Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side effect of many antineoplastic agents, but the mechanisms underlying the toxicities of certain drugs are unclear. At their maximal tolerated doses (MTD), the microtubule-binding drugs paclitaxel and ixabepilone induce more severe neuropathy in mice relative to eribulin mesylate, paralleling their toxicity profiles in clinic. We hypothesized that the severity of their neurotoxic effects might be explained by the levels at which they accumulate in the peripheral nervous system. To test this hypothesis, we compared their pharmacokinetics and distribution in peripheral nerve tissue. After administration of a single intravenous dose, each drug was rapidly cleared from plasma but all persisted in the dorsal root ganglia (DRG) and sciatic nerve (SN) for up to 72 hours. Focusing on paclitaxel and eribulin, we performed a two week MTD-dosing regimen followed by a determination of drug pharmacokinetics, tissue distribution and multiple functional measures of peripheral nerve toxicity for four weeks. Consistent with the acute dosing study, both drugs persisted in peripheral nervous tissues for weeks, in contrast to their rapid clearance from plasma. Notably, although eribulin exhibited greater DRG and SN penetration than paclitaxel, the neurotoxicity produced biologically was consistently more severe with paclitaxel. Overall, our results argued that sustained exposure of microtubule-binding chemotherapeutic agents in peripheral nerve tissues cannot by itself account for their associated neurotoxicity.
Keywords: eribulin mesylate, paclitaxel, ixabepilone, microtubules, chemotherapy-induced neuropathy, tissue distribution
INTRODUCTION
Chemotherapy-induced peripheral neurotoxicity (CIPN) is a major clinical problem representing a significant dose-limiting side-effect of many anti-neoplastic drugs. Even when neuropathy is not dose-limiting, residual neuropathy can severely affect quality of life in cancer survivors. The severity of neuropathy caused by a particular chemotherapeutic drug is dependent on multiple factors including mechanism of action, frequency of administration, duration of treatment and cumulative dose (1,2). Within the class of microtubule targeting drugs, high grade neuropathy occurs more frequently with paclitaxel and epothilones, such as ixabepilone, while eribulin is less likely to produce dose-limiting neuropathy. Animal models of CIPN have provided data that is in broad agreement with clinical results (3–7) and thus provide an opportunity to explore the relationship between exposure and toxicity for both existing and new chemotherapies. For example, we have reported that paclitaxel and ixabepilone produce more severe neuropathy in mice compared to eribulin mesylate at their respective maximal tolerated doses (MTD) (3), findings that replicate the frequency of severe neuropathy reported in comparative clinic studies (1,8). However, it is not known whether these differences are due to the intrinsic toxicity of these agents or due to differences in exposure of the vulnerable peripheral nervous system.
It has long been recognized that cumulative chemotherapy dose over time is the most important clinical predictor of neuropathy. This dependence on dose could either be due to an accumulation of injury produced by the toxicity of each administration or be due to an accumulation of drug in peripheral nerve tissues that reach toxic levels only after multiple doses. Peripheral nervous tissue exposure is difficult to quantitate clinically, and is likely poorly predicted by plasma concentration because of variable distribution, often strongly influenced by factors such as formulation or route of administration (9–12). In addition, clearance mechanisms from tissues also varies, determined both by binding of chemotherapeutic agents within tissues and efflux mediated by transport mechanisms such as P-glycoproteins (13,14).
In order to investigate further the potential relationship between peripheral nervous tissue accumulation and neuropathy it would be useful to compare chemotherapeutic agents with similar mechanisms but different neurotoxic potential. In the experiments described here, we determined the relationship between plasma pharmacokinetics, peripheral nervous system tissue concentration and development of neuropathy using a single administration and a two-week, MTD model for three microtubule targeting chemotherapies. We report for the first time that all three drugs show dramatic and sustained accumulation in DRG and SN, with measureable levels maintained for weeks after the last dose. However, at least in the case of paclitaxel and eribulin, the degree of tissue penetration and accumulation does not correlate with development of neuropathy.
METHODS
Animals
Female BALB/c mice (approximately 7–8 weeks old at onset of dosing) were obtained from Harlan Laboratories (Indianapolis, IN) and maintained with free access to water and a standardized synthetic diet (Harlan Teklab). Animal housing and procedure room temperature and humidity were maintained at 20 ± 2°C and 55 ± 10% respectively. Artificial lighting provided a 12h light/12h dark cycle (light 7am–7pm). All experimental protocols were approved by the Institutional Animal Care and Use Committee of Sobran Inc and adhered to all of the applicable institutional and governmental guidelines for the humane treatment of laboratory animals.
