Abstract
Adeno-associated virus (AAV) vector-based gene therapy is a promising treatment strategy for delivery of neurotrophic transgenes to retinal ganglion cells (RGCs) in glaucoma patients. Retinal distribution of transgene expression following intravitreal injection (IVT) of AAV is variable in animal models and the vitreous humor may represent a barrier to initial vector penetration. The primary goal of our study was to investigate the effect of prior core vitrectomy with posterior hyaloid membrane peeling on pattern and efficiency of transduction of a capsid amino acid substituted AAV2 vector, carrying the green fluorescent protein (GFP) reporter transgene following IVT in dogs. When progressive intraocular inflammation developed starting 4 weeks post IVT, the study plan was modified to allow detailed characterization of the etiology as a secondary goal. Unexpectedly, surgical vitrectomy was found to significantly limit transduction, whereas in non-vitrectomized eyes transduction efficiency reached upwards to 37.3% of RGC layer cells. The developing retinitis was characterized by mononuclear cell infiltrates resulting from a delayed-type hypersensitivity reaction, which we suspect was directed at the GFP transgene. Our results, in a canine large animal model, support caution when considering surgical vitrectomy before IVT for retinal gene therapy in patients, as prior vitrectomy appears to significantly reduce transduction efficiency and may predispose the patient to development of vector-induced immune reactions.
INTRODUCTION
Neuroprotective strategies are a critical focus in the development of novel therapeutic interventions to prevent retinal ganglion cell (RGC) loss secondary to glaucoma.1 Direct supplementation of exogenous neurotrophic factors via intravitreal injection (IVT) results in RGC preservation in rodent glaucoma models. Unfortunately, these effects are short-lived, typically persisting less than 2 weeks.2–5 Although significant advancement has been made in sustained-release device technology, the steady-state delivery of neurotrophic support remains limited to between 6 months and 3 years.6 As an alternative to sustained-release devices, adeno-associated virus (AAV)-mediated gene therapy appears promising. Since AAV vectors can stably transduce post-mitotic inner retinal cells, long-term transgene expression resulting in a lifetime therapeutic effect becomes a theoretical possibility.7 Evaluation of IVT-delivered AAV vectors in small animal models has demonstrated rescue of RGCs following axonal insult; however, this effect appears to be variable by retinal region.8 AAV delivered via IVT in large animal models also produces variable RGC transduction between retinal regions.9–11 We recently reported a similar finding when using photoreceptor-targeted AAV vectors in dogs, where retinal tissue penetrating ability was significantly inhibited over the temporal retina.12 Development of a method that generates consistent, widespread retinal transduction is pivotal to the advancement of AAV gene therapy in management of glaucoma as well as outer retinal diseases.
Immune reactions following the administration of viral vectors reduce therapeutic benefits while simultaneously inflicting damage on transduced cells and harming the patient.13 The eye has long been recognized as an immune-privileged site, limiting the development of immune reactions following both subretinal and IVT delivery.14 However, reports of immune reactions in animal models following intraocular delivery have become more frequent as dosing strategies are modified to employ more efficient, engineered AAV vectors with escalation of administration titers.11,15,16 When an unexpected immune reaction occurs, it is paramount to determine whether it is directed against vector capsid or the expressed transgene. Animal models appear to be resistant to the development of cell-mediated capsid reactions; however, transgene reactions have been described.13,17 Risk of these reactions is significantly increased with gene therapy for diseases caused by recessive null mutations, since a novel transgene is expressed to which the host immune system has never been exposed.18
Core vitrectomy is routinely used in human patients before subretinal injection of AAV vectors, which provides space for the advancing subretinal bleb and limits elevation of intraocular pressure immediately post injection.19 It is also commonly used in management of retinal detachments and proliferative diabetic retinopathy. A primary aim of our study was to evaluate the effect of vitrectomy on the regional distribution of transduction following IVT of AAV in dogs, as well as a qualitative assessment of its effect on retinal penetration ability. A secondary aim of our study was to characterize the efficiency of AAV capsid subtype 2 with three capsid tyrosine-to-phenylalanine substitutions and one threonine-to-valine substitution [AAV2 (triple Y-F+T-V)], carrying the green fluorescent protein (GFP) reporter transgene driven by the constitutive chicken-β-actin promoter, for transduction of RGCs in the dog model. AAV2 (triple Y-F) has been previously shown to increase transduction efficiency in RGCs >30-fold when compared with wild-type AAV2 in mice.20 A preliminary dose-range finding and safety study in our laboratory suggested that AAV2 (triple Y-F+T-V) is more efficient than AAV2 (triple Y-F) following IVT in dogs, and we hypothesized that core vitrectomy with posterior hyaloid membrane peeling may further potentiate this transduction efficiency. When a marked intraocular, presumed immune reaction occurred part way through the study, focus was redirected toward characterization of the reaction, including procedural and/or vector-induced variables responsible for the immune response.
RESULTS AND DISCUSSION
Preliminary safety and dose-range finding study
The vector dose used in our study was selected based on results from a preliminary dose-range finding and safety pilot study utilizing the same vector (unpublished data). In the pilot study, eight normal dog eyes were administered either AAV2 (triple Y-F+T-V) or AAV2 (triple Y-F), both utilizing chicken-β-actin promoter to drive GFP expression. Four eyes received IVT of 2.6 × 1011 vector genomes and had higher transduction rates compared with four eyes administered a total of 2.6 × 1010 vector genomes. AAV2 (triple Y-F+T-V) transduced cells within the GCL, our intended cellular target, more efficiently than AAV2 (triple Y-F) (Supplementary Table 1). Aside from expected mild post-operative intraocular inflammation, characterized by grade 1 out of four aqueous humor flare (Supplementary Table 2) in all eyes, which cleared within 48 h post IVT, no adverse effects were noted in any of the eight eyes. On the basis of these findings, AAV2 (triple Y-F+T-V) at a dose of 2.6 × 1011 vector genomes was selected for the study described below.
Ophthalmic examinations and GFP fluorescence
Core vitrectomy with posterior hyaloid membrane peeling was performed in the right eye of three dogs, 4 weeks before vector injection. Twelve days after vitrectomy, Dog 1 developed endophthalmitis of the right eye (grade 2/4 aqueous humor flare (Supplementary Table 2), miosis and vitreal cloudiness) and was treated with systemic corticosteroids and antibiotics. The endophthalmitis resolved within 1 week. Regional retinal thinning, evidenced by patchy tapetal hyperreflectivity, was the only abnormality present on ophthalmic examination at the time of vector injection. AAV2 (triple Y-F+T-V) was delivered via IVT into both vitrectomized and non-vitrectomized eyes of all three dogs immediately adjacent to the retinal surface along the visual streak. A total of 2.6 × 1011 vector genomes were injected in a volume of 200 µl. During the first 4 weeks post IVT, all eyes were normal aside from expected post-procedural mild anterior uveitis (grade 1/4 aqueous humor flare) at examination 24 h following injection, with resolution by 48 h.
