Abstract
The human growth factor receptor type 2 (HER2) is overexpressed in breast as well as other types of cancer. ImmunoPET, a noninvasive imaging procedure that could assess HER2 status in both primary and metastatic lesions simultaneously, could be a valuable tool for optimizing application of HER2-targeted therapies in individual patients. Herein, we have evaluated the tumor targeting potential of the 5F7 anti-HER2 Nanobody (single-domain antibody fragment; ~13 kDa) after 18F labeling by two methods.
Methods
The 5F7 Nanobody was labeled with 18F using the novel residualizing label N-succinimidyl 3-((4-(4-18F-fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate (18F-SFBTMGMB; 18F-RL-I) and also via the most commonly utilized 18F protein labeling prosthetic agent, N-succinimidyl 3-18F-fluorobenzoate (18F-SFB). For comparison, 5F7 Nanobody was also labeled using the residualizing radioiodination agent N-succinimidyl 4-guanidinomethyl-3-125I-iodobenzoate (125I-SGMIB). Paired label (18F/125I) internalization assays and biodistribution studies were performed on HER2-expressing BT474M1 breast carcinoma cells and in mice with BT474M1 subcutaneous xenografts, respectively. Micro positron emission tomography/computed tomography (microPET/CT) imaging of 5F7 Nanobody labeled using 18F-RL-I also was performed.
Results
Internalization assays indicated that intracellularly retained radioactivity for 18F-RL-I-5F7 was similar to that for co-incubated 125I-SGMIB-5F7, while that for 18F-SFB-5F7 was lower than co-incubated 125I-SGMIB-5F7 and decreased with time. BT474M1 tumor uptake of 18F-RL-I-5F7 was 28.97 ± 3.88 %ID/g at 1 h and 36.28 ± 14.10 %ID/g at 2 h, reduced by >90% trastuzumab blocking, indicating HER2-specificity of uptake, and also 26–28% higher (P < 0.05) than that of 18F-SFB-5F7. At 2 h, the tumor-to-blood ratio for 18F-RL-I-5F7 (47.4 ± 13.1) was significantly higher (P < 0.05) than for 18F-SFB-5F7 (25.4 ± 10.3); however, kidney uptake was 28–36-fold higher for 18F-RL-I-5F7.
Conclusion
18F-RL-I-5F7 is a promising tracer for evaluating HER2 status by immunoPET; however, in settings where renal background is problematic, strategies for reducing its kidney uptake may be needed.
Keywords: HER2, Nanobody, 18F, ImmunoPET, Residualizing label
INTRODUCTION
Despite the introduction of molecularly targeted therapies, death rates in women from breast cancer remain higher than those from any other malignancy except lung cancer (1,2). Because the human epidermal growth factor receptor type 2 (HER2, ErbB2/neu) is associated with tumor aggressiveness and poor prognosis, a variety of HER2-targeted therapies have been developed (3). A notable example is trastuzumab, a monoclonal antibody reactive with the extracellular domain of HER2, which can significantly increase survival in the 25–30% of breast cancer patients with HER2-positive disease. As with other molecularly targeted therapies, those directed against HER2 are largely ineffective in patients that are HER2-negative at the time of treatment. Moreover, those patients not likely to benefit would be needlessly subjected to treatment related side effects such as the cardiotoxicity associated with trastuzumab treatment (4), which could be avoided if their HER2 status was known. Thus, it is imperative to assess the HER2 levels in tumors of individual patients before administering trastuzumab or other HER2-targeted therapy. Indeed, evaluation of HER2 expression in every primary breast cancer has been recommended by both the American Society of Clinical Oncology and the European Group of Tumor Markers (5,6).
The two primary techniques for assaying HER2 levels, immunohistochemical staining and fluorescence in situ hybridization (7) are problematic because they are invasive and may not be representative due to heterogeneous HER2 expression within the primary tumor. Moreover, they are not informative about differences in HER2 levels between primary lesion and metastases or among different metastatic sites (8–10), leading to recommendations that a biopsy be obtained to evaluate target status in metastases before selecting an appropriate therapy (11). This need has provided motivation for the evaluation of HER2-specific antibodies, antibody fragments and affibodies labeled with positron emitters for assessment of global HER2 expression by immunoPET (12–14).
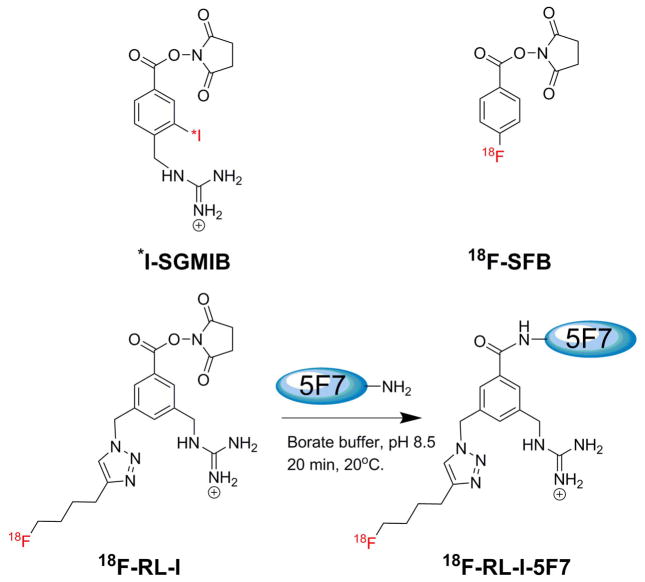
Herein we explore the feasibility of utilizng 18F-labeled Nanobodies as probes for evaluating HER2 status by immunoPET. Nanobodies (a.k.a. VHH; 12–15 kDa) are antigen-binding fragments of heavy-chain-only antibodies from Camelidae (15,16) having biological half-lives (1–2 h) that are ideal for labeling with 18F (t½ = 1.8 h). Our 18F labeling strategy is based on our previous studies with radioiodine labeling of the anti-HER2 Nanobody 5F7 using the residualizing label N-succinimidyl 4-guanidinomethyl-3-*I-iodobenzoate (*I-SGMIB) (16). The *I-SGMIB-5F7 conjugate exhibited substantially higher uptake in HER2 expressing xenografts than those reported previously for any combination of Nanobody, radionuclide, and tumor model. Herein, the 5F7 Nanobody was labeled with 18F using both an agent conceptually analogous to *I-SGMIB, N-succinimidyl 3-((4-(4-18F-fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate (18F-SFBTMGMB; 18F-RL-I) (Fig. 1) (17), and with 18F-SFB (18), and then evaluated in HER2 positive BT474M1 breast carcinoma cells and xenograft models.