Mice were treated with single and multiple doses of a previously determined 6 dose MTD regimen administered intravenously on a Q2Dx3 × 2 week schedule (for method see (3)). MTD was defined as the maximal dose of eribulin mesylate, ixabepilone or paclitaxel administered at which no more than one animal in the treatment group, died spontaneously. In addition, this was the maximal dose tested at which no mice in the dose group required euthanasia due to >20% individual weight loss, showing overt clinical signs of distress or inability to eat and/or drink. The MTD dose when administered IV 6 times on a Q2Dx3 × 2 week schedule was found to be 1.125 mg/kg for eribulin, 2 mg/kg for ixabepilone and 30 mg/kg for paclitaxel.
Drugs and Formulations
Eribulin mesylate (synthesized at Eisai Research Institute and stored at −80 degrees in the dark) was dissolved in 100% anhydrous DMSO (Sigma-Aldrich, St. Louis, MO) to produce a 10 mg/ml stock solution, which was separated into aliquots and stored at −80°C until day of administration. Each administration day the stock solution was thawed and diluted with saline to a final concentration of 0.112 mg/mL in 2.5% DMSO/97.5% and administered in a 10 mL/kg volume.
Paclitaxel (purchased from LC Laboratories, Woburn, MA and stored at −20 degrees C, in the dark) was dissolved in ethanol (100%) at 10% of final volume. An equal volume of cremophor (10% of final volume) was then added and the mixture re-vortexed for about 10 min. Immediately prior to injection, ice cold saline was added to final volume (as 80% of final) and the solution was maintained on ice during dosing. Dosing solutions of 3 mg/mL were made fresh daily and administered in a 10 mL/kg volume.
Ixabepilone (Ixempra, Bristol-Myers Squibb, N.J.), was prepared according to the package insert. The formulated ixabepilone stock solution (2 mg/mL) was immediately aliquoted and stored at 80 degrees C until use. On each experimental day, the stock solution was diluted by adding 50% ethanol/50% cremophor with subsequent vortexing to yield a resultant solution that was 5 times the required dosing concentration. Finally, 4 volumes of PBS were added, while vortexing, to achieve a final dosing concentration of 10 mL/kg.
Pharmacokinetic Studies
Pharmacokinetic (PK) studies were performed in female BALB/c mice to determine the plasma, DRG and SN exposure of eribulin and paclitaxel after single and multiple MTD administrations as described above. For the single dose PK, plasma and tissue were taken at the following time points: time zero (no treatment), 0.25h, 0.5h, 1h, 3h, 6h, 24h, 72h, and 7d post drug administration. For PK following multiple doses, plasma and tissues were taken at the following time points during/after the Q2Dx3 × 2 week 6 dose administrations: 1 h before 3rd, 4th, 5th, 6th dose and then 24 h, 7 and 14 days following the last (6th) dose. At sacrifice, blood was removed via cardiac puncture. Plasma was derived from the whole blood by centrifugation at 3000 RPM at 4 degrees C in plasma separator tubes for 10 min. The SN and DRG were removed and pooled from three mice per time point and homogenized with three times their respective weights of mouse plasma using a MiniBead Beater-96. All samples were stored at −80 degrees C until subsequent analysis. Samples were analyzed for eribulin, ixabepilone and paclitaxel using reversed phase chromatography on a LC/MS/MS (API-4000 with a Shimadzu autosampler) using methods based on procedures previously described (15–17). The lower limit of quantifications were 0.5, 2, and 10 ng/ml in plasma and 5, 10 and 50 ng/g in DRG, for eribulin, paclitaxel and ixabepilone respectively, and 8mg/g for each analyte in SN. PK parameters were calculated using noncompartmental analysis in WinNonLin v 5.0.3.
Electrophysiology
Electrophysiological measurements were performed as previously described (3,18). In brief, baseline caudal and digital nerve conduction velocities (NCVs) and amplitudes were measured in all mice one week prior to initiation of dosing. Mice were anesthetized with 2% isoflurane (by inhalation, for induction and maintenance) and placed on a heating pad with rectal temperature monitored and maintained between 37.0 – 40.0°C. Platinum subdermal needle electrodes (Grass Technologies, West Warwick, RI) were used. Caudal NCV was recorded from electrodes in a bipolar configuration at the base of the tail (at the hair line); the stimulating cathode being positioned 35 mm further distal. Digital NCV was recorded using stimulation at the base of the second toe and recording at the level of the lateral malleolus. Amplitudes were measured as the baseline to peak neural response. Each nerve segment stimulation was repeated at least 3 times, up to a maximum of 6 times, with increasing voltage until the maximal response was achieved, using AcqKnowledge software version 3.7.3 (BIOPAC Systems Inc.). Mice were assigned into a vehicle, paclitaxel or eribulin treatment group (10 mice/group). Following MTD dosing on a Q2Dx3 for 2 weeks schedule, mice were again tested for NCV at 24 h, 7 days and 14 days following the last dose.