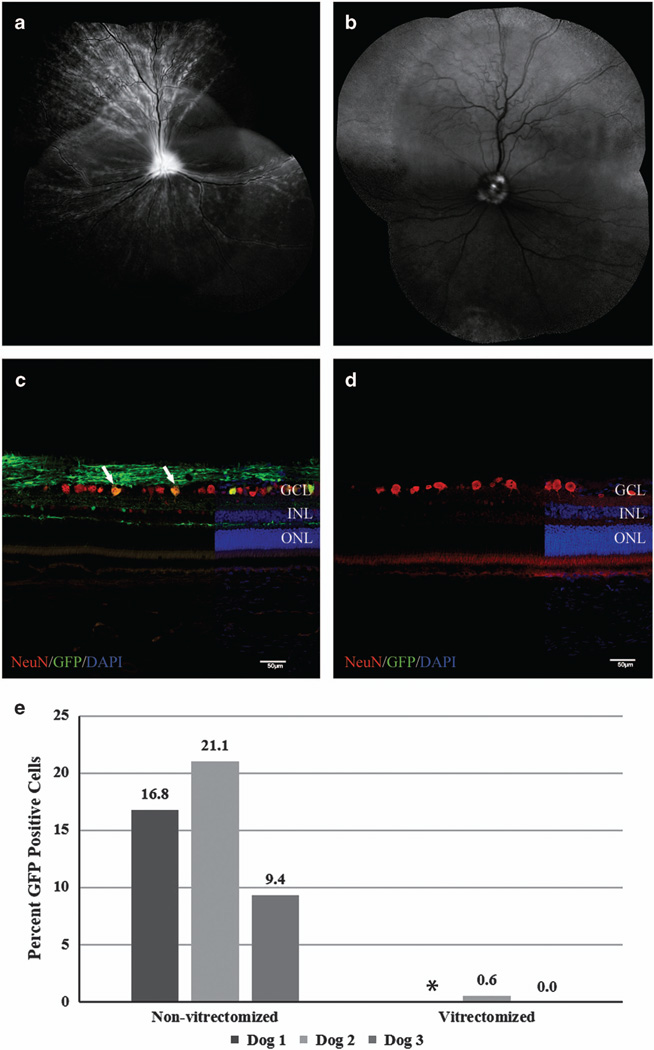
At 2 weeks post IVT, confocal scanning laser ophthalmoscopy showed widespread increased GFP fluorescence above background tapetal autofluorescence in the non-vitrectomized eye of all three dogs. The multifocal pattern with curvilinear streaks coursing toward the optic nerve head suggested the fluorescence was mostly resultant from RGC and nerve fiber layer expression of GFP (Figure 1a). Subjectively, the intensity and distribution of retinal fluorescence increased in the non-vitrectomized eye of all three dogs as the study progressed. The vitrectomized eyes of Dogs 2 and 3 were grossly devoid of retinal GFP fluorescence apart from focal fluorescence involving the optic nerve head beginning at 2 weeks post IVT (Figure 1b). Dog 1 showed weak multifocal GFP fluorescence in the inferotemporal fundus region and adjacent to the retinal vasculature in the vitrectomized eye, which had previously been affected by endophthalmitis following vitrectomy.
Figure 1.
Comparison of ganglion cell layer transduction efficiency of non-vitrectomized and vitrectomized eyes following IVT of AAV2 (triple Y-F+T-V). Representative confocal scanning laser ophthalmoscopy images (a, b) obtained at 5 weeks post injection demonstrate widespread fluorescence in non-vitrectomized eye (a) versus fluorescence limited to the optic nerve head of the vitrectomized eye. (b) The low level of background autofluorescence in the superior retina of image (b) is the expected appearance of the canine tapetum. Immunohistochemical labeling of retinal cryosections with NeuN antibody (c, d) demonstrating a higher number of cells colabeling with GFP in non-vitrectomized eye (c, white arrows) compared with absent transduction in vitrectomized eye (d). (e) Overall ganglion cell layer transduction efficiency for non-vitrectomized eyes and vitrectomized eyes 6 weeks post IVT. Non-vitrectomized eyes had significantly higher levels of ganglion cell layer transduction (P=0.04; unpaired t-test). DAPI, 4',6-diamidino-2-phenylindole; GCL, ganglion cell layer; GFP, green fluorescent protein; INL, inner nuclear layer; IVT, intravitreal injection; ONL, outer nuclear layer. Scale bar, 50 µm. *There is no transduction efficiency calculation for the vitrectomized eye of Dog 1 due to loss of sample for histopathological analysis.
