2 = 48%; p = 0.12) confirming the appropriateness of a fixed effects model. Consistent with previous meta-analyses (Munafo et al., 2008), the effect size of the first published paper (Lancaster et al., 2012) was much larger than the effect size for the subsequent studies suggesting an overestimation of the effect in first published investigations (Ioannidis et al., 2001). Additionally, an analysis including participants from all ethnicities within the present sample (n=526) revealed consistent results (MD: −0.04; 95% CI −0.08 to −0.003; p < 0.05; See Supplemental Figure 3).
Figure 3. Forest plot of the pooled effect of COMT genotype on total response bias.
Size of square is proportional to sample size. CI: confidence interval; df: degrees of freedom; IV: Inverse Variance (statistical method). Lancaster et al., 2015 report a significant COMT rs4680 genotype x block interaction; here we depict the effect for the main effect of COMT rs4680 genotype on response bias.
Discussion
This study sought to replicate recently reported associations between COMT Val158Met genotype and behavioral reward learning ((Lancaster et al., 2015, Lancaster et al., 2012); Table 2). Consistent with these prior findings, our data suggest that individuals homozygous for the low enzymatic activity Met allele have relatively increased reward learning (as reflected by heightened response bias toward a stimulus more frequently associated with reward; Figure 2). Further, a meta-analysis of four studies ((Goetz et al., 2013, Lancaster et al., 2015, Lancaster et al., 2012) and the present study), using the same probabilistic reward learning task ((Pizzagalli et al., 2005); n=431), also produced a significant association between response bias and rs4680 genotype (Figure 3). Consistent with our findings, recent complementary evidence suggests that COMT rs4680 genotype is also associated with other aspects of reward function, including positive affect in response to rewarding experiences (Wichers et al., 2007) and reward seeking behavior (Lancaster et al., 2012). Collectively, these findings across studies provide evidence for an association between COMT rs4680 genotype and individual differences in reward processing, which may in turn, confer variability in vulnerability to a host of psychopathologies.
Putative Neural Mechanisms
While this study did not examine putative neural mechanisms through which COMT rs4680 genotype may be associated with individual differences in reward learning, emerging literature probing associations between COMT rs4680 genotype and neural phenotypes allows for informed speculation. This research suggests differential DA function associated with COMT rs4680 genotype wherein Met-allele homozygosity results in higher PFC DA levels relative to Val-allele homozygosity due to 40% fold reduction in COMT activity (Chen et al., 2004). This Met-allele driven variability in PFC DA may influence phasic reward prediction signals, stimulus signal-to-noise ratios as well as working memory to produce differences in reward learning. However, as noted below, each of these interpretations is also challenged by conflicting evidence.
Phasic changes in subcortical DA neuron firing are thought to encode differences in reward prediction errors (i.e., difference between expected and observed value), which are crucial signals for reward learning (Bayer & Glimcher, 2005, Garris et al., 1999, Schultz, 2002, Schultz, 2007). The expression of COMT in subcortical regions is minimal and the direct effect on subcortical DA cell activity is unknown. However, midbrain and striatal dopaminergic neurons are regulated by the PFC (Seamans & Yang, 2004), and the COMT Val allele is associated with increased tyrosine hydroxylase expression within the midbrain and, hence, presumably increased DA synthesis (Akil et al., 2003, Meyer-Lindenberg et al., 2005). This putative increase in DA synthesis may decrease the ability to detect phasic activity necessary for reward prediction, leading to decreased reward learning in Val-allele carriers (Pizzagalli et al., 2008, Santesso et al., 2009). Moreover, rs4680 genotype has been associated with individual differences in the functional interactions of subcortical and prefrontal regions during a working memory task (Meyer-Lindenberg et al., 2005) suggesting that COMT rs4680 genotype-related differences in PFC-subcortical interactions may contribute to reward learning.
Notably however, while this interpretation is consistent with our understanding of the role of subcortical DA and reward learning, direct neuroimaging studies of COMT rs4680 genotype associations with reward-related brain activation, which is believed to be tied to DA signaling (Knutson & Gibbs, 2007), have yielded conflicting evidence (Antypa et al., 2013, Camara et al., 2009, Dreher et al., 2009, Forbes et al., 2009, Schmack et al., 2008, Yacubian et al., 2007). For instance, some studies have shown increased reward-related ventral striatum reactivity in Met homozygotes (Dreher et al., 2009, Schmack et al., 2008, Yacubian et al., 2007) while others have shown increased activation in Val homozygotes (Camara et al., 2009). While these contradictory findings call into question the potential impact of COMT genotype on reward function through its effect on subcortical DA, it is important to note that none of these tasks were designed to evaluate reward learning specifically (as opposed to other forms of reward processing such as anticipating or receiving money). It is also possible that false positive associations (Farrell et al., 2015, Lee & Song, 2015, Munafò et al., 2005, Nickl-Jockschat et al., 2015) may contribute to these equivocal results.