RESULTS
Pharmacokinetic Studies
Following intravenous administration, plasma concentrations of eribulin, paclitaxel and ixabepilone declined rapidly, presumably due to rapid distribution to peripheral compartments (19–21). Limitations of assay sensitivity prevented characterization of terminal elimination. After a single infusion, all three drugs rapidly distributed into DRG and SN, remaining above the limit of detection for over 72 hrs. Concentration-time analyses of eribulin, paclitaxel and ixabepilone in their respective matrices after single dose are depicted in Figure 1A–C.
Figure 1(A–C).
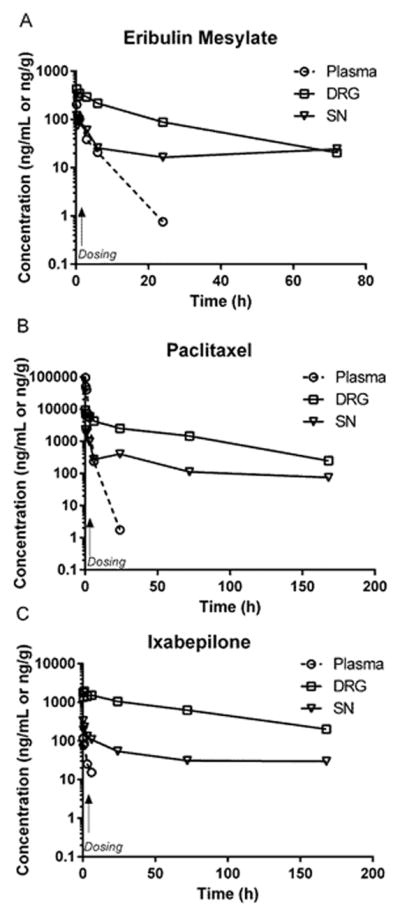
Pharmacokinetic profile of paclitaxel, eribulin mesylate and ixabepilone in plasma, DRG, and SN from mice receiving a single intravenous dose (marked with arrow) of 30, 1.125 and 2.0 mg/kg, respectively. In all cases, plasma samples collected after 24h post dose were below lower levels of quantification although DRG and SN levels remained quantifiable to 72 h post dose. (Symbols: Plasma ⦵, DRG
 , SN
, SN
 ).
).
As shown by the pharmacokinetic parameters (Table 1), the three drugs varied widely in relative penetration into tissue after intravenous administration. Accumulation during the early tissue distribution, reflected as maximal exposure (Cmax) reached in tissue compared to plasma, was greatest for ixabepilone followed by eribulin. Peak paclitaxel concentration in tissue was lower than peak plasma concentration, while eribulin and ixabepilone displayed greater Cmax in tissue than in plasma. Peak exposure was greater in DRG than sciatic nerve for all three compounds. The overall DRG and SN exposure relative to plasma were characterized by calculating a tissue penetration index (TPI) for both Cmax and AUC. As shown in Table 1, the relative exposure by either measure was greater in DRG than sciatic nerve for all three drugs with ixabepilone having the highest exposure, eribulin being intermediate, and paclitaxel relatively lower.
Table 1.
Pharmacokinetic parameters of a single intravenous dose of eribulin mesylate, paclitaxel and ixabepilone in mice.