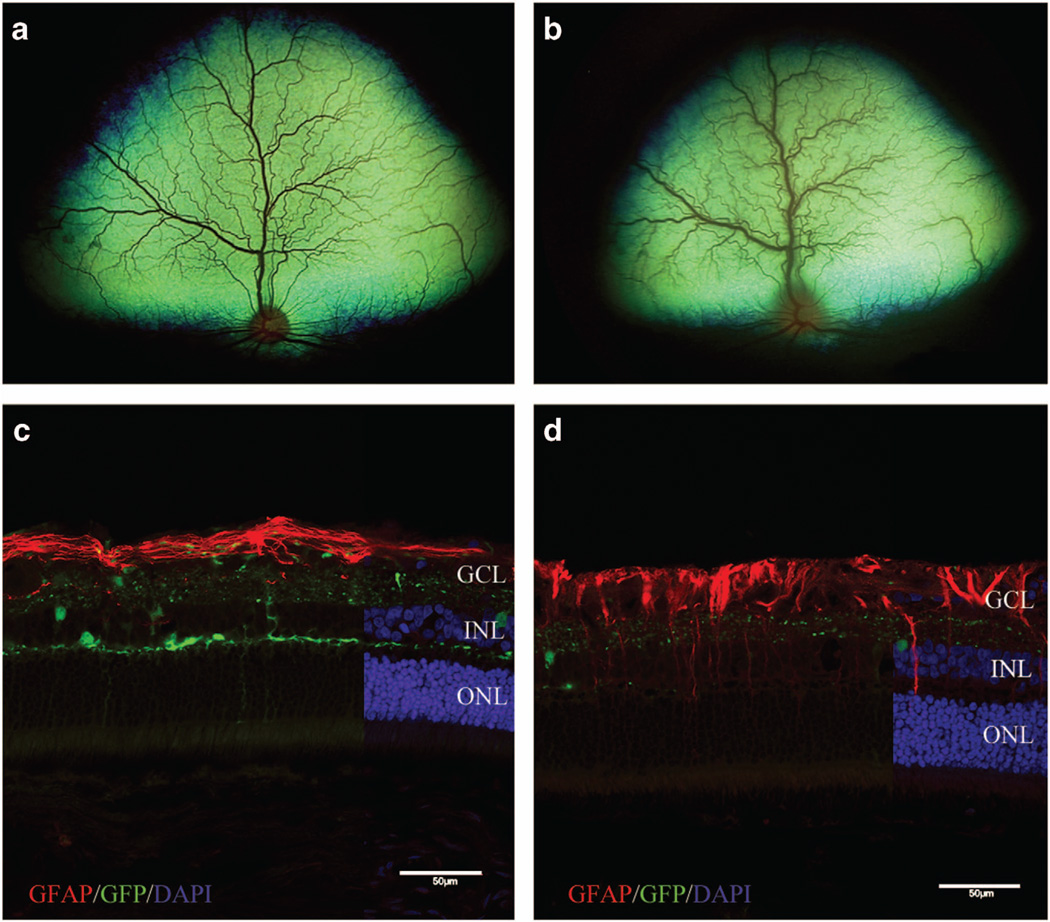
At 4 weeks post IVT, Dog 3 developed acute bilateral endophthalmitis (grade 3/4 aqueous flare, miosis and vitreal cloudiness) and was treated with topical and systemic corticosteroids and systemic antibiotics. The inflammation improved within 1 week but did not fully resolve, with vitreal cloudiness persisting. It was unclear what caused this inflammation; the acute and bilateral nature of the inflammation was suggestive of an immune reaction to the AAV capsid or transgene product; however, a lack of inflammatory signs in the other two dogs precluded the omission of inflammation or infection secondary to the IVT surgical procedure as a possible etiology, so the study was continued. At 6 weeks post IVT, Dog 1 developed acute endophthalmitis in the vitrectomized eye (grade 3/4 aqueous flare, miosis and vitreal cloudiness), and retinitis (diffuse tapetal hyporeflectivity and increased vessel tortuosity) in the non-vitrectomized eye (Figure 2b). Timing of the inflammatory reactions observed at 4 and 6 weeks post IVT is similar to that reported for both capsid and transgene-specific cytotoxic T-lymphocyte reactions in humans and large animal models.13,21 This led us to hypothesize the inflammation was vector-induced, and a decision was made to terminate the study to collect samples critical for definitive determination of the etiology and to preserve retinal tissues necessary for evaluation of vector transduction distribution and efficiency. Dog 1 was killed immediately because of the rapidly progressive inflammation, and Dogs 2 and 3 were killed the following day. Unfortunately, the abrupt decision to kill Dog 1 for humane reasons before planning assays for characterization of the immune reaction and vector biodistribution precluded collection of some samples from this animal. Aside from the described intraocular inflammation, all three dogs were systemically healthy throughout the course of the study.
Figure 2.
White-light fundoscopic imaging showing the normal fundus of the non-vitrectomized eye of Dog 1 at 4 weeks post IVT (a) and acute retinitis that developed during week 6 (b). Fluorescent microscopic images of retinas from non-vitrectomized eyes labeled with GFAP shown in c and d to demonstrate glial activation of Müller cells as a result of inflammation. (c) Retina from Dog 2 without gross evidence of inflammation, showing expected strong GFAP labeling limited predominantly to the nerve fiber layer. (d) Retina from Dog 3 with chronic inflammation, showing GFAP labeling extending from the nerve fiber layer into the inner and outer retinal layers. DAPI, 4',6-diamidino-2-phenylindole; GCL, ganglion cell layer; GFP, green fluorescent protein; INL, inner nuclear layer; IVT, intravitreal injection; ONL, outer nuclear layer. Scale bar, 50 µm.
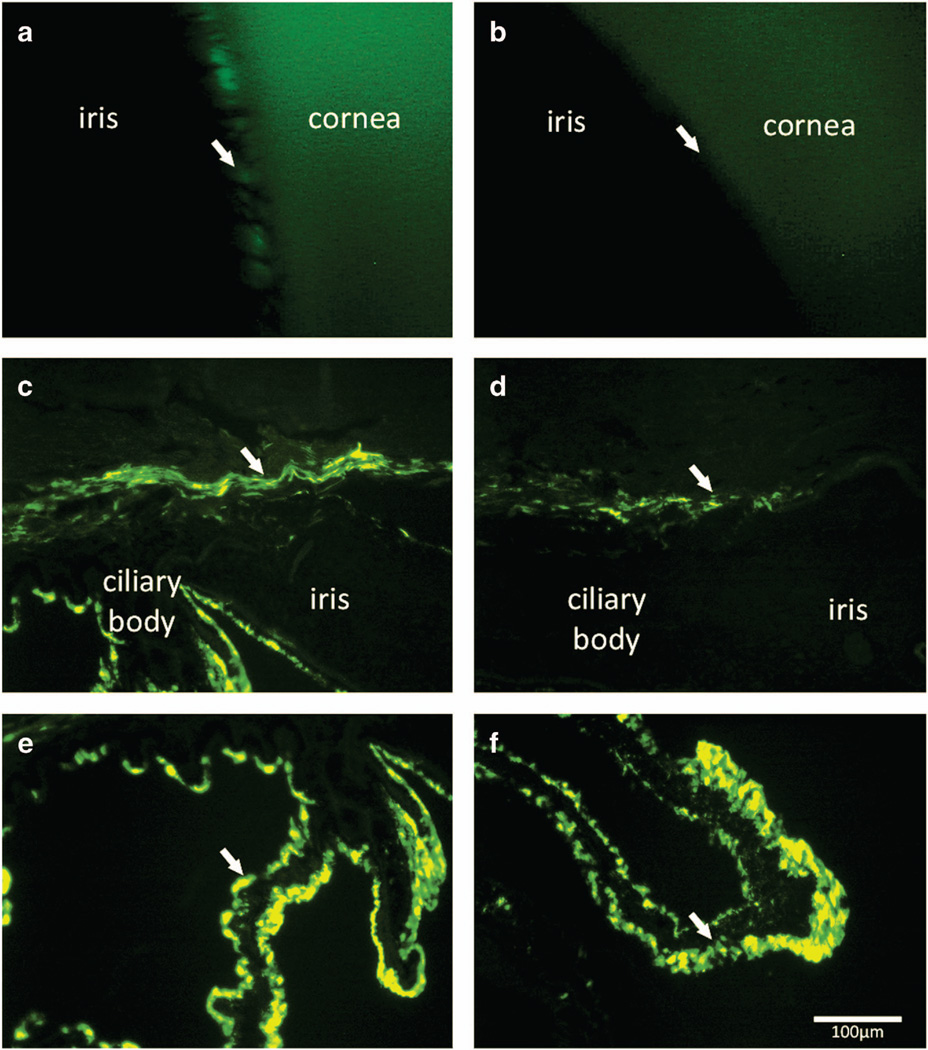
Gonioscopic imaging (488 nm blue light) was performed on Dog 2 at 6 weeks post IVT. Fluorescence was observed within the ciliary cleft of the vitrectomized eye, while the non-vitrectomized eye did not have appreciable fluorescence (Figures 3a and b). At the time of eyecup collection six weeks post IVT, the cornea, iris and ciliary body from Dogs 2 and 3 were also collected and cryosections were prepared. Both vitrectomized and non-vitrectomized eyes expressed GFP within the ciliary body (Figures 3e and f). Vitrectomized eyes had subjectively increased GFP expression within the iris and trabecular meshwork (Figures 3c and d).
Figure 3.
Fluorescent gonioscopic imaging showing fluorescence within the ciliary cleft of vitrectomized eye of Dog 2 at 6 weeks post IVT (a, arrow). Note the pectinate ligament fibrils demonstrated by the dark bands crossing the region of fluorescence. Non-vitrectomized eye of Dog 2 shows no appreciable fluorescence within the cliary cleft (b, arrow). Immunohistochemical labeling of cryosections of the anterior segment of vitrectomized eye (c, e) showed GFP expression within the trabecular meshwork outflow pathway (c, arrow), and expression within the ciliary body epithelium (e, arrow). The anterior segment sections from non-vitrectomized eye (d, f) showed less prominent GFP expression within the trabecular meshwork outflow pathway (d, arrow), but comparable expression within the ciliary body epithelium (f, arrow). GFP, green fluorescent protein; IVT, intravitreal injection. Scale bar, 100 µm.
The lack of appreciable retinal GFP expression in vitrectomized eyes was unexpected. We originally hypothesized that vector solution injected within an intact vitreous humor could be sequestered by the gel-like collagen matrix, preventing widespread exposure of the retina. Our results suggest that the intact vitreous humor is actually beneficial for retinal exposure to the vector solution. Flow of aqueous humor within the posterior segment of an eye with intact vitreous humor is in a posterior direction (from the hyaloid membrane toward the retina).22 We now postulate that loss of the posterior vitreous humor disrupts this flow and creates increased reflux of vector solution across the anterior hyaloid membrane into the anterior chamber. This is supported by the increased level of GFP expression within anterior segment structures in vitrectomized eyes (Figure 3).
AAV vector retinal transduction efficiency and tropism
At 6 weeks post IVT, sagittal eyecup cryosections were taken from all eyes through the optic nerve head as well as 2–4 mm nasal and temporal of the optic nerve head. Quantification of cells expressing GFP within the RGC layer (GCL) revealed vitrectomized eyes had either very limited or absent GFP expression (Figure 1d). Non-vitrectomized eyes showed an overall mean transduction rate of 15.7 ± 5.9% of cells within the GCL (Figure 1c). The transduction rate difference between vitrectomized eyes and non-vitrectomized eyes was significant (P = 0.04). Variation in GCL transduction rates was observed between sagittal retinal regions, with a mean of 20.8 ± 4.5% and 21.8 ± 14.7% cells transduced in the nasal and central sagittal planes, respectively, versus 4.6 ± 4.6% cells transduced in the temporal sagittal plane (Supplementary Figure 1). This difference was not statistically significant (P = 0.13). Peak transduction rates were as high as 25.8% in the nasal plane and 37.3% in the central plane. We recently reported a similar pattern following IVT of photoreceptor-specific AAV vectors in dogs.12 Transduction occurred within all other retinal cellular layers predominantly in regions underneath the retinal vasculature in non-vitrectomized eyes, with both rod and cone photoreceptors as well as rod bipolar cells expressing GFP (Supplementary Figure 2). This pattern of increased AAV penetration at the site of retinal vessels has been described previously following IVT.10,12 These retinal cell populations are important targets for retinal gene therapy.
Direct comparison of our GCL transduction efficiency results to other reports utilizing IVT of AAV vectors in large animal models is challenging, as quantification of GCL transduction rate has not been previously reported. By subjectively comparing our in vivo imaging to images published from other studies, our results appear to suggest AAV2 (triple Y-F+T-V) generates higher GCL transduction compared with both wild-type AAV2 and AAV2 (quad Y-F) vectors previously used in dogs and primates.9–11 Optimization of capsid amino acid substitutions has been shown to decrease intracellular proteasomal degradation of AAV vectors.23,24 Confocal scanning laser ophthalmoscopy images from non-vitrectomized eyes in our study seem to demonstrate comparable levels of in vivo GFP expression to the novel 7m8 vector used in primates as reported by Dalkara et al.,11 although cross-species comparisons should be interpreted with caution.
Microscopic evaluation of retinitis
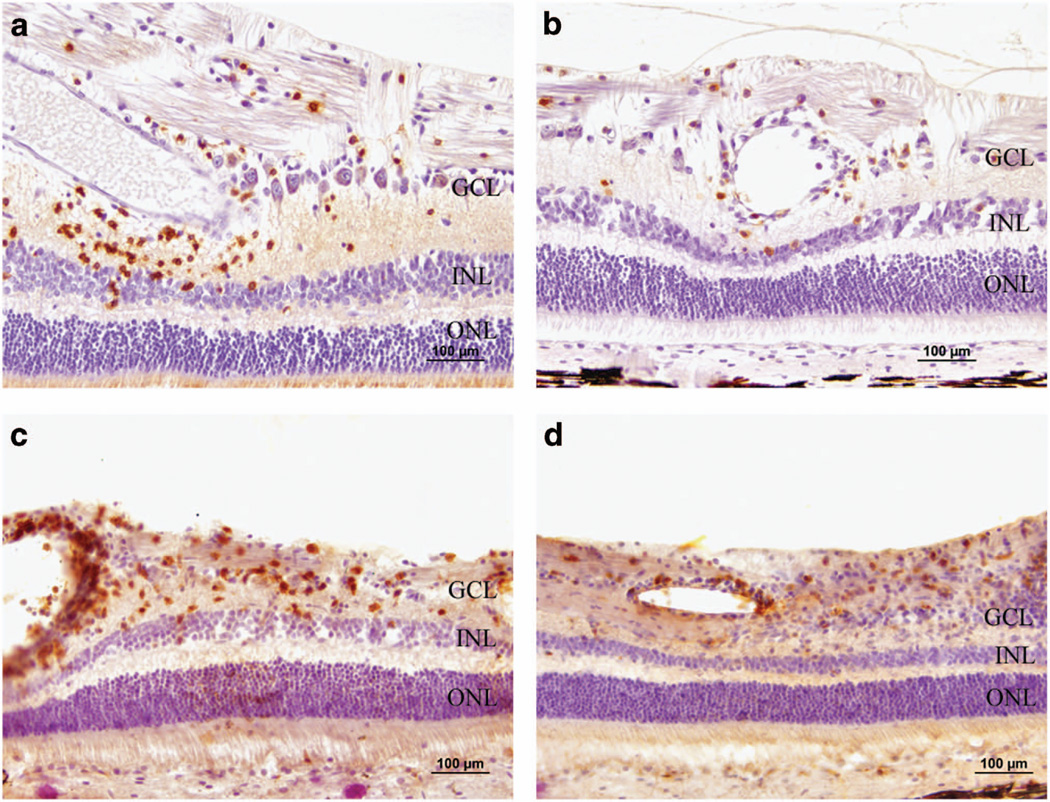
The vitrectomized eye of Dog 1 was fixed in Bouin’s solution and submitted for histopathological evaluation. Hematoxylin and eosin-stained retinal sections revealed that the clinically acute retinitis was characterized by mononuclear inflammatory cells, suggestive of an immune-mediated reaction as opposed to an infectious etiology. Retinal sections were labeled with cluster of differentiation (CD) 3 and 20 antibodies to identify T- and B-lymphocytes, respectively. Mononuclear inflammatory cells within the retina labeled positive with both CD3 and CD20 antibodies, indicating a heterogeneous lymphocytic inflammatory response (Figures 4a and b). Paraformaldehyde-fixed retinal cryosections from the non-vitrectomized eye of Dog 1 and both eyes of Dogs 2 and 3 were labeled with CD4 and CD8 antibodies to differentiate between T-helper and cytotoxic T-lymphocytes, respectively. Dog 2 did not have any labeled cells. Positive labeling for both antibodies was observed in both eyes of Dog 3 (Figures 4c and d), as well as the non-vitrectomized eye of Dog 1. Subjectively, CD-positive inflammatory cells were more abundant within the retina of the non-vitrectomized eye of Dog 3 compared with the vitrectomized eye. The vitrectomized eye of Dog 1 could not be labeled with CD4 and CD8 because of the use of Bouin’s fixative. Labeling of retinal cryosections with glial fibrillary acidic protein antibody showed increased expression deep to the nerve fiber layer in Dog 3 compared with Dog 2, indicating glial transformation of retinal Müller cells secondary to chronic retinal inflammation (Figures 2c and d).
Figure 4.
Photomicrographs characterizing the cellular component of the retinal inflammatory response from vitrectomized eye of Dog 1 (a, b) and non-vitrectomized eye of Dog 3 (c, d) through the use of CD labeling. Positive immunolabeling of mononuclear inflammatory cells with CD20 (a) and CD3 (b) demonstrates a mixed response involving both B cells and T cells, respectively. Positive immunolabeling of inflammatory cells with CD4 (c) and CD8 (d) demonstrates that both T-helper and cytotoxic T cells, respectively, were present. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 100 µm.
Collectively, these results are consistent with a delayed-type hypersensitivity immune reaction, similar to that reported following systemic and intraocular AAV delivery in large animal models.13,25 Presence of both CD4+ and CD8+ T-lymphocytes as well as B-lymphocytes within the inflamed retinas is reminiscent of the hepatic inflammatory cell profile described by Gao et al.13 following intraportal injection of AAV7 vectors driving GFP expression in primates. AAV2 and AAV8 vectors driving GFP expression are capable of producing a dose-dependent adaptive immune response following subretinal delivery in primates.25 This not only leads to suppression of transgene expression but also destruction of the affected retinal tissues.11,25 The timing of the reaction in our study is slightly later than described following intraportal injection of AAV, but much earlier than that reported following subretinal injection or IVT in non-vitrectomized eyes.11,13,25
Immune response assays
A neutralizing antibody (NAb) assay was performed four weeks post IVT to detect humoral responses directed against AAV2 vector. A robust NAb response occurred in all dogs. Reciprocal serum dilutions required to achieve <50% neutralization of AAV transduction measured between 20 480 and 81 920 in Dogs 2 and 3, and >81 920 (the highest dilution tested) in Dog 1. Serum from a naive animal housed in the same vivarium tested <5 (the lowest dilution tested). The generation of a humoral immune response is expected following IVT. A study in mice demonstrated IVT generates capsid-specific NAb capable of suppressing transgene expression when the same vector was injected via IVT into the contralateral eye.26
Peripheral blood mononuclear cells (PBMCs) were isolated from Dogs 2 and 3 at 6 weeks post IVT and challenged against vector-specific antigens. PBMCs from Dog 2 exhibited a robust stimulatory response to GFP antigen, but not to AAV2 (triple Y-F) capsid antigen (Table 1). The reactivity of PBMCs from Dog 2 to GFP is consistent with previous studies where subretinal, intraportal and intravenous AAV injection in primates and dogs generated an adaptive immune response to GFP.13,25,27 Capsid-directed T-lymphocyte responses were not detected in any of these studies. The presence of GFP-primed PBMCs in Dog 2 suggests that a T-lymphocyte inflammatory reaction would have eventually developed in this animal.13 Dog 3 did not generate a significant stimulatory response to either test antigen or the control antigen Candida albicans. We suspect this is an effect of immunosuppressive corticosteroid treatment (oral prednisone 1 mg kg−1 day−1) it received for bilateral endophthalmitis for 2 weeks before PBMC collection. The PBMC results from Dog 2 are highly suggestive of a GFP transgene-specific T-lymphocyte response; however, a direct link cannot be definitively made between this response and the immune reactions seen in Dogs 1 and 3 due to absence of a PBMC sample from Dog 1. Future experiments in our laboratory will include PBMC collections in all animals before and serially following AAV injection, in order to definitively characterize any immune responses that may occur.
Table 1.
PBMC stimulation index following challenge with vector-derived antigens
| Subject | Positive control antigens | Vector capsid | Transgene | |
|---|---|---|---|---|
| PHA | Candida albicans | AAV2 | GFP | |
| Dog 2 | 80.55 | 19.67 | 0.61 | 4.60 |
| Dog 3a | 21.36 | 1.81 | 1.35 | 0.84 |
Abbreviations: AAV, adeno-associated virus; GFP, green fluorescent protein; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin.
Dog 3 had received 2 weeks of immunosuppressive prednisone therapy before PBMC collection.
The bold values in the table represent positive result >2.
The eye is considered an immune-privileged site, capable of generating an immune deviant response to intraocular antigens, although this process can be overcome by inflammation or a strong antigen.28–31 We suspect the vitrectomy procedure greatly increased the amount of AAV vector transiting into the anterior chamber and aqueous humor outflow pathways, resulting in significant transduction of off-target cell populations and exposure of GFP antigen to the systemic immune system. AAV is unable to efficiently transduce antigen presenting cells (APCs); therefore, GFP antigens must be exogenously taken up by APCs in order to be presented for T-lymphocyte activation, a process known as ‘cross-presentation’.17 The increased level of immunogenic GFP antigen within the anterior segment of vitrectomized eyes likely overwhelmed existing immune-deviation mechanisms, resulting in a delayed-type hypersensitivity response instead of immune tolerance.28 A lack of response to capsid antigen may have resulted from evasion of proteasomal degradation due to the presence of Y-F and T-V substitutions, decreasing the amount of capsid antigen available for cross-presentation.
Biodistribution of AAV vector
Ocular and non-ocular tissue samples were collected from Dogs 2 and 3 for quantitative PCR biodistribution analysis of AAV vector genomes (Table 2). AAV vector genomes were present in the aqueous humor and optic nerves of vitrectomized and non-vitrectomized eyes. A much greater quantity of vector genomes was present in the aqueous humor of the non-vitrectomized eye of Dog 2 compared with the vitrectomized eye. We suspect this indicates a slow ‘extended release’ phenomenon, where the intact vitreous sequesters the AAV vectors, resulting in a much slower clearance of vector across the anterior hyaloid membrane. The vitrectomized eye, however, may have had more rapid clearance of vector into the aqueous humor post IVT, and the lower number of genomes in the fluid aqueous humor present 6 weeks later represents a steady-state level within the eye, with most of vector already within anterior uveal tissues or filtered out of aqueous drainage pathways. This theory is supported by the increased level of GFP expression within the anterior uveal tissues and trabecular meshwork of the vitrectomized eyes (Figure 3). To explore this theory, future studies in our laboratory will include serial aqueous humor samples following IVT of AAV vectors, as well as the collection of the ciliary body and trabecular meshwork tissues, for quantitative PCR analysis.
Table 2.
Biodistribution of AAV genomes determined by qPCR (number of vector genomes per µg DNA
| Tissue | Dog 2 | Dog 3 |
|---|---|---|
| Aqueous humor OD | 54 275 | 2505 |
| Aqueous humor OS | 6 220 225 | 1525 |
| Optic nerve OD | 90 | 145 |
| Optic nerve OS | 710 | 10 |
| Optic chiasm | 31 | 52 |
| Cerebrum | 0 | 0 |
| Cerebellum | 0 | 14 |
| Parotid gland | 0 | 0 |
| Submandibular lymph node | 0 | 0 |
| Lung | 0 | 0 |
| Heart | 10 | 0 |
| Liver | 53 | 3 |
| Pancreas | 0 | 0 |
| Spleen | 611 | 581 |
| Kidney | 6 | 19 |
Abbreviations: AAV, adeno-associated virus; OD, right eye; OS, left eye; qPCR, quantitative PCR.
The bold values in the table represent positive results >2.
Quantitative PCR also detected vector DNA in splenic tissue, but not in any other sampled non-ocular tissues. This trafficking of vector genomes to the spleen has been described after intravenous or intraportal injection of AAV.13,27 Following exposure to an intraocular antigen, specialized APCs responsible for immune deviation preferentially migrate to the spleen, where they generate regulatory T-lymphocytes to promote antigen tolerance.32 We suspect this established mechanism is responsible for vector genomes collected within splenic tissue but not in other organs.
Our study had a number of limitations. The unexpected and acute nature of the immune reactions resulted in expedited changes in the study protocol. The immediate killing of Dog 1 due to welfare concerns over the rapidly developing severe intraocular inflammation resulted in a lack of collecting valuable immune assay samples for that animal. Use of Bouin’s solution for fixation of the vitrectomized eye of Dog 1 to achieve better histopathological characterization of the inflammatory response led to an inability to label sections from that eye with CD4 and CD8 antibodies as well as exclusion of the eye from transduction efficiency assessments, which in turn reduced the statistical power. The immunosuppressive corticosteroid treatment of Dog 3 likely inhibited the expected PBMC reactivity to GFP antigen. Therefore, our conclusions regarding the specificity of the immune responses are based on results from a very limited number of animals. In addition, the GFP-specific PBMC result from Dog 2 could have been further characterized using methods such as intracellular cytokine staining and flow cytometry to verify the effector functions of the T-lymphocytes.18
In conclusion, our study demonstrates that core vitrectomy with posterior hyaloid membrane peeling occurring before IVT delivery of AAV vectors does not enhance retinal transduction in the dog, but instead significantly reduces transduction. We also demonstrated that the dog is capable of generating a delayed-type hypersensitivity response to the GFP transgene following IVT of an AAV vector. On the basis of comparison with previous studies in our laboratory in which similar doses of the same vector were used without adverse complications, development of this immune reaction appears to be potentiated by the vitrectomy procedure. Therefore, we urge caution when considering IVT delivery of AAV vectors for retinal gene therapy in patients that have had a prior vitrectomy. Additional studies are warranted to evaluate whether a similar pattern would occur in eyes affected by advanced vitreal syneresis. There was no evidence of a capsid-directed T-lymphocyte response, a finding that is promising for future studies utilizing AAV2 (triple Y-F+T-V), although the absence of a collected PBMC sample from Dog 1 compels us to make this statement with some reservation. The vector was able to transduce inner retinal neurons, including RGCs and bipolar cells, and may be considered for use in future efficacy studies in animal models of inner retinal disease.
MATERIALS AND METHODS
Animals
Three purpose-bred 10-month-old Beagle dogs (Marshall BioResources, North Rose, NY, USA) were used in the study. Power analysis using data from a preliminary pilot study revealed that an N of three dogs would be required to achieve a power of >0.8 when using a paired one-tailed t-test. All dogs were socially housed with a 12-hour light:dark cycle. Animal care was in compliance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research, and all procedures were performed following approval by the Michigan State University Institutional Animal Care and Use Committee.
Vitrectomy
Core vitrectomy with posterior hyaloid membrane peeling was performed in the right eye via three-port pars plana approach in all three dogs 1 month before vector injection. Briefly, three 23-gauge scleral ports (Alcon, Fort Worth, TX, USA) were placed to facilitate infusion, illumination and vitrectomy probe insertion into the vitreous humor. A 23-gauge vitrectomy probe (Alcon Accurus) was used to remove the posterior half of the vitreous humor and replace it with balanced salt solution (BSS, Alcon) under direct visualization through an operating microscope (Zeiss S8, Thornwood, NY, USA) with noncontact, indirect visualization system (Oculus BIOM, Port St Lucie, FL, USA) installed. Microfiltered triamcinolone crystals (Bristol-Myers Squibb, New York, NY, USA) were injected to facilitate visualization and removal of posterior vitreous cortical fibers, followed by placement of the vitrector over the optic nerve head to induce detachment and peeling of the posterior hyaloid membrane.
AAV vector
The recombinant AAV vector construct containing capsid amino acid substitutions was manufactured and purified at the University of Florida College of Medicine using previously described methods.33,34 All AAV vectors underwent testing for endotoxin and were determined to contain <5 EU ml−1. The AAV2 based capsid was mutated by substitution of three surface-exposed capsid tyrosine residues with phenylalanine and one threonine residue with valine (Y444F, Y500F, Y730F and T491V; referred to as AAV2 (triple Y-F+T-V)). This capsid variant has previously been shown to maximally avoid proteasomal degradation with up to 90% of vector genomes reaching the nucleus of infected cells.35 The full-length, ubiquitous chicken-β-actin promoter was used to drive expression of the reporter, GFP.
Intravitreal injections
Vectors were prepared for injection by diluting stock supplies to a titer of 1.3 × 1012 vg ml−1 using sterile BSS. One month after the vitrectomy was performed in the right eye, 200 µl of vector solution was injected into the vitreous humor of both eyes immediately anterior to the retinal surface in a transverse plane along the visual streak using a RetinaJect injector (SurModics, Inc., Eden Prairie, MN, USA) as previously described.10,36 Post-operative treatment included antibacterial and anti-inflammatory medications as previously described.10
Ophthalmic examinations and imaging
Post IVT, all dogs received regular ophthalmic examinations and fluorescence fundus imaging as previously described.10 Confocal scanning laser ophthalmoscopy utilizing 488 nm laser-induced fluorescence (confocal scanning laser ophthalmoscopy, Spectralis; Heidelberg Engineering, Carlsbad, CA, USA) was performed weekly post injection under general anesthesia.
Immune reaction assays
Before killing, serum was collected from all dogs for analysis of NAb titers and whole-blood samples were collected from Dogs 2 and 3 for PBMC isolation. A control serum sample was also obtained from an age-matched AAV-naive, colony dog. NAb titers were determined via NAb assay utilizing ARPE-19 cells and an AAV2 (triple Y-F+T-V)-mCherry vector at 5000 particles per cell, as previously described.37
PBMCs were isolated as previously described,38 then immediately frozen at − 80 °C to maintain cell viability before antigen challenge analysis. Anti-AAV2 (triple Y-F+T-V) and GFP antigen-specific lymphocyte proliferation responses were assessed as previously described.39 Briefly, lymphocytes were cultured and separated into four groups with three (controls) and six (unknowns) cultures per group: unstimulated (as negative control), stimulated with AAV2 (triple Y-F+T-V) (5000, 500 and 50 particles per cell), and stimulated with GFP (10, 1.0 and 0.1 µg ml−1). After 5 days of incubation, the stimulation index was defined as: (mean counts per minute of [3H]thymidine from stimulated cells)/(mean counts per minute of [3H]thymidine from unstimulated cells). On the basis of antigen-specific lymphocyte proliferation response (ASR) results at baseline in other non-ocular studies, stimulation index values greater than 2.0(ref. 40) or 3.0(ref. 39) have been considered significant. The viability of each lymphocyte culture was confirmed by positive controls with mitogen-induced proliferation in response to phytohemagglutinin (PHA) (10, 1.0 and 0.1 µg ml−1) and recall antigen induced proliferation to C. albicans (10, 1.0 and 0.1 µgml−1).
Eyecup collection and sectioning
Following the development of bilateral endophthalmitis in two out of the three dogs, all dogs were killed 6 weeks post IVT with an intravenous injection of sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA). Eyes were immediately enucleated and processed as previously described,41 with the exception of the right eye of Dog 1, which was fixed in Bouin’s solution for histopathological processing to characterize the inflammatory response. Twenty micron-thick sagittal cryosections, three sections per slide, were collected (Leica CM3050-S cryostat, Leica Microsystems, Buffalo Grove, IL, USA) and stored at − 20 °C.
Immunohistochemistry and cell quantification
Details of primary and secondary antibodies used for immunohistochemical labeling can be found in Supplementary Table 3. Bouin’s solution-fixed retinal sections from the vitrectomized eye of Dog 1 were used for labeling with CD 3 and CD 20 antibodies, all other antibodies were used to label paraformaldehyde-fixed sections. Unfortunately, this meant the vitrectomized eye of Dog 1 was excluded from analysis of vector transduction efficiency. Sagittal sections labeled with Neuronal nuclei (NeuN) antibody were used for counting purposes. Identifying information was masked, and NeuN positive cells in the GCL counted at × 20 magnification by a single observer (RFB). Cells co-labeled with NeuN and GFP were also quantified for each section. Representative images were captured using a confocal laser scanning microscope (Olympus FluoView fv1000 Confocal, Center Valley, PA, USA) at × 20 magnification.
AAV biodistribution analysis
Following killing, ocular and non-ocular tissue samples (see Table 2) were collected from Dogs 2 and 3 and frozen at − 80 °C. Ocular and non-ocular tissue biodistribution of chicken-β-actin-GFP vector genomes was determined via quantitative PCR analysis as previously described.42
Statistical analysis
Differences in overall GCL transduction efficiency between vitrectomized (Dogs 2 and 3) and non-vitrectomized eyes (all dogs) were compared using an unpaired Student’s t-test. Differences in GCL transduction efficiency between sagittal regions within non-vitrectomized eyes of all three dogs were compared using analysis of variance (Excel, Microsoft, Redmond, WA, USA). Results were considered significant if P<0.05. Data are displayed as mean± s.d.
Supplementary Material
Acknowledgments
We thank Janice Querbin and Kristin Koehl for animal care assistance, Laurence Occelli for imaging assistance and the laboratory of Lorraine Sordillo for PBMC collection. We also thank Cheryl Craft for hCAR antibody production, Jingfen Sun for performing NAb assays as well as Vince Chiodo and the Retinal Gene Therapy Vector lab for AAV purification. JTB and RFB acknowledge the following funding source: American College of Veterinary Ophthalmologists Vision for Animals Foundation. SLB, SEB, WWH and AMK acknowledge the following funding source: Foundation Fighting Blindness and an unrestricted grant to the UF Department of Ophthalmology from Research to Prevent Blindness. SEB acknowledges the following funding source: NIH Grant R01 EY024280. SEB, SLB and WWH acknowledge funding support from NIH Grant P30EY021721. AMK acknowledges the following funding source: NIH Grant R01 EY019304. SMP-J acknowledges the following funding source: Myers-Dunlap Endowment for Canine Health. WWH acknowledges the following funding sources: NIH grants R01 EY017549, R24 EY022023, and R24 EY022012.
Footnotes
CONFLICT OF INTEREST
WWH and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The remaining authors declare no conflict of interest.
Supplementary Information accompanies this paper on Gene Therapy website (http://www.nature.com/gt)
REFERENCES
- 1.Chader GJ. Advances in glaucoma treatment and management: neurotrophic agents. Invest Ophthalmol Vis Sci. 2012;53:2501–2505. doi: 10.1167/iovs.12-9483n. [DOI] [PubMed] [Google Scholar]
- 2.Hellstrom M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma. 2011;28:2475–2483. doi: 10.1089/neu.2011.1928. [DOI] [PubMed] [Google Scholar]
- 3.van Adel BA, Kostic C, Deglon N, Ball AK, Arsenijevic Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–115. doi: 10.1089/104303403321070801. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011;11:116–131. doi: 10.2174/156652311794940746. [DOI] [PubMed] [Google Scholar]
- 5.Harvey AR, Hellström M, Rodger J. Gene therapy and transplantation in the retinofugal pathway. Prog Brain Res. 2009;175:151–161. doi: 10.1016/S0079-6123(09)17510-6. [DOI] [PubMed] [Google Scholar]
- 6.Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs Aging. 2010;27:117–134. doi: 10.2165/11530970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Wilson AM, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Therapy. 2012;19:127–136. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- 8.Kwong JMK, Gu L, Nassiri N, Bekerman V, Kumar-Singh R, Rhee KD, et al. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene Therapy. 2015;22:138–145. doi: 10.1038/gt.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, et al. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci. 2011;52:2775–2783. doi: 10.1167/iovs.10-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM, et al. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Therapy. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 12.Boyd RF, Sledge DG, Boye SL, Boye SE, Hauswirth WW, Komaromy AM, et al. Photoreceptor-targeted gene delivery using intravitreally administered AAV vectors in dogs. Gene Therapy. 2015;23:223–230. doi: 10.1038/gt.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett K, Bennett J. Immunology of AAV-mediated gene transfer in the eye. Front Immunol. 2013;4:261. doi: 10.3389/fimmu.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minella AL, Mowat FM, Willett KL, Sledge D, Bartoe JT, Bennett J, et al. Differential targeting of feline photoreceptors by recombinant adeno-associated viral vectors: implications for preclinical gene therapy trials. Gene Therapy. 2014;21:913–920. doi: 10.1038/gt.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran WA, Boye SL, Boye SE, Chiodo VA, Lewin AS, Hauswirth WW, et al. rAAV2/5 gene-targeting to rods:dose-dependent efficiency and complications associated with different promoters. Gene Therapy. 2010;17:1162–1174. doi: 10.1038/gt.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JM. Autoimmunity, recessive diseases, and gene replacement therapy. Mol Ther. 2010;18:2045–2047. doi: 10.1038/mt.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Lizak MJ, Tansey G, Csaky KG, Robinson MR, Yuan P, et al. Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann Biomed Eng. 2005;33:150–164. doi: 10.1007/s10439-005-8974-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabriel N, Hareendran S, Sen D, Gadkari RA, Sudha G, Selot R, et al. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Methods. 2013;24:80–93. doi: 10.1089/hgtb.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 27.Bell P, Gao G, Haskins ME, Wang L, Sleeper M, Wang H, et al. Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs. Hum Gene Ther. 2011;22:985–997. doi: 10.1089/hum.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 29.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 30.Ohta K, Yamagami S, Taylor AW, Streilein JW. IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2000;41:2591–2599. [PubMed] [Google Scholar]
- 31.Saban DR, Elder IA, Nguyen CQ, Smith WC, Timmers AM, Grant MB, et al. Characterization of intraocular immunopathology following intracameral inoculation with alloantigen. Mol Vis. 2008;14:615–624. [PMC free article] [PubMed] [Google Scholar]
- 32.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4:130–137. [PubMed] [Google Scholar]
- 33.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 34.Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, et al. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One. 2013;8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One. 2013;8:e59142. doi: 10.1371/journal.pone.0059142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM. Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol. 2010;128:1442–1448. doi: 10.1001/archophthalmol.2010.210. [DOI] [PubMed] [Google Scholar]
- 37.Boye SE, Alexander JJ, Boye SL, Witherspoon CD, Sandefer KJ, Conlon TJ, et al. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther. 2012;23:1101–1115. doi: 10.1089/hum.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Boyle NJ, Contreras GA, Mattmiller SA, Sordillo LM. Changes in glucose transporter expression in monocytes of periparturient dairy cows. J Dairy Sci. 2012;95:5709–5719. doi: 10.3168/jds.2012-5327. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez YJ, Wang J, Kearns WG, Loiler S, Poirier A, Flotte TR. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol. 1999;73:8549–8558. doi: 10.1128/jvi.73.10.8549-8558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- 41.Mowat FM, Breuwer AR, Bartoe JT, Annear MJ, Zhang Z, Smith AJ, et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Therapy. 2012;20:545–555. doi: 10.1038/gt.2012.63. [DOI] [PubMed] [Google Scholar]
- 42.Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013;24:23–28. doi: 10.1089/humc.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.