FIGURE 1.
Structures of *I-SGMIB and 18F-SFB and the scheme for labeling 5F7 Nb using 18F-RL-I
MATERIALS AND METHODS
Nanobody, cells, and culture conditions
Production, purification and characteristics of anti-HER2 5F7 Nanobody, obtained from Ablynx (Ghent, Belgium), in the format lacking the CysCysGly tail, have been described (19). HER2-expressing BT474M1 human breast carcinoma cells (20) were cultured in DMEM/F12 medium containing 10% fetal calf serum, streptomycin (100 μg/mL), and penicillin (100 IU/mL) (Sigma Aldrich, MO). Cells were cultured at 37°C in a humidified incubator under 5% CO2 with media changed every two days. When about 80% confluent, cells were sub-cultured by trypsinization (0.05 % Trypsin-EDTA).
Radiolabeling Nanobody 5F7
Details of the synthesis of 18F-RL-I-5F7, 18F-SFB-5F7 and 125I-SGMIB-5F7, as well as the affinity and immunoreactivity of these immunoconjugates, have been reported in a recent publication (17) (see Supplementary Materials for additional synthetic details).
Internalization assays
Two sets of internalization assays were performed on BT474M1 cells comparing the behavior of 18F-RL-I-5F7 or 18F-SFB-5F7 with co-incubated 125I-SGMIB-5F7 as described (19) but only at 1, 2 and 4 h (detailed in Supplementary Materials).
Biodistribution studies
Paired label studies were performed in mice with BT474M1 subcutaneous xenografts (detailed in Supplementary Materials) following protocols approved by the Duke University IACUC (16,19). In Experiment 1, two groups of five mice were injected via the tail vein with 148 kBq (4 μCi; 0.9 μg) 125I-SGMIB-5F7 and 370 kBq (10 μCi; 5.9 μg) 18F-RL-I-5F7 in 100 μL of PBS. In Experiment 2, two groups of five mice received 148 kBq (4 μCi; 0. 5 μg) 125I-SGMIB-5F7 and 555 kBq (15 μCi; 3.8 μg) 18F-SFB-5F7. At 1 h and 2 h post injection, five mice were killed with an overdose of isofluorane, dissected, and tissues and blood were harvested. Tissues, blood, and urine were weighed and counted for 125I and 18F activity in an automated gamma counter. From these data, percentage of injected dose per gram of tissue (%ID/g) and tumor-to-normal tissue ratios were calculated.
MicroPET/CT imaging
Imaging was performed on a Siemens Inveon microPET/CT system (Malvern, PA) in groups of 4 mice with BT474M1 xenografts with and without HER2 blocking. For HER2 blocking, mice were injected i.v. trastuzumab in phosphate buffer (4.4 mg in 200 μL; 220 mg/kg) 24 h before injection of 3.0–4.5 MBq (80–120 μCi; ~10 μg) 18F-RL-I-5F7 in 100 μL PBS. Mice were anesthetized using 2–3% isoflurane in oxygen and placed prone in the scanner gantry for a 5 min PET acquistion followed by a 5 min CT scan. Control mice were imaged at 1 and 2 h while HER2-blocked mice were imaged at 1 h. List mode PET data were histogram-processed and the images reconstructed using standard OSEM3D/MAP algorithm—2 OSEM3D iterations, and 18 MAP iterations—with a cutoff (Nyquist) of 0.5. Images were corrected for attenuation (CT-based) and radioactive decay. Image analysis was performed using Inveon Research Workplace software. Regions of interest (ROI) were drawn around tumors on the co-registered PET and CT images and 18F uptake was expressed as SUV and %ID/g.
Statistical Analyses
Results are presented as mean ± SD and statistical significance of differences in uptake between two tracers that were co-injected (18F vs 125I) was calculated with a 2-tailed, paired Student t test using Microsoft Excel, while an 2-tailed unpaired Student t test was used to compare the results obtained for the two 18F labeling methods in different groups of animals. A P value of <0.05 was considered statistically significant.