| Analyte | Dose (mg/kg) | Matrix | Cmax (ng/g) | AUC (0-t) (hr.ng/g)a | AUC (0-inf) (hr.ng/g) | TPIb Cmax | TPIc AUC |
|---|---|---|---|---|---|---|---|
| Eribulin mesylate | 1.125 | Plasma | 203 | 487 | 491 | --- | --- |
| Eribulin mesylate | 1.125 | Dorsal Root Ganglia | 428 | 4270 | 7080 | 2.1 | 8.8 |
| Eribulin mesylate | 1.125 | Sciatic Nerve | 121 | 731 | NR | 0.6 | 1.5 |
| Ixabepilone | 2.0 | Plasma | 117 | 260 | 304 | --- | --- |
| Ixabepilone | 2.0 | Dorsal Root Ganglia | 1940 | 9250 | 125000 | 16.6 | 35.6 |
| Ixabepilone | 2.0 | Sciatic Nerve | 333 | 920 | NR | 2.8 | 3.5 |
| Paclitaxel | 30.0 | Plasma | 95000 | 114000 | 114000 | --- | --- |
| Paclitaxel | 30.0 | Dorsal Root Ganglia | 9280 | 94000 | 279000 | 0.1 | 0.8 |
| Paclitaxel | 30.0 | Sciatic Nerve | 2250 | 12100 | 60200 | 0.02 | 0.1 |
: AUC(0-t)=0–24h for eribulin mesylate and paclitaxel; 0–6h for ixabepilone
: TPI= Cmax(tissue)/Cmax (plasma)
: TPI= AUC(0-t tissue)/AUC(0-t plasma)
NR= not reported because extrapolation area >30%
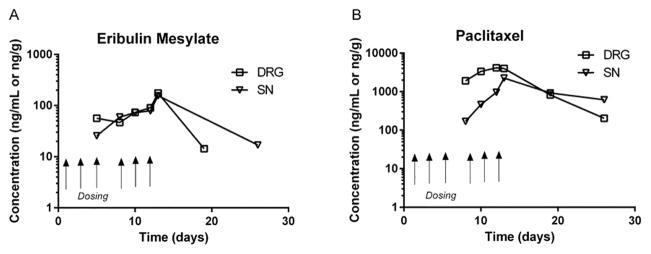
Since CIPN develops in the mouse model only after repeated administration, the tissue exposure was subsequently examined following a multiple MTD dosing paradigm of eribulin and paclitaxel (Figure 2A and B, respectively). Similar to observations after acute dosing, the chemotherapies rapidly entered SN and DRG from plasma. In all cases, plasma concentrations declined within 24h of last dosing and were not detectable thereafter. In contrast, concentrations of eribulin and paclitaxel were maintained in SN and DRG for up 26 days after completion of dosing (Figure 2A and B). As shown in Figure 2, paclitaxel exposure reached maximal levels in the DRG after the first dose, while in the sciatic nerve there was accumulation with multiple doses. Eribulin exposure showed increasing accumulation in both DRG and sciatic nerve with multiple administrations.
Figure 2(A and B).
Pharmacokinetic profile of paclitaxel and eribulin mesylate in plasma, DRG and SN from mice receiving mice receiving Q2D ×3 for 2 week MTD dosing regimen of 1.125 mg/kg per dose for eribulin mesylate and 30 mg/kg per dose for paclitaxel. Plasma levels were below detection at all time points. In contrast, eribulin mesylate was quantifiable in DRG and SN samples up to 19 and 26 days post initial dose respectively. Paclitaxel remained quantifiable in the DRG and SN up to 26 days post dose. NOTE: Eribulin data for Day 20 was BLQ. Arrows depict actual dosing days (on Day 1, 3, 5, 8, 10 and 12). (Symbols: Plasma ⦵, DRG
 , SN
, SN
 ).
).
Electrophysiology
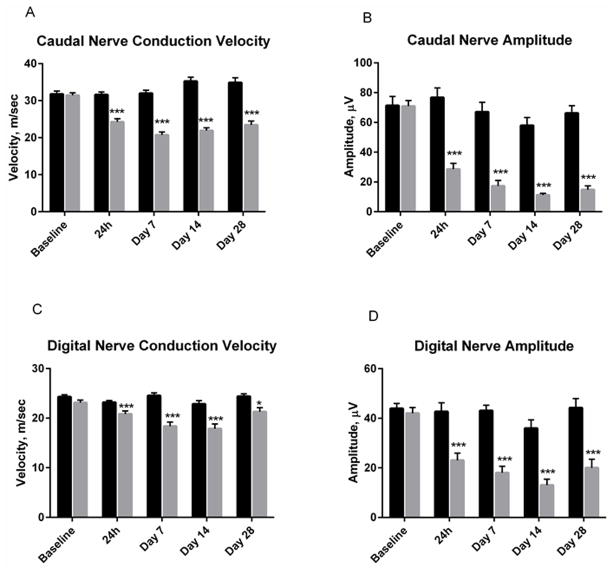
Following the 2 week MTD dosing regimen, caudal nerve conduction velocity was significantly slowed by paclitaxel at every time point measured (ranging from 23–38% of vehicle; Figure 3A). Similarly, caudal amplitude was significantly reduced by paclitaxel administration, remaining suppressed by 77 ± 4% (mean ± SEM) at 28 days post first dosing (Figure 3B). Digital nerve conduction velocity also showed significant deficits at every time point measured (between 10 and 25% of vehicle; Figure 3C). Paclitaxel also significantly affected digital nerve amplitude at all time points, remaining suppressed by 53 ± 7.8% at 28 days post dosing (Figure 3D). Overall, the pattern of deficit was present the first day following dosing, showing a trend toward recovery for the conduction velocity measurements but mild gradual worsening for the sensory nerve amplitude measures. These patterns did not appear to be related to the prolonged tissue exposure.