Alternatively, though not mutually exclusive, the effects of rs4680 genotype on reward learning may arise from its effects on prefrontal DA function and related behaviors. In addition to striatal reward prediction errors, a wide variety of higher order brain function, including executive control and working memory, likely contributes to reward learning (Collins & Frank, 2012). Extensive working memory research suggests COMT-related effects on prefrontal DA may modulate signal-to-noise ratio allowing task-related information to be prioritized, potentially facilitating learning of novel stimulus-reward pairings [(Akil et al., 2003, Meyer-Lindenberg & Weinberger, 2006, Seamans & Yang, 2004) but see also (Nickl-Jockschat et al., 2015)]. Further, this literature suggests that in the context of working memory, an optimum level of DA stimulation is necessary to reach the highest signal-to-noise ratio, placing Met homozygotes at the height of this inverted u-curve (Meyer-Lindenberg et al., 2005). Moreover, behavioral and in silico experiments suggest that prefrontal function may contribute to reward learning by influencing initial learning acquisition rate (Collins & Frank, 2012). Supporting this interpretation, genetic association studies have linked polymorphisms associated with variability in subcortical DA signaling (e.g. DARPP-32 and DRD2) to individual differences in learning rates after initial learning has occurred, and polymorphisms associated with cortical DA function (e.g. COMT) with learning rates during initial acquisition (Frank et al., 2007). Thus, although the effects of COMT rs4680 genotype on subcortical function have been hypothesized (Bilder et al., 2004), evidence (Huotari et al., 2002) suggests that an explanation based on prefrontal regulation of striatal DA metabolism via top–down projections may also be important (Matsumoto et al., 2003).
While this interpretation could potentially account for the behavioral effects observed here, it is challenged by recent meta-analyses suggesting that COMT rs4680 genotype may have no main effect on higher order executive function such as working memory (Nickl-Jockschat et al., 2015). Notably, it is possible that Val allele-specific patterns of methylation may contribute to this contradictory literature, as the Val-allele homozygotes have a CpG methylation site that Met-allele carriers do not. Moreover, methylation at this site is related to stress exposure and variability in behavioral and neural working memory phenotypes (Ursini et al., 2011). Specifically, Val-allele homozygotes with low stress levels and heightened methylation in this region have working memory-related neural function and behavior comparable to Met-allele carriers highlighting the importance of considering methylation and stress in future COMT rs4680 genotype research (Ursini et al., 2011).
Vulnerability to Psychopathology
Recent theoretical and empirical evidence suggests that reward processing deficits within psychiatric disorders may be closely linked to motivation, reward learning and reward decision making, rather than hedonic response (Barch et al., 2015, Pizzagalli, 2014). Since positive reinforcement increases the likelihood of behaviors linked to them, reward learning dysfunction may reduce motivation to pursue rewards, thus increasing the probability of symptom persistence or even exacerbation of psychopathology (Pizzagalli, 2014). Consistent with this hypothesis, behavioral reward learning, as measured by the task used in this study, has been associated with anhedonic symptoms and depression (Luking et al., 2015a, Luking et al., 2015b, Pizzagalli et al., 2005) as well as chronicity of symptoms after antidepressant treatment (Vrieze et al., 2013). Accordingly, reward learning deficits observed in COMT rs4680 Val-allele carriers may place them at greater risk for psychopathology characterized by deficient reward processing as has been reported in some (Baune et al., 2008, Benedetti et al., 2009, Benedetti et al., 2010, Spronk et al., 2011, Yoshida et al., 2008), but not all (Szegedi et al., 2005), studies.