RESULTS
Internalization Assays
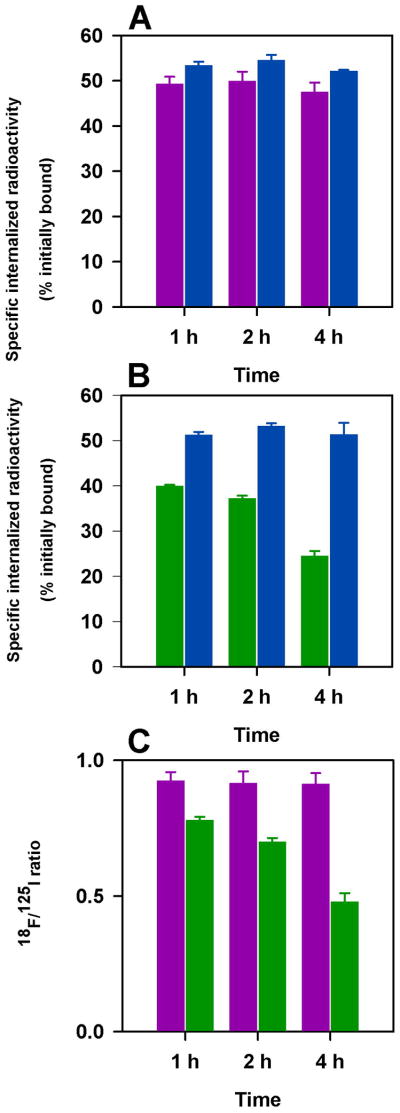
In the first study (Fig. 2A), intracellularly trapped 18F-RL-I-5F7 activity was 49.3 ± 1.6%, 49.9 ± 2.1%, and 47.5 ± 2.1%, of initially cell-bound levels, at 1 h, 2 h and 4 h, respectively, values slightly lower than those for co-incubated 125I-SGMIB-5F7 (53.4 ± 0.8%, 55.0 ± 1.2%, and 52.1 ± 0.3%). In contrast, intracellular counts from 18F-SFB-5F7 decreased from 39.9 ± 0.3% at 1 h to 24.5 ± 1.1% 4 h (Fig. 2B), values significantly lower (P<0.05) than those for co-incubated 125I-SGMIB-5F7 (1 h, 51.2 ± 0.7%; 4 h, 51.3 ± 2.6%). Normalizing to co-administered 125I-SGMIB-5F7 was performed for the two experiments and the resultant 18F/125I ratios shown in Figure 2C, further demonstrate the advantage of 18F-RL-I-5F7 over 18F-SFB-5F7 with regard to intracellular trapping of 18F activity. These results suggest that, RL-I, like SGMIB, helps retain radioactivity in BT474M1 cells in vitro after internalization of labeled Nanobody.
FIGURE 2.
Paired label internalization of 125I-SGMIB-5F7 (blue bars) versus 18F-RL-I-5F7 (magenta bars) (A) and 125I-SGMIB-5F7 (blue bars) versus 18F-SFB-5F7 (green bars) (B) in BT-471M1 breast cancer cells in vitro. Ratio of 18F/125I obtained from the two experiments (C).
Biodistribution Studies
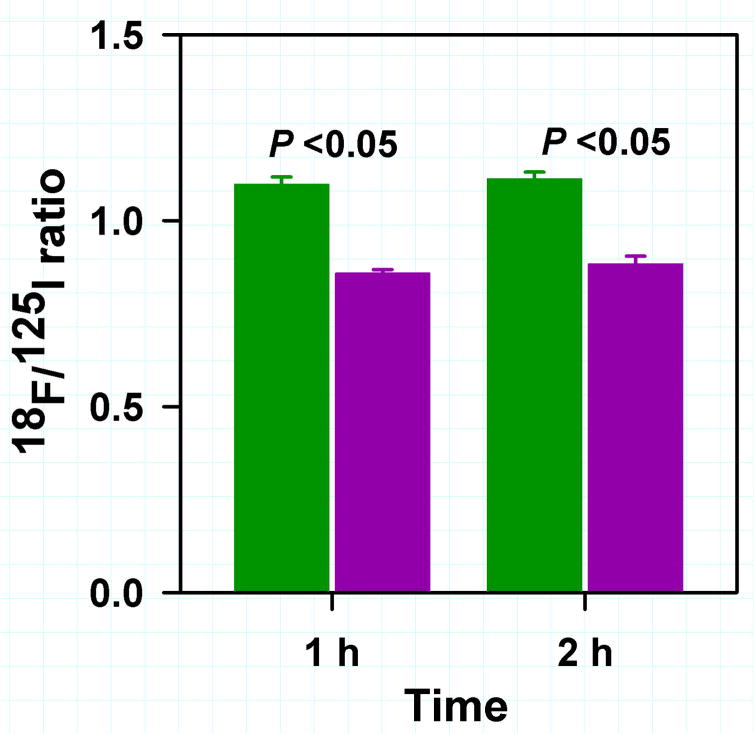
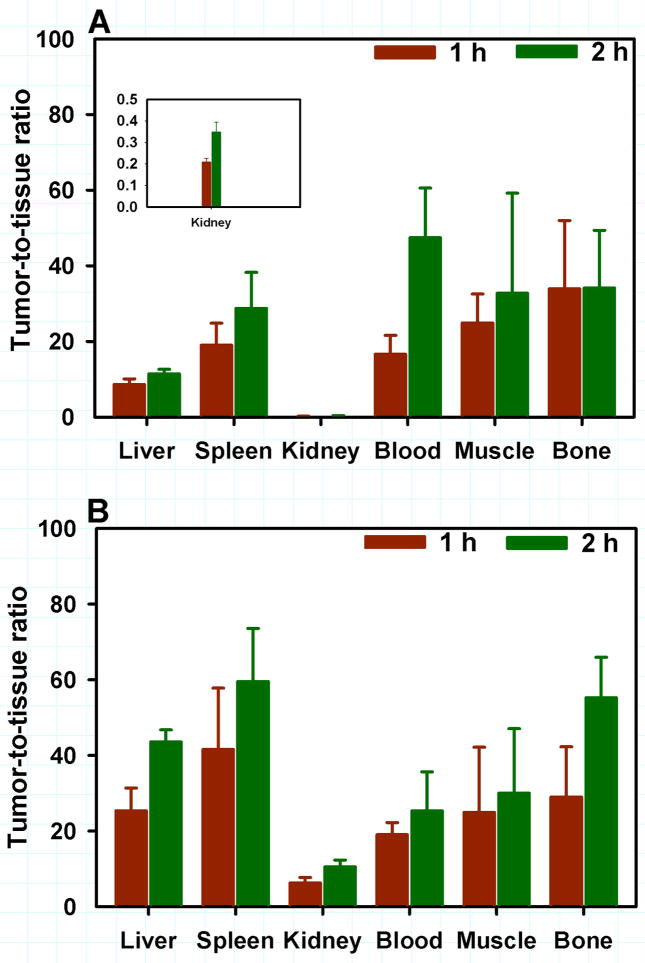
The tissue distribution of 18F-RL-I-5F7 and 18F-SFB-5F7 were compared to that of co-administered 125I-SGMIB-5F7 in mice with BT474M1 xenografts at 1 and 2 h and the results are presented in Tables 1 and 2, respectively. Tumor uptake of 18F-RL-I-5F7 increased from 29.0 ± 3.9% ID/g at 1 h to 36.3 ± 14.1% ID/g at 2 h and was significantly higher (P<0.05) than that of co-administered 125I-SGMIB-5F7. In contrast, tumor uptake of 18F-SFB-5F7 was also >20% ID/g but significantly lower (P<0.05) than that of co-administered 125I-SGMIB-5F7 at all time points. When normalized to 125I-SGMIB-5F7 levels (Fig. 3), tumor uptake of 18F-RL-I-5F7 was 26–28% higher (P<0.05) than that of 18F-SFB-5F7, consistent with the hypothesized