Figure 3(A–D).
Mice receiving a 2 week MTD dosing regimen of paclitaxel (Q2D ×3 dosing of 30 mg/kg iv for 2 weeks) exhibited significant deficits in caudal and digital nerve conduction velocity and amplitude. The deficits were maintained for up to 28 days after completion of dosing. Figure depicts mean ± SEM values at each time point. Vehicle (black). Paclitaxel (gray). P values are noted as follows *p=<0.05; **p=<0.01; ***P=<0.001
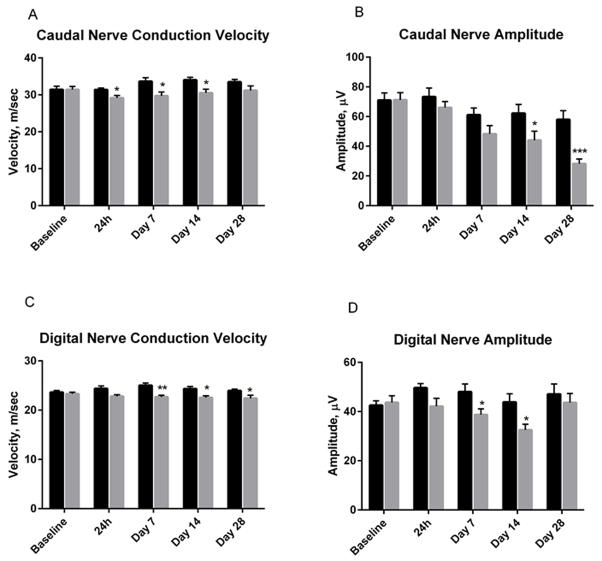
In contrast, eribulin had no significant effect on caudal velocity at any time point tested, although a non-significant deficit, (ranging between 7 and 17%), was consistently observed (Figure 4A). Eribulin-treated mice showed a less severe, but statically significant suppression of caudal amplitude at 14 and 28 days following the last dose (30 ± 9.8 and 52 ± 5.0%, respectively) (Figure 4B). Similarly, eribulin produced small but significant deficits in digital nerve velocity from 7 days post dose, ranging from 8–12% of vehicle (Figure 4C). Digital amplitude in eribulin - treated mice was significantly attenuated at 7 days (19 ± 4.9%) and 14 days (26 ± 5.2%) post dosing, but recovered back to pre-dosing values at the 28 day time point (Figure 4D). As observed for paclitaxel, the time course of functional deficit was not directly correlated with the drug exposure time-course, particularly the late decline in caudal nerve amplitude that occurred when tissue exposure was no longer detectable.
Figure 4(A–D) .
Mice receiving a 2 week MTD dosing regimen of eribulin mesylate (Q2D ×3 dosing of 1.125 mg/kg iv for 2 weeks) exhibited significant deficits in caudal and digital nerve conduction velocity and amplitude. Figure depicts mean ± SEM values at each time point. Vehicle (black). Eribulin mesylate (gray). P values are noted as follows *p=<0.05; **p=<0.01; ***P=<0.001
DISCUSSION
After a single i.v. dose, paclitaxel, ixabepilone, and eribulin cleared rapidly from plasma, but accumulated and dramatically persisted in DRG and SN with limited clearance three days post administration. In repeated dose studies, eribulin and paclitaxel also rapidly declined in plasma but accumulated and persisted in peripheral nervous tissues for up to 26 days after dosing. This neurotoxic class of drugs appears to exert toxic effects directly through prolonged exposure within sensitive tissues. However, although eribulin consistently showed greater tissue penetration than paclitaxel, neurotoxicity was minimal and less severe than with paclitaxel. This is in concordance with the milder morphological deficits induced by eribulin in this paradigm compared to paclitaxel reported previously (3). Together, these data suggest that dramatic and sustained exposure of chemotherapeutic agents in peripheral nerve tissues alone cannot account for their neurotoxicity profiles.