Limitations and Conclusions
Interpretation of the current results should be considered in the context of study limitations. First, our sample (even the pooled meta-analytic data) is small for a genetic association study making our estimated effect imprecise. Second, our results were only significant when all subsamples were combined (or meta-analyzed), and in our largest dataset (Sample 2). The relationship was not significant in our other samples, though it approached a trending relationship in our Sample 3 and showed a similar directional effect across all. The non-significant results from Sample 1 may be partially attributable to the original design of the study. Here participants performed the probabilistic reward learning task under stress and no-stress conditions, where the order was counterbalanced across subjects (Bogdan et al., 2010, Bogdan et al., 2011). While this study did not use data from the stress condition, given preliminary evidence of Gene x Environment interactions at this locus (Craddock et al., 2006), it is possible that this study design and the presence of the stress condition weakened the link between COMT genotype and reward learning. Moreover, our 3 samples differed in sex, ethnicity and age distribution as well as the version of the probabilistic reward task used. These study-related differences may have added variability to our reported effects. In an attempt to account for this possibility, study differences including, the study of origin, sex, and age, were included as covariates in our analysis. Further, we analyzed each sample independently and conducted a meta-analysis, which resulted in the same conclusion.
Third, results were only significant when analyses were constrained to European-American individuals. However, the directionality of the effect was consistent when all ethnicities were included in analyses (Supplemental Figure 2) and a meta-analysis across published studies (including the present data) also yielded evidence of significant association when including individuals of all ancestral origins (Supplemental Figure 3). It is important to note that this is the only study to date to contain a sample of mixed ethnicities so the role of ancestral origin in COMT Val158Met genotype–response bias phenotype associations is unclear. Notably, among other phenotypes, there is evidence of differential association according to ancestry (e.g.,(Domschke et al., 2007, Hosak, 2007).
Fourth, our meta-analysis only included data from previously published research. In light of publication bias for positive as opposed to null findings (Hirschhorn et al., 2002, Munafo et al., 2004), it is possible that additional data are available which do not report the associations described herein and may have led to a biased meta-analysis. Along with these previous reports, our study thus highlights the importance of not only replicating genetic association studies but also performing meta-analyses in an attempt to more accurately measure effect sizes (Munafo et al., 2008). Lastly, meta-analyses were conducted using a fixed effects model. While the heterogeneity observed in the data support such a model, the larger studies included within the meta-analysis by definition contributes more to the weighted average. Notably, a random effects model showed trending effects (p=.08) in the same direction as the fixed effects model.
These limitations notwithstanding, the present study suggests that a common genetic variant within the COMT gene (rs4680) is associated with individual differences in reward learning. Our study further highlights the importance of replication and meta-analyses in genetic association studies. While these findings shed light on how this functional genetic polymorphism is important in the appearance of individual differences in reward learning, further research is needed to elucidate the potential neural mechanisms underlying these behavioral associations and to trace such associations to the development of psychopathology.
Supplementary Material
Acknowledgments
We are thankful to the Duke Neurogenetics Study (DNS) and Center for Depression, Anxiety and Stress Research (CDASR) participants and staff. Studies conducted at Harvard University were supported by NIMH Grant R01-MH068376 (awarded to DAP), and a Sackler Scholar in Psychobiology (awarded to RB). DAP was also partially supported by NIMH grant MH101521. The Duke Neurogenetics Study is supported by Duke University and the National Institutes of Health (NIDA R01-DA033369). ARH receives additional support from the National Institutes of Health (NIDA R01-DA031579). NCS was supported by NIMH (T32-MH014677). RB was supported by the Klingenstein Third Generation Foundation and receives additional support from the National Institutes of Health (NIA R01-AG045231). LJM was supported by the National Institutes of Health (T32-GM081739).
Footnotes
In sample 1, two different stimuli were used: mouth and nose. In this sample participants had to indicate whether a long (mouth, 11.00 mm; nose, 5.31 mm) or short (mouth, 10.00 mm; nose, 5.00 mm) stimulus was presented. In sample 2, only mouth stimuli (long mouth: 13 mm, short mouth: 11.5 mm) were presented. In sample 3, only mouth stimuli were presented, and these were the same length as in sample 1.
The task in sample 1 and 3 consisted of 80 blocks whereas in sample 2 it consisted of 100 blocks. Accordingly, 32 of the trials in sample 1 and 3 were rewarded and 40 trials in sample 2 were rewarded.
Participants in samples 1 and 3 received reward for 24 and 8 of the rich and lean stimulus trials, respectively, whereas those in sample 2 received 30 and 10 reward for the rich and lean stimulus, respectively.
Conflict of Interest
In the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Otsuka America Pharmaceutical and Pfizer for activities unrelated to this project. All other authors report no conflict of interest.