effects of charged guanidine (17) and polar triazole (21) moieties in 18F-RL-I on trapping of labeled catabolites, thereby enhancing tumor uptake. Generally, uptake of the two 18F-5F7 conjugates in normal tissues was more than an order of magnitude lower than that in tumor, with the exception of renal levels for 18F-RL-I-5F7, which were comparable to those for 125I-SGMIB-5F7. Tumor-to-normal tissue ratios for both 18F-labeled 5F7 conjugates increased from 1 to 2 h after injection (Fig. 4). With 18F-RL-I-5F7, the tumor-to-blood and tumor-to-muscle ratios at 2 h reached 47 ± 13 and 38 ± 21, respectively, values that were higher than those observed for 18F-SFB-5F7 (25 ± 10; P<0.02 and 30 ± 17; P>0.05, respectively). In contrast, tumor-to-tissue ratios for 18F-SFB-5F7 were higher than those for 18F-RL-I-5F7 in liver, spleen, bone and most notably, kidneys (P <0.04–0.001).
TABLE 1.
Paired Label Biodistribution of 18F-RL-I-5F7 and 125I-SGMIB-5F7 in SCID Mice Bearing BT474M1 Xenografts.
| Tissue | %Injected Dose/ga | |||
|---|---|---|---|---|
| 1 h | 2 h | |||
| 125I-SGMIB | 18F-RL-I-5F7 | 125I-SGMIB | 18F-RL-I-5F7 | |
| Liver | 3.27 ± 0.61 | 3.42 ± 0.57b | 3.12 ± 0.94 | 3.17 ± 1.13b |
| Spleen | 2.01 ± 0.62 | 1.63 ± 0.51 | 1.85 ± 0.49 | 1.31± 0.40 |
| Lung | 10.47 ± 2.27 | 9.07 ± 1.06b | 8.03 ± 0.83 | 6.63 ± 1.46b |
| Heart | 1.43 ± 0.47 | 1.30 ± 0.53b | 0.62 ± 0.15 | 0.54 ± 0.14b |
| Kidney | 122.68 ± 18.03 | 139.45 ± 20.54 | 96.92 ± 30.55 | 105.02 ± 39.52b |
| Stomach | 1.91 ± 0.77 | 1.97 ± 0.78b | 0.59 ± 0.28 | 0.54 ± 0.23b |
| Sm. Intestine | 1.71 ± 0.52 | 2.10 ± 0.59 | 0.94 ± 0.30 | 1.28 ± 0.42 |
| Lg. Intestine | 1.36 ± 0.31 | 1.68 ± 0.37 | 1.89 ± 1.30 | 2.47 ± 1.63 |
| Muscle | 1.16 ± 0.48 | 1.27 ± 0.50 | 0.91 ± 0.30 | 1.10 ± 0.38b |
| Blood | 1.75 ± 0.84 | 1.95 ± 0.87 | 0.65 ± 0.41 | 0.83 ± 0.43 |
| Bone | 0.76 ± 0.37 | 0.97 ± 0.31 | 0.78 ± 0.23 | 1.13 ± 0.28 |
| Brain | 0.10 ± 0.04 | 0.11 ± 0.04 | 0.07 ± 0.01 | 0.07 ± 0.02b |
| Tumor | 26.36 ± 3.12 | 28.97 ± 3.88 | 32.52 ± 12.11 | 36.28 ± 14.10 |
Mean ± SD (n = 5).
Difference in uptake not statistically significant.
TABLE 2.