The paclitaxel concentrations we observed in DRG using a two-week, maximum tolerated dose model of paclitaxel are significantly higher than those previously reported by Cavaletti et al (22) and Xiao et al (4), both performed in rats. Cavaletti reported a mean tissue concentration in DRG of 366 ng/g after 5 mg/kg/day i.v. on five alternating days. Xiao reported concentrations of 446 ng/g 24h after 7 days of administration (2 mg/kg i.p. on four alternate days) with no morphological deficits, while in our current study we observed 4000 ng/g 24h after 6 injections of 30 mg/kg i.v. (Q2Dx3 for 2 wks), a concentration that also induced morphological deficits in our earlier study (3). Differences in paclitaxel administration route and well as cumulative dose may underlie these reported differences. Also, given the reported higher paclitaxel exposures from a cremophor formulation (31) versus ethanol/tween formulation (4), some accumulation in tissue may occur due to the depot effect of the formulation (23,24). In addition to the higher DRG concentration, we observed a concentration in sciatic nerve that was approximately 50% of that measured in DRG, while Xiao et al reported sciatic nerve concentrations only 10% of that measured in DRG. This is consistent with our observation that paclitaxel accumulates in sciatic nerve with repeated doses. Interestingly, in the weeks after administration, the tissue concentration of paclitaxel in sciatic nerve was actually higher than that in DRG, reversing the relative tissue concentration during the period of administration. This pattern of gradual accumulation in nerve might explain why cumulative dose of paclitaxel is the critical clinical metric for neuropathy risk (25), if nerve rather than DRG exposure in vulnerable patients is responsible for toxicity. Correlation in actual clinical samples would be needed to further explore this relationship.
The pharmacokinetics observed in these experiments can be understood as the sum of three dynamic processes: 1) distribution of drug into tissue, 2) retention of drug in tissue, likely at specific binding sites, and 3) efflux from tissue by active transport. Regarding uptake into tissues, paclitaxel, eribulin and ixabepilone all exhibited a similar pattern of rapid peripheral nerve tissue distribution, even though they each differ in lipophilicity and formulation. Tissue distribution of these drugs is complex and can depend on formulation. For example, the distribution of paclitaxel in cremophor differs substantially from albumin-bound paclitaxel pharmacokinetics (9,26). Tissue distribution is not purely passive; active uptake into tumors by carriers (27) such as OATP1B-mediated transport (11,28) have been described. However, the cellular pharmacokinetics and specific transport mechanisms of these drugs in cultured peripheral neurons or glial cells are completely unexplored. Such studies are warranted to elucidate the potential for unique accumulation and retention of microtubule targeting chemotherapies in peripheral neural cells versus tumor cells.
The sustained retention of all three drugs in DRG and SN is most likely due to a combination of intracellular binding and lack of efflux mechanisms. Complex computational models of intracellular pharmacokinetics of paclitaxel have been developed in vitro using cell lines (29–31) in which intracellular target binding is a critical factor in the absence of active efflux mechanisms because of the high concentration of their binding target, microtubules. Although the binding sites of paclitaxel and eribulin on microtubules vary (32,33), as do their effects on microtubule function (34,35), their affinity and the density of binding sites appear to be sufficient to result in prolonged intracellular DRG and SN retention.
Cellular efflux mechanisms, if present, appear to be insufficient to overcome retention through target binding. While extensive work has been completed to understand how efflux through P-glycoproteins affects exposure of chemotherapeutics in solid tumors (36,37) little is known regarding efflux mechanisms in peripheral nervous system tissue. It is known that paclitaxel and ixabepilone do not achieve high levels of exposures in the brain in patients (19,38) but this is presumed due to active efflux by P-glycoproteins across the blood brain barrier, not lack of CNS distribution (14,39,40). While the efflux transporters found in the blood-brain barrier have been found in blood vessels in peripheral nerve (41,42), the DRG appear to exist outside the blood brain barrier, and thus are exposed to greater plasma components (43), possibly leading to the observed rapid accumulation and retention. We are not aware of in vitro studies of intracellular binding and efflux of these microtubule binding drugs in cultured neurons or DRGs. Such studies might illuminate mechanisms of peripheral nervous system vulnerability to toxicity and development of better-tolerated agents.
Even though persistent levels of the chemotherapies were achieved in the mice PNS, the results do not provide a pharmacokinetic explanation for the relative neurotoxicity of these agents. The more subtle functional and pathological changes produced by eribulin compared to paclitaxel and ixabepilone is not predicted by relative exposure, accumulation or retention in PNS tissues. While tissue accumulation may be necessary to produce the profound neurotoxicity of paclitaxel and ixabepilone, differential interaction with microtubules must explain differences in neurotoxicity. Eribulin may have less neurotoxic potential because it primarily affects microtubule growth in contrast to paclitaxel and ixabepilone which affect microtubule function more broadly (34,44). Differences in microtubule binding properties may have significant effects on the toxicity profile of each microtubule targeting agent (45,46). In this context, eribulin mesylate has a 10 fold lower affinity for microtubule sides than its positive ends (47), possibly resulting in a lesser disruption of axonal transport of essential molecules which may be the underlying reason for its lower propensity to induce neuropathy (48). In addition to the functional and pharmacokinetic findings reported, we recently showed differential effects of paclitaxel and eribulin on α-tubulin expression, tubulin acetylation, and EB1 abundance in the peripheral nerve following acute dosing (44), and are currently investigating the longitudinal course of these effects. This type of comparative study may prove useful in the design of more potent chemotherapeutic agents with less neurotoxicity. In conclusion, the dramatic and sustained exposure of chemotherapeutic agents in peripheral nervous tissue itself cannot account for their ensuing neurotoxicity profiles, and other factors must be implicated.