References
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: Bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev. 2013;37:1597–1610. doi: 10.1016/j.neubiorev.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, Luking K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2015_376. [DOI] [PubMed] [Google Scholar]
- Baune BT, Hohoff C, Berger K, Neumann A, Mortensen S, Roehrs T, Deckert J, Arolt V, Domschke K. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33:924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Pirovano A, Marino E, Smeraldi E. The catechol-O-methyltransferase Val(108/158)Met polymorphism affects antidepressant response to paroxetine in a naturalistic setting. Psychopharmacology (Berl) 2009;203:155–160. doi: 10.1007/s00213-008-1381-7. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Lorenzi C, Pirovano A, Smeraldi E. Effect of catechol-O-methyltransferase Val(108/158)Met polymorphism on antidepressant efficacy of fluvoxamine. Eur Psychiatry. 2010;25:476–478. doi: 10.1016/j.eurpsy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol Dis. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Perlis RH, Fagerness J, Pizzagalli DA. The impact of mineralocorticoid receptor ISO/VAL genotype (rs5522) and stress on reward learning. Genes Brain Behav. 2010;9:658–667. doi: 10.1111/j.1601-183X.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychol Med. 2009;39:211–218. doi: 10.1017/S0033291708003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31:13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Camara E, Kramer UM, Cunillera T, Marco-Pallares J, Cucurell D, Nager W, Mestres-Misse A, Bauer P, Schule R, Schols L, Tempelmann C, Rodriguez-Fornells A, Munte TF. The Effects of COMT (Val108/158Met) and DRD4 (SNP -521) Dopamine Genotypes on Brain Activations Related to Valence and Magnitude of Rewards. Cereb Cortex. 2009;20:1985–1996. doi: 10.1093/cercor/bhp263. [DOI] [PubMed] [Google Scholar]
- Carey CE, Agrawal A, Zhang B, Conley ED, Degenhardt L, Heath AC, Li D, Lynskey MT, Martin NG, Montgomery GW, Wang T, Bierut LJ, Hariri AR, Nelson EC, Bogdan R. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol. 2015;124:860–877. doi: 10.1037/abn0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AG, Frank MJ. How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis. Eur J Neurosci. 2012;35:1024–1035. doi: 10.1111/j.1460-9568.2011.07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frias NS, Nikolova YS, Michalski LJ, Baranger DA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45:2605–2617. doi: 10.1017/S0033291715000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AR, Sponheim SR. Anhedonia as a phenotype for the Val158Met COMT polymorphism in relatives of patients with schizophrenia. J Abnorm Psychol. 2008;117:788–798. doi: 10.1037/a0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Deckert J, O’Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:667–673. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’Donovan MC, Corvin A, Cichon S, Sullivan PF. Evaluating historical candidate genes for schizophrenia. Mol Psychiatry. 2015;20:555–562. doi: 10.1038/mp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) American Psychiatric Press, Inc; Washington, D.C: 1997. [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci U S A. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield JBB, Lubman DI, Yucel M. Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Australian & New Zealand Journal of Psychiatry. 2014;48:36–51. doi: 10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Goetz EL, Hariri AR, Pizzagalli DA, Strauman TJ. Genetic moderation of the association between regulatory focus and reward responsiveness: a proof-of-concept study. Biol Mood Anxiety Disord. 2013;3:3. doi: 10.1186/2045-5380-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Hosak L. Role of the COMT gene Val158Met polymorphism in mental disorders: a review. Eur Psychiatry. 2007;22:276–281. doi: 10.1016/j.eurpsy.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Lancaster TM, Heerey EA, Mantripragada K, Linden DE. Replication study implicates COMT val158met polymorphism as a modulator of probabilistic reward learning. Genes Brain Behav. 2015;14:486–492. doi: 10.1111/gbb.12228. [DOI] [PubMed] [Google Scholar]
- Lancaster TM, Linden DE, Heerey EA. COMT val158met predicts reward responsiveness in humans. Genes Brain Behav. 2012;11:986–992. doi: 10.1111/j.1601-183X.2012.00838.x. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Lee LO, Prescott CA. Association of the catechol-O-methyltransferase val158met polymorphism and anxiety-related traits: a meta-analysis. Psychiatr Genet. 2014;24:52–69. doi: 10.1097/YPG.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Song GG. BDNF 196 G/A and COMT Val158Met Polymorphisms and Susceptibility to ADHD: A Meta-Analysis. J Atten Disord. 2015 doi: 10.1177/1087054715570389. [DOI] [PubMed] [Google Scholar]