Paired Label Biodistribution of 18F-SFB-5F7 and 125I-SGMIB-5F7 in SCID Mice Bearing BT474M1 Xenografts.
| Tissue | %Injected Dose/ga | |||
|---|---|---|---|---|
| 1 h | 2 h | |||
| 125I-SGMIB | 18F-SFB | 125I-SGMIB | 18F-SFB | |
| Liver | 2.02 ± 0.47 | 0.95 ± 0.21 | 2.18 ± 0.55 | 0.68 ± 0.13 |
| Spleen | 1.08 ± 0.33 | 0.67 ± 0.37 | 1.28 ± 0.22 | 0.52± 0.13 |
| Lung | 2.96 ± 0.39 | 2.31 ± 0.40 | 2.56 ± 0.70 | 2.00 ± 0.38b |
| Heart | 0.76 ± 0.20 | 0.62 ± 0.15 | 0.62 ± 0.13 | 0.54 ± 0.15b |
| Kidney | 102.50 ± 26.35 | 3.90 ± 1.13 | 93.00 ± 18.82 | 2.90 ± 0.77 |
| Stomach | 1.32 ± 1.17 | 1.43 ± 1.69b | 1.98 ± 1.12 | 3.21 ± 4.62b |
| Sm. Intestine | 1.17 ± 0.55 | 0.69 ± 0.33 | 1.32 ± 0.46 | 0.59 ± 0.19 |
| Lg. Intestine | 1.18 ± 0.47 | 0.69 ± 0.50 | 3.86 ± 5.47 | 1.22 ± 1.66b |
| Muscle | 1.14 ± 0.27 | 1.16 ± 0.37b | 1.21 ± 0.89 | 1.43 ± 1.20b |
| Blood | 0.96 ± 0.26 | 1.25 ± 0.29 | 0.67 ± 0.51 | 1.44 ± 0.88 |
| Bone | 0.88 ± 0.36 | 0.97 ± 0.55b | 0.51 ± 0.09 | 0.54 ± 0.08b |
| Brain | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.08 ± 0.05 | 0.12 ± 0.06 |
| Tumor | 27.88 ± 8.08 | 23.94 ± 7.00 | 33.59 ± 6.24 | 29.59 ± 5.10 |
Mean ± SD (n = 5).
Difference in uptake not statistically significant.
FIGURE 3.
18F/125I Ratio in tumor from the paired label biodistribution of 18F-RL-I5F7 and 125I-SGMIB-5F7 and 18F-SFB-5F7 and 125I-SGMIB-5F7in SCID mice bearing BT474M1 xenografts. Green bars-18F-RL-I-5F7; Magenta bars-18F-SFB-5F7
FIGURE 4.
Tumor-to-tissue ratios for selected tissues obtained from the biodistribution of 18F-RL-I-5F7 (A) and 18F-SFB-5F7 (B)
MicroPET/CT imaging
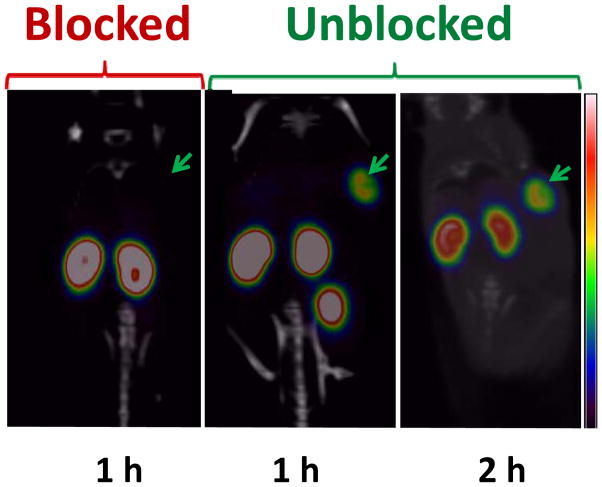
Representative microPET/CT whole body coronal images of the mice with BT474M1 xenografts obtained 1 and 2 h after injection of 18F-RL-I-5F7 as well as for a mouse receiving a blocking dose of trastuzumab 24 h prior to tracer injection are shown in Figure 5. SUV and %ID/g values calculated from the imaging data are presented in Table 3; consistent with the necropsy experiments, high tumor uptake was observed at both time points. No significant uptake was seen in normal organs other than the kidneys and bladder, resulting in high contrast images. Pre-injection of trastuzumab reduced tumor accumulation of 18F-RL-I-5F7 by more than 90%, confirming that tumor localization was HER2 specific.
FIGURE 5.
PET/CT images of mice bearing BT474M1 xenografts after injection of 18F-RL-I-5F7. Images were obtained at 1 and 2 h without and at 1 h with blocking of HER2 receptors by pre-administration of trastuzumab.
TABLE 3.
Tumor SUV and %ID/g from MicroPET/CT Imaging of SCID Mice Bearing BT474M1 Xenografts after Injection of 18F-RL-I-5F7
| Mouse Number | 1 h | 2 h | ||
|---|---|---|---|---|
|
| ||||
| SUV | %ID/g | SUV | %ID/g | |
| Unblocked | ||||
| 1a | 4.2 ± 0.7 | 22.4 ± 3.8 | 4.3 ± 0.8 | 23.0 ± 4.2 |
| 2a | 3.3 ± 0.4 | 17.8 ± 2.1 | 3.6 ± 0.7 | 19.4 ± 4.1 |
| 3b | 4.9 ± 0.7 | 26.4 ± 3.9 | 4.9 ± 0.8 | 26.2 ± 4.2 |
| 4b | 4.4 ± 0.6 | 24.9 ± 3.3 | 4.6 ± 0.7 | 26.1 ± 3.7 |
| Blocked | ||||
| 1a | 0.4 ± 0.1 | 2.1 ± 0.1 | ND | |
| 2a | 0.4 ± 0.1 | 2.1 ± 0.8 | ND | |
| 3b | 0.3 ± 0.1 | 1.3 ± 0.5 | ND | |
| 4b | 0.2 ± 0.1 | 1.0 ± 0.5 | ND | |
Batch1:
Batch2;
ND = not determined.