Acknowledgments
GRANT SUPPORT
This research was supported by a sponsored research grant from Eisai, Inc (to B.S.S), the Johns Hopkins Brain Science Institute, and the NIH R01CA19389501 (to B.S.S.).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. 2011;11(2):73–81. doi: 10.1016/j.clbc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Balayssac D, Ferrier J, Descoeur J, Ling B, Pezet D, Eschalier A, et al. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf. 2011;10(3):407–17. doi: 10.1517/14740338.2011.543417. [DOI] [PubMed] [Google Scholar]
- 3.Wozniak KM, Nomoto K, Lapidus RG, Wu Y, Carozzi V, Cavaletti G, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res. 2011;71(11):3952–62. doi: 10.1158/0008-5472.CAN-10-4184. [DOI] [PubMed] [Google Scholar]
- 4.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33(9):1667–76. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011;232(2):154–61. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1–2):150–61. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chen N, Palmisano M, Zhou S. Pharmacologic sensitivity of paclitaxel to its delivery vehicles drives distinct clinical outcomes of paclitaxel formulations. Mol Pharm. 2015;12(4):1308–17. doi: 10.1021/acs.molpharmaceut.5b00026. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Albrecht BJ, Yan X, Gao M, Weng HR, Bartlett MG. A rapid analytical method for the quantification of paclitaxel in rat plasma and brain tissue by high-performance liquid chromatography and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2013;27(19):2127–34. doi: 10.1002/rcm.6671. [DOI] [PubMed] [Google Scholar]
- 11.Nieuweboer AJ, Hu S, Gui C, Hagenbuch B, Ghobadi Moghaddam-Helmantel IM, Gibson AA, et al. Influence of drug formulation on OATP1B-mediated transport of paclitaxel. Cancer Res. 2014;74(11):3137–45. doi: 10.1158/0008-5472.CAN-13-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liu J, Zhang J, Wang L, Chan J, Wang H, et al. A cell-based pharmacokinetics assay for evaluating tubulin-binding drugs. Int J Med Sci. 2014;11(5):479–87. doi: 10.7150/ijms.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taur JS, DesJardins CS, Schuck EL, Wong YN. Interactions between the chemotherapeutic agent eribulin mesylate (E7389) and P-glycoprotein in CF-1 abcb1a-deficient mice and Caco-2 cells. Xenobiotica. 2011;41(4):320–6. doi: 10.3109/00498254.2010.542256. [DOI] [PubMed] [Google Scholar]
- 14.Gallo JM, Li S, Guo P, Reed K, Ma J. The effect of P-glycoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res. 2003;63(16):5114–7. [PubMed] [Google Scholar]
- 15.Mortier KA, Renard V, Verstraete AG, Van Gussem A, Van Belle S, Lambert WE. Development and validation of a liquid chromatography-tandem mass spectrometry assay for the quantification of docetaxel and paclitaxel in human plasma and oral fluid. Anal Chem. 2005;77(14):4677–83. doi: 10.1021/ac0500941. [DOI] [PubMed] [Google Scholar]
- 16.Desjardins C, Saxton P, Lu SX, Li X, Rowbottom C, Wong YN. A high-performance liquid chromatography-tandem mass spectrometry method for the clinical combination study of carboplatin and anti-tumor agent eribulin mesylate (E7389) in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):373–82. doi: 10.1016/j.jchromb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Xu XS, Zeng J, Mylott W, Arnold M, Waltrip J, Iacono L, et al. Liquid chromatography and tandem mass spectrometry for the quantitative determination of ixabepilone (BMS-247550, Ixempra) in human plasma: method validation, overcoming curve splitting issues and eliminating chromatographic interferences from degradants. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(5–6):525–37. doi: 10.1016/j.jchromb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Wozniak KM, Wu Y, Farah MH, Littlefield BA, Nomoto K, Slusher BS. Neuropathy-inducing effects of eribulin mesylate versus paclitaxel in mice with preexisting neuropathy. Neurotox Res. 2013;24(3):338–44. doi: 10.1007/s12640-013-9394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peereboom DM, Supko JG, Carson KA, Batchelor T, Phuphanich S, Lesser G, et al. A phase I/II trial and pharmacokinetic study of ixabepilone in adult patients with recurrent high-grade gliomas. J Neurooncol. 2010;100(2):261–8. doi: 10.1007/s11060-010-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen A, Warren DJ, Brunsvig PF, Aamdal S, Kristensen GB, Olsen H. High sensitivity assays for docetaxel and paclitaxel in plasma using solid-phase extraction and high-performance liquid chromatography with UV detection. BMC Clin Pharmacol. 2006;6:2. doi: 10.1186/1472-6904-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, et al. A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res. 2009;15(12):4207–12. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 22.Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, et al. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 2000;21(3):389–93. [PubMed] [Google Scholar]