- Liverant GI, Sloan DM, Pizzagalli DA, Harte CB, Kamholz BW, Rosebrock LE, Cohen AL, Fava M, Kaplan GB. Associations among smoking, anhedonia, and reward learning in depression. Behav Ther. 2014;45:651–663. doi: 10.1016/j.beth.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Luking KR, Neiman JS, Luby JL, Barch DM. Reduced Hedonic Capacity/Approach Motivation Relates to Blunted Responsivity to Gain and Loss Feedback in Children. J Clin Child Adolesc Psychol. 2015a:1–13. doi: 10.1080/15374416.2015.1012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, Barch DM. Child Gain Approach and Loss Avoidance Behavior: Relationships With Depression Risk, Negative Mood, and Anhedonia. J Am Acad Child Adolesc Psychiatry. 2015b;54:643–651. doi: 10.1016/j.jaac.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Morris BH, Rottenberg J. Heightened reward learning under stress in generalized anxiety disorder: a predictor of depression resistance? J Abnorm Psychol. 2015;124:115–127. doi: 10.1037/a0036934. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res. 2004;129:39–44. doi: 10.1016/j.psychres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Janouschek H, Eickhoff SB, Eickhoff CR. Lack of meta-analytic evidence for an impact of COMT Val158Met genotype on brain activation during working memory tasks. Biol Psychiatry. 2015;78:e43–46. doi: 10.1016/j.biopsych.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Koenen KC, Galea S, Wang CM, Seney ML, Sibille E, Williamson DE, Hariri AR. Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nat Neurosci. 2014;17:1153–1155. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Der-Avakian A, D’Souza MS, Madden PA, Heath AC, Shiffman S, Markou A, Pizzagalli DA. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71:1238–1245. doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Ann Rev Clin Psych. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Top 10 Replicated Findings From Behavioral Genetics. Perspect Psychol Sci. 2016;11:3–23. doi: 10.1177/1745691615617439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30:1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, Pizzagalli DA. Enhanced negative feedback responses in remitted depression. Neuroreport. 2008;19:1045–1048. doi: 10.1097/WNR.0b013e3283036e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack K, Schlagenhauf F, Sterzer P, Wrase J, Beck A, Dembler T, Kalus P, Puls I, Sander T, Heinz A, Gallinat J. Catechol-O-methyltransferase val158met genotype influences neural processing of reward anticipation. NeuroImage. 2008;42:1631–1638. doi: 10.1016/j.neuroimage.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting Formal with Dopamine and Reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornaiencki R, Cantrup R, Rushlow WJ, Rajakumar N. Prefrontal cortical D1 dopamine receptors modulate subcortical D2 dopamine receptor-mediated stress responsiveness. Int J Neuropsychopharmacol. 2009;12:1195–1208. doi: 10.1017/S1461145709000121. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Frankle WG, Weinberger DR, Laruelle M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Spronk D, Arns M, Barnett KJ, Cooper NJ, Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: a pilot study. J Affect Disord. 2011;128:41–48. doi: 10.1016/j.jad.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Rujescu D, Tadic A, Muller MJ, Kohnen R, Stassen HH, Dahmen N. The catechol-O-methyltransferase Val108/158Met polymorphism affects short-term treatment response to mirtazapine, but not to paroxetine in major depression. Pharmacogenomics J. 2005;5:49–53. doi: 10.1038/sj.tpj.6500289. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Witte JS. Point: population stratification: a problem for case-control studies of candidate-gene associations? Cancer Epidemiol Biomarkers Prev. 2002;11:505–512. [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, Sinibaldi L, Gelao B, Romano R, Rampino A, Taurisano P, Mancini M, Di Giorgio A, Popolizio T, Baccarelli A, De Blasi A, Blasi G, Bertolino A. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, Peeters F, Derom C, Vlietinck R, Mengelers R, Delespaul P, van Os J. The Catechol-O-Methyl Transferase Val158Met Polymorphism and Experience of Reward in the Flow of Daily Life. Neuropsychopharmacology. 2007;33:3030–3036. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, Braus DF, Buchel C. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Higuchi H, Takahashi H, Kamata M, Sato K, Inoue K, Suzuki T, Itoh K, Ozaki N. Influence of the tyrosine hydroxylase val81met polymorphism and catechol-O-methyltransferase val158met polymorphism on the antidepressant effect of milnacipran. Hum Psychopharmacol. 2008;23:121–128. doi: 10.1002/hup.907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.