DISCUSSION
Nanobodies are an attractive platform for use in tandem with short-lived positron emitters because of their rapid tumor uptake and normal tissue clearance (15). A recent Phase 1 clinical study with a 68Ga-labeled Nanobody (68Ga-NOTA-2Rs15d) demonstrated the feasibility of evaluating HER2 status in patients with breast carcinoma metastases by immunoPET (22). Although encouraging results were reported, 18F might be an even more attractive radionuclide for labeling Nanobodies for several reasons. Compared with 68Ga, 18F has a more than threefold lower energy and tissue range, resulting in improved spatial resolution. Moreover, its longer physical half life provides the option for delayed imaging in circumstances where background activity may be problematic, and also allows radiopharmaceutical distribution from regional production sites, facilitating widespread use. In the present study, we have evaluated two approaches for labeling Nanobodies with 18F – a novel residualizing label that we developed for 18F-labeling of proteins and peptides targeting internalizing receptors such as HER2 (17) as well as 18F-SFB, the most widely utilized protein/peptide radiofluorination agent for which several automated procedures have already been developed (23).
Our previous studies with radioiodinated anti-HER2 5F7 Nanobody documented the importance of using a residualizing labeling approach for maximizing retention of radioactivity in HER-2 expressing tumors (16,19). Because peak and cumulative tumor radioactivity levels with 131I-SGMIB-5F7 were considerably higher than previously reported for any Nanobody radionuclide combination (15), SGMIB was selected as the design template for creating an 18F-labeled residualizing label. 18F-RL-I was synthesized and used to label the 5F7 Nanobody in reasonable radiochemical yield with preservation of immunoreactivity (62–80%) and affinity (4.7 ± 0.9 nM) for HER2 after labeling (17).
The potential advantage of the residualizing labeling agent was first evaluated in internalization assays performed with HER2-expressing BT474M1 breast carcinoma cells. Because there is no suitable fluorine radionuclide to use in tandem with 18F, direct paired label comparisons of Nanobody labeled with 18F-RL-I and 18F-SFB cannot be performed. Instead, indirect comparison was made by performing two paired label studies with 125I-SGMIB-5F7 serving as a common reference. Intracellularly trapped radioactivity levels for 5F7 labeled with 18F-RL-I remained constant at >47% of initial cell-bound radioactivity over the 4-h experiment and were similar to those for co-incubated 125I-SGMIB-5F7. In contrast, intracellular radioactivity levels for 18F-SFB-5F7 were lower and decreased with time, and exhibited similar behavior on this cell line as 5F7-GGC Nanobody labeled using Iodogen (16), demonstrating the residualizing capability of the 18F-RL-I moiety.
Biodistribution and microPET imaging experiments in severe combined immunodeficiency (SCID) mice with HER2-expressing BT474M1 xenografts demonstrated rapid tumor accumulation and blood pool clearance of 18F-RL-I-5F7. Pretreatment with trastuzumab reduced tumor levels more than tenfold, confirming that uptake was HER2 specific. When normalized to co-administered 125I-SGMIB-5F7, tumor accumulation of 18F-RL-I-5F7 was 26–28% higher than that observed with 18F-SFB-5F7 at 1 and 2 h, consistent with the 16–24% higher normalized intracellular activity measured with BT474M1 cells in vitro for 18F-RL-I-5F7. By 4 h, the intracellular retention advantage increased to 47%, suggesting that the residualizing ability of the RL-I prosthetic group might be even more pronounced in vivo at later time points.
It is worth noting that the tumor accumulation of 5F7 after labeling with both 18F-labeled prosthetic groups was higher than that observed in this xenograft model when this Nanobody was radioiodinated using either the Iodogen or IB-Mal-D-GEEEK methods (16,19) and considerably higher than that reported for any other combination of Nanobody, radionuclide and xenograft model (15,24,25). With regard to other studies with 18F, tumor accumulation of Nanobodies labeled using 18F-SFB and targeting the macrophage mannose receptor (26) and HER2 (27) were reported to be 2.40 ± 0.46% ID/g (3 h) and 3.09 ± 0.02% ID/g (1 h), respectively, about tenfold lower than observed in the current study. Utilization of a sortase based site-specific method involving a click reaction for labeling Nanobodies with 18F also has been reported (28); however, the goal was imaging immune response to tumor, not a cancer cell surface molecular target.
It is also relevant to compare the tumor targeting of these 18F-labeled 5F7 conjugates to 18F-labeled anti-HER2 affibodies because of the similarity in molecular weight (6.5 vs. 12–15 kDa) and intended clinical application for these labeled proteins. In studies with HER2 specific ZHER2:342 affibody labeled via N-2-(-4-18F-fluorobenzamido)ethyl]maleimide performed in mice with xenografts expressing high levels of HER2, peak tumor uptake occurred at 1 h and ranged from about 10–22% ID/g (29,30). A second generation affibody, ZHER2:2891 (GE-226) with improved HER2 affinity (76 pM) was evaluated in mice with HER2 expressing NCI-N87 xenografts after labeling with 18F by three methods; optimal tumor accumulation was obtained (7.15 ± 0.69% ID/g at 90 min) when labeling was performed using 4-18F-fluorobenzaldehyde (FBA) (31). In a subsequent PET imaging study with 18F-FBA-GE-226, peak tumor uptake in three high HER2-expressing cell lines ranged from 10.9 ± 1.5% ID/mL for MCF7-HER2 cells to 18.7 ± 2.4% ID/mL for SKOV-3 cells (14). Although differences in variables such as animal model, protein dose and internalization rate could play a role (32), the results obtained in the current study with 18F-labeled anti-HER2 5F7 Nanobody compare favorably with those reported for 18F-labeled affibodies.
Normal tissue clearance of the labeled Nanobody conjugates was quite rapid except from the kidneys for 18F-RL-I-5F7 and 125I-SGMIB-5F7. This behavior is consistent with the high degree of renal retention observed with other proteins with molecular weights less than 60 kDa (33) as well as Nanobodies labeled with radiometals (15), other residualizing radiohalgen moietes (19), and those containing polar amino acid residues at the C-terminal (24,25). Exceptions to this behavior are Nanobodies labeled with radioiodine using Iodogen (16,19), presumably reflecting their rapid dehalogenation in vivo, and the about 30-fold lower kidney uptake observed in the current study for 18F-SFB-5F7 compared to 18F-RL-I-5F7 and 125I-SGMIB-5F7. A factor that could contribute to the low renal activity levels seen with 18F-SFB-5F7 is the formation of 4-18F-fluorohippuric acid, the primary metabolite reported from other proteins labeled using the 18F-SFB method (34). On the other hand, the polar triazole (21) and especially the guanidine moieties in 18F-RL-I might have contributed to its high renal retention as seen with Nanobodies bearing other polar functionalities (24,25). Future studies are planned to determine whether the radioactivity retained in kidneys is due to intact 18F-RL-I-5F7 or trapping of lower molecular weight catabolites generated by its lysosomal proteolysis (33,35).
From an imaging perspective, high renal activity levels should not interfere with lesion detection both for primary and the most common sites of metastases for HER2-positive cancers as was demonstrated in a recent study with a 68Ga-NOTA anti-HER2 Nanobody (22). If necessary, significant reduction in kidney uptake of radiolabeled Nanobodies can be achieved though the use of positively-charged amino acids or the plasma expander Gelofusin (24). It also may be possible to decrease kidney uptake by introducing brush border enzyme-cleavable linkers in the prosthetic moiety (35,36) and efforts in this direction are under way in our laboratories. In addition, these 18F-labeled 5F7 conjugates are not retained in the liver, a frequent site of metastases for HER2-positive breast cancers. This is a potential advantage compared with other HER2-specific immunoPET agents such as 89Zr-DFO-trastuzumab that exhibit significant accumulation in the liver (37).
CONCLUSION
The results of this study demonstrate the feasibility of utilizing 18F-labeled anti-HER2 Nanobodies for the evaluation of HER2 expressing cancers. Excellent tumor targeting was observed with both reagents; however, use of the recently developed residualizing agent 18F-RL-I resulted accumulation and retention of significantly higher 18F levels in BT474M1 human breast carcinoma cells and xenografts compared with 18F-SFB. As has been observed previously with Nanobodies labeled with residualizing radiometals, renal uptake of 18F-RL-I-5F7 was high, which if problematic, might require compensatory strategies such as Gelofusin administration. In conclusion, both 18F-RL-I-5F7 and 18F-SFB-5F7 warrant further evaluation as tracers for the evaluation of HER2 expressing cancers using immunoPET.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants CA188177, CA42324 and for microPET imaging, S10RR31792.
The authors want to thank Hilde Revets (Ablynx, Belgium) for providing the 5F7 Nanobody, Xiao-Guang Zhao for biodistribution studies and Thomas Hawk for help with microPET imaging studies.
Footnotes
DISCLOSURE
This work was supported in part by National Institutes of Health grants CA188177, CA42324 and for microPET imaging, S10RR31792. No other potential conflict of interest relevant to this article was reported.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Chen VW, Ries LA, et al. Overview of breast cancer collaborative stage data items--their definitions, quality, usage, and clinical implications: a review of SEER data for 2004–2010. Cancer. 2014;120(Suppl):3771–3780. doi: 10.1002/cncr.29059. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen DL, Kumler I, Palshof JA, Andersson M. Efficacy of HER2-targeted therapy in metastatic breast cancer. monoclonal antibodies and tyrosine kinase inhibitors. Breast. 2013;22:1–12. doi: 10.1016/j.breast.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A ‘dual-hit’. Exp Clin Cardiol. 2011;16:70–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical practice Guideline Update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 6.Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 7.Minot DM, Voss J, Rademacher S, et al. Image analysis of HER2 immunohistochemical staining. reproducibility and concordance with fluorescence in situ hybridization of a laboratory-validated scoring technique. Am J Clin Pathol. 2012;137:270–276. doi: 10.1309/AJCP9MKNLHQNK2ZX. [DOI] [PubMed] [Google Scholar]
- 8.Fabi A, Di Benedetto A, Metro G, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res. 2011;17:2055–2064. doi: 10.1158/1078-0432.CCR-10-1920. [DOI] [PubMed] [Google Scholar]
- 9.Sapino A, Goia M, Recupero D, Marchio C. Current challenges for HER2 testing in diagnostic pathology: state of the art and controversial Issues. Front Oncol. 2013;3:129. doi: 10.3389/fonc.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Breast. 2014;23:489–502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Mortimer JE, Bading JR, Colcher DM, et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55:23–29. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olafsen T, Sirk SJ, Olma S, Shen CK, Wu AM. ImmunoPET using engineered antibody fragments: fluorine-18 labeled diabodies for same-day imaging. Tumour Biol. 2012;33:669–677. doi: 10.1007/s13277-012-0365-8. [DOI] [PubMed] [Google Scholar]
- 14.Trousil S, Hoppmann S, Nguyen QD, et al. Positron emission tomography imaging with 18F-labeled ZHER2:2891 affibody for detection of HER2 expression and pharmacodynamic response to HER2-modulating therapies. Clin Cancer Res. 2014;20:1632–1643. doi: 10.1158/1078-0432.CCR-13-2421. [DOI] [PubMed] [Google Scholar]
- 15.D’Huyvetter M, Xavier C, Caveliers V, Lahoutte T, Muyldermans S, Devoogdt N. Radiolabeled nanobodies as theranostic tools in targeted radionuclide therapy of cancer. Expert Opin Drug Deliv. 2014;11:1939–1954. doi: 10.1517/17425247.2014.941803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruszynski M, Koumarianou E, Vaidyanathan G, et al. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J Nucl Med. 2014;55:650–656. doi: 10.2967/jnumed.113.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidyanathan G, McDougald D, Choi J, et al. N-Succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate ([18F]SFBTMGMB): a residualizing label for 18F-labeling of internalizing biomolecules. Org Biomol Chem. 2016;14:1261–1271. doi: 10.1039/c5ob02258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat protoc. 2006;1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
- 19.Pruszynski M, Koumarianou E, Vaidyanathan G, et al. Targeting breast carcinoma with radioiodinated anti-HER2 nanobody. Nucl Med Biol. 2013;40:52–59. doi: 10.1016/j.nucmedbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Z, Xia W, Wang HY, et al. Antitumor activity of an Ets protein, PEA3, in breast cancer cell lines MDA-MB-361DYT2 and BT474M1. Mol Carcinog. 2006;45:667–675. doi: 10.1002/mc.20212. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann CM, Hermann S, Faust A, et al. Novel fluorine-18 labeled 5-(1-pyrrolidinylsulfonyl)-7-azaisatin derivatives as potential PET tracers for in vivo imaging of activated caspases in apoptosis. Bioorg Med Chem. 2015;23:5734–5739. doi: 10.1016/j.bmc.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-Nanobody for PET/CT assessment of HER2-expression in breast carcinoma. J Nucl Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 23.Richter S, Wuest F. 18F-Labeled peptides: The future is bright. Molecules. 2014;19:20536–20556. doi: 10.3390/molecules191220536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Huyvetter M, Vincke C, Xavier C, et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4:708–720. doi: 10.7150/thno.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xavier C, Vaneycken I, D’Huyvetter M, et al. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013;54:776–784. doi: 10.2967/jnumed.112.111021. [DOI] [PubMed] [Google Scholar]
- 26.Blykers A, Schoonooghe S, Xavier C, et al. PET Imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18F-radiolabeled camelid single-domain antibody fragments. J Nucl Med. 2015;56:1265–1271. doi: 10.2967/jnumed.115.156828. [DOI] [PubMed] [Google Scholar]
- 27.Vaneycken I, Xavier C, Blykers A, Devoogdt B, Caveliers V, Lahoutte T. Synthesis and first in vivo evaluation of 18F-anti-HER2-nanobodies: a new probe for PET imaging of HER2 expression in breast cancer. J Nucl Med. 2011;52:664. Abstract. [Google Scholar]
- 28.Rashidian M, Keliher EJ, Bilate AM, et al. Noninvasive imaging of immune responses. Proc Nat Acad Sci USA. 2015;112:6146–6151. doi: 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and 18F-labeled affibody molecules. J Nucl Med. 2009;50:1131–1139. doi: 10.2967/jnumed.108.057695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008;35:1008–1018. doi: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser M, Iveson P, Hoppmann S, et al. Three methods for 18F labeling of the HER2-binding affibody molecule ZHER2:2891 including preclinical assessment. J Nucl Med. 2013;54:1981–1988. doi: 10.2967/jnumed.113.122465. [DOI] [PubMed] [Google Scholar]
- 32.Malmberg J, Sandström M, Wester K, Tolmachev V, Orlova A. Comparative biodistribution of imaging agents for in vivo molecular profiling of disseminated prostate cancer in mice bearing prostate cancer xenografts: focus on 111In- and 125I-labeled anti-HER2 humanized monoclonal trastuzumab and ABY-025 affibody. Nucl Med Biol. 2011;38:1093–1102. doi: 10.1016/j.nucmedbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Akizawa H, Uehara T, Arano Y. Renal uptake and metabolism of radiopharmaceuticals derived from peptides and proteins. Adv Drug Delivery Rev. 2008;60:1319–1328. doi: 10.1016/j.addr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Pietzsch J, Bergmann R, Wuest F, Pawelke B, Hultsch C, van den Hoff J. Catabolism of native and oxidized low density lipoproteins: in vivo insights from small animal positron emission tomography studies. Amino acids. 2005;29:389–404. doi: 10.1007/s00726-005-0203-z. [DOI] [PubMed] [Google Scholar]
- 35.Akizawa H, Imajima M, Hanaoka H, Uehara T, Satake S, Arano Y. Renal brush border enzyme-cleavable linkages for low renal radioactivity levels of radiolabeled antibody fragments. Bioconjugate Chem. 2013;24:291–299. doi: 10.1021/bc300428b. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Olafsen T, Anderson AL, Wu A, Raubitschek AA, Shively JE. Reduction of kidney uptake in radiometal labeled peptide linkers conjugated to recombinant antibody fragments. Site-specific conjugation of DOTA-peptides to a Cys-diabody. Bioconjugate Chem. 2002;13:985–995. doi: 10.1021/bc025565u. [DOI] [PubMed] [Google Scholar]
- 37.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharm Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.