- 23.Au JL, Li D, Gan Y, Gao X, Johnson AL, Johnston J, et al. Pharmacodynamics of immediate and delayed effects of paclitaxel: role of slow apoptosis and intracellular drug retention. Cancer Res. 1998;58(10):2141–8. [PubMed] [Google Scholar]
- 24.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17(5):735–49. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 25.Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology. 2014;76(Pt A):175–83. doi: 10.1016/j.neuropharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 27.Sprowl JA, Sparreboom A. Uptake carriers and oncology drug safety. Drug Metab Dispos. 2014;42(4):611–22. doi: 10.1124/dmd.113.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sissung TM, Reece KM, Spencer S, Figg WD. Contribution of the OATP1B subfamily to cancer biology and treatment. Clin Pharmacol Ther. 2012;92(5):658–60. doi: 10.1038/clpt.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuh HJ, Jang SH, Wientjes MG, Au JL. Computational model of intracellular pharmacokinetics of paclitaxel. J Pharmacol Exp Ther. 2000;293(3):761–70. [PubMed] [Google Scholar]
- 30.Fransson MN, Brugard J, Aronsson P, Green H. Semi-physiologically based pharmacokinetic modeling of paclitaxel metabolism and in silico-based study of the dynamic sensitivities in pathway kinetics. Eur J Pharm Sci. 2012;47(4):759–67. doi: 10.1016/j.ejps.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Jang SH, Wientjes MG, Au JL. Kinetics of P-glycoprotein-mediated efflux of paclitaxel. J Pharmacol Exp Ther. 2001;298(3):1236–42. [PubMed] [Google Scholar]
- 32.Vahdat L. Ixabepilone: a novel antineoplastic agent with low susceptibility to multiple tumor resistance mechanisms. Oncologist. 2008;13(3):214–21. doi: 10.1634/theoncologist.2007-0167. [DOI] [PubMed] [Google Scholar]
- 33.Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086–95. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 34.LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–9. doi: 10.1016/j.neuro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Rourke B, Yang CP, Sharp D, Horwitz SB. Eribulin disrupts EB1-microtubule plus-tip complex formation. Cell Cycle. 2014;13(20):3218–21. doi: 10.4161/15384101.2014.950143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang SH, Wientjes MG, Au JL. Interdependent effect of P-glycoprotein-mediated drug efflux and intracellular drug binding on intracellular paclitaxel pharmacokinetics: application of computational modeling. J Pharmacol Exp Ther. 2003;304(2):773–80. doi: 10.1124/jpet.102.044172. [DOI] [PubMed] [Google Scholar]
- 37.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–50. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 38.Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, et al. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst. 1995;87(14):1077–81. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- 39.Narayan S, Carlson EM, Cheng H, Condon K, Du H, Eckley S, et al. Novel second generation analogs of eribulin. Part III: Blood-brain barrier permeability and in vivo activity in a brain tumor model. Bioorg Med Chem Lett. 2011;21(6):1639–43. doi: 10.1016/j.bmcl.2011.01.096. [DOI] [PubMed] [Google Scholar]
- 40.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110(9):1309–18. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, et al. Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct. 2007;32(2):139–47. doi: 10.1247/csf.07015. [DOI] [PubMed] [Google Scholar]
- 42.Yosef N, Ubogu EE. An immortalized human blood-nerve barrier endothelial cell line for in vitro permeability studies. Cell Mol Neurobiol. 2013;33(2):175–86. doi: 10.1007/s10571-012-9882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105(1):146–53. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Benbow SJ, Cook BM, Reifert J, Wozniak KM, Slusher BS, Littlefield BA, et al. Effects of Paclitaxel and Eribulin in Mouse Sciatic Nerve: A Microtubule-Based Rationale for the Differential Induction of Chemotherapy-Induced Peripheral Neuropathy. Neurotox Res. 2015 doi: 10.1007/s12640-015-9580-6. [DOI] [PubMed] [Google Scholar]
- 45.Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6(4):620–9. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez EA. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8(8):2086–95. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- 47.Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49(6):1331–7. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17(3):1061–